Abstract
Ibrutinib (Imbruvica): a novel targeted therapy for chronic lymphocytic leukemia
INTRODUCTION
Chronic lymphocytic leukemia (CLL) remains the most common adult leukemia in Western countries. In the United States alone, an estimated 15,720 new cases of CLL and 4,600 deaths will occur in 2014.1 CLL is a neoplasm of lymphoid origin; more specifically, it is a malignancy of mature B lymphocytes. It is characterized by progressive accumulation of these leukemic cells in the peripheral blood, bone marrow, and lymphoid tissues.2 CLL is most prevalent in the elderly, with a median age at diagnosis of 72 years. The disease is twice as common in males as females and occurs more frequently in Caucasians compared with African-Americans. It is a rare diagnosis in Hispanics and Native Americans, and much rarer in the Asian population.3
There are no clearly discernible occupational or environmental risk factors that predispose patients to CLL. However, a family history of the disease or other lymphoid malignancies is among the strongest risk factors for the development of CLL. Several familial clusters of CLL have been reported, and 5% to 10% of CLL patients will have another family member with the disease. In fact, CLL is diagnosed, on average, 14 years earlier in patients with a family history, there-by confirming a genetic predisposition that exists. Despite familial occurrence and commonality of disease, no specific screening recommendations exist.4
CLL is extremely heterogeneous in its clinical course. Some patients live for decades with indolent disease and do not require treatment, whereas others suffer an aggressive course with intrinsic resistance to chemotherapy, eventually leading to death.2,3 In the latter case, patients experience rapidly deteriorating blood counts (i.e., anemia and thrombocytopenia) and organomegaly. Typical “B” symptoms of lymphoma may be present, which include: unintentional loss of 10% or more of body weight within the previous six months, fevers greater than 38 degrees Celsius for at least two weeks without evidence of infection, drenching night sweats for more than four weeks unrelated to an infectious process, and significant fatigue.5,6 Hypogammaglobulinemia and recurrent infection are common due to inherent impairment in humoral and cellular immunity.7
In addition to the patient’s clinical presentation, laboratory tests, such as a complete blood count with differential and a comprehensive metabolic panel, should be performed. During physical examination, attention to node-bearing areas and to size of liver and spleen is essential. Performance status should be recorded at diagnosis. Furthermore, other tests may be warranted, such as hepatitis B screening if CD20 monoclonal antibody therapy is considered and multiple gated acquisition scan/echo-cardiogram if an anthracycline-based regimen is indicated. Pregnancy testing in women of childbearing age should be completed if chemotherapy is planned. The aforementioned diagnostic workup is not comprehensive, and other tests may be useful under certain circumstances (such as a chest/abdominal/pelvic computed tomography scan prior to initiation of therapy, particularly when peripheral adenopathy is present and symptoms suggest bulky lymph nodes). The diagnosis of CLL requires the presence of at least 5,000 clonal B cells/mcL in the peripheral blood. The presence of fewer B cells in the absence of lymphadenopathy or other clinical features characteristic of a lymphoproliferative disorder is defined as monoclonal B-lymphocytosis, a distinct entity from CLL.3,6
There are two widely accepted staging methods for use in both patient care and clinical trials: the Rai (stage 0–IV) and the Binet (stage A–C) systems. In general, the higher the stage, the poorer the clinical outcome and the shorter the median overall survival (OS). These staging systems are simple, inexpensive, and can be applied by physicians worldwide. In addition to clinical staging, over the past decade numerous prognostic factors have been identified in patients with CLL. These factors include serum markers, such as thymidine kinase and beta-2 microglobulin; genetic markers, including immunoglobulin heavy chain variable region (IgHV) mutational status by molecular analysis and cytogenetic abnormalities detected by fluorescence in situ hybridization (FISH) such as del(13q), trisomy 12, del(11q), and del(17p); and CD38 and ZAP-70 expression detected by flow cytometry or immunohistochemistry.
