Abstract
The biotrophic pathogen Ustilago maydis causes tumors by redirecting vegetative and floral development in maize (Zea mays L.). After fungal injection into immature tassels, tumors were found in all floral organs, with a progression of organ susceptibility that mirrors the sequential location of foci of cell division in developing spikelets. There is sharp demarcation between tumor-forming zones and areas with normal spikelet maturation and pollen shed; within and immediately adjacent to the tumor zone, developing anthers often emerge precociously and exhibit a range of developmental defects suggesting that U. maydis signals and host responses are restricted spatially. Male-sterile maize mutants with defects in anther cell division patterns and cell fate acquisition prior to meiosis formed normal adult leaf tumors, but failed to form anther tumors. Methyl jasmonate and brassinosteroid phenocopied these early-acting anther developmental mutants by generating sterile zones within tassels that never formed tumors. Although auxin, cytokinin, abscisic acid and gibberellin did not impede tassel development, the Dwarf8 mutant defective in gibberellin signaling lacked tassel tumors; the anther ear1 mutant reduced in gibberellin content formed normal tumors; and Knotted1, in which there is excessive growth of leaf tissue, formed much larger vegetative and tassel tumors. We propose the hypothesis that host growth potential and tissue identity modulate the ability of U. maydis to redirect differentiation and induce tumors.
Keywords: Anther, Brassinosteroid, Male-sterile, Methyl jasmonate, Ustilago maydis
Introduction
Although the biotrophic fungal pathogen Ustilago maydis grows on many flowering plants (León-Ramírez et al. 2004), it exhibits exquisite host specificity in eliciting tumors. This capacity is restricted to domesticated maize (Zea mays L.) and its wild progenitor teosinte (Banuett 2002). In natural infections, haploid fungal sporidia elon-gate on maize surfaces, complementary mating types fuse to form a dikaryon, and the resulting filament enters the plant by direct penetration. Fungal hyphae proliferate rapidly in intimate contact with invaginations of the host plasma membrane within the infection zone (Doehlemann et al. 2009). Unlike oncogenic agents in animals that reactivate cell division, tumors induced by U. maydis require dividing host cells, subverting controls on normal maize cell proliferation to promote excessive cell division, polyploidization and enormous cell expansion (Callow and Ling 1973; Callow 1975; Banuett 2002). Within tumors, diploid fungal cells mature into teliospores, the primary agent of fungal dispersal. Altogether, fungal infection, growth and spore differentiation require about 2 weeks and culminate in the release of billions of diploid teliospores (Banuett and Herskowitz 1996).
Based on gene deletion assays and analysis of the U. maydis genome, the current hypothesis to explain tumor induction is that this pathogen secretes a suite of effector proteins that trigger abnormal host cell differentiation. Tumor expansion undoubtedly depends on plant hormones. Although U. maydis produces significant amounts in planta, fungal-derived auxin is not essential for pathogenicity (Kämper et al. 2006; Reineke et al. 2008). Recent sequencing of the U. maydis genome demonstrated that this pathogen encodes 386 predicted secreted proteins, some of which are required for inciting seedling leaf tumors by unknown mechanisms (Mueller et al. 2008). Maize and other flowering plants encode hundreds of predicted membrane-localized receptor kinases, but only a few have defined ligands. These and other poorly defined systemic and cell-to-cell signaling networks in maize are likely to be targets of U. maydis proteins that redirect host development leading to tumorogenesis.
To date, the requirements for tumor formation have been investigated by manipulating the pathogen, using the host plant, typically a seedling, as a substrate. Therefore, many basic questions on tumor growth have not been explored from the plant perspective. For example, are specific plant genes required for tumor formation in different plant organs? Would maize mutants altered in hormone biosyn-thesis, perception, or signal transduction form larger or fewer tumors? To explore these and related questions, we have surveyed the impact of plant hormones and maize mutants defective in growth control. The tassel was chosen because it can be carefully staged, and it has a well-established progression of foci of cell division. The tassel is a complex inflorescence containing well-synchronized cohorts of developing flowers that collectively span about 1 week of floral development. Additionally, numerous male-sterile (ms) mutants have defects that disrupt the cell division patterns and acquisition of cell fate in anthers, providing the opportunity to explore whether U. maydis can elicit tumors in host organs with altered development. Collectively, these studies initiate a genetic analysis of the host requirements for tumor formation.
Materials and methods
Plant material and growth conditions
Inbred line stocks are maintained by the Walbot Laboratory and include A619, B73, Ky21 and W23 (recessive for bz2, a gene in the anthocyanin biosynthetic pathway). Other Walbot Laboratory stocks include the homozygous an1 bz2 deletion mutant in W23, and the ms lines msca1, mac1 and ms26 backcrossed twice into W23 and maintained as 1:1 segregating families. Kn1-OL (segregating 1:1 normal:mutant), was obtained from Sarah Hake (Plant Gene Expression Center, USDA, Albany CA). The spi1 stock (segregating 1:1 normal:mutant) was obtained from Paula McSteen (Pennsylvania State University, University Park PA). The Dwarf8 stock was obtained from the Maize Genetics Cooperation Stock Center (http://maizecoop.cropsci.uiuc.edu/ and maintained by crossing onto inbred lines to generate families segregating 1:1 normal:mutant.
The ms and inbred lines were grown outdoors in summer 2007 at Stanford CA. Families of 25 (uniform) or 50 (segregating stocks) were planted weekly for U. maydis injections to define the period of tumor susceptibility in specific tassel structures; primarily, data from W23 are reported, but the experiments also included Ky21, A619 and B73, all of which gave similar results. In summer 2008, these inbred lines were grown again for evaluation of hormone treatments. The W23 bz2 tester line was grown in multiple plantings in a greenhouse equipped with lights providing ~50% of summer noon solar fluence (16-h light/8-h dark) at Stanford CA. A subset of inbred and ms lines were grown under these conditions, as well as all of the developmental mutants. Greenhouse families typically contained 36–40 individuals in uniform stocks and 80 individuals in segregating stocks grown in two ranks of pots with automatic watering and fertilizer treatments with cohorts of 8–10 individuals used in each treatment. There was a slight, but consistent, temperature gradient across the greenhouse space resulting in a 1–2 days of developmental tassel stage difference between the first (advanced) and last (slowest) plants. To minimize age effects, in experiments with two or more treatments, every nth plant down a row was assigned to the same treatment cohort.
Ustilago maydis culture and injections
The FB1 and FB2 haploid mating types of U. maydis were obtained from Flora Banuett (Long Beach State University, Long Beach, CA) and grown as described in Banuett and Herskowitz (1994, 1996). Briefly, strains were streaked out separately on solid media and grown for 2 days at 27–28°C. Individual colonies were picked and streaked out for a second 2-day growth period. From these second plates, a loop full of FB1 or FB2 was inoculated into 15– 25-mL liquid media in a 125-mL Erlenmeyer flask for growth at 27–28°C on a rotary shaker set at 125 rpm for 16–18 h. To initiate fungal fusion, the overnight liquid FB1 and FB2 cultures were mixed, and within 45 min injected into plants. In the experiments involving maize hormone mutants, the solopathogenic strain SG200 (supplied by Regine Kahmann, Max Planck Institut, Marburg Germany) was used (Kämper et al. 2006); the strain was grown following the procedures of Krüger et al. (2000). Cells were collected by centrifugation at 3,000×g for 15 min, and then resuspended in sterile water at A600nm = 1.0 prior to injection.
