Previews
One of the biggest challenges in biology is to understand how mitochondria influence aging and age-related diseases. Chin et al. (2014) reveal how a mitochondrial metabolite (mitobolite) inhibits mitochondrial ATPase and extends lifespan by mimicking dietary restriction in worms. One of the biggest challenges in biology is to understand how mitochondria influence aging and age-related diseases. Chin et al. (2014) reveal how a mitochondrial metabolite (mitobolite) inhibits mitochondrial ATPase and extends lifespan by mimicking dietary restriction in worms.
The free radical theory of aging proposes that accumulating macromolecular damage due to increased reactive oxygen species over time causes aging. As mitochondria are believed to be the main contributors of free radicals, reducing mitochondrial electron transport chain function would be expected to increase lifespan. Several studies in nematodes, flies, and mice have corroborated this idea by demonstrating that genetic inhibition of several mitochondrial components, especially those of the electron transport chain (ETC) complexes, can extend lifespan (Aguilaniu et al., 2005). However, several findings in the field have diminished the enthusiasm for the idea that simply the inhibition of mitochondrial function or even free radicals will extend lifespan in mammals. Furthermore, restricting nutrients in mammals or flies is accompanied by enhancement of mitochondrial biogenesis and function (Guarente, 2008; Zid et al., 2009). This in principle opposes the ‘Warburg effect’, as the enhancement of mitochondrial function helps the organism switch from glycolysis to oxidative metabolism, which mediates the protective effects of dietary restriction, such as slowing aging and decreased cancer rates. How and when an organism reduces mitochondrial function to extend life span however remains unclear. Recent work by Jing Huang and colleagues suggests that a mitochondrial metabolite (mitobolite) extends lifespan by inhibition of mitochondrial ATPase through mechanisms overlapping with dietary restriction in Caenorhabditis elegans(Chin et al., 2014).
Mitochondrial tricarboxylic acid cycle(TCA) metabolites, such as pyruvate, fumarate, malate and oxaloacetate, have been previously shown to extend lifespan upon feeding in C. elegans(Mouchiroud et al., 2011; Williams et al., 2009), though the mechanistic underpinnings of the pro-longevity effects remain to be delineated. Chin et al. now add alpha keto-glutarate (KG) to the list of “youthful” TCA intermediates. Using a novel unbiased technique, DARTS (drug affinity responsive target stability), they identify the binding proteins of KG that may explain its effects on aging. In this assay, cell lysates were incubated in varying concentrations of KG, followed by a complete proteolysis by a mixture of proteases, with the assumption that if KG is binding to a protein, that protein or the associated fragment will be protected from protease action. Uncleaved fragments were separated on a gel and further identified by liquid chromatography-tandem mass spectrometry. Chin et al. found that KG binds to several proteins, including two subunits of mitochondrial ATPase. Using several biochemical assays, including measurement of ATPase activity, respiration in isolated mitochondria, and measurement of ATP levels in worms after KG feeding, the authors confirmed that KG indeed inhibits mitochondrial ATPase activity. Consistently, reduction of atp2 (C. elegans mitochondrial ATPase b subunit) by RNAi led to increased lifespan, which was not further increased by KG feeding.
One consequence of inhibition of mitochondrial ATPase is reduction in cellular ATP levels. This in turn could lead to increased AMP and activation of the energy sensing kinases such as AMP kinase. The authors observed a reduction in ATP levels, though the lifespan extension upon KG feeding was not dependent on AMPK or hypoxia-inducible factor-1α. Inhibition of target of rapamycin (TOR), which mediates the effects of dietary restriction in multiple species (Kapahi et al. 2010), did not further extend lifespan upon KG feeding. However, the longevity effects were dependent on pha-4, known to be critical for lifespan extension by dietary restriction in C. elegans (Panowski, et al. 2007). The authors also demonstrated that KG inhibits TOR in both human and mouse cell lines, though the mechanisms of this interaction remain to be elucidated. Consistent with the effects of inhibition of TOR, autophagy was enhanced in worms fed with KG, treated with oligomycin (ATPase inhibitor) or carrying atp-2 mutations. Together, these results suggest that KG dependent-ATPase inhibition modulates autophagy in a TOR-dependent manner to extend lifespan.
