Abstract
Dental and orthopaedic implants have been under continuous advancement to improve their interactions with bone and ensure a successful outcome for patients. Surface characteristics such as surface topography and surface chemistry can serve as design tools to enhance the biological response around the implant, with in vitro, in vivo and clinical studies confirming their effects. However, the comprehensive design of implants to promote early and long-term osseointegration requires a better understanding of the role of surface wettability and the mechanisms by which it affects the surrounding biological environment. This review provides a general overview of the available information about the contact angle values of experimental and of marketed implant surfaces, some of the techniques used to modify surface wettability of implants, and results from in vitro and clinical studies. We aim to expand the current understanding on the role of wettability of metallic implants at their interface with blood and the biological milieu, as well as with bacteria, and hard and soft tissues.
Keywords: surface energy, hydrophilicity, contact angle, titanium implant roughness, surface conditioning, osteoblast, keratinocyte differentiation, bone to implant contact, osseointegration
1. Introduction
The continuous improvements in the performance of titanium (Ti) dental implants, with 10-year success rates higher than 95% in recent years [1] up from 66% or less in the early years of the technology [2], has made them one of the most viable options to replace missing teeth and has expanded the profile of the patient population. However, success rates are far from ideal in riskier patient populations, whose bone is compromised by disease or age [3]. In the case of orthopaedic applications, Ti alloy implants are also employed to provide an important solution for the treatment of degenerated joints but suffer from short lifetimes, especially with younger patients receiving these procedures [4]. Major enhancements in the performance of Ti implants have been achieved by targeting the surface of the device [5, 6]. Recent studies suggest that surfaces mimicking bone innate characteristics lead to enhanced osteoblast maturation [7, 8], increased bone-to-implant contact [9] and improved success rates clinically [10, 11]. The impact of surface characteristics such as surface roughness, chemistry and wettability have been well established in the short-term interactions between implants and the biological milieu, as well as in the long-term outcome of the device.
Surface chemistry is, in many cases, determined by the nature of the bulk material used, which depends on the mechanical properties required for the application. Interestingly, the surface chemical composition can differ widely from the bulk due to surface reactivity and preferential presentation of certain elements. For dental implant applications, titanium (Ti) and its alloys, including commercially pure (cp) Ti, titanium –aluminum-vanadium (Ti6Al4V) and, more recently, titanium-zirconium (TiZr), are used due to their favorable weight-to-strength ratio, good biological performance, and adequate corrosion resistance under normal conditions [12, 13]. Remarkably, their corrosion resistance stems from the high affinity of passive metals such as Ti towards oxygen, resulting in the formation of an inherent thin oxide layer on the surface that protects the bulk material from the environment and any further corrosion [14]. Indeed, the good biological performance of Ti implants has been attributed to this passive oxide layer that could be mimicking the ceramic nature of bone [15], which is up to 65% composed mainly of inorganic phosphates [16].
Surface topography can also have a direct effect on the biological response of bone, as microrough implants have proven to be superior to their smooth counterparts [17]. In the regular cycle of bone remodeling, the surface topography in areas that support new bone formation contains a high degree of structural complexity generated after bone resorption, including features at the micro-, submicro- and nanoscale [18, 19]. The presence of these features on the bone surfaces that experience new growth has led to the hypothesis that these structural cues can direct osteoblast response and tissue regeneration, which has been since thoroughly established [20–22]. Consequently, most clinically available implants try to mimic bone’s hierarchical structure by incorporating some type of surface modification at the microscale or, more recently, a combination of micro- and nanoscale surface features [10, 23].
The surface energy of an implant, indirectly measured by liquid-solid contact angle (CA) and thus related to wettability, is another surface characteristic known to affect the biological response to the implant. However, less is known about the intrinsic wettability of bone and how best to mimic such a property. Thus, surface wettability is not a focus of most surface characterization studies of implants [24–26] and the values of CAs of clinically marketed implants range widely [27]. Most studies have found that hydrophilic surfaces tend to enhance the early stages of cell adhesion, proliferation, differentiation and bone mineralization compared to hydrophobic surfaces [28, 29]. However, opposite results have been found in studies using different chemistries [30] and it is possible that extremely high surface energies promote cell adhesion but hinder cell motility and subsequent cell functions [31]. Therefore, the comprehensive design of implants to ensure successful outcomes for patients requires a better understanding of the role of surface wettability and the mechanisms by which it affects the surrounding biological environment.
This review provides a general overview of the available information about the CA values of experimental and of marketed dental implant surfaces, techniques used to modify surface wettability of implants, and results from in vitro and clinical studies. The aim is to expand the current understanding on the role of wettability of metallic implants at their interface with blood clots and the biological milieu.
2. Experimental and marketed dental implant surfaces: Wetting behavior and approaches for hydrophilization
Even though wetting has been recognized as an important surface property of implants [32], published data are very sparse. Generally, wettability is quantified by the CA, which is the angle between the tangent line to a liquid drop’s surface at the three-phase boundary, and the horizontal solid’s surface. In principle, the CA can range from 0 to 180 degrees (°). Water CAs lower than 90° designate surfaces as hydrophilic, and CAs very close to 0° as superhydrophilic. Surfaces with water CAs above 90° are considered hydrophobic, and those with CAs above 150° are termed superhydrophobic.
Whereas the wetting behavior of dental implant screws has recently been reported for nine currently marketed implant systems [27], most available publications until now that include some information on surface wettability have reported CA data on experimental implant surfaces, in many cases flat discs, where the reliability of the results translated to the corresponding marketed implants is not fully assured [33, 34]. Most implant surfaces currently in clinical use are hydrophobic, according to our findings and those of other groups [27, 32]. However, the range of CAs found on different dental implant surfaces varies widely, from superhydrophobic angles around 150° to superhydrophilic ones of 0°.
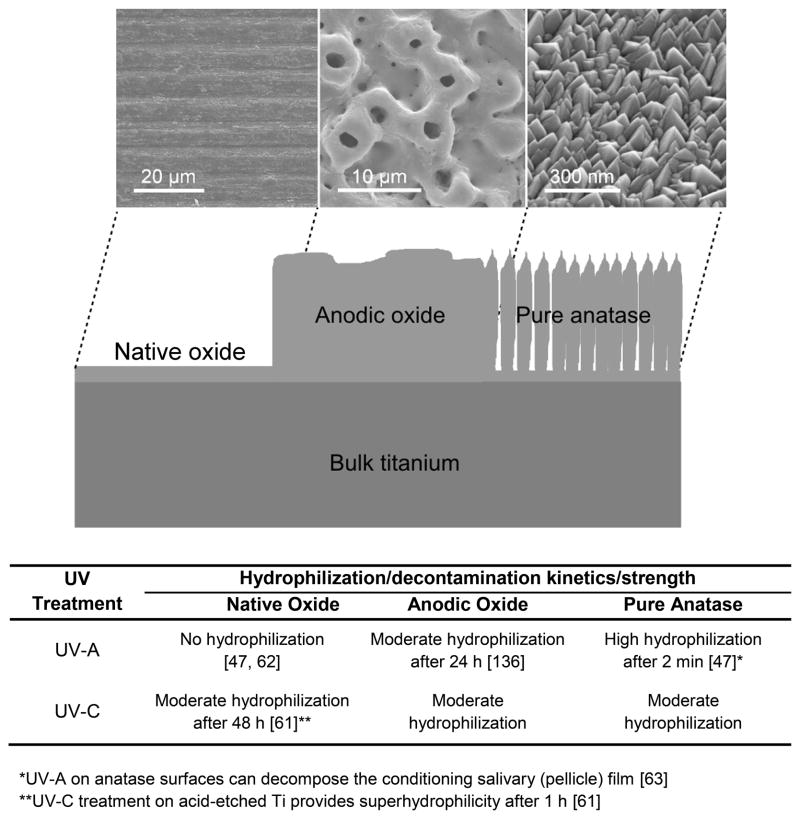
In general, wetting is reduced on microstructured surfaces [35], created by blasting, etching, or anodization. The presence of microscale structures and superimposed nanoscale features, as found on newly developed surface modifications, might also modulate wetting and the corresponding biological response (Figure 1) [7]. Thorough description of the wetting behavior should therefore include macroscopic, microscopic and, ideally, nanoscopic approaches. Still, many systems lack any data on wettability. Table 1 summarizes available published data on the wettability of cpTi and of commercial dental implant surfaces.
Figure 1.
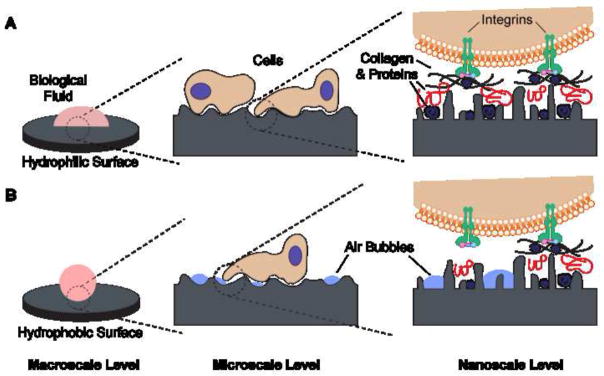
Schematic of the possible interactions with (A) hydrophilic and (B) hydrophobic surfaces at different length scales. (A) Hydrophilic surfaces interact closely with biological fluids, allowing normal protein adsorption to the surface and subsequent interactions with cell receptors. (B) Hydrophobic surfaces are prone to hydrocarbon contamination, leading to entrapment of air bubbles that can interfere with protein adsorption and cell receptor adhesion/activation.
Table 1.
Wettability of machined cpTi and of commercial, roughened dental implant surfaces.
| Surface/Company | Static contact angle (sessile drop) [°] | 1st advancing contact angle (tensiometry) [°] |
|---|---|---|
| cpTi | 97 [67], E 81 [132], E 88 [53], E 89 [133], E >70 [62], E 64 [121], E 24 [134] 58 [135], E* |
91 [43], E 92 [67], E |
| NanoTite/3i | 122 [132], E | 100 [27] |
| SLA/Institut Straumann | 126 [27] 117 [53], E 140 [43], E >150 [7], E 50 [7], E** |
124 [27] >150 [7], E |
| SLActive/Institut Straumann | 0 [27] 5 [53], E 0 [43], E |
0 [27] |
| Osseospeed/AstraTech | 138 [27] | |
| TiUnite/Nobel Biocare | 44 [62], E 37 [136], E |
125 [27] |
| Promote/Camlog | 110 [27] | |
| Plus/Dentsply Friadent | 103 [27] | |
| GS II/Osstem | 27 [27] | |
| TiOblast/AstraTech | 54 [121], E |
Ti-alloy (TiAl6V4);
condensed submicron droplet; E=experimental sample
As shown in Table 1, two main methods for the quantification of wettability through CAs have been applied: first, the sessile drop method, where liquid drops are set on a surface and the CA is directly measured from the drop shape surface [36]. Second, tensiometry, where CAs are measured indirectly according to the Wilhemy balance technique [27]. In this case, samples have to be fixed at an electrobalance and the forces detected during continuously immersing and withdrawing the samples into the wetting liquid, respectively, allow the calculation of advancing and receding CAs. Generally, dynamic CAs can be measured if there is a relative movement between the material and the wetting liquid. Without such movement, static CAs can be analyzed.
Among the many surface modifications of Ti implants [37], several have been developed to increase surface hydrophilicity. Most cases involve experimental implant surfaces that have yet to reach the market, although some have proven to be successful clinically. An example of a clinically relevant surface is the hydrophilic alternative to a hydrophobic blasted and acid-etched (BAE) surface. BAE surfaces have strongly outperformed relatively smooth, machined surfaces [17, 38, 39]. The main difference during processing of the hydrophobic BAE surface and its modified, superhydrophilic (modBAE) version is that surface neutralization after acid etching occurs in a contaminant-free, protective nitrogen environment and the implant is finally stored in neutral saline solution instead of air. Recent reviews highlight numerous in vitro, in vivo and clinical studies focusing on this hydrophilic surface [40, 41].
