Abstract
Intercellular chemical signaling in bacteria, commonly referred to as quorum sensing (QS), relies on the production and detection of compounds known as pheromones to elicit coordinated responses among members of a community. Pheromones produced by Gram-positive bacteria are comprised of small peptides. Based on both peptide structure and sensory system architectures, Gram-positive bacterial signaling pathways may be classified into one of four groups with a defining hallmark: cyclical peptides of the Agr type, peptides that contain Gly-Gly processing motifs, sensory systems of the RNPP family, or the recently characterized Rgg-like regulatory family. The recent discovery that Rgg family members respond to peptide pheromones increases substantially the number of species in which QS is likely a key regulatory component. These pathways control a variety of fundamental behaviors including conjugation, natural competence for transformation, biofilm development, and virulence factor regulation. Overlapping QS pathways found in multiple species and pathways that utilize conserved peptide pheromones provide opportunities for interspecies communication. Here we review pheromone signaling identified in the genera Enterococcus and Streptococcus, providing examples of all four types of pathways.
Keywords: intercellular communication, Firmicutes, competence, biofilms, virulence, gene regulation
INTRODUCTION
Quorum sensing
For many years, bacteria were thought to be isolated single-celled organisms lacking social abilities. Secreted signaling molecules were identified (Tomasz & Hotchkiss, 1964), although infrequently, and the full extent of bacterial communication was not appreciated until much later. Only recently have researchers come to understand bacterial populations as communities of organisms in which signals serve as communication devices, sharing information within and between bacterial populations. The study of this process, termed quorum sensing (QS), has provided important insight into how bacteria regulate behaviors in synchrony with members of their community. QS-mediated processes include biofilm formation and dispersal (Davies et al., 1998, Hancock & Perego, 2004, Parsek & Greenberg, 2005, Rickard et al., 2006, Waters et al., 2008, Ueda et al., 2009, Chang et al., 2011), virulence factor regulation (Winzer & Williams, 2001, Zhu et al., 2002, Rutherford & Bassler, 2012, Subramoni & Sokol, 2012), competence development (Håvarstein et al., 1995, Fontaine et al., 2010, Mashburn-Warren et al., 2010), sporulation (Perego & Hoch, 1996, Steiner et al., 2012), and many others.
In Gram-negative bacteria, the QS signals are often acyl-homoserine lactone molecules that have been extensively discussed and reviewed elsewhere (Fuqua et al., 2001, Schauder et al., 2001, Fuqua & Greenberg, 2002, Waters & Bassler, 2005, Ng & Bassler, 2009). Gram-positive bacteria, on the other hand, are found to use small peptides, commonly termed pheromones, as signals to mediate QS behaviors. In this review, we use the terms QS signal and pheromone interchangeably, recognizing these compounds serve a variety of purposes that may provide a means to measure population density or as a mechanism to signal from one individual to another. These signaling peptides regulate a wide array of processes, including many related to host-microbe interactions, and thus may provide novel targets for therapies that interfere with communication to disrupt bacterial infection. Disrupting virulence without directly killing or inhibiting growth of bacterial pathogens is expected to place lower selective pressure on bacteria to evolve mechanisms that would overcome such treatments. Quorum-quenching strategies may therefore provide an alternative method of treatment against antibiotic-resistant pathogens by means that are less likely to perpetuate resistance. To advance development of such therapeutic methodologies, a deeper understanding of the mechanisms by which bacteria regulate behaviors by intercellular signaling should be pursued. This review focuses on the current understanding of pheromone pathways used by Gram-positive bacteria of the genera Streptococcus and Enterococcus.
Gram-positive pheromone systems
Recent years have seen a dramatic increase in studies revealing new pheromone pathways among Gram-positive bacteria that expand upon the understanding of peptide signaling described for model organisms like Bacillus subtilis, Staphylococcus aureus, and Streptococcus pneumoniae. It is our aim to categorize fundamental attributes of these pheromone signaling pathways. Although all of the QS peptides described to date among Firmicutes are ribosomally synthesized, their processing, secretion, and signaling abilities differ widely (Figure 1). Gram-positive QS pathways fall into four general groups based on features of the pheromones and their receptors: 1) members of the RNPP (Rap, NprR, PlcR, and PrgX) family of regulators; 2) Agr-type cyclical pheromones; 3) peptides with double-glycine (Gly-Gly) processing motifs; and 4) regulators of the Rgg family. It is becoming evident that Gram-positive bacteria often utilize multiple types of QS pathways within a species to control a wide variety of processes.
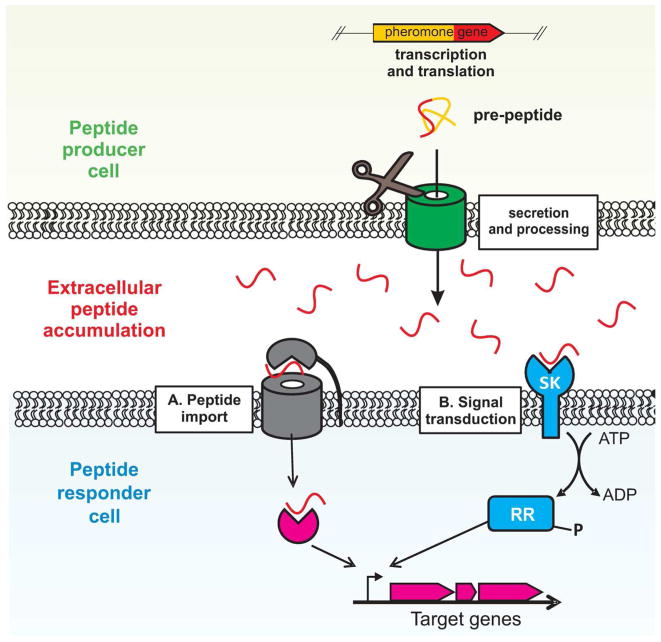
Figure 1. Peptide signaling in Gram-positive bacteria.
Production of Gram-positive peptide pheromones involves transcription and translation of precursors followed by processing and secretion. Once in the extracellular environment, peptides are often further processed before interacting with surrounding cells. To exert effects on neighboring cells, peptides are either A. directly imported into the cell where they interact with their cognate receptor or B. interact with a surface exposed senor kinase (SK). Following peptide interaction with an SK, a signal is transmitted intracellularly in the form of phosphorylation of a response regulator (RR). The phosphorylated RR or peptide/receptor combination can then alter gene expression by either directly binding DNA or interacting with transcriptional regulators such as sigma factors and RNA polymerase.
Pheromones that bind to RNPP regulators are transported to the cytoplasm where they directly interact with an RNPP family member to modulate gene expression. The RNPP family was named for the Rap auxiliary regulatory proteins of B. subtilis, the neutral protease regulator, NprR, found in Bacillus species, the phospholipase C regulator, PlcR, of the Bacillus cereus family, and the pheromone responsive gene regulator, PrgX, of Enterococcus faecalis (Declerck et al., 2007). The group has grown to contain many orthologs of these regulators as well. Often the RNPP family member and its cognate peptide are encoded adjacent to one another. Following transcription and translation, pre-peptides are secreted and processed into mature pheromones where they can encounter other cells in the population. The pheromones then are taken into the cell by transporters of the oligopeptide permease (Opp) family and subsequently bind to the RNPP cytoplasmic regulators. In the case of Rap proteins, pheromone binding disrupts the interaction of Rap with response regulator proteins that control gene expression (Core & Perego, 2003, Baker & Neiditch, 2011, Parashar et al., 2013). NprR, on the other hand, contains a helix-turn-helix (HTH) DNA binding domain. Binding of the NprX peptide activates interaction of the NprR HTH domain with DNA, thus activating transcriptional activity (Zouhir et al., 2013). Similarly, PrgX, a repressor of gene transcription, and PlcR, an activator, are bound by their cognate peptide (or multiple peptides in the case of PrgX) to exert a conformational change in the proteins thus modulating DNA binding and transcriptional regulation of target genes (Bae et al., 2002, Declerck et al., 2007). The RNPP family of quorum sensing systems have been recently reviewed (Rocha-Estrada et al., 2010).
Agr-type peptides are named for accessory gene regulator, consisting of the genes agrABCD, that stands as a well-studied QS circuit controlling virulence factor expression in S. aureus. A hallmark feature of this pathway is the utilization of a cyclical peptide pheromone encoded by agrD in S. aureus. The peptide is proposed to be exported and processed via a dedicated transport protein termed AgrB (Saenz et al., 2000, Nakayama et al., 2001). AgrB contains a putative cysteine endopeptidase domain (Qiu et al., 2005) and cyclization of the peptide is thought to be assisted by the transporter. Pheromone detection occurs by a two-component signal transduction system (TCSTS) at the cellular surface whereby the peptide binds to a dedicated histidine kinase, AgrC, that transmits the signal via phosphorylation of the cytoplasmic response regulator AgrA (Sturme et al., 2002). Orthologous signaling pathways have been identified in Enterococcus (Fsr, discussed below), Clostridium, and Listeria (Agr).
Competence-stimulating peptides (CSPs) of streptococci and class II bacteriocins belong to the Gly-Gly-type peptide family. As their name suggests, these peptides contain a double glycine motif in their conserved leader sequence (LSX2ELX2IXGG) (Havarstein et al., 1994). Gly-Gly peptides are secreted via a transporter containing an accessory domain that proteolytically processes the leader sequence at a site in the polypeptide immediately following the conserved Gly-Gly motif (Havarstein et al., 1995). As seen in Agr-type systems, peptides of the double glycine family are sensed via a TCSTS that transmits a signal internally via phosphorylation of cognate response regulators.
Like the RNPP family, regulators of the Rgg family directly bind to pheromones that are internalized subsequent to their export and maturation. Although secretion and processing of these peptides is not fully understood, several reports have found a role for the Eep (enhanced expression of pheromone) metalloprotease, which also cleaves the signal sequence of the enterococcal sex pheromones (An et al., 1999, Chang et al., 2011). These peptides are potentially processed further upon reaching the extracellular milieu, and mature peptides are internalized via Opp or Ami peptide uptake systems prior to interaction with Rggs. Not every Rgg-type regulator has been shown to interact with a peptide pheromone, although as this family of proteins has continued to receive attention, more peptide interactions have been found or are hypothesized to be present (Fleuchot et al., 2011, Shelburne et al., 2011, Cook et al., 2013).. Structural information on Rgg proteins remains elusive; however, structure prediction algorithms suggest that Rgg proteins contain similar tricopeptide repeat (TPR)-like domains responsible for peptide interactions in the RNPP family leading some to propose that Rgg proteins should be included in the RNPP family (Mashburn-Warren et al., 2010, Fleuchot et al., 2011). For the purposes of this review, we will consider them as separate groups while still highlighting similarities between the two.
RNPP-REGULATED CELL COMMUNICATION
The conjugative peptides of Enterococcus faecalis
Enterococci are well known for their ability to undergo conjugation, horizontally transferring genes within and between species via conjugative plasmids. Conjugation in enterococci is generally controlled via two counteracting peptide pheromones where one peptide serves as an inducer of the signaling pathway leading to conjugation, and the other as an inhibitor that prevents unnecessary mating between cells that already contain the plasmid. Several conjugative plasmids have been identified (e.g. pAD1, pCF10, pAM373, etc.) along with the regulatory peptides controlling their transfer, whose names follow the plasmid nomenclature (e.g. cCF10 and iCF10 for conjugation agonist and inhibitor, respectively, of plasmid pCF10; Table 1). The inducing peptide precursors are encoded on the chromosome of E. faecalis, while the inhibitor peptides are encoded on the plasmid. Expression of the inhibitor peptide gene from the plasmid ensures that conjugation will not occur between two cells harboring the same conjugative plasmid. Ratios of inducer to inhibitor concentration are tipped in favor of the inducer when plasmid-free cells, which cannot generate the inhibitor, are present, allowing plasmid-free recipient cells to induce plasmid-containing donor cells to conjugate. In general, the conjugative peptides of E. faecalis are highly specific, only stimulating conjugation of their cognate plasmid. Addition of exogenous peptide analogs differing in only one amino acid also fail to activate conjugation of the cognate plasmid, further demonstrating the high specificity of conjugative peptides (Dunny, 2001). Although the peptide binding proteins encoded on the conjugative plasmids are highly homologous, some variability exists in the peptide binding pocket, presumably contributing to specificity (Dunny, 2001).
