Abstract
The use of spinal implants for spine fusion has been steadily increasing to avoid the risks of complications and donor site morbidity involved when using autologous bone. A variety of fusion cages are clinically available, with different shapes and chemical compositions. However, detailed information about their surface properties and the effects of such properties on osteogenesis is lacking in the literature. Here we evaluate the role of surface properties for spinal implant applications, covering some of the key biological processes that occur around an implant and focusing on the role of surface properties, specifically surface structure, on osseointegration, drawing examples from other implantology fields when required. Our findings revealed that surface properties such as micro-roughness and nanostructures can directly affect early cell behavior and long-term osseointegration. Micro-roughness has been well established in the literature to have a beneficial effect on osseointegration of implants. In the case of the role of nanostructures, the number of reports is increasing and most studies reveal a positive effect from the nanostructures alone and a synergistic effect when combined with micro-rough surfaces. Still, long-term clinical results are necessary to establish the full implications of surface nanomodifications.
Keywords: (4 to 6) spine fusion, spinal implants, osseointegration, titanium, bone, nano structures, surface roughness, microrough
1 Introduction
Musculoskeletal diseases such as back pain, arthritis and bone fractures, have been recognized as the most reported health condition in the United States, amounting to almost 8% of the US gross domestic product in lost wages and healthcare related costs [1]. In the case of chronic back pain, spinal fusions have become a viable treatment to eliminate pain and restore a patient's quality of life [2-4]. Autologous bone grafts are the gold standard filler for orthopaedic surgeries because of their osteogenic capabilities, but increased complications and morbidity of the donor site have shifted the focus to graft substitutes and spinal implant devices [5, 6]. With an aging population in the United States, there is a pressing need for surgical approaches that can capitalize on the intrinsic regenerative capacity of mineralized tissues to provide a more permanent treatment.
The modern use of metallic and polymeric implants for orthopaedic and dental applications has been evolving for the last 60 years, with major advances coming from the dental implant field [7-10]. Originally, endosseous implants were expected to perform their job simply through a mechanical anchorage with bone. Early efforts had relatively high failure rates, in part due to a layer of fibrous connective tissue that grows between the bone and the implant [11] (Fig. 1B). The formation of the fibrous capsule, thought to be an inevitable consequence of the implantation procedure [12, 13], can start a vicious cycle of micromotion and inflammation around the implant that eventually leads to osteolysis and implant failure [14-16]. To achieve long-lasting and successful outcomes, strong and direct interaction between bone and the implant surface is required [7, 17]. Such direct contact between bone and the implant surface defines osseointegration and is the current goal of a successful bone implantation procedure (Fig. 1C).
Figure 1.
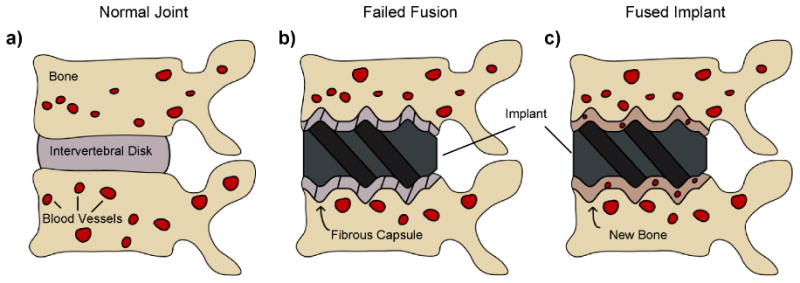
Schematic of (A) a normal joint, (B) a failed implant and (C) a fused and osseointegrated implant.
In the orthopaedic implant field, several reports have found fibrous capsules around implants of metallic [18, 19] or polymeric nature [20-22]. This type of failure is commonly attributed to toxic wear debris phagocytosed by macrophages and other cells of the surrounding tissue [23-25]. However, several cases that resulted in fibrous encapsulation of implants did not present detectable traces of wear debris [26, 27] and still elicited an aseptic inflammatory response that can lead to osteolysis [14]. Most of these cases involve polymeric or metallic implants with smooth surfaces [27-29]. Yet from experiences in the dental field, it is now well accepted that the presence of a fibrous layer can be avoided by controlling the surface properties of the implant, such as increasing surface microroughness, to promote bone apposition directly onto the implant surface [30-33].
The process of osseointegration involves a complex chain of events, from protein adsorption and blood clotting at the implant surface to site infiltration and biological recognition of the surface by mesenchymal stem cells (MSCs) and osteoblasts, finally leading to bone formation by these cells at the interface, thus creating an intimate bond between the bone and implant [32, 34]. These events are directly and indirectly affected by the surface properties of the device, making them key determinants of the implant's outcome in vitro, in vivo and clinically [35-37] (Fig. 2).
Figure 2.
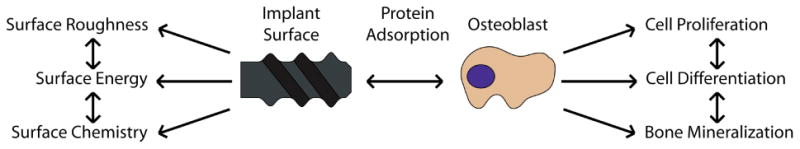
Diagram showing the direct and indirect interactions between surface properties (e.g., surface roughness, surface energy, surface chemistry) and biological events, such as protein adsorption and osteoblast response (e.g., proliferation, differentiation, bone mineralization).
A variety of fusion cages are clinically available, with different shapes and chemical compositions. However, detailed information about their surface properties and the effects of such properties on osteogenesis is lacking in the literature. Additionally, no systematic reviews are available on the role of surface properties for spinal implant applications. Thus, this review will cover some of the key biological processes that occur around an implant focusing on the role of surface properties, specifically surface structure, on osseointegration, drawing examples from other implantology fields when required. Other factors that may influence the outcome of the implant, such as surgical technique, patient's record and implant shape have been reviewed elsewhere [38-40].
2 Osseointegration: Key Biological Processes
2.1 Wound Healing and Fibrin Clot Formation
The process of osseointegration involves several biological events that determine the mechanical stability and outcome of the implant. One of the first events to occur when an implant is placed in the body is the adsorption of water molecules, proteins and lipids from the blood to the surface of the device [41, 42]. The specific protein profile presented on the surface will depend on the surface properties of the implant. Many proteins present in blood may interact with the implant's surface, some of which are associated with the host inflammatory response, such as fibrinogen and complement molecules, as well as other proteins involved in cell attachment, such as fibronectin and vitronectin [42-44]. The attachment of blood platelets and the subsequent release of their inner contents promotes the formation of fibrin clots that serve as an immature meshwork to fill voids and facilitate cell migration towards the surface of the implant [45] (Fig. 3). The surface coverage and strength of attachment of the fibrin clots to the implant surface depends on implant surface properties [46, 47]. One hypothesis suggests that increasing surface roughness enhances the strength of fibrin clot attachment, which is important to withstand the forces of cells moving along and pulling these fibrin fibers to promote wound contraction [48]. Other reports propose that increasing surface roughness supports higher amounts of fibrin clot extension on the surface, promoting a better wound healing response [46].
Figure 3.
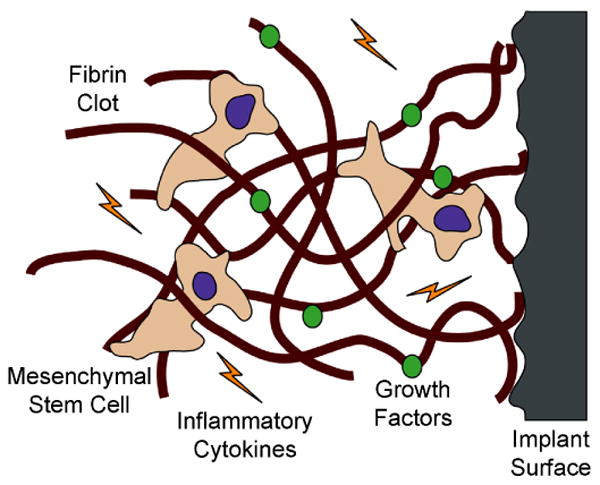
Schematic depicting fibrin clot adhesion to a rough surface and mesenchymal stem cell (MSC) migration through the clot. The MSCs pull on the fibrin clot to reach the surface of the implant, and at the same time are exposed to several inflammatory cytokines and growth factors that can influence their differentiation state.