Initial studies confirmed that the microstructure of BAE and modBAE surfaces was identical, however, the wettability was quite different [42–44]. Whereas BAE implants exhibited limited wetting, modBAE were superhydrophilic and less contaminated by hydrocarbon contamination [43]. In contrast to BAE surfaces, which can adsorb contaminants while exposed to ambient atmosphere during processing, modBAE surfaces are maintained clean, with high surface energy and good wetting. This allows the formation of a strong interface with bone [42], demonstrating the impact of surface chemical interactions, including those with hydrocarbon contaminants, on the wetting behavior [43, 45–47]. A recent publication, however, could not confirm statistically significant differences in the carbon content of BAE and modBAE surfaces, although hydroxylation had higher levels on the modBAE surface [45].
The different biological and clinical performance of modBAE surfaces compared to BAE is likely related to the different surface chemistry and hydrophilicity [48]. However, every surface treatment may lead to variations both in the chemical composition and in the topography [37]. Indeed, recent reports suggest that the process of producing superhydrophilic modBAE surfaces generates nanostructures along the microrough topography [23, 49]. In vitro, the combination of micro- and nanostructures coupled with higher dynamic wettability has proven to enhance osteoblast differentiation and local factor production [7]. Recently, osseointegration was considerably improved in a rabbit tibial model using implants that combined nanostructured and hydrophilic surfaces [50].
Other more active methods of cleaning and hydrophilizing the surface of Ti samples are available. Baier et al. have shown that radio frequency glow discharge (RFGD) treatment is an effective method to clean and sterilize inorganic surfaces and elevate them to a high-energy state, correlating with greater cellular adhesion [51] possibly due to the reduction of contaminants from the surface [52]. More recently, atmospheric pressure plasma successfully cleaned and hydrophilized Ti implant surfaces [53]. The CA of BAE surfaces was reduced from 117° to 0° directly after short-time plasma treatment, as well as the CAs of other Ti surfaces that could be similarly hydrophilized, independently of their respective surface roughness. The results also showed that the size of osteoblasts growing on the hydrophilized surfaces was larger. Unfortunately, the authors did not consider the re-hydrophobization (i.e., loss of hydrophilicity) of the surface after such treatment, which occurs over a relatively short period in air. Ti, once hydrophilized by chemical etching or by plasma treatments, does not sustain a long-lasting superhydrophilic effect but instead re-hydrophobizes [54]. Similar results were shown by Scharnweber et al. with blasted and acid etched Ti surfaces that preserved hydrophilicity during storing in dry methanol but lost their good wetting properties after exposure to air [55]. The stability of the hydrophilization effect in air and in aqueous systems, and the kinetics of re-contamination may be critical factors dependent on the final clinical application.
The discovery of photoinduced water splitting on titanium dioxide (TiO2) electrodes [56] opened the door for broad research on photocatalysis, and has led to important technical developments and applications such as self-cleaning surfaces [57–59]. Because anatase TiO2, a very effective photocatalyst, is a semiconductor with a band gap of 3.2 eV, ultra violet (UV) light with wavelengths shorter than 400 nm is necessary for activation. Light-generated radicals and anionic oxygen species resulting from the anatase activation are able to decompose organic compounds found on the surface [59]. Using this principle, Wang et al. [60] were the first to report on the increased hydrophilicity of crystalline TiO2 surfaces upon UV irradiation. Indeed, anatase irradiated with UV-A becomes superhydrophilic, as numerous studies have shown since then [52, 61, 62]. Re-hydrophobization is also a well-known phenomenon of photocatalytic TiO2 once hydrophilized by UV-A irradiation. However, compared to plasma treatments the hydrophilic effect appears relatively long lasting as superhydrophilicity in ambient atmosphere may exist for hours and a hydrophilized surface state remains for days or weeks [47].
Recently in the dental field, decomposition of proteinaceous and salivary pellicle films could be demonstrated on UV-A activated nanocrystalline anatase thin films [47, 63]. Based on those studies, our team postulated a pre-determined breaking point for plaque biofilms located at the pellicle, which is the macromolecular thin film that serves as a conditioning layer between the implant surface and the bacterial overlayer [63]. Therefore, surface coating with crystalline TiO2 and irradiation by UV-A can be an advanced method to clean and hydrophilize implant surfaces. Interestingly, Ti specimens with native oxide surfaces are not activated by UV-A irradiation but can be hydrophilized using higher energy UV-C rays, which when applied do not necessarily trigger photocatalysis but more likely lead to a direct decomposition of the contaminating organic layers by photolysis [64]. Most methods to modify the surface oxide layer of a Ti implant result in widely different surface micro- and nanotopographies that further complicate the evaluation of surface wettability on the biological response. Figure 2 highlights different types of Ti surface oxides and their respective possibilities of hydrophilization.
Figure 2.
Efficiency of hydrophilization upon UV-A or UV-C treatment, respectively, on three different Ti surface oxides: the native passive layer, a thicker electrochemically manufactured anodic oxide, and a pulse magnetron sputtered layer of polycrystalline pure anatase. The efficiency classification used in the figure is as follows: no hydrophilization means the CA did not change compared to an untreated specimen; moderate hydrophilization reflects CAs that decreased but without reaching superhydrophilicity; high hydrophilization indicates the wetting liquid spread on the surface, forbidding CA measurements and signaling superhydrophilicity. Hydrophilization from UV-A irradiation is mediated by the formation of hydroxyl groups and by cleansing of the surface from organic contaminants due to photocatalytic formation of radical and anionic species at the material/organic contamination interface. Hydrophilization by UV-C irradiation also depends on cleansing of the surface from organic contaminants, but in this case due to direct photolytic decomposition.
The approaches described so far result in a clean surface that becomes the working principle to their enhanced hydrophilicity. Yet, as outlined by Hashimoto et al., a clean surface is in a metastable state and, thus, a completely clean surface cannot be obtained because ambient conditions will easily cause re-contamination [59]. The dynamic nature of a biomaterial interface is highly relevant for a dental implant’s surface and its condition at the time of clinical application.
Other Ti surface modifications can improve its hydrophilic properties by changing the surface chemistry. Park et al. [65] recently reported that different polyelectrolyte modifications prepared on the native oxide layers of machined and BAE Ti surfaces enhanced the hydrophilicity of the surface without changing its microtopography. Polyelectrolyte coatings shifted the hydrophobicity of the original Ti surfaces to a moderate hydrophilic state with a water CA between 40° and 60°, in contrast to the superhydrophilic modifications described previously (i.e., modBAE, UV-A treated anatase, RFGD plasma treated and UV-C treated Ti).
Additional Ti surface modifications exist with a hydrophilic characteristic. For example, acid etching Ti followed by alkali treatment resulted in microstructured, bioactive, hydrophilized surfaces with very low static and advancing CAs [66, 67]. However, the change of two or more surface characteristics at the same time, such as surface roughness and chemistry, either deliberately or not, complicates the evaluation of the roles of the parameters on the wetting behavior and the biological performance.
3. Impact of wettability: In vitro and in vivo studies
Surface wettability can affect four major aspects of the biological system: (1) adhesion of proteins and other macromolecules onto the surface (conditioning), (2) hard and soft tissue cell interactions with the pre-conditioned surfaces, (3) bacterial adhesion and subsequent biofilm formation, and (4) rate of osseointegration in the clinic (in vivo).
Surface conditioning by biomolecules derived from blood, interstitial fluid or saliva is a fast process, initiated within milliseconds after implant insertion [68]. Consequently, it is not possible to investigate cellular or bacterial interactions with biomaterials without considering the initial conditioning process. The impact of hydrophilicity on protein adhesion has been intensively studied using model proteins (e.g. albumin), or biological liquids such as blood, serum, plasma, or sterile filtered saliva (for in-depth reviews on the topic see [68, 69]).
Generally, hydrophilicity can influence the bonding strength and the total amount of proteins bound to a surface, the conformation and orientation of individual protein molecules, and the overall composition of the macromolecular film forming on a surface by selective adhesion from the respective bioliquid [69, 70]. These processes will result in an altered surface charge and wettability compared to the original implant surface, as well as a specific pattern of protein motifs or other guidance cues on the biomaterial surface (Figure 3). The adsorbed biological signals can activate receptors located on the outer membrane of cells. The expression of receptors on the cell surface varies with cell type and their differentiation stage. Subsequently, these receptors will determine the initial cellular attachment as well as short- and long-term processes like proliferation and differentiation.
Figure 3.
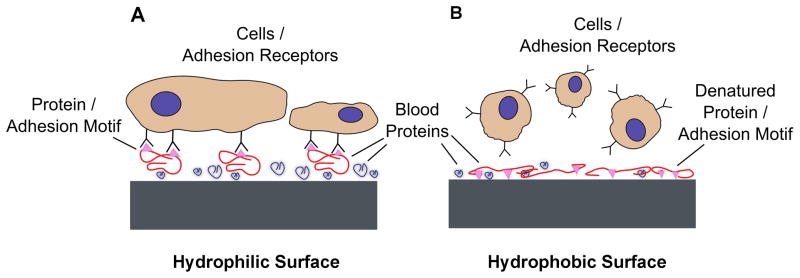
Schematic depicting the effects of (A) hydrophilic and (B) hydrophobic surfaces on protein adsorption and conformation. (A) Hydrophilic surfaces in contact with blood and biological fluids promote protein adsorption in a conformation that exposes adhesion motifs and enhances cell adhesion. (B) Hydrophobic surfaces can partially denature proteins, disturbing their tertiary structure and causing cell-binding sites to be less accessible, which results in diminished cell adhesion.
Thus, the impact of hydrophilicity on cellular and tissue reactions towards biomaterials is largely mediated through the initial protein interactions with the respective surface. For implant applications, the biological milieu encountered initially will be, in most cases, blood.
3.1. Interactions with blood
The impact of hydrophilicity on blood/biomaterial interactions has been reviewed by Spijker et al. [71]. The selective adhesion of blood proteins can activate different immunological signaling cascades triggering, for example, the activation of the complement system or adhesion and activation of thrombocytes that will lead eventually to the formation of a blood clot between the implant and the surrounding tissue [72]. Other hydrophilicity-dependent blood cell interactions include differences in adhesion and receptor expression of polymorphonuclear leucocytes (PMNL) and maturation of dendritic cells (DC). On hydrophilic surfaces, Eriksson et al. found that anti-CD16 antibodies could block adhesion of PMNLs, whereas anti-CD162 antibodies blocked adhesion to the hydrophobic surface [73]. The expression of CD11b, CD16 and CD62L by PMNL showed different kinetics depending on hydrophilicity of the biomaterial. In another study using dendritic cells, Kou et al. demonstrated that human dendritic cells remained in a more immature state on superhydrophilic modBAE specimens in comparison to standard BAE samples [74]. They concluded that modBAE surfaces are able to promote a non-inflammatory environment by a less pronounced stimulatory effect on DC phenotype, thereby reducing the innate immune response and promoting peri-implant bone formation clinically.
Extracellular matrix proteins found in blood that promote non-hematopoietic cell adhesion can have very different functionality following adsorption to hydrophilic or hydrophobic surfaces. One typical example is fibronectin, which is present in human blood in concentrations of ca. 0.4 mg/ml and, thus, becomes part of the conditioning layer of dental implants when inserted into a blood-filled bone cavity during implantation. The effects of hydrophilicity on protein adhesion, orientation and changes in substructure by partial unfolding have been reviewed by Wilson et al. [69]. According to Wilson, fibronectin adsorbed to hydrophobic surfaces shows a marked reduction in cell-adhesive function. The ability of fibronectin to retain its functionality on hydrophilic surfaces is another contributing factor to improved cell response. Similarly, other proteins such as collagen type I and vitronectin, important for osteoblast differentiation [75], may also be influenced by the surface wettability.