Criteria for initiating treatment may vary depending on whether or not the patient is part of a clinical trial. In general practice, newly diagnosed patients with asymptomatic early-stage disease (i.e., Rai 0, Binet A) should be monitored without therapy unless they have evidence of disease progression.6 Therapy for symptomatic CLL has comprised predomi nantly chemotherapeutic agents, including chlorambucil (Leukeran, Aspen Global), cyclophosphamide (Cytoxan, Baxter), fludarabine, bendamustine (Treanda, Cephalon), and combinations of these agents. These therapies are effective for palliation but have not been shown to improve survival.
Combining rituximab (Rituxan, Genentech), a monoclonal antibody directed against the CD20 antigen on B lymphocytes, to chemotherapy (fludarabine in combination with cyclophosphamide) results in higher response rates, prolonged remissions, and improved OS. However, certain subsets of CLL patients (such as those with del[17p]/TP53 mutations) have a poor response to chemoimmunotherapy. Furthermore, these treatments are not curative and therapy options for relapsed disease tend to have increased toxicity and reduced antitumor efficacy.8
In an effort to optimize treatment outcomes, novel molecularly targeted therapies have been developed. Unlike cytotoxic chemotherapy, which attacks all rapidly dividing cells, targeted therapy is directed toward precise pathways thought to contribute to the growth of cancer cells. This modality of treating cancer allows for more specific antitumor activity while avoiding normal cells and thus decreases traditional toxicity seen with chemotherapy, such as alopecia, gastrointestinal distress, and myelosuppression.9 In February 2014, the Food and Drug Administration (FDA) granted accelerated approval to ibrutinib (Imbruvica, Pharmacyclics), an innovative, promising targeted treatment option for patients with CLL.
INDICATIONS AND USAGE10
Ibrutinib is approved for the treatment of patients with CLL who have received at least one prior therapy. It is also approved for relapsed/refractory mantle-cell lymphoma patients.
PHARMACOLOGY
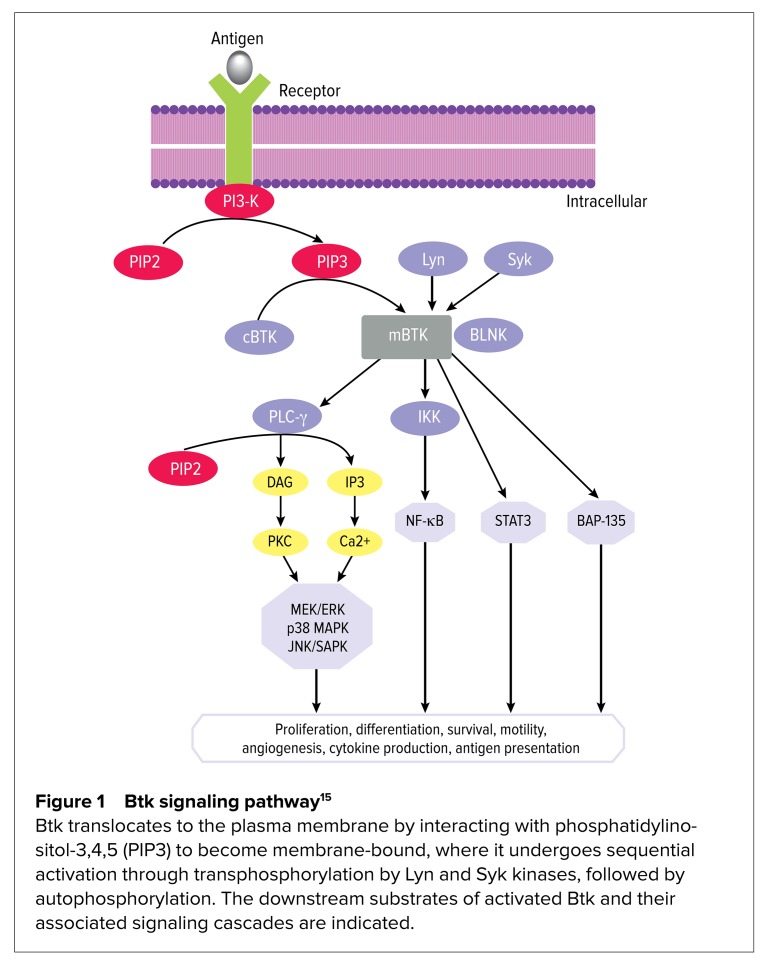
The B cell receptor (BCR) pathway regulates multiple cellular processes (such as proliferation, differentiation, and apoptosis) that are essential for the functioning and survival of both normal and malignant B cells.2 In B cell malignancies, such as CLL, aberrant BCR signaling plays a critical role in the pathogenesis of disease.11 The BCR pathway is responsible for the phosphorylation of numerous protein tyrosine kinases (PTKs), including Lyn, Syk, and Bruton’s tyrosine kinase (Btk).12 These PTKs have been found to be constitutively active and over-expressed in CLL, leading to uncontrolled proliferation and survival of malignant B cells.9 Consequently, there has been rapid clinical development of inhibitors targeting these PTKs.11 Among the many PTKs involved in BCR signaling, Btk, a tyrosine kinase member of the Tec kinase family, is a distinctive therapeutic target.13 Upon BCR activation, Btk becomes activated by other PTKs, such as Lyn and Syk, resulting in activation of downstream transcription factors necessary for B cell proliferation and differentiation (demonstrated in Figure 1).11
