Abstract
Our translational research group focuses on addressing the problem of exercise defects in diabetes with basic research efforts in cell and rodent models and clinical research efforts in subjects with diabetes mellitus. CREB (cAMP-response-element-binding protein) regulates cellular differentiation of neurons, β-cells, adipocytes and smooth muscle cells; it is also a potent survival factor and an upstream regulator of mitochondrial biogenesis. In diabetes and cardiovascular disease, CREB protein content is decreased in the vascular media, and its regulation in aberrant in β-cells, neurons and cardiomyocytes. Loss of CREB content and function leads to decreased vascular target tissue resilience when exposed to stressors such as metabolic, oxidative or sheer stress. This basic research programme set the stage for our central hypothesis that diabetes-mediated CREB dysfunction predisposes the diabetes disease progression and cardiovascular complications. Our clinical research programme revealed that diabetes mellitus leads to defects in functional exercise capacity. Our group has determined that the defects in exercise correlate with insulin resistance, endothelial dysfunction, decreased cardiac perfusion and diastolic dysfunction, slowed muscle perfusion kinetics, decreased muscle perfusion and slowed oxidative phosphorylation. Combined basic and clinical research has defined the relationship between exercise and vascular function with particular emphasis on how the signalling to CREB and eNOS [endothelial NOS (nitric oxide synthase)] regulates tissue perfusion, mitochondrial dynamics, vascular function and exercise capacity. The present review summarizes our current working hypothesis that restoration of eNOS/NOS dysfunction will restore cellular homoeostasis and permit an optimal tissue response to an exercise training intervention.
Keywords: cAMP-response-element-binding protein (CREB), cardiovascular disease, diabetes, exercise, mitochondrion, nitric oxide synthase (NOS)
Introduction
Diabetes is a leading cause of death and disability worldwide and a leading cause of blindness, end-stage renal disease, non-traumatic amputation and CVD (cardiovascular disease). Interventions to optimize glucose control, blood pressure and lipids dramatically decrease microvascular and macrovascular events, yet excess burden remains. For example, even with optimal risk factor reduction, cardiovascular events and death is 3–5-fold higher in people with diabetes [1]. The goal of the research ongoing in our laboratory is to define novel strategies for reduction of CVD in diabetes. In our larger clinical research groups, we made the concerning observation that people with diabetes have decreased functional exercise capacity [2]. Specifically, adults and adolescents with either Type 2 or Type 1 diabetes have decreased cardiovascular fitness as measured by O2 consumption (V̇o2 max) and slowed oxygen uptake kinetics [2–5]. The decreased V̇o2 peak is associated with excess CVD and all-cause mortality [6,7]. V̇o2 kinetics measures the acceleration of oxygen uptake and consumption at the onset of exercise and, when slowed, represents a defect in submaximal exercise function (relevant for activities of daily living). These defects in functional exercise capacity have implications for day-to-day life and longevity.
Our translational research group focuses on addressing the problem of exercise defects in diabetes with basic research efforts in cell and rodent models and clinical research efforts in subjects with diabetes mellitus. The present review outlines the basic observations that have led to our current working model starting with our observation that the transcription factor CREB (cAMP-response-element-binding protein), is a crucial modulator of cellular homoeostasis. CREB regulates cellular differentiation of neurons, β-cells, adipocytes and SMCs (smooth muscle cells); it is also a potent survival factor and an upstream regulator of mitochondrial biogenesis. In diabetes and CVD, CREB protein content is decreased in the vascular media and its regulation in aberrant in β-cells, neurons and cardiomyocytes [8–12]. Loss of CREB content and function leads to decreased vascular target tissue resilience when exposed to stressors such as metabolic, oxidative or sheer stress. This basic research programme set the stage for our central hypothesis that diabetes-mediated CREB dysfunction predisposes the diabetes disease progression and cardiovascular complications. The second line of research revealed that diabetes mellitus (Type 1 and Type 2 in youth and adults) leads to defects in functional exercise capacity [13]. This defect manifests in a 20–30% decrease in maximal O2 consumption and slowed oxygenation and heart rate kinetics at submaximal exercise. Our group has determined that the defects in exercise correlate with insulin resistance, endothelial dysfunction, decreased cardiac perfusion and diastolic dysfunction, slowed muscle perfusion kinetics, decreased muscle perfusion [4,13–15] and slowed oxidative phosphorylation (M. Cree-Green and K.J. Nadeau, unpublished work). In the last few years, we have begun to weave these two threads together to define the relationship between exercise and vascular function with particular emphasis on how the signalling to CREB and eNOS [endothelial NOS (nitric oxide synthase)] regulates tissue perfusion, mitochondrial dynamics, vascular function and exercise capacity. This review summarizes our published work on CREB, mitochondrial function, cell-specific eNOS/NOS and diabetes mediated exercise impairments to set the stage for our current working hypothesis that restoration of eNOS/NOS dysfunction will restore cellular homoeostasis and permit optimal response to an exercise training intervention.
