Abstract
Background
Very few studies have been performed to understand the underlying neural substrates of adolescent major depressive disorder (MDD). Studies in depressed adults have demonstrated that the subgenual anterior cingulate cortex (sgACC) plays a pivotal role in depression and have revealed aberrant patterns of resting-state functional connectivity (RSFC). Here, we examine the RSFC of the sgACC in medication-naïve first-episode adolescents with MDD.
Methods
Twenty-three adolescents with MDD and 36 well-matched control subjects underwent functional magnetic resonance imaging to assess the RSFC of the sgACC.
Results
We observed elevated connectivity between the sgACC and the insula and between the sgACC and the amygdala in the MDD group compared with the control subjects. Decreased connectivity between the sgACC and the precuneus was also found in the MDD group relative to the control subjects. Within the MDD group, higher levels of depression significantly correlated with decreased connectivity between the sgACC and left precuneus. Increased rumination was significantly associated with reduced connectivity between sgACC and the middle and inferior frontal gyri in the MDD group.
Conclusions
Our study is the first to examine sgACC connectivity in medication-naïve first-episode adolescents with MDD compared with well-matched control participants. Our results suggest aberrant functional connectivity among the brain networks responsible for salience attribution, executive control, and the resting-state in the MDD group compared with the control participants. Our findings raise the possibility that therapeutic interventions that can restore the functional connectivity among these networks to that typical of healthy adolescents might be a fruitful avenue for future research.
Keywords: Adolescent major depression, amygdala, default mode network, insula, resting-state, subgenual anterior cingulate
Adolescence is a crucial developmental period when the incidence of psychiatric illnesses, such as depression and other mood disorders, significantly increases (1). Epidemio-logical studies indicate that up to 8.3% of adolescents in the United States suffer from depression (2). Moreover, adolescent-onset depression is often recurrent and persists into adulthood, leading to elevated rates of divorce, loss of work, illness, and death (2). Vulnerability to the development of depression might be related to atypical maturational changes in the adolescent brain (3). Thus, understanding how departure from typical brain development patterns might influence the incidence of depression is particularly important, because it might ultimately improve our ability to prevent its emergence or lead to more effective treatments for those affected.
Despite the significance of this crucial developmental period, few studies have been performed to understand the underlying neurobiological substrates of adolescent depression. In contrast, much more work has been done in depressed adults. This work has led to the development of several models of adult depression. One such model hypothesizes that a network of cortical regions and associated limbic structures is differentially affected by the disorder (4,5). Within this network the subgenual region of the anterior cingulate cortex (sgACC) is thought to be pivotal to affective regulation and depression (6–14).
Another model, the triple network model (TNM), suggests that major neuropsychiatric disorders including depression might be explained in part by the relationship between three core intrinsic connectivity networks (ICN) of the brain that can be identified in resting-state functional magnetic resonance imaging scans (15). The ICNs are interdependent distributed networks of brain regions observed in the human brain at rest that show strong correspondence with task-related connectivity patterns (16). The three core ICNs are the default mode network (DMN), the salience network (SN), and the central executive network (CEN). The DMN is anchored in the posterior cingulate cortex and ventromedial prefrontal cortex (PFC) (17,18) and extends into the sgACC (15,16,19,20). In this network, the ventromedial PFC node is involved in self-referential processing, social cognitive processes, value-based decision making, and emotion regulation (21–24). The SN involves the cingulate-frontal operculum system and often the amygdala (25). It is implicated in detecting, integrating, and filtering relevant interoceptive, autonomic, and emotional information (25). Finally, the CEN encompasses dorsolateral prefrontal and lateral posterior parietal cortical regions and is thought to be critical to higher-level cognitive effort (26). A core hypothesis of the TNM is that neuropsychiatric disorders, such as depression, might be associated with aberrant functional connectivity (AFC) within and between these three core networks (15). Of note, although AFC has been reported in many neuropsychiatric disorders [see (15) for review], DMN “functional connectivity in depression is disproportionately driven by activation in the sgACC” (27).
Investigations of sgACC resting-state functional connectivity (RSFC) have been conducted in adults, adolescents, and children with major depressive disorder (MDD). Studies of adult depression have demonstrated elevated connectivity between the sgACC and dorsolateral PFC (28) that moderates with treatment (29) as well as increased connectivity between sgACC and dorsomedial PFC (30). In adolescents, reduced functional connectivity (FC) between the sgACC and insula as well as the inferior and superior PFC have been reported (31). More recently, increased FC between the sgACC and dorsomedial PFC has also been found in medicated depressed adolescents (32). Finally, in children with preschool-onset MDD, reduced FC between the sgACC and PFC regions has been documented (33). These differences in FC displayed by children (33), adolescents (32), and adults (28,30) might be related to developmental changes. For example, large scale changes in FC have been reported over the course of development from childhood to adulthood (34), with changes in the FC of the sgACC being related to maturation (35). Differences among these studies could also be attributed to medication status. In adults, it has been suggested that antidepressant medication can affect brain activation (11), with recent preliminary evidence indicating that medication might affect the FC of the sgACC (36). Finally, the study by Cullen et al.(31) is unique among those reviewed in that they permitted adolescents to listen to music of their choice while being scanned. Therefore it is possible that differences in the “emotional import” of the music selected by each participant might modulate the FC of the sgACC (31).