Injections were performed using 3 or 12-mL sterile plastic syringes with 25 or 26 gauge needles. Typically, eight to ten biological replicates were performed with each injection treatment type, and in most cases entire experiments were repeated for evaluation of plant response and tumor formation from a few days through 2 weeks after injection. Based on extensive experience in dissecting immature tassels, the location of this organ could be reliably identified without cutting into the plant. Above the tassel, which terminates the shoot, the leaf sheaths overlap, but there is no solid center, and this region contains the inflorescence. Prior to injection, one or two plants of each genotype were cut open with a sharp knife, penetrating just deep enough to see the tassel wrapped in the flag leaf. Based on this height and palpation to find the end of the stem, a 2–3-cm circle was drawn with a black marker at the identified tassel location on all plants. The standard injection protocol involved 1 mL of the mixed U. maydis culture injected all at once, taking care to penetrate only halfway across the plant diameter. If there was strong back pressure indicative of a needle inserted into the stem, the needle was retracted and reinserted a few mm higher on the plant. Modifications to the protocol involved injecting 1 mL in several positions along the immature tassel, or reducing the total volume to 0.1–0.2 mL. For dilution trials, after 5 min of co-incubation of FB1 with FB2, 1/10 and 1/100 dilution stocks were prepared using fungal growth media. The injected fluids collected in the air space around the tassel; after injection, needle tracks in leaves verified that injection had occurred (Fig. 1a); rarely, there was a small injection wound site on the tassel for the media and fungal injections. Cohorts of untreated plants were used to assess normal development and date of pollen shed.
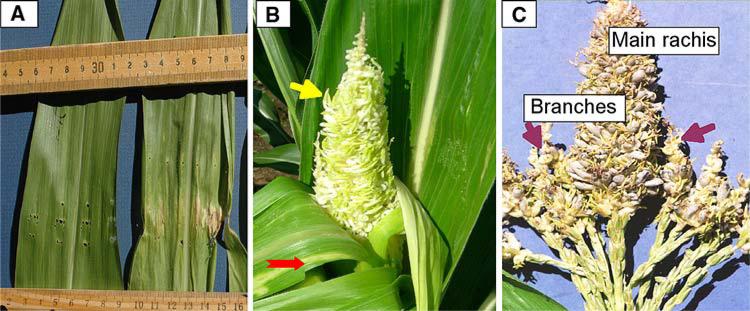
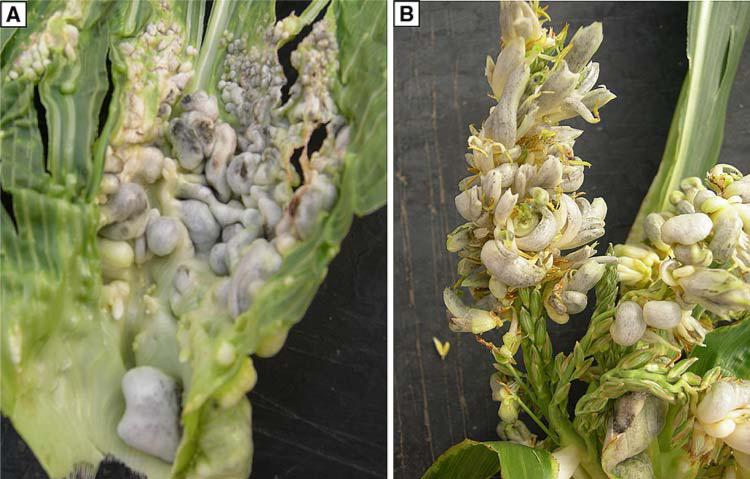
Fig. 1.
Ustilago maydis causes leaf chlorosis and induces tumors in maize tassel organs. Six-week-old field-grown plants (bz2 in the W23 inbred line) were injected with U. maydis. Tassels were evaluated at 10–14 dpi by peeling back the topmost leaves. a Comparison of mock-infected leaves receiving fungal growth media (left leaf) to the chlorotic and necrotic regions induced after U. maydis injection through leaves (right leaf) into the whorl. Leaves were photographed at 7 dpi, and tumors were visible by 12 dpi on the infected leaf (not shown). b The tassel was photographed in situ and was 100% converted to a tumorous condition. Note the precocious emergence and greening of anthers intermingled with immature, white tumors (upper arrow). There are chlorotic and necrotic zones on the leaf blades of the upper five leaves (all pierced by the injection needle) and small tumors are visible on the penultimate leaf (lower arrow). c This tassel was photographed at 14 dpi; tumors are restricted to the upper half of the central rachis and to branch tips (arrows). The branches ring the base of the central rachis and are tightly compressed against it in immature tassels. Approximately, 50% of the tassel has tumors, and these gray growths are filled with black teliospores. Palea, lemma and stamens were converted to tumors. There is a sharp boundary between the tumor and normal zone, and within the tumor zone, lower floret anthers have emerged precociously. Fertile anthers were produced in the normal area below the tumor zone 3 days later, at the normal flowering time for W23. The inverse pattern (not illustrated), in which the lower half of the central spike and branches are affected and the upper half of the tassel is normal, was also observed. Note that the tumors in the central rachis align with the positions of tumors in the branches
Hormone and other chemical treatments
Chemicals were obtained from Sigma (St. Louis, MO) with the exception of brassinosteroid (gift of Zhi-Yong Wang, Carnegie Institution, Stanford, CA) and the cyclopropyl propane dione (CPD) auxin transport inhibitor (Caisson Labs, Rexburg ID). Stock solutions were prepared and kept at 4°C. Approximately 1 mL was delivered to tassels by injection. Track marks and chlorotic zones developed on leaves after some hormone injections. Cohorts were injected with the same hormone for comparison to mock (water) injection; a complete set of experiments was performed with the bz2 W23 line and a subset of hormones was tested on B73, Ky21 and A619.
Treatment evaluations
Approximately 10–14 days post-injection (dpi), the tip of the tassel was visible in most genotypes, and the leaves could be peeled back to observe the entire structure. Tassels were photographed in situ and then dissected from the plant for more detailed observations and photography, including tassel length, the distribution of tumors after U. maydis injection and developmental alterations after hormone treatments. Spikelets were dissected to determine if anthers were maturing normally and contained developing pollen.
Results
Tumor formation in tassel tissues reflects the timing of cell proliferative capacity
The corn smut fungus U. maydis causes sporadic tumors in field-grown maize from haploid spores present in soil or deposited by wind. To examine infection of maize tassel tissues systematically, field-grown W23, Ky21, A619 and B73 plants were injected with freshly mixed haploid FB1 and FB2 U. maydis strains. These strains carry different alleles that define the two mating types and therefore can form dikaryons (Banuett 2002). Injections were performed into the apical region of maize plants using a hypodermic syringe after locating the apex by palpation; the whorl of immature leaf bases above the shoot apical region is more readily compressed because there is no stem. This region contains the vegetative shoot apical meristem and, after the induction of flowering, the tassel inflorescence (Danilevskaya et al. 2008).
The standard protocol involved injecting 1 mL of U. maydis culture into the apical region. As summarized in Table 1, control injections with distilled water caused no visible plant damage; the injection holes in the leaf whorl healed within a few days and could not be located at 10– 14 dpi. Fungal growth media resulted in visible 1–2-mm holes in leaves ringed by chlorotic or necrotic zones a few mm wide that are likely the result of a pathogen-associated molecular pattern response to the sugar and yeast extract in the media (Fig. 1a, left leaf). Because the blades of immature leaves are tightly wrapped encircling the apex, individual leaves had four to six such tiny lesions representing a single passage of the injection needle through the overlapped leaf blades. Neither water (N = 30) nor media injections (N = 60) ever resulted in tumor formation in any line, indicating that wounding of plant leaves in the field or greenhouse did not result in U. maydis infections, even if fungal injections were occurring simultaneously in neighboring plants.