Some of the first cells to arrive at the implantation site include neutrophils and macrophages, which clean the wound site from possible pathogens and necrotic tissue [49, 50]. Other important cell types to colonize the implantation site include MSCs from blood and bone marrow [47, 51]. These cells have the motility and enzymatic activity to travel through dense fibrin clots on their way to the surface of the implant [52], where they will be exposed to inflammatory cytokines and growth factors conducive to wound healing and tissue regeneration [53] (Fig. 3). MSCs have the potential to differentiate into several cell types, such as osteoblasts, chondrocytes, and fibroblasts, depending on the biological environment and the implant surface properties [53, 54]. However, the fate of stem cells around osseous implants seems to be biased towards the formation of bone tissue, with some soft tissue being formed at the interface between bone and the implant depending on the surface characteristics of the implant. Thus, by the time MSCs reach the surface of the implant, they might have already set in motion the differentiation machinery necessary to become pre-osteoblasts and start forming bone.
2.2 Mimicking Bone Structure: Bone Remodeling
Once the implant has been stably fixed in the bone with the fibrin meshwork firmly established, bone can form on two different fronts: on the surface of the bone surrounding the implant (distance osteogenesis) and directly on the surface of the implant (contact osteogenesis) [48]. Depending on the surface characteristics, the differentiating osteoblasts reaching these two fronts will either proliferate for a few cycles or begin laying down a noncollagenous assortment of proteins that initiates mineralization called lamina limitans, or “cement line” [55-57]. The cement line, rich in proteins like osteopontin, bone sialoprotein and proteoglycans [34, 58, 59], further promotes osteoblast recruitment and maturation. For successful osseointegration, contact osteogenesis is required and should be promoted by the implant.
The next stage in the osseointegration process requires a bone remodeling cycle in which osteoclasts resorb the newly formed bone to resolve microcracks and prime the surface for new bone formation [60, 61]. Osteoclasts acidify the mineralized matrix just underneath their ruffled membranes to dissolve calcium phosphate crystals and create microscale resorption lacunae that are 30 to 100 μm in diameter [62, 63]. Osteoclasts, however, do not produce collagenase, an enzyme required to degrade collagen [60]. Thus, resorption lacunae have various submicro- and nanoscale features created by the collagen tufts and fibers left by osteoclasts, giving bone a high degree of structural complexity. This nanotopography, with its inherent biochemical information, could be the signal that osteoblasts require when looking for a surface that requires new bone formation. The concept of mimicking the hierarchical structure of bone on implant surfaces by including nanostructures on commercially available devices originates from this observation (Fig. 4).
Figure 4.
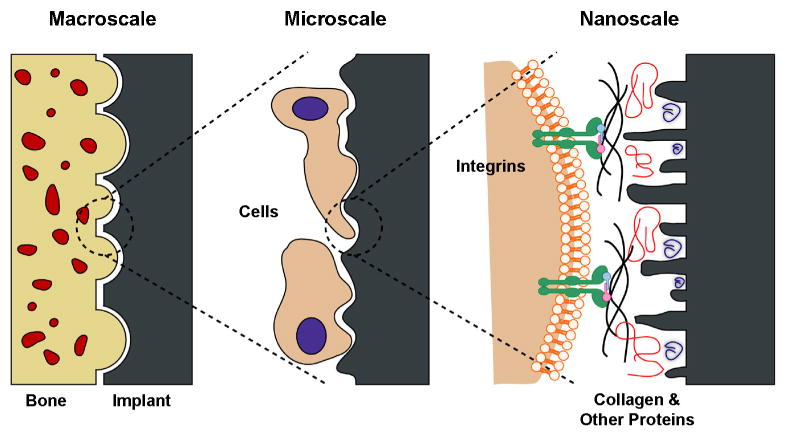
Interactions between bone and the implant surface at different length-scales. At the macroscale, the implant should provide a good mechanical fixation with bone. At the microscale, micro- and submicro-features presented on the surface can directly interact with osteoblasts and mesenchymal stem cells. At the nanoscale, cell membrane receptors, such as integrins, can recognize proteins adsorbed on the surface, which in turn are modulated by the nanostructures on the surface. Figure used with permission from [115].
If the surface properties of the implant are not selected appropriately, the invading cells can form a layer of fibrous tissue between the implant and the bone that jeopardizes the outcome of the procedure. The lack of bone attachment to such an implant generates a vicious cycle that starts with micromotion and inflammation, and ends up with thickening of the fibrous layer, degradation of the surrounding bone and loosening of the implanted device [14, 26, 27]. Interestingly, tailoring the surface properties of implants can help avoid these failed outcomes.
3 Osseointegration and Implant Surface Structure
Because certain patient conditions such as old age, poor bone quality, and smoking can jeopardize the success of the implantation surgery [40], the goal is to design implants in such a way as to minimize the effect of patient variables and improve the success rate. Much attention is paid to the macro shape of an implant to produce good primary fixation and to the bulk chemical composition of the implant to provide the mechanical properties required for the application. While these macroscale aspects are important, surface characteristics at the micro-, submicro- and nanoscale must be considered at the same time to ensure successful and long-term osseointegration. A loose definition of micro, submicro, and nano applies to features having at least one of their dimensions (i.e., height, length, width) smaller than 100 μm, 1 μm, or 100 nm, respectively. More stringent evaluations apply the aforementioned thresholds to all dimensions of the feature. Notably, such small surface structures are invisible to the naked eye and require specialized equipment to quantify them, such as electron microscopy [64], laser confocal microscopy [65] or atomic force microscopy [66].
In the dental and orthopaedic fields, implants are commonly made out of metals, with titanium and its alloys being widely used for dental implant applications due to their suitable weight-to-strength ratio and good biological performance. Interestingly, the surface chemistry of an implant can be quite different from its bulk chemistry. Titanium spontaneously forms a thin oxide layer that inhibits further corrosion of the implant. This oxide layer, which is ceramic in nature, is suggested to provide titanium's good biological performance by mimicking the ceramic properties of hydroxyapatite in bone [30]. However, the topography of the surface, regardless of the chemistry, still requires attention to enhance the process of osseointegration.
In the case of spinal implants, poly-ether-ether ketone (PEEK) has become a popular bulk material for spinal cage manufacturing due to its mechanical properties, which can be tailored to resemble those of bone, and its low radio-opacity when compared to metals [20]. Although attractive, these properties are not required for successful osseointegration. Furthermore, PEEK promotes the formation of a fibrous layer between bone and the implant [21, 22]. The orthopedic industry has attempted to overcome this fibrous encapsulation through the use of different surface modifications such as coating the PEEK surface with titanium [67-69]. A vast literature related to surface modification of Ti and Ti alloy implants supports its importance for successful osseointegration, as discussed below. However, reports on surface modification of PEEK are not as readily available in the literature or are still proprietary, so the clinical value of these modifications is not yet established.
3.1 Micro-Roughness Effect in Vivo
Most commercially-available implants in the dental field contain some type of surface modification to increase their surface roughness. This is in part due to the large number of studies showing beneficial results of microroughness in vitro, in vivo and clinically [10, 31, 36]. Several surface modification techniques exist to increase microroughness such as acid etching, sand blasting, heat treatments, anodic oxidation, as well as the combination of any of these treatments (Fig. 5). The surface topography created by these different microstructuring treatments will vary greatly and, although seldom compared among each other, they commonly enhance the process of osseointegration when compared to relatively smooth surfaces [70].
Figure 5.
