Abstract
Microbial community structures in methane seep sediments in the Nankai Trough were analyzed by tag-sequencing analysis for the small subunit (SSU) rRNA gene using a newly developed primer set. The dominant members of Archaea were Deep-sea Hydrothermal Vent Euryarchaeotic Group 6 (DHVEG 6), Marine Group I (MGI) and Deep Sea Archaeal Group (DSAG), and those in Bacteria were Alpha-, Gamma-, Delta- and Epsilonproteobacteria, Chloroflexi, Bacteroidetes, Planctomycetes and Acidobacteria. Diversity and richness were examined by 8,709 and 7,690 tag-sequences from sediments at 5 and 25 cm below the seafloor (cmbsf), respectively. The estimated diversity and richness in the methane seep sediment are as high as those in soil and deep-sea hydrothermal environments, although the tag-sequences obtained in this study were not sufficient to show whole microbial diversity in this analysis. We also compared the diversity and richness of each taxon/division between the sediments from the two depths, and found that the diversity and richness of some taxa/divisions varied significantly along with the depth.
Keywords: methane seep, sediment, deep-sea, tag-sequencing
In deep-sea anoxic methane seep sediments, methanotrophic Euryarchaeota and syntrophic sulfate-reducing Deltaproteobacteria oxidize methane anaerobically with the following equation:
| (4) |
and this syntrophic reaction is one of the most abundant processes of methane seep ecosystems (16). In contrast to the subsurface sediments, sulfur oxidizers belong to Gammaproteobacteria and Epsilonproteobacteria that utilize the high sulfide fluxes derived from anaerobic oxidation of methane (AOM) (14, 16, 20, 31), aerobic methanotroph in Gammaproteobacteria (15, 40), and ammonia-oxidizing marine group I (MGI) Archaea ‘Nitrosopumilales’ (3, 9, 11, 14) distributed on the seafloor. Therefore, the ecosystem is regarded as a chemosynthetic oasis in the deep-sea floor environment that is supported by these primary producers in comparison to the ambient seafloor sediment ecosystem that is restricted by the input of fresh detritus (16, 34). In addition, other uncultivated lineages of Archaea such as the Deep-sea Archaeal Group (DSAG, also known as Marine Benthic Group B), Marine Benthic Group D (MBGD) and Miscellaneous Crenarchaeotic Group (MCG, also known as Marine Benthic Group C) and diverse bacterial groups such as the Chloroflexi, Planctomycetes, WS1, Bacteroidetes, Firmicutes, JS1, OP11 and other taxa/divisions have been observed in methane seep sediments (1, 9, 11, 15, 17, 20, 22, 29, 31).
In this study, we found atypical microbial communities in a sediment core from a microbial mat site on a deep-sea methane seep in the Nankai Trough by quantitative PCR techniques and geochemical analyses, and then applied SSU rRNA gene tag-sequencing analysis. Pyrosequencing technique applied to SSU rRNA gene tag-sequencing analysis of marine and soil biospheres, including hydrocarbon seep sediments, retrieved more than 5,000 sequences and revealed a ‘rare biosphere’ (e.g., 12, 18, 21, 25, 32, 35). Here, we applied this technique to elucidate the richness of the microbial diversity and rare members in atypical microbial communities of methane seep sediments. In this study, oligonucleotide primer sequences of 530F and 907R were also reexamined, and novel primer sets of 530F and 907R were applied to the amplification of SSU rRNA gene fragments from DNA assemblages. The tag-sequences obtained were used for statistic analyses that demonstrate the richness of the microbial diversity in methane seep sediments. Furthermore, we compared the diversity and richness of each phylogroup according to the depth of the methane seep sediment.
Materials and Methods
Site description, sampling and geochemistry of core sample
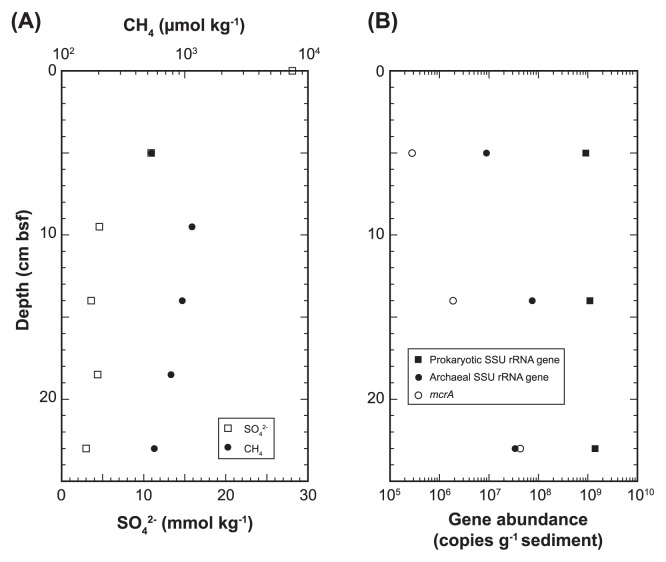
Sediment core 949C3 (25 cm in length) was taken by a push corer from a bacterial mat in an active methane seep site at the Omine Ridge (2,519 m below the sea surface) (33°7.33′N, 136°28.77′E), Nankai Trough off Kumano area with a manned submersible ‘Shinkai 6500’ (dive #6K949) on the YK06-03 cruise (April 2006) of R/V Yokosuka (JAMSTEC, Japan). The sediments consisted of blackish-gray sandy-silt and contained hydrogen sulfide. The core was subsampled by sterilized top-cut syringes and spatulas at 5 cm intervals on board, stored at −80°C for microbiology and used for interstitial water geochemistry. Sulfate concentration in core 949C3 decreased with increasing depth and more than 5.4×102 μmol kg−1 methane was detected through the sediment core column (Fig. 1A). Geochemical data were provided by Toki et al. (personal communication) and will be published elsewhere.
Fig. 1.
Concentrations of sulfate and methane (A), and abundances of genes for all prokaryotic and archaeal SSU rRNA, and mcrA (B) in sediment core 949C3. Methane concentration at sediment-water interface was not determined.
Nucleic acid extraction and quantitative PCR analyses
DNA was extracted from approx. 10 g sediments using an Ultra Clean Power Max Soil DNA Purification Kit (Mo Bio Laboratories, Carlsbad, CA, USA). The archaeal and total prokaryotic SSU rRNA genes from environmental microbial DNA were quantified using a quantitative fluorescent PCR method with a 7500 Real Time PCR System (PE Applied Biosystems, Foster City, CA, USA) described previously (38) with minor modification following Nunoura et al. (26). Quantitative PCR for mcrA with a primer set of ME3MF and ME2r’ was conducted using SYBR Premix Ex Taq II (Takara, Otsu, Japan) in the presence of MgCl2 (final 2.5 mM) as described previously (26).