Table 1.
|
Red residues indicate conservation among the peptide family
indicates cleavage site
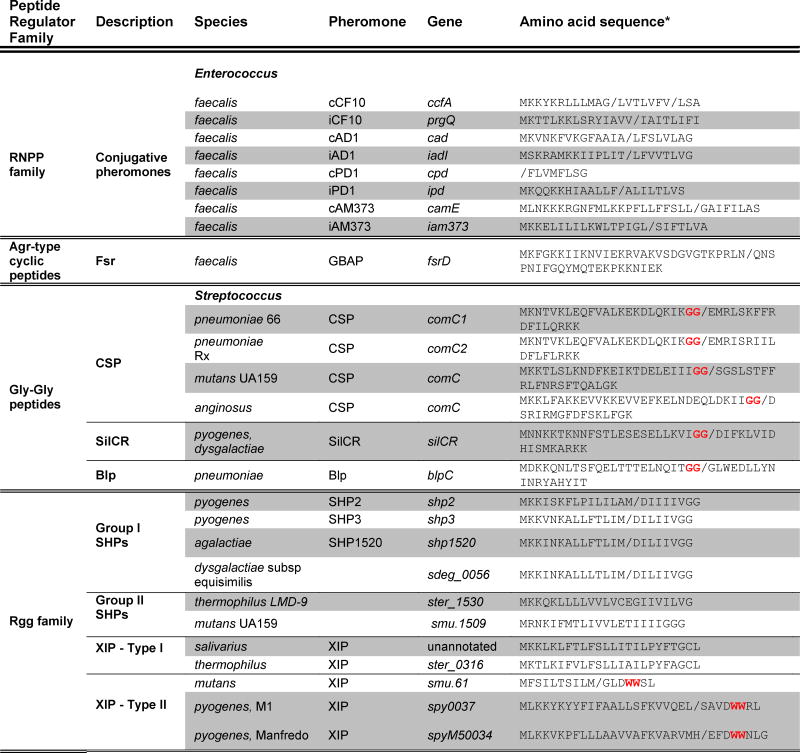
For this review, we focus on the pCF10 conjugative plasmid system, although the pAD1, pPD1, and pAM373 regulatory systems have also been described and reviewed in varying detail and share common aspects of signaling (Wirth, 1994, Nakayama et al., 1995, De Boever et al., 2000, Clewell, 2007). The inducer pheromone of pCF10 is expressed constitutively as part of a lipoprotein, CcfA (Antiporta & Dunny, 2002), whose function remains unclear. The pheromone peptide is located within the secretion signal-sequence region of CcfA, and is released from the polypeptide by the signal peptidase II (SPII) enzyme (Figure 2). Full processing of the precursor peptide to the mature pheromone also requires the action of the intramembrane Eep metalloprotease, either before or during the secretion process (Chandler et al., 2005, Chandler & Dunny, 2008). Finally, an exopeptidase is predicted to cleave the remaining three C-terminal amino acids resulting in mature peptide (Antiporta & Dunny, 2002) (Figure 2). Given that cCF10 was originally identified as a secreted molecule (Dunny et al., 1978) and that inducing activity is found in culture supernatant (Chandler & Dunny, 2008), it is reasoned that the cell dissociates the highly hydrophobic peptides (e.g. cCF10, LVTLVFV) from the membrane by a yet to be identified transport system.
Figure 2. Processing of the E. faecalis conjugation peptide cCF10.
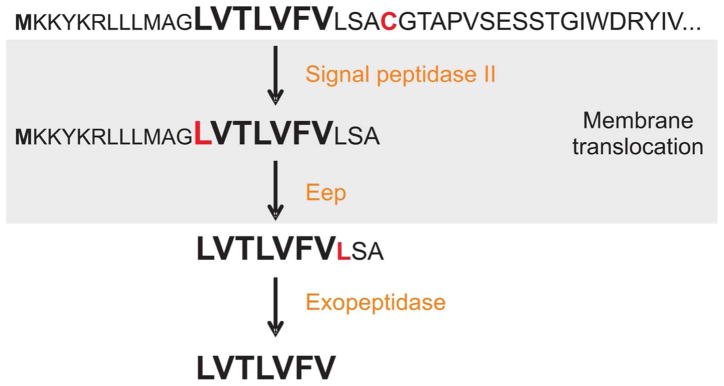
Processing of the CcfA lipoprotein involves at least three separate cleavage events that occur upstream of the amino acids indicated in red. First, Signal peptidase II cleaves the lipoprotein upstream of a cysteine residue. The peptide precursor is then cleaved sometime during the transport process, likelyby a metalloprotease, Eep, possibly assisted by other proteases. The pro-peptide is secreted into the extracellular environment where it is further cleaved by an exopeptidase, removing the terminal three amino acids to give the mature cCF10 peptide pheromone which is now free to interact with neighboring cells.
Cellular detection of mature cCF10 peptide requires importation of the peptide to the cytosol and is facilitated by PrgZ, a plasmid-encoded protein with homology to OppA, the substrate-binding subunit of the oligopeptide permease (Opp) system (Leonard et al., 1996). PrgZ is thought to recruit the chromosomally encoded permease subunits (OppBCDF) forming a transport complex. PrgZ then substitutes for OppA by binding extracellular pheromone prior to transport through the Opp system. Although the presence of PrgZ increases cell sensitivity to the pheromone, it is not required for pheromone import, presumably because OppA can fulfill this role, albeit with lower affinity (Leonard et al., 1996). Following import, mature cCF10 binds to the master regulator of conjugation, PrgX, a member of the RNPP family of regulators. In the absence of cCF10, PrgX binds to two target sites on pCF10 upstream of the PQ promoter and represses transcription (Bae et al., 2002). One of the PrgX binding sites is an inverted repeat while the other only contains a portion of this palindromic sequence. cCF10 binding to PrgX alleviates repression and allows transcription of downstream genes encoding conjugation factors such as aggregation substance (AS) (Figure 3).
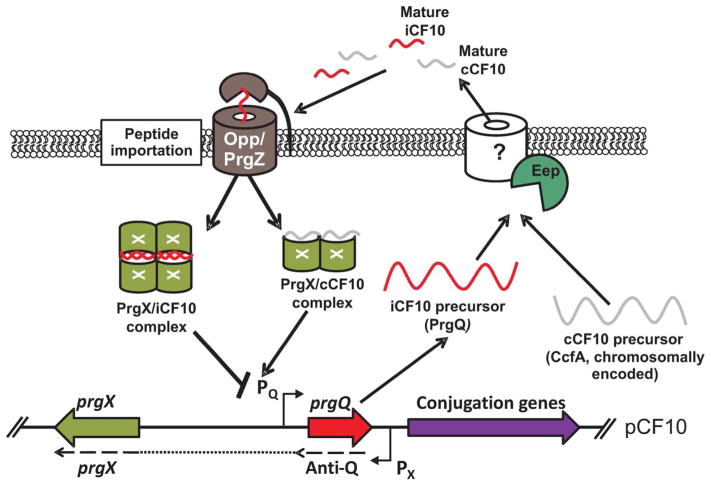
Figure 3. Peptide regulation of pCF10 conjugation in E. faecalis.
During non-inducing conditions, PrgQ is produced constitutively from the PQ promoter. PrgQ, the precursor for the iCF10 peptide, is processed by Eep immediately before or during secretion. Mature iCF10 pheromone in the extracellular milieu is sensed by surrounding cells and taken up via PrgZ and the Opp system where the peptide interacts with the PrgX regulator. PrgX-iCF10 complexes are thought to form a tetrameric structure allowing binding of PrgX to DNA adjacent to PQ, effectively shutting off transcription by blocking RNA polymerase access. Concurrently, cells are constitutively producing CcfA from the chromosome. CcfA, the cCF10 peptide precursor, is processed, secreted, and reimported similarly to iCF10. Once exported, mature cCF10 pheromone has been re-internalized, it competes with iCF10 to bind to PrgX. When cCF10 is in abundance, the system favors PrgX-cCF10 complexes destabilizing the DNA binding tetramer and allowing for increased RNA polymerase access to PQ and transcription of the downstream conjugation genes.
pCF10-containing cells have several mechanisms to prevent auto-induction of conjugation. The antagonist to cCF10 is the inhibitor pheromone iCF10, which is encoded by prgQ on pCF10. Like CcfA, processing of PrgQ to the mature iCF10 pheromone also occurs in an Eep-dependent manner (Chandler & Dunny, 2008). Like cCF10, iCF10 is re-imported to the cytoplasm via the Opp system in conjunction with PrgZ (Leonard et al., 1996) and the peptides compete to interact with PrgX (Shi et al., 2005). Binding of iCF10 stabilizes a tetrameric form of PrgX that is required for optimal interactions with each PrgX binding site on the DNA adjacent to the conjugation promoter, effectively blocking access of RNA polymerase to initiate transcription of conjugation genes. Displacement of iCF10 by cCF10 is thought to destabilize the dimer-dimer interface of the tetramer, and therefore DNA binding, allowing transcription of the conjugation genes (Shi et al., 2005). Thus, the ratio of the inhibitor and inducer peptides determines the conjugative state of the cell (Chatterjee et al., 2013) (Figure 3).
Another mechanism preventing conjugation is mediated by the plasmid-encoded protein PrgY, whose effects are seen in decreasing the availability of endogenous cCF10 (Chandler et al., 2005). The full mechanism of PrgY-dependent sequestration (or possible degradation) of self-produced cCF10 is not fully understood. It is known that this process is Eep-independent and involves a direct interaction between PrgY and cCF10, although whether cCF10 has been fully processed at this point is also unknown (Chandler & Dunny, 2008). These events are among the first steps involved in the conjugation process. Several additional elements, beyond PrgX and the pheromones, contribute to a complex regulatory pathway that provides precise control of plasmid transfer. For example, transcriptional interference between divergent PQ and PX promoters causes reciprocal repression on one another and the small RNAs produced from these promoters (QS and Anti-Q, respectively) interact to form a transcriptional terminator upstream of the conjugation genes (Johnson et al., 2010, Shokeen et al., 2010, Chatterjee et al., 2011). The QS RNA also negatively regulates PrgX levels by targeting prgX mRNA for degradation by RNaseIII (Johnson et al., 2011). Overall, the regulation of this system responds to several levels of transcriptional and post-transcriptional control.
The enterococcal sex pheromones not only serve as mediators of a cell-to-cell interaction that initiates conjugation but also serve as an ongoing tally system for the community that indicates the probability of potential “mates” remaining in a group (Chatterjee et al., 2013). The use of iCF10 as a means to count donor cells provides a method for the bacteria to down-regulate conjugation genes and prevent the expenditure of energy when the majority of the population already contains the plasmid (Chatterjee et al., 2013). Using this signal for a dual purpose allows cells to tightly control the spread of genes throughout a population and illustrates flexibility in purpose of signaling peptides while maintaining their specificity.
Components of the conjugation pathway have also been associated with the regulation of virulence behavior in E. faecalis. Aggregation substance allows clumping of cells to facilitate efficient conjugation and has also been shown to be involved in fibrin adhesion (Hirt et al., 2000), increased vegetation formation in a rabbit model of endocarditis, (Chuang et al., 2009) and increased biofilm formation and colonization on ex vivo cardiac valves (Chuang-Smith et al., 2010). Expression of AS is highly upregulated in plasma (Hirt et al., 2002), possibly due to plasma interference with iCF10 signaling (Chandler et al., 2005).
The metalloprotease Eep, which is critical in the processing of conjugative peptides as well as Rgg-dependent pheromones (see below), has been implicated in contributing to the virulence of E. faecalis in a rabbit model of endocarditis. A Δeep strain is severely attenuated in its ability to form endocarditis vegetations, exhibits an altered cellular distribution in an in vitro biofilm assay (Frank et al., 2011), and is slightly attenuated in a mouse model of catheter-associated urinary tract infection (Frank et al., 2013).
The production of conjugative pheromones has not been limited to Enterococcus. pAM373, another conjugative plasmid first discovered in enterococci, is of interest to researchers because its cognate pheromone, cAM373, is also produced by S. aureus and Streptococcus gordonii. Although the cAM373 produced by the these species differs slightly from the enterococcal version of cAM373 (S. gordonii by 3 amino acids and S. aureus by 1 amino acid), the variant peptides are able to induce conjugation in E. faecalis, indicating a possible role in interspecies signaling and gene transfer (Clewell et al., 1985). Though transfer of pAM373 to S. aureus and S. gordonii was not reported at the time of discovery of the variant peptides (Clewell et al., 1985), transfer to S. gordonii was shown in a follow-up study (Vickerman et al., 2010). This is especially significant as it is believed that vancomycin-resistant enterococci (VRE) are responsible for transferring vancomycin resistance genes to S. aureus via conjugation, creating vancomycin-resistant S. aureus (VRSA) (Weigel et al., 2007). Few cases of VRSA have been reported in the United States (Sievert et al., 2008) and the first reported case in Europe was only identified in 2013 (Melo-Cristino, 2013) but the spread of antibiotic resistance is a dangerous problem and dissecting the mechanisms of transfer will be essential in preventing further dissemination of resistance determinants.
AGR-TYPE CYCLIC PHEROMONES
Virulence regulation by the GBAP QS peptide in enterococci
Virulence in E. faecalis is also controlled by a peptide-based QS system encoded by the fsr gene locus. The fsr locus is comprised of four genes, fsrABDC, that were originally identified as homologues of the S. aureus Agr QS system (Qin et al., 2000). The Agr system controls several virulence factors and has been extensively studied and reviewed elsewhere (Novick & Geisinger, 2008, Thoendel et al., 2011). The fsr QS system is controlled by a peptide, known as gelatinase biosynthesis-activating pheromone (GBAP), and is associated with virulence in rabbit models of endophthalmitis and endocarditis, as well as biofilm formation in vitro (Mylonakis et al., 2002, Hancock & Perego, 2004, Thurlow et al., 2010).