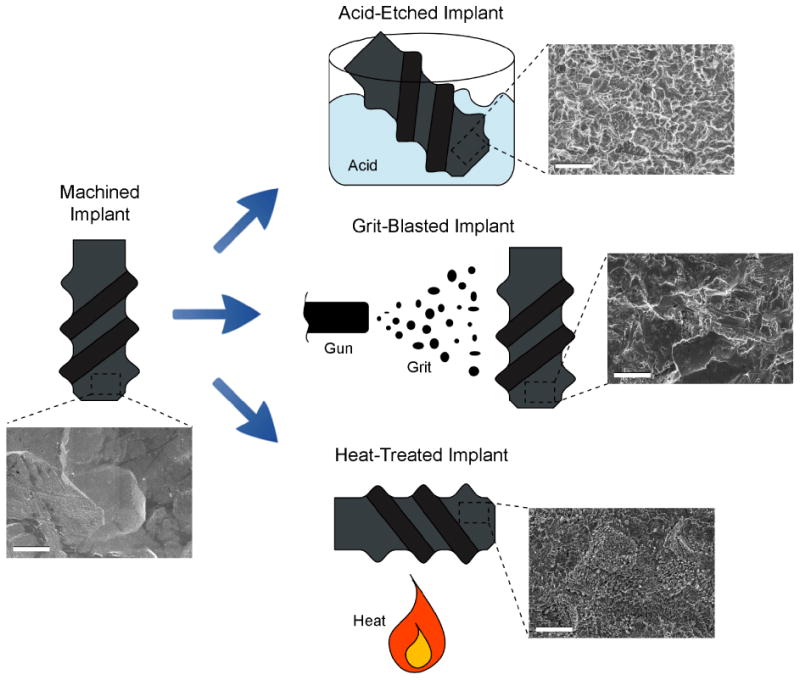
Schematic and SEM images of different examples of surface modification treatments that can be applied to machined implants, including acid etching, grit blasting and heat treatment. SEM scale bar = 3 μm.
In one study, machined, relatively smooth pedicle screws were compared to grit-blasted, microrough screws, both made out of titanium alloy (Ti6Al4V), in a sheep spine model after 12 weeks of healing [70]. Implant osseointegration was assessed by micro-computed tomography (micro-CT) and histomorphometry. The results from micro-CT showed that both machined and grit-blasted implants were surrounded by bone. However, the resolution of the micro-CT was not sufficient for detailed judgment of the bone-implant interface. Through histomorphometrical analysis, higher incidence of soft tissue between bone and the machined surface was found when compared to the grit-blasted surface, and this observation was correlated to a higher bone-to-implant contact (BIC) percentage for grit-blasted implants (73.5 ± 28.5 %) versus machined ones (59.6 ± 25.8 %). Moreover, the force necessary to pull-out the screws was four times as great for the grit blasted implants versus the machined implants.
Similar results are abundant in the literature and show enhanced osseointegration on microrough surfaces with very different topography, from simple uniform micropatterns [71] to more complex restructured surfaces [72], compared to machined surfaces as measured by BIC and mechanical testing [73, 74]. However, the type (e.g., sharp peaks, grooves, pores) and degree of microroughness (i.e., as quantified by surface roughness measurements) can affect the early healing and long-term success of the implant [75, 76].
Surfaces with complex microtopography appear to be even more osteogenic than surfaces with only one type of roughness. Acid etched titanium surfaces were compared to sand-blasted and acid-etched surfaces in a pig maxilla model after 10 weeks of healing [77]. Both treatments increased surface microroughness, but sand-blasted and acid-etched surfaces had a considerably higher roughness average (Ra = 1.53 ± 0.11 μm) than just acid etched surfaces (Ra = 0.90 ± 0.11 μm). The authors reported that both surfaces had the ability to interlock with bone, but the removal torque force on the sand-blasted and acid-etched implant was significantly higher (157.29 ± 38.04 N) than on the acid-etched implants (105.33 ± 25.12 N).
3.2 Microroughness Effect in Vitro
A series of well controlled studies using Ti substrates generated using photolithography to create microscale craters and modified using acid etching or anodization to create submicron scale and nanoscale features, showed that osteoblast lineage cells exhibit specific preferences for microstructure and nanostructure elements with respect to osteoblastic differentiation [78, 79]. These in vitro experiments indicated that a complex topography characterized by 30 μm diameter craters superimposed with irregular pits and peaks approximately 3 μm in diameter elicited the most differentiated osteoblast phenotype. Moreover, cells could discriminate between the more pointed nano-architecture created by acid etching and the more rounded nanofeatures created by electrochemical anodization, even though peak heights were comparable, exhibiting a more differentiated phenotype on the acid-etched surface.
The favorable response elicited by microrough implants at the in vivo level has been attributed to the activation of several important signaling pathways in osteoblasts and MSCs in vitro. Once these cells come in contact with a surface, either a bone surface that has been resorbed by osteoclasts or an implant surface, they go through a progression of well-defined phases including proliferation, differentiation and, in some cases, apoptosis. These phases are transcriptionally regulated, meaning that genetic and protein profiles during each phase are distinct [80]. The duration of each phase and the cell response with respect to osteoblastic differentiation is determined by the surface properties of the device.
A key observation in vitro has been that osteoblasts and MSCs after 5 to 7 days of culture on microrough surfaces in vitro have lower cell numbers and higher levels of differentiation markers, such as alkaline phosphatase and osteocalcin among others, when compared to relatively smooth surfaces [70, 79, 81]. Alkaline phosphatase is an enzyme produced early during osteoblast differentiation and is important for the onset of mineralization; while osteocalcin is a late differentiation marker produced at high levels during the mature state of the osteoblast [82, 83]. The decrease in cell number and increase in differentiation markers agree with the normal progression of osteoblast differentiation, indicating that cells growing on the microrough surfaces exit the proliferation phase earlier to start differentiating and producing the proteins necessary for bone formation.
Osteoblasts do not interact directly with the surface of the implant but can sense the changes in surface properties by identifying the layer of adsorbed proteins from the surrounding environment using cell membrane receptors such as integrins [84, 85]. Integrins are composed of α and β subunits that can bind specific proteins in the extracellular matrix and start signaling cascades within the cell [85]. Microroughness has been shown to influence the types of integrins that are produced by the cells, promoting those subunits associated with bone proteins, such as α2 and β1, but not those subunits associated with soft tissue proteins, such as α5 or αv [86]. Thus, microroughness can affect the progression of the osteoblast phenotype by upregulating integrins such as α2β1, which directly regulates osteoblast differentiation and local factor production [86].
Additionally, healthy bone growth and regeneration requires a healthy vasculature that develops in intimate association with osteoblasts to supply oxygen, nutrients and other factors that can enhance bone formation [87, 88]. In turn, osteoblasts can promote the formation of blood vessels through secretion of angiogenic factors, such as vascular endothelial growth factor (VEGF) and fibroblast growth factor 2 (FGF-2), which can be enhanced by an increase in surface microroughness [89]. At the same time, other important factors secreted by osteoblasts during implant osseointegration that can be enhanced by adjusting surface microroughness include: bone morphogenetic proteins (BMPs) [90], transforming growth factor-β 1 and 2 (TGF-β) [91], and Wnts [92]. Importantly, these factors are soluble and their signaling is required for bone development [93-95]. Experiments in which osteoblasts are grown on Ti substrates in a co-culture system where MSCs are grown on standard tissue culture plastic, show that the soluble factors produced by the osteoblasts are able to induce MSC osteoblastic differentiation [54]. These observations help explain how the formation of bone on a microtextured implant in vivo can impact bone formation within the bone bed distal to the implant.
Cells on microtextured surfaces also produce factors that modulate bone resorption. By producing increased levels of osteoprotegerin, the cells can control the number and activity of osteoclasts [96], thereby promoting net new bone formation over bone remodeling during healing. In addition, cells that are cultured on microtextured Ti produce higher levels of anti-inflammatory cytokines and lower levels of pro-inflammatory cytokines than cells on smooth surfaced Ti [97]. Thus, cells on an implant surface can also impact the overall environment in which bone healing is occurring.
Unfortunately, even with an increase in surface microroughness, implant failure still occurs in challenging cases such as those with patients compromised by disease or age [98]. Thus, other key characteristics such as surface energy and surface nanotopography may be manipulated and when combined with surface microroughness can synergistically promote bone formation in direct contact with the implant, especially in cases of patients with compromised bone [31, 99].
3.3 Role of Nanostructures in Vivo
In recent years, a few studies have been published that report the beneficial effects of adding nanostructures to implants in vivo [100-102]. However, most surface nanostructural modifications introduce changes to other implant characteristics, such as surface chemistry and surface energy, thus complicating the evaluation of the influence of these nanostructures on cell response [103, 104]. Regardless, we will focus on the outcomes of reports suggesting that nanostructures can be attractive features to incorporate into clinical implants, highlighting these limitations when necessary.