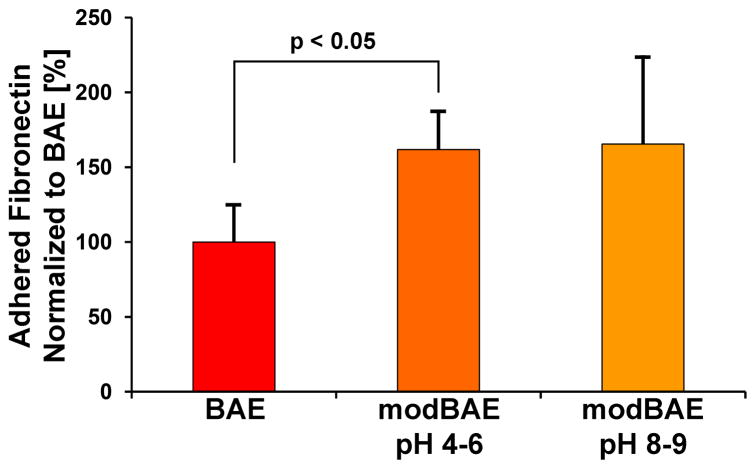
Studies performed by our team are in agreement with these results, where fibronectin adsorption to hydrophobic BAE surfaces was compared to that on superhydrophilic modBAE implant surfaces stored in isotonic sodium chloride (NaCl) at different pH (Figure 4) [76]. Fibronectin conditioning was determined by ELISA following incubation in a solution of plasma-fibronectin at physiological concentration for 1 h. Adsorbed fibronectin was quantified by antibodies directed against the cell-binding domain. The tested hydrophilic modification stored in basic pH had similar amounts of functional fibronectin cell-binding domains detected on the surface as when stored in acidic pH, which was 1.5-fold higher than hydrophobic BAE surfaces (Figure 4).
Figure 4.
Fibronectin adhesion on a hydrophobic, blasted and acid-etched (BAE) Ti implant surface, compared to adhesion on different hydrophilic modifications (modBAE stored at pH 4–6 or 8–9) of the original surface. Samples were incubated with a physiological concentration (0.4mg/ml) of human plasma fibronectin for 1h. Bound fibronectin was determined by immunological quantification using an antibody directed against the fibronectin cell-binding domain. Data represent means and standard deviations (n = 3) from three independent experiments. All data are expressed as % related to BAE.
3.2. Interactions with cells from hard and soft tissues
After implant surface conditioning by proteins and small molecules found in blood, successful bone-implant integration requires surface colonization by osteoblast precursor cells, followed by differentiation, extracellular matrix synthesis and tissue restoration. These different cell processes are also influenced by the highly dynamic biological cues that originate from the conditioning layer, including the initial processes of cell adhesion and spreading. The influence of hydrophilicity on the adsorption of diverse biological mediators also has an impact on all subsequent steps in the healing cascade leading, at last, to long-term tissue integration.
The connection between hydrophilicity, surface conditioning by serum proteins and the adhesion of different cells was recently demonstrated by Huang et al. [77]. Protein conditioning and cell adhesion/spreading were investigated on micropatterned Ti samples showing a superhydrophilic surface with a superimposed hydrophobic grid. On these patterned surfaces, the overall amount of total adhered fluorescein isothiocyanate (FITC)-labeled serum proteins from fetal bovine serum (FBS) was similar on super-hydrophilic and -hydrophobic areas. Cell adhesion and spreading, however, was almost totally inhibited on the hydrophobic grid for periods of up to 2 days, indicating a distinctly different protein composition and/or different orientation of single proteins on hydrophobic versus hydrophilic areas that lead to the enhanced cell adhesion on hydrophilic regions.
Numerous reports have corroborated the influence of hydrophilicity on cell responses such as adhesion, spreading, proliferation and several aspects of differentiation. Most of the work has been done on hard tissue cells such as osteoblasts [44, 61], but soft tissue cells such as fibroblasts or keratinocytes are influenced as well [78, 79].
3.2.1. Influence of wettability on hard tissue cells
As mentioned above, an important step in the wound healing process around the implant is the formation of a fibrin blood clot that serves as a bridging scaffold for migrating cells. The moderate immune response [74] and lower activation of thrombocytes [80], found on hydrophilic surfaces compared to hydrophobic ones can facilitate the invasion and mobilization of the blood clot by mesenchymal stem cells (MSCs) [81], considered as one of the initial non-hematopoietic cell types to colonize an implantation site [82, 83].
Few studies have directly evaluated the role of surface wettability on MSC response [7, 84–87]. Some of these studies focused mainly on cell morphology and the initial cell processes of cell attachment and proliferation, and found that hydrophilic Ti/TiO2 surfaces that had been photocatalytically hydrophilized promoted flattened morphologies and higher proliferation rates [84, 85]. Our team has performed experiments to evaluate the osteoblastic differentiation of MSCs on hydrophobic BAE surfaces compared to superhydrophilic modBAE surfaces and found that MSC cell numbers were lower on the superhydrophilic surfaces [86]. These results on the superhydrophilic surfaces correlated to synergistically higher levels of the osteoblast late differentiation marker osteocalcin and the local factor osteoprotegerin, as well as higher mRNA expression of the transcription factor RUNX2, the genes OCN and DKK2, and integrins ITGA2 and ITGB1, all elements that together favor osteoblastic differentiation of mesenchymal stem cells.
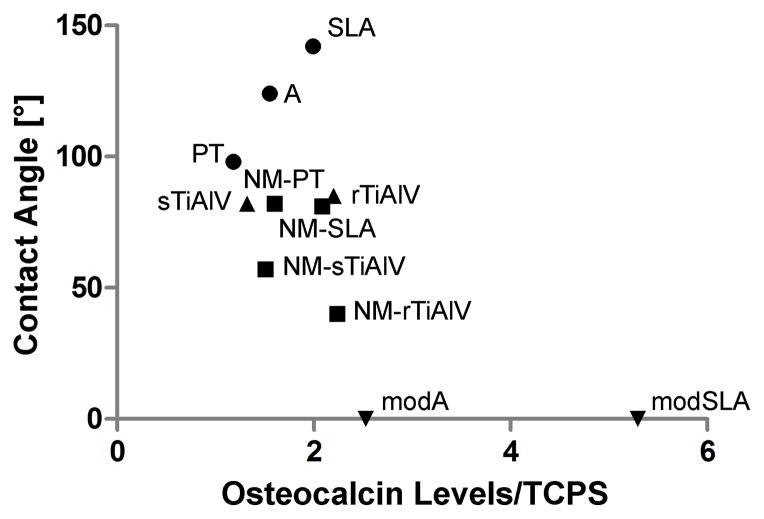
Osteoblasts resulting from the differentiation of MSCs or that come from the surrounding bone are the next cell type in line to respond to the wettability characteristics of the surface. Hydrophilic surfaces have been shown to enhance osteoblast maturation [44], production of local factors [44, 88] and mineralization [89] compared to hydrophobic surfaces (Figure 5). In addition, other surface characteristics such as surface topography coupled to surface wettability could cause a synergistic effect on cell response. Osteoblast response has been evaluated on BAE specimens compared to nanomodified BAE specimens, the former exhibiting static and dynamic hydrophobicity while the latter had similar static hydrophobicity but presented hydrophilic characteristics when evaluated by dynamic CA [7]. Osteoblast differentiation markers and local factor production were promoted by both microrough groups when compared to a microsmooth negative control. However, these markers and factors were synergistically enhanced on the dynamically hydrophilic, nanomodified BAE specimens compared to hydrophobic BAE surfaces, which could be due to the nanotopography or the enhanced wettability, but most probably a combination of both (Figure 5).
Figure 5.
Change in response of MG63 cells to differences in surface wettability. MG63 cells were plated on Ti or Ti6Al4V specimens with different surface modifications (machined, nanomodified, or micromodified) and covering a wide range of CAs, and cultured to confluence on TCPS [22, 90, 137]. Data represent the levels of osteocalcin secreted into the conditioned media (n=6 independent samples) normalized to production on tissue culture polystyrene surfaces. The specimen abbreviations from the respective references were maintained: PT = machined, pre-treatment Ti; A = acid-etched Ti; SLA = sandblasted with large grit and acid-etched Ti; modA = hydrophilized A; modSLA = hydrophilized SLA; NM-PT = heat-treated, nanomodified Ti; NM-SLA = heat-treated, nanomodified SLA; sTiAlV = smooth Ti6Al4V; rTiAlV = (micro) rough, double acid-etched Ti6Al4V; NM-sTiAlV = nanomodified sTiAlV; NM-rTiAlV = nanomodified rTiAlV.
Evidently, osteoblast maturation is strongly influenced by microtopography, but the maturation path seems mostly unidirectional and can be synergistically enhanced indistinctly by other surface characteristics such as hydrophilicity and nanotopography [7, 90]. In the case of MSC osteoblastic differentiation, microtopography also plays a key role [86] but the stem cell response to other superimposed surface characteristics can be far more complex. Superhydrophilic modBAE surfaces synergistically enhanced the MSC osteoblastic differentiation [86] compared to hydrophobic BAE surfaces, as described above. However, MSCs exposed to the dynamically hydrophilic, nanostructured BAE surfaces exhibited cell numbers and production of differentiation markers and local factors similar to those on microsmooth controls, except for increased production of vascular endothelial growth factor (VEGF) [7]. Differentiation paths other than osteoblastic were not considered in the study, but MSC responses on these multifaceted surfaces that attempt to mimic the in vivo environment more closely provide new insights and possibly attest to the diversity of cellular differentiation states required around the implantation site for the appropriate osseointegration.
3.2.2. Influence of wettability on keratinocytes and oral fibroblasts
The response of soft tissue cells such as keratinocytes and fibroblasts to the surface wettability of transmucosal (dental) and transcutaneous (orthopaedic) osseous implants may prove to be important for long-term success. However, only a few studies have explored this idea [78, 79, 91]. Keratinocytes are the major constituents of the epidermis, the outermost layer of the skin, and of the stratified squamous epithelial layer in the oral mucosa [92]. Fibroblasts, instead, are responsible for producing the supportive extracellular matrix in the dermis in the case of the skin and in the lamina propria, which is the connective tissue layer underneath the stratified squamous epithelial layer, in the case of the oral mucosa [93]. Intimate integration of these two layers of soft tissue cells with the implanted device is required to seal the implantation site from the external environment, avoiding infections and peri-implantitis, and ensuring a successful outcome [94, 95].
Besides hydrophilicity, keratinocytes and fibroblasts have been found to respond to surface characteristics such as surface chemistry [96, 97] and surface topography [78, 98]. In a study that controlled surface roughness and surface wettability to isolate the effects of surface chemistry on mouse fibroblasts, Ti surfaces that were coated with TiN by physical vapor deposition were compared to surfaces that were either thermally oxidized, laser irradiated or unmodified controls [97]. Fibroblast morphology and activity were enhanced on TiN surfaces compared to other groups, indicating that tailoring the surface chemistry of the implant can support soft tissue growth.
Surface nanotopography can modulate soft tissue cell response and this response may be specific to the type of nanostructures present on the surface. Puckett et al. evaluated the effects of surface nanomodification and wettability of Ti surfaces on keratinocyte function for transcutaneous osseous implant applications [78]. Keratinocyte adhesion, spreading and filopodia extension increased on the nanomodified surfaces, by either electron beam (e-beam) evaporation or anodization, compared to unmodified Ti controls after 4 h of culture. After 3 and 5 days of culture, keratinocyte spreading and proliferation was enhanced on the e-beam-evaporated (nanorough) surfaces compared to anodized (nanotubular) surfaces and unmodified controls. Thus, the authors suggest that nanomodifications could promote skin growth and limit the risk of infection, improving the longevity of transcutaneous osseointegrated implants.