Originally, the 3′ end of the fsrB gene was thought to encode the GBAP pheromone (Nakayama et al., 2001). Later studies revealed that the GBAP propeptide was actually encoded by another gene, fsrD, immediately downstream and in-frame with fsrB but translated independently (Figure 4) (Nakayama et al., 2006). FsrB is involved in processing the FsrD propeptide to its final active form (Figure 4) (Qin et al., 2000, Nakayama et al., 2001). Interestingly, unlike other linear peptides described in this review, GBAP has a cyclic structure. Autoinducers (AIs) from the Agr and Agr-like systems, including AgrD of S. aureus and LamD of Lactobacillus plantarum, form cyclic peptides with thiolactone rings (Lyon & Novick, 2004, Sturme et al., 2005). GBAP, instead, forms a lactone ring using a serine residue rather than the cysteine residue found in most AgrD-like peptides. It is hypothesized that FsrB has cysteine-protease activity, allowing it to process FsrD to the active GBAP, much like AgrB processes AgrD (Nakayama et al., 2006).
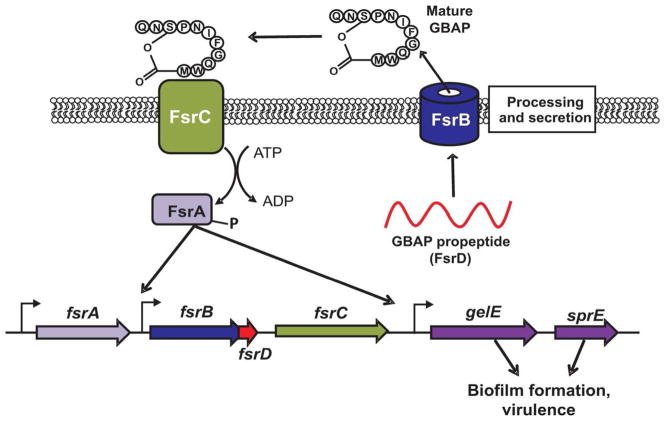
Figure 4. The Fsr QS system of E. faecalis.
FsrD, the gelatinase biosynthesis activating pheromone (GBAP) precursor, is processed to a cyclical peptide during secretion by FsrB. Mature GBAP pheromone interacts with the FsrC sensor kinase on the surface of surrounding cells causing phosphorylation of the DNA-binding response regulator, FsrA. Phosphorylated FsrA binds to promoters, including those of fsrB and gelE/sprE, and upregulates gene expression.
An important difference between GBAP and conjugation-peptide signaling can be found in signal detection. As shown in Figure 4, GBAP is sensed outside the cell by interacting with the trans-membrane histidine kinase FsrC. FsrC is modulated by the binding of GBAP as the bound ligand promotes kinase activity. FsrC is then able to phosphorylate the DNA binding response regulator FsrA, effectively controlling gene expression (Del Papa & Perego, 2011). As mentioned above, conjugative pheromones are detected in the cytoplasm following their import and interact directly with transcriptional regulators, rather than acting through a TCSTS.
The fsrABDC locus is located immediately upstream of two genes, gelE and sprE, both of which are positively controlled by the fsr system. GelE and SprE have each been identified as regulators of biofilm formation (Qin et al., 2000, Sifri et al., 2002, Hancock & Perego, 2004). FsrB also regulates a variety of other genes important in biofilm formation and E. faecalis surface protein expression (Bourgogne et al., 2006). As biofilm formation is an essential factor in the development of enterococcal diseases such as endocarditis (Nallapareddy et al., 2006, Thurlow et al., 2010) and infections on indwelling catheters (Donlan, 2001), the Fsr pathway plays an important role in the virulence of E. faecalis.
GLY-GLY PEPTIDES
Competence pheromones of Mitis and Anginosus streptococci
One of the first-discovered and best-studied peptide-controlled bacterial processes is competence development in Streptococcus. Natural genetic transformation has long been known as a behavior responsive to a “communicable” signal among cells of a population, and the mode of communication was first proposed to be a macromolecular, proteinaceous substance (Pakula & Walczak, 1963, Tomasz & Hotchkiss, 1964, Tomasz & Mosser, 1966). The full characterization of this factor as a 17-residue peptide pheromone was completed some thirty years after its initial descriptions and it was named competence stimulating peptide (CSP) (Håvarstein et al., 1995). CSP is a processed form of ComC, part of the ComAB/ComCDE system found in the Mitis and Anginosus groups of streptococci (Havarstein et al., 1996, Pestova et al., 1996). Although all members of these groups contain functional ComAB/ComCDE systems, not all of them are naturally transformable in laboratory conditions. CSP is processed from the precursor ComC during export by ComAB (also known as NlmTE in some streptococci) (Hui & Morrison, 1991, Håvarstein et al., 1995). Like GBAP, CSP is not imported into the cell but rather interacts with a TCSTS comprised of a membrane-spanning histidine kinase, ComD, and a cytoplasmic response regulator ComE. The precursor peptide ComC contains a conserved N-terminal double-glycine leader sequence motif characteristic of Gram-positive bacteriocins (discussed below) (Havarstein et al., 1994). This Gly-Gly sequence is important for cleavage of the leader sequence by the N-terminal peptidase region of ComA (Ishii et al., 2006). Interestingly, unlike the leader sequence, the mature CSP peptide sequence is not highly conserved between streptococcal species and even in some cases, divergent variants are observed within a species ((Whatmore et al., 1999) and Table 1). In S. pneumoniae, for example, two distinct allelic variants of comC have been described, comC1 and comC2, encoding CSP-1 and CSP-2 peptide subtypes (Pozzi et al., 1996) (Table 1). Heterogeneity is also seen in the CSP receptor, ComD, where four distinct allelic variations have been described, each binding in varying degrees to different CSP types (Iannelli et al., 2005). Not surprisingly, the highest level of amino acid variability is localized to the sensor domain in the N-terminal portion of ComD which is the domain thought to interact with CSP (Pozzi et al., 1996).
Following CSP interaction with ComD, ComE is phosphorylated and subsequently activates late competence genes (Figure 5). Phosphorylated ComE binds to a direct repeat sequence conserved in S. pneumoniae, S. mitis, S. oralis, S. crista, S. gordonii, and S. sanguis (Ween et al., 1999). This direct repeat is also found upstream of the comAB and comCDE operons, ensuring a positive feedback loop in competence development.
Figure 5. Competence development in streptococci.
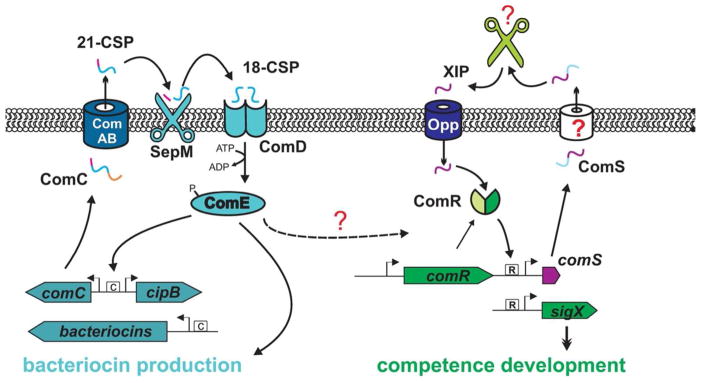
Left panel: Competence development in Anginosus and Mitis groups of streptococci uses the ComAB/ComCDE system and requires production and processing of ComC to the competence stimulating peptide CSP. CSP interaction with the surface sensor kinase ComD allows phosphorylation of ComE. Phosphorylated ComE binds to conserved CIN (C) box motifs upstream of late competence genes in streptococci of the Mitis and Anginosus groups as well as bacteriocins in S. mutans.
Right panel: In the Pyogenic, Bovis, Salivarius and Mutans groups of streptococci, the most proximal regulator of competence genes is the ComRS system. ComS is secreted and processed to the SigX inducing peptide (XIP) via an unknown mechanism. XIP is imported into the cell by the Opp transporter system where it directly interacts with ComR. ComR binds to DNA and upregulates expression of comS as well as sigX, an alternative sigma factor controlling expression of later competence genes. In S. mutans, these two pathways interact although the mechanism and reason for this interaction is not understood. The figure is a modified reproduction (Federle & Morrison, 2012)
Two important ComE-regulated competence genes are the alternative sigma factor, sigX (also known as comX), and a gene encoding an accessory protein comW (Peterson et al., 2000, Luo et al., 2004). SigX is highly conserved among streptococci and is even found in species that do not contain the comAB/comCDE genes, such as species of the Pyogenic, Bovis and Salivarius groups (Havarstein, 2010). It acts to control competence by directing RNA polymerase to a conserved competence induced (cin) box consensus sequence found at the 5′-end of “late” competence gene operons, which are responsible for DNA uptake and genomic integration (Lee & Morrison, 1999, Luo & Morrison, 2003). In pneumococci, the period of time that cells remain in the competent state is short, averaging about 20 minutes. During this period of time, levels of SigX mRNA and protein rise and fall dramatically (Luo & Morrison, 2003). Though ComAB/ComCDE positive feedback accounts for the rapid increase in SigX production, the late gene dprA mediates negative feedback on the system and shuts down the competent state (Mirouze et al., 2013).
CSP in S. pneumoniae has primarily been studied as an inducer of competence, but in recent years has been shown to control many other bacterial processes through the activation of ComE or SigX. One such process, allolysis or autolysis, involves the lysing and release of virulence factors and DNA by noncompetent cells, allowing predation by competent neighbors (Guiral et al., 2005). Additionally, S. pneumoniae comE mutants display reduced lung colonization in animal studies, widening the effects associated with CSP regulatory pathways (Kowalko & Sebert, 2008).
Bacteriocin regulation
Systematic evaluation of TCSTSs in S. pneumoniae identified genes with significant homology to comCDE that contain a small peptide with a double-glycine leader sequence encoded by blpC (Throup et al., 2000). This quorum-sensing circuit regulates bacteriocin-like peptides (Blps) (de Saizieu et al., 2000) whose sequences and activities are diverse among pneumococcal species (Lux et al., 2007), and can impact competition among them within a host (Dawid et al., 2007). Similar genetic loci are found among species of S. thermophilus (Fontaine et al., 2007), as well as pyogenic species S. pyogenes, S. agalactiae and S. dysgalactiae. Blps are also found in S. mutans but have been labeled comCDE, leading to some confusion as to the primary role of these genes. The BlpABCRH orthologous system of S. mutans was first recognized for its involvement in competence induction in this organism, and has therefore been referred to as Com and the signaling pheromone as CSP. Clear genomic comparisons (Martin et al., 2006) and demonstration that CSP induces promoters located upstream of bacteriocins (van der Ploeg, 2005) confirm that this system is more analogous to the Blp regulatory pathways seen in S. pneumoniae and salivarius species. Effects imposed by induction of bacteriocins, however, have led to intriguing phenotypes in regards to competence induction, most clearly exemplified in S. mutans. CSP induction has a clear effect on culture viability and, through an indirect and undetermined mechanism, requiring induction of mutacin V (CipB), is able to upregulate SigX expression and SigX-dependent genes, including the murein hydrolase LytF, which promotes cell lysis (Perry et al., 2009, Dufour & Levesque, 2013). CSP also plays a role in the development of biofilms in S. mutans, likely using an autolysis pathway to provide nutrients and extracellular DNA (eDNA) for production of the biofilm matrix (Perry et al., 2009).
The streptococcal invasion locus, or Sil, is another bacteriocin-like peptide based QS system found in approximately 25% of S. pyogenes strains and >80% of Group G streptococci (GGS) (Michael-Gayego et al., 2013). The sil locus was originally identified using a transposon screen for virulence in a mouse model of necrotizing fasciitis. A transposon insertion into an open reading frame, silC, showed attenuated virulence, leading researchers to believe that the sil locus may play a role in controlling invasiveness of S. pyogenes (Hidalgo-Grass et al., 2002). The Sil regulon is composed of silAB which encode a TCSTS, silDE which encode the ATP-binding transporter system, and the silC/silCR locus. The sil locus is organized similarly to the blp system of S. pneumoniae and SilAB are homologous to BlpRH (Hidalgo-Grass et al., 2002). Overlapping a majority of the silC gene, but transcribed from the reverse strand, is a gene encoding a QS peptide pheromone, silCR. Like CSP and bacteriocin peptides, the full length SilCR peptide contains a Gly-Gly processing motif (Hidalgo-Grass et al., 2002, Hidalgo-Grass et al., 2004, Eran et al., 2007). silAB and silDE/CR appear to be co-transcribed from two promoters, termed P1 and P3 respectively. Upon addition of SilCR peptide, induction of the P3 promoter occurs in a SilA-dependent fashion, controlling several putative bacteriocin-related genes (Eran et al., 2007, Belotserkovsky et al., 2009).
The Sil system is not present or functional in all strains of S. pyogenes; SilC is not found in M1 or M3 strains, the ATG start codon has been mutated in M14 strains and the putative pheromone transporter SilD is truncated in M18 strains (Hidalgo-Grass et al., 2004). Although the frequency of the sil locus in S. pyogenes is fairly low, it is far more frequent in S. dysgalactiae subsp. equisimilis (SDSE or Group G Streptococcus), and the SilCR peptide can facilitate cross-species communication between S. pyogenes and SDSE (Belotserkovsky et al., 2009). Interestingly, sil has a high degree of homology to comCDE of S. pneumoniae and a putative com-box promoter is located upstream of silC (Hidalgo-Grass et al., 2002), although SigX was not found to influence Sil regulation (Eran et al., 2007).