Figure 1.
Btk signaling pathway15
Btk translocates to the plasma membrane by interacting with phosphatidylinositol-3,4,5 (PIP3) to become membrane-bound, where it undergoes sequential activation through transphosphorylation by Lyn and Syk kinases, followed by autophosphorylation. The downstream substrates of activated Btk and their associated signaling cascades are indicated.
Ibrutinib is an orally administered, highly potent, selective, and irreversible small-molecule inhibitor of Btk.14 It forms a covalent bond with a cysteine residue (CYS-481) at the active site of Btk, leading to inhibition of Btk enzymatic activity.10 Ibrutinib also abrogates the full activation of Btk by inhibiting its autophosphorylation at Tyr-223.15 This inhibition prevents downstream activation of the BCR pathway and subsequently blocks cell growth, proliferation, and survival of malignant B cells.9
PHARMACOKINETICS
Absorption
Ibrutinib is rapidly absorbed after oral administration, with peak plasma concentrations observed one to two hours after dosing. Its exposure increases with doses up to 840 mg/day. The steady-state area under the curve (AUC) (mean ± standard deviation) observed in patients at 560 mg/day is 953 ± 705 ng*h/mL and in patients at 420 mg/day is 680 ± 517 ng*h/mL.10 A phase 1, open-label, dose-escalation trial demonstrated that administration of ibrutinib with food increases exposure approximately twofold compared with administration after overnight fasting.16 Further pharmacokinetic data showed 30% higher AUC values in patients ages 65 years and older at a dose of 420 mg/day.17 However, greater concentrations did not confer accumulation or increased toxicity; therefore, ibrutinib may be administered with or without food and dose reductions are not advised in elderly patients. Ibrutinib exposure in males and females is similar. No substantial difference in systemic exposure was found between patients with del(17p) versus those who were negative for this cytogenetic abnormality.16,17
Distribution10
Reversible binding of ibrutinib to human plasma protein in vitro was 97.3% with no concentration dependence in the range of 50 to 1,000 ng/mL. The apparent volume of distribution at steady state is approximately 10,000 L.
Metabolism10
Metabolism is the main route of elimination for ibrutinib. It is converted to several metabolites, primarily by the cytochrome P450 (CYP) enzyme CYP3A4, and to a minor extent by CYP2D6. The active metabolite, the dihydrodiol PCI-45227, has inhibitory activity towards Btk approximately 15 times lower than that of ibrutinib. The range of the mean metabolite to parent ratio for PCI-45227 at steady state is 1:2.8.
Elimination10
Ibrutinib plasma concentrations follow a biphasic elimination pattern, resulting in an apparent terminal half-life of four to six hours. Ibrutinib is eliminated primarily via the feces (approximately 80%), mainly in the form of metabolites (only 1% as unchanged drug). The elimination of ibrutinib in the urine is less than 10% and in the form of metabolites.