Surface wettability can also affect keratinocyte response, albeit fewer studies have looked into this question [78, 79, 91]. In the same study described above by Puckett et al., surface energy for the different surfaces was calculated using CA measurements with different liquids [78]. Nanotubular surfaces had the highest surface energy, while the unmodified controls had the lowest surface energy. Interestingly, the strongest keratinocyte response occurred on the nanorough surfaces with an intermediate surface energy compared to the other groups.
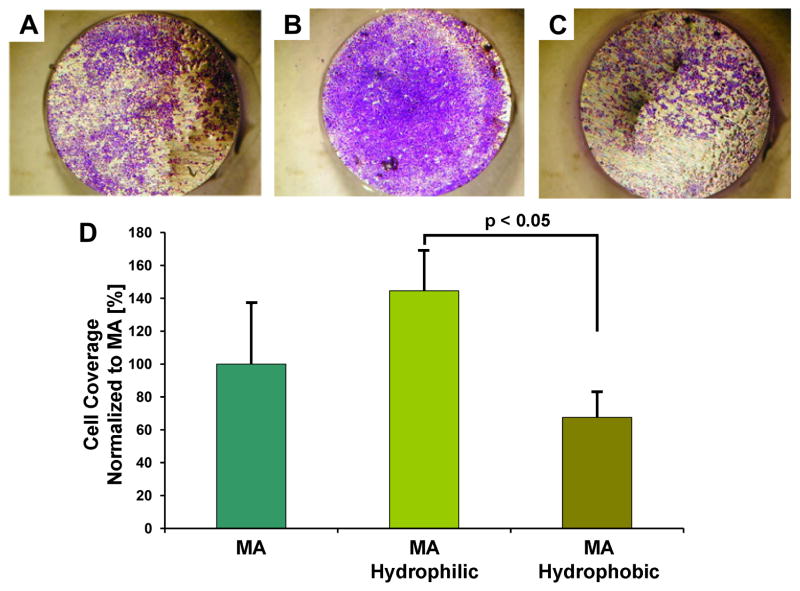
Conversely, in a study performed by our team, human oral keratinocytes cultivated for 3 days on machined, relatively micro-smooth Ti samples with controlled wettability presented markedly enhanced proliferation on cold plasma-treated, superhydrophilic samples compared to silane-coupled, hydrophobic ones (Figure 6) [79]. Higher keratinocyte proliferation on superhydrophilic surfaces resulted in faster surface coverage, suggesting that implants with superhydrophilic surface could lead to faster restoration of a tight epithelial seal.
Figure 6.
Photometric quantification of crystal violet bound to the different experimental surfaces for human oral keratinocyte surface coverage evaluation. Keratinocytes were incubated on (A) control machined (MA) surfaces or (B) MA surfaces hydrophilized by cold plasma treatment or (C) samples hydrophobized by silane coupling to the surface, for 3 to 5 days, stained with crystal violet, and photodocumented. (D) The bound crystal violet was eluted and quantified photometrically to determine surface coverage. Data represent means and standard deviations (n = 3) from three independent experiments. All data are expressed as % related to MA.
More recently, An et al. evaluated the response of the oral squamous carcinoma cell line HSC-2 to hydrophobic acid-etched and BAE surfaces, as well as to their superhydrophilic counterparts (modA, modBAE) [91]. The HSC-2 epithelial cells exhibited increased cell spreading and higher motility, as evaluated by time-lapse microscopy, on the hydrophilic surfaces compared to the hydrophobic ones. Smoother acid-etched surfaces also favored this enhanced response compared to the rougher blasted ones. The study also looked at the mRNA expression of differentiation markers such as KRT14, ITGA6, ITGB4, and local factors such as TGF-β1 and TGF-β3, without significant differences among the different Ti surfaces. The authors suggested that smoother, superhydrophilic surfaces might positively influence the epithelial seal around the implant.
3.3. Influence of hydrophilicity on bacterial colonization
Biomaterial-associated infections represent a small percentage but result in serious complications in the orthopaedic field [99, 100]. In dentistry, bacteria-caused complications in the oral cavity, such as periodontitis or dental implant-related peri-implantitis, may cause tooth or implant loss [101, 102]. In recent years, it became evident that bacteria entering the bloodstream during tooth treatment or dental implant placement can cause life-threatening infections not related to their infiltration sites, such as infective endocarditis by bacteria-platelet thrombi [103].
Since bacteria within a biofilm community are difficult to eradicate by systemic antibiotics, several anti-adhesive and antibacterial strategies have been developed to prevent the formation of mature biofilms on biomaterials surface by impeding the initial and most vulnerable stages of bacterial adhesion [104]. These strategies include surface coatings with dendrimers or polymeric brushes such as poly(ethylene glycol) layers [105, 106], surface modifications using positively charged molecules or zwitterionic coatings such as phosphorylcholine [107, 108], as well as surface application of silver nanoparticles or photocatalysts [63, 104, 109].
Even though the mechanisms by which bacteria adhere to surfaces are not fully understood, several physicochemical surface properties of the material as well as of the bacteria themselves have been described to contribute to the initial adhesion process [110]. On the materials side, surface roughness as well as hydrophilicity and surface free energy are known to influence bacterial adhesion [111, 112]. However, it is not easy to predict bacterial interfacial behavior for each single possible factor.
Regarding surface roughness, threshold values that may modulate bacterial adhesion are not clearly defined. In dentistry, increased plaque formation has been reported above roughness values of 0.2 μm [113]. However, three-dimensional surface nanomodifications that can satisfy the roughness threshold to prevent bacterial colonization and simultaneously improve soft and hard tissue integration, as required in transgingival dental implant sites, have been difficult to achieve. Regarding surface free energy, bacterial adhesion may decrease or increase with increasing surface energy of substrates, depending on the physicochemical properties not only of the substrate, but also of the bacterial strains tested and the aqueous solution used [114]. Thus, surface hydrophilicity plays a role in biomaterial/bacterial interaction, but the systems are complex and influenced by a pattern of different factors, depending on the implant site and, correspondingly, the bacterial species and strains of interest.
In theory, hydrophobic bacterial strains will more likely adhere to biomaterials with hydrophobic surface properties, and correspondingly hydrophilic species will preferentially adhere to hydrophilic surfaces. Hydrophobic interactions are widespread and involved in the mechanism of action of different microbial adhesion elements including hydrophobic cell membrane components as well as adhesins located on fimbria or pili [115]. Several studies have confirmed that adhesion of the human pathogens S. aureus and S. epidermidis is correlated to increasing hydrophobicity of the biomaterial surface, and that hydrophobicity in general is a main driving force for bacterial adhesion [116].
In the clinical setting, however, the situation may be quite different. Quirynen et al. [117] compared oral biofilm development on hydrophobic (surface free energy of 20 erg/cm2) and more hydrophilic (58 erg/cm2) specimens in a clinical study over periods of up to 6 days. In this study, the hydrophobic material had significantly less plaque after 6 days. One of the main reasons for the poor correlation between theoretical thermodynamics or in vitro basic models, and the more complex clinical situation is the formation of conditioning films in vivo that alter the surface properties of biomaterials as soon as they encounter biofluids.
Wettability differences between biomaterials are often lessened by this conditioning process. Bruinsma et al. [118] reported that the initial difference in wettability between hydrophilic and hydrophobic contact lenses, with water CAs of 57° and 106° respectively, was reduced to 57° and 69° after contact with human tear liquid. The adsorbed tear films, however, showed a different protein composition as analyzed by SDS-PAGE, thus influencing subsequent bacterial adhesion. In another study, MacKintosh et al. [119] investigated the adhesion of S. epidermidis to polyethylene terephtalate (PET) samples with hydrophilic, hydrophobic, and ionic charge surface modifications. When the adhesion experiments were performed in phosphate-buffered saline (PBS), where the original surface state was maintained, nonspecific bacterial adhesion after 24 h was lower by more than one order of magnitude on the hydrophilic modification in comparison to the unmodified control and other modified surfaces. After incubation in serum, however, control, hydrophilic and hydrophobic surfaces showed comparable, very low bacterial adhesion, while the cationic and anionic modifications instead showed a substantially higher amount of bacterial adhesion. As stated by the authors, serum proteins are more likely to exert a greater effect in vivo on the surface adhesion and biofilm formation of S. epidermidis than nonspecific interactions.
Since S. epidermidis and many other bacteria have developed specific adhesion mechanisms to certain blood proteins like fibrinogen or fibronectin [119], biomaterial surface properties that lead to conformational changes of these proteins during surface adsorption [120] will probably also influence bacterial adhesion. Hence, even if surface free energy and surface charge are in fact the determinants of bacterial adhesion, the indirect influences of these parameters coupled to changes in chemical composition, molecule conformation in the conditioning film and the presence of nanoscale features on the surface are still not fully understood or predictable. As a result, anti-adhesive strategies based on physicochemical and thermodynamic approaches have only been partly successful so far, but still represent one of the most attractive tools to combat the costly and burdensome bacterial complications.
3.4. Wettability role in animal and clinical studies
The influence of implant surface wettability transcends its role at the protein and cellular level, and has been confirmed in vivo and in the clinic [11, 28, 42, 61, 85, 121–123]. Hydrophilic surfaces of experimental, relatively smooth (Ra < 0.8 μm) disk-shaped Ti implants were shown to promote early bone formation compared to hydrophobic surfaces [28]. In a 3-week rat tibial model, cell viability of the tissue covering the explanted devices revealed lower cell viability and higher immunofluorescent positive cells for alkaline phosphatase and bone morphogenetic protein 2 (BMP-2) after eight days on the hydrophilic surfaces compared to hydrophobic ones. VEGF-positive cells were found in similar numbers on both surfaces after 8 days, and comparable osteocalcin and alkaline phosphatase positive cells were measured on both surfaces after 14 days up to 21 days, suggesting that hydrophilicity had a greater role in the early stages promoting osteoblastic differentiation. Similar results have been found on experimental Ti surfaces coated with anatase TiO2 and irradiated with UV-A for 24 h to enhance hydrophilicity [121]. Early osseointegration was enhanced in a 4-week dog mandibular model, with bone-to-implant contact significantly higher (42.7%) on the UV-hydrophilized surfaces compared to non-coated, hydrophobic controls (28.4%) after 2 weeks.
Complex surfaces with roughened surface topography and varied surface wettability have also validated the key role of surface hydrophilicity. Experimental surfaces modified by blasting, acid etching and anodization were evaluated in a 12-week rabbit tibial model [122]. The anodized surfaces presented the lowest surface roughness and the highest hydrophilicity among the different groups, and promoted the highest removal torque in vivo, suggesting that the effect of hydrophilicity could elicit a stronger response than surface roughness by itself in vivo. Noteworthy is the fact that the anodized surfaces were the only ones to contain well-defined nanostructures on its surface.
Interestingly, other studies have correlated improved osseointegration to wettability modification characteristics other than hydrophilicity. Aita et al. showed that the osteoconductive capacity of Ti implants increased after 4 weeks in a rat femoral model as a function of hydrocarbon removal after UV treatment rather than with the level of hydrophilicity, and indicated that the amount of hydrocarbon adsorbed on the TiO2 surface at the time of implantation was crucial in determining the bone-to-implant integration [61]. This implies a critical role for surface contamination of Ti implants not only for the wetting behavior but also for the biological outcome. Wettability reflects hydrocarbon contamination, but even if superhydrophilicity is acquired, this does not guarantee a surface free of any contamination [59].