Reconstruction of primer set
In order to retrieve environmental SSU rRNA gene fragments from all phylogenetic groups (phylogroups), we surveyed 530F and 907R primer sites (19) corresponds to V4 and V5 regions in 105,346 bacterial and 6,517 archaeal SSU rRNA gene sequences by ARB version 20030822 in 2007 (23) (Table 1). Based on this survey, we reconstructed 7 and 6 primer sequences for 530F and 907R sites targeting all prokaryotic SSU rRNA genes (Table 2) and 3 and 5 primer sequences for 530F and 907R sites targeting the archaeal SSU rRNA gene (Table S1). Mixtures of 530F and 907R sites were prepared (Table 2 and S1) in order to broaden the taxonomic range of SSU rRNA genes and minimize PCR amplification bias. The mixing ratio for all prokaryotic primer sets was determined as follows: 1) ratio between Archaea and Bacteria should be 1:1; 2) based on the population of sequences covered by each primer sequence, predominant primer sequences were determined; 3) based on the number of mismatch positions between the predominant primer sequence and minor sequences, the coverage of each primer sequence ratio between the predominant primer and minor primers was assessed. The coverage of the primer set was reconfirmed at the end of 2011 targeting sequences longer than 1.2 and 0.9 kb in length of bacterial and archaeal sequences, respectively, in SILVA SSU Ref NR database (http://www.arb-silva.de/download/arb-files/) (30). The SILVA SSU Ref NR database is based on the full Ref 108 and HSM/MWM datasets with a 99% criterion applied to remove the redundant sequence described in http://www.arb-silva.de/projects/ssu-ref-nr/ (30). Group specific mismatches between 530F and 907R primer sequences, and the coverage of primer sequences targeting SSU rRNA gene sequences in SILVA SSURef database are summarized in Table 3, S2 and S3. The primer set for all prokaryotic SSU rRNA genes covers 92.6, 84.1 and 86.3% of bacterial, archaeal and eukaryotic SSU rRNA genes without mismatch residues, respectively.
Table 1.
Comparison of sequences between previouly constructed primers and sequences in database at 2007
| Seaquence (5′ to 3′) | No. of sequences | Coverage | ||
|---|---|---|---|---|
|
| ||||
| Archaea | Bacteria | |||
| 530F | GTGCCAGCMGCCGCGG | |||
| 1 | GTGCCAGCAGCCGCGG | 113 | 101,157 | Most of the Bacteria, Methanococcales, some uncultured Archaea |
| 2 | GTGCCAGCCGCCGCGG | 3,518 | 32 | diverse uncultured Archaea* |
| 3 | GTGTCAGCCGCCGCGG | 2,087 | 0 | Crenarchaeota |
| 4 | GTGGCAGCCGCCGCGG | 291 | 0 | Archaeoglobales, Thermococcales, some Korarchaeota |
| 5 | CTGCCAGCCGCCGCGG | 20 | 0 | Methanopyrus, uncultured Euryarchaeota |
| 6 | TTGCCAGCCGCCGCGG | 49 | 0 | some uncultured Halobacteriales & diverse uncultured Euryarchaeota |
| 7 | GTGCCAGCAGCTGCGG | 0 | 390 | Chlamydiaceae & OP5 & diverce bacterial groups |
| 8 | GTGCCAGAAGCGTCGG | 0 | 59 | OP11 |
| 9 | GTGCCAGCAGTCGCGG | 0 | 244 | OP11 & OD1 & diverce bacterial groups |
| 10 | GTGCCAGAAGCCTCGG | 0 | 32 | OP11 & WS6 & OD1 |
| 11 | GTGCCAGAAGAATCGG | 0 | 12 | WS6 |
| 12 | GTGCCAGAAGCATCGG | 0 | 33 | WS6 & OD1 |
| 13 | GTGCCAGCAGCAGCGG | 0 | 132 | WS6 & OP11 & OD1 |
| 14 | GTGCCAGCAGGAGCGG | 0 | 15 | OD1 |
| 15 | GTGGCAGTCGCCACGG | 2 | 0 | Nanoarchaeota |
| 6,080 | 102,106 | |||
| 907R | CCGTCAATTCMTTTRAGTTT | |||
| 1 | CCGTCAATTCCTTTGAGTTT | 0 | 80,234 | Most of Bacteria |
| 2 | CCGTCAATTCCTTTAAGTTT | 37 | 889 | some Korarchaeota & diverse archaeal group |
| 3 | CCGTCAATTCATTTGAGTTT | 1 | 14,881 | diverse bacterial groups |
| 4 | CCGCCAATTCCTTTAAGTTT | 5,877 | 8 | most of Archaea* |
| 5 | CCGCCAATTTCTTTAAGTTT | 73 | 0 | SAGMEG & diverse archaeal group |
| 6 | CCGCCAATTCCTTTGAGTTT | 9 | 740 | some Desulfurococcalesspecies, Aquificae, Thermomicrobia & OP11 |
| 7 | CCGTCAATTCCCTTGAGTTT | 0 | 107 | OP1 & diverse bacterial groups |
| 8 | CCGTCAATTCCTTTATGTTT | 0 | 169 | TM7 & diverse bacterial groups |
| 9 | CCGCCAATTCCTTAAAGTTT | 40 | 0 | uncultured Halobacteriales & several uncultured Archaea |
| 10 | CCGCCAATTCCCTTAAGTTT | 43 | 0 | DSEG & several uncultured archaeal group |
| 11 | CCGTCTATTCCTTTGAGTTT | 0 | 1,809 | Desulfurobacteriaceae & OD1 |
| 12 | CCGTCAATTCATTTAAGTTT | 3 | 24 | Ancient Archaeal Group, uncultured Gammaproteobacteria |
| 13 | CCGTCAATTCCTTCAAGTTT | 31 | 2 | almost Korarchaeota |
| 14 | CCGCCTATTCCTTTAAGTTT | 16 | 0 | Nanoarchaeota |
| 15 | CCGCCAATTCCTTCAAGTTT | 7 | 0 | diverse uncultured archaeal group |
| 16 | CCGCCAATTCCTTTGAATTT | 0 | 11 | OP11 |
| 17 | CCGTCAATTCCTTTGAGCTT | 0 | 83 | diverse bacterial groups |
| 18 | CCGCCAATTCCTTTAAGTAT | 6 | 0 | diverse archaeal groups |
| 19 | CCGCCTATTCCTTTGAGTTT | 0 | 21 | OD1 & some bacterial groups |
| 20 | CCGCCTATCCCTTTGAGTTT | 0 | 11 | OP11 |
| 21 | CCGCCAATTCGTTTGAGTTT | 0 | 26 | OP11 |
| 22 | CCGCCAATTCATTTGAGTTT | 0 | 27 | WS6 |
| 23 | CCGTCAATTTCTTTGAGTTT | 0 | 2,580 | Some of Deltaproteobacteria & diverse bacterial groups |
| 24 | CCGTCAATTCTTTTGAGTTT | 0 | 572 | Planctomycetes, Chlomadiae |
| 25 | CCGTCAATTCGTTTGAGTTT | 0 | 142 | EM19, uncultured Chloroflexi |
| 26 | CCGCCAATTCCCTTAAGTTT | 43 | 0 | SAGMEG |
| 6,186 | 102,336 | |||
All of the ANME related seqences are included in these group without any mismatch residues.
Table 2.