Table 1.
Impact of small molecule injections on maize plants at the 2–5-cm tassel stage
| Treatment | Injection site | Upper leaves | Tassel | Notes |
|---|---|---|---|---|
| Water | Holes heal | No effect | No effect | |
| Mock infection | Tiny chlorosis | Chlorosis | No effect | See Fig. 1a |
| 1 μM Auxin | Tiny chlorotic halo | No effect | No effect | |
| 1 μM CPD | Tiny chlorotic halo | No effect | No effect | |
| 1 μM Gibberellic acid | Tiny chlorotic halo | No effect | No effect | |
| 1 μM Cytokinin | Tiny chlorotic halo | No effect | No effect | |
| 1 μM Abscisic acid | Tiny chlorotic halo | No effect | No effect | |
| 1 μM Methyl jasmonate | Extensive chlorosis and necrosis | Chlorosis and necrosis | Pale tissue zone with arrested anthers | See Fig. 5b |
| 1 nM Brassinosteroid | Extensive chlorosis | Extensive chlorosis | Browning zone with arrested anthers | See Fig. 5c |
Greenhouse and field-grown W23 plants were used for each treatment (N = 8–36 per compound) with three trials of MeJ and BR on W23 and one trial on A619 (N = 50 plants each). CPD is a non-hydrolyzable 1-naphthylphthalamic acid analog that inhibits polar auxin transport (Geisler et al. 2005)
Maize leaf blade growth is generated by a basal proliferation zone near the junction with the leaf sheath (the ligular region), and in a plant at the transition to tassel production, approximately six immature leaves are present in the whorl surrounding the apex. Ustilago maydis induced a strong and reproducible response in these leaves: large chlorotic zones extended from the site of injection toward the base of the blade approaching the zone of cell proliferation (Fig. 1a, right leaf). In the youngest leaf in a whorl, the injection sites were near the leaf tip and more of the leaf surface was affected as expected if this leaf was a small primordium at the time of injection. The chlorotic zone was visible within 2 dpi, and leaves developed tumors in these regions, visible approximately at 10 dpi. Ustilago maydis synthesizes a toxin that directly or indirectly elicits this damage response (Abou-Zeid 1995), but fungal infection in proliferating zones is required to redirect plant growth to tumor formation (Banuett 2002). On penetrating the leaf whorl, the injected fluid fills or partially fills an air pocket surrounding the immature tassel, saturating the developing organ. Most injections pierced the tassel, however, no wound site was found on the tassel from water, mock or most fungal injections. Final tassel length of ~30 cm and branch number 10–14 dpi were indistinguishable from non-injected sibling plants (data not shown).
In vegetative plants up to 5 weeks old, U. maydis caused leaf and stem tumors (N = 30), primarily at the base of blades and the top of the sheath, centered on the ligular region (data not shown) in the zones of maximal cell division. The shoot apex was typically arrested and no tassel formed. Plants injected after 7–8 weeks of maturity (N = 37), at the stage when leaves were post-mitotic and meiosis was completed in the tassel, formed no tumors in the multiple organs tested. Collectively, these observations confirm those of numerous studies on maize seedlings and juvenile plants that only proliferative regions form tumors (Banuett and Herskowitz 1996) and extend this principle to the adult leaves above the ear node and to the tassel.
For plants injected between 5 and 6 weeks, when the shoot apex just changed from a vegetative to an inflorescence meristem (days to this change were established for each inbred line) and the tassel was small (<5 cm), copious tumor formation was observed in tassels (Fig. 1b, c) and the upper three to six leaves. The external staging method was highly reproducible for ascertaining tassel position. Over 93% of the injected plants, summing all experiments with a single 1-mL fungal culture injection into the tassel of inbred line plants, had tassel tumors (N = 230/246). Minor variation in field or greenhouse growth conditions can shift tassel maturation several days in a group of inbred plants (see “Materials and methods”). Tassel evaluation at 10 –14 dpi indicated that plants lacking tassel tumors were likely to have been either too immature (still a vegetative apex at the time of injection) or too old (tassel cells had ceased mitosis) based on the timing of pollen shed of untreated plants. In 10.5% (N = 246) of U. maydis injections at the susceptible stage, all visible florets on the tassel were converted to tumorous growth or had stamen abnormalities (Fig. 1b). In almost all tumor zones, stamens of normal gross morphology or with various defects emerged. Anthers exposed to light are green, and the precociously exerted anthers are sterile. The filaments subtending such anthers elongate ~10 days earlier than normal; some filaments contain U. maydis hyphae, but others appear to lack fungal cells, especially the filaments in the region between the zone of tumors and the area with normal spikelet development.
The most common result was that only a portion of the tassel has organs converted to tumors (Fig. 1c). In these partially converted tassels, there is a sharp demarcation between the zone of tumor formation and precocious anther exertion and a zone of normal anthers that shed fertile pollen. Within the tumor zone, entire spikelets or individual floral parts can be converted to tumors. For example, the upper floret within a spikelet can have severe tumors, while the less mature lower floret exhibits precocious filament elongation and sterile anther emergence. Closer inspection of the emerged anthers indicates that many stamens have severe morphological defects (Fig. 2), particularly the unrolling of locules (or failure to form them properly) and asymmetric growth in the absence of apparent U. maydis infection.
Fig. 2.
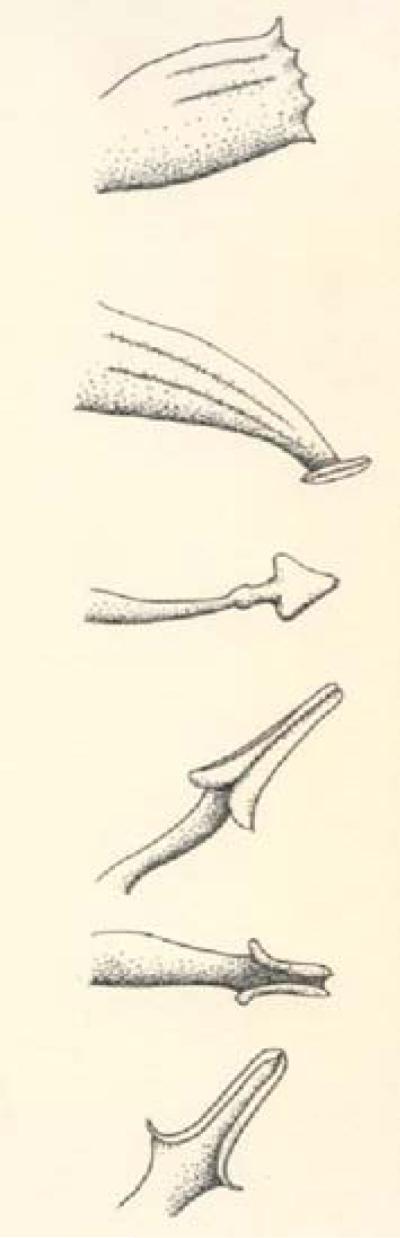
Common anther abnormalities in tumor zones. Anther abnormalities proceeding from the top: fusion of three anthers, flat plate of tissue at the tip of three fused anthers, individual spade-shaped anther lacking locules, slightly unrolled locules, more extensive locule deformation and failure of locule formation
Tumor development occurs in a different pattern than the order of spikelet maturation
Maize tassels have ranks of paired spikelets along the central rachis with branches attached to the base of the rachis. Development is highly synchronized within cohorts of spikelets along the rachis and branches, and collectively the flowers span about 1 week of development (Ma et al. 2008). Each spikelet has two florets: the upper exerts anthers 1 day earlier than the lower. As shown in Fig. 1c, and observed consistently, the zone of tumor formation on the rachis is large, typically involving 50–75% of the tassel. Tumor positions on all or nearly all the lateral branches align with the tumor zone on the central rachis, whether apical, central or basal. In tassel primordia, branches are short and ring the base of the main spike. A subset could be pierced during injection; however, the entire ring of branches was not injected or wounded directly.