When defining the impact of modulating mitochondrial function, there tends to be an overemphasis in measuring ROS and ATP levels, which fails to take into consideration the dynamic role of ‘mitobolites’. Mitochondria originated by endosymbiosis and, over time, they lost most of their DNA, which normally plays an important role in responding to environmental perturbations. However, mitobolites may act as the key sensors of mitochondrial perturbations and mediate nuclear-mitochondrial interactions. The study by Chin et al adds to the emerging evidence on the growing role of such mitobolites in modulating metabolism, growth, disease processes and longevity (Chandel, 2014). Many unanswered questions remain about mitobolites. How is the mitochondrial metabolite flux regulated? How are levels of various metabolites integrated to code for certain responses? What are the different targets, and their tissue specificity, that are regulated by the secreted mitobolites? Also, what is the role of non-TCA mitobolites in aging such as those generated by fatty acid oxidation, urea cycle, cardiolipin synthesis, heme, quinone and steroid biosynthesis? Recent studies have indicated that the exometabolome (metabolite excreted in the environment by the worm) of long-lived mitochondrial mutants is significantly different from short-lived mitochondrial mutants or other animals (such as insulin receptor mutant, daf-2), supporting the possibility that mitobolites can further extend lifespan in certain long-lived mutants (Butler et al., 2013; Butler et al., 2010). Thus, one may have to integrate the levels of various mitobolites and the cellular state to decipher how each perturbation will influence the downstream events to modulate aging.
The elucidation of the mechanism by which KG inhibits mitochondrial ATPase and mimics the effects of dietary restriction underscores the important role mitobolites play as potential signaling molecules. Wider appreciation of the role of mitobolites will not only help resolve some of the paradoxes in the field but also provide excellent therapeutic targets to counteract aging and age-related diseases in mammals.
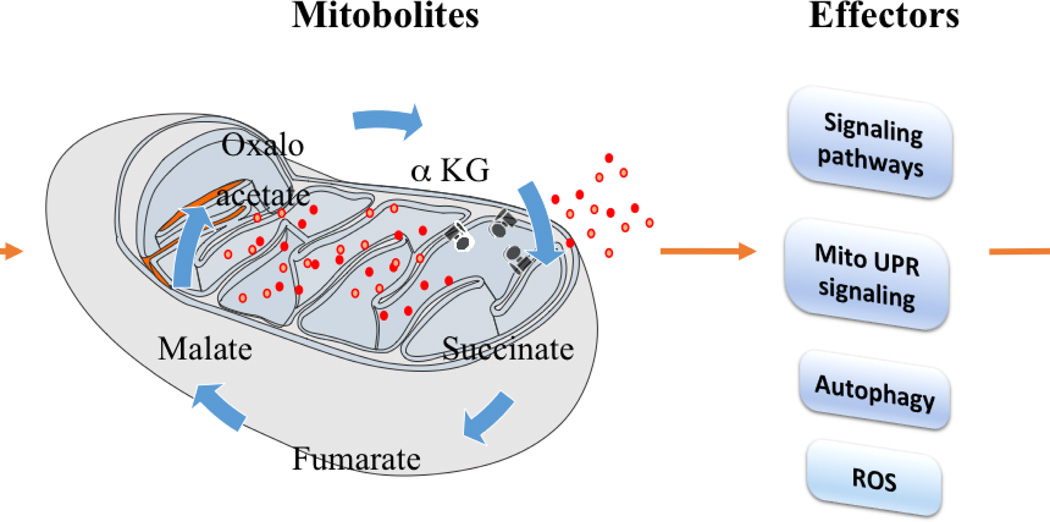
Figure 1. The role of mitochondria-derived metabolites, ‘mitobolites’, in modulating metabolism, growth and longevity.