Prokaryotic SSU rRNA gene primer sequences constructed in this study
| Name | Sequence (5′-3′) | Group coverage | Proportion in mixtures |
|---|---|---|---|
| Prokaryotic set | |||
| 30F mixture | |||
| Bac 530F | GTGCCAGCAGCCGCGG | 1 | 30 |
| Arch 530F | GTGBCAGCCGCCGCGG | 2, 3, 4 | 24 |
| Arch2 530F | YTGCCAGCCGCCGCGG | 5, 6 | 6 |
| Bac2 530F | GTGCCAGCAGCWGCGG | 7, 13, 14 | 1 |
| Bac3 530F | GTGCCAGCAGTCGCGG | 9 | 1 |
| Bac4 530F | GTGCCAGAAGMMTCGG | 10, 11, 12, (8) | 1 |
| Nano 530F | GTGGCAGTCGCCACGG | 15 | 3 |
| 907R mixture | |||
| Uni 907R | CCGYCAATTCMTTTRAGTTT | 1, 2, 3, 4, 6, 12, 19, 22, (8, 17, 18, 23, 24, 25) | 20 |
| DeepAB 907R | CCGYCTATTCCTTTGAGTTT | 11, (14), 20 | 1 |
| SAG-Del 907R | CCGYCAATTTCTTTRAGTTT | 5, 23 | 1 |
| DeepAB2 907R | CCGYCAATTCCCTTRAGTTT | 7, 10, 26 | 1 |
| Arch2 907R | CCGYCAATTCCTTMAAGTTT | 9, 13, 15 | 1 |
| OP11 907R | CCGCCAATTCCTTTGAATTT | 16, 21 | 1 |
Numbers in group coverage are refered from Table 1.
Groups in parentheses harbor 1 base mismatch with primer sequence.
Table 3.
Coverage of 530F and 907R primers targeting SILVA SSURef database at the end of 2011. In the database, we identified archaeal 13,022, badterial 282,285 and eukariotic 36,054 sequences that harbor both 530F and 907R regions
| (A) Number of sequences without mismatch residues compared to 530F primers. | |||
|---|---|---|---|
|
| |||
| 530F primers | Archaea | Bacteria | Eukarya |
| Arch_530F | 12,062 | 63 | 78 |
| Arch2_530F | 90 | 3 | 0 |
| Nano_530F | 38 | 0 | 0 |
| Bac_530F | 214 | 271,887 | 33,165 |
| Bac2_530F | 0 | 1,031 | 502 |
| Bac3_530F | 0 | 562 | 38 |
| Bac4_530F | 0 | 188 | 1 |
|
| |||
| Total | 12,404 | 273,734 | 33,784 |
|
| |||
| Coverage (%) without mismatch residues | 95.3 | 97.0 | 93.7 |
|
| |||
| (B) Number of sequences with 1 mismatch residues compared to 530F primers. | |||
|
| |||
| 530F primers | Archaea | Bacteria | Eukarya |
|
| |||
| Arch_530F | 298 | 490 | 49 |
| Arch2_530F | 12 | 222 | 16 |
| Nano_530F | 9 | 0 | 0 |
| Bac_530F | 19 | 3,979 | 1,056 |
| Bac2_530F | 7 | 174 | 39 |
| Bac3_530F | 2 | 151 | 39 |
| Bac4_530F | 0 | 169 | 14 |
|
| |||
| Total | 347 | 5,185 | 1,213 |
|
| |||
| Coverage (%) with 1 mismatch residue | 2.7 | 1.8 | 3.4 |
|
| |||
| (C) Number of sequences without mismatch residues compared to 907R primers. | |||
|
| |||
| 907R primers | Archaea | Bacteria | Eukarya |
|
| |||
| Uni_907R | 11,166 | 256,453 | 27,714 |
| DeepAB_907R | 0 | 3,835 | 0 |
| SAG-Del_907R | 158 | 6,020 | 5,679 |
| DeepAB2_907R | 138 | 428 | 25 |
| Arch2_907R | 159 | 4 | 368 |
| OP11_907R | 0 | 7 | 0 |
|
| |||
| Total sequences | 11,621 | 266,747 | 33,786 |
|
| |||
| Coverage (%) without mismatch residues | 89.2 | 94.5 | 93.7 |
|
| |||
| (D) Number of sequences with 1 mismatch residues compared to 907R primers. | |||
|
| |||
| 907R primers | Archaea | Bacteria | Eukarya |
|
| |||
| Uni_907R | 363 | 7,790 | 622 |
| DeepAB_907R | 1 | 760 | 31 |
| SAG-Del_907R | 17 | 437 | 420 |
| DeepAB2_907R | 27 | 225 | 7 |
| Arch2_907R | 13 | 256 | 72 |
| OP11_907R | 6 | 279 | 0 |
|
| |||
| Total sequences | 427 | 9,747 | 1,152 |
|
| |||
| Coverage (%) with 1 mismatch residue | 3.3 | 3.5 | 3.2 |
Amplification of SSU rRNA gene and pyrosequencing
DNA assemblages for SSU rRNA gene tag-sequencing analysis were extracted from sediments at 5 and 25 cm below the seafloor (cmbsf). PCR amplification of SSU rRNA gene fragments using the primer set of ‘530F’ and ‘907R’ described above was conducted under the following conditions. The PCR mixture contained 0.5 volumes of 2×GC buffer I for LA Taq polymerase (Takara), 0.1 U μL−1 LA Taq, 0.4 pmol μL−1 of each primer mixture and 1.6 nmol μL−1 dNTP. The DNA amplification condition was 5 min of denaturation at 96°C, and 25 cycles at 96°C for 20 s, 48°C for 70 s and 72°C for 30 s. Each of the ‘530F’ and ‘907R’ oligonucleotide primers harbored 24 bases of non-palindromic sequencing “key” sequences ‘CCATCTGTTCCCTCCCTGTCTCAG’ and ‘CCTATCCCCTGTTGCGTGTCTCAG’ for pyrosequencing at their 5′ terminal end, respectively. These primers were mixed as shown in Table 2 and applied to PCR amplification of SSU rRNA gene fragments. The amplified SSU rRNA gene fragments that were purified by agarose gel electrophoresis were applied to another 3 rounds of amplification to add the sequence for emulsion PCR and labeling biotin required for 454 pyrosequencing. The primers used in the other PCR amplification were adaptor A ‘CCATCTCATCCCTGCGTGTCCCATCTGTTCCCTCCCTGTCTCAG’ and adaptor B ‘BioTEG/CCTATCCCCTGTGTGCCTTGCCTATCCCCTGTTGCGTGTCTCAG’ to extend the “key” sequences described above. These adaptors are necessary to capture single-strand DNA for emulsion PCR. Primer sequence for pyrosequencing was ‘CCATCTGTTCCCTCCCTGTC’ encoded in adaptor A. The PCR mixture consisted of 0.1 volumes of 10×Ex Taq buffer, 0.05 U μL−1 of Ex Taq (Takara), 0.4 pmol μL−1 of each primer, 0.8 nmol μL−1 dNTP and 0.01 pmol SSU rRNA gene fragments. The amplification condition was 4 min of denaturation at 96°C, and 4 cycles at 96°C for 30 s, 64°C for 30 s and 72°C for 1 min. The biotin-labeled SSU rRNA gene fragments were purified by gel electrophoresis. The SSU rRNA gene fragments were analyzed by 454 pyrosequencer GS20 (Roche, Basel, Switzerland) in Takara. A gasket separated into 16 regions was used for pyrosequencing and we applied one sample per region in this study.