Pharmacodynamics8
Post-treatment assessments of ibrutinib in CLL patients showed full inhibition of Btk at doses of 420 mg and 840 mg per day. The median level of Btk occupancy was 96% to 99%, which was seen as early as four hours after administration and maintained for 24 hours at both dose levels.
PIVOTAL CLINICAL TRIALS
Clinical studies of ibrutinib in patients with CLL started in May 2010. The published literature includes its use as initial therapy in elderly patients and in the relapsed/refractory patient population, in both settings as monotherapy. There are ongoing trials utilizing ibrutinib in combination with monoclonal antibodies and chemoimmunotherapy.2 This article will highlight the use of ibrutinib as single-agent therapy for patients with relapsed/refractory CLL.
A phase 1, open-label, dose-escalation study was conducted to determine the dose, safety profile, pharmacokinetics/pharmacodynamics, and tumor response in patients with relapsed/refractory non-Hodgkin lymphoma, CLL, and Waldenström macroglobulinemia. Patients who failed at least one previous therapy were eligible. Fifty-six patients received escalating weight-based doses of ibrutinib, ranging from 1.25 mg/kg to 12.5 mg/kg per day on a 28-days-on, seven-days-off schedule (35-day cycle), or continuous daily dosing of 8.3 mg/kg, or a fixed dose of 560 mg until disease progression, unacceptable toxicity, or patient/investigator decision to terminate therapy.
Greater than 95% Btk occupancy was observed at ibrutinib doses of at least 2.5 mg/kg per day, and a 420-mg dose was chosen for future studies. The most common adverse events (AEs) observed were typically grade 1 or 2 in severity; grade 3 or 4 events were infrequent and independent of dose. Grade 3 or 4 hematological toxicities included neutropenia (12.5%), thrombocytopenia (7.2%), and anemia (7.1%). No evidence of cumulative hematological or nonhematological toxicity was observed in patients with prolonged dosing. Ibrutinib was well tolerated, and no consistent relationship between dose level and AEs was evident. The low rate of AEs reflects the high specificity of ibrutinib for the Btk receptor with marginal off-target toxicity. Pharmacokinetic studies demonstrated that ibrutinib is rapidly absorbed and eliminated, with mean peak plasma concentrations observed one to two hours after dosing. The reported overall response rate (ORR) was 60%, with two CLL patients achieving a complete response. Responses were durable, with median progression-free survival (PFS) of 13.6 months at the time of data cutoff.16
Promising results from the phase 1 trial prompted a phase 1b/2 study evaluating the efficacy of ibrutinib exclusively in patients with relapsed/refractory CLL. Eighty-five patients received ibrutinib 420 mg/day (n = 51) or 840 mg/day (n = 34). Both doses were administered on a continuous schedule until the onset of disease progression or unacceptable toxicity. The median age of the study population was 66 years (range: 37–82 years), and 35% of the study participants were age 70 years or older. Patients included in this study were generally considered to have high-risk disease and had received a median of four previous therapies.
The primary endpoint was safety of the two fixed-dose regimens. Secondary endpoints included ORR, PFS, and pharmacokinetics/pharmacodynamics. No differences in the time to the peak ibrutinib blood concentration (median Tmax, two hours) or the terminal half-life (7.8 ± 3.6 hours with 420 mg/day and 8.1 ± 3.4 hours with 840 mg/day) were apparent between doses. Post-treatment assessments demonstrated full occupancy of Btk by ibrutinib at both dose levels.
Long-term therapy with ibrutinib was associated with modest toxicity, with most AEs being grade 1 or 2. The most common AEs were diarrhea, fatigue, and upper respiratory tract infection; the majority of these resolved without the need for suspension of treatment with ibrutinib. The most common AEs of grade 3 or higher were pneumonia (12%) and dehydration (6%). Infections that were at least grade 3 in severity occurred most frequently early in the course of treatment. Grade 3 or 4 hematological AEs were infrequent: anemia (6%), neutropenia (15%), and thrombocytopenia (6%). Bleeding of at least grade 3 severity occurred in four patients. Eight patients died within 30 days of receiving the last dose of ibrutinib; causes included pneumonia, systemic inflammatory response syndrome, sarcoma, and progression of CLL.