The RSFC and task-based studies of depression have identified functional changes that are associated with clinical measures of relevance in both adults and adolescents. In adults, sgACC FC was positively correlated with length of depressive episode (27). In adolescents, the strength of correlation between sgACC and dorsomedial PFC was positively correlated with depression severity (32). Finally, in a task-based study using psychophysiological interaction in adolescents with MDD, insula activity was associated with psychosocial function (37). These results suggest that clinical measures of depression and their relationship with FC should be investigated in depressed adolescents, given the role played by the insula and sgACC in depression (37–39).
Rumination is a prominent aspect of depression that might manifest in the resting-state (27). Recent studies have begun to elucidate the neural substrate of ruminative thought processes in adult depression. These studies have shown elevated sgACC activity in the DMN in depressed adults and that the degree of activation is modulated by the level of maladaptive rumination (40-42). The right anterior insula, a component of the SN, has also been associated with maladaptive rumination in depressed adults (40). Furthermore, the amygdala, another element of the SN, has been associated with rumination in depressed adults, with activation positively correlated with rumination (43,44). These observations are important, because the insula and the amygdala are thought to play key roles in depression (37-39). To date, however, no RSFC studies have investigated rumination in adolescents with MDD.
The aim of the present study was to examine the RSFC of the sgACC in a large sample of medication-naïve first-episode depressed adolescents compared with a group of well-matched healthy control subjects. Furthermore, we wished to examine the relationships between depression, rumination, and the FC of the sgACC. On the basis of the reviewed literature and the triple network model, we hypothesized that AFC within and between the core networks would be observed in the MDD group relative to the healthy control subjects. More specifically, we hypothesized that AFC in the resting state would manifest in the MDD group compared with control subjects in a network of brain regions involving the amygdala and insula. Finally, we hypothesized that these differences in FC in the depressed adolescents would be significantly correlated with clinical measures of depression and rumination.
Methods and Materials
Participants
The institutional review boards of University of California San Diego, University of California San Francisco, Rady Children's Hospital, and the county of San Diego all approved this study. Seventy-five participants were scanned for the present study: 45 healthy control subjects; and 30 with MDD. Participants gave written informed assent, and their parent/legal guardian provided written informed consent. Participants were financially compensated for their time.
Assessment
All healthy adolescents were administered the computerized Diagnostic Interview Schedule for Children version 4.0 (45) and the Diagnostic Predictive Scale (46), to determine the presence of any Axis I disorders.
The Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (47) was administered to all potentially depressed adolescents.
Depressive symptoms were scored by the Children's Depression Rating Scale-Revised (CDRS-R) (48) and Beck Depression Inventory-II (BDI-II) (49). Rumination was assessed by the Ruminative Responses Scale (RRS) of the Response Styles Questionnaire (50). Psychosocial functioning was assessed with the Children's Global Assessment Scale (CGAS) (51).
All participants were right-handed, and the groups were well-matched for IQ, socioeconomic status, age, gender, ethnicity, and pubertal stage. Additional questionnaires and inclusion/exclusion criteria are detailed in Supplement 1.
MR Data Acquisition and Preprocessing
Scans were acquired on a 3T GE MR750 System (GE Healthcare, Milwaukee, Wisconsin). One 8-min, 32-sec resting-state scan (256 volumes repetition time/echo time = 2 sec/30 msec, flip angle = 90°, 64 × 64 matrix, 3×3×3 mm voxels, 40 axial slices) was acquired. A high-resolution T1-weighted scan (repetition time/echo time = 8.1 msec/3.17 msec, flip angle = 12°, 256 × 256 matrix, 1×1×1 mm voxels, 168 sagittal slices) was acquired to permit functional localization.
Analyses were conducted with AFNI (52) and FSL software (53). Detailed methods are in Supplement 1. The T1-weighted images were skull stripped and transformed to MNI152 space (Montreal Neurological Institute, Montreal, Quebec, Canada) with an affine transform (54,55) followed by nonlinear refinement (56,57). Tissue components (gray matter, white matter, and cerebrospinal fluid) were segmented (58). Echo-planar images were motion corrected and aligned to the T1 images (59), convolved with a 4.2-mm full-width-at-half-maximal isotropic Gaussian filter, grand-mean scaled, and transformed to MNI152 space at 3 × 3 × 3 mm resolution. Bandpass filtering (.01−.1 Hz), censoring of outlier volumes and those with excessive motion, and removal of physiological noise (motion and average signal from white matter and cerebrospinal fluid) were accomplished in a single generalized least squares regression step, which required that no fewer than 177 time-points remained after censoring.