Phylogenetic assignment and clustering of SSU rRNA gene fragments, and statistics
Sequences shorter than 90 bp were removed from the analysis. Sequencing tags were aligned using the partial order algorithm (POA) (SINA, http://www.arb-silva.de/aligner/) with a reference multiple alignment SILVA SSU Ref NR. Then, tags were clustered into operational taxonomic units (OTU) by 98% sequence identity using MOTHUR 3.6 with default parameters (9). The taxonomic position of each OTU was automatically assigned based on Blast analysis in the QIIME software package (6) using SILVA Ref NR as a reference dataset of SSU rRNA gene sequences. Sequences presenting with a relatively high E-value (>1.0E-30) or low identity (<90%) to best match the reference sequence were designated as other archaea or bacteria, and sequences that did not harbor significant identity to any reference sequences were excluded from the analysis. Alpha diversity indexes (rarefaction curves, Chao1, ACE, Shannon, Shannon evenness and Shimpson) in each of the libraries and taxa/divisions were also calculated using MOTHUR 3.6 (33).
Accession numbers
Sequences and quality from pyrosequencing runs were deposited in the DDBJ/EMBL/GenBank database under accession number DRA000449.
Results
Quantitative PCR of genes for SSU rRNA and mcrA
The copy numbers of total prokaryotic and archaeal SSU rRNA genes in DNA assemblages from anoxic methane seep sediments ranged from 7.8×107 to 1.2×108 copies g−1 sediments and 1.4×106 to 3.8×106 copies g−1 sediment, respectively (Fig. 1B). The abundance of mcrA, a key gene of AOM, was 2.8×105 to 4.3×107 copy numbers g−1 sediment (Fig. 1B). Archaeal abundance was 1.0, 6.8 and 2.4% at 5, 15 and 25 cmbsf, respectively, in the SSU rRNA gene population, and the ratio of mcrA to archaeal SSU rRNA gene was 3.1, 2.5 and 126% at 5, 15 and 25 cmbsf, respectively.
Microbial community composition in methane seep sediments
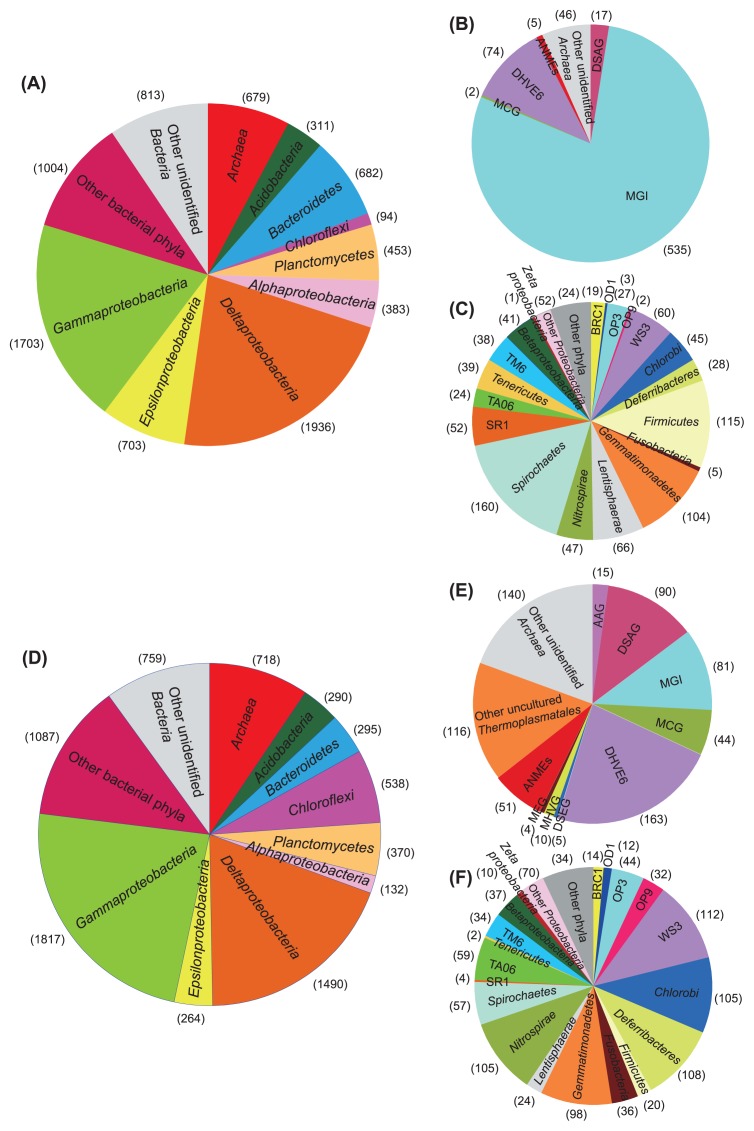
Each 8,709 and 7,690 SSU rRNA gene tag-sequence was obtained from methane seep sediments at 5 and 25 cmbsf, respectively. These 679 and 718 tag-sequences at 5 and 25 cmbsf, respectively, were identified as the archaeal SSU rRNA gene (Fig. 2).
Fig. 2.
Composition of abundant phyla/classes/divisions in SSU rRNA gene communities at 5 and 25 cmbsf in methane seep sediment core 949C3 (A and D, respectively). Compositions of archaeal SSU rRNA gene tags at 5 and 25 cmbsf are presented in B and E, respectively, and minor bacterial populations presented as ‘other bacterial phyla’ in A and D are demonstrated in C and F, respectively. ‘Other phyla’ in C and F include Armatimonadetes (OP10), OP11, TM7, Chlamidiae, Elusimicrobia (TM1), Fibrobacteres and Verrucomicrobia while OP11 was detected only at 5 cmbsf. Numbers in parentheses indicate tag numbers for each phylum/class/division.
The phylum Proteobacteria shared 55.3 and 49.7% of total tag-sequences from sediments at 5 and 25 cmbsf, respectively (Fig. 2). Deltaproteobacteria and Gammaproteobacteria were the predominant proteobacterial classes at both depths. Sequences related to the sulfate-reducing family Desulfobacteraceae predominated in the deltaproteobacterial populations (Fig. 3 and Table S4). Among the gamma-proteobacterial sequences, sequences related to sulfur oxidizers were more abundant than those related to heterotrophs. The dominant gammaproteobacterial sulfur-oxidizing family detected in this study was Acidithiobacillaceae, and sequences similar to other sulfur-oxidizing families, such as Ectothiorhodospiraceae and Chromatiaceae in the Chromatiales, Piscirickettsiaceae and Thiotrichaceae in the Thiotrichales, and the Thiohalophilus-related group were also detected. Sequences related to the methanotrophic gammaproteobacteial order Methylococcales were also found as one of the dominant members of the gammaproteobacterial sequences at 5 cmbsf and its abundance remarkably decreased at 25 cmbsf (Fig. 3 and Table S4). Abundance of epsilon-and alphaproteobacterial sequences decreased with the depth increased. The dominant populations in Epsilonproteobacteria were closely related to sulfur-oxidizing genera such as Arcobacter in Campylobacteraceae, and Sulfurimonas and Sulfurovum in Helicobacteraceae. The predominance of Campylobacteraceae sequences in the epsilonproteobacterial tags was only found at 5 cmbsf (Fig. 3 and Table S4). As minor populations among proteobacterial sequences, we found 1 and 10 sequences related to the marine Fe-oxidizing ‘Zetaproteobacteria’ from 5 and 25 cmbsf, respectively (7) (Fig. 2). SSU rRNA gene similarity between “Mariprofundus ferrooxydans” and four zetaproteobacterial OTUs obtained in this study (P9_5856, P9_1214, P9_1934 and P9_3677) ranged from 92 to 97%. Most of the zetaproteobacterial SSU rRNA gene sequences have been detected from hydrothermal environments with a few exceptions, such as the brine-seawater interface in the Red Sea (8), a salt marsh environment (24) and biocorroding microbiota in coastal seawaters (7). This is the first observation of zetaproteobacterial sequences in methane seep sediments. The other abundant bacterial phyla were Acidobacteria, Bacteroidetes, Chloroflexi and Planctomycetes, up to 7.0% of bacterial sequences in each depth. In addition to these phyla, Armatimonadetes (OP10), Actinobacteria, Chlamidiae, Chlorobi, Deferribacteres, Elusimicrobia (TM1), Firmicutes, Fusobacteria, Gemmatimonadetes, Lentisphaerae, Nitrospirae, Spirochaetes, Tenericutes and Verrucomicrobia were detected as minor populations (Fig. 2 and Table S4). Tag-sequences related to previously uncultivated candidatus bacterial divisions such as BRC1, OD1, OP3, OP9, OP11, SR1, TA06, TM6, TM7 and WS3 were also observed in minor populations.