Machined, relatively smooth titanium surfaces have been compared to nanostructured surfaces in a rat tibial model for up to 56 days [101]. The nanomodification process used for this study involved depositing oxide nanoparticles on the surface of the implant through a sol-gel technique without affecting the overall microroughness. The oxide nanoparticles used for the coating included different crystalline phases of TiO2 (i.e., anatase, rutile), as well as zirconia (ZrO2), introducing changes to either crystal structure or chemistry, respectively, when compared to the machined control. No differences were found between the nanostructured implants compared to the machined control when evaluating removal torque forces up to 56 days after implantation. However, the BIC for all nanomodified implants was higher than the machined control. These results were correlated to quantitative polymerase chain reaction (qPCR) data that showed higher mRNA levels of osteoblastic differentiation markers, such as osteocalcin and osteopontin, in the bone surrounding the nanostructured implants.
Nanomodified implant materials have also been compared to microrough implants in vivo. For these experiments, nanomodified coin-shaped implants were assessed against grit-blasted implants in a rabbit tibial model after 4 weeks [100]. Electrochemical anodization in hydrofluoric acid (HF) and annealing (550 °C) was used to create well-defined, anatase nanotubes on the surface of the test implants. The nanomodification altered the crystal structure, as reported, and possibly the surface chemistry by incorporating F traces from the anodization treatment, but the latter was not evaluated. In addition to possible differences in surface chemistry resulting from the anodization process, other differences related to the initial surface microtexture of the test materials were not controlled. Within the constraints of these experimental parameters, however, the results showed that the pull-out force for nanotube implants was considerably higher than for control implants, and these results were corroborated by histological sections that showed increased BIC percentage on nanotube surfaces when compared to controls. Chemical mapping of the pulled-out surfaces by energy dispersive x-ray (EDX) spectroscopy also provided confirmation of greater bone being retained on the nanomodified surfaces, confirming that there was greater BIC causing the fracture to occur within the peri-implant bone and not at the bone/implant interface.
The ultimate goal in implant design is to mimic bone hierarchical structure at all different length scales (i.e., macro, micro and nano) and this has also been assessed by adding nanostructure to already microrough implants. The performance of sand-blasted Ti alloy (Ti-15Mo-5Zr-3Al) implants was compared to that of sandblasted and nanomodified implants in a rat femoral model for up to 8 weeks [102]. In this particular case, the nanomodification was termed nanobimorphic for the presence of what the authors called nanotrabecular and nanotuftlike structures on the surface, created by alkali (NaOH) and heat (600 °C) treatments. The modification introduced surface chemical changes by increasing the oxygen content and the O/Ti ratio. Biomechanical evaluation found that push-in forces for the sand-blasted, alkali and heat-treated implants were significantly higher after 1, 2, 4 and 8 weeks when compared to sand-blasted-only implants. These results were also confirmed by greater CaP content and by histomorphometrical analysis showing more BIC after 4 weeks of implantation, on the surface of the extracted nanomodified implants.
These different studies taken together support the concept of adding nanostructures to both microsmooth and microrough implants to improve the early healing and long-term osseointegration of implants for bone applications.
3.4 In Vitro Response to Nanostructures
The phenomena seen in vivo of more BIC and higher forces during biomechanical testing on nanostructured implants have been attributed to enhanced activity at the cellular level by osteoblasts and MSCs. Although few studies have been published questioning the influence of nanostructures on cell behavior [105], many other reports have shown that osteoblasts are indeed sensitive to these small features and can respond strongly to them. Morphological evaluations of cells growing on nanomodified substrates compared to nanosmooth controls show more filopodia extensions and actin cytoskeletal alignment [106, 107], as well as enhanced cell adhesion [108]. This response can be associated with the fact that the spacing of adhesion sites on a surface can regulate integrin binding to the extracellular matrix (ECM), with a spacing of less than 54 nm promoting the formation of focal adhesion complexes important for cell signaling and recognition of the ECM [109].
Cell spreading and attachment assays by themselves, however, are not sufficient to establish the beneficial role of nanostructures for bone formation. Studies looking at the differentiation state of osteoblasts growing on nanostructured surfaces have found higher mRNA production of osteoblast markers, such as osterix, alkaline phosphatase and osteocalcin [110]. The final protein levels of these markers have also been shown to increase on nanomodified surfaces when compared to nanosmooth surfaces, confirming the influence of nanostructures on osteoblast phenotype [79, 111].
For clinical applications, the addition of nanostructures to microrough implants is the most attractive option for surface modifications to take advantage of the already demonstrated enhancements of microroughness and to couple them to the improvements generated by nanostructures. Yet, cellular response is rarely linear, thus requiring assessment of the effects of such a combination of microroughness and nanostructures at the cellular level. Indeed, reports show synergistic effects in terms of enhanced osteoblast interactions with the surface, as well as higher mRNA and protein production of markers for osteoblast differentiation on the combined microrough and nanostructured surfaces when compared to just microrough surfaces [112-115].
Studies performed by our group using a heat treatment modification to superimpose nanostructures onto relatively flat or microrough Ti substrates corroborated these findings [115]. Our results showed a modest effect on osteoblast response by nanostructures on flat substrates compared to unmodified flat controls, while the superposition of nanostructures onto microrough surfaces synergistically enhanced the production of osteoblast differentiation markers and local factors important for bone formation, compared to unmodified microrough controls. These results suggest that osteoblasts are very sensitive to the hierarchical structure of their surface for the production of new bone.
Osteoblasts have been consistently shown to respond to nanostructures by increasing production of differentiation markers and other local factors [114, 115]. MSCs usually isolated from bone marrow and treated with osteogenic induction media to drive them into osteoblastic differentiation, have also been assessed and confirmed to respond to nanostructures [113]. In addition, MSCs have been shown to be directed towards osteoblastic differentiation by microstructures even when not exposed to osteogenic media or other inductive factors in the culture medium [54]. Interestingly, when MSCs are cultured without osteogenic media on nanostructured surfaces, their fate seems to depend on other factors. Randomly displaced patterns of nanostructures on polycaprolactone (PCL) substrates, without the use of soluble factors, can drive MSCs to produce osteogenic markers to similar levels as those treated with osteogenic media on flat substrates, while highly ordered patterns may prevent spontaneous MSC osteoblastic differentiation and promote the maintenance of MSC stemness rather than differentiation [116].
The idea that such small changes can be so influential is fascinating and the concept of maintaining MSC stemness can be extensively exploited in the field of tissue regeneration and the manipulation of stem cells. However, these results also indicate that many questions remain to be answered in the quest to incorporate nanostructures in clinical implants. While in vivo studies in the literature report that topographically hierarchical surfaces promote osseointegration [102] and our previous results have shown that combined micro/nanorough surfaces synergistically enhance osteoblast differentiation [115], other studies by our group on either commercially pure Ti or Ti alloy (Ti6Al4V) have shown that MSCs may be behaving differently than committed osteoblasts on these hierarchical surfaces [117, 118]. These results may be providing an insight into the biological complexity surrounding a healing implant. The positive in vivo results can be considered a good first step to bring these surface modifications closer to the clinics, but not until long-term clinical studies are performed will the full implications of these different surface features on the performance of implants for bone applications be completely understood.
4 Summary
In summary, the success of spinal implants is largely dependent on the surface characteristics of the device (e.g., surface roughness, surface chemistry, surface energy), as much as it is dependent on the macro design of the implant, the experience of the physician and patient variables. A complex chain of biological effects that ends with the differentiation of osteoblasts and the production of bone on the surface of the implant is required to achieve successful osseointegration, and this biological response can be modulated by the properties of the implant surface. Surface microroughness is one of the surface characteristics of an implant that has been well established as a tool to achieve better osseointegration, which has also been confirmed and explained in vitro. More recently, nanotopography has been evaluated and applied to implants to better mimic the endogenous structure of bone, with very promising results in terms of osteoblast maturation and bone formation. Experiments with MSCs on nanostructured surfaces without osteogenic media are providing great insights and revealing a complex story of cellular diversity for the success of an implant. Understanding the effects of surface properties on cell response is of utmost importance to design implants that can provide a robust solution by minimizing patient and clinical variables.