Also intriguing, the patterns of tumor growth do not reflect the timing of spikelet maturation, judged by the well-established patterns of anther development and emergence (Cheng and Pareddy 1994). Within the inflorescence spikelet, primordia develop acropetally, from base to tip, over several days. Once formed, however, floral maturation follows a different pattern: central rachis florets mature more quickly than the “older” basal spikelets and several days ahead of the younger, more apical spikelets; the lateral branches have a similar pattern with anthers maturing most quickly centrally followed by anther emergence at the tips and finally the bases. Anthers in the central zone of the lateral branches are about 1 day delayed relative to the initial exerted anthers of the central rachis. This maturation pattern is established when anthers are <1-mm long during the period of rapid mitotic proliferation (Fig. 3a), and this pattern persists through anther emergence.
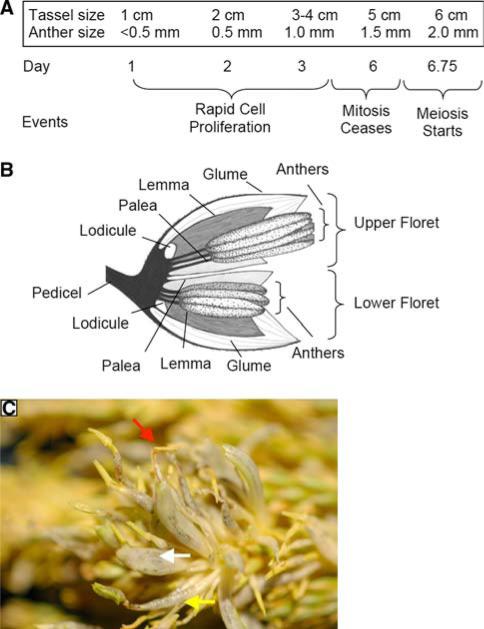
Fig. 3.
Landmark development stages in initial tassel and anther ontogeny. a Approximately 29 days are required for anther initiation within spikelets to pollen shed (Ma et al. 2008). Over the course of approximately 1 week, tassels grow from 1 to 6 cm in length. At the earliest stage, anther primordia are present, and these expand to 0.5 mm by the next day. Within anthers, mitotic proliferation continues until day 6, the 1.5-mm stage, when all cell types are present in a normal anther. After 18 h, meiosis starts in the central cells (Ma et al. 2008). b Progression of tumor location within a spikelet. Spikelets are encased by a pair of leaf-like glumes and contain two florets. Each floret contains three stamens (subtending filament plus anther), encased by a palea and a lemma. The lodicules are generally considered to be modified petals (Bommert et al. 2005); their expansion at spikelet maturity separates the glumes, permitting emergence of mature anthers. Infections of tassels <1 cm results in conversion of the entire spikelet to a tumor, with few recognizable maize structures. Infections at the 1-cm tassel stage result in tumors primarily in the palea and lemma plus stamen filaments, and from the 2–4-cm-tassel stage, upper floret filaments and anthers are converted to tumorous growths. Infections after cessation of cell division in anthers (day 6 in a) can cause tumors in the lodicules. c Tumors and abnormal stamens in the apical region of a W23 tassel induced by injection at the ~1-cm tassel stage. Entire spikelets are converted to tumors (middle arrow), and in some cases recognizable stamens emerge from the tip of such converted spikelets (upper arrow). In some florets, U. maydis infection in the filament is readily visualized (lower arrow), and the anther has an abnormal morphology
The U. maydis injections were timed to occur when spikelets contained 0.5–1-mm anthers at the period of maximal cell proliferation (Fig. 3a) in the upper florets of the central zone of the main spike, or within 2 days after this point, when most spikelets have anthers with mitotically active cells. When the entire upper half of a tassel exhibits tumors (Fig. 1c), this represents conversion of spikelets that differ in maturation by about 5 days and that range from the most mature (slightly larger than 1-mm anthers in the upper florets) to some of the least mature spikelets (some lack anther primordia). Also puzzling is that the entire tassel was exposed to millions of U. maydis cells, yet tumor formation was in one contiguous zone. We hypothesize that fungal infection is, in fact, very rare or uses the injection site. As the entire tassel contains proliferative cells albeit in different floral organs depending on developmental stage, the zone of tumor formation appears to be set by U. maydis growth within the plant, and not by where tissue was exposed externally to the fungus.
To test this hypothesis, cohorts of plants were injected one or three times, delivering <0.1 mL into the tassel with each injection. Another cohort received the standard 1 mL that fills the air space surrounding the apex. Other groups were treated with either 1/10 or 1/100 dilutions of fungi. As shown in Table 2, the average percentage area containing tumors with the standard protocol was 75% (N = 10, all plants had at least some tumors and several plants were 100% converted to tumors). With less than one-tenth as much inoculum, tumor formation was less extensive, but still affected nearly half of the tassel area and was very similar to a 1/10 dilution given in 1 mL (42% tassel conversion). Although these treatments are significantly different from the standard protocol at the P < 0.05 (Student's t test), each had at least one plant that was 100% converted to tumors. We conclude that U. maydis is not “rate-limiting” for tumor formation.
Table 2.
Percentage of tassel converted to tumors in W23 after different injection protocols
| Treatment | Injections | Tassel area with tumors (%) |
|---|---|---|
| 1 mL undiluted (standard) | 1 | 75 |
| <0.1 mL undiluted | 1 | 39 |
| 0.1 mL undiluted, plus 0.7-mL culture media | 3 | 57 |
| ~0.2 mL undiluted | 3 | 70 |
| 1 mL 1/10 diluted | 1 | 42 |
| 1 mL 1/100 diluted | 1 | 66 |
Fungal cultures are grown to A600nm = 1, and 1/10 and 1/100 dilutions of the mixed FB1 plus FB2 strains were tested
Conversion of tassel organs into tumors is developmental stage specific
To analyze the spatial distribution of tumors in distinct tassel organs, standard U. maydis injections were performed daily into cohorts of eight W23 individuals, beginning with plants that had tassels of <0.5 cm and proceeding through the 6-cm tassel stage. This staging was performed by slicing through the leaf whorl at the tassel position and measuring one plant in each cohort. The distribution of tumors was scored after tassel emergence in each cohort. As summarized in Fig. 3a, b, the tissues that converted into tumors depended on the developmental age. Considering tumor formation only in the central part of the rachis (the first part of the tassel to mature), tumors formed predominantly in the spikelet glumes, leaf-life organs encasing the florets, at the earliest stage injected, followed sequentially by tumors involving primarily the lemma, the palea, the upper floret stamens, the lower floret stamens and finally the lodicules over the 7-day span of injections. In each tassel, there was a complex mixture of spikelet tumor types, but the developmental progression was clear. Tumors often involved post-genital fusion of parts, i.e., palea to glume, palea to lemma at the tips, stamen filaments to palea or other filaments, and anther fusion.