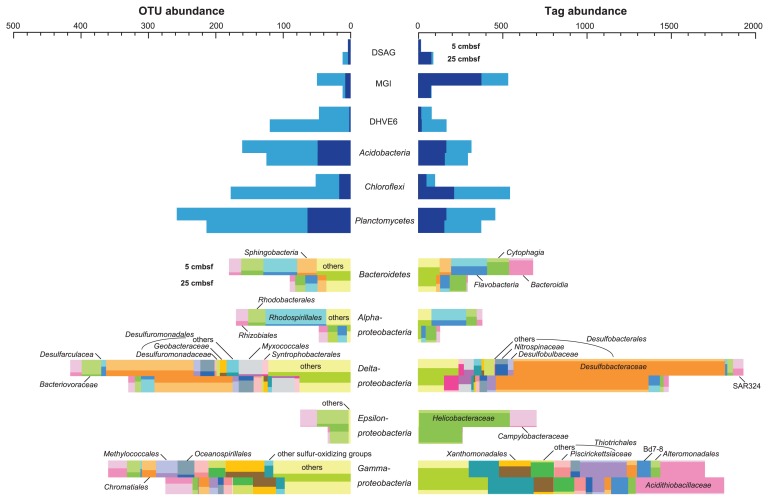
Fig. 3.
Abundance of shared tags and OTUs in each taxon/division between depths of 5 and 25 cmbsf in sediment core 949C3, and the order or family-level composition of bacterial major phyla/classes based on top hit sequences of each tag sequence in Blast analysis. Darker area for each taxon/division indicates shared population.
In contrast to bacterial populations, significant differences in archaeal communities were observed between the depths of 5 and 25 cmbsf (Fig. 2 and 3). In this study, groupings of uncultivated archaeal lineages followed the SILVA database. The Deep Sea Euryarchaeotic Group (DSEG) in the SILVA database includes Deep-sea Hydrothermal Vent Euryarchaeotic Group 8 (DHVEG 8) and a part of DHVEG 4, the DHVEG 6 (SILVA) contains DHVEG 5, and the Miscellaneous Euryarchaeotic Group (MEG) (SILVA) also harbors DHVEG 3 and a part of DHVEG 4. In the sediments at 5 cmbsf, the MGI predominated with 78.8% of archaeal sequences, and the next abundant group was the DHVEG 6 with 10.9% of archaeal sequences. On the other hand, at a depth of 25 cmbsf, the abundance of MGI-related sequences decreased to 11.3%, and those of other uncultivated lineages such as the DHVEG 6, DSAG and other uncultivated Thermoplasmatales increased to more than 12.5% of archaeal sequences. The unexpected dearth of potential anaerobic methanotrophs (ANME) was observed even at 25 cmbsf. At 5 cmbsf, only one each of the clones of ANME-1 and Lost City Methanosarcinales sequences and 3 ANME-3/Methanococcoides sequences was found, and 2 ANME-1, 13 ANME-2a, 14 ANME-2c and 21 ANME-3/Methanococcoides sequences were detected at 25 cmbsf. The ANME-3 and Methanococcoides methanogens could not be distinguished by short tag-sequences. In addition, the DSEG, MEG, MCG, Ancient Archaeal Group (AAG), Marine Hydrothermal Vent Group (MHVG) and Marine Benthic Group D (MBGD) were detected as minor populations in the archaeal tag population (Table S4).
Diversity and richness
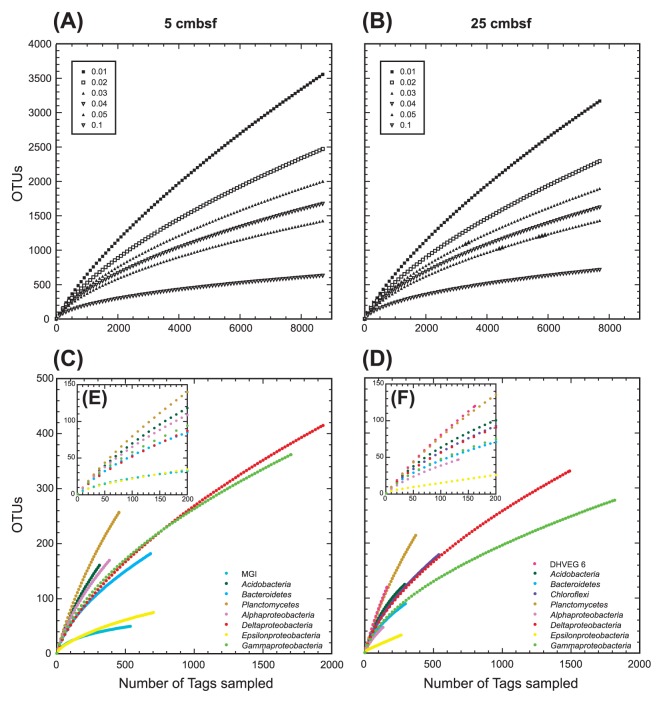
The richness of microbial diversity in each sample was assessed by rarefaction curves, and Chao1 and ACE indexes were calculated by MOTHUR. Both rarefaction curves of 5 and 25 cmbsf present with similar diversity richness and revealed that we could not retrieve whole microbial diversity by this tag-sequencing analysis (Fig. 4). We identified al least 2,467 and 2,290 OTUs (<2% dissimilarity) from sediments at 5 and 25 cmbsf, respectively. Chao1 index at 5 and 25 cmbsf was 6,558 and 6,373, and ACE index at 5 and 25 cmbsf was 11,118 and 11,269, respectively (Table S5).
Fig. 4.
Rarefaction analysis for methane seep sediment samples from 5 and 25 cmbsf of sediment core 949C3 based on MOTHUR program. Rarefaction of whole microbial community is shown for OTUs with differences that do not exceed 1, 2, 3, 4, 5 and 10% (A and B). C and D show taxon/division-specific rarefaction for OTUs with <2% dissimilarity. E and F show rarefaction at <200 tags in C and D, respectively.
In this study, we also examined the microbial diversity of each abundant archaeal and bacterial taxon/division that harbors more than 100 tags, such as the MGI, DHVEG 6, Acidobacteria, Bacteroidetes, Chloroflexi, Planctomycetes, and Alpha-, Gamma-, Delta- and Epsilonproteobacteria (Fig. 4 and Table S5). The Shannon index of diversity varied from 1.68 to 5.21 in bacterial taxa and 2.22 to 4.65 in archaeal divisions while similar uniformity of the population was observed in both domains; 0.55 to 0.94 and 0.83 to 0.97 of Shannon evenness among bacterial and archaeal taxa/divisions, respectively (Table S5). The highest diversity was observed in the Planctomycetes population from both libraries, and the diversity of DHVEG 6 at 25 cmbsf was comparable to that of Planctomycetes (Fig. 4 and Table S5), while the archaeal diversity in previous studies was usually lower than bacterial diversity (1, 2, 11, 31). Both the diversity and richness of Epsilonproteobacteria, and MGI archaea were significantly smaller than in other taxa/divisions.