CREB regulates vascular SMC phenotype and is degraded in vascular disease
CREB is a transcription factor that plays an integral role in cellular proliferation, differentiation and adaptive responses [16–18]. CREB is regulated by G-protein-coupled receptors, tyrosine kinase receptors, oxidant and osmotic stress and Ca2+. Pivotal targets of CREB include genes important for survival, differentiation and metabolic adaptation (Figure 1). CREB is an integrator or hub protein that plays a role in life/death decisions of the cell and it is dysfunctional in the vasculature, heart, brain and β-cell in diabetes [8,11,12,20]. Our laboratory first identified CREB as a determinant of the quiescent SMC phenotype in a series of companion papers [10,11]. We demonstrated that overexpression of active CREB in SMCs decreased proliferative potential, whereas dominant-negative CREB increased SMC activation [10]. Diabetes mellitus induces phenotypic modulation of SMCs in the vasculature, leading to aberrant proliferation and migration of SMCs and vascular dysfunction [11]. Vascular CREB content is decreased in a spectrum of rodent models of CVD risk (hypertension, obesity, diabetes, the metabolic syndrome, pulmonary hypertension and dyslipidaemia) [21]. Using an atherosclerosis model of LDL (low-density lipoprotein) receptor-null mice and in vitro studies of SMCs exposed to LDL and oxLDL (oxidized LDL), we showed that both forms of LDL induce an acute activation of CREB. However, only oxLDL leads to CREB down-regulation [21]. We showed further that SMCs exposed to a panel of non-esterified (‘free’) fatty acids exhibited an acute activation of CREB via PKC (protein kinase C) activation. Only saturated fatty acids triggered the down-regulation of CREB [22]. CREB protein content is also reduced in the SMCs of hypertensive pulmonary arteries (PA SMCs) in animals exposed to chronic hypoxia. Hypoxia-induced PA SMCs produce a growth factor called PDGF (platelet-derived growth factor)-BB. We defined that CREB down-regulation by chronic PDGF-BB is mediated through chronic activation of PI3K (phosphoinositide 3-kinase)/Akt and induction of a novel downstream target: protein kinase CK2 [23]. CK2 augments CREB phosphorylation at Ser103 and Ser107, enhancing the nuclear export and proteasomal degration of CREB [23]. In the systemic vasculature, TZDs (thiazolidinediones) prevent arterial remodelling and vasoconstriction. TZDs block induction of CK2 and interfere with PDGF-mediated CREB degradation [24]. The physiological relevance of the TZD/Akt/CK2/CREB SMC protection pathway is supported by our recent publications demonstrating the ability of rosiglitazone, PI3K inhibitors and antioxidants to block the proliferation of PA SMCs and stimulate regression of arterial remodelling [24–26]. Collectively, these data support a model wherein CREB serves as a regulator of the quiescent SMC phenotype. Models of vascular disease including diabetes mellitus, hyperlipidaemia, aging and pulmonary hypertension consistently show that loss of SMC CREB via degradation or nuclear export is permissive for the proliferative SMC phenotype, ultimately promoting disease progression.
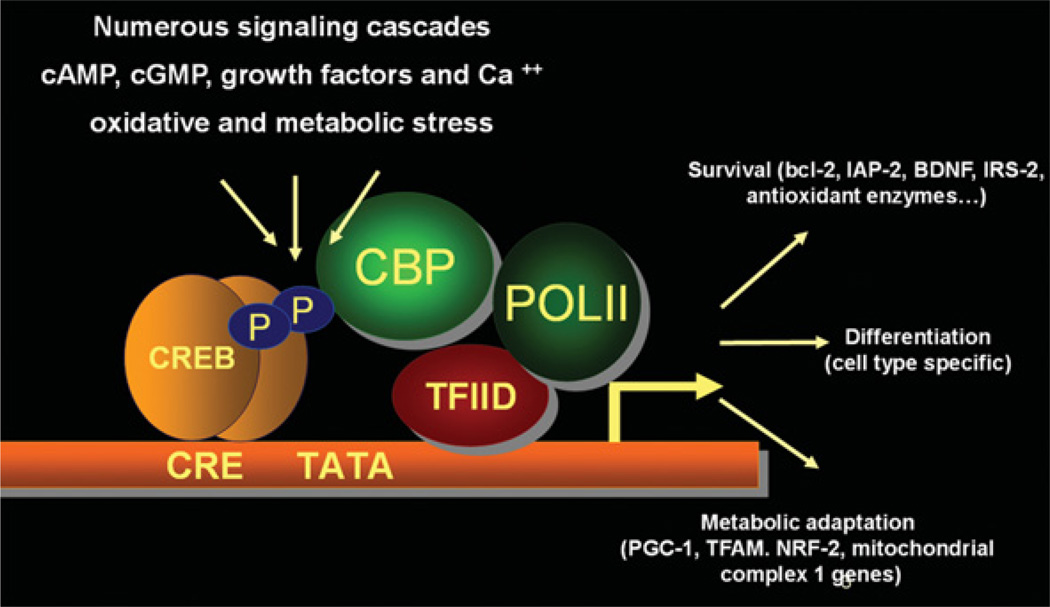
Figure 1. Targets of CREB regulation.
CREB is a transcriptional hub integrating signals from G-protein-coupled receptors, tyrosine kinase receptors, Ca2+ and oxidant injury to regulate genes essential for survival, differentiation and metabolic adaptation. BDNF, brain-derived neurotrophic factor; CBP, CREB-binding protein; CRE, cAMP-response element; IAP-2, inhibitor of apoptosis 2; IRS-2, insulin receptor substrate 2; NRF-2, nuclear factor-erythroid 2-related factor 2; POLII, RNA polymerase II; TFAM, transcription factor A, mitochondrial; TFIID, transcription factor IID.