Anthers with pre-meiotic defects fail to form tumors
Many unusual anther morphologies were found within and near the tumor area (Fig. 3c). Most of these defects involve unrolled anther locules or a spade-shaped anther. These defective anthers could reflect failure of locules to form properly or to maintain their structure. Within the tumor zone, highly elongated anther filaments often contain maturing teliospores, indicating a local impact on anther growth, but there was no evidence of teliospore or tumor formation within the unrolled anthers.
The observation that U. maydis could cause abnormal anther morphologies suggested that the fungal infection can have a local, but indirect, impact on normal development. To further explore this, three male-sterile mutants of maize with well-characterized developmental defects were tested for the ability to support anther tumor formation or to exhibit abnormal morphological development. The msca1 mutant has anthers of normal external morphology, but all cell types differentiate as leaf cell types rather than anther cells (Chaubal et al. 2003). These anthers fail to suppress leaf development genes that are active during very early stages of normal anther development (Ma et al. 2007). In the case of mac1 (Sheridan et al. 1999), the tapetal and middle cell layers fail to form and there is an excess number of meiotic cells. Both of these mutants have proliferating, albeit abnormal, cells, during the stage when U. maydis infections normally result in anther tumors. In contrast, the ms26 mutant has a normal pattern of cell proliferation, but fails post-meiotically, approximately 7 days after the cessation of mitosis (Albertsen et al. 2009).
As much as 1 mL of U. maydis suspension was injected into plants of families segregating 1:1 (ms//ms = sterile and ms//+ = fertile). This was a blind test, because the injections occurred 2 weeks before fertility could be scored on emerging tassels. The ms26 sterile tassels formed tumors and had abnormal, emerged anthers (Fig. 4a) indistinguishable from those observed in fertile siblings and inbred lines (Fig. 2). In contrast, neither the msca1 (Fig. 4b) nor mac1 tassels formed anther tumors or exerted abnormal anthers. These tassels, at the stage when copious anther tumors formed on normal siblings (Fig. 3c), instead, formed tumors primarily in the stem tissue at the tip and base of the central rachis. When very small (1–2 cm) msca1 or mac1 tassels were injected, a subset of glumes were converted to tumors; when older tassels (~6 cm) were injected, lodicules formed a few tumors (data not shown). These results confirm the progression of sensitivity to U. maydis outlined for normal development (Fig. 3a) and indicate that glumes and lodicules retain susceptibility to U. maydis infection and can support tumor formation. In contrast, the internal floral tissues (palea, lemma, stamens) of msca1 and mac1 did not support tumor formation, although both mutants had anthers with many proliferative cells at the time of infection. Collectively these results indicate that factors present during normal development of specific organs are required by U. maydis for the redirection of maize cells into the tumor pathway.
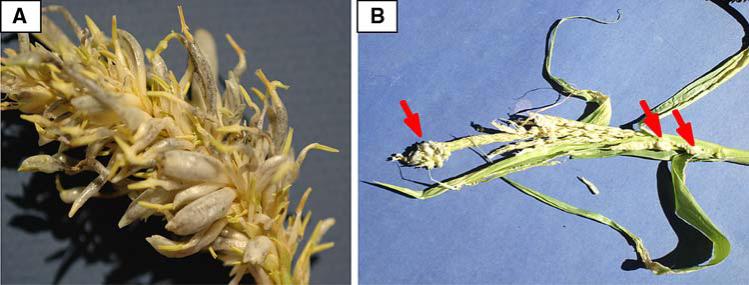
Fig. 4.
Male sterile mutants differ in the ability to support tumor formation. a Fused organ tumors and abnormal filament elongation and anther unrolling in ms26 were identical to fertile siblings in the same family. b msca1 tassel has tumors in the stem at the base and tip of the tassel (arrows), but not in anthers
Hormone injections can cause leaf damage and developmental arrest in maize tassels
The chlorotic and necrotic leaf zones near the sites of U. maydis inoculation could be produced by either direct fungal action or by plant responses. In an effort to phenocopy host responses, 5–6-week old field- and greenhouse-grown maize in several inbred lines were injected with various agents. As shown in Table 1, the injection sites from introducing water healed rapidly and could not be found at 10 dpi, while fungal growth media yielded tiny holes with a necrotic edge and slight chlorosis at the injection site (Figs. 1a, 5a), and limited chlorosis on the younger leaves pierced by the needle. The plant hormones auxin, gibberellic acid, abscisic acid and cytokinin, as well as an auxin transport inhibitor (CPD), at 1-μM concentrations resulted in tiny chlorotic lesions around the injection sites. No phenotypes were observed on the younger leaves, which were assumed to have healed as in the water control. None of these treatments had any impact on tassels; therefore, wounding a tassel does not elicit host responses that cause the stamen abnormalities observed during fungal infection.
Fig. 5.

Impact of injections of fungal growth media, methyl jasmo-nate and BR on adult leaves. Forty-day-old plants (bz2 in the W23 inbred line) were injected with the compounds listed in Table 1. a Mock infections with fungal growth media produce small holes that do not heal, surrounded by small chlorotic zones. This leaf was injected multiple times with a total volume of 1.5 mL at a stage when the leaf was tightly wrapped, so that one injection produced three holes in the blade. b Two injections, totaling 1.5 mL of 1 lM MeJ, produced holes that did not heal with a halo of chlorosis plus long necrotic streaks surrounded by extensive chlorosis. This photograph is of the abaxial surface. The affected tissue is distal to the injection sites. c One injection of 1-nM BR, totaling 1.5 mL, yielded small holes that did not heal with chlorotic halos and a proximal zone of very fine chlorotic dots starting ~1 cm from the injection site
Dramatically different results were obtained with 1 μM of methyl jasmonate (MeJ, Fig. 5b) and 1 nM of brassinosteroid (BR, Fig. 5c). MeJ injections phenocopied the U. maydis symptoms of necrotic lesions adjacent to the injection site and chlorotic striping along major and minor leaf veins. The BR injections produced chlorotic stripes near the injection site, but no necrosis was observed. Both BR and MeJ affected the entire whorl of injected leaves, as is found with an infection. Because BR and MeJ elicit leaf symptoms similar to those found a few days after fungal infection, it is likely that U. maydis induces local production of one or both of these hormones. Support for this conjecture comes from microarray profiling: Doehlemann et al. (2008) found that transcripts for several MeJ bio-synthetic genes are up-regulated over the course of 0.5–8 dpi in seedling leaves. The array platform employed did not contain BR biosynthetic or signal transduction components.
Surprisingly, both BR and MeJ injections caused developmental arrest in the tassel. The affected zone from a single 1-lM MeJ treatment was 3–8 cm of very pale, almost white, tissue on the central rachis and shorter zones on the lateral branches (Fig. 6). The single MeJ treatment arrested spikelets when anthers were <2 mm, and no anthers were ever exerted from the pale zone. After 1-nM BR treatment, a pale zone of 3–6 cm, with slight browning of the glumes, was observed (data not shown). Spikelet development was also arrested with anthers <2 mm. Therefore, these two hormones can disrupt spikelet development during the period of rapid cell proliferation in anthers. Strikingly, spikelets were completely normal adjacent to the pale zones with fertile anthers produced at the expected time (Fig. 6). This reinforces the observations made after U. maydis infection that parts of the tassel can proceed through development normally, despite massive disruption nearby.