Discussion
Function and adaptation of the dominant microbial components in tag-sequencing
In order to know the distribution patterns within each dominant taxon/division along the depth, we examined the abundance of shared OTUs and tags in each taxon/division between sediments at 5 and 25 cmbsf (Fig. 3 and Table S4). Shared tags and OTUs at both depths suggest the presence of species or strains that may grow in both environments. In tag populations of Desulfobacteraceae, Helicobacteraceae and some gammaproteobacterial groups, most of the tags were shared at both depths while the abundance of shared OTUs in each OTU population was relatively low. This pattern indicates the presence of a depth-specific highly diverse minor population in these taxa. Low abundance of shared OTUs and shared tags in both depths was observed in the DHVEG 6 and Planctomycetes populations. The trend could be explained by the markedly high OTU richness (Fig. 4 and Table S5), and the presence of adapted populations in each environment. In tag populations of the DSAG, MGI and Flavobacteria, tag numbers at the two depths differed significantly, and most of the tags in the smaller tag population were also found in the larger tag population at other depths. The results suggest that specific populations in each class/division can survive and grow in their hostile environments in a relatively small population, such as the oxidative stress-tolerant population of anaerobic DSAG in a relatively oxidative environment at 5 cmbsf, and reductive stress-tolerant population of obligately aerobic or facultatively anaerobic MGI archaea at 25 cmbsf. In the epsilon-proteobacterial population, Campylobacteraceae tags almost disappeared at 25 cmbsf while Helicobacteraceae tags still dominated at 25 cmbsf. Considering the versatile energy metabolisms observed in both families (5), such family-specific distribution found in this study is unexpected. Among bacterial phyla that harbor fermentation metabolism such as Acidobacteria, Bacteroidetes and Chloroflexi, each phylum presents with different distribution patterns. The abundance of Acidobacteria was constant along the depth while that of Bacteroidetes, especially the classes Bacteroidia and Flavobacteria, decreased and Chloroflexi increased at 25 cmbsf. The growth of Bacteroidetes might be inhibited by higher sulfide concentration at 25 cmbsf, and Chlorofelxi species may prefer more reduced environments than Acidobacteria and Bacteroidetes species.
Abundance and functions of ANME and other Archaea
The proportion of archaeal SSU rRNA gene tag sequences was 7.8 and 9.3% in the tag populations from 5 and 25 cmbsf, respectively, while that estimated by quantitative PCR for SSU rRNA genes was 1.8% and 2.4%, respectively. The archaeal population in all prokaryotic SSU rRNA genes shown by tag-sequencing is different from that observed by quantitative PCR, but the trend for the abundance of Archaea in the sediments at 25 cmbsf to be larger than that at 5 cmbsf is consistent in both analyses. A similar trend between quantitative PCR and SSU rRNA gene clone analysis using the same primer sets was also observed in other experiences (13).
Comparing the tag-populations from the two depths, the abundance of tags from MGI decreased and those from DHVEG 6 and DSAG increased at 25 cmbsf. At 5 cmbsf, DHVEG 6 shared relatively high abundance while DSAG was just a minor population (Fig. 2 and Table S4). DHVEG 6 has been detected from relatively anoxic terrestrial soils and marine sediments (10, 37, 39), and DSAG has been observed in anoxic marine sediments from diverse environments, such as the ambient seafloor, methane seep and hydrothermal field (37, 39). On the other hand, inhabitance of DHVEG 6 in shallower sediments than DSAG was also found in the archaeal community structure associated with ambient sediments in a hydrothermal field (27). These distribution patterns suggest that DHVEG 6 can grow in relatively oxidative environments while other anaerobic archaeal groups such as DSAG prefer more reductive environments.
The geochemical profile of sulfate and methane concentrations in sediment core 949C3 suggested the occurrence of AOM (Fig. 1A), and SSU rRNA gene tag sequencing revealed the presence of ANME (Fig. 2). Copy numbers of mcrA at 25 cmbsf were about 150-fold larger than those at 5 cmbsf, and the ratio of mcrA to the prokaryotic SSU rRNA gene copy number was 0.06 and 3.1% at 5 and 25 cmsf, respectively (Fig. 1B). On the other hand, ANME-related sequences shared 0.07 and 0.66% of SSU rRNA gene tags at 5 and 25 cmbsf, respectively, and no SSU rRNA gene tags from methanogens distant from ANME were detected in the tag libraries. These results suggest that the abundance of the ANME SSU rRNA gene sequences was likely underestimated in the SSU rRNA gene tag population although no mismatch residue was found between ANME SSU rRNA gene sequences in the public database and primer sequences used in this study (Table 1 and 2).
Overview of microbial community in methane seep sediments
Dominant archaeal phylogroups found in tag populations were not related to the sequences from ANME or methanogens, and could not be signatures of methane seep environment as described above; however, the high abundance of mcrA in the archaeal population and geochemical profiles suggest the occurrence of AOM in sediment core 949C3 (Fig. 1, 2 and Table S4). Furthermore, the high tag abundance of sulfur oxidizers belong to Gammaproteobacteria and Epsilonproteobacteria, and that of sulfate-reducing Deltaproteobacteria in SSU rRNA gene tag populations at both 5 and 25 cmbsf suggests the co-occurrence of sulfur oxidation and sulfate reduction through the core column (Fig. 3). Consequently, we conclude that the microbial communities found in this study were associated with methane seep sediments but not ambient seafloor sediments.
In the previous study of hydrocarbon seep deep-sea sediment in the Gulf of Mexico by SSU rRNA gene V6 region tag-sequencing analysis, community structures of sediments at 13.5 cmbsf from within and outside a mat formation site were examined (21). In the case of the Gulf of Mexico hydrocarbon seep site, higher AOM activity and low microbial diversity was observed in sediments within a mat formation while higher diversity was observed in sediments from outside a mat formation at the same seep site. Higher microbial diversity in sediments from outside the mat formation was also confirmed by rRNA cDNA clone analysis. Compared with the microbial diversity at the Gulf of Mexico site, the microbial diversity found in this study (Chao1 6,558 and 6,374) is similar to that from outside the mat formation (Chao1 <5,909) rather than that from the mat formation (Chao1 <58) at the Gulf of Mexico site, although the geochemical profile of core 949C3 is similar to that in the core from the microbial mat formation. The richness of the anoxic methane seep sediment observed in the rarefaction curves and the richness of anoxic methane seep sediment in this study is likely similar to those in soils (32) and deep-sea hydrothermal environments (12, 35).