FC Analysis
Four subgenual seed locations, two in each hemisphere, were chosen on the basis of a prior exploration of anterior cingulate connectivity in the resting state (60). Seeds were 3 mm in radius, occupied 189 μL, and located at ±5, −34, −4 and ±5, −25, −10. For each seed, the Pearson correlation between the whole brain four-dimensional residuals and the average seed time-series was computed. Correlation coefficients were converted to Z scores with Fisher's r-to-z transform (61).
Group Analysis
Voxel-wise between-group analyses for each seed were accomplished with linear mixed effects models implemented in R software (62) where participant was treated as a random effect. Voxels were thresholded (F1,58 = 4.01, p = .05) and, to control for multiple comparisons, were required to be part of a cluster of at least 2000 μL. Bonferroni correction was used to correct for the number of seeds, and the corrected p was set to .05/4 = .0125. A Monte Carlo simulation was used to identify the volume threshold and, together with the voxel-wise threshold, resulted in a 5% probability of a significant cluster surviving due to chance across all four seeds.
Demographic and Clinical Scales Analysis
All statistical analyses were conducted with R software (62). Between-group differences were assessed by means of Welch T tests for age, Wechsler Abbreviated Scale of intelligence, BDI-II, and CDRS-R. Group differences in gender and number of participants/group were assessed with χ2 test of equal proportions. Effect sizes for these tests were computed with Hedge's g (63). The Wilcoxon rank-sum test determined group differences in the Hollingshead socioeconomic scale, CGAS, RRS, and Tanner Stage. Effect sizes for these tests were computed with the probability of superiority (PS), which ranges from 0 to 1 and represents the probability that a randomly selected control reports a greater value on the corresponding measure than a randomly selected MDD participant (64).
Correlational Analysis
Within the MDD group, the relationships between CGAS, CDRS-R, BDI-II, RRS, and the average Z score within each of the regions of interest identified by the between-group whole-brain analyses were examined with Spearman's rank correlation test.
Results
Demographic and Clinical Scales
As expected, given the matching criteria, the groups did not significantly differ in age, gender, socioeconomic status, Tanner pubertal stage, or IQ (all p > .05). Similarly, all of the depression scales (CDRS-R and BDI-II) showed that the MDD group endorsed significantly greater levels of depression than the control subjects (Hedge's g for CDRS-R = −4.99 and for BDI-II = −2.94). Additionally, on the CGAS, the MDD group demonstrated lower psychosocial function than the healthy adolescents (PS = .99). The MDD adolescents demonstrated greater rumination than the control subjects as measured by the RRS (PS = .06) (Table 1).
Table 1. Participant Characterization: Demographic Data and Clinical Rating Scales.
| Characteristic | Control | MDD | df | Statistic | Effect Size |
|---|---|---|---|---|---|
| Number of Participants Recruited (n) | 45 | 30 | |||
| Overall Recruitment Gender (M/F) | 17/28 | 11/19 | 1 | ≈0 | ≈0 |
| Rejected Due to Excessive Motion and Outlier Count (n)a | 9 | 7 | 1 | .0033 | ≈0 |
| Number of Participants Surviving Motion and Outlier Correction (n) | 36 | 23 | |||
| Gender (M/F)a | 11/25 | 7/16 | 1 | ≈0 | ≈0 |
| Age at Time of Scan (yrs) | 16.1 ± .2 (13.1-17.9) | 16 ± .3 (13.1-17.8) | 41.35 | .45 | .12 |
| Hollingshead Socioeconomic Scoreb | 29 ± 16.3 (0-77) | 33 ± 26.7 (11-70) | NA | 348.5 | .42 |
| Tanner Scoreb | 4 ± .7 (3-5) | 4 ± .7 (2.5-5) | NA | 446 | .54 |
| Wechsler Abbreviated Scale of Intelligence | 106.4 ± 2.1 (84-133) | 101.5 ± 2.2 (85-120) | 51.97 | 1.61 | .42 |
| Children's Global Assessment Scaleb,c | 90 ± 7.4 (75-100) | 65 ± 14.8 (40-85) | NA | 817.5 | .99 |
| Ruminative Responses Styles Questionnaireb,c | 22 ± 13.3 (0-68) [7] | 57 ± 23.7 (14-101) [2] | NA | 50.5 | .06 |
| Children's Depression Rating Scale (Standardized)c | 33.4 ± .9 (30-55) | 71.2 ± 1.8 (55-85) | 33.34 | −18.96 | −4.99 |
| Beck Depression Inventory IIc | 3 ± .6 (0-12) [1] | 27.3 ± 2.1 (4-47) | 25.72 | −11.16 | −2.94 |
| Comorbid Diagnoses in the MDD Group | |||||
| No comorbid diagnoses | 5 | ||||
| Generalized anxiety disorder | 12 | ||||
| Specific phobia | 2 | ||||
| Anxiety disorder not otherwise specified | 1 | ||||
| Posttraumatic stress disorder | 2 | ||||
| Enuresis | 1 |
Entries are of the form: mean ± SEM (minimum - maximum) unless otherwise stated. The optional number in square brackets indicates the number of missing items of data. Effect size is Hedges' g unless otherwise indicated. Statistical comparisons were by means of Welch t tests unless otherwise indicated.