Furthermore, studies have found a synergistic effect between high surface roughness and surface hydrophilicity. Using a split-mouth maxillary in vivo implantation model with domestic pigs, increased bone-to-implant contacts have been observed for clinically relevant, superhydrophilic modBAE surfaces compared to machined surfaces [67] and to hydrophobic BAE controls [42]. In 8-week studies, the significant differences were found in the initial 2- or 4-week time points, indicating early loading capacity for combined microrough, bioactive and hydrophilic surfaces.
Interestingly, other hydrophilic treatments involving alkali etching of microstructured Ti surfaces have found contrasting results. Implants with the alkali treatment on acid etched surfaces have promoted secondary stability in the early stages of healing in animal models compared to the control [67, 124]. The control implants, however, had a machined surface, so beneficial effects of hydrophilicity could not be separated from the influence of the altered structure of the etched surfaces. No meaningful differences were found in a mini pig model using the alkali treatment on BAE surfaces, compared to hydrophobic BAE and hydrophilic modBAE controls [125].
Schwarz et al. evaluated the performance of BAE and modBAE implants in the lower and upper jaw of dogs for 4 weeks [126]. The results confirmed the superior performance of superhydrophilic surfaces over hydrophobic surfaces, with higher bone-to-implant contacts after 1 week and up to 2 weeks before reaching similar levels, in the case of the lower jaw, or until the end of the study, in the case of the upper jaw. In the same study, the non-submerged surface of the implants was treated in different ways to investigate soft tissue integration to different surface finishes: relatively smooth machined, hydrophobic BAE, superhydrophilic acid-etched (modA) and superhydrophilic modBAE. Analyses showed that junctional epithelium was commonly separated from machined and BAE surfaces that had not been chemically treated to achieve superhydrophilicity, while epithelial cells seemed to be in close contact with modA surfaces after 2 weeks and both modA and modBAE surfaces appeared to promote attachment of well-vascularized subepithelial connective tissue.
Finally, Olivares-Navarrete et al. studied if the effects of surface characteristics such as surface roughness and wettability could be affected by age in a novel murine femoral intramedullary bone formation model using machined, BAE and modBAE implants [123]. The results revealed that after 4 weeks the old mice (9-month old) developed significantly higher bone-to-implant contact on modBAE surfaces compared to machined surfaces, in contrast to younger mice (2-month old), which presented similarly high levels on all three surfaces. Taken together, these results suggest that older mice have lower regenerative capacity than younger mice, but osseointegration similar to that in young mice can be achieved when given the appropriate surface properties. However, these findings have not been verified with humans in the clinic.
What has been verified clinically is that the synergistic effects of combined surface microroughness and superhydrophilicity can lead to enhanced osseointegration during the early phases of healing in a 6-week submerged retro-molar human model comparing hydrophobic BAE implants to superhydrophilic modBAE ones [11]. The surgical technique to remove the implant from the patients allowed the circumferential sampling of 1 mm of tissue surrounding the device for histomorphometric analyses. The percentage of bone-to-implant contact in the initial 2-week and 4-week time points was more prominent on the superhydrophilic surface (14.8% vs. 12.2% and 48.3% vs. 32.4%, respectively), but after 6 weeks at the end of the study the two surfaces supported similar levels of contact (61.6% vs. 61.5%).
The apparent early osseointegration found on superhydrophilic modBAE implants has been put to test in a two-center posterior maxillary/mandibular clinical study in which the devices were loaded 21 days after placement [127]. Using a non-submerged protocol, 89 implants in 56 patients were evaluated in total. After 21 days of healing, the devices were loaded with provisional restorations in full occlusion, and definitive metal ceramic restorations were given after 6 months of healing. The patients were continuously monitored for 24 months after implantation. Two implants failed to integrate during healing and two other implants required a longer healing time, while 85 implants (95.6%) were loaded without incident after 21 days of healing. No other implants were lost during the course of the study but one more was lost for follow-up. The success rate 2 years after implantation reached 97.7%, with all implants exhibiting favorable radiographic and clinical findings. Other studies with similar hydrophilized modBAE surfaces using different cleaning protocols have also reported favorable short term 1-year clinical outcomes [128] and increased survival rate in a 4- to 5-year retrospective study [129], although lack of hydrophobic controls and low participation at the 1-year follow up, respectively, limit the impact of their results.
Another hydrophilization technique evaluated clinically used phosphonic acid coupled to blasted and acid-etched surfaces to generate a “biomimetic” surface with increased hydrophilicity [130]. One-year results from a controlled clinical trial comparing the phosphonic acid-treated implants versus untreated controls in a split-mouth model with 23 patients confirmed the biocompatibility of the new surface modification. However, similar outcomes with the untreated controls suggested limited effectiveness for the phosphonic acid modification.
Finally, the alkali-treatment used to hydrophilize hydrophobic BAE surfaces has also been tested in a clinical case series [131]. For this study, 35 implants were placed in 10 patients with compromised bone density (class 3 and 4). Although no controls were included in the experimental design, the authors concluded the implants exhibited good results, in terms of implant stability quotient (ISQ) and vertical bone volume, up to one year after loading.
The superposition of hydrophilicity on marketed implants becomes very attractive when considering the enhancement in early osseointegration. Reducing the healing times and, thus, the time it takes for patients to return to their normal lifestyles, from several months to weeks, underscores the importance of hydrophilicity. Furthermore, it is reasonable to suggest that earlier, more stable osseointegration could extend coverage of compromised patients and also promote the long-term health of the implantation site, possibly prolonging the lifetime of the implant, which is difficult to evaluate in vitro, in vivo, or even in the clinic.
4. Conclusions
The roles of surface properties such as roughness and chemistry have been thoroughly evaluated in osseointegration; however, relatively few studies in the literature have investigated the effects of surface wettability on key biological aspects. Recent studies suggest a general stimulating effect of higher surface hydrophilicity on hard and soft tissue integration with the implant, yielding accelerated healing and early osseointegration. Experimental designs that would allow a more definitive correlation of observed biological responses to single surface parameters, such as hydrophilicity, charge, specific functional groups, or nanoroughness are challenging, as are designs that would allow investigators to further identify and quantify the role of synergistic effects. The optimal degree of hydrophilicity for best biological and clinical outcomes remains unclear. While several recent hydrophilized implant systems favor superhydrophilicity, it is unclear if a more moderate hydrophilicity would further optimize interfacial reactions. Although openly expressed in papers and by manufacturers, current knowledge can only hypothesize that earlier, more stable osseointegration can also promote the long-term health of the implantation site, possibly extending the lifetime of the implant.
Acknowledgments
Support for LS, FR and JGG from the ITI Foundation (Basel, Switzerland), the German Research Foundation (DFG, Deutsche Forschungsgemeinschaft, Bonn, Germany) and the Baden-Wuerttemberg Stiftung (Stuttgart, Germany) is gratefully acknowledged. RAG is thankful for the support of the Government of Panama (IFARHU-SENACYT) and the IMI Program of the National Science Foundation (ICMR Program, Award No. DMR04-09848). BDB, ZS and RAG are supported by the National Institutes of Health (US PHS Grant AR052102).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Karoussis IK, Bragger U, Salvi GE, Burgin W, Lang NP. Effect of implant design on survival and success rates of titanium oral implants: a 10-year prospective cohort study of the ITI Dental Implant System. Clin Oral Implants Res. 2004;15:8–17. doi: 10.1111/j.1600-0501.2004.00983.x. [DOI] [PubMed] [Google Scholar]
- 2.Albrektsson T, Zarb G, Worthington P, Eriksson AR. The long-term efficacy of currently used dental implants: a review and proposed criteria of success. Int J Oral Maxillofac Implants. 1986;1:11–25. [PubMed] [Google Scholar]
- 3.Fransson C, Wennstrom J, Berglundh T. Clinical characteristics at implants with a history of progressive bone loss. Clin Oral Implants Res. 2008;19:142–7. doi: 10.1111/j.1600-0501.2007.01448.x. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs JJ, Andersson GBJ, Bell JE, Weinstein SL, Dormans JP, Gnatz SM, et al. United States Bone and Joint Decade: The burden of musculoskeletal diseases in the United States. 1. Rosemont: AAOS; 2008. [Google Scholar]
- 5.Schwartz Z, Boyan BD. Underlying Mechanisms at the Bone-Biomaterial Interface. J Cell Biochem. 1994;56:340–7. doi: 10.1002/jcb.240560310. [DOI] [PubMed] [Google Scholar]
- 6.Puleo DA, Nanci A. Understanding and controlling the bone-implant interface. Biomaterials. 1999;20:2311–21. doi: 10.1016/s0142-9612(99)00160-x. [DOI] [PubMed] [Google Scholar]
- 7.Gittens RA, Olivares-Navarrete R, Cheng A, Anderson DM, McLachlan T, Stephan I, et al. The roles of titanium surface micro/nanotopography and wettability on the differential response of human osteoblast lineage cells. Acta Biomater. 2013;9:6268–77. doi: 10.1016/j.actbio.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mendonca G, Mendonca DBS, Aragao FJL, Cooper LF. The combination of micron and nanotopography by H2SO4/H2O2 treatment and its effects on osteoblast-specific gene expression of hMSCs. J Biomed Mater Res A. 2010;94A:169–79. doi: 10.1002/jbm.a.32701. [DOI] [PubMed] [Google Scholar]