Acknowledgments
5 DISCLOSURES: This research was supported by USPHS AR052102, the ITI Foundation (Basel, Switzerland), and Titan Spine LLC (Mequon, WI). RAG was partially supported by a fellowship from the Government of Panama (IFARHU-SENACYT). Support for the work of KHS was provided by the U.S. Department of Energy, Office of Basic Energy Sciences (Award No. DESC0002245). BDB is a consultant for Titan Spine LLC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jacobs JJ, Andersson GBJ, Bell JE, Weinstein SL, Dormans JP, Gnatz SM, et al. United States Bone and Joint Decade: The burden of musculoskeletal diseases in the United States. 1st. Rosemont: AAOS; 2008. [Google Scholar]
- 2.Hanley EN. The indications for lumbar spinal fusion with and without instrumentation. Spine. 1995;20:S143–S53. [PubMed] [Google Scholar]
- 3.Hacker RJ, Cauthen JC, Gilbert TJ, Griffith SL. A prospective randomized multicenter clinical evaluation of an anterior cervical fusion cage. Spine. 2000;25:2646–54. doi: 10.1097/00007632-200010150-00017. [DOI] [PubMed] [Google Scholar]
- 4.Zdeblick TA, Phillips FM. Interbody cage devices. Spine. 2003;28:S2–S7. doi: 10.1097/01.BRS.0000076841.93570.78. [DOI] [PubMed] [Google Scholar]
- 5.Agrawal CM, Attawia M, Borden MD, Boyan BD, Bruder SP, Bucholz RW, et al. Bone graft substitutes. 1st. West Conshohocken: ASTM International / AAOS; 2003. [Google Scholar]
- 6.Ray CD. Threaded fusion cages for lumbar interbody fusions - An economic comparison with 360 degrees fusions. Spine. 1997;22:681–5. doi: 10.1097/00007632-199703150-00021. [DOI] [PubMed] [Google Scholar]
- 7.Branemark PI, Hansson BO, Adell R, Breine U, Lindstrom J, Hallen O, et al. Osseointegrated implants in the treatment of the edentulous jaw Experience from a 10-year period. Scand J Plast Reconstr Surg Suppl. 1977;16:1–132. [PubMed] [Google Scholar]
- 8.Bobbio A. The first endosseous alloplastic implant in the history of man. Bull Hist Dent. 1972;20:1–6. [PubMed] [Google Scholar]
- 9.Lang BR, Chiappa AA. Mandibular Implants - a New Method of Attachment. J Prosthet Dent. 1969;22:261–7. doi: 10.1016/0022-3913(69)90255-8. [DOI] [PubMed] [Google Scholar]
- 10.Sykaras N, Iacopino AM, Marker VA, Triplett RG, Woody RD. Implant materials, designs, and surface topographies: their effect on osseointegration. A literature review Int J Oral Maxillofac Implants. 2000;15:675–90. [PubMed] [Google Scholar]
- 11.Beck PM. Endosseous implants: a review of current advances and a case report. Oral Health. 1970;60:19–21. [PubMed] [Google Scholar]
- 12.Southam JC, Selwyn P. Structural changes around screws used in the treatment of fractured human mandibles. Br J Oral Surg. 1971;8:211–21. doi: 10.1016/s0007-117x(70)80082-8. [DOI] [PubMed] [Google Scholar]
- 13.Picton DC, Johns RB, Wills DJ, Davies WI. The relationship between the mechanisms of tooth and implant support. Oral Sci Rev. 1974;5:3–22. [PubMed] [Google Scholar]
- 14.Athanasou NA, Quinn J, Bulstrode CJ. Resorption of bone by inflammatory cells derived from the joint capsule of hip arthroplasties. J Bone Joint Surg Br. 1992;74:57–62. doi: 10.1302/0301-620X.74B1.1732267. [DOI] [PubMed] [Google Scholar]
- 15.Lassus J, Salo J, Jiranek WA, Santavirta S, Nevalainen J, Matucci-Cerinic M, et al. Macrophage activation results in bone resorption. Clin Orthop Relat Res. 1998:7–15. [PubMed] [Google Scholar]
- 16.Szmukler-Moncler S, Salama H, Reingewirtz Y, Dubruille JH. Timing of loading and effect of micromotion on bone-dental implant interface: Review of experimental literature. J Biomed Mater Res. 1998;43:192–203. doi: 10.1002/(sici)1097-4636(199822)43:2<192::aid-jbm14>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 17.Cook HP. Immediate reconstruction of the mandible by metallic implant following resection for neoplasm. Ann R Coll Surg Engl. 1968;42:233–59. [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas KA, Kay JF, Cook SD, Jarcho M. The Effect of Surface Macrotexture and Hydroxylapatite Coating on the Mechanical Strengths and Histologic Profiles of Titanium Implant Materials. J Biomed Mater Res. 1987;21:1395–414. doi: 10.1002/jbm.820211205. [DOI] [PubMed] [Google Scholar]
- 19.Hallab NJ, Cunningham BW, Jacobs JJ. Spinal implant debris-induced osteolysis. Spine (Phila Pa 1976) 2003;28:S125–38. doi: 10.1097/00007632-200310151-00006. [DOI] [PubMed] [Google Scholar]
- 20.Kurtz SM, Devine JN. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials. 2007;28:4845–69. doi: 10.1016/j.biomaterials.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Santos ER, Goss DG, Morcom RK, Fraser RD. Radiologic assessment of interbody fusion using carbon fiber cages. Spine (Phila Pa 1976) 2003;28:997–1001. doi: 10.1097/01.BRS.0000061988.93175.74. [DOI] [PubMed] [Google Scholar]
- 22.Anjarwalla NK, Morcom RK, Fraser RD. Supplementary stabilization with anterior lumbar intervertebral fusion - A radiologic review. Spine. 2006;31:1281–7. doi: 10.1097/01.brs.0000217692.90624.ab. [DOI] [PubMed] [Google Scholar]
- 23.Cunningham BW, Orbegoso CM, Dmitriev AE, Hallab NJ, Sefter JC, Asdourian P, et al. The effect of spinal instrumentation particulate wear debris. an in vivo rabbit model and applied clinical study of retrieved instrumentation cases. Spine J. 2003;3:19–32. doi: 10.1016/s1529-9430(02)00443-6. [DOI] [PubMed] [Google Scholar]
- 24.Lohmann CH, Schwartz Z, Koster G, Jahn U, Buchhorn GH, MacDougall MJ, et al. Phagocytosis of wear debris by osteoblasts affects differentiation and local factor production in a manner dependent on particle composition. Biomaterials. 2000;21:551–61. doi: 10.1016/s0142-9612(99)00211-2. [DOI] [PubMed] [Google Scholar]
- 25.Pioletti DP, Takei H, Kwon SY, Wood D, Sung KLP. The cytotoxic effect of titanium particles phagocytosed by osteoblasts. J Biomed Mater Res. 1999;46:399–407. doi: 10.1002/(sici)1097-4636(19990905)46:3<399::aid-jbm13>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 26.Maniatopoulos C, Pilliar RM, Smith DC. Threaded versus porous-surfaced designs for implant stabilization in bone-endodontic implant model. J Biomed Mater Res. 1986;20:1309–33. doi: 10.1002/jbm.820200907. [DOI] [PubMed] [Google Scholar]
- 27.Piattelli A, Scarano A, Favero L, Iezzi G, Petrone G, Favero GA. Clinical and histologic aspects of dental implants removed due to mobility. J Periodontol. 2003;74:385–90. doi: 10.1902/jop.2003.74.3.385. [DOI] [PubMed] [Google Scholar]
- 28.Olivares-Navarrete R, Gittens RA, Schneider JM, Hyzy SL, Haithcock DA, Ullrich PF, et al. Osteoblasts exhibit a more differentiated phenotype and increased bone morphogenetic protein production on titanium alloy substrates than on poly-ether-ether-ketone. Spine J. 2012;12:265–72. doi: 10.1016/j.spinee.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olivares-Navarrete R, Hyzy SL, Gittens RAs, Schneider JM, Haithcock DA, Ullrich PF, et al. Rough titanium alloys regulate osteoblast production of angiogenic factors. Spine J. 2013;13:1563–70. doi: 10.1016/j.spinee.2013.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sul YT, Johansson C, Wennerberg P, Cho LR, Chang BS, Albrektsson P. Optimum surface properties of oxidized implants for reinforcement of osseointegration: Surface chemistry, oxide thickness, porosity, roughness, and crystal structure. Int J Oral Maxillofac Implants. 2005;20:349–59. [PubMed] [Google Scholar]