Statistic is the W, χ2, or t value. Statistics for clinical scales and demographics refer only to participants surviving motion and outlier correction.
F, female; M, male; MDD, major depressive disorder; NA, not applicable.
χ2 test for equality of proportions.
Median ± median (minimum - maximum) or median ± median absolute deviation (minimum-maximum).The optional number in brackets indicates the number of missing items of data. Wilcox rank sum test. The effect size is the probability of superiority.
p < .001.
Rsfc
The regions identified in the whole-brain between-group analysis (Table 2) were consistent with those identified as being part of the salience, central executive, and default mode networks (15). The left inferior seed demonstrated greater positive connectivity to bilateral inferior frontal gyrus (IFG) and bilateral insula in the MDD group compared with control subjects (Figure 1) as well other regions listed in Table 2. The right inferior seed showed greater positive connectivity in the MDD group than the control subjects to right cuneus, right lentiform nucleus, bilateral superior temporal gyrus, and left claustrum.
Table 2. Seed Locations and Regions Showing Between-Group Differences in Mean RSFC.
| Structure | Hemisphere | BA | Volume μL | Center of Mass | Mean RSFC | |||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| X | Y | Z | MDD | NCL | ||||
| Left Superior Seed | 189 | 5 | −34 | −4 | ||||
| MDD > control subjects | ||||||||
| Cuneus | R | 19 | 2052 | −13 | 80 | 29 | .17 | .02 |
| Left Inferior Seed | 189 | 5 | −25 | −10 | ||||
| MDD > control subjects | ||||||||
| Insula | L | 13 | 19,548 | 38 | −18 | 8 | .17 | .01 |
| Insula | R | 13 | 13,905 | −37 | 4 | −6 | .17 | .00 |
| Middle frontal gyrus | R | 10 | 6345 | −37 | −41 | 11 | .13 | −.04 |
| Inferior parietal lobule | L | 40 | 5076 | 53 | 42 | 42 | .14 | .00 |
| Superior temporal gyrus | R | 22 | 4401 | −51 | 57 | 14 | .19 | .04 |
| Inferior parietal lobule | R | 40 | 3780 | −58 | 36 | 44 | .11 | −.02 |
| Paracentral lobule | R | 31 | 2673 | −4 | 15 | 47 | .20 | .05 |
| Precentral gyrus | L | 6 | 2511 | 42 | −2 | 37 | .19 | .03 |
| Inferior frontal gyrus | L | 47 | 2241 | 34 | −33 | −9 | .24 | .08 |
| Right Superior Seed | 189 | −5 | −34 | −4 | ||||
| MDD < control subjects | ||||||||
| Precuneus | L | 7 | 15,228 | 5 | 60 | 51 | −.14 | .04 |
| Middle frontal gyrus | L | 6 | 4482 | 28 | 2 | 52 | −.14 | .02 |
| Middle occipital gyrus | L | 18 | 2349 | 28 | 92 | 2 | −.09 | .09 |
| MDD > control subjects | ||||||||
| Amygdala/parahippocampal | R | 20 | 3429 | −33 | 4 | −29 | .17 | .01 |
| Gyrus | ||||||||
| Culmen | L | 3186 | 16 | 37 | −34 | .10 | −.04 | |
| Amygdala/uncus | L | 21 | 2160 | 34 | 6 | −31 | .15 | −.02 |
| Right Inferior Seed | 189 | −5 | −25 | −10 | ||||
| MDD > control subjects | ||||||||
| Cuneus | R | 18 | 14,094 | −7 | 75 | 12 | .18 | .02 |
| Lentiform nucleus | R | 6777 | −21 | −9 | 13 | .15 | −.01 | |
| Inferior frontal gyrus | L | 45 | 6426 | 48 | −21 | 14 | .19 | .02 |
| Claustrum | L | 4455 | 27 | 8 | 17 | .18 | .00 | |
| Inferior frontal gyrus | R | 46 | 2322 | −43 | −40 | 4 | .14 | −.02 |
| Superior temporal gyrus | R | 22 | 2106 | −47 | 27 | 0 | .21 | .04 |
| Superior temporal gyrus | L | 22 | 2106 | 51 | 49 | 12 | .22 | .04 |
As measured by Fisher Z-transformed Pearson correlation. Center-of-mass coordinates are in the MNI152 (Montreal Neurological Institute, Montreal, Quebec, Canada) (right anterior inferior) standard and structure labels from the Talairach and Tournoux atlas.