- 9.Kubo K, Tsukimura N, Iwasa F, Ueno T, Saruwatari L, Aita H, et al. Cellular behavior on TiO2 nanonodular structures in a micro-to-nanoscale hierarchy model. Biomaterials. 2009;30:5319–29. doi: 10.1016/j.biomaterials.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 10.Mendes VC, Moineddin R, Davies JE. Discrete calcium phosphate nanocrystalline deposition enhances osteoconduction on titanium-based implant surfaces. J Biomed Mater Res A. 2009;90:577–85. doi: 10.1002/jbm.a.32126. [DOI] [PubMed] [Google Scholar]
- 11.Lang NP, Salvi GE, Huynh-Ba G, Ivanovski S, Donos N, Bosshardt DD. Early osseointegration to hydrophilic and hydrophobic implant surfaces in humans. Clin Oral Implants Res. 2011;22:349–56. doi: 10.1111/j.1600-0501.2011.02172.x. [DOI] [PubMed] [Google Scholar]
- 12.Grandin HM, Berner S, Dard M. A Review of Titanium Zirconium (TiZr) Alloys for Use in Endosseous Dental Implants. Materials. 2012;5:1348–60. [Google Scholar]
- 13.Rack HJ, Qazi JI. Titanium alloys for biomedical applications. Mater Sci Eng C Mater Biol Appl. 2006;26:1269–77. [Google Scholar]
- 14.Landolt D. Corrosion and Surface Chemistry of Metals. 1. Lausanne, Switzerland: EPFL Press; 2007. [Google Scholar]
- 15.Sul YT, Johansson C, Wennerberg P, Cho LR, Chang BS, Albrektsson P. Optimum surface properties of oxidized implants for reinforcement of osseointegration: Surface chemistry, oxide thickness, porosity, roughness, and crystal structure. Int J Oral Maxillofac Implants. 2005;20:349–59. [PubMed] [Google Scholar]
- 16.Bigi A, Cojazzi G, Panzavolta S, Ripamonti A, Roveri N, Romanello M, et al. Chemical and structural characterization of the mineral phase from cortical and trabecular bone. J Inorg Biochem. 1997;68:45–51. doi: 10.1016/s0162-0134(97)00007-x. [DOI] [PubMed] [Google Scholar]
- 17.Wennerberg A, Albrektsson T. Effects of titanium surface topography on bone integration: a systematic review. Clin Oral Implants Res. 2009;20:172–84. doi: 10.1111/j.1600-0501.2009.01775.x. [DOI] [PubMed] [Google Scholar]
- 18.Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–8. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 19.Mulari MTK, Qu Q, Harkonen PL, Vaananen HK. Osteoblast-like cells complete osteoclastic bone resorption and form new mineralized bone matrix in vitro. Calcif Tissue Int. 2004;75:253–61. doi: 10.1007/s00223-004-0172-3. [DOI] [PubMed] [Google Scholar]
- 20.Boyan BD, Schwartz Z, Lohmann CH, Sylvia VL, Cochran DL, Dean DD, et al. Pretreatment of bone with osteoclasts affects phenotypic expression of osteoblast-like cells. J Orthop Res. 2003;21:638–47. doi: 10.1016/S0736-0266(02)00261-9. [DOI] [PubMed] [Google Scholar]
- 21.Davies JE. Bone bonding at natural and biomaterial surfaces. Biomaterials. 2007;28:5058–67. doi: 10.1016/j.biomaterials.2007.07.049. [DOI] [PubMed] [Google Scholar]
- 22.Gittens RA, Olivares-Navarrete R, McLachlan T, Cai Y, Hyzy SL, Schneider JM, et al. Differential responses of osteoblast lineage cells to nanotopographically-modified, microroughened titanium-aluminum-vanadium alloy surfaces. Biomaterials. 2012;33:8986–94. doi: 10.1016/j.biomaterials.2012.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wennerberg A, Svanborg LM, Berner S, Andersson M. Spontaneously formed nanostructures on titanium surfaces. Clin Oral Implants Res. 2013;24:203–9. doi: 10.1111/j.1600-0501.2012.02429.x. [DOI] [PubMed] [Google Scholar]
- 24.Sittig C, Textor M, Spencer ND, Wieland M, Vallotton PH. Surface characterization of implant materials cp Ti, Ti-6Al-7Nb and Ti-6Al-4V with different pretreatments. J Mater Sci Mater Med. 1999;10:35–46. doi: 10.1023/a:1008840026907. [DOI] [PubMed] [Google Scholar]
- 25.Massaro C, Rotolo P, De Riccardis F, Milella E, Napoli A, Wieland M, et al. Comparative investigation of the surface properties of commercial titanium dental implants. Part I: chemical composition. J Mater Sci Mater Med. 2002;13:535–48. doi: 10.1023/a:1015170625506. [DOI] [PubMed] [Google Scholar]
- 26.Le Guehennec L, Soueidan A, Layrolle P, Amouriq Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent Mater. 2007;23:844–54. doi: 10.1016/j.dental.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 27.Rupp F, Scheideler L, Eichler M, Geis-Gerstorfer J. Wetting Behavior of Dental Implants. Int J Oral Maxillofac Implants. 2011;26:1256–66. [PubMed] [Google Scholar]
- 28.Eriksson C, Nygren H, Ohlson K. Implantation of hydrophilic and hydrophobic titanium discs in rat tibia: cellular reactions on the surfaces during the first 3 weeks in bone. Biomaterials. 2004;25:4759–66. doi: 10.1016/j.biomaterials.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 29.Bornstein MM, Valderrama P, Jones AA, Wilson TG, Seibl R, Cochran DL. Bone apposition around two different sandblasted and acid-etched titanium implant surfaces: a histomorphometric study in canine mandibles. Clin Oral Implants Res. 2008;19:233–41. doi: 10.1111/j.1600-0501.2007.01473.x. [DOI] [PubMed] [Google Scholar]
- 30.Kennedy SB, Washburn NR, Simon CG, Jr, Amis EJ. Combinatorial screen of the effect of surface energy on fibronectin-mediated osteoblast adhesion, spreading and proliferation. Biomaterials. 2006;27:3817–24. doi: 10.1016/j.biomaterials.2006.02.044. [DOI] [PubMed] [Google Scholar]
- 31.Qiu Q, Sayer M, Kawaja M, Shen X, Davies JE. Attachment, morphology, and protein expression of rat marrow stromal cells cultured on charged substrate surfaces. J Biomed Mater Res. 1998;42:117–27. doi: 10.1002/(sici)1097-4636(199810)42:1<117::aid-jbm15>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 32.Palmquist A, Engqvist H, Lausmaa J, Thomsen P. Commercially available dental implants: Review of their surface characteristics. J Biomater Tissue Eng. 2012;2:112–24. [Google Scholar]
- 33.Kilpadi DV, Lemons JE. Surface energy characterization of unalloyed titanium implants. J Biomed Mater Res. 1994;28:1419–25. doi: 10.1002/jbm.820281206. [DOI] [PubMed] [Google Scholar]
- 34.Kohavi D, Badihi HL, Rosen G, Steinberg D, Sela MN. Wettability versus electrostatic forces in fibronectin and albumin adsorption to titanium surfaces. Clin Oral Implants Res. 2012 doi: 10.1111/j.1600-0501.2012.02508.x. [DOI] [PubMed] [Google Scholar]
- 35.Rupp F, Scheideler L, Rehbein D, Axmann D, Gels-Gerstorfer J. Roughness induced dynamic changes of wettability of acid etched titanium implant modifications. Biomaterials. 2004;25:1429–38. doi: 10.1016/j.biomaterials.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 36.Mittal KL, Good RJ. Contact angle, wettability and adhesion: Festschrift in honor of Professor Robert J. Good. Utrecht, The Netherlands: VSP; 1993. [Google Scholar]
- 37.Junker R, Dimakis A, Thoneick M, Jansen JA. Effects of implant surface coatings and composition on bone integration: a systematic review. Clin Oral Implants Res. 2009;20 (Suppl 4):185–206. doi: 10.1111/j.1600-0501.2009.01777.x. [DOI] [PubMed] [Google Scholar]
- 38.Sykaras N, Iacopino AM, Marker VA, Triplett RG, Woody RD. Implant materials, designs, and surface topographies: their effect on osseointegration. A literature review. Int J Oral Maxillofac Implants. 2000;15:675–90. [PubMed] [Google Scholar]
- 39.Schwartz Z, Raz P, Zhao G, Barak Y, Tauber M, Yao H, et al. Effect of Micrometer-Scale Roughness of the Surface of Ti6Al4V Pedicle Screws in Vitro and in Vivo. J Bone Jt Surg (Am) 2008;90A:2485–98. doi: 10.2106/JBJS.G.00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwarz F, Wieland M, Schwartz Z, Zhao G, Rupp F, Geis-Gerstorfer J, et al. Potential of chemically modified hydrophilic surface characteristics to support tissue integration of titanium dental implants. J Biomed Mater Res B Appl Biomater. 2009;88B:544–57. doi: 10.1002/jbm.b.31233. [DOI] [PubMed] [Google Scholar]
- 41.Wennerberg A, Galli S, Albrektsson T. Current knowledge about the hydrophilic and nanostructured SLActive surface. Clin Cosmet Investig Dent. 2011;3:59–67. doi: 10.2147/CCIDEN.S15949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buser D, Broggini N, Wieland M, Schenk RK, Denzer AJ, Cochran DL, et al. Enhanced bone apposition to a chemically modified SLA titanium surface. J Dent Res. 2004;83:529–33. doi: 10.1177/154405910408300704. [DOI] [PubMed] [Google Scholar]
- 43.Rupp F, Scheideler L, Olshanska N, deWild M, Wieland M, Geis-Gerstorfer J. Enhancing surface free energy and hydrophilicity through chemical modification of microstructured titanium implant surfaces. J Biomed Mater Res A. 2006;76A:323–34. doi: 10.1002/jbm.a.30518. [DOI] [PubMed] [Google Scholar]
- 44.Zhao G, Schwartz Z, Wieland M, Rupp F, Geis-Gerstorfer J, Cochran DL, et al. High surface energy enhances cell response to titanium substrate microstructure. J Biomed Mater Res. 2005;74A:49–58. doi: 10.1002/jbm.a.30320. [DOI] [PubMed] [Google Scholar]
- 45.Park JH, Wasilewski CE, Almodovar N, Olivares-Navarrete R, Boyan BD, Tannenbaum R, et al. The responses to surface wettability gradients induced by chitosan nanofilms on microtextured titanium mediated by specific integrin receptors. Biomaterials. 2012;33:7386–93. doi: 10.1016/j.biomaterials.2012.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marshall SJ, Bayne SC, Baier R, Tomsia AP, Marshall GW. A review of adhesion science. Dent Mater. 2010;26:E11–E6. doi: 10.1016/j.dental.2009.11.157. [DOI] [PubMed] [Google Scholar]
- 47.Rupp F, Haupt M, Klostermann H, Kim HS, Eichler M, Peetsch A, et al. Multifunctional nature of UV-irradiated nanocrystalline anatase thin films for biomedical applications. Acta Biomater. 2010;6:4566–77. doi: 10.1016/j.actbio.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 48.Zinelis S, Silikas N, Thomas A, Syres K, Eliades G. Surface characterization of SLActive dental implants. Eur J Esthet Dent. 2012;7:72–92. [PubMed] [Google Scholar]
- 49.Wennerberg A, Albrektsson T. On implant surfaces: a review of current knowledge and opinions. Int J Oral Maxillofac Implants. 2010;25:63–74. [PubMed] [Google Scholar]
- 50.Wennerberg A, Jimbo R, Stübinger S, Obrecht M, Dard M, Berner S. Nanostructures and hydrophilicity influence osseointegration: a biomechanical study in the rabbit tibia. Clin Oral Implants Res. 2013 doi: 10.1111/clr.12213. [DOI] [PubMed] [Google Scholar]
- 51.Baier RE, Meyer AE, Natiella JR, Natiella RR, Carter JM. Surface properties determine bioadhesive outcomes: methods and results. J Biomed Mater Res. 1984;18:337–55. doi: 10.1002/jbm.820180404. [DOI] [PubMed] [Google Scholar]
- 52.Park JH, Olivares-Navarrete R, Baier RE, Meyer AE, Tannenbaum R, Boyan BD, et al. Effect of cleaning and sterilization on titanium implant surface properties and cellular response. Acta Biomater. 2012;8:1966–75. doi: 10.1016/j.actbio.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duske K, Koban I, Kindel E, Schroder K, Nebe B, Holtfreter B, et al. Atmospheric plasma enhances wettability and cell spreading on dental implant metals. J Clin Periodontol. 2012;39:400–7. doi: 10.1111/j.1600-051X.2012.01853.x. [DOI] [PubMed] [Google Scholar]