Interventions that modulate mitochondrial functions regulate the flux of mitobolites. Mitobolites (red dots) potentially regulate mitochondrial proteins, including mitochondrial ATPase (black structures), and once released from mitochondria, these mitobolites may also regulate cellular signaling pathways. This in turn modulates various physiological processes like metabolism, growth and longevity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguilaniu H, Durieux J, Dillin A. Metabolism, ubiquinone synthesis, and longevity. Genes Dev. 2005;19:2399–2406. doi: 10.1101/gad.1366505. [DOI] [PubMed] [Google Scholar]
- Butler JA, Mishur RJ, Bhaskaran S, Rea SL. A metabolic signature for long life in the Caenorhabditis elegans Mit mutants. Aging Cell. 2013;12:130–138. doi: 10.1111/acel.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JA, Ventura N, Johnson TE, Rea SL. Long-lived mitochondrial (Mit) mutants of Caenorhabditis elegans utilize a novel metabolism. FASEB J. 2010;24:4977–4988. doi: 10.1096/fj.10-162941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandel NS. Mitochondria as signaling organelles. BMC Biol. 2014;12:34. doi: 10.1186/1741-7007-12-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin RM, Fu X, Pai MY, Vergnes L, Hwang H, Deng G, Diep S, Lomenick B, Meli VS, Monsalve GC, Hu E, Whelan SA, Wang JX, Jung G, Solis GM, Fazlollahi F, Kaweeteerawat C, Quach A, Nili M, Krall AS, Godwin HA, Chang HR, Faull KF, Guo F, Jiang M, Trauger SA, Saghatelian A, Braas D, Christofk HR, Clarke CF, Teitell MA, Petrascheck M, Reue K, Jung ME, Frand AR, Huang J. The metabolite alpha-ketoglutarate extends lifespan by inhibiting ATP synthase and TOR. Nature. 2014 doi: 10.1038/nature13264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L. Mitochondria--a nexus for aging, calorie restriction, and sirtuins? Cell. 2008;132:171–176. doi: 10.1016/j.cell.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapahi P, Chen D, Rogers AN, Katewa SD, Li PW, Thomas EL, Kockel L. With TOR, less is more: a key role for the conserved nutrient-sensing TOR pathway in aging. Cell Metab. 2010;11:453–465. doi: 10.1016/j.cmet.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouchiroud L, Molin L, Kasturi P, Triba MN, Dumas ME, Wilson MC, Halestrap AP, Roussel D, Masse I, Dalliere N, Segalat L, Billaud M, Solari F. Pyruvate imbalance mediates metabolic reprogramming and mimics lifespan extension by dietary restriction in Caenorhabditis elegans. Aging Cell. 2011;10:39–54. doi: 10.1111/j.1474-9726.2010.00640.x. [DOI] [PubMed] [Google Scholar]
- Panowski SH, Wolff S, Aguilaniu H, Durieux J, Dillin A. PHA-4/Foxa mediates diet-restriction-induced longevity of C. elegans. Nature. 2007;447:550–555. doi: 10.1038/nature05837. [DOI] [PubMed] [Google Scholar]
- Williams DS, Cash A, Hamadani L, Diemer T. Oxaloacetate supplementation increases lifespan in Caenorhabditis elegans through an AMPK/FOXO-dependent pathway. Aging Cell. 2009;8:765–768. doi: 10.1111/j.1474-9726.2009.00527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zid BM, Rogers AN, Katewa SD, Vargas MA, Kolipinski MC, Lu TA, Benzer S, Kapahi P. 4E-BP extends lifespan upon dietary restriction by enhancing mitochondrial activity in Drosophila. Cell. 2009;139:149–160. doi: 10.1016/j.cell.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]