Fig. 6.
MeJ causes developmental arrest in tassels. Greenhouse-grown bz2 plants in the W23 inbred line were injected with 1 mL of 1-μM MeJ at the 2–6-cm stage of tassel development, and the tassels were observed at maturity. Zones of 3–8 cm of pale tissue with anthers arrested at the 2 mm size were observed. The glumes had not matured or formed chlorophyll, resulting in a pale, nearly white zone of the tassel. In this example, the pale zone is distal on the central rachis and on all of the branch tips. BR treatment resulted in a browned area of 3–6 cm, which similarly could be in any part of the tassel (not shown)
Pretreatment with MeJ or BR limits tumor formation in tassels
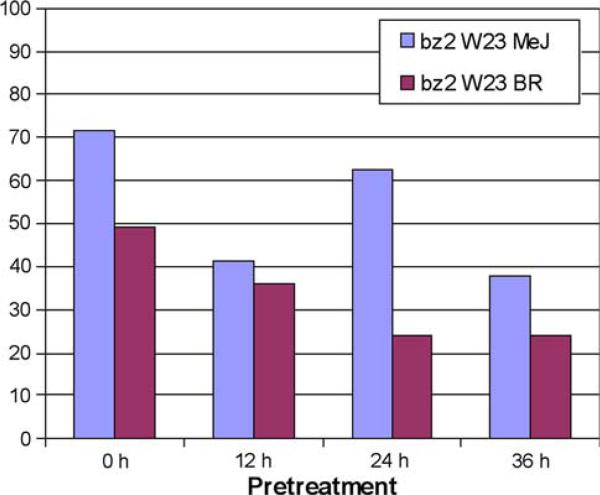
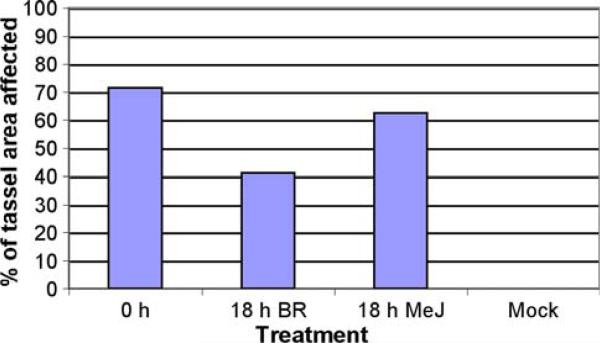
Increased levels of both MeJ and BR are associated with host defenses against pathogens, albeit in often opposing pathways within the network of plant responses (Pieterse et al. 2009). The specific roles, if any, of these hormones have not been established during U. maydis infections in maize. Therefore, an experiment was devised to test whether hormone pre-treatment would decrease the spread of U. maydis. As shown in Fig. 7, in the initial experiment more than 70% of the tassel area of plants receiving the standard 1 mL of fungal culture injection was converted to tumors. Cohorts pre-treated with 1-μM MeJ at 12, 24 or 36 h prior to U. maydis injection showed decreased tumor formation. Hormone pre-treatments were given at the same time early in the morning; consequently, the 12 and 36-h U. maydis injections were given in the early evening in a cooler greenhouse than the morning infection at 24 h. We have noted greater tumor proliferation in morning infections in other trials and attribute the greater tumor formation 24 h after MeJ injection to better fungal growth in a warm greenhouse. In a separate planting of W23, the protective effect of BR pre-treatment was evaluated using a single 1-nM injection of 1 mL. In this trial, ~50% of the tassel area was converted to tumors, and BR 24 and 36 h of pre-treatments halved the percentage area.
Fig. 7.
Extent of U. maydis tumor formation in W23 after MeJ or BR pre-treatment. Groups of ten plants received no pre-treatment (positive control for calculating the tassel area impacted by fungal infection) or a single hormone treatment (1 mL, 1-μM MeJ or 1-nM BR) at the times indicated prior to U. maydis infection
No tumors were ever observed in the pale zones of a MeJ- or BR-injected tassel. Therefore, MeJ and BR treatments generate chemical phenocopies of early-acting ms mutants in which anthers arrest prior to meiosis and cannot form tumors. Do these hormone pre-treatments suppress tumor formation only in the zone of developmental arrest? Tassels were approximately 30 cm at the time of evaluation. The standard injection protocol in the MeJ experiment converted more than 21 cm to tumors, on average. MeJ injection caused a zone of developmental arrest averaging ~6 cm, but tumors were found in an area less than 12 cm, on average. The suppression of tumors encompassed a larger area than can be explained solely by the area lacking maturing spikelets. In the BR trials, plants receiving the standard inoculation had 15 cm of tassel, on average, converted to tumors, and this was reduced to about 7 cm by BR. Hormone caused spikelet arrest in a zone of ~4 cm, considerably smaller than the area lacking tumors. Therefore, both MeJ and BR impede U. maydis tumor formation in an area greater than the ms area. Indeed, in many cases, the tumor-free zone, centered around the region of developmental arrest, was bracketed by tumor zones distally and proximally, which in plants with no hormone pre-treatment would most likely have been a zone of continuous tumors based on the distribution of tumors on plants receiving the standard infection.
To determine if the MeJ and BR effects are general, a second trial was performed with an 18-h hormone pre-treatment of inbred A619 (Fig. 8). In this experiment, the MeJ impact was modest (~15% reduction in tumor area), but BR was nearly as effective as observed in the W23 inbred line. A mock treatment with water was included as a control, and no wounding response of chlorosis or necrosis was observed. However, U. maydis alone and both hormones elicited leaf symptoms similar to those observed with W23 (Figs. 1a, 5b, c).
Fig. 8.
Extent of U. maydis tumor formation in the A619 genotype after MeJ or BR pre-treatment of tassels. Groups of ten plants received either no pretreatment (positive control for establishing the extent of tumor formation) or a single mock (water) or hormone injection (as defined in Fig. 7) 18 h prior to fungal infection using the standard 1 mL of U. maydis suspension
Tumor formation in maize mutants with hormone or growth abnormalities
Given the mounting evidence that plant genotype and hormone status plays a role in responses to U. maydis, we next tested the ability of the fungus to elicit adult leaf and tassel tumors in several maize mutants defective in hormone biosynthesis or response. The developmental defects for most mutants are reviewed by Barazesh and McSteen (2008), plus a description of an1 is given by Bensen et al. (1995) and that of Kn1 by Smith et al. (1992).
anther ear1 bz2 is a semi-dwarf, ms mutant whose gibberellin deficiency and filament elongation defect can be rescued by the exogenous application of GA. Mutant plants infected with U. maydis formed unusually large tumors on the leaf sheaths and ligules. The most common phenotypes on tassels were adherent tassel branches and tumors that resulted in the fusion of two or more lateral branches. The filaments in U. maydis-infected anther ear1 bz2 individuals also were elongated, although this is a defining mutant characteristic. The “selected line” in which plants were slightly taller and some filament elongation occurred gave the same tumor phenotypes as the standard an1 line.
Dwarf8 encodes a DELLA-like transcription factor that negatively regulates GA responses. In contrast to the anther ear1, Dwarf8 failed to produce any tumors on adult leaves or tassel in all but one individual (N = 8).