CREB regulation of mitochondrial function
Mitochondria are critical sensors of cellular environment involved in cellular homoeostatic decision making. In the context of cellular stress (either toxic or physiological), mitochondrial adaptation is at the centre of cell fate. The decision to increase or decrease metabolism, adjust fuel partitioning and efficiency, and support survival are each, in part, regulated by the mitochondria. Early work from our group and others demonstrated that CREB is a critical regulator of cell survival and mitochondrial integrity via stimulation of Bcl-2 expression [27]. We reported redundant signalling downstream of the insulin receptor via p38 MAPK (mitogen-activated protein kinase), Akt and ERK (extracellular-signal-regulated kinase) to CREB and Bcl-2 [27–29]. In addition, we demonstrated a potent role for CREB in the context of diabetes-mediated oxidant or cytokine stress [8,20,30]. More recently, we and others reported that CREB is an upstream regulator of mitochondrial biogenesis via regulation of PGC1α (peroxisome-proliferator-activated receptor γ co-activator 1α) and ERRα (oestrogen-related receptor α) [31]. We demonstrated that exercise stimulated CREB activity and Bcl-2 decreased caspase 3 activation and decreased heart failure in the SHHF (spontaneous hypertensive heart failure) rat [12]. Consistent with this observation, CREB activity (not protein content) is decreased across a spectrum of cardiac heart failure models [32]. Fentzke et al. [33] generated a cardiac-specific transgenic mouse with dominant-negative CREB under the MHC promoter (MHC DNCREB). This mouse develops dilated cardiomyopathy [33]. Watson et al. [31] showed abnormal mitochondrial structure and function plus abnormal fuel partitioning in the MHC DNCREB mouse. In addition, this mouse fails to respond to exercise training with physiological remodelling [34]. Taken together, this study indicates that CREB is essential for heart mitochondrial function and contributes to physiological remodelling. These observations led us to postulate that CREB dysregulation in the diabetic heart and vasculature may contribute to, or exacerbate, the well-established mitochondrial dysfunction in diabetes. We are currently pursuing aspects of this question in our basic and clinical studies. In the next section, we provide an overview of our work in the diabetic vasculature evaluating the mitochondrial adaptation to exercise intervention and close with a summary of our ongoing and published clinical trials targeting insulin action, CREB and the mitochondria as strategies to improve exercise capacity in diabetes and enhance the physiological impact of exercise in the diabetes mellitus host.
Mitochondrial adaptation
CREB content and nuclear localization are decreased in vascular diseases including diabetes, dyslipidaemia, hypertension, aging and pulmonary hypertension [9,21,35]. In the light of the impact of CREB on mitochondrial function, we evaluated the mitochondrial content and function in diabetic rodent models and observed decreased mitochondrial protein profiles and enzyme activities [30]. We next tested whether we could restore vascular CREB content or CREB mitochondrial targets with exercise intervention [36]. In control rats, we observed a significant increase in mitochondrial protein profiles with a short-term exercise intervention which was, unexpectedly, absent from hypertensive or hypertensive diabetes mellitus rats [36,37] (Figure 2). These unanticipated results led to a series of studies to define the molecular determinants of vascular mitochondrial response to exercise and to evaluate these targets in diabetes mellitus.
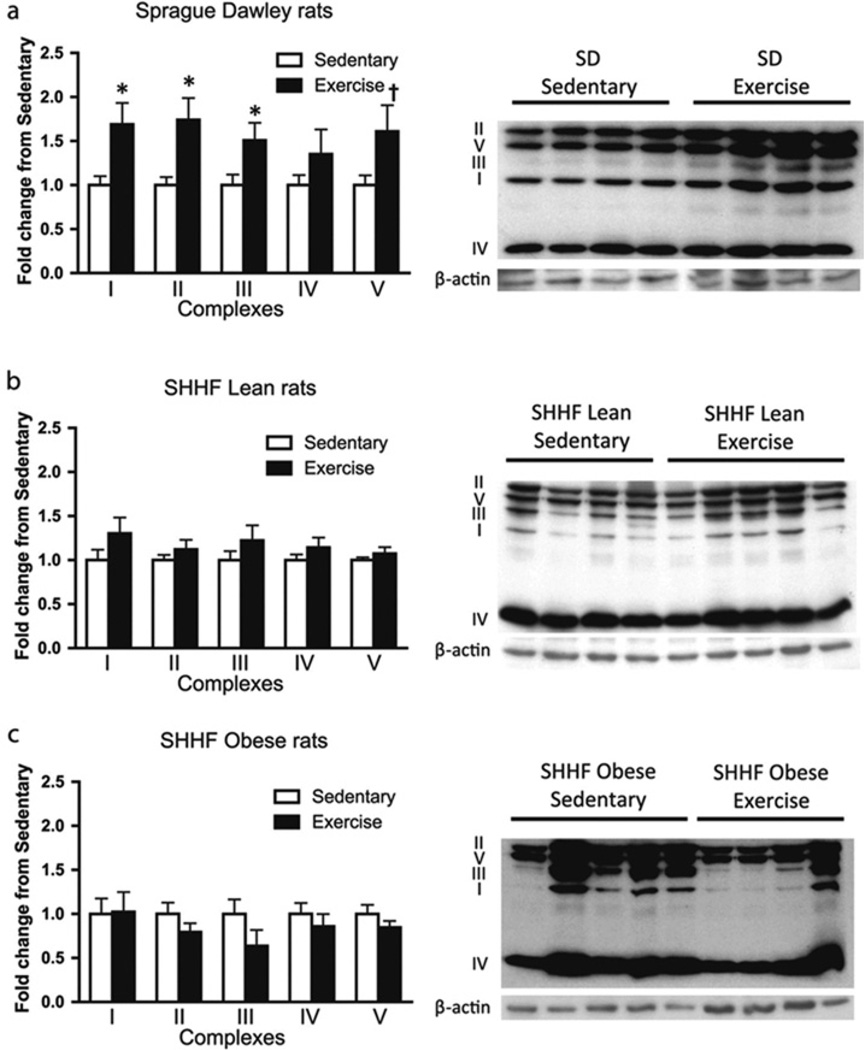
Figure 2. Aortic mitochondrial protein content with exercise intervention.