- 31.Schwarz F, Wieland M, Schwartz Z, Zhao G, Rupp F, Geis-Gerstorfer J, et al. Potential of chemically modified hydrophilic surface characteristics to support tissue integration of titanium dental implants. J Biomed Mater Res B Appl Biomater. 2009;88B:544–57. doi: 10.1002/jbm.b.31233. [DOI] [PubMed] [Google Scholar]
- 32.Puleo DA, Nanci A. Understanding and controlling the bone-implant interface. Biomaterials. 1999;20:2311–21. doi: 10.1016/s0142-9612(99)00160-x. [DOI] [PubMed] [Google Scholar]
- 33.Kokubo T, Kim HM, Kawashita M, Nakamura T. Bioactive metals: preparation and properties. J Mater Sci Mater Med. 2004;15:99–107. doi: 10.1023/b:jmsm.0000011809.36275.0c. [DOI] [PubMed] [Google Scholar]
- 34.Davies JE. Bone bonding at natural and biomaterial surfaces. Biomaterials. 2007;28:5058–67. doi: 10.1016/j.biomaterials.2007.07.049. [DOI] [PubMed] [Google Scholar]
- 35.Albrektsson T, Wennerberg A. Oral implant surfaces: Part 1--review focusing on topographic and chemical properties of different surfaces and in vivo responses to them. Int J Prosthodont. 2004;17:536–43. [PubMed] [Google Scholar]
- 36.Wennerberg A, Albrektsson T. Effects of titanium surface topography on bone integration: a systematic review. Clin Oral Implants Res. 2009;20:172–84. doi: 10.1111/j.1600-0501.2009.01775.x. [DOI] [PubMed] [Google Scholar]
- 37.Mendonca G, Mendonca DBS, Aragao FJL, Cooper LF. Advancing dental implant surface technology - From micron- to nanotopography. Biomaterials. 2008;29:3822–35. doi: 10.1016/j.biomaterials.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 38.Regan JJ, Yuan H, McAfee PC. Laparoscopic fusion of the lumbar spine: minimally invasive spine surgery. A prospective multicenter study evaluating open and laparoscopic lumbar fusion Spine (Phila Pa 1976) 1999;24:402–11. doi: 10.1097/00007632-199902150-00023. [DOI] [PubMed] [Google Scholar]
- 39.McAfee PC. Interbody fusion cages in reconstructive operations on the spine. J Bone Joint Surg Am. 1999;81:859–80. doi: 10.2106/00004623-199906000-00014. [DOI] [PubMed] [Google Scholar]
- 40.Baelum V, Ellegaard B. Implant survival in periodontally compromised patients. J Periodontol. 2004;75:1404–12. doi: 10.1902/jop.2004.75.10.1404. [DOI] [PubMed] [Google Scholar]
- 41.Andrade JD, Hlady V. Protein adsorption and materials biocompatibility - A tutorial review and suggested hypotheses. Adv Polym Sci. 1986;79:1–63. [Google Scholar]
- 42.Wilson CJ, Clegg RE, Leavesley DI, Pearcy MJ. Mediation of biomaterial-cell interactions by adsorbed proteins: A review. Tissue Eng. 2005;11:1–18. doi: 10.1089/ten.2005.11.1. [DOI] [PubMed] [Google Scholar]
- 43.Jansson E, Tengvall P. In vitro preparation and ellipsometric characterization of thin blood plasma clot films on silicon. Biomaterials. 2001;22:1803–8. doi: 10.1016/s0142-9612(00)00359-8. [DOI] [PubMed] [Google Scholar]
- 44.Keselowsky BG, Collard DM, Garcia AJ. Surface chemistry modulates fibronectin conformation and directs integrin binding and specificity to control cell adhesion. J Biomed Mater Res A. 2003;66A:247–59. doi: 10.1002/jbm.a.10537. [DOI] [PubMed] [Google Scholar]
- 45.Marx RE. Platelet-rich plasma: Evidence to support its use. J Oral Maxillofac Surg. 2004;62:489–96. doi: 10.1016/j.joms.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 46.Di Iorio D, Traini T, Degidi M, Caputi S, Neugebauer J, Piattelli A. Quantitative evaluation of the fibrin clot extension on different implant surfaces: An in vitro study. J Biomed Mater Res B Appl Biomater. 2005;74B:636–42. doi: 10.1002/jbm.b.30251. [DOI] [PubMed] [Google Scholar]
- 47.Davies JE. Understanding peri-implant endosseous healing. J Dent Educ. 2003;67:932–49. [PubMed] [Google Scholar]
- 48.Davies JE. Mechanisms of endosseous integration. Int J Prosthodont. 1998;11:391–401. [PubMed] [Google Scholar]
- 49.Babensee JE, Anderson JM, McIntire LV, Mikos AG. Host response to tissue engineered devices. Adv Drug Del Rev. 1998;33:111–39. doi: 10.1016/s0169-409x(98)00023-4. [DOI] [PubMed] [Google Scholar]
- 50.Schindeler A, McDonald MM, Bokko P, Little DG. Bone remodeling during fracture repair: The cellular picture. Semin Cell Dev Biol. 2008;19:459–66. doi: 10.1016/j.semcdb.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 51.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–84. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 52.Neuss S, Schneider RK, Tietze L, Knuchel R, Jahnen-Dechent W. Secretion of fibrinolytic enzymes facilitates human mesenchymal stem cell invasion into fibrin clots. Cells Tissues Organs. 2010;191:36–46. doi: 10.1159/000215579. [DOI] [PubMed] [Google Scholar]
- 53.Bruder SP, Fink DJ, Caplan AI. Mesenchymal stem cells in bone development, bone repair, and skeletal regeneration therapy. J Cell Biochem. 1994;56:283–94. doi: 10.1002/jcb.240560809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Olivares-Navarrete R, Hyzy SL, Hutton DL, Erdman CP, Wieland M, Boyan BD, et al. Direct and indirect effects of microstructured titanium substrates on the induction of mesenchymal stem cell differentiation towards the osteoblast lineage. Biomaterials. 2010;31:2728–35. doi: 10.1016/j.biomaterials.2009.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saruwatari L, Aita H, Butz F, Nakamura HK, Ouyang J, Yang Y, et al. Osteoblasts generate harder, stiffer, and more delamination-resistant mineralized tissue on titanium than on polystyrene, associated with distinct tissue micro- and ultrastructure. J Bone Miner Res. 2005;20:2002–16. doi: 10.1359/JBMR.050703. [DOI] [PubMed] [Google Scholar]
- 56.Owen TA, Aronow M, Shalhoub V, Barone LM, Wilming L, Tassinari MS, et al. Progressive development of the rat osteoblast phenotype invitro - Reciprocal relationships in expression of genes associated with osteoblast proliferation and differentiation during formation of the bone extracellular-matrix. J Cell Physiol. 1990;143:420–30. doi: 10.1002/jcp.1041430304. [DOI] [PubMed] [Google Scholar]
- 57.Davies JE, Baldan N. Scanning electron microscopy of the bone bioactive implant interface. J Biomed Mater Res. 1997;36:429–40. doi: 10.1002/(sici)1097-4636(19970915)36:4<429::aid-jbm1>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 58.McKee MD, Nanci A. Osteopontin at mineralized tissue interfaces in bone, teeth, and osseointegrated implants: Ultrastructural distribution and implications for mineralized tissue formation, turnover, and repair. Microsc Res Tech. 1996;33:141–64. doi: 10.1002/(SICI)1097-0029(19960201)33:2<141::AID-JEMT5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 59.Thurner PJ, Lam S, Weaver JC, Morse DE, Hansma PK. Localization of Phosphorylated Serine, Osteopontin, and Bone Sialoprotein on Bone Fracture Surfaces. J Adhes. 2009;85:526–45. [Google Scholar]
- 60.Mulari MTK, Qu Q, Harkonen PL, Vaananen HK. Osteoblast-like cells complete osteoclastic bone resorption and form new mineralized bone matrix in vitro. Calcif Tissue Int. 2004;75:253–61. doi: 10.1007/s00223-004-0172-3. [DOI] [PubMed] [Google Scholar]
- 61.Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat Rev Genet. 2003;4:638–49. doi: 10.1038/nrg1122. [DOI] [PubMed] [Google Scholar]