Knotted1 is characterized by excessive, ectopic growth of leaf tissue that results from the mis-expression of a KNOX transcription factor in leaves rather than restriction to the shoot apex. We hypothesized that these ectopic growths of rapidly expanding cells would be “hotspot sites” of U. maydis action. On adult leaves, tumors formed preferentially along leaf veins on the adaxial surface, which was the same surface as the ectopic knots in Kn1. Tumors that formed on leaves surrounding the tassel were the largest observed in any genotype. Surprisingly, the tassels in 75% of the U. maydis-infected individuals were completely converted into large tumor masses, though there was no apparent phenotype in Kn1 tassels (Fig. 9). A subset of the remaining Kn1 tassels were “arrested” in development, a phenotype similar to the MeJ and BR treatments.
Fig. 9.
The Kn1-dominant mutant produces exceptionally large tumors. a Adaxial leaf blade; pale knots are tissue outgrowths on veins. b Tassel at 13 dpi
sparse inflorescence1 (spi1) is defective in auxin bio-synthesis. There are few lateral branches and a sparse distribution of spikelets along the central rachis and branches. Because the tassel defect was very early in development, we hypothesized that few or no tumors would form in spi1 plants. The adult leaves of ~50% of the spi1 mutants formed leaf tumors, but no tumors formed on the tassel except for sporadic small tumors at the base of the tassel stem. The most common tassel phenotypes were severely arrested tassels (i.e., <1 vs. 30 cm for wild type) and excessive elongation of the spikelet pedicel and bracts.
Discussion
To better understand the role of the maize host in tumori-genesis induced by U. maydis, we developed an efficient injection protocol for infecting immature tassels and the whorl of leaves surrounding the inflorescence. Initial experiments demonstrated that tumor formation required cell proliferation in adult leaves and tassel organs, as was already established for seedling and juvenile leaves (Banuett 2002), and that standard inbred lines showed similar tumor formation. All floral organs can form tumors, but there is a progression of which parts are most prominently involved that follows the chronological pattern of organ maturation in spikelets. Although a substantial inoculum was delivered to each tassel and the area converted to tumors averaged more than half the tassel, tumor distribution in the central rachis and branches more likely reflected hyphal growth within the tissues than external exposure to fungi. The zone of tumors contained developmentally abnormal maize organs, particularly precociously extended filaments and anthers with poorly formed locules. Except in cases where the entire tassel was converted to tumors, tumor-free tassel areas continued normal development and shed fertile pollen at the expected time. These observations indicate that despite the excessive growth sustained by nutrients and water delivered to tumors, normal development can proceed in uninfected areas. At the junction of the normal and tumor areas, some spikelets had precociously exerted anthers lacking any evidence of fungi. Thus, we conclude that fungal factors and host responses to them can impact nearby florets, but the responses are highly localized and do not reprogram the entire tassel.
Male-sterile mutants with defects in anther cell proliferation and differentiation prior to meiosis failed to form anther tumors. In these lines, tumors were generally restricted to stem tissue at the base of the tassel and at the tips of the rachis and branches. All of the ms mutants formed normal leaf tumors. Based on these observations, we hypothesize that U. maydis interacts differently with vegetative and reproductive organs, possibly by expressing different genes in specific plant parts, and that the ms mutants provide either a confounding environment (msca1 expresses a combination of leaf and anther gene products) or lack normal cell types and gene products (mac1) required for tumor progression in the tassel. The ms26 mutant, which develops normally during the period of mitotic proliferation in spikelets, forms tumors similar to wild-type siblings, indicating that later defects that truncate development do not interfere with tumor formation. These observations raise important questions about what types of cells can be colonized by Ustilago and in what specific ways host differentiation status influences fungal success.
Because plant hormones are undoubtedly involved in supporting tumor growth, we investigated whether these agents can cause any of the phenotypes observed after U. maydis infection. Surprisingly auxin, an auxin transport inhibitor, gibberellic acid, cytokinin or abscisic acid delivered in single 1-μM injections failed to manifest any symptoms on leaves or inflorescences. In contrast, both MeJ and BR caused leaf symptoms similar to U. maydis, with the MeJ-treated leaves showing chlorotic and necrotic patterns virtually identical to fungal injections. These two hormones also phenocopied the early-acting ms mutants by generating tassel regions in which spikelets ceased maturing, with anthers arrested at <2 mm. Pre-treatment with MeJ and BR prevented U. maydis-induced tumor formation within and adjacent to the ms zone. The MeJ and BR experiments confirm that tassel development is highly compartmentalized in that failure in one area does not impede normal development in the remaining area. Furthermore, the chemical sterilization zone and only the immediately adjacent 1–2 cm of spikelets lacked tumors: the hormone block to tumors was also highly localized. Both the distribution of the applied hormone and maize cell responses to the treatment fail to elicit systemic responses that impact tassel development.
Little is known about the role(s) of BR in maize, particularly in floral development. However, MeJ is implicated as a key regulator in the abortion of pistils in the initially perfect flowers of the tassel (Acosta et al. 2009) and required for stamen development in Arabidopsis (Mandaokar and Browse 2009). When the MeJ levels or signalling is disrupted, female floral parts form in the tassel, and these are fertile resulting in the tasselseed phenotype; anther development is suppressed. Application of MeJ can suppress female development in tasselseed1, permitting normal anther maturation (Acosta et al. 2009). One puzzling aspect is that in our experiments, a single application of 1-μM MeJ to young tassels resulted in blocks of spikelet failure in otherwise normal inbred lines, while in the tasselseed1 study three applications of 1-mM MeJ restored normal anther development. It is unclear why a 3,000-fold higher treatment of MeJ permitted normal anther maturation, but it is possible that in the tasselseed1 mutant, sensitivity to the hormone was substantially lower.
The MeJ and BR experiments establish that host physiology is crucial to the establishment of Ustilago-induced tumors in maize tassels. Analysis of tumor formation in a range of developmental mutants with altered growth control demonstrates that host genotype is also critical. Decreased GA levels in the an1 mutant prevent normal filament elongation, but infection with U. maydis causes the complete suite of tumor types plus precociously elon-gated filaments and unrolled anther locules as observed in inbred lines. Disruption of GA responses in Dwarf8, however, eliminated tumor formation in both leaves and tassels. The excess leaf growth mutant Kn1 induced exceptionally large tumors in both leaf and tassel tissues. These observations demonstrate that a genetic analysis of host requirements for tumor formation will be fruitful and provide the foundation for future tests of the hypotheses that specific U. maydis genes are required to elicit tumors in individual organs and that maize mis-differentiation mutants fail to provide plant factors required for tumor progression in adult leaves and tassels.
Acknowledgments
Many thanks are due to Flora Banuett for traveling to Stanford with the FB1 and FB2 strains and demonstrating fungal injection. Allison Stegner assisted with the hormone experiments and sketched the interpretative drawings. Regine Kahmann provided additional strains and useful advice on tumor formation. Gunther Doehlemann commented on a draft of the manuscript. DSS was supported by a National Institute of Health Ruth L. Kirschstein National Research Service post-doctoral award (1 F32 GM076968-01). Research was supported in part by a National Science Foundation award to VW (IOS-0852788).
Footnotes
Communicated by Sheila McCormick.
References
- Abou-Zeid AM. Effect of Ustilago maydis (DC) Corda and its toxin on some maize cultivars. J Phytopathol (Berlin) 1995;143:577–580. [Google Scholar]
- Acosta IF, Laparra H, Romero SP, Schmelz E, Hamberg M, Mottinger JP, Moreno MA, Dellaporta SL. tasselseed1 is a lipoxygenase affecting jasmonic acid signaling in sex determination of maize. Science. 2009;323:262–265. doi: 10.1126/science.1164645. [DOI] [PubMed] [Google Scholar]
- Albertsen MC, Fox T, Huffman G, Trimnell M. Nucleotide sequences mediating male fertility and method of using same. 2009 Patent number US 7,517,975 B2.