Mitochondrial protein expression profile in SD (Sprague–Dawley) and SHHF lean and obese rats with and without exercise training. Aortic lysates were generated from SD (n = 8 and 9 for sedentary and exercise respectively), SHHF lean (n = 8 and 10 for sedentary and exercise respectively) and SHHF obese (n = 9 for both sedentary and exercise) rats as labelled: 30 µg of protein was run on SDS/PAGE (10% gels), transferred on to nitrocellulose membranes and Western blot analysis was carried out. Results are means±S.E.M. *P < 0.05; †P=0.05–0.10 (Student’s t test). Reproduced with permission from [36]: Knaub, L.A., McCune, S., Chicco, A.J., Miller, M., Moore, R.L., Birdsey, N., Lloyd, M.I., Villarreal, J., Keller, A.C., Watson, P.A. and Reusch, J.E. (2013) Impaired response to exercise intervention in the vasculature in metabolic syndrome. Diab. Vasc. Dis. Res. 10, 222–238. © 2013 SAGE Publications.
Mitochondrial dynamics
Mitochondrial content, structure and function are coordinated by an agile intracellular machinery regulating mitochondrial biogenesis, mitochondrial dynamics (fission and fusion), mitochondrial localization and autophagy (reviewed in [38]). This machinery enables the homoeostatic plasticity needed for rapid adaptation to physiological challenges. In the vasculature, mitochondria serve a number of functions in addition to ATP production, including the regulation of Ca2+ sparks and currents, regulation of ROS (reactive oxygen species), and regulation of cell proliferation and survival [39,40]. Early studies by Taggart and Wray [41] and Sward et al. [42] demonstrated a seminal role for mitochondria in control of vascular contractile function (constriction and relaxation); more recent work defined their importance for calcium regulation and control of vascular tone (stiffness) [43]. We were interested in defining the determinants of basal and adaptive mitochondrial function in the vasculature.
Role of NOS in vascular mitochondrial content and adaptation
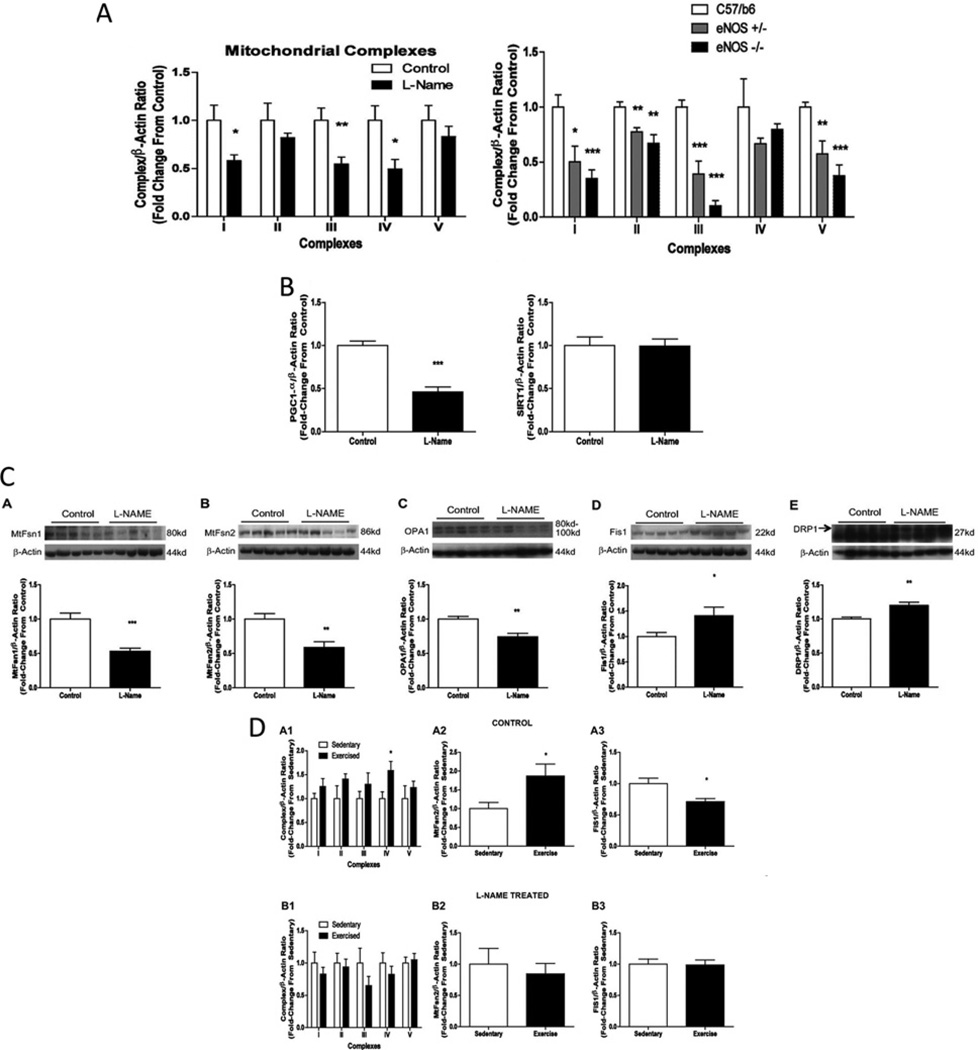
To better understand the underpinnings of decreased vascular mitochondrial content and adaptation in diabetes, we explored the connections between known mitochondrial regulatory pathways and established vascular consequences of diabetes. Endothelial dysfunction, defined by decreased flow-mediated vasodilatation and vascular insulin resistance (specifically to eNOS regulation) are established vascular abnormalities in diabetes [44]. The work of Nisoli’s group firmly established the role of NO [generated by eNOS and nNOS (neuronal NOS)] as an upstream regulator of mitochondrial biogenesis [45]. We therefore examined the impact of eNOS deletion or pharmacological inhibition of NOS on baseline and exercise-stimulated vascular mitochondrial biogenesis and dynamics. Miller et al. [37] reported decreased aortic mitochondrial protein profiles in aortic tissues of eNOS-null and l-NAME (NG-nitro-l-arginine methyl ester)-treated rodents (similar to our observations in diabetes mellitus) [37]. In addition to decreased CREB and PGC1α in these models, we also observed increased expression of fission proteins (Fis1 and Drp1) and decreased expression of fusion proteins (Opa1, Mfn1 and Mfn2) [37] (Figure 3). These data are consistent with NOS as a biologically important modulator of basal vascular mitochondrial function and structure. In addition, we demonstrated that exercise increased Mfn2 and decreased Fis1 in control rats [37]. This pattern would predict a more fused and networked mitochondrial structure. In contrast, l-NAME prohibited the increase in mitochondrial protein expression and the changes in Mfn2 and Fis1 with exercise [37]. These findings have informed our current working hypothesis that diabetes mellitus-mediated vascular NOS dysfunction (particularly with exercise) contributes to failed mitochondrial plasticity and vascular dysfunction.