When examined in the context of virulence, SilCR was originally reported to control the development of necrotic lesions in mice and the authors hypothesized that SilCR could act as a therapeutic to control invasive S. pyogenes infections (Hidalgo-Grass et al., 2004). A conflicting study later examined the role of the Sil system in expression of genes involved in the formation of necrotic lesions by S. pyogenes: sagA encoding streptolysin O which has been shown to play a role in virulence and invasion (Betschel et al., 1998, Datta et al., 2005), spyCEP (also known as scpC), a serine protease that also contributes to necrotic lesion formation (Hidalgo-Grass et al., 2006, Sumby et al., 2008), and siaA, a gene involved in iron regulation and the formation of murine necrotic lesions (Montanez, 2005). This study found that addition of the SilCR peptide influenced expression of these genes in a growth-phase-dependent manner. In contrast to earlier data, this study found no therapeutic benefit of addition of the SilCR peptide in a murine necrotic lesion model, and instead reported impaired lesion healing following addition of the peptide (Salim et al., 2008). The presence of the SilCR peptide appears to attenuate virulence in a mouse model of infection with GGS and vaccination against SilCR in this model actually results in a more severe infection (Michael-Gayego et al., 2013). It appears from these conflicting results that SilCR action may be species, strain or serotype dependent and further research must be done to determine the role of this QS system in streptococcal infections.
RGG REGULATORS AND SHORT HYDROPHOBIC PEPTIDES
Competence Pheromones of Pyogenic, Bovis, Salivarius and Mutans streptococci
As genome sequences of many streptococcal species were completed around the turn of the century it was recognized that virtually all retained intact DNA uptake and integration genes, along with the alternative sigma factor gene sigX, required for natural transformation (Martin and Claverys, 2003). Less clear were methodologies or conditions that would favor natural transformation in laboratory settings for most species, and genome sequences did not provide clear indications that the QS pathways found in Mitis and Anginosus groups, encoding comAB/comCDE, were present in Salivarius, Pyogenic, or Bovis groups. Though transformation had become possible for S. thermophilus by over-expressing SigX (Blomqvist et al., 2006), mechanisms for natural induction in these groups remained poorly understood. In a similar vein, when a pathway hypothesized to be the comCDE equivalent in S. mutans was disrupted, measurable levels of transformation continued (Ahn et al., 2006), indicating that other regulatory components controlling the competent state remained undiscovered. Such a pathway was recently identified and found to employ a separate family of regulators and peptides (Fontaine et al., 2010, Mashburn-Warren et al., 2010). This pathway, termed ComRS, responds to a short linear peptide, encoded by comS and called XIP for sigX inducing peptide. ComR is the receptor for XIP, and is a member of the Rgg family of transcriptional regulators. The ComRS pathway appears to be the proximal regulator of SigX (and thus, the late competence genes) among the Pyogenic, Salivarius, Bovis and Mutans groups, and serves as a functional alternative to the pneumococcal ComAB/ComCDE pathway since ComRS is not present in the Anginosus or Mitis groups.
Two classes of ComRS are recognized, based on amino acid sequences of ComR and ComS and the predicted DNA binding sites of ComR. Type I ComRS variants are found in species of the Salivarius group, and Type II in Pyogenic, Mutans and Bovis species (Fontaine et al., 2010, Mashburn-Warren et al., 2010, Fleuchot et al., 2011, Fontaine et al., 2013). The clearest difference between these groups can be seen at the C-terminus of ComS, which encodes the mature pheromone; Type II peptides contain a double tryptophan motif while Type I variants do not (Table 1).
The XIP peptides differ from bacteriocin-like CSP peptides in that they do not contain a Gly-Gly motif in the leader sequence. In fact, leader sequences of ComS peptides vary greatly in their length and properties. In some pyogenic species like S. pyogenes, ComS is 32 amino acids with a leader that shares some properties with classic signal sequences (von Heijne, 1986) whereas the S. mutans ComS is only 17 amino acids and the leader bears no resemblance to a signal sequence. Production of mature XIP does not require the Eep protease (Khan et al., 2012) and specific processing and secretion machinery has not yet been identified. Once mature XIP is produced outside of the cell, it is re-imported via the Opp system where it interacts directly with ComR to bind promoter sequences upstream of comS and sigX (Mashburn-Warren et al., 2010). Auto-regulation of comS generates a positive feedback loop that results in a robust cellular response to pheromone and a vigorous induction of sigX and competence-related genes.
In S. mutans, the comRS pathway was shown to be epistatic to the pathway named comCDE (a blp-like locus named for its homology to the S. pneumoniae comCDE genes, see below) in its ability to regulate sigX. Unlike comE, deletion of comR, comS, or the pheromone uptake apparatus, opp, completely abolished competence (Mashburn-Warren et al., 2010). Nonetheless, the comCDE genes and CSP do have an important impact on competence, and the interaction between the comCDE and comRS pathways remains an intriguing puzzle (Figure 5). Recently, it was shown that environment, and growth media in particular, play an important role in competence development in the laboratory. Results from studies using a gfp reporter of sigX expression demonstrated that S. mutans responded to exogenous CSP in complex medium (BHI) but not in a peptide-free chemically defined medium (CDM). In contrast, S. mutans did not respond to XIP in peptide-rich medium, but in CDM XIP stimulated cells at sub-micromolar concentrations (Son et al., 2012). CSP’s effect on competence occurs only in a subpopulation of cells, displaying a bimodal distribution in sigX expression across the population, whereas XIP induction in CDM is unimodal, occurring in all cells (Aspiras et al., 2004, Lemme et al., 2011, Son et al., 2012). Interestingly, the effects of CSP require the presence of comS and presumably the production of XIP; however, an opp deletion mutant did not affect bimodal induction (Son et al., 2012). These perplexing findings highlight the interplay between two intertwined pheromone pathways and provide a fine illustration of the complexities that signaling circuits can have when coupled together (Figure 5).
Although all species of streptococci contain at least one of these competence regulatory systems, most remain recalcitrant to methods that would stimulate the competent state in the laboratory. It was not until the past year that natural transformation was demonstrated in members of the Bovis group, including Streptococcus infantarius and macedonicus species (Morrison et al., 2013). In the human pathogen S. pyogenes, natural transformation has not been demonstrated but the expression of SigX and downstream competence genes was shown to be dependent on the ComRS system (Mashburn-Warren et al., 2012). Though all genes hypothesized to be required for transformation are apparently intact in this species, it is currently unclear whether some streptococci have lost the ability to become competent or if the optimal conditions have not yet been determined.
Emerging Rgg pathways
The Rgg family of pheromone-responsive transcription factors, which includes ComR, is increasingly recognized as having a widespread presence among many species of Firmicutes. Sulavik et al. first defined the role of Rgg in S. gordonii as a regulator gene of glucosyltransferases (Sulavik et al., 1992, Sulavik & Clewell, 1996). Rgg-like regulators (sometimes called MutR) have since been found in a wide range of Gram-positive bacteria (Sanders et al., 1998, Chaussee et al., 1999, Qi et al., 1999, Samen et al., 2006, Ibrahim et al., 2007a, Chang et al., 2011, Dumoulin et al., 2013) and control a variety of bacterial processes, including production of the cysteine proteinase SpeB in S. pyogenes (Lyon et al., 1998, Chaussee et al., 1999), S. mutans lantibiotic bacteriocin production (Qi et al., 1999), and virulence gene regulation in S. agalactiae (Samen et al., 2006), among others. Only in recent years were Rgg members, once considered to be ‘stand-alone regulators’ (Kreikmeier, McIver, 2003), shown to respond to short hydrophobic peptides (SHPs) that serve as pheromones. In a bioinformatics study that analyzed intergenic regions of select streptococcal genomes, it was found that rgg-like genes were frequently found adjacent to small genes encoding SHPs (Ibrahim et al., 2007a). This study identified 10 rgg/shp pairs in 5 different species of streptococci, and none of the SHPs described had been previously annotated. Recent work by the same investigators expanded the analysis to identify rgg-like genes for 90 genomes, including Lactobacillale and Listeriaceae species (Fleuchot et al., 2011). A total of 494 rgg genes were identified, along with 61 adjacent shp genes that were categorized separately from 27 putative XIP-encoding open reading frames.
The first clear indication that an Rgg protein responded to a SHP peptide was reported in S. thermophilus, where gene regulation by rgg1358 was found to rely on the adjacent shp1358, the eep protease, and the ami oligopeptide transport system (Ibrahim et al., 2007b, Fleuchot et al., 2011). The primary target of this regulatory pathway is a gene encoding a cyclical peptide, Pep1357C. Although the function of this cyclic peptide remains unclear, it is speculated to regulate another signaling pathway or possibly act as an antimicrobial peptide.
Additional evidence that Rgg proteins make up a wide-spread QS family was provided by work in other streptococci. All S. pyogenes strains sequenced to date contain four rgg paralogs, named rgg1 through rgg4 based on homology scores to the S. gordonii rgg (Figure 6). In 2011, it was demonstrated that two Rgg/SHP pairs, Rgg2/SHP2 and Rgg3/SHP3, constituted the first conserved QS system identified in this species (Chang et al., 2011). In this system, Rgg2 and Rgg3 act as competing regulators with Rgg3 as a repressor and Rgg2 as an activator, both working on the promoters for the two adjacent shp genes. SHP2 and SHP3 require Eep for processing, although possible additional processing and export elements have yet to be identified. The mature peptides are re-imported into the cell via Opp where each interacts with both Rgg2 and Rgg3. In the absence of pheromone, Rgg3 serves to block transcription of target promoters, and the effect of SHP binding is to displace Rgg3 from the operator site (Chang et al., 2011, LaSarre et al., 2012). This event alone is not enough for robust expression from the regulated promoters; Rgg2 also needs to engage a SHP peptide for full activation of Rgg targets (Chang et al., 2011). Two target promoters of Rgg2 and Rgg3 have been identified and shown to regulate operons that include each shp gene along with several genes of unknown function. Why two Rgg proteins and two separate pheromones are used by S. pyogenes to control expression of these genes is not clear, but it could provide a mechanism to ensure tight repression in the absence of pheromone and robust activation when induced. Rgg3 bound to DNA in the absence of pheromone sterically blocks the promoter from RNA polymerase and maintains a repressed state of transcription. However, elimination of Rgg3 by mutatgenesis does not result in high levels of transcription; this only occurs with the ability of Rgg2 to activate when bound to a SHP pheromone. Evidence suggests that production of SHP2 involves a different pathway from SHP3, a process that may be determined by the peptide leader sequence (Lasarre et al., 2013). Having separate secretion or processing pathways for pheromones that regulate identical targets suggests that S. pyogenes finds it beneficial to control pheromone production by at least two different mechanisms. For instance, the differential activity observed in SHP production may be dictated by environmental conditions. In such a scenario, coupling environmental conditions to quorum sensing would offer a simple method for the cell to determine if two conditions, in this case the culture environment and the culture density, are satisfactory to carry out a process. Since NZ131 uses the Rgg2/3 signaling pathway to regulate biofilm growth (Chang et al., 2011), it stands to reason that cells in the population monitor environmental conditions and then decide as a group whether to form a biofilm.
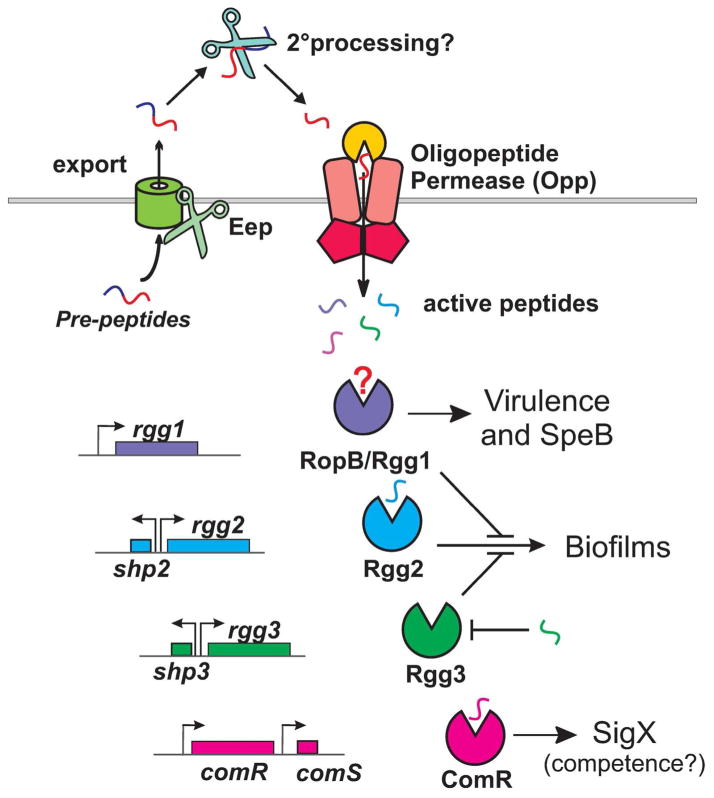
Figure 6. Rgg/SHP pathways in S. pyogenes.