- 54.Rupp F, Axmann D, Ziegler C, Geis-Gerstorfer J. Adsorption/desorption phenomena on pure and Teflon((R)) AF-coated titania surfaces studied by dynamic contact angle analysis. J Biomed Mater Res. 2002;62:567–78. doi: 10.1002/jbm.10198. [DOI] [PubMed] [Google Scholar]
- 55.Scharnweber D, Schlottig F, Oswald S, Becker K, Worch H. How is wettability of titanium surfaces influenced by their preparation and storage conditions? J Mater Sci Mater Med. 2010;21:525–32. doi: 10.1007/s10856-009-3908-9. [DOI] [PubMed] [Google Scholar]
- 56.Fujishima A, Honda K. Electrochemical photolysis of water at a semiconductor electrode. Nature. 1972;238:37–8. doi: 10.1038/238037a0. [DOI] [PubMed] [Google Scholar]
- 57.Fujishima A, Rao TN, Tryk DA. Titanium dioxide photocatalysis. J Photochem Photobiol C:Photochem Rev. 2000;1:1–21. [Google Scholar]
- 58.Fujishima A, Zhang XT, Tryk DA. TiO2 photocatalysis and related surface phenomena. Surf Sci Rep. 2008;63:515–82. [Google Scholar]
- 59.Hashimoto K, Irie H, Fujishima A. TiO2 photocatalysis: A historical overview and future prospects. Jpn J Appl Phys, Part 1. 2005;44:8269–85. [Google Scholar]
- 60.Wang R, Hashimoto K, Fujishima A, Chikuni M, Kojima E, Kitamura A, et al. Light-induced amphiphilic surfaces. Nature. 1997;388:431–2. [Google Scholar]
- 61.Aita H, Hori N, Takeuchi M, Suzuki T, Yamada M, Anpo M, et al. The effect of ultraviolet functionalization of titanium on integration with bone. Biomaterials. 2009;30:1015–25. doi: 10.1016/j.biomaterials.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 62.Sawase T, Jimbo R, Wennerberg A, Suketa N, Tanaka Y, Atsuta M. A novel characteristic of porous titanium oxide implants. Clin Oral Implants Res. 2007;18:680–5. doi: 10.1111/j.1600-0501.2007.01404.x. [DOI] [PubMed] [Google Scholar]
- 63.Rupp F, Haupt M, Eichler M, Doering C, Klostermann H, Scheideler L, et al. Formation and Photocatalytic Decomposition of a Pellicle on Anatase Surfaces. J Dent Res. 2012;91:104–9. doi: 10.1177/0022034511424901. [DOI] [PubMed] [Google Scholar]
- 64.Gallardo-Moreno AM, Pacha-Olivenza MA, Fernandez-Calderon MC, Perez-Giraldo C, Bruque JM, Gonzalez-Martin ML. Bactericidal behaviour of Ti6Al4V surfaces after exposure to UV-C light. Biomaterials. 2010;31:5159–68. doi: 10.1016/j.biomaterials.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 65.Park JH, Schwartz Z, Olivares-Navarrete R, Boyan BD, Tannenbaum R. Enhancement of Surface Wettability via the Modification of Microtextured Titanium Implant Surfaces with Polyelectrolytes. Langmuir. 2011;27:5976–85. doi: 10.1021/la2000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jonasova L, Muller FA, Helebrant A, Strnad J, Greil P. Biomimetic apatite formation on chemically treated titanium. Biomaterials. 2004;25:1187–94. doi: 10.1016/j.biomaterials.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 67.von Wilmowsky C, Muller L, Lutz R, Lohbauer U, Rupp F, Neukam FW, et al. Osseointegration of Chemically Modified Titanium Surfaces: An in Vivo Study. Adv Eng Mater. 2008;10:B61–B6. [Google Scholar]
- 68.Vogler EA. Protein adsorption in three dimensions. Biomaterials. 2012;33:1201–37. doi: 10.1016/j.biomaterials.2011.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wilson CJ, Clegg RE, Leavesley DI, Pearcy MJ. Mediation of biomaterial-cell nteractions by adsorbed proteins: A review. Tissue Eng. 2005;11:1–18. doi: 10.1089/ten.2005.11.1. [DOI] [PubMed] [Google Scholar]
- 70.Andrade JD, Hlady V. Protein adsorption and materials biocompatibility - A tutorial review and suggested hypotheses. Adv Polym Sci. 1986;79:1–63. [Google Scholar]
- 71.Spijker HT, Graaff R, Boonstra PW, Busscher HJ, van Oeveren W. On the influence of flow conditions and wettability on blood material interactions. Biomaterials. 2003;24:4717–27. doi: 10.1016/s0142-9612(03)00380-6. [DOI] [PubMed] [Google Scholar]
- 72.Keselowsky BG, Bridges AW, Burns KL, Tate CC, Babensee JE, LaPlaca MC, et al. Role of plasma fibronectin in the foreign body response to biomaterials. Biomaterials. 2007;28:3626–31. doi: 10.1016/j.biomaterials.2007.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eriksson C, Nygren H. Polymorphonuclear leukocytes in coagulating whole blood recognize hydrophilic and hydrophobic titanium surfaces by different adhesion receptors and show different patterns of receptor expression. J Lab Clin Med. 2001;137:296–302. doi: 10.1067/mlc.2001.114066. [DOI] [PubMed] [Google Scholar]
- 74.Kou PM, Schwartz Z, Boyan BD, Babensee JE. Dendritic cell responses to surface properties of clinical titanium surfaces. Acta Biomater. 2011;7:1354–63. doi: 10.1016/j.actbio.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Salasznyk RM, Williams WA, Boskey A, Batorsky A, Plopper GE. Adhesion to Vitronectin and Collagen I Promotes Osteogenic Differentiation of Human Mesenchymal Stem Cells. J Biomed Biotechnol. 2004;2004:24–34. doi: 10.1155/S1110724304306017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Scheideler L, Rupp F, Wieland M, Geis-Gerstorfer J. Storage conditions of titanium implants influence molecular and cellular interactions. J Dent Res. 2005;84(Spec Iss A) Abstract No. 0870. [Google Scholar]
- 77.Huang QL, Lin LX, Yang Y, Hu R, Vogler EA, Lin CJ. Role of trapped air in the formation of cell-and-protein micropatterns on superhydrophobic/superhydrophilic microtemplated surfaces. Biomaterials. 2012;33:8213–20. doi: 10.1016/j.biomaterials.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 78.Puckett SD, Lee PP, Ciombor DM, Aaron RK, Webster TJ. Nanotextured titanium surfaces for enhancing skin growth on transcutaneous osseointegrated devices. Acta Biomater. 2010;6:2352–62. doi: 10.1016/j.actbio.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 79.Scheideler L, Rupp F, Heigert D, de Wild M, Wieland M, Geis-Gerstorfer J. Influence of roughness and wettability of titanium implants on keratinocytes. J Dent Res. 2007;86(Spec Iss A) Abstract No. 1301. [Google Scholar]
- 80.Brogren H, Karlsson L, Andersson M, Wang L, Erlinge D, Jern S. Platelets synthesize large amounts of active plasminogen activator inhibitor 1. Blood. 2004;104:3943–8. doi: 10.1182/blood-2004-04-1439. [DOI] [PubMed] [Google Scholar]
- 81.Neuss S, Schneider RK, Tietze L, Knuchel R, Jahnen-Dechent W. Secretion of fibrinolytic enzymes facilitates human mesenchymal stem cell invasion into fibrin clots. Cells Tissues Organs. 2010;191:36–46. doi: 10.1159/000215579. [DOI] [PubMed] [Google Scholar]
- 82.Davies JE. In vitro modeling of the bone/implant interface. Anat Rec. 1996;245:426–45. doi: 10.1002/(SICI)1097-0185(199606)245:2<426::AID-AR21>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 83.Dalby MJ, Gadegaard N, Tare R, Andar A, Riehle MO, Herzyk P, et al. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat Mater. 2007;6:997–1003. doi: 10.1038/nmat2013. [DOI] [PubMed] [Google Scholar]
- 84.Jimbo R, Sawase T, Baba K, Kurogi T, Shibata Y, Atsuta M. Enhanced initial cell responses to chemically modified anodized titanium. Clinical implant dentistry and related research. 2008;10:55–61. doi: 10.1111/j.1708-8208.2007.00061.x. [DOI] [PubMed] [Google Scholar]
- 85.Sawase T, Jimbo R, Baba K, Shibata Y, Ikeda T, Atsuta M. Photo-induced hydrophilicity enhances initial cell behavior and early bone apposition. Clin Oral Implants Res. 2008;19:491–6. doi: 10.1111/j.1600-0501.2007.01509.x. [DOI] [PubMed] [Google Scholar]
- 86.Olivares-Navarrete R, Hyzy SL, Hutton DL, Erdman CP, Wieland M, Boyan BD, et al. Direct and indirect effects of microstructured titanium substrates on the induction of mesenchymal stem cell differentiation towards the osteoblast lineage. Biomaterials. 2010;31:2728–35. doi: 10.1016/j.biomaterials.2009.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Aita H, Att W, Ueno T, Yamada M, Hori N, Iwasa F, et al. Ultraviolet light-mediated photofunctionalization of titanium to promote human mesenchymal stem cell migration, attachment, proliferation and differentiation. Acta Biomater. 2009;5:3247–57. doi: 10.1016/j.actbio.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 88.Lim JY, Taylor AF, Li Z, Vogler EA, Donahue HJ. Integrin expression and osteopontin regulation in human fetal osteoblastic cells mediated by substratum surface characteristics. Tissue Eng. 2005;11:19–29. doi: 10.1089/ten.2005.11.19. [DOI] [PubMed] [Google Scholar]
- 89.Lim JY, Shaughnessy MC, Zhou Z, Noh H, Vogler EA, Donahue HJ. Surface energy effects on osteoblast spatial growth and mineralization. Biomaterials. 2008;29:1776–84. doi: 10.1016/j.biomaterials.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 90.Zhao G, Raines AL, Wieland M, Schwartz Z, Boyan BD. Requirement for both micron- and submicron scale structure for synergistic responses of osteoblasts to substrate surface energy and topography. Biomaterials. 2007;28:2821–9. doi: 10.1016/j.biomaterials.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.An N, Rausch-fan X, Wieland M, Matejka M, Andrukhov O, Schedle A. Initial attachment, subsequent cell proliferation/viability and gene expression of epithelial cells related to attachment and wound healing in response to different titanium surfaces. Dent Mater. 2012;28:1207–14. doi: 10.1016/j.dental.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 92.Presland RB, Dale BA. Epithelial structural proteins of the skin and oral cavity: function in health and disease. Crit Rev Oral Biol Med. 2000;11:383–408. doi: 10.1177/10454411000110040101. [DOI] [PubMed] [Google Scholar]
- 93.Nolte SV, Xu W, Rennekampff HO, Rodemann HP. Diversity of fibroblasts--a review on implications for skin tissue engineering. Cells Tissues Organs. 2008;187:165–76. doi: 10.1159/000111805. [DOI] [PubMed] [Google Scholar]
- 94.Rompen E, Domken O, Degidi M, Pontes AE, Piattelli A. The effect of material characteristics, of surface topography and of implant components and connections on soft tissue integration: a literature review. Clin Oral Implants Res. 2006;17 (Suppl 2):55–67. doi: 10.1111/j.1600-0501.2006.01367.x. [DOI] [PubMed] [Google Scholar]
- 95.Quirynen M, De Soete M, van Steenberghe D. Infectious risks for oral implants: a review of the literature. Clin Oral Implants Res. 2002;13:1–19. doi: 10.1034/j.1600-0501.2002.130101.x. [DOI] [PubMed] [Google Scholar]
- 96.Kimura Y, Matsuzaka K, Yoshinari M, Inoue T. Initial attachment of human oral keratinocytes cultured on zirconia or titanium. Dent Mater J. 2012;31:346–53. doi: 10.4012/dmj.2011-189. [DOI] [PubMed] [Google Scholar]
- 97.Groessner-Schreiber B, Neubert A, Muller WD, Hopp M, Griepentrog M, Lange KP. Fibroblast growth on surface-modified dental implants: an in vitro study. J Biomed Mater Res A. 2003;64:591–9. doi: 10.1002/jbm.a.10417. [DOI] [PubMed] [Google Scholar]