Figure 3. Impact of NOS/eNOS on aortic mitochondrial protein content and dynamics baseline and with exercise intervention.
eNOS deletion and NOS disruption mimic diabetes and disrupt adaptive mitochondrial dynamics. (A) eNOS deletion or pharmacological NOS inhibition decrease aortic mitochondrial content. (B) NOS inhibition decreases mitochondrial regulator PGC1α without changing SIRT1 (sirtuin 1) protein content. (C) NOS inhibition decreases mitochondrial fusion proteins (Mfn1, Mfn2 and OPA1) and increases mitochondrial fission proteins (Fis1 and Drp1). (D) Exercise intervention increases mitochondrial protein and Mfn2 and decreases Fis1 in control rat aorta, whereas no change is observed in rats exercising with the NOS inhibitor treatment. Adapted from [37]: Miller, M.W., Knaub, L.A., Olivera-Fragoso, L.F., Keller, A.C., Balasubramaniam, V., Watson, P.A. and Reusch, J.E. (2013) Nitric oxide regulates vascular adaptive mitochondrial dynamics. Am. J. Physiol. Heart Circ. Physiol. 304, H1624–H1633. © 2013 The American Physiological Society.
Bridging clinical therapeutics to new molecular targets
Type 2 diabetes affects more than 300 million people worldwide and its pathogenesis is a combination of insulin resistance and relative β-cell deficiency. Many agents exist to manage Type 2 diabetes, including agents that augment or replace defective insulin secretion and agents that improve or mimic insulin action on target tissues (decrease hepatic glucose output or improve glucose utilization). We are intrigued that the incretin class of insulin secretogogues, GLP1 (glucagon-like peptide 1) receptor analogues or DPP4 (dipeptidyl peptidase 4) inhibitors act via G-protein-coupled receptors that are highly expressed in the vasculature [46,47]. In addition, we closely follow the work of Lui’s group who has demonstrated that incretin agents stimulate eNOS and PKA (protein kinase A) and enhance endothelial function and tissue perfusion leading to improved muscle glucose utilization [48,49]. We therefore examined the impact of the DPP4 inhibitor saxagliptin on vascular mitochondrial adaptation in diabetes. In these studies, which have been published only in abstract form to date [50], we observed restoration of the exercise-mediated increase in mitochondrial protein profiles in the diabetes mellitus aorta, increased expression of eNOS, nNOS and PGC1α. Studies are underway to examine further the impact of the incretins on target tissue response to exercise in health and diabetes.
Clinical implications
People with diabetes have decreased functional exercise capacity that may be important in the premature mortality in diabetes. We have defined a set of physiological parameters that predict or correlate with exercise defects. As outlined in the Introduction, our groups have determined that the defects in exercise correlate with insulin resistance, endothelial dysfunction, diastolic dysfunction, decreased cardiac perfusion, slowed muscle perfusion kinetics [3–5,14,15] and slowed oxidative phosphorylation (J. Reusch, K. Nadeau and M. Cree-Green, unpublished work). Over the last decade, we have sought to target physical inactivity, insulin-sensitivity and excess oxidant burden as ways to improve exercise function in people with diabetes. As expected, exercise training can improve fitness in people with diabetes, but it is not normalized to the level of their matched non-diabetic counterparts [3]. In a series of proof-of-concept studies, we and others demonstrated that improving insulin-sensitivity and endothelial function with TZDs enhances exercise capacity in Type 2 diabetes [50]. Similarly, vitamin E plus C also improved endothelial function and exercise capacity (published so far only in abstract form [52]). Ongoing investigations (see http://www.clintrials.gov) are examining the impact of the incretin class of drugs on these end points.
Summary
Diabetes, via many pathways including glucose elevation, insulin resistance, oxidant burden and low-grade inflammation, generates persistent target organ stress that overwhelms the innate homoeostatic machinery and leads to target organ dysfunction. Our work has defined a unique consequence of this homoeostatic failure: loss of adaptive mitochondrial plasticity in the vasculature. Exploration of this defect has revealed the interesting roles of eNOS and nNOS that may be amenable to pharmacological targeting.
Acknowledgments
Funding
Our work is funded by a Veterans’ Administration Merit Award (Basic and Clinical), Denver Research Institute, National Institutes of Health [grant numbers RO1-DK064741, T32HL007171, P01HL014985 and UL1RR025780], Bristol-Myers Squibb, the University of Colorado Center for Women’s Health Research, American Diabetes Association, American Heart Association and Juvenile Diabetes Research Foundation International.
Abbreviations
- CREB
cAMP-response-element-binding protein
- CVD
cardiovascular disease
- DPP4
dipeptidyl peptidase 4
- eNOS
endothelial nitric oxide synthase
- LDL
low-density lipoprotein
- l-name
NG-nitro-l-arginine methyl ester
- MHC DNCREB
dominant-negative CREB under the MHC promoter
- nNOS
neuronal NOS
- NOS
nitric oxide synthase
- oxLDL
oxidized LDL
- PA SMC
SMC of hypertensive pulmonary arteries
- PDGF
platelet-derived growth factor
- PGC1α
peroxisome-proliferator-activated receptor γ co-activator 1α
- PI3K
phosphoinositide 3-kinase
- SHHF
spontaneous hypertensive heart failure
- SMC
smooth muscle cell
- TZD
thiazolidinedione.