BA, Brodmann area; MDD, major depressive disorder; NCL, normal control subjects; RSFC, resting-state functional connectivity.
Figure 1.
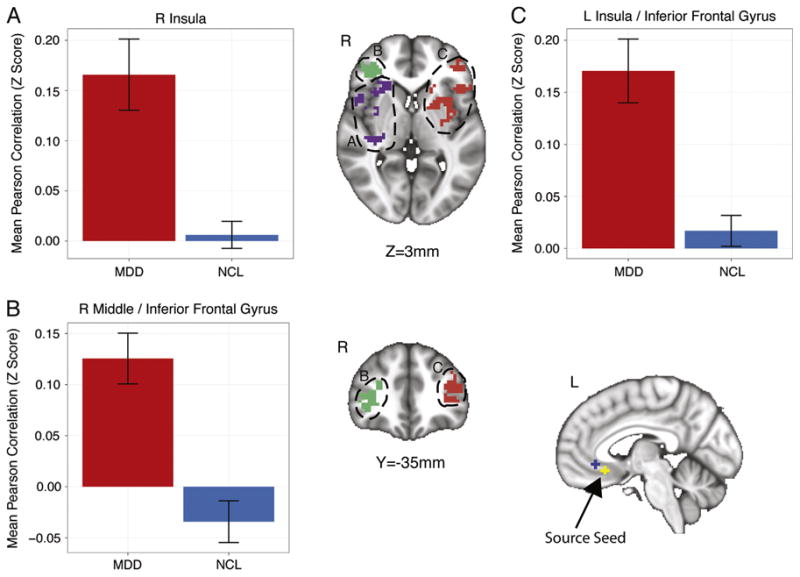
Regions showing group differences in the correlation with a subgenual anterior cingulate cortex seed in the left hemisphere. Error bars indicate the SEM. L, left; MDD, major depressive disorder; NCL, normal control subjects; R, right.
The left superior seed showed connectivity differences in only one region in the right cuneus that was more strongly positively connected to the sgACC in the MDD group than the control participants. The right superior seed displayed negative connectivity to the left precuneus and middle frontal and middle occipital gyri in the MDD group relative to the control participants who showed positive connectivity to these three regions. We observed increased positive connectivity between the right superior seed and the right amygdala extending caudally into the parahippocampal gyrus in the MDD group relative to control participants (Figure 2). The right superior seed also demonstrated increased FC with the left culmen of the cerebellum and the left amygdala extending ventrally into the uncus in the MDD group compared with the control participants (Figure 2). In the case of these three regions (the right amygdala/parahippocampa gyrus, left culmen, and left amygdala/uncus), the MDD group demonstrated positive connectivity to the sgACC, whereas the control group showed positive connectivity for the right amygdala/parahippocampal gyrus and negative connectivity for the left culmen and left amygdala/uncus.
Figure 2.
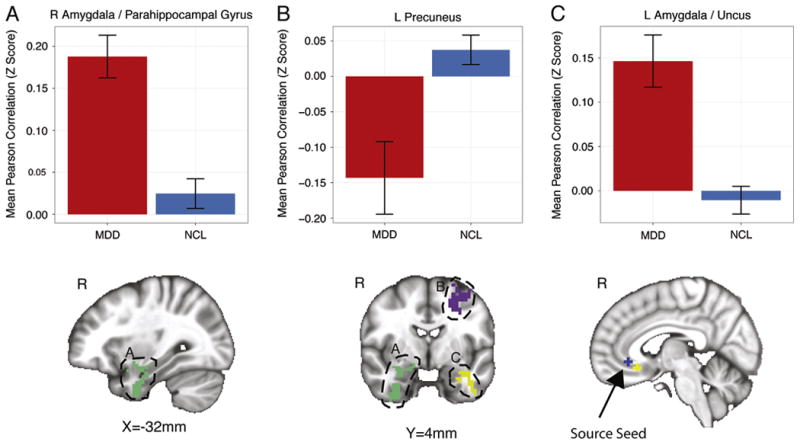
Regions showing group differences in the correlation with a subgenual anterior cingulate cortex seed in the right hemisphere. Error bars indicate the SEM. L, left; MDD, major depressive disorder; NCL, normal control subjects; R, right.