- 98.den Braber ET, Jansen HV, de Boer MJ, Croes HJ, Elwenspoek M, Ginsel LA, et al. Scanning electron microscopic, transmission electron microscopic, and confocal laser scanning microscopic observation of fibroblasts cultured on microgrooved surfaces of bulk titanium substrata. J Biomed Mater Res. 1998;40:425–33. doi: 10.1002/(sici)1097-4636(19980605)40:3<425::aid-jbm13>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 99.Arciola CR, Campoccia D, Speziale P, Montanaro L, Costerton JW. Biofilm formation in Staphylococcus implant infections. A review of molecular mechanisms and implications for biofilm-resistant materials. Biomaterials. 2012;33:5967–82. doi: 10.1016/j.biomaterials.2012.05.031. [DOI] [PubMed] [Google Scholar]
- 100.Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med. 2004;350:1422–9. doi: 10.1056/NEJMra035415. [DOI] [PubMed] [Google Scholar]
- 101.Pye AD, Lockhart DE, Dawson MP, Murray CA, Smith AJ. A review of dental implants and infection. J Hosp Infect. 2009;72:104–10. doi: 10.1016/j.jhin.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 102.Norowski PA, Jr, Bumgardner JD. Biomaterial and antibiotic strategies for peri-implantitis: a review. J Biomed Mater Res B Appl Biomater. 2009;88:530–43. doi: 10.1002/jbm.b.31152. [DOI] [PubMed] [Google Scholar]
- 103.Kerrigan SW, Cox D. Platelet-bacterial interactions. Cell Mol Life Sci. 2010;67:513–23. doi: 10.1007/s00018-009-0207-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Knetsch ML, Koole LH. New strategies in the development of antimicrobial coatings: the example of increasing usage of silver and silver nanoparticles. Polymers. 2011;3:340–66. [Google Scholar]
- 105.Eichler M, Katzur V, Scheideler L, Haupt M, Geis-Gerstorfer J, Schmalz G, et al. The impact of dendrimer-grafted modifications to model silicon surfaces on protein adsorption and bacterial adhesion. Biomaterials. 2011;32:9168–79. doi: 10.1016/j.biomaterials.2011.08.063. [DOI] [PubMed] [Google Scholar]
- 106.Muller R, Eidt A, Hiller KA, Katzur V, Subat M, Schweikl H, et al. Influences of protein films on antibacterial or bacteria-repellent surface coatings in a model system using silicon wafers. Biomaterials. 2009;30:4921–9. doi: 10.1016/j.biomaterials.2009.05.079. [DOI] [PubMed] [Google Scholar]
- 107.Roosjen A, Norde W, van der Mei HC, Busscher HJ. The use of positively charged or low surface free energy coatings versus polymer brushes in controlling biofilm formation. Prog Colloid Polym Sci. 2006:132. [Google Scholar]
- 108.Hsiue GH, Lee SD, Chang PC, Kao CY. Surface characterization and biological properties study of silicone rubber membrane grafted with phospholipid as biomaterial via plasma induced graft copolymerization. J Biomed Mater Res. 1998;42:134–47. doi: 10.1002/(sici)1097-4636(199810)42:1<134::aid-jbm17>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 109.Vasilev K, Sah VR, Goreham RV, Ndi C, Short RD, Griesser HJ. Antibacterial surfaces by adsorptive binding of polyvinyl-sulphonate-stabilized silver nanoparticles. Nanotechnology. 2010;21:215102. doi: 10.1088/0957-4484/21/21/215102. [DOI] [PubMed] [Google Scholar]
- 110.An YH, Friedman RJ. Concise review of mechanisms of bacterial adhesion to biomaterial surfaces. J Biomed Mater Res. 1998;43:338–48. doi: 10.1002/(sici)1097-4636(199823)43:3<338::aid-jbm16>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 111.Drake DR, Paul J, Keller JC. Primary bacterial colonization of implant surfaces. Int J Oral Maxillofac Implants. 1999;14:226–32. [PubMed] [Google Scholar]
- 112.Quirynen M, Bollen CM. The influence of surface roughness and surface-free energy on supra- and subgingival plaque formation in man. A review of the literature. J Clin Periodontol. 1995;22:1–14. doi: 10.1111/j.1600-051x.1995.tb01765.x. [DOI] [PubMed] [Google Scholar]
- 113.Bollen CM, Lambrechts P, Quirynen M. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: a review of the literature. Dent Mater. 1997;13:258–69. doi: 10.1016/s0109-5641(97)80038-3. [DOI] [PubMed] [Google Scholar]
- 114.Liu Y, Zhao Q. Influence of surface energy of modified surfaces on bacterial adhesion. Biophys Chem. 2005;117:39–45. doi: 10.1016/j.bpc.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 115.Doyle RJ. Contribution of the hydrophobic effect to microbial infection. Microb Infect. 2000;2:391–400. doi: 10.1016/s1286-4579(00)00328-2. [DOI] [PubMed] [Google Scholar]
- 116.Oliveira R, Azeredo J, Teixeira P, Fonseca AP. The role of hydrophobicity in bacterial adhesion. In: Gilbert P, Allison D, Brading M, Verran J, Walker J, editors. Biofilm community interactions: chance or necessity? Cardiff; 2001. pp. 11–22. [Google Scholar]
- 117.Quirynen M, Marechal M, Busscher HJ, Weerkamp AH, Darius PL, van Steenberghe D. The influence of surface free energy and surface roughness on early plaque formation. An in vivo study in man. J Clin Periodontol. 1990;17:138–44. doi: 10.1111/j.1600-051x.1990.tb01077.x. [DOI] [PubMed] [Google Scholar]
- 118.Bruinsma GM, van der Mei HC, Busscher HJ. Bacterial adhesion to surface hydrophilic and hydrophobic contact lenses. Biomaterials. 2001;22:3217–24. doi: 10.1016/s0142-9612(01)00159-4. [DOI] [PubMed] [Google Scholar]
- 119.MacKintosh EE, Patel JD, Marchant RE, Anderson JM. Effects of biomaterial surface chemistry on the adhesion and biofilm formation of Staphylococcus epidermidis in vitro. J Biomed Mater Res A. 2006;78:836–42. doi: 10.1002/jbm.a.30905. [DOI] [PubMed] [Google Scholar]
- 120.Keselowsky BG, Collard DM, Garcia AJ. Surface chemistry modulates fibronectin conformation and directs integrin binding and specificity to control cell adhesion. J Biomed Mater Res A. 2003;66A:247–59. doi: 10.1002/jbm.a.10537. [DOI] [PubMed] [Google Scholar]
- 121.Hirakawa Y, Jimbo R, Shibata Y, Watanabe I, Wennerberg A, Sawase T. Accelerated bone formation on photo-induced hydrophilic titanium implants: an experimental study in the dog mandible. Clin Oral Implants Res. 2012 doi: 10.1111/j.1600-0501.2011.02401.x. [DOI] [PubMed] [Google Scholar]
- 122.Elias CN, Oshida Y, Lima JH, Muller CA. Relationship between surface properties (roughness, wettability and morphology) of titanium and dental implant removal torque. J Mech Behav Biomed Mater. 2008;1:234–42. doi: 10.1016/j.jmbbm.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 123.Olivares-Navarrete R, Raines AL, Hyzy SL, Park JH, Hutton DL, Cochran DL, et al. Osteoblast maturation and new bone formation in response to titanium implant surface features are reduced with age. J Bone Miner Res. 2012;27:1773–83. doi: 10.1002/jbmr.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Strnad J, Urban K, Povysil C, Strnad Z. Secondary stability assessment of titanium implants with an alkali-etched surface: a resonance frequency analysis study in beagle dogs. Int J Oral Maxillofac Implants. 2008;23:502–12. [PubMed] [Google Scholar]
- 125.Vasak C, Busenlechner D, Schwarze UY, Leitner HF, Munoz Guzon F, Hefti T, et al. Early bone apposition to hydrophilic and hydrophobic titanium implant surfaces: a histologic and histomorphometric study in minipigs. Clin Oral Implants Res. 2013 doi: 10.1111/clr.12277. [DOI] [PubMed] [Google Scholar]
- 126.Schwarz F, Ferrari D, Herten M, Mihatovic I, Wieland M, Sager M, et al. Effects of surface hydrophilicity and microtopography on early stages of soft and hard tissue integration at non-submerged titanium implants: an immunohistochemical study in dogs. J Periodontol. 2007;78:2171–84. doi: 10.1902/jop.2007.070157. [DOI] [PubMed] [Google Scholar]
- 127.Morton D, Bornstein MM, Wittneben JG, Martin WC, Ruskin JD, Hart CN, et al. Early loading after 21 days of healing of nonsubmerged titanium implants with a chemically modified sandblasted and acid-etched surface: two-year results of a prospective two-center study. Clin Implant Dent Relat Res. 2010;12:9–17. doi: 10.1111/j.1708-8208.2009.00204.x. [DOI] [PubMed] [Google Scholar]
- 128.Degasperi W, Andersson P, Verrocchi D, Sennerby L. One-Year Clinical and Radiographic Results with a Novel Hydrophilic Titanium Dental Implant. Clin Implant Dent Relat Res. 2012 doi: 10.1111/cid.12022. [DOI] [PubMed] [Google Scholar]
- 129.Zumstein T, Billstrom C, Sennerby L. A 4- to 5-year retrospective clinical and radiographic study of Neoss implants placed with or without GBR procedures. Clin Implant Dent Relat Res. 2012;14:480–90. doi: 10.1111/j.1708-8208.2010.00286.x. [DOI] [PubMed] [Google Scholar]
- 130.Esposito M, Dojcinovic I, Germon L, Levy N, Curno R, Buchini S, et al. Safety and efficacy of a biomimetic monolayer of permanently bound multi-phosphonic acid molecules on dental implants: 1 year post-loading results from a pilot quadruple-blinded randomised controlled trial. Eur J Oral Implantol. 2013;6:227–36. [PubMed] [Google Scholar]
- 131.Held U, Rohner D, Rothamel D. Early loading of hydrophilic titanium implants inserted in low-mineralized (D3 and D4) bone: one year results of a prospective clinical trial. Head Face Med. 2013;9:37. doi: 10.1186/1746-160X-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gubbi P, Towse R, Berckmans B. Hydrophobic/hydrophilic characteristic of titanium surfaces: Machined, dual etched (OsseoTite), and dual acid etched with nanometer-scale CaP (NanoTite). Society for Biomaterials; 32nd Annual Meeting; Chicago, IL, USA. 2007. Abstract No. 796. [Google Scholar]
- 133.Taborelli M, Jobin M, Francois P, Vaudaux P, Tonetti M, Szmukler-Moncler S, et al. Influence of surface treatments developed for oral implants on the physical and biological properties of titanium. (I) Surface characterization. Clin Oral Implants Res. 1997;8:208–16. doi: 10.1034/j.1600-0501.1997.080307.x. [DOI] [PubMed] [Google Scholar]
- 134.Sardin S, Morrier JJ, Benay G, Barsotti O. In vitro streptococcal adherence on prosthetic and implant materials. Interactions with physicochemical surface properties. J Oral Rehabil. 2004;31:140–8. doi: 10.1046/j.0305-182x.2003.01136.x. [DOI] [PubMed] [Google Scholar]
- 135.Sela MN, Badihi L, Rosen G, Steinberg D, Kohavi D. Adsorption of human plasma proteins to modified titanium surfaces. Clin Oral Implants Res. 2007;18:630–8. doi: 10.1111/j.1600-0501.2007.01373.x. [DOI] [PubMed] [Google Scholar]
- 136.Jimbo R, Xue Y, Hayashi M, Schwartz HO, Andersson M, Mustafa K, et al. Genetic Responses to Nanostructured Calcium-phosphate-coated Implants. J Dent Res. 2011;90:1422–7. doi: 10.1177/0022034511422911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gittens RA, McLachlan T, Olivares-Navarrete R, Cai Y, Berner S, Tannenbaum R, et al. The effects of combined micron-/submicron-scale surface roughness and nanoscale features on cell proliferation and differentiation. Biomaterials. 2011;32:3395–403. doi: 10.1016/j.biomaterials.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jimbo R, Ono D, Hirakawa Y, Odatsu T, Tanaka T, Sawase T. Accelerated photo-induced hydrophilicity promotes osseointegration: an animal study. Clin Implant Dent Relat Res. 2011;13:79–85. doi: 10.1111/j.1708-8208.2009.00179.x. [DOI] [PubMed] [Google Scholar]