References
- 1.Vamos EP, Millett C, Parsons C, Aylin P, Majeed A, Bottle A. Nationwide study on trends in hospital admissions for major cardiovascular events and procedures among people with and without diabetes in England 2004–2009. Diabetes Care. 2012;35:265–272. doi: 10.2337/dc11-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Regensteiner JG, Sippel J, McFarling ET, Wolfel EE, Hiatt WR. Effects of non-insulin-dependent diabetes on oxygen consumption during treadmill exercise. Med Sci. Sports Exerc. 1995;27:661–667. [PubMed] [Google Scholar]
- 3.Brandenburg SL, Reusch JE, Bauer TA, Jeffers BW, Hiatt WR, Regensteiner JG. Effects of exercise training on oxygen uptake kinetic responses in women with type 2 diabetes. Diabetes Care. 1999;22:1640–1646. doi: 10.2337/diacare.22.10.1640. [DOI] [PubMed] [Google Scholar]
- 4.Nadeau KJ, Zeitler PS, Bauer TA, Brown MS, Dorosz JL, Draznin B, Reusch JE, Regensteiner JG. Insulin resistance in adolescents with type 2 diabetes is associated with impaired exercise capacity. J. Clin. Endocrinol. Metab. 2009;94:3687–3695. doi: 10.1210/jc.2008-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nadeau KJ, Regensteiner JG, Bauer TA, Brown MS, Dorosz JL, Hull A, Zeitler P, Draznin B, Reusch JE. Insulin resistance in adolescents with type 1 diabetes and its relationship to cardiovascular function. J. Clin. Endocrinol. Metab. 2010;95:513–521. doi: 10.1210/jc.2009-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Church TS, Thomas DM, Tudor-Locke C, Katzmarzyk PT, Earnest CP, Rodarte RQ, Martin CK, Blair SN, Bouchard C. Trends over 5 decades in U.S. occupation-related physical activity and their associations with obesity. PLoS ONE. 2012;6:e19657. doi: 10.1371/journal.pone.0019657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katzmarzyk PT, Church TS, Janssen I, Ross R, Blair SN. Metabolic syndrome, obesity, and mortality: impact of cardiorespiratory fitness. Diabetes Care. 2005;28:391–397. doi: 10.2337/diacare.28.2.391. [DOI] [PubMed] [Google Scholar]
- 8.Jambal P, Masterson S, Nesterova A, Bouchard R, Bergman B, Hutton JC, Boxer LM, Reusch JE, Pugazhenthi S. Cytokine-mediated downregulation of the transcription factor CREB in pancreatic β-cells. J. Biol. Chem. 2003;278:23055–23065. doi: 10.1074/jbc.M212450200. [DOI] [PubMed] [Google Scholar]
- 9.Klemm DJ, Leitner JW, Watson P, Nesterova A, Reusch JE, Goalstone ML, Draznin B. Insulin-induced adipocyte differentiation: activation of CREB rescues adipogenesis from the arrest caused by inhibition of prenylation. J. Biol. Chem. 2001;276:28430–28435. doi: 10.1074/jbc.M103382200. [DOI] [PubMed] [Google Scholar]
- 10.Klemm DJ, Watson PA, Frid MG, Dempsey EC, Schaack J, Colton LA, Nesterova A, Stenmark KR, Reusch JE. cAMP response element-binding protein content is a molecular determinant of smooth muscle cell proliferation and migration. J. Biol. Chem. 2001;276:46132–46141. doi: 10.1074/jbc.M104769200. [DOI] [PubMed] [Google Scholar]
- 11.Watson PA, Nesterova A, Burant CF, Klemm DJ, Reusch JE-B. Diabetes-related changes in cAMP response element-binding protein content enhance smooth muscle cell proliferation and migration. J. Biol. Chem. 2001;276:46142–46150. doi: 10.1074/jbc.M104770200. [DOI] [PubMed] [Google Scholar]
- 12.Watson PA, Reusch JE, McCune SA, Leinwand LA, Luckey SW, Konhilas JP, Brown DA, Chicco AJ, Sparagna GC, Long CS, Moore RL. Restoration of CREB function is linked to completion and stabilization of adaptive cardiac hypertrophy in response to exercise. Am J. Physiol. Heart Circ. Physiol. 2007;293:H246–H259. doi: 10.1152/ajpheart.00734.2006. [DOI] [PubMed] [Google Scholar]
- 13.Regensteiner JG, Bauer TA, Reusch JE, Brandenburg SL, Sippel JM, Vogelsong AM, Smith S, Wolfel EE, Eckel RH, Hiatt WR. Abnormal oxygen uptake kinetic responses in women with type II diabetes mellitus. J. Appl. Physiol. 1998;85:310–317. doi: 10.1152/jappl.1998.85.1.310. [DOI] [PubMed] [Google Scholar]
- 14.Bauer TA, Reusch JE, Levi M, Regensteiner JG. Skeletal muscle deoxygenation following the onset of moderate exercise suggests slowed microvascular blood flow kinetics in Type 2 diabetes. Diabetes Care. 2007;11:2880–2885. doi: 10.2337/dc07-0843. [DOI] [PubMed] [Google Scholar]
- 15.Regensteiner JG, Bauer TA, Reusch JE, Quaife RA, Chen MY, Smith SC, Miller TM, Groves BM, Wolfel EE. Cardiac dysfunction during exercise in uncomplicated type 2 diabetes. Med. Sci. Sports Exerc. 2009;41:977–984. doi: 10.1249/MSS.0b013e3181942051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dworkin S, Mantamadiotis T. Targeting CREB signalling in neurogenesis. Expert Opin. Ther. Targets. 2010;14:869–879. doi: 10.1517/14728222.2010.501332. [DOI] [PubMed] [Google Scholar]