All sequenced species of S. pyogenes contain four Rgg paralogs. Rgg1 (RopB) controls expression of SpeB and other virulence factors. The putative Rgg1 cognate peptide is unknown. Divergently transcribed from rgg2 and rgg3 are the short hydrophobic peptide genes shp2 and shp3, respectively. The comS gene is located downstream of comR, encoded on the same DNA strand. The pheromones are translated as pre-peptides prior to processing by Eep, secretion, and further extracellular processing. Mature peptides are imported into the cell where they can interact with their Rgg receptor to influence cellular behaviors such as biofilm formation and competence development. The figure is a modified reproduction (Federle, 2012).
Among the Rgg proteins of S. pyogenes, Rgg1, better known as the regulator of proteinase B (RopB), is best understood in its relationship to pathogenesis, since it is known to activate SpeB, an important virulence factor (Neely et al., 2003). Three lines of reasoning sustain the hypothesis that RopB may use a secreted peptide as a signal to activate speB. First, protein structure-prediction algorithms PHYRE (Kelley & Sternberg, 2009) and I-TASSER (Zhang, 2008), suggest that RopB shares structural attributes consistent with the RNPP family members PlcR and PrgX. Secondly, deletion of the Opp system in S. pyogenes significantly lowered the levels of SpeB produced (Podbielski et al., 1996), and finally, ectopic expression of RopB alone was not sufficient to promote speB expression (Neely et al., 2003, Shelburne et al., 2011). Unlike typical Rgg/SHP pairs, RopB is not divergently transcribed from a shp-like gene and peptide identification has thus far eluded scientists. Recent evidence suggests that RopB-dependent activation of SpeB in late exponential growth is negatively affected by a peptide originating from a protein, Vfr (Shellburne, 2011). Clear parallels can be drawn to the enterococcal conjugation inhibitory peptides and it will be interesting to learn if a corresponding activating pheromone is implicated to interact with RopB.
Quorum sensing functionality of Rgg proteins continues to expand to other species. An Rgg system previously shown to regulate virulence factor production in S. agalactiae, termed RovS (Samen et al., 2006), was examined prior to the 2007 characterization of the adjacent shp gene, shp1520 (Ibrahim et al., 2007a). Recently, it was demonstrated that SHP1520 and RovS comprise a quorum sensing circuit in S. agalactiae and also serve as a cross-species signaling system together with the SHP/Rgg circuit of S. pyogenes (Cook et al., 2013). The terminal eight amino acids of SHP1520 and SHP2 are identical, leading investigators to examine whether production of the peptide by one species would influence gene expression in the other. Unlike in S. pyogenes where both activator (Rgg2) and repressor (Rgg3) control expression of the shp genes, S. agalactiae only contains RovS, a homolog of Rgg2. Addition of spent culture supernatants as well as co-culture studies demonstrated that the production of SHP1520 as well as its activator, RovS, could induce the expression of SHP2 and SHP3 in S. pyogenes and vice versa. S. agalactiae is not the only species to contain SHP2 homologs and S. dysgalactiae subsp. equisimilis and Streptococcus porcinus peptides also induced peptide expression in both S. pyogenes and S. agalactiae (Cook et al., 2013). Synthetic SHPs from similar classes of Rgg could also induce gene expression responses in S. mutans, and S. thermophilus (Fleuchot et al., 2013). These initial examples of cross-species signaling among streptococci provide important new insights into the extensive role Rgg/SHP pairs may play in regulating bacterial behaviors.
Pheromone quenching
Clearly peptide pheromones play a key role in bacterial gene regulation and virulence behaviors and recent studies have begun to focus on peptide inhibitors as a means to combat these QS-controlled processes. Ostensibly, inhibition of QS-mediated pathogenesis could provide an important alternative to antibiotics with the benefit of decreased pressure to evolve resistance. Recent comprehensive reviews discuss the current research on QS inhibitors (QSIs) and the potential they present for therapeutics and drug design (Gonzalez & Keshavan, 2006, LaSarre & Federle, 2013). Most of the recent research in QSIs has focused on Gram-negative bacteria and research in Gram-positive QSIs has mostly been centered around S. aureus AIP although research into other species is beginning to emerge.
A 2007 study examined actinomycete metabolites for their ability to inhibit the production of GBAP and fsr signaling in E. faecalis without inhibiting growth. This study identified a peptide antibiotic, siamycin I as inhibiting GBAP production as well as gelatinase at sub-lethal concentrations (Nakayama et al., 2007). The authors suggest that this compound could be used as a therapeutic if it is further developed. A 2009 screen by the same group of fungal butanol extracts found that ambuic acid also inhibited gelatinase production without inhibiting growth of E. faecalis (Nakayama et al., 2009). More recently, a high-throughput screen (HTS) was developed to identify compounds that inhibit the agr and fsr QS systems. This HTS has already resulted in four compounds found to inhibit QS but not growth (Desouky et al., 2013). Addition of mutated peptides has been shown to decrease QS ability in S. pneumoniae. Addition of CSP-E1A, a mutant CSP where the first glutamic acid residue was changed to an alanine inhibited competence development, likely by competition, and also reduced virulence factor expression (Zhu & Lau, 2011). These screens strongly suggest that a myriad of compounds with QSI activity likely exist and may be the key to avoiding the ever-increasing danger of antibiotic resistance in bacterial pathogens.
It may also be possible to exploit bacterially derived peptides to quench signaling systems. In the case of E. faecalis, bacterially produced inhibitor peptides can prevent the transfer of genes via conjugation. Similarly, S. aureus produces four types of AIPs, each of which can activate their cognate receptor AgrC and concurrently block signaling from other AIPs by competitive inhibition (Lyon et al., 2002). Use of bacterially-produced inhibitor peptides or pheromone analogs to directly inhibit or competitively block bacterial signals could serve as a means to interfere with the virulence and resistance phenotypes associated with QS.
Conclusion
A myriad of pheromone-based systems exist in both Gram-positive and Gram-negative bacteria controlling a wide array of bacterial behaviors. In Gram-positive bacteria, peptide pheromones and their cognate receptors fall into four important groups; Agr-like peptides, peptides containing Gly-Gly leader sequences, RNPP family regulators and Rgg regulators. Examples of all four of these groups can be found in the genera Streptococcus and Enterococcus. New peptides are still being described, many of which have currently unknown functions (Ibrahim et al., 2007a, Ibrahim et al., 2007b). It is a strong possibility that many more bacterial processes are controlled by pheromones than scientists currently realize. As new peptide systems are discovered and characterized, and if found to influence pathogenic attributes of host-microbe interactions, then QSIs may become an increasingly important source of novel therapeutics against bacterial infections, while providing treatments that are less likely to impose the evolutionary constraints associated with the development of antibiotic resistance.
Summary.
This report summarizes current knowledge on peptide pheromones found in the genera Streptococcus and Enterococcus and reviews the four major categories of gram-positive bacterial signaling pathways.
References
- Ahn SJ, Wen ZT, Burne RA. Multilevel control of competence development and stress tolerance in Streptococcus mutans UA159. Infect Immun. 2006;74:1631–1642. doi: 10.1128/IAI.74.3.1631-1642.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An F, Sulavik M, Clewell D. Identification and characterization of a determinant (eep) on the Enterococcus faecalis chromosome that is involved in production of the peptide sex pheromone cAD1. Journal of Bacteriology. 1999;181:5915–5921. doi: 10.1128/jb.181.19.5915-5921.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An FY, Clewell DB. Identification of the cAD1 sex pheromone precursor in Enterococcus faecalis. J Bacteriol. 2002;184:1880–1887. doi: 10.1128/JB.184.7.1880-1887.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antiporta M, Dunny G. ccfA, the genetic determinant for the cCF10 peptide pheromone in Enterococcus faecalis. J Bacteriol. 2002;184:1155–1162. doi: 10.1128/jb.184.4.1155-1162.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspiras MB, Ellen RP, Cvitkovitch DG. ComX activity of Streptococcus mutans growing in biofilms. FEMS Microbiol Lett. 2004;238:167–174. doi: 10.1016/j.femsle.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Bae T, Kozlowicz T, Dunny G. Two targets in pCF10 DNA for PrgX binding: their role in production of Qa and prgX mRNA and in regulation of pheromone-inducible conjugation. Journal of Molecular Biology. 2002;315:995–1007. doi: 10.1006/jmbi.2001.5294. [DOI] [PubMed] [Google Scholar]
- Baker MD, Neiditch MB. Structural basis of response regulator inhibition by a bacterial anti-activator protein. PLoS Biol. 2011;9:e1001226. doi: 10.1371/journal.pbio.1001226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belotserkovsky I, Baruch M, Peer A, Dov E, Ravins M, Mishalian I, Persky M, Smith Y, Hanski E. Functional Analysis of the Quorum-Sensing Streptococcal Invasion Locus (sil) PLoS Pathog. 2009:5. doi: 10.1371/journal.ppat.1000651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betschel SD, Borgia SM, Barg NL, Low DE, De Azavedo JC. Reduced virulence of group A streptococcal Tn916 mutants that do not produce streptolysin S. Infect Immun. 1998;66:1671–1679. doi: 10.1128/iai.66.4.1671-1679.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomqvist T, Steinmoen H, Havarstein LS. Natural genetic transformation: A novel tool for efficient genetic engineering of the dairy bacterium Streptococcus thermophilus. Appl Environ Microbiol. 2006;72:6751–6756. doi: 10.1128/AEM.01156-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgogne A, Hilsenbeck SG, Dunny GM, Murray BE. Comparison of OG1RF and an isogenic fsrB deletion mutant by transcriptional analysis: the Fsr system of Enterococcus faecalis is more than the activator of gelatinase and serine protease. J Bacteriol. 2006;188:2875–2884. doi: 10.1128/JB.188.8.2875-2884.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler J, Flynn A, Bryan E, Dunny G. Specific control of endogenous cCF10 pheromone by conserved domain of the pCF10-encoded regulatory protein PrgY in Enterococcus faecalis. Journal of Bacteriology. 2005;187:13. doi: 10.1128/JB.187.14.4830-4843.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler JR, Dunny GM. Characterization of the sequence specificity determinants required for processing and control of sex pheromone by the intramembrane protease Eep and the plasmid-encoded protein PrgY. Journal of Bacteriology. 2008;190:1172–1183. doi: 10.1128/JB.01327-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler JR, Hirt H, Dunny GM. A paracrine peptide sex pheromone also acts as an autocrine signal to induce plasmid transfer and virulence factor expression in vivo. Proc Natl Acad Sci. 2005;102:15617–15622. doi: 10.1073/pnas.0505545102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JC, LaSarre B, Jimenez JC, Aggarwal C, Federle MJ. Two Group A Streptococcal Peptide Pheromones Act through Opposing Rgg Regulators to Control Biofilm Development. PLoS Pathog. 2011:7. doi: 10.1371/journal.ppat.1002190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A, Johnson C, Shu C-C, Kaznessis Y, Ramkrishna D, Dunny G, Hu W-S. Convergent transcription confers a bistability switch in Enterococcus faecalis conjugation. Proceedings of the National Academy of Sciences. 2011;108:9721–9726. doi: 10.1073/pnas.1101569108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A, Cook LC, Shu CC, Chen Y, Manias DA, Ramkrishna D, Dunny GM, Hu WS. Antagonistic self-sensing and mate-sensing signaling controls antibiotic resistance transfer. Proc Natl Acad Sci U S A. 2013;110:7086–7090. doi: 10.1073/pnas.1212256110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaussee MS, Ajdic D, Ferretti JJ. The rgg Gene of Streptococcus pyogenes NZ131 Positively Influences Extracellular SPE B Production. Infect Immun. 1999;67:1715–1722. doi: 10.1128/iai.67.4.1715-1722.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang O, Schlievert P, Wells C, Manias D, Tripp T, Dunny G. Multiple functional domains of Enterococcus faecalis aggregation substance Asc10 contribute to endocarditis virulence. Infection and Immunity. 2009;77:539–548. doi: 10.1128/IAI.01034-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang-Smith O, Wells C, Henry-Stanley M, Dunny G. Acceleration of Enterococcus faecalis biofilm formation by aggregation substance expression in an ex vivo model of cardiac valve colonization. PLoS One. 2010;5:e15798. doi: 10.1371/journal.pone.0015798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. Properties of Enterococcus faecalis plasmid pAD1, a member of a widely disseminated family of pheromone-responding, conjugative, virulence elements encoding cytolysin. Plasmid. 2007;58:205–227. doi: 10.1016/j.plasmid.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Clewell DB, An FY, White BA, Gawron-Burke C. Streptococcus faecalis sex pheromone (cAM373) also produced by Staphylococcus aureus and identification of a conjugative transposon (Tn918) J Bacteriol. 1985;162:1212–1220. doi: 10.1128/jb.162.3.1212-1220.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook LC, LaSarre B, Federle MJ. Interspecies Communication among Commensal and Pathogenic Streptococci. mBio. 2013;4:e00382–00313. doi: 10.1128/mBio.00382-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Core L, Perego M. TPR-mediated interaction of RapC with ComA inhibits response regulator-DNA binding for competence development in Bacillus subtilis. Mol Microbiol. 2003;49:1509–1522. doi: 10.1046/j.1365-2958.2003.03659.x. [DOI] [PubMed] [Google Scholar]