- Banuett F. Pathogenic development in Ustilago maydis. In: Osiewacz HD, editor. Molecular biology of fungal development. Marcel Dekker; New York: 2002. pp. 349–398. [Google Scholar]
- Banuett F, Herskowitz I. Identification of fuz7, an Ustilago maydis MEK/MAPKK homolog required for A locus-dependent and A locus-independent steps in the fungal life cycle. Genes Dev. 1994;8:1367–1378. doi: 10.1101/gad.8.12.1367. [DOI] [PubMed] [Google Scholar]
- Banuett F, Herskowitz I. Discrete developmental stages during teliospore formation in the corn smut fungus, Ustilago maydis. Development. 1996;122:2965–2976. doi: 10.1242/dev.122.10.2965. [DOI] [PubMed] [Google Scholar]
- Barazesh S, McSteen P. Hormonal control of grass inflorescence development. Trends Plant Sci. 2008;13:656–662. doi: 10.1016/j.tplants.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Bensen RJ, Johal GS, Crane VC, Tossberg JT, Schnable PS, Meeley RB, Briggs SP. Cloning and characterization of the maize an1 gene. Plant Cell. 1995;7:75–84. doi: 10.1105/tpc.7.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bommert P, Satoh-Nagasawa N, Jackson D, Hirano H. Genetics and evolution of inflorescence and flower development in grasses. Plant Cell Physiol. 2005;46:69–78. doi: 10.1093/pcp/pci504. [DOI] [PubMed] [Google Scholar]
- Callow JA. Endopolyploidy in maize smut neoplasms induced by maize smut fungus, Ustilago maydis. New Phytol. 1975;75:253–257. [Google Scholar]
- Callow JA, Ling IT. Histology of neoplasms and lesions in maize seedlings following the infection of sporidia of Ustilago maydis (DC) Corda. Physiol Plant Path. 1973;3:489–494. [Google Scholar]
- Chaubal R, Anderson JR, Trimnell MR, Fox TW, Albertsen MC, Bedinger P. The transformation of anthers in the msca1 mutant of maize. Planta. 2003;216:778–788. doi: 10.1007/s00425-002-0929-8. [DOI] [PubMed] [Google Scholar]
- Cheng P-C, Pareddy DR. Morphology and development of the tassel and ear. In: Freeling M, Walbot V, editors. The maize handbook. Springer; New York: 1994. pp. 37–47. [Google Scholar]
- Danilevskaya ON, Meng X, Selinger DA, Deschamps S, Hermon P, Vansant G, Gupta R, Ananiev EV, Muszynski MG. Involvement of the MADS-box gene ZMM4 in floral induction and inflorescence development in maize. Plant Physiol. 2008;147:2054–2069. doi: 10.1104/pp.107.115261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doehlemann G, Wahl R, Horst RJ, Voll LM, Usadel B, Poree F, Stitt M, Pons-Kühnemann J, Sonnewald U, Kahmann R, Kämper J. Reprogramming a maize plant: transcriptional and metabolic changes induced by the fungal biotroph Ustilago maydis. Plant J. 2008;56:181–195. doi: 10.1111/j.1365-313X.2008.03590.x. [DOI] [PubMed] [Google Scholar]
- Doehlemann G, van der Linde K, Aßmann D, Schwammbach D, Hof A, Mohanty A, Jackson D, Kahmann R. Pep1, a secreted effector protein of Ustilago maydis, is required for successful invasion of plant cells. PLoS Pathog. 2009;5:e1000290. doi: 10.1371/journal.ppat.1000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler M, Blakeslee JJ, Bouchard R, Lee OR, Vincenzetti V, Bandyopadhyay A, Titapiwatanakun B, Peer WA, Bailly A, Richards EL, Ejendal KFK, Smith AP, Baroux C, Grossniklaus U, Müller A, Hrycyna CA, Dudler R, Murphy AS, Martinoia E. Cellular efflux of auxin catalyzed by the Arabidopsis MDR/PGP transporter AtPGP1. Plant J. 2005;44:179–194. doi: 10.1111/j.1365-313X.2005.02519.x. [DOI] [PubMed] [Google Scholar]
- Kämper J, Kahmann R, Bölker M, et al. Insights from the genome of the biotrophic fungal plant pathogen Ustilago maydis. Nature. 2006;444:97–101. doi: 10.1038/nature05248. [DOI] [PubMed] [Google Scholar]
- Krüger J, Loubradou G, Wanner G, Regenfelder E, Feldbrügge M, Kahmann R. Activation of the cAMP pathway in Ustilago maydis reduces fungal proliferation and teliospore formation in plant tumors. Mol Plant Microbe Interact. 2000;13:1034–1040. doi: 10.1094/MPMI.2000.13.10.1034. [DOI] [PubMed] [Google Scholar]
- León-Ramírez CG, Cabrera-Ponce JL, Martínez-Espinoza AD, Herrera-Estrella L, Méndez L, Reynaga-Peña CG, Ruiz-Herrera J. Infection of alternative host plant species by Ustilago maydis. New Phytol. 2004;164:337–346. doi: 10.1111/j.1469-8137.2004.01171.x. [DOI] [PubMed] [Google Scholar]
- Ma J, Duncan D, Morrow DJ, Fernandes J, Walbot V. Transcriptome profiling of maize anthers using genetic ablation to analyze pre-meiotic and tapetal cell types. Plant J. 2007;50:637–648. doi: 10.1111/j.1365-313X.2007.03074.x. [DOI] [PubMed] [Google Scholar]
- Ma J, Skibbe DS, Fernandes JF, Walbot V. Male reproductive development: gene expression profiling of maize anther and pollen ontogeny. Genome Biology. 2008;9:R181. doi: 10.1186/gb-2008-9-12-r181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandaokar A, Browse J. MYB108 acts together with MYB24 to regulate jasmonate-mediated stamen maturation in Arabidopsis. Plant Physiol. 2009;149:851–862. doi: 10.1104/pp.108.132597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller O, Kahmann R, Aguilar G, Trejo-Aguilar B, Wu A, de Vries RP. The secretome of the maize pathogen Ustilago maydis. Fungal Genet Biol. 2008;45:S63–70. doi: 10.1016/j.fgb.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Pieterse CMJ, Leon-Reyes A, Van der Ent S, Van Wees SCM. Networking by small-molecule hormones in plant immunity. Nature Chem Biol. 2009;5:308–316. doi: 10.1038/nchembio.164. [DOI] [PubMed] [Google Scholar]
- Reineke G, Heinze B, Schirawski J, Buettner H, Kahmann R, Basse CW. Indole-3-acetic acid (IAA) biosynthesis in the smut fungus Ustilago maydis and its relevance for increased IAA levels in infected tissue and host tumour formation. Mol Plant Path. 2008;9:339–355. doi: 10.1111/j.1364-3703.2008.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan WF, Golubeva EA, Abrhamova LI, Golubovskaya IN. The mac1 mutation alters the developmental fate of the hypodermal cells and their cellular progeny in the maize anther. Genetics. 1999;153:933–941. doi: 10.1093/genetics/153.2.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LG, Greene BA, Veit B, Hake S. A dominant mutation in the maize homeobox gene, Knotted-1, causes its ectopic expression in leaf cells with altered fates. Development. 1992;116:21–30. doi: 10.1242/dev.116.1.21. [DOI] [PubMed] [Google Scholar]