- 62.Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–8. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 63.Chambers TJ, Revell PA, Fuller K, Athanasou NA. Resorption of bone by isolated rabbit osteoclasts. J Cell Sci. 1984;66:383–99. doi: 10.1242/jcs.66.1.383. [DOI] [PubMed] [Google Scholar]
- 64.Svanborg LM, Andersson M, Wennerberg A. Surface characterization of commercial oral implants on the nanometer level. J Biomed Mater Res B Appl Biomater. 2010;92:462–9. doi: 10.1002/jbm.b.31538. [DOI] [PubMed] [Google Scholar]
- 65.Wennerberg A, Ohlsson R, Rosen BG, Andersson B. Characterizing three-dimensional topography of engineering and biomaterial surfaces by confocal laser scanning and stylus techniques. Med Eng Phys. 1996;18:548–56. doi: 10.1016/1350-4533(95)00005-4. [DOI] [PubMed] [Google Scholar]
- 66.Wieland M, Textor M, Spencer ND, Brunette DM. Wavelength-dependent roughness: A quantitative approach to characterizing the topography of rough titanium surfaces. Int J Oral Maxillofac Implants. 2001;16:163–81. [PubMed] [Google Scholar]
- 67.Wang H, Xu M, Zhang W, Kwok DT, Jiang J, Wu Z, et al. Mechanical and biological characteristics of diamond-like carbon coated poly aryl-ether-ether-ketone. Biomaterials. 2010;31:8181–7. doi: 10.1016/j.biomaterials.2010.07.054. [DOI] [PubMed] [Google Scholar]
- 68.Sagomonyants KB, Jarman-Smith ML, Devine JN, Aronow MS, Gronowicz GA. The in vitro response of human osteoblasts to polyetheretherketone (PEEK) substrates compared to commercially pure titanium. Biomaterials. 2008;29:1563–72. doi: 10.1016/j.biomaterials.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 69.Dennes TJ, Schwartz J. A Nanoscale Adhesion Layer to Promote Cell Attachment on PEEK. JACS. 2009;131:3456–7. doi: 10.1021/ja810075c. [DOI] [PubMed] [Google Scholar]
- 70.Schwartz Z, Raz P, Zhao G, Barak Y, Tauber M, Yao H, et al. Effect of Micrometer-Scale Roughness of the Surface of Ti6Al4V Pedicle Screws in Vitro and in Vivo. J Bone Jt Surg (Am) 2008;90A:2485–98. doi: 10.2106/JBJS.G.00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hallgren C, Reimers H, Chakarov D, Gold J, Wennerberg A. An in vivo study of bone response to implants topographically modified by laser micromachining. Biomaterials. 2003;24:701–10. doi: 10.1016/s0142-9612(02)00266-1. [DOI] [PubMed] [Google Scholar]
- 72.Giavaresi G, Fini M, Chiesa R, Giordano C, Sandrini E, Bianchi AE, et al. A novel multiphase anodic spark deposition coating for the improvement of orthopedic implant osseointegration: An experimental study in cortical bone of sheep. J Biomed Mater Res A. 2008;85:1022–31. doi: 10.1002/jbm.a.31566. [DOI] [PubMed] [Google Scholar]
- 73.Simmons CA, Valiquette N, Pilliar RM. Osseointegration of sintered porous-surfaced and plasma spray-coated implants: An animal model study of early postimplantation healing response and mechanical stability. J Biomed Mater Res. 1999;47:127–38. doi: 10.1002/(sici)1097-4636(199911)47:2<127::aid-jbm3>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 74.Buser D, Nydegger T, Hirt HP, Cochran DL, Nolte LP. Removal torque values of titanium implants in the maxilla of miniature pigs. Int J Oral Maxillofac Implants. 1998;13:611–9. [PubMed] [Google Scholar]
- 75.Buser D, Broggini N, Wieland M, Schenk RK, Denzer AJ, Cochran DL, et al. Enhanced bone apposition to a chemically modified SLA titanium surface. J Dent Res. 2004;83:529–33. doi: 10.1177/154405910408300704. [DOI] [PubMed] [Google Scholar]
- 76.He FM, Yang GL, Li YN, Wang XX, Zhao SF. Early bone response to sandblasted, dual acid-etched and H2O2/HCl treated titanium implants: an experimental study in the rabbit. Int J Oral Maxillofac Surg. 2009;38:677–81. doi: 10.1016/j.ijom.2009.03.716. [DOI] [PubMed] [Google Scholar]
- 77.Szmukler-Moncler S, Perrin D, Ahossi V, Magnin G, Bernard JP. Biological properties of acid etched titanium implants: Effect of sandblasting on bone anchorage. J Biomed Mater Res B Appl Biomater. 2004;68B:149–59. doi: 10.1002/jbm.b.20003. [DOI] [PubMed] [Google Scholar]
- 78.Zinger O, Zhao G, Schwartz Z, Simpson J, Wieland M, Landolt D, et al. Differential regulation of osteoblasts by substrate microstructural features. Biomaterials. 2005;26:1837–47. doi: 10.1016/j.biomaterials.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 79.Zhao G, Zinger O, Schwartz Z, Wieland M, Landolt D, Boyan BD. Osteoblast-like cells are sensitive to submicron-scale surface structure. Clin Oral Implants Res. 2006;17:258–64. doi: 10.1111/j.1600-0501.2005.01195.x. [DOI] [PubMed] [Google Scholar]
- 80.Stein GS, Lian JB. Molecular mechanisms mediating proliferation/differentiation interrelationships during progressive development of the osteoblast phenotype. Endocr Rev. 1993;14:424–42. doi: 10.1210/edrv-14-4-424. [DOI] [PubMed] [Google Scholar]
- 81.Kim MJ, Kim CW, Lim YJ, Heo SJ. Microrough titanium surface affects biologic response in MG63 osteoblast-like cells. J Biomed Mater Res A. 2006;79:1023–32. doi: 10.1002/jbm.a.31040. [DOI] [PubMed] [Google Scholar]
- 82.Boyan BD, Schwartz Z, Bonewald LF, Swain LD. Localization of 1,25-(Oh)2d3-responsive alkaline-phosphatase in osteoblast-like cells (Ros 17/2.8, Mg-63, and Mc-3t3) and growth cartilage cells in culture. J Biol Chem. 1989;264:11879–86. [PubMed] [Google Scholar]
- 83.Hauschka PV, Lian JB, Cole DEC, Gundberg CM. Osteocalcin and matrix gla protein -Vitamin K-dependent proteins in bone. Physiol Rev. 1989;69:990–1047. doi: 10.1152/physrev.1989.69.3.990. [DOI] [PubMed] [Google Scholar]
- 84.Petrie TA, Reyes CD, Burns KL, Garcia AJ. Simple application of fibronectin-mimetic coating enhances osseointegration of titanium implants. J Cell Mol Med. 2009;13:2602–12. doi: 10.1111/j.1582-4934.2008.00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.van der Flier A, Sonnenberg A. Function and interactions of integrins. Cell Tissue Res. 2001;305:285–98. doi: 10.1007/s004410100417. [DOI] [PubMed] [Google Scholar]
- 86.Olivares-Navarrete R, Raz P, Zhao G, Chen J, Wieland M, Cochran DL, et al. Integrin alpha 2 beta 1 plays a critical role in osteoblast response to micron-scale surface structure and surface energy of titanium substrates. Proc Natl Acad Sci U S A. 2008;105:15767–72. doi: 10.1073/pnas.0805420105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Villanueva JE, Nimni ME. Promotion of Calvarial Cell Osteogenesis by Endothelial-Cells. J Bone Miner Res. 1990;5:733–9. doi: 10.1002/jbmr.5650050710. [DOI] [PubMed] [Google Scholar]
- 88.Maes C, Kobayashi T, Selig MK, Torrekens S, Roth SI, Mackem S, et al. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev Cell. 2010;19:329–44. doi: 10.1016/j.devcel.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Raines AL, Olivares-Navarrete R, Wieland M, Cochran DL, Schwartz Z, Boyan BD. Regulation of angiogenesis during osseointegration by titanium surface microstructure and energy. Biomaterials. 2010;31:4909–17. doi: 10.1016/j.biomaterials.2010.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Balloni S, Calvi EM, Damiani F, Bistoni G, Calvitti M, Locci P, et al. Effects of Titanium Surface Roughness on Mesenchymal Stem Cell Commitment and Differentiation Signaling. Int J Oral Maxillofac Implants. 2009;24:627–35. [PubMed] [Google Scholar]
- 91.Donos N, Retzepi M, Wall I, Hamlet S, Ivanovski S. In vivo gene expression profile of guided bone regeneration associated with a microrough titanium surface. Clin Oral Implants Res. 2011;22:390–8. doi: 10.1111/j.1600-0501.2010.02105.x. [DOI] [PubMed] [Google Scholar]