- Datta V, Myskowski SM, Kwinn LA, Chiem DN, Varki N, Kansal RG, Kotb M, Nizet V. Mutational analysis of the group A streptococcal operon encoding streptolysin S and its virulence role in invasive infection. Mol Microbiol. 2005;56:681–695. doi: 10.1111/j.1365-2958.2005.04583.x. [DOI] [PubMed] [Google Scholar]
- Davies D, Parsek M, Pearson J, Iglewski B, Costerton J, Greenberg E. The invovlement of cell-to-cell signals in the development of a bacterial biofilm. Science. 1998;280:295–297. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- Dawid S, Roche AM, Weiser JN. The blp bacteriocins of Streptococcus pneumoniae mediate intraspecies competition both in vitro and in vivo. Infect Immun. 2007;75:443–451. doi: 10.1128/IAI.01775-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Boever EH, Clewell DB, Fraser CM. Enterococcus faecalis conjugative plasmid pAM373: complete nucleotide sequence and genetic analyses of sex pheromone response. Mol Microbiol. 2000;37:1327–1341. doi: 10.1046/j.1365-2958.2000.02072.x. England. [DOI] [PubMed] [Google Scholar]
- de Saizieu A, Gardes C, Flint N, Wagner C, Kamber M, Mitchell TJ, Keck W, Amrein KE, Lange R. Microarray-based identification of a novel Streptococcus pneumoniae regulon controlled by an autoinduced peptide. J Bacteriol. 2000;182:4696–4703. doi: 10.1128/jb.182.17.4696-4703.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Declerck N, Bouillaut L, Chaix D, Rugani N, Slamti L, Hoh F, Lereclus D, Arold ST. Structure of PlcR: Insights into virulence regulation and evolution of quorum sensing in Gram-positive bacteria. Proc Natl Acad Sci U S A. 2007;104:18490–18495. doi: 10.1073/pnas.0704501104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Papa MF, Perego M. Enterococcus faecalis Virulence Regulator FsrA Binding to Target Promoters. J Bacteriol. 2011;193:1527–1532. doi: 10.1128/JB.01522-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desouky SE, Nishiguchi K, Zendo T, Igarashi Y, Williams P, Sonomoto K, Nakayama J. High-Throughput Screening of Inhibitors Targeting Agr/Fsr Quorum Sensing in Staphylococcus aureus and Enterococcus faecalis. Biosci Biotechnol Biochem. 2013;77:923–927. doi: 10.1271/bbb.120769. [DOI] [PubMed] [Google Scholar]
- Donlan RM. Biofilms and device-associated infections. Emerg Infect Dis. 2001;7:277–281. doi: 10.3201/eid0702.010226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour D, Levesque CM. Cell death of Streptococcus mutans induced by a quorum-sensing peptide occurs via a conserved streptococcal autolysin. J Bacteriol. 2013;195:105–114. doi: 10.1128/JB.00926-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumoulin R, Cortes-Perez N, Gaubert S, Duhutrel P, Brinster S, Torelli R, Sanguinetti M, Posteraro B, Repoila F, Serror P. The enterococcal Rgg-like regulator ElrR activates expression of the elrA operon. J Bacteriol. 2013 doi: 10.1128/JB.00121-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunny G, Brown B, Clewell D. Induced cell aggregation and mating in Streptococcus faecalis: evidence for a bacterial sex pheromone. Proceedings of the National Academy of Sciences. 1978;75:3479–3483. doi: 10.1073/pnas.75.7.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunny GM, Antiporta MH, Hirt H. Peptide pheromone-induced transfer of plasmid pCF10 in Enterococcus faecalis: probing the genetic and molecular basis for specificity of the pheromone response. Peptides. 2001;22:1529–1539. doi: 10.1016/s0196-9781(01)00489-2. [DOI] [PubMed] [Google Scholar]
- Eran Y, Getter Y, Baruch M, Belotserkovsky I, Padalon G, Mishalian I, Podbielski A, Kreikemeyer B, Hanski E. Transcriptional regulation of the sil locus by the SilCR signalling peptide and its implications on group A streptococcus virulence. Mol Microbiol. 2007;63:1209–1222. doi: 10.1111/j.1365-2958.2007.05581.x. England. [DOI] [PubMed] [Google Scholar]
- Federle M. Pathogenic streptococci speak, but what are they saying? Virulence. 2012;3:92–94. doi: 10.4161/viru.3.1.18652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federle MJ, Morrison DA. One if by land, two if by sea: signalling to the ranks with CSP and XIP. Mol Microbiol. 2012;86:241–245. doi: 10.1111/mmi.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleuchot B, Guillot A, Mezange C, Besset C, Chambellon E, Monnet V, Gardan R. Rgg-Associated SHP Signaling Peptides Mediate Cross-Talk in Streptococci. PLoS One. 2013;8:e66042. doi: 10.1371/journal.pone.0066042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleuchot B, Gitton C, Guillot A, et al. Rgg proteins associated with internalized small hydrophobic peptides: a new quorum-sensing mechanism in streptococci. Mol Microbiol. 2011;80:1102–1119. doi: 10.1111/j.1365-2958.2011.07633.x. [DOI] [PubMed] [Google Scholar]
- Fontaine L, Boutry C, Guedon E, Guillot A, Ibrahim M, Grossiord B, Hols P. Quorum-sensing regulation of the production of Blp bacteriocins in Streptococcus thermophilus. J Bacteriol. 2007;189:7195–7205. doi: 10.1128/JB.00966-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine L, Boutry C, de Frahan MH, Delplace B, Fremaux C, Horvath P, Boyaval P, Hols P. A novel pheromone quorum-sensing system controls the development of natural competence in Streptococcus thermophilus and Streptococcus salivarius. J Bacteriol. 2010;192:1444–1454. doi: 10.1128/JB.01251-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine L, Goffin P, Dubout H, Delplace B, Baulard A, Lecat-Guillet N, Chambellon E, Gardan R, Hols P. Mechanism of competence activation by the ComRS signalling system in streptococci. Mol Microbiol. 2013;87:1113–1132. doi: 10.1111/mmi.12157. [DOI] [PubMed] [Google Scholar]
- Frank K, Barnes A, Grindle S, Manias D, Schlievert P, Dunny G. Use of recombinase-based in vivo expression technology to characterize Enterococcus faecalis gene expression during infection identifies in vivo-expressed antisense RNAs and implicates the protease Eep in pathogenesis. Infect Immun. 2011;80:539–549. doi: 10.1128/IAI.05964-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank KL, Guiton PS, Barnes AM, Manias DA, Chuang-Smith ON, Kohler PL, Spaulding AR, Hultgren SJ, Schlievert PM, Dunny GM. AhrC and Eep are biofilm infection-associated virulence factors in Enterococcus faecalis. Infect Immun. 2013;81:1696–1708. doi: 10.1128/IAI.01210-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuqua C, Greenberg EP. Listening in on bacteria: acyl-homoserine lactone signalling. Nat Rev Mol Cell Biol. 2002;3:685–695. doi: 10.1038/nrm907. [DOI] [PubMed] [Google Scholar]
- Fuqua C, Parsek M, Greenberg E. Regulation of gene expression by cell-to-cell communication: acyl-homoserine lactone quorum sensing. Annu Rev Genet. 2001;35:439–468. doi: 10.1146/annurev.genet.35.102401.090913. [DOI] [PubMed] [Google Scholar]
- Gonzalez JE, Keshavan ND. Messing with bacterial quorum sensing. Microbiol Mol Biol Rev. 2006;70:859–875. doi: 10.1128/MMBR.00002-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiral S, Mitchell T, Martin B, Claverys J-P. Competence-programmed predation of noncompetent cells in the human pathogen Streptococcus pneumoniae: Genetic requirements. Proc Natl Acad Sci. 2005;102:8710–8715. doi: 10.1073/pnas.0500879102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock L, Perego M. The Enterococcus faecalis fsr two-component system controls biofilm development through production of gelatinase. J Bacteriol. 2004;186:5629–5639. doi: 10.1128/JB.186.17.5629-5639.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havarstein L. Increasing competence in the genus Streptococcus. Mol Microbiol. 2010;78:541–544. doi: 10.1111/j.1365-2958.2010.07380.x. [DOI] [PubMed] [Google Scholar]
- Havarstein LS, Holo H, Nes IF. The leader peptide of colicin V shares consensus sequences with leader peptides that are common among peptide bacteriocins produced by gram-positive bacteria. Microbiology. 1994;140 ( Pt 9):2383–2389. doi: 10.1099/13500872-140-9-2383. [DOI] [PubMed] [Google Scholar]
- Havarstein LS, Diep DB, Nes IF. A family of bacteriocin ABC transporters carry out proteolytic processing of their substrates concomitant with export. Mol Microbiol. 1995;16:229–240. doi: 10.1111/j.1365-2958.1995.tb02295.x. [DOI] [PubMed] [Google Scholar]
- Havarstein LS, Gaustad P, Nes IF, Morrison DA. Identification of the streptococcal competence-pheromone receptor. Mol Microbiol. 1996;21:863–869. doi: 10.1046/j.1365-2958.1996.521416.x. [DOI] [PubMed] [Google Scholar]
- Hidalgo-Grass C, Ravins M, Dan-Goor M, Jaffe J, Moses AE, Hanski E. A locus of group A streptococcus involved in invasive disease and DNA transfer. Molecular Microbiology. 2002;46:87–99. doi: 10.1046/j.1365-2958.2002.03127.x. [DOI] [PubMed] [Google Scholar]
- Hidalgo-Grass C, Mishalian I, Dan-Goor M, Belotserkovsky I, Eran Y, Nizet V, Peled A, Hanski E. A streptococcal protease that degrades CXC chemokines and impairs bacterial clearance from infected tissues. Embo j. 2006;25:4628–4637. doi: 10.1038/sj.emboj.7601327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo-Grass C, Dan-Goor M, Maly A, et al. Effect of a bacterial pheromone peptide on host chemokine degradation in group A streptococcal necrotising soft-tissue infections. Lancet. 2004:363. doi: 10.1016/S0140-6736(04)15643-2. [DOI] [PubMed] [Google Scholar]
- Hirt H, Erlandsen SL, Dunny GM. Heterologous inducible expression of Enterococcus faecalis pCF10 aggregation substance asc10 in Lactococcus lactis and Streptococcus gordonii contributes to cell hydrophobicity and adhesion to fibrin. J Bacteriol. 2000;182:2299–2306. doi: 10.1128/jb.182.8.2299-2306.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt H, Schlievert P, Dunny G. In vivo induction of virulence and antibiotic resistance transfer in Enterococcus faecalis mediated by the sex pheromone-sensing system of pCF10. Infection and Immunity. 2002;70:716–723. doi: 10.1128/iai.70.2.716-723.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MS, Biswas I. An Extracelluar Protease, SepM, Generates Functional Competence-Stimulating Peptide in Streptococcus mutans UA159. J Bacteriol. 2012;194:5886–5896. doi: 10.1128/JB.01381-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui FM, Morrison DA. Genetic transformation in Streptococcus pneumoniae: nucleotide sequence analysis shows comA, a gene required for competence induction, to be a member of the bacterial ATP-dependent transport protein family. J Bacteriol. 1991;173:372–381. doi: 10.1128/jb.173.1.372-381.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Håvarstein L, Coomaraswamy G, Morrison D. An unmodified heptadecapeptide pheromone induces competence for genetic transformation in Streptococcus pneumoniae. Proc Natl Acad Sci. 1995 doi: 10.1073/pnas.92.24.11140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannelli F, Oggioni MR, Pozzi G. Sensor domain of histidine kinase ComD confers competence pherotype specificity in Streptoccoccus pneumoniae. FEMS Microbiol Lett. 2005;252:321–326. doi: 10.1016/j.femsle.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Ibrahim M, Nicolas P, Bessieres P, Bolotin A, Monnet V, Gardan R. A genome-wide survey of short coding sequences in streptococci. Microbiology. 2007a;153:3631–3644. doi: 10.1099/mic.0.2007/006205-0. [DOI] [PubMed] [Google Scholar]