The ORR was 71% in both dosing cohorts. Two patients in the 420-mg group attained a complete response; no complete responses were seen in the 840-mg group. Blood lymphocytosis generally occurred by day 7 after initiation of ibrutinib (in 78% of patients), with a peak in the absolute lymphocyte count at a median of four weeks, and then slowly declined with continuation of therapy (resolution by a median of 23 weeks). It is important to note that lymphocytosis occurred concomitantly with a notable reduction in lymph node and spleen size, as well as frequent improvement in cytopenias. Treatment-related lymphocytosis has been seen with other agents that target B cell receptor signaling; CLL experts conclude that this is not a sign of progressive disease.
The response to ibrutinib did not appear to vary according to traditional high-risk prognostic features, suggesting the encouraging activity of this agent in a difficult-to-treat population. Treatment with ibrutinib promoted durable responses regardless of dose. The 26-month estimated rates of PFS and OS were 75% and 83%, respectively. The results of this trial led to the accelerated FDA approval of ibrutinib for relapsed/refractory patients with CLL.8
Results from the randomized, multi-center, phase 3 RESONATE trial will act as confirmation for the accelerated approval. The purpose of this study is to evaluate whether treatment with single-agent ibrutinib at 420 mg/day results in a clinically significant improvement in PFS as compared to treatment with ofatumumab (Arzerra, GlaxoSmithKline), a monoclonal antibody directed against the CD20 antigen on B lymphocytes, similar to rituximab, in patients with relapsed/refractory CLL. Secondary endpoints include OS, hematological improvements, and improvement of disease-related symptoms (i.e., fatigue, night sweats, and splenomegaly). A total of 391 patients who had received at least one prior therapy were enrolled. This study was initiated in June 2012; in January 2014, Pharmacyclics notified the FDA that the Data Monitoring Committee had stopped the trial early based on favorable results of a planned interim analysis (significant improvement in PFS and OS in patients treated with ibrutinib). Full data from this investigation have not yet been released.18
ADVERSE DRUG REACTIONS
The AEs reported below reflect exposure to ibrutinib in the phase 1b/2 trial discussed earlier; these patients were treated with 420 mg/day of ibrutinib for a median of 15.6 months.8 Adverse reactions leading to dose reduction occurred in 13% of patients. Ten percent of patients discontinued treatment due to AEs of infection (6%) and subdural hematomas (4%).
The most commonly occurring nonhematological AEs of any grade severity (20% or more) included diarrhea (63%), bruising (54%), upper respiratory tract infection (48%), hyperuricemia (38%), fatigue (31%), musculoskeletal pain (27%), skin rash (27%), pyrexia (25%), elevated serum creatinine (23%), peripheral edema (23%), constipation (23%), arthralgia (23%), stomatitis (21%), nausea (21%), sinusitis (21%), and dizziness (21%). The most common grade 3 or higher nonhematological AEs (5% or more) were pneumonia (8%), hypertension (8%), sinusitis (6%), skin infection (6%), dehydration (6%), musculoskeletal pain (6%), and atrial fibrillation (5%). The most common hematological AEs (20% or more of any grade severity) included thrombocytopenia (71%), neutropenia (47–54%), and anemia (41–44%). Grade 3 or higher neutropenia and thrombocytopenia were seen in 27% and 10% of patients, respectively.10
DOSAGE AND ADMINISTRATION10
The recommended dose of ibrutinib for CLL is 420 mg/day administered orally with water at approximately the same time each day. If a dose is not taken at the scheduled time, it can be taken as soon as possible on the same day with a return to the normal schedule the following day. Patients should be advised that extra doses of ibrutinib should not be taken to make up for the missed dose.