- 92.Olivares-Navarrete R, Hyzy S, Wieland M, Boyan BD, Schwartz Z. The roles of Wnt signaling modulators Dickkopf-1 (Dkk1) and Dickkopf-2 (Dkk2) and cell maturation state in osteogenesis on microstructured titanium surfaces. Biomaterials. 2010;31:2015–24. doi: 10.1016/j.biomaterials.2009.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Karsenty G, Wagner EF. Reaching a genetic and molecular understanding of skeletal development. Dev Cell. 2002;2:389–406. doi: 10.1016/s1534-5807(02)00157-0. [DOI] [PubMed] [Google Scholar]
- 94.Kato M, Patel MS, Levasseur R, Lobov I, Chang BH, Glass DA, 2nd, et al. Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J Cell Biol. 2002;157:303–14. doi: 10.1083/jcb.200201089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Centrella M, Horowitz MC, Wozney JM, McCarthy TL. Transforming growth factor-beta gene family members and bone. Endocr Rev. 1994;15:27–39. doi: 10.1210/edrv-15-1-27. [DOI] [PubMed] [Google Scholar]
- 96.Lossdorfer S, Schwartz Z, Wang L, Lohmann CH, Turner JD, Wieland M, et al. Microrough implant surface topographies increase osteogenesis by reducing osteoclast formation and activity. J Biomed Mater Res A. 2004;70:361–9. doi: 10.1002/jbm.a.30025. [DOI] [PubMed] [Google Scholar]
- 97.Hyzy SL, Olivares-Navarrete R, Hutton DL, Tan C, Boyan BD, Schwartz Z. Microstructured titanium regulates interleukin production by osteoblasts, an effect modulated by exogenous BMP-2. Acta Biomater. 2013;9:5821–9. doi: 10.1016/j.actbio.2012.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Olivares-Navarrete R, Raines AL, Hyzy SL, Park JH, Hutton DL, Cochran DL, et al. Osteoblast maturation and new bone formation in response to titanium implant surface features are reduced with age. J Bone Miner Res. 2012;27:1773–83. doi: 10.1002/jbmr.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Elias CN, Meirelles L. Improving osseointegration of dental implants. Expert Rev Med Devices. 2010;7:241–56. doi: 10.1586/erd.09.74. [DOI] [PubMed] [Google Scholar]
- 100.Bjursten LM, Rasmusson L, Oh S, Smith GC, Brammer KS, Jin S. Titanium dioxide nanotubes enhance bone bonding in vivo. J Biomed Mater Res A. 2010;92:1218–24. doi: 10.1002/jbm.a.32463. [DOI] [PubMed] [Google Scholar]
- 101.Mendonca G, Mendonca DBS, Simoes LGP, Araujo AL, Leite ER, Golin AL, et al. Nanostructured implant surface effect on osteoblast gene expression and bone-to-implant contact in vivo. Mater Sci Eng C Mater Biol Appl. 2011;31:1809–18. [Google Scholar]
- 102.Tsukimura N, Ueno T, Iwasa F, Minamikawa H, Sugita Y, Ishizaki K, et al. Bone integration capability of alkali- and heat-treated nanobimorphic Ti-15Mo-5Zr-3Al. Acta Biomater. 2011;7:4267–77. doi: 10.1016/j.actbio.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 103.Jimbo R, Xue Y, Hayashi M, Schwartz HO, Andersson M, Mustafa K, et al. Genetic Responses to Nanostructured Calcium-phosphate-coated Implants. J Dent Res. 2011;90:1422–7. doi: 10.1177/0022034511422911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mendonca G, Mendonca DBS, Simoes LGP, Araujo AL, Leite ER, Duarte WR, et al. Nanostructured alumina-coated implant surface: Effect on osteoblast-related gene expression and bone-to-implant contact in vivo. Int J Oral Maxillofac Implants. 2009;24:205–15. [PubMed] [Google Scholar]
- 105.Cai KY, Bossert J, Jandt KD. Does the nanometre scale topography of titanium influence protein adsorption and cell proliferation? Colloids Surf B Biointerfaces. 2006;49:136–44. doi: 10.1016/j.colsurfb.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 106.Webster TJ, Ejiofor JU. Increased osteoblast adhesion on nanophase metals: Ti, Ti6Al4V, and CoCrMo. Biomaterials. 2004;25:4731–9. doi: 10.1016/j.biomaterials.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 107.Biggs MJP, Richards RG, Gadegaard N, Wilkinson CDW, Dalby MJ. The effects of nanoscale pits on primary human osteoblast adhesion formation and cellular spreading. J Mater Sci Mater Med. 2007;18:399–404. doi: 10.1007/s10856-006-0705-6. [DOI] [PubMed] [Google Scholar]
- 108.Khang D, Lu J, Yao C, Haberstroh KM, Webster TJ. The role of nanometer and submicron surface features on vascular and bone cell adhesion on titanium. Biomaterials. 2008;29:970–83. doi: 10.1016/j.biomaterials.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 109.Arnold M, Cavalcanti-Adam EA, Glass R, Blummel J, Eck W, Kantlehner M, et al. Activation of integrin function by nanopatterned adhesive interfaces. Chemphyschem. 2004;5:383–8. doi: 10.1002/cphc.200301014. [DOI] [PubMed] [Google Scholar]
- 110.Mendonca G, Mendonca DBS, Simoes LGP, Araujo AL, Leite ER, Duarte WR, et al. The effects of implant surface nanoscale features on osteoblast-specific gene expression. Biomaterials. 2009;30:4053–62. doi: 10.1016/j.biomaterials.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 111.de Oliveira PT, Nanci A. Nanotexturing of titanium-based surfaces upregulates expression of bone sialoprotein and osteopontin by cultured osteogenic cells. Biomaterials. 2004;25:403–13. doi: 10.1016/s0142-9612(03)00539-8. [DOI] [PubMed] [Google Scholar]
- 112.Kubo K, Tsukimura N, Iwasa F, Ueno T, Saruwatari L, Aita H, et al. Cellular behavior on TiO2 nanonodular structures in a micro-to-nanoscale hierarchy model. Biomaterials. 2009;30:5319–29. doi: 10.1016/j.biomaterials.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 113.Mendonca G, Mendonca DBS, Aragao FJL, Cooper LF. The combination of micron and nanotopography by H2SO4/H2O2 treatment and its effects on osteoblast-specific gene expression of hMSCs. J Biomed Mater Res A. 2010;94A:169–79. doi: 10.1002/jbm.a.32701. [DOI] [PubMed] [Google Scholar]
- 114.Wilkinson A, Hewitt RN, McNamara LE, McCloy D, Dominic Meek RM, Dalby MJ. Biomimetic microtopography to enhance osteogenesis in vitro. Acta Biomater. 2011;7:2919–25. doi: 10.1016/j.actbio.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 115.Gittens RA, McLachlan T, Olivares-Navarrete R, Cai Y, Berner S, Tannenbaum R, et al. The effects of combined micron-/submicron-scale surface roughness and nanoscale features on cell proliferation and differentiation. Biomaterials. 2011;32:3395–403. doi: 10.1016/j.biomaterials.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.McMurray RJ, Gadegaard N, Tsimbouri PM, Burgess KV, McNamara LE, Tare R, et al. Nanoscale surfaces for the long-term maintenance of mesenchymal stem cell phenotype and multipotency. Nat Mater. 2011;10:637–44. doi: 10.1038/nmat3058. [DOI] [PubMed] [Google Scholar]
- 117.Gittens RA, Olivares-Navarrete R, McLachlan T, Cai Y, Hyzy SL, Schneider JM, et al. Differential responses of osteoblast lineage cells to nanotopographically-modified, microroughened titanium-aluminum-vanadium alloy surfaces. Biomaterials. 2012;33:8986–94. doi: 10.1016/j.biomaterials.2012.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gittens RA, Olivares-Navarrete R, Cheng A, Anderson DM, McLachlan T, Stephan I, et al. The roles of titanium surface micro/nanotopography and wettability on the differential response of human osteoblast lineage cells. Acta Biomater. 2013;9:6268–77. doi: 10.1016/j.actbio.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