Correlational Analysis
Within the MDD group, several regions (Table 3) demonstrated significant relationships with the clinical scales. Those regions showing negative correlations with measures of depression (BDI-II and CDRS-R) included left middle frontal and left occipital gyri, left precuneus, and the left precentral gyrus (all p < .05). Positive correlations between the CGAS and regions in the left precuneus and left middle frontal and left occipital gyri were observed (all p < .05). One region, the left claustrum, showed a negative correlation with the CGAS (p < .05). Negative correlations with RRS were observed in the right middle frontal gyrus (MFG) and right IFG (all p < .05).
Table 3. Table of Regions Showing Correlations with Clinical Rating Scales.
| Cluster | Regression Variable | S | ρ | p |
|---|---|---|---|---|
| Superior Right Seed | ||||
| L precuneus | Children's Global Assessment Scale | 948.94 | .53 | <.01 |
| L middle frontal gyrus | Children's Global Assessment Scale | 769.10 | .62 | <.01 |
| L middle occipital gyrus | Children's Global Assessment Scale | 649.53 | .68 | <.001 |
| L middle frontal gyrus | Children's Depression Rating Scale-Revised (Standardized) | 2937.71 | −.45 | <.05 |
| L middle occipital gyrus | Children's Depression Rating Scale-Revised (Standardized) | 3183.44 | −.57 | <.01 |
| L precuneus | Beck Depression Inventory II | 2880.06 | −.42 | <.05 |
| L middle frontal gyrus | Beck Depression Inventory II | 3081.31 | −.52 | <.05 |
| Inferior Left Seed | ||||
| R middle frontal gyrus | Ruminative Responses Scale | 2370.27 | −.54 | <.05 |
| L precentral gyrus | Children's Depression Rating Scale-Revised (Standardized) | 3108.22 | −.54 | <.01 |
| Inferior Right Seed | ||||
| L claustrum | Children's Global Assessment Scale | 2925.24 | −.45 | <.05 |
| R inferior frontal gyrus | Ruminative Responses Scale | 2227.22 | −.45 | <.05 |
L, left; R, right;
Spearman's correlation coefficient;
Spearman's rank sum.
Discussion
This study compared the RSFC of the sgACC in medication-naïve first-episode adolescents with a primary diagnosis of MDD with a group of well-matched control subjects. Our study yielded four main results. Firstly, we observed several brain regions coactive with the sgACC that are not typically considered part of the DMN. This result might reflect AFC within and between the ICNs of the brain. Secondly, our results further support the body of research indicating that the sgACC is an important node in a network of limbic and paralimbic regions that have previously been shown to be dysfunctional in depressed adults (9,11,65–70), adolescents (31,32), and children (33). Thirdly, our correlational analysis suggests that greater connectivity between regions of the DMN might be associated with better psychosocial function in depressed adolescents and that more severe depression might be related to reduced connectivity between these nodes. Finally, we observed that increased levels of rumination were associated with decreased FC between the sgACC and both the IFG and MFG, which are components of the CEN (15).
We observed elevated positive connectivity with the sgACC in the bilateral insulae (Figure 1) as well as greater negative connectivity between the sgACC and the left precuneus (Figure 2) in the MDD group compared with the control subjects. These observations might reflect FC changes between the ICNs of the brain. In the TNM, the SN is thought to be centered on the anterior cingulate and insular cortex (15). The insula is thought to play a role in the integration of autonomic, visceral, and hedonic information (71). Indeed, the insula has been proposed to be critical to selecting from among internally and externally available homeostatically relevant information to guide behavior (71). Furthermore, the right anterior insula is thought to play a key role in switching from a predominantly CEN/SN dominated brain state to the default mode state (72). Our observation of increased connectivity between the sgACC and both the right anterior insula and left middle insula (Figure 1) in the MDD group might be significant insofar as it might be indicative of AFC between the SN and DMN. This AFC might be due to the strong connectivity we observed between the sgACC and right anterior insula, which might preclude a successful transition into the pattern of neural activity characteristic of a normal DMN (72).
Although we observed greater positive connectivity between the sgACC and insula in the depressed adolescents compared with control subjects, Cullen et al.(31) reported decreased connectivity between these two regions. This difference could be due to issues such as medication status, sample size, or the “emotional import” of the music participants listened to while being scanned (31). In the present study, we examined a larger sample (MDD: 23 vs. 12; control: 36 vs. 14) of medication-naïve first-episode participants who were not permitted to listen to music while being scanned. In the Cullen et al.(31) study, participants on several different types of psychiatric medications were scanned while listening to music of the participants' choice. Although playing music during the scan might be regarded as uncommon (32), recent evidence suggests that this might not appreciably affect the topology of the DMN but rather might enhance connectivity among the nodes thereof (73). Therefore, it is possible that medicated depressed adolescents scanned while listening to music might demonstrate elevated FC between the sgACC and insula, whereas unmedicated depressed adolescents scanned while not listening to music might show reduced FC between these two structures.