Capsules should be swallowed whole and should not be opened, broken, or chewed. Ibrutinib is classified as a hazardous agent; appropriate precautions for handling and disposal should be employed.
DOSAGE ADJUSTMENT FOR TOXICITY10
Treatment with ibrutinib should be interrupted for any grade 3 or higher nonhematological toxicities, grade 3 or higher neutropenia with infection or fever, or grade 4 hematological toxicities. Recommended dose modifications for these toxicities are shown in Table 1.
Table 1.
Dosage Adjustment for Ibrutinib Toxicities10
| Dose modification for nonhematological and hematological toxicities should take place upon improvement to grade 1 toxicity or return to baseline (recovery). | |
| First occurrence | Restart at 420 mg/day |
| Second occurrence | Restart at 280 mg/day |
| Third occurrence | Restart at 140 mg/day |
| Fourth occurrence | Discontinue ibrutinib |
SPECIFIC POPULATIONS10
Pregnancy
Ibrutinib was administered orally to pregnant rats at daily doses of 10, 40, and 80 mg/kg. A daily dose of 80 mg/kg in animals results in approximately 20 times greater exposure than that seen in CLL patients administered a dose of 420 mg/day. Ibrutinib at daily doses of 40 mg/kg or more was correlated with decreased fetal weight in rats. At 80 mg/kg daily in rats, heart and major vessel malformations occurred, along with increased post-implantation loss.
Based on these findings in animal reproduction studies, it can be extrapolated that ibrutinib can cause fetal harm when administered to pregnant women. The FDA has designated this medication Pregnancy Category D. If ibrutinib is used during pregnancy or if the patient becomes pregnant while taking ibrutinib, a discussion of the potential hazards to the fetus should take place.
Nursing Mothers
It is not known whether ibrutinib is excreted in human breast milk. Therefore, the risk versus benefit should be assessed prior to making a decision on whether to discontinue the medication or to discontinue nursing.
Pediatric Use
The safety and efficacy of ibrutinib in the pediatric patient population have not been established.
Geriatric Use
In clinical trials, no overall difference was observed in terms of efficacy between younger patients and elderly patients (65 years of age and older). However, a higher frequency and severity (grade 3 or higher) of AEs were reported in elderly patients. Despite these findings, the manufacturer does not recommend any dosage adjustments in older patients.
Renal Impairment
Due to minimal renal clearance of ibrutinib, a creatinine clearance (CrCL) of 25 mL/min or more has no influence on drug exposure and dosage adjustments are not recommended for these patients. Ibrutinib has not been studied in patients with severe renal impairment (CrCL less than 25 mL/min) or end-stage renal disease requiring dialysis; thus, no dosage adjustments are provided in the manufacturer’s labeling.
Hepatic Impairment
Due to extensive hepatic metabolism of ibrutinib, significant increases in drug exposure are anticipated in patients with hepatic impairment. Patients with serum aspartate transaminase (AST/SGOT) or alanine transaminase (ALT/SGPT) of three times or more the upper limit of normal (ULN) were excluded from clinical trials. No dosage adjustment is provided in the manufacturer’s labeling for any grade of hepatic impairment. There is insufficient data to recommend an initial dose of ibrutinib in patients with baseline hepatic impairment.
DRUG AND FOOD INTERACTIONS10
Ibrutinib is predominantly metabolized by CYP3A4. Co-administration of ketoconazole, a strong CYP3A4 inhibitor, increased Cmax (the maximum concentration of a drug after its administration) and AUC of ibrutinib by 29-fold and 24-fold, respectively, in healthy volunteers. Consequently, it is advised to avoid concomitant administration of ibrutinib with moderate or strong inhibitors of CYP3A4. If a moderate CYP3A4 inhibitor must be utilized, the manufacturer recommends reducing the dose of ibrutinib to 140 mg/day. If short-term therapy (seven days or less) with a strong CYP3A4 inhibitor (i.e., ketoconazole, itraconazole, voriconazole, posaconazole, clarithromycin, telithromycin) is necessary, the clinician may carefully consider interrupting ibrutinib therapy during the duration of inhibitor usage based on risk versus benefit. Chronic therapy with strong CYP3A4 inhibitors should be avoided. Patients should be advised to avoid grapefruit and Seville oranges during ibrutinib therapy, as these contain moderate inhibitors of CYP3A4.