- 17.Knoll B, Nordheim A. Functional versatility of transcription factors in the nervous system: the SRF paradigm. Trends Neurosci. 2009;32:432–442. doi: 10.1016/j.tins.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Lopez-Lluch G, Irusta PM, Navas P, de Cabo R. Mitochondrial biogenesis and healthy aging. Exp. Gerontol. 2008;43:813–819. doi: 10.1016/j.exger.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reference deleted. [Google Scholar]
- 20.Pugazhenthi S, Nesterova A, Jambal P, Audesirk G, Kern M, Cabell L, Eves E, Rosner MR, Boxer LM, Reusch JE. Oxidative stress-mediated down-regulation of bcl-2 promoter in hippocampal neurons. J. Neurochem. 2003;84:982–996. doi: 10.1046/j.1471-4159.2003.01606.x. [DOI] [PubMed] [Google Scholar]
- 21.Schauer IE, Knaub LA, Lloyd M, Watson PA, Gliwa C, Lewis KE, Chait A, Klemm DJ, Gunter JM, Bouchard R, et al. CREB downregulation in vascular disease: a common response to cardiovascular risk. Arterioscler. ThrombVasc. Biol. 2010;30:733–741. doi: 10.1161/ATVBAHA.109.199133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schauer IE, Reusch JE. Nonesterified fatty acid exposure activates protective and mitogenic pathways in vascular smooth muscle cells by alternate signaling pathways. Metabolism. 2009;58:319–327. doi: 10.1016/j.metabol.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garat CV, Fankell D, Erickson PF, Reusch JE, Bauer NN, McMurtry IF, Klemm DJ. Platelet-derived growth factor BB induces nuclear export and proteasomal degradation of CREB via phosphatidylinositol 3-kinase/Akt signaling in pulmonary artery smooth muscle cells. Mol. Cell. Biol. 2006;26:4934–4948. doi: 10.1128/MCB.02477-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crossno JT, Jr, Garat CV, Reusch JE, Morris KG, Dempsey EC, McMurtry IF, Stenmark KR, Klemm DJ. Rosiglitazone attenuates hypoxia-induced pulmonary arterial remodeling. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007;292:L885–L897. doi: 10.1152/ajplung.00258.2006. [DOI] [PubMed] [Google Scholar]
- 25.Klemm DJ, Majka SM, Crossno JT, Jr, Psilas JC, Reusch JE, Garat CV. Reduction of reactive oxygen species prevents hypoxia-induced CREB depletion in pulmonary artery smooth muscle cells. J. Cardiovasc. Pharmacol. 2011;58:181–191. doi: 10.1097/FJC.0b013e31821f2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garat CV, Crossno JT, Jr, Sullivan TM, Reusch JE, Klemm DJ. Inhibition of phosphatidylinositol-3-kinase/Akt signaling attenuates hypoxia-induced pulmonary artery remodeling and suppresses CREB depletion in arterial smooth muscle cells. J. Cardiovasc. Pharmacol. 2013;6:539–548. doi: 10.1097/FJC.0000000000000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pugazhenthi S, Miller E, Sable C, Young P, Heidenreich KA, Boxer LM, Reusch JE. Insulin-like growth factor-I induces bcl-2 promoter through the transcription factor cAMP-response element-binding protein. J. Biol. Chem. 1999;274:27529–27535. doi: 10.1074/jbc.274.39.27529. [DOI] [PubMed] [Google Scholar]
- 28.Pugazhenthi S, Boras T, O’Connor D, Meintzer MK, Heidenreich KA, Reusch JE. Insulin-like growth factor I-mediated activation of the transcription factor cAMP response element-binding protein in PC12 cells: involvement of p38 mitogen-activated protein kinase-mediated pathway. J. Biol. Chem. 1999;274:2829–2837. doi: 10.1074/jbc.274.5.2829. [DOI] [PubMed] [Google Scholar]
- 29.Pugazhenthi S, Nesterova A, Sable C, Heidenreich KA, Boxer LM, Heasley LE, Reusch JE. Akt/protein kinase B up-regulates Bcl-2 expression through cAMP-response element-binding protein. J. Biol. Chem. 2000;275:10761–10766. doi: 10.1074/jbc.275.15.10761. [DOI] [PubMed] [Google Scholar]
- 30.Sarkar SA, Gunter J, Bouchard R, Reusch JE, Wiseman A, Gill RG, Hutton JC, Pugazhenthi S. Dominant negative mutant forms of the cAMP response element binding protein induce apoptosis and decrease the anti-apoptotic action of growth factors in human islets. Diabetologia. 2007;50:1649–1659. doi: 10.1007/s00125-007-0707-z. [DOI] [PubMed] [Google Scholar]
- 31.Watson PA, Birdsey N, Huggins GS, Svensson EC, Heppe DB, Knaub LA. Cardiac-specific overexpression of dominant-negative CREB leads to increased mortality and mitochondrial dysfunction in female mice. Am. J. Physiol. Heart Circ. Physiol. 2010;293:H246–H259. doi: 10.1152/ajpheart.00394.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Konhilas JP, Watson PA, Maass A, Boucek DM, Horn T, Stauffer BL, Luckey SW, Rosenberg P, Leinwand LA. Exercise can prevent and reverse the severity of hypertrophic cardiomyopathy. Circ. Res. 2006;98:540–548. doi: 10.1161/01.RES.0000205766.97556.00. [DOI] [PubMed] [Google Scholar]
- 33.Fentzke RC, Korcarz CE, Lang RM, Lin H, Leiden JM. Dilated cardiomyopathy in transgenic mice expressing a dominant-negative CREB transcription factor in the heart. J. Clin. Invest. 1998;101:2415–2426. doi: 10.1172/JCI2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spencer KT, Collins K, Korcarz C, Fentzke R, Lang RM, Leiden JM. Effects of exercise training on LV performance and mortality in a murine model of dilated cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2000;279:H210–H215. doi: 10.1152/ajpheart.2000.279.1.H210. [DOI] [PubMed] [Google Scholar]