We also observed increased FC between the sgACC and right amygdala in the MDD group relative to the control subjects (Figure 2). Previous studies have reported hyperactivation of the amygdala in both adults (39,44,74–76) and adolescents (37,77–81) with major depression. Amygdalar activation has been shown to predict likelihood of positive treatment outcome in depressed adults (82). Consequently, the amygdala has been proposed to play a key role in depression (38,39). Similarly, sgACC hyperactivation has also been observed in both depressed adults (10,66) and adolescents (83). Therefore, it has been hypothesized that the sgACC plays a pivotal role in depression (38,67,84). Effective treatment has been shown to reduce activity levels toward that typical of healthy individuals in both the amygdala (66) and sgACC (11). However, it is unclear whether the FC between these two regions might alter with treatment. Future longitudinal studies are necessary to address this question and identify whether the connectivity between these regions might be a potential biomarker of depression and an apt target for treatment. Our results also suggest that these two regions are functionally connected. Consistent with our FC findings, it has been shown that the amygdala and sgACC are anatomically connected by white matter fibers in the uncinate fasciculus (85). Furthermore, structural alterations in the connection between sgACC and amygdala have been reported in depressed adolescents (86), but whether these predate development of depression and might have a causative effect or are a consequence of the illness is unclear. Future studies in adolescents at risk for depression might help to address these issues.
Within the MDD group, we hypothesized that the clinical measures of depression would be associated with the strength of connectivity between the sgACC and other brain regions. We observed that higher BDI-II scores were significantly correlated with decreased FC in the MDD group between the sgACC and left precuneus, which are components of the DMN (15,19,87) (Table 3). Consistent with this observation, greater psychosocial function was significantly correlated with increased connectivity between the sgACC and left precuneus. We also observed that greater CDRS-R scores were significantly correlated with decreased connectivity between the sgACC and left MFG in the MDD group. Because the MFG is a component of the CEN (15), this observation might indicate an impairment of top-down regulation of emotion by the CEN. These findings suggest that increased depression and decreased psychosocial functioning are associated with AFC of the sgACC and are consistent with our hypothesis that differences in FC within the depressed adolescents would be significantly correlated with measures of clinical depression. Overall, the MDD group displayed more negative connectivity than the control subjects, with respect to the sgACC-left MFG connectivity. We also found negative correlation between depression scores (CDRS-R, BDI-II) and sgACC-left MFG FC in the MDD group. These results suggest that the CEN of depressed adolescents with less negative FC between these regions might be more effective at regulating depressive symptoms. Conversely, more negative sgACC-left MFG FC might indicate inadequate regulation of depressive symptoms.
Finally, within the MDD group, we investigated whether rumination correlated with connection strength from the sgACC. As shown in Table 3, increased rumination was associated with decreased FC between the sgACC and both the right MFG and right IFG. Both the MFG and IFG are thought to be components of the CEN (88–92), with right IFG important to emotion regulation in both healthy and depressed adults (93–95). Overall, the MDD group displayed greater positive sgACC-MFG and sgACC-IFG connectivity than the control subjects. We also observed a negative correlation between rumination and sgACC-MFG and sgACC-IFG connectivity in the MDD group. These results suggest that the CEN of depressed adolescents with lower FC between these regions might be inadequately regulating negative emotional thoughts (96). Conversely, individuals with greater sgACC-MFG and sgACC-IFG connectivity might be better regulating negative emotional thoughts.
The results of this study must be interpreted in light of its limitations. The current study is cross-sectional and therefore cannot address whether or not these observations are a consequence of MDD. Future longitudinal studies should be performed to address this question. Given the high rates of comorbid diagnoses in this sample of depressed adolescents, future studies are still required to investigate the specificity of these findings and how they might be influenced by comorbidity. Notwithstanding, adolescent depression is a highly comorbid disorder (97–99), and inclusion of participants with comorbid diagnoses arguably makes our sample more representative of the patients typically seen in clinics and thus contributes to the generalizability of our results. The issue of gender differences is important, especially given the higher rates of depression in female adolescents than male adolescents (1,100). Although we conducted a preliminary investigation of the effect of gender in the current sample (Supplement 1), we were limited in this investigation by the small number of depressed male adolescents (n = 7). Future studies are required to investigate whether and, if so, how FC varies by gender in depressed adolescents. Finally, we used the TNM as a theoretical basis to explain our findings. However, it is possible that other theories might be equally applicable to the results reported herein. Although we have attempted to explain most of our findings with the TNM, the FC of the depressed adolescent brain might be more complex and involve additional networks than the three central to the TNM. The application of the TNM might therefore be regarded as preliminary, and future studies are required to confirm the applicability of the TNM to the study of adolescent depression.