- Ibrahim M, Guillot A, Wessner F, Algaron F, Besset C, Courtin P, Gardan R, Monnet V. Control of the Transcription of Short Gene Encoding a Cyclic Peptide in Streptococcus thermophilus: a New Quorum-Sensing System? J Bacteriol. 2007b;189:8844–8854. doi: 10.1128/JB.01057-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii S, Yano T, Hayashi H. Expression and characterization of the peptidase domain of Streptococcus pneumoniae ComA, a bifunctional ATP-binding cassette transporter involved in quorum sensing pathway. J Biol Chem. 2006;281:4726–4731. doi: 10.1074/jbc.M512516200. [DOI] [PubMed] [Google Scholar]
- Johnson C, Haeming H, Chatterjee A, Weaver K, Hu W-S, Dunny G. RNA-mediated reciprocal regulation between two bacterial operons is RNase III dependent. mBio. 2011;2:00189–00111. doi: 10.1128/mBio.00189-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C, Manias D, Haemig H, Shokeen S, Weaver K, Henkin T, Dunny G. Direct evidence for control of the pheromone-inducible prgQ operon of Enterococcus faecalis plasmid pCF10 by a counter transcript-driven attenuation mechanism. Journal of Bacteriology. 2010;192:1634–1642. doi: 10.1128/JB.01525-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley LA, Sternberg MJ. Protein structure prediction on the Web: a case study using the Phyre server. Nat Protoc. 2009;4:363–371. doi: 10.1038/nprot.2009.2. [DOI] [PubMed] [Google Scholar]
- Khan R, Rukke HV, Ricomini Filho AP, Fimland G, Arntzen MO, Thiede B, Petersen FC. Extracellular identification of a processed type II ComR/ComS pheromone of Streptococcus mutans. J Bacteriol. 2012;194:3781–3788. doi: 10.1128/JB.00624-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalko J, Sebert M. The Streptococcus pneumoniae Competence Regulatory System Influences Respiratory Tract Colonization. 2008 doi: 10.1128/IAI.01696-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaSarre B, Federle MJ. Exploiting quorum sensing to confuse bacterial pathogens. Microbiol Mol Biol Rev. 2013;77:73–111. doi: 10.1128/MMBR.00046-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaSarre B, Aggarwal C, Federle MJ. Antagonistic Rgg Regulators Mediate Quorum Sensing via Competitive DNA Binding in Streptococcus pyogenes. mBio. 2012:3. doi: 10.1128/mBio.00333-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasarre B, Chang JC, Federle MJ. Redundant GAS Signaling Peptides Exhibit Unique Activation Potentials. J Bacteriol. 2013;195:4310–4318. doi: 10.1128/JB.00684-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MS, Morrison DA. Identification of a new regulator in Streptococcus pneumoniae linking quorum sensing to competence for genetic transformation. J Bacteriol. 1999;181:5004–5016. doi: 10.1128/jb.181.16.5004-5016.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemme A, Grobe L, Reck M, Tomasch J, Wagner-Dobler I. Subpopulation specific transcriptome analysis of CSP induced Streptococcus mutans. Journal of Bacteriology. 2011;8:1863–1877. doi: 10.1128/JB.01363-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard B, Podbielski A, Hedberg P, Dunny G. Enterococcus faecalis pheromone binding protein, PrgZ, recruits a chromosomal oligopeptide permease system to import sex pheromone cCF10 for induction of conjugation. Proc Natl Acad Sci. 1996;93:260–264. doi: 10.1073/pnas.93.1.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Lau P, Lee J, Ellen R, Cvitkovitch D. Natural genetic transformation of Streptococcus mutans growing in biofilms. Journal of Bacteriology. 2001;183:897–908. doi: 10.1128/JB.183.3.897-908.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo P, Morrison DA. Transient Association of an Alternative Sigma Factor, ComX, with RNA Polymerase during the Period of Competence for Genetic Transformation in Streptococcus pneumoniae. J Bacteriol. 2003;185:349–358. doi: 10.1128/JB.185.1.349-358.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo P, Li H, Morrison DA. Identification of ComW as a new component in the regulation of genetic transformation in Streptococcus pneumoniae. Mol Microbiol. 2004;54:172–183. doi: 10.1111/j.1365-2958.2004.04254.x. [DOI] [PubMed] [Google Scholar]
- Lux T, Nuhn M, Hakenbeck R, Reichmann P. Diversity of bacteriocins and activity spectrum in Streptococcus pneumoniae. J Bacteriol. 2007;189:7741–7751. doi: 10.1128/JB.00474-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon GJ, Novick RP. Peptide signaling in Staphylococcus aureus and other Gram-positive bacteria. Peptides. 2004;25:1389–1403. doi: 10.1016/j.peptides.2003.11.026. [DOI] [PubMed] [Google Scholar]
- Lyon GJ, Wright JS, Muir TW, Novick RP. Key determinants of receptor activation in the agr autoinducing peptides of Staphylococcus aureus. Biochemistry. 2002;41:10095–10104. doi: 10.1021/bi026049u. [DOI] [PubMed] [Google Scholar]
- Lyon WR, Gibson CM, Caparon MG. A role for trigger factor and an rgg-like regulator in the transcription, secretion and processing of the cysteine proteinase of Streptococcus pyogenes. Embo j. 1998;17:6263–6275. doi: 10.1093/emboj/17.21.6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B, Quentin Y, Fichant G, Claverys JP. Independent evolution of competence regulatory cascades in streptococci? Trends Microbiol. 2006;14:339–345. doi: 10.1016/j.tim.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Mashburn-Warren L, Morrison DA, Federle MJ. A novel double-tryptophan peptide pheromone is conserved in mutans and pyogenic Streptococci and Controls Competence in Streptococcus mutans via an Rgg regulator. Mol Microbiol. 2010;78:589–606. doi: 10.1111/j.1365-2958.2010.07361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashburn-Warren L, Morrison DA, Federle MJ. The Cryptic Competence Pathway in Streptococcus pyogenes Is Controlled by a Peptide Pheromone. J Bacteriol. 2012;194:4589–4600. doi: 10.1128/JB.00830-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo-Cristino J, Resina C, Manuel V, Lito L, Ramirez M. First case of infection with vancomycin-resistant Staphylococcus aureus in Europe. The Lancet. 2013;382:205. doi: 10.1016/S0140-6736(13)61219-2. [DOI] [PubMed] [Google Scholar]
- Michael-Gayego A, Dan-Goor M, Jaffe J, Hidalgo-Grass C, Moses AE. Characterization of the sil in invasive groups A and G streptococci; antibodies against bacterial pheromone peptide SilCR result in severe infection. Infect Immun. 2013 doi: 10.1128/IAI.00359-13. epub ahead. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirouze N, Berge MA, Soulet AL, et al. Direct involvement of DprA, the transformation-dedicated RecA loader, in the shut-off of pneumococcal competence. Proc Natl Acad Sci U S A. 2013;110:E1035–1044. doi: 10.1073/pnas.1219868110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montanez G. Thesis. Georgia State University; Atlanta, GA: 2005. The Role of Sia and Siu ABC-Type Transporters in Iron Utilization and Virulence in Streptococcus pyogenes. [Google Scholar]
- Mori M, Isogai A, Sakagami Y, Fujino M, Kitada C, Clewell DB, Suzuki A. Isolation and structure of Streptococcus faecalis sex pheromone inhibitor, iAD1, that is excreted by donor strains harboring plasmid pAD1. Agric Biol Chem. 1986;50:539–541. [Google Scholar]
- Mori M, Sakagami Y, Ishii Y, Isogai A, Kitada C, Fujino M, Adsit JC, Dunny GM, Suzuki A. Structure of ccf10, a Peptide Sex-Pheromone Which Induces Conjugative Transfer of the Streptococcus faecalis Tetracycline Resistance Plasmid, pCF10. Journal of Biological Chemistry. 1988;263:14574–14578. [PubMed] [Google Scholar]
- Mori M, Tanaka H, Sakagami Y, Isogai A, Fujino M, Kitada C, White BA, An FY, Clewell DB, Suzuki A. Isolation and structure of the Streptococcus faecalis sex pheromone, cAM373. FEBS Lett. 1986;206:69–72. doi: 10.1016/0014-5793(86)81342-4. Netherlands. [DOI] [PubMed] [Google Scholar]
- Morrison DA, Guedon E, Renault P. Competence for Natural Genetic Transformation in the Streptococcus bovis Group Streptococci S. infantarius and S. macedonicus. J Bacteriol. 2013;195:2612–2620. doi: 10.1128/JB.00230-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylonakis E, Engelbert M, Qin X, Sifri CD, Murray BE, Ausubel FM, Gilmore MS, Calderwood SB. The Enterococcus faecalis fsrB gene, a key component of the fsr quorum-sensing system, is associated with virulence in the rabbit endophthalmitis model. Infect Immun. 2002;70:4678–4681. doi: 10.1128/IAI.70.8.4678-4681.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama J, Ruhfel R, Dunny G, Isogai A, Suzuki A. The prgQ gene of Enterococcus faecalis tetracycline resistance plasmid pCF10 encodes a peptide inhibitor, iCF10. Journal of Bacteriology. 1994;176:4. doi: 10.1128/jb.176.23.7405-7408.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama J, Yoshida K, Kobayashi H, Isogai A, Clewell DB, Suzuki A. Cloning and characterization of a region of Enterococcus faecalis plasmid pPD1 encoding pheromone inhibitor (ipd), pheromone sensitivity (traC), and pheromone shutdown (traB) genes. J Bacteriol. 1995;177:5567–5573. doi: 10.1128/jb.177.19.5567-5573.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama J, Uemura Y, Nishiguchi K, Yoshimura N, Igarashi Y, Sonomoto K. Ambuic acid inhibits the biosynthesis of cyclic peptide quormones in gram-positive bacteria. Antimicrob Agents Chemother. 2009;53:580–586. doi: 10.1128/AAC.00995-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama J, Cao Y, Horii T, Sakuda S, Akkermans AD, de Vos WM, Nagasawa H. Gelatinase biosynthesis-activating pheromone: a peptide lactone that mediates a quorum sensing in Enterococcus faecalis. Mol Microbiol. 2001;41:145–154. doi: 10.1046/j.1365-2958.2001.02486.x. England. [DOI] [PubMed] [Google Scholar]
- Nakayama J, Chen S, Oyama N, Nishiguchi K, Azab EA, Tanaka E, Kariyama R, Sonomoto K. Revised model for Enterococcus faecalis fsr quorum-sensing system: the small open reading frame fsrD encodes the gelatinase biosynthesis-activating pheromone propeptide corresponding to staphylococcal agrd. J Bacteriol. 2006;188:8321–8326. doi: 10.1128/JB.00865-06. United States. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama J, Tanaka E, Kariyama R, Nagata K, Nishiguchi K, Mitsuhata R, Uemura Y, Tanokura M, Kumon H, Sonomoto K. Siamycin attenuates fsr quorum sensing mediated by a gelatinase biosynthesis-activating pheromone in Enterococcus faecalis. J Bacteriol. 2007;189:1358–1365. doi: 10.1128/JB.00969-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nallapareddy S, Singh K, Sillanpaa J, Garsin D, Hook M, Erlandsen S, Murray B. Endocarditis and biofilm-associated pili of Enterococcus faecalis. J Clin Invest. 2006;116:2799–2807. doi: 10.1172/JCI29021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely MN, Lyon WR, Runft DL, Caparon M. Role of RopB in growth phase expression of the SpeB cysteine protease of Streptococcus pyogenes. J Bacteriol. 2003;185:5166–5174. doi: 10.1128/JB.185.17.5166-5174.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng W-L, Bassler BL. Bacterial Quorum-Sensing Network Architectures. Annual Review of Genetics. 2009;43:197–222. doi: 10.1146/annurev-genet-102108-134304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick RP, Geisinger E. Quorum sensing in staphylococci. Annu Rev Genet. 2008;42:541–564. doi: 10.1146/annurev.genet.42.110807.091640. [DOI] [PubMed] [Google Scholar]
- Pakula R, Walczak W. On the nature of competence of transformable streptococci. J Gen Microbiol. 1963;31:125–133. doi: 10.1099/00221287-31-1-125. [DOI] [PubMed] [Google Scholar]
- Parashar V, Konkol MA, Kearns DB, Neiditch MB. A plasmid-encoded phosphatase regulates Bacillus subtilis biofilm architecture, sporulation, and genetic competence. J Bacteriol. 2013;195:2437–2448. doi: 10.1128/JB.02030-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsek MR, Greenberg EP. Sociomicrobiology: the connections between quorum sensing and biofilms. Trends Microbiol. 2005;13:27–33. doi: 10.1016/j.tim.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Perego M, Hoch J. Cell-cell communication regulates the effects of protein aspartate phosphatases on the phosphorelay controlling development in Bacillus subtilis. PNAS. 1996;93:1549–1553. doi: 10.1073/pnas.93.4.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JA, Cvitkovitch DG, Levesque CM. Cell death in Streptococcus mutans biofilms: a link between CSP and extracellular DNA. FEMS Microbiol Lett. 2009;299:261–266. doi: 10.1111/j.1574-6968.2009.01758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestova EV, Havarstein LS, Morrison DA. Regulation of competence for genetic transformation in Streptococcus pneumoniae by an auto-induced peptide pheromone and a two-component regulatory system. Mol Microbiol. 1996;21:853–862. doi: 10.1046/j.1365-2958.1996.501417.x. [DOI] [PubMed] [Google Scholar]