Co-administration of ibrutinib with strong inducers of CYP3A4 decreases ibrutinib plasma concentrations by nearly 10-fold. Therapy with strong CYP3A inducers (i.e., carbamazepine, rifampin, phenytoin, St. John’s wort) should be avoided. Alternative agents with lower CYP3A4 induction properties should be considered.
CONTRAINDICATIONS10
There are no contraindications to ibrutinib listed in the manufacturer’s labeling.
WARNINGS AND PRECAUTIONS10
Although ibrutinib has no contraindications for use, hemorrhage, infection, myelosuppression, renal toxicity, occur-rence of new malignancies, and embryo-fetal toxicities are all possible AEs that require monitoring.
Of the patients studied in clinical trials, 6% experienced grade 3 or higher bleeding events, including subdural hematoma, ecchymoses, gastrointestinal bleeding, and hematuria. Bleeding events of any grade occurred in 63% of patients treated with 420 mg/day of ibrutinib. The mechanism of bleeding is not well understood, and the risk may be elevated in those receiving antiplatelet or anticoagulant therapies. Withholding ibrutinib for at least three to seven days before and after surgery should be considered.
Health care professionals should use caution when administering ibrutinib to patients with active infection. Both fatal and nonfatal infections have occurred. Grade 3 or greater infections were seen in 35% of patients. Patients taking ibrutinib should be monitored for fever and infection and treated promptly.
Because myelosuppression is a concern, complete blood counts should be monitored monthly in patients taking ibrutinib. Treatment-emergent grade 3 or 4 cytopenias were reported in 35% of patients. These included neutropenia (27%) and thrombocytopenia (10%).
Ibrutinib can cause renal failure, which in some cases can be serious or fatal. Treatment-emergent increases in creatinine levels up to 1.5 times the ULN were seen in 23% of patients. Increases in creatinine 1.5 to three times the ULN occurred in 4% of patients. Appropriate hydration and routine monitoring of kidney function is recommended.
Ibrutinib may increase the risk for second primary malignancies. The overall incidence of other malignancies was 10% in clinical trials, with 8% of patients developing skin cancers and 2% of patients developing other carcinomas.
COST
Ibrutinib is available as white opaque 140-mg capsules in packages of 90 ($9,840) and 120 ($13,120) capsules per bottle, reflecting an average wholesale unit price of $109.33 per capsule.19 Select specialty pharmacies are authorized to dispense ibrutinib (a list is available on the manufacturer’s website, www.imbruvica.com). This network of pharmacies will assist eligible patients in obtaining access to the medication through services such as prior authorization assistance and co-pay support. Patients who are not eligible for co-pay support can be referred to the YOU&i AccessTM Program, a personalized support program from Pharmacyclics.
P&T COMMITTEE CONSIDERATIONS
Ibrutinib is an orally available, first-in-class inhibitor of Btk for management of patients with CLL. For patients who are admitted to an inpatient facility, therapy with ibrutinib should be continued.
Utilizing ibrutinib as a treatment option can have a significant pharmacoeconomic impact on the treatment of CLL. Patients can take their medication orally and do not require hospitalization for administration. In addition, side effects are minimal. Unlike traditional cytotoxic chemotherapy, ibrutinib does not seem to involve serious hematological or nonhematological AEs that often require hospitalization for management.
In the long term, the cost of ibrutinib may be considerably less than the overall costs of CLL and its complications.
CONCLUSION
Ibrutinib is a breakthrough treatment option for patients with CLL. Given its excellent toxicity profile, it could be an appropriate choice for those patients in whom chemotherapy is otherwise contraindicated (i.e., advanced age, multiple comorbidities, poor performance status). Published clinical trials suggest ibrutinib is highly active in the treatment of CLL in the relapsed/refractory setting and induces durable remissions in predominantly elderly, heavily pre-treated patients with high-risk disease characteristics. Use of ibrutinib is expected to dramatically change the treatment paradigm of CLL in the years to come. Numerous studies are in the pipeline, all of which show exciting and promising results thus far.