Unexpectedly, the abundance of genes for archaeal SSU rRNA and mcrA in the methane seep sediments (core 949C3) was apparently smaller than those in previously analyzed methane seep sediments (core 744C1) taken from the other microbial mat formation site on the same ridge in April 2003; archaeal SSU rRNA gene abundance at 5 cmbsf was 9.0×106 and 4.6×108 copies g−1 sediment and at 25 cmbsf was 2.9×109 and 3.4×107 copies g−1 sediment, in cores 949C3 and 744C1, respectively, and mcrA gene abundance at 5 cmbsf was 2.8×105 and 4.6×107 copies g−1 sediment, and at 25 cmbsf was 3.4×107 and 2.6×109 copies g−1 sediment, in cores 949C3 and 744C1, respectively (26, 28). In addition, quantitative PCR analysis revealed that the archaeal SSU rRNA gene population in all prokaryotic SSU rRNA genes in core 949C3 (1.7 to 6.7%) was smaller than that in core 744C1 (27 to 39%). Furthermore, the predominance of MGI in the archaeal population at 5 cmbsf in core 949C3 was observed in SSU rRNA gene tag-sequencing while MGI was not detected in archaeal SSU rRNA gene clone analysis at the same depth in core 744C1 (28). On the other hand, the geochemical profile of core 744C1 also suggests the occurrence of AOM, as in the case of core 949C3, while core 744C1 bore lower concentrations of methane; methane concentrations in core 949C3 (5.4×102-1.2×103 μmol kg−1) were higher than those of 744C1 (4.2–6.0×101 μmol kg−1) (26, 36). The typical geochemical profiles of active AOM and increasing mcrA population with increasing depth in core 949C3 (Fig. 1) suggest the presence of a typical mature AOM microbial community predominated by ANME and Deltaproteobacteria (16) below the sediments analyzed in this study. Accordingly, we conclude that ANME communities in core 949C3 have not matured yet. We assume two potential geological reasons for the undeveloped ANME community in core 949C3, although we cannot conclude the geological systems of the methane seep site without long-term monitoring of methane flux and/or other hydrological survey at the methane seep site. One potential reason is the recent upwelling of a high concentration of methane in the sediments, and the AOM-dependent microbial community was at the developmental stage. In this case, a mature AOM microbial community would occur just beneath the seafloor, as in the case of core 744C1. The other is that the input of oxidative seawater inhibits the development of AOM suggested by the co-occurrence of potential sulfur oxidizers and sulfate reducers at 25 cmbsf.
From another aspect, the microbial communities analyzed are likely analogues of microbial communities just beneath the seafloor under the microbial mat formation site that harbors typical mature AOM-dependent microbial communities. In typical methane seep sediments presenting geochemical signatures of sulfate reduction and methane oxidation, the transition of the sulfur-oxidizing community to sulfate-reducing ANME community occurs within a few cm, and the ANME community predominates in the core column (4, 11, 15, 17, 29). Thus, it is intractable to observe such community transition because of the difficulty of sampling such thin layers from the sediment core. In contrast to such typical methane seep sediments, the microbial communities observed in this study were not predominated by the ANME community, although typical geochemical signatures of sulfate reduction and methane oxidation were observed. By demonstrating SSU rRNA gene tag-sequencing analysis of atypical microbial communities associated with methane seep sediments, we can observe the unseen distribution pattern of microbes in relatively oxidative environments, especially the family-specific distribution patterns found in the Epsilonproteobacteria (Fig. 3).
Supplementary Material
Acknowledgements
We would like to express our appreciation of the R/V Yokosuka and Shinkai 6500 operation team during cruises YK06-03 (JAMSTEC) for their assistance in collecting samples. We are also grateful to the on-board scientists on the YK06-03 cruise.
References
- 1.Alain K, Holler T, Musat F, Elvert M, Treude T, Krüger M. Microbiological investigation of methane- and hydrocarbon-discharging mud volcanoes in the Carpathian Mountains, Romania. Environ Microbiol. 2006;8:574–590. doi: 10.1111/j.1462-2920.2005.00922.x. [DOI] [PubMed] [Google Scholar]
- 2.Aller JY, Kemp PF. Are Archaea inherently less diverse than Bacteria in the same environments? FEMS Microbiol Ecol. 2008;65:74–87. doi: 10.1111/j.1574-6941.2008.00498.x. [DOI] [PubMed] [Google Scholar]
- 3.Bidle KA, Kastner M, Bartlett DH. A phylogenetic analysis of microbial communities associated with methane hydrate containing marine fluids and sediments in the Cascadia margin (ODP site 892B) FEMS Microbiol Lett. 1999;177:101–108. doi: 10.1111/j.1574-6968.1999.tb13719.x. [DOI] [PubMed] [Google Scholar]
- 4.Boetius A, Ravenschlag K, Schubert CJ, et al. A marine microbial consortium apparently mediating anaerobic oxidation of methane. Nature. 2000;407:623–626. doi: 10.1038/35036572. [DOI] [PubMed] [Google Scholar]
- 5.Campbell BJ, Engel AS, Porter ML, Takai K. The versatile epsilon-proteobacteria: key players in sulphidic habitats. Nat Rev Microbiol. 2006;4:458–468. doi: 10.1038/nrmicro1414. [DOI] [PubMed] [Google Scholar]
- 6.Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dang H, Chen R, Wang L, Shao S, Dai L, Ye Y, Guo L, Huang G, Klotz MG. Molecular characterization of putative biocorroding microbiota with a novel niche detection of Epsilon- and Zetaproteobacteria in Pacific Ocean coastal seawaters. Environ Microbiol. 2011;13:3059–3074. doi: 10.1111/j.1462-2920.2011.02583.x. [DOI] [PubMed] [Google Scholar]
- 8.Emerson D, Rentz JA, Lilburn TG, Davis RE, Aldrich H, Chan C, Moyer CL. A novel lineage of Proteobacteria involved in formation of marine Fe-oxidizing microbial mat communities. PLoS ONE. 2007;2:e667. doi: 10.1371/journal.pone.0000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang J, Shizuka A, Kato C, Schouten S. Microbial diversity of cold-seep sediments in Sagami Bay, Japan, as determined by 16S rRNA gene and lipid analyses. FEMS Microbiol Ecol. 2006;57:429–441. doi: 10.1111/j.1574-6941.2006.00126.x. [DOI] [PubMed] [Google Scholar]
- 10.Großkopf R, Janssen PH, Liesack W. Diversity and structure of the methanogenic community in anoxic rice paddy soil microcosms as examined by cultivation and direct 16S rRNA gene sequence retrieval. Appl Environ Microbiol. 1998;64:960–969. doi: 10.1128/aem.64.3.960-969.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heijs SK, Haese RR, van der Wielen PW, Forney LJ, van Elsas JD. Use of 16S rRNA gene based clone libraries to assess microbial communities potentially involved in anaerobic methane oxidation in a Mediterranean cold seep. Microb Ecol. 2007;53:384–398. doi: 10.1007/s00248-006-9172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huber JA, Welch DB, Morrison HG, Huse SM, Neal PR, Butterfield DA, Sogin ML. Microbial population structures in the deep marine biosphere. Science. 2007;318:97–100. doi: 10.1126/science.1146689. [DOI] [PubMed] [Google Scholar]