- 35.Garat CV, Crossno JT, Jr, Sullivan TM, Reusch JE, Klemm DJ. Thiazolidinediones prevent PDGF-BB-induced CREB depletion in pulmonary artery smooth muscle cells by preventing upregulation of casein kinase 2α’ catalytic subunit. J. Cardiovasc. Pharmacol. 2010;55:469–480. doi: 10.1097/FJC.0b013e3181d64dbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knaub LA, McCune S, Chicco AJ, Miller M, Moore RL, Birdsey N, Lloyd MI, Villarreal J, Keller AC, Watson PA, Reusch JE. Impaired response to exercise intervention in the vasculature in metabolic syndrome. Diab. Vasc. Dis. Res. 2013;10:222–238. doi: 10.1177/1479164112459664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller MW, Knaub LA, Olivera-Fragoso LF, Keller AC, Balasubramaniam V, Watson PA, Reusch JE. Nitric oxide regulates vascular adaptive mitochondrial dynamics. Am. J. Physiol. Heart Circ. Physiol. 2013;304:H1624–H1633. doi: 10.1152/ajpheart.00987.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dorn GW., 2nd Mitochondrial dynamics in heart disease. Biochim. Biophys. Acta. 2013;1833:233–241. doi: 10.1016/j.bbamcr.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chalmers S, Saunter C, Wilson C, Coats P, Girkin JM, McCarron JG. Mitochondrial motility and vascular smooth muscle proliferation. Arterioscler. Thromb. Vasc. Biol. 2012;32:3000–3011. doi: 10.1161/ATVBAHA.112.255174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCarron JG, Olson ML, Wilson C, Sandison ME, Chalmers S. Examining the role of mitochondria in Ca2+ signaling in native vascular smooth muscle. Microcirculation. 2013;20:317–329. doi: 10.1111/micc.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taggart MJ, Wray S. Hypoxia and smooth muscle function: key regulatory events during metabolic stress. J. Physiol. 1998;509:315–325. doi: 10.1111/j.1469-7793.1998.315bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sward K, Dreja K, Lindqvist A, Persson E, Hellstrand P. Influence of mitochondrial inhibition on global and local [Ca2+]I in rat tail artery. Circ. Res. 2002;90:792–799. doi: 10.1161/01.res.0000015214.40360.84. [DOI] [PubMed] [Google Scholar]
- 43.Katakam PV, Wappler E, Katz P, Rutkai I, Institoris A, Domoki F, Gaspar T, Grovenburg SM, Snipes JA, Busija DW. Depolarization of mitochondria in endothelial cells promotes cerebral vascular vasodilation by activation of nitric oxide synthase. Arterioscler. Thromb. Vasc. Biol. 2013;33:752–759. doi: 10.1161/ATVBAHA.112.300560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Montagnani M, Chen H, Barr VA, Quon MJ. Insulin-stimulated activation of eNOS is independent of Ca2+ but requires phosphorylation by Akt at Ser1179. J. Biol. Chem. 2001;276:30392–30398. doi: 10.1074/jbc.M103702200. [DOI] [PubMed] [Google Scholar]
- 45.Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, Bracale R, Valerio A, Francolini M, Moncada S, Carruba MO. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science. 2003;299:896–899. doi: 10.1126/science.1079368. [DOI] [PubMed] [Google Scholar]
- 46.Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation. 2008;117:2340–2350. doi: 10.1161/CIRCULATIONAHA.107.739938. [DOI] [PubMed] [Google Scholar]
- 47.Gros R, You X, Baggio LL, Kabir MG, Sadi AM, Mungrue IN, Parker TG, Huang Q, Drucker DJ, Husain M. Cardiac function in mice lacking the glucagon-like peptide-1 receptor. Endocrinology. 2003;144:2242–2252. doi: 10.1210/en.2003-0007. [DOI] [PubMed] [Google Scholar]
- 48.Chai W, Dong Z, Wang N, Wang W, Tao L, Cao W, Liu Z. Glucagon-like peptide 1 recruits microvasculature and increases glucose use in muscle via a nitric oxide-dependent mechanism. Diabetes. 2012;61:888–896. doi: 10.2337/db11-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dong Z, Chai W, Wang W, Zhao L, Fu Z, Cao W, Liu Z. Protein kinase A mediates glucagon-like peptide 1-induced nitric oxide production and muscle microvascular recruitment. Am. J. Physiol. Endocrinol. Metab. 2013;304:E222–E228. doi: 10.1152/ajpendo.00473.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Keller AC, Knaub LA, Miller MW, Reusch JE. Saxagliptin restores vascular mitochondrial exercise response in diabetes. Arterioscler. Thromb. Vasc. Biol. 2013;33:A165. [Google Scholar]
- 51.Regensteiner JG, Bauer TA, Reusch JE. Rosiglitazone improves exercise capacity in individuals with type 2 diabetes. Diabetes Care. 2005;28:2877–2883. doi: 10.2337/diacare.28.12.2877. [DOI] [PubMed] [Google Scholar]
- 52.Gill E, Weil J, Reusch JEB, Wolfel E, Hiatt W, Lindenfeld J, Quaife R, Regensteiner JA. Administration of l-arginine and antioxidants increase vasodilator function in women with type 2 DM. Circulation. 1999;100(Suppl.):I.757. [Google Scholar]