- Petersen FC, Fimland G, Scheie AA. Purification and functional studies of a potent modified quorum-sensing peptide and a two-peptide bacteriocin in Streptococcus mutans. Mol Microbiol. 2006;61:1322–1334. doi: 10.1111/j.1365-2958.2006.05312.x. [DOI] [PubMed] [Google Scholar]
- Peterson S, Cline RT, Tettelin H, Sharov V, Morrison DA. Gene expression analysis of the Streptococcus pneumoniae competence regulons by use of DNA microarrays. J Bacteriol. 2000;182:6192–6202. doi: 10.1128/jb.182.21.6192-6202.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podbielski A, Pohl B, Woischnik M, Korner C, Schmidt KH, Rozdzinski E, Leonard BA. Molecular characterization of group A streptococcal (GAS) oligopeptide permease (opp) and its effect on cysteine protease production. Mol Microbiol. 1996;21:1087–1099. doi: 10.1046/j.1365-2958.1996.661421.x. [DOI] [PubMed] [Google Scholar]
- Pozzi G, Masala L, Iannelli F, Manganelli R, Havarstein LS, Piccoli L, Simon D, Morrison DA. Competence for genetic transformation in encapsulated strains of Streptococcus pneumoniae: two allelic variants of the peptide pheromone. J Bacteriol. 1996;178:6087–6090. doi: 10.1128/jb.178.20.6087-6090.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi F, Chen P, Caufield PW. Functional analyses of the promoters in the lantibiotic mutacin II biosynthetic locus in Streptococcus mutans. Appl Environ Microbiol. 1999;65:652–658. doi: 10.1128/aem.65.2.652-658.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X, Singh KV, Weinstock GM, Murray BE. Effects of Enterococcus faecalis fsr genes on production of gelatinase and a serine protease and virulence. Infect Immun. 2000;68:2579–2586. doi: 10.1128/iai.68.5.2579-2586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu R, Pei W, Zhang L, Lin J, Ji G. Identification of the putative staphylococcal AgrB catalytic residues involving the proteolytic cleavage of AgrD to generate autoinducing peptide. J Biol Chem. 2005;280:16695–16704. doi: 10.1074/jbc.M411372200. [DOI] [PubMed] [Google Scholar]
- Rickard AH, Palmer RJ, Jr, Blehert DS, Campagna SR, Semmelhack MF, Egland PG, Bassler BL, Kolenbrander PE. Autoinducer 2: a concentration-dependent signal for mutualistic bacterial biofilm growth. Mol Microbiol. 2006;60:1446–1456. doi: 10.1111/j.1365-2958.2006.05202.x. [DOI] [PubMed] [Google Scholar]
- Rocha-Estrada J, Aceves-Diez A, Guarneros G, de la Torre M. The RNPP family of quorum-sensing proteins in Gram-positive bacteria. Applied Microbiology and Biotechnology. 2010;87:913–923. doi: 10.1007/s00253-010-2651-y. [DOI] [PubMed] [Google Scholar]
- Rutherford ST, Bassler BL. Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harb Perspect Med. 2012:2. doi: 10.1101/cshperspect.a012427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenz HL, Augsburger V, Vuong C, Jack RW, Gotz F, Otto M. Inducible expression and cellular location of AgrB, a protein involved in the maturation of the staphylococcal quorum-sensing pheromone. Arch Microbiol. 2000;174:452–455. doi: 10.1007/s002030000223. [DOI] [PubMed] [Google Scholar]
- Salim KY, de Azavedo JC, Bast DJ, Cvitkovitch DG. Regulation of sagA, siaA and scpC by SilCR, a putative signaling peptide of Streptococcus pyogenes. FEMS Microbiol Lett. 2008;289:119–125. doi: 10.1111/j.1574-6968.2008.01375.x. [DOI] [PubMed] [Google Scholar]
- Samen UM, Eikmanns BJ, Reinscheid DJ. The transcriptional regulator RovS controls the attachment of Streptococcus agalactiae to human epithelial cells and the expression of virulence genes. Infect Immun. 2006;74:5625–5635. doi: 10.1128/IAI.00667-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders JW, Leenhouts K, Burghoorn J, Brands JR, Venema G, Kok J. A chloride-inducible acid resistance mechanism in Lactococcus lactis and its regulation. Mol Microbiol. 1998;27:299–310. doi: 10.1046/j.1365-2958.1998.00676.x. [DOI] [PubMed] [Google Scholar]
- Schauder S, Shokat K, Surette MG, Bassler BL. The LuxS family of bacterial autoinducers: biosynthesis of a novel quorum-sensing signal molecule. Mol Microbiol. 2001;41:463–476. doi: 10.1046/j.1365-2958.2001.02532.x. [DOI] [PubMed] [Google Scholar]
- Shelburne SA, 3rd, Olsen RJ, Makthal N, Brown NG, Sahasrabhojane P, Watkins EM, Palzkill T, Musser JM, Kumaraswami M. An amino-terminal signal peptide of Vfr protein negatively influences RopB-dependent SpeB expression and attenuates virulence in Streptococcus pyogenes. Mol Microbiol. 2011;82:1481–1495. doi: 10.1111/j.1365-2958.2011.07902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi K, Brown C, Gu Z, Kozlowicz B, Dunny G, Ohlendorf D, Earhart C. Structure of peptide sex pheromone receptor PrgX and PrgX/pheromone complexes and regulation of conjugation in Enterococcus faecalis. Proceedings of the National Academy of Sciences. 2005;102:18596–18601. doi: 10.1073/pnas.0506163102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shokeen S, Johnson C, Greenfield T, Manias D, Dunny G, Weaver K. Structural analysis of the Anti-Q-Qs interaction: RNA-mediated regulation of E. faecalis plasmid pCF10 conjugation. Plasmid. 2010;64:26–35. doi: 10.1016/j.plasmid.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievert DM, Rudrik JT, Patel JB, McDonald LC, Wilkins MJ, Hageman JC. Vancomycin-resistant Staphylococcus aureus in the United States, 2002–2006. Clin Infect Dis. 2008;46:668–674. doi: 10.1086/527392. [DOI] [PubMed] [Google Scholar]
- Sifri CD, Mylonakis E, Singh KV, Qin X, Garsin DA, Murray BE, Ausubel FM, Calderwood SB. Virulence effect of Enterococcus faecalis protease genes and the quorum-sensing locus fsr in Caenorhabditis elegans and mice. Infect Immun. 2002;70:5647–5650. doi: 10.1128/IAI.70.10.5647-5650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son M, Ahn SJ, Guo Q, Burne RA, Hagen SJ. Microfluidic study of competence regulation in Streptococcus mutans: environmental inputs modulate bimodal and unimodal expression of comX. Mol Microbiol. 2012;86:258–272. doi: 10.1111/j.1365-2958.2012.08187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner E, Scott J, Minton NP, Winzer K. An agr quorum sensing system that regulates granulose formation and sporulation in Clostridium acetobutylicum. Appl Environ Microbiol. 2012;78:1113–1122. doi: 10.1128/AEM.06376-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturme MH, Kleerebezem M, Nakayama J, Akkermans AD, Vaugha EE, de Vos WM. Cell to cell communication by autoinducing peptides in gram-positive bacteria. Antonie Van Leeuwenhoek. 2002;81:233–243. doi: 10.1023/a:1020522919555. [DOI] [PubMed] [Google Scholar]
- Sturme MH, Nakayama J, Molenaar D, Murakami Y, Kunugi R, Fujii T, Vaughan EE, Kleerebezem M, de Vos WM. An agr-like two-component regulatory system in Lactobacillus plantarum is involved in production of a novel cyclic peptide and regulation of adherence. J Bacteriol. 2005;187:5224–5235. doi: 10.1128/JB.187.15.5224-5235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramoni S, Sokol PA. Quorum sensing systems influence Burkholderia cenocepacia virulence. Future Microbiol. 2012;7:1373–1387. doi: 10.2217/fmb.12.118. [DOI] [PubMed] [Google Scholar]
- Sulavik MC, Clewell DB. Rgg is a positive transcriptional regulator of the Streptococcus gordonii gtfG gene. J Bacteriol. 1996;178:5826–5830. doi: 10.1128/jb.178.19.5826-5830.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulavik MC, Tardif G, Clewell DB. Identification of a gene, rgg, which regulates expression of glucosyltransferase and influences the Spp phenotype of Streptococcus gordonii Challis. J Bacteriol. 1992;174:3577–3586. doi: 10.1128/jb.174.11.3577-3586.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumby P, Zhang S, Whitney AR, Falugi F, Grandi G, Graviss EA, Deleo FR, Musser JM. A chemokine-degrading extracellular protease made by group A Streptococcus alters pathogenesis by enhancing evasion of the innate immune response. Infect Immun. 2008;76:978–985. doi: 10.1128/IAI.01354-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Mori M, Sakagami Y, Isogai A, Fujino M, Kitada C, Craig RA, Clewell DB. Isolation and structure of bacterial sex pheromone, cPD1. Science. 1984;226:849–850. doi: 10.1126/science.6436978. [DOI] [PubMed] [Google Scholar]
- Thoendel M, Kavanaugh JS, Flack CE, Horswill AR. Peptide signaling in the staphylococci. Chem Rev. 2011;111:117–151. doi: 10.1021/cr100370n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Throup JP, Koretke KK, Bryant AP, et al. A genomic analysis of two-component signal transduction in Streptococcus pneumoniae. Mol Microbiol. 2000;35:566–576. doi: 10.1046/j.1365-2958.2000.01725.x. [DOI] [PubMed] [Google Scholar]
- Thurlow LR, Thomas VC, Narayanan S, Olson S, Fleming SD, Hancock LE. Gelatinase contributes to the pathogenesis of endocarditis caused by Enterococcus faecalis. Infect Immun. 2010;78:4936–4943. doi: 10.1128/IAI.01118-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A, Hotchkiss RD. REGULATION OF THE TRANSFORMABILITY OF PHEUMOCOCCAL CULTURES BY MACROMOLECULAR CELL PRODUCTS. Proc Natl Acad Sci U S A. 1964;51:480–487. doi: 10.1073/pnas.51.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A, Mosser JL. On the nature of the pneumococcal activator substance. Proc Natl Acad Sci U S A. 1966;55:58–66. doi: 10.1073/pnas.55.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda A, Attila C, Witeley M, Wood T. Uracil influences quorum sensing and biofilm formation in Pseudomonas aeruginosa and fluorouracil is an antagonist. Microbial Biotechnology. 2009;2:62–74. doi: 10.1111/j.1751-7915.2008.00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ploeg J. Regulation of Bacteriocin Production in Streptococcus mutans by the Quorum-Sensing System Required for Development of Genetic Competence. J Bacteriol. 2005;187:3980–3989. doi: 10.1128/JB.187.12.3980-3989.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickerman MM, Flannagan SE, Jesionowski AM, Brossard KA, Clewell DB, Sedgley CM. A genetic determinant in Streptococcus gordonii Challis encodes a peptide with activity similar to that of enterococcal sex pheromone cAM373, which facilitates intergeneric DNA transfer. J Bacteriol. 2010;192:2535–2545. doi: 10.1128/JB.01689-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986;14:4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters CM, Bassler BL. Quorum sensing: Cell-to-cell communication in bacteria. Annual Review of Cell and Developmental Biology. 2005;21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- Waters CM, Lu W, Rabinowitz JD, Bassler BL. Quorum sensing controls biofilm formation in Vibrio cholerae through modulation of cyclic di-GMP levels and repression of vpsT. J Bacteriol. 2008;190:2527–2536. doi: 10.1128/JB.01756-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ween O, Gaustad P, Havarstein L. Identification of DNA binding sites for ComE, a key regulator of natural competence in Streptococcus pneumoniae. Mol Microbiol. 1999;33:817–827. doi: 10.1046/j.1365-2958.1999.01528.x. [DOI] [PubMed] [Google Scholar]
- Weigel L, Donlan R, Shin D, et al. High-level vancomycin-resistant Staphylococcus aureus isolates associated with a polymicrobial biofilm. Antimicrobial Agents and Chemotherapy. 2007;51:231–238. doi: 10.1128/AAC.00576-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whatmore AM, Barcus VA, Dowson CG. Genetic diversity of the streptococcal competence (com) gene locus. J Bacteriol. 1999;181:3144–3154. doi: 10.1128/jb.181.10.3144-3154.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winzer K, Williams P. Quorum sensing and the regulation of virulence gene expression in pathogenic bacteria. Int J Med Microbiol. 2001;291:131–143. doi: 10.1078/1438-4221-00110. [DOI] [PubMed] [Google Scholar]
- Wirth R. The sex pheromone system of Enterococcus faecalis. More than just a plasmid-collection mechanism? Eur J Biochem. 1994;222:235–246. doi: 10.1111/j.1432-1033.1994.tb18862.x. [DOI] [PubMed] [Google Scholar]
- Zhang Y. I-TASSER server for protein 3D structure prediction. BMC Bioinformatics. 2008;9:40. doi: 10.1186/1471-2105-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Miller MB, Vance RE, Dziejman M, Bassler BL, Mekalanos JJ. Quorum-sensing regulators control virulence gene expression in Vibrio cholerae. Science Signaling. 2002;99:3129. doi: 10.1073/pnas.052694299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Lau G. Inhibition of Competence Development, Horizontal Gene Transfer and Virulence in Streptococcus pneumoniae by a Modified Competence Stimulating Peptide. PLOS Pathogens. 2011:7. doi: 10.1371/journal.ppat.1002241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouhir S, Perchat S, Nicaise M, Perez J, Guimaraes B, Lereclus D, Nessler S. Peptide-binding dependent conformational changes regulate the transcriptional activity of the quorum-sensor NprR. Nucleic Acids Res. 2013 doi: 10.1093/nar/gkt546. epub ahead. [DOI] [PMC free article] [PubMed] [Google Scholar]