- 13.Igisu M, Takai K, Ueno U, et al. Domain-level identification and quantification of relative prokaryotic cell abundance in microbial communities by Micro-FTIR spectroscopy. Environ Microbiol Rep. 2012;4:42–49. doi: 10.1111/j.1758-2229.2011.00277.x. [DOI] [PubMed] [Google Scholar]
- 14.Inagaki F, Sakihama Y, Inoue A, Kato C, Horikoshi K. Molecular phylogenetic analyses of reverse-transcribed bacterial rRNA obtained from deep-sea cold seep sediments. Environ Microbiol. 2002;4:277–286. doi: 10.1046/j.1462-2920.2002.00294.x. [DOI] [PubMed] [Google Scholar]
- 15.Inagaki F, Tsunogai U, Suzuki M, et al. Characterization of C1-metabolizing prokaryotic communities in methane seep habitats at the Kuroshima Knoll, southern Ryukyu Arc, by analyzing pmoA, mmoX, mxaF, mcrA, and 16S rRNA genes. Appl Environ Microbiol. 2004;70:7445–7455. doi: 10.1128/AEM.70.12.7445-7455.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jørgensen BB, Boetius A. Feast and famine-microbial life in the deep-sea bed. Nat Rev Microbiol. 2007;5:770–781. doi: 10.1038/nrmicro1745. [DOI] [PubMed] [Google Scholar]
- 17.Knittel K, Lösekann T, Boetius A, Kort R, Amann R. Diversity and distribution of methanotrophic archaea at cold seeps. Appl Environ Microbiol. 2005;71:467–479. doi: 10.1128/AEM.71.1.467-479.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krause L, Diaz NN, Goesmann A, Kelley S, Nattkemper TW, Rohwer F, Edwards RA, Stoye J. Phylogenetic classification of short environmental DNA fragments. Nucleic Acids Res. 2008;36:2230–2239. doi: 10.1093/nar/gkn038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lane DJ. 16S-23S rRNA sequencing. In: Stackebrandt E, Goodfellow M, editors. Nucleic acid techniques in bacterial systematics. John Wiley and Sons; New York, NY: 1985. pp. 115–175. [Google Scholar]
- 20.Li L, Kato C, Horikoshi K. Microbial diversity in sediments collected from the deepest cold-seep area, the Japan Trench. Mar Biotechnol. 1999;1:391–400. doi: 10.1007/pl00011793. [DOI] [PubMed] [Google Scholar]
- 21.Lloyd KG, Albert DB, Biddle JF, Chanton JP, Pizarro O, Teske A. Spatial structure and activity of sedimentary microbial communities underlying a Beggiatoa spp. mat in a Gulf of Mexico hydrocarbon seep. PLoS One. 2010;5:e8738. doi: 10.1371/journal.pone.0008738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lösekann T, Knittel K, Nadalig T, Fuchs B, Niemann H, Boetius A, Amann R. Diversity and abundance of aerobic and anaerobic methane oxidizers at the Haakon Mosby Mud Volcano, Barents Sea. Appl Environ Microbiol. 2007;73:3348–3362. doi: 10.1128/AEM.00016-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ludwig W, Strunk O, Westram R, et al. ARB: a software environment for sequence data. Nucleic Acids Res. 2004;32:1363–1371. doi: 10.1093/nar/gkh293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McBeth JM, Little BJ, Ray RI, Farrar KM, Emerson D. Neutrophilic iron-oxidizing “Zetaproteobacteria” and mild steel corrosion in nearshore marine environments. Appl Environ Microbiol. 2011;77:1405–1412. doi: 10.1128/AEM.02095-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKenna P, Hoffmann C, Minkah N, et al. The macaque gut microbiome in health, lentiviral infection, and chronic enterocolitis. PLoS Pathog. 2008;4:e20. doi: 10.1371/journal.ppat.0040020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nunoura T, Oida H, Miyazaki J, Miyashita A, Imachi H, Takai K. Quantification of mcrA by fluorescent PCR in methanogenic and anaerobic methanotrophic microbial communities. FEMS Microbiol Ecol. 2008;64:240–247. doi: 10.1111/j.1574-6941.2008.00451.x. [DOI] [PubMed] [Google Scholar]
- 27.Nunoura T, Oida H, Nakaseama M, et al. Archaeal diversity and distribution along thermal and geochemical gradients in hydrothermal sediments at the Yonaguni Knoll IV hydrothermal field in the Southern Okinawa Trough. Appl Environ Microbiol. 2010;76:1198–1211. doi: 10.1128/AEM.00924-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nunoura T, Oida H, Toki T, Ashi J, Takai K, Horikoshi K. Quantification of mcrA by quantitative fluorescent PCR in sediments from methane seep of the Nankai Trough. FEMS Microbiol Ecol. 2006;57:149–157. doi: 10.1111/j.1574-6941.2006.00101.x. [DOI] [PubMed] [Google Scholar]
- 29.Orphan VJ, Hinrichs KU, Ussler W, III, Paull CK, Taylor LT, Sylva SP, Hayes JM, Delong EF. Comparative analysis of methane-oxidizing archaea and sulfate-reducing bacteria in anoxic marine sediments. Appl Environ Microbiol. 2001;67:1922–1934. doi: 10.1128/AEM.67.4.1922-1934.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pruesse E, Quast C, Knittel K, Fuchs B, Ludwig W, Peplies J, Glöckner FO. SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 2007;35:7188–7196. doi: 10.1093/nar/gkm864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reed AJ, Lutz RA, Vetriani C. Vertical distribution and diversity of bacteria and archaea in sulfide and methane-rich cold seep sediments located at the base of the Florida Escarpment. Extremophiles. 2006;10:199–211. doi: 10.1007/s00792-005-0488-6. [DOI] [PubMed] [Google Scholar]
- 32.Roesch LFW, Fulthorpe RR, Riva A, et al. Pyrosequencing enumerates and constrasts soil microbial diversity. ISME J. 2007;1:283–290. doi: 10.1038/ismej.2007.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schloss PD, Westcott SL, Ryabin T, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith CR, Berelson W, Demaster DJ, Dobbs FC, Hammond D, Hoover DJ, Pope RH, Stephens M. Latitudinal variations in benthic processes in the abyssal equatorial Pacific: control by biogenic particle flux. Deep-Sea Res, Part II. 1997;44:2295–2317. [Google Scholar]
- 35.Sogin ML, Morrison HG, Huber JA, Welch DM, Huse SM, Neal PR, Arrieta JM, Herndl GJ. Microbial diversity in the deep sea and the underexplored “rare biosphere”. Proc Natl Acad Sci USA. 2006;103:12115–12120. doi: 10.1073/pnas.0605127103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Toki T, Tsunogaia U, Gamo T, Kuramoto S, Ashi J. Detection of low-chloride fluids beneath a cold seep field on the Nankai accretionary wedge off Kumano, south of Japan. Earth Planet Sci Lett. 2004;228:37–47. [Google Scholar]
- 37.Takai K, Horikoshi K. Genetic diversity of archaea in deep-sea hydrothermal vent environments. Genetics. 1999;152:1285–1297. doi: 10.1093/genetics/152.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takai K, Horikoshi K. Rapid detection and quantification of members of the archaeal community by quantitative PCR using fluorogenic probes. Appl Environ Microbiol. 2000;66:5066–5072. doi: 10.1128/aem.66.11.5066-5072.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teske A, Sørensen KB. Uncultured archaea in deep marine subsurface sediments: have we caught them all? ISME J. 2008;2:3–18. doi: 10.1038/ismej.2007.90. [DOI] [PubMed] [Google Scholar]
- 40.Yan T, Ye Q, Zhou J, Zhang CL. Diversity of functional genes for methanotrophs in sediments associated with gas hydrates and hydrocarbon seeps in the Gulf of Mexico. FEMS Microbiol Ecol. 2006;57:251–259. doi: 10.1111/j.1574-6941.2006.00122.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.