REFERENCES
- 1.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Chavez JC, Sahakian E, Pinilla-Ibarz J. Ibrutinib: an evidence-based review of its potential in the treatment of advanced chronic lymphocytic leukemia. Core Evid. 2013;8:37–45. doi: 10.2147/CE.S34068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gribben JG. How I treat CLL up front. Blood. 2010;115:187–197. doi: 10.1182/blood-2009-08-207126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slager SL, Kay NE. Familial CLL: What does it mean to me? Clin Lymphoma Myeloma. 2009;9(Suppl 3):S194–S197. doi: 10.3816/CLM.2009.s.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byrd JC, Stilgenbauer S, Flinn IW. Chronic lymphocytic leukemia. Hematology Am Soc Hematol Educ Program. 2004;2004:163–183. doi: 10.1182/asheducation-2004.1.163. [DOI] [PubMed] [Google Scholar]
- 6.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia. Blood. 2008;111:5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamblin AD, Hamblin TJ. The immuno-deficiency of chronic lymphocytic leukemia. Br Med Bull. 2008;87:49–62. doi: 10.1093/bmb/ldn034. [DOI] [PubMed] [Google Scholar]
- 8.Byrd JC, Furman RR, Coutre SE, et al. Targeting Btk with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369:32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhatt V, Alejandro L, Michael A, et al. The promising impact of ibrutinib, a Bruton’s tyrosine kinase inhibitor, for the management of lymphoid malignancies. Pharmacotherapy. 2014;34:303–314. doi: 10.1002/phar.1366. [DOI] [PubMed] [Google Scholar]
- 10.Imbruvica [package insert] Sunnyvale, California: Pharmacyclics, Inc.; Feb, 2014. [Google Scholar]
- 11.Burger JA, Chiorazzi N. B cell receptor signaling in chronic lymphocytic leukemia. Trends Immunol. 2013;34:592–601. doi: 10.1016/j.it.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan WN. Regulation of B lymphocyte development and activation by Bruton’s tyrosine kinase. Immunol Res. 2001;23:147–156. doi: 10.1385/IR:23:2-3:147. [DOI] [PubMed] [Google Scholar]
- 13.Aalipour A, Advani RH. Bruton tyrosine kinase inhibitors: a promising novel targeted treatment for B cell lymphomas. Br J Haematol. 2013;163:436–443. doi: 10.1111/bjh.12573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herman SE, Gordon AL, Hertlein E, et al. Bruton’s tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood. 2011;117:6287–6296. doi: 10.1182/blood-2011-01-328484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akinleye A, Chen Y, Mukhi N, et al. Ibrutinib and novel Btk inhibitors in clinical development. J Hematol Oncol. 2013;6:59. doi: 10.1186/1756-8722-6-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Advani RH, Buggy JJ, Sharman JP, et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B cell malignancies. J Clin Oncol. 2013;31:88–94. doi: 10.1200/JCO.2012.42.7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sukbuntherng J, Jejurkar P, Chan S, et al. Pharmacokinetics (PK) of ibrutinib in patients with chronic lymphocytic leukemia (CLL) [abstract 7056] J Clin Oncol. 2013:31. [Google Scholar]
- 18.Pharmacyclics, Inc . ClinicalTrials.gov. Bethesda, Maryland: US National Library of Medicine; 2014. A phase 3 study of ibrutinib versus ofatumumab in patients with relapsed or refractory CLL (RESONATE) Available at: http://clinicaltrials.gov/show/NCT01578707. Accessed March 8, 2014. [Google Scholar]
- 19.Red Book Online. Ann Arbor, Michigan: Truven Health Analytics; Available at: www.micromedexsolutions.com. Accessed June 5, 2014. [Google Scholar]