In summary, the present study examined the RSFC of the sgACC in medication-naïve first-episode adolescents with MDD compared with a group of well-matched healthy control participants. Relative to control participants, the depressed adolescents demonstrated greater sgACC-amygdala and sgACC-insula connectivity, suggesting AFC between the SN and DMN in the resting state. Furthermore, adolescents with greater levels of depression and lower levels of psychosocial function demonstrated weaker sgACC-precuneus connectivity. Taken together, these results suggest that adolescent depression might be related to or accompanied by AFC between the DMN and SN that might be underpinned by elevated connectivity between the sgACC and both the insula and amygdala. Our results are consistent with and further support prior reports of elevated FC in depressed adolescents (32) and adults (27,28,30) rather than reduced connectivity (31). We also observed increased rumination as a function of decreased connectivity between the sgACC and both the right MFG and right IFG, suggesting impaired top-down modulation by the CEN of negative emotional thoughts. Finally, our findings further support the model that the sgACC plays a key role in major depression (4,5) and are consistent with the TNM of neuropsychi-atric disorders (15). Collectively, our results raise the possibility that potential therapeutic interventions that can restore the FC within and between the SN, CEN, and DMN to that typical of healthy adolescents might be a fruitful avenue for future research in the treatment and prevention of adolescent depression.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institute of Mental Health (7R01MH085734-04 and 3R01MH085734-02S1) and from the National Alliance for Research in Schizophrenia and Affective Disorders Foundation to TTY.
The funding agency played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
We are deeply grateful to the anonymous reviewers for their thoughtful comments on this article.
Dr. Fumiko Hoeft receives grants or research support from the National Institutes of Health. Dr. Owen Wolkowitz receives grants or research support from the National Institutes of Health and the Department of Defense. He is also on the Scientific Advisory Board for Telome Health, Incorporated. Dr. Stuart Eisendrath receives grant or research support from the National Institutes of Health and the Mellam Foundation. Dr. Guido Frank receives grant or research support from the National Institutes of Health. He also serves as a consultant to the Eating Disorder Center of Denver. Dr. Robert Hendren has received grants or research support from Forest Pharmaceuticals, Inc., Curemark, BioMarin Pharmaceutical, Roche, Autism Speaks, the Vitamin D Council, and the National Institutes of Mental Health within the past two years. He is also on the Advisory Boards for Biomarin, Forest, and Janssen. Dr. Jeffrey Max receives grant or research support from the National Institutes of Health. He also provides expert testimony in cases of traumatic brain injury on an ad hoc basis for plaintiffs and defendants on a more or less equal ratio. This activity constitutes approximately 5% of his professional activities. Dr. Martin Paulus receives grant or research support from the National Institutes of Health. Dr. Susan Tapert receives grant or research support from the National Institutes of Health and the Veterans Affairs. Dr. Alan Simmons receives grant or research support from the Veterans Affairs and National Institutes of Mental Health. Dr. Tony Yang receives grant or research support from the National Institutes of Health. Dr. Colm Connolly, Mr. Jing Wu, Dr. Tiffany Ho, and Mr. Dipavo Banerjee report no biomedical financial interests or potential conflicts of interests.
Footnotes
Supplementary material cited in this article is available online at http://dx.doi.org/10.1016/j.biopsych.2013.05.036.
References
- 1.Costello EJ, Pine DS, Hammen C, March JS, Plotsky PM, Weissman MM, et al. Development and natural history of mood disorders. Biol Psychiatry. 2002;52:529–542. doi: 10.1016/s0006-3223(02)01372-0. [DOI] [PubMed] [Google Scholar]
- 2.Birmaher B, Ryan ND, Williamson DE, Brent DA, Kaufman J, Dahl RE, et al. Childhood and adolescent depression: A review of the past 10 years. Part I. J Am Acad Child Adolesc Psychiatry. 1996;35:1427–1439. doi: 10.1097/00004583-199611000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: Towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull. 2003;65:193–207. doi: 10.1093/bmb/65.1.193. [DOI] [PubMed] [Google Scholar]
- 5.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: Implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bush G, Luu P, Posner M. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 7.Freedman LJ, Insel TR, Smith Y. Subcortical projections of area 25 (subgenual cortex) of the macaque monkey. J Comp Neurol. 2000;421:172–188. [PubMed] [Google Scholar]
- 8.Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005;6:533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Osuch EA, Ketter TA, Kimbrell TA, George MS, Benson BE, Willis MW, et al. Regional cerebral metabolism associated with anxiety symptoms in affective disorder patients. Biol Psychiatry. 2000;48:1020–1023. doi: 10.1016/s0006-3223(00)00920-3. [DOI] [PubMed] [Google Scholar]
- 10.Kennedy SH, Konarski JZ, Segal ZV, Lau MA, Bieling PJ, McIntyre RS, Mayberg HS. Differences in brain glucose metabolism between responders to CBT and venlafaxine in a 16-week randomized controlled trial. Am J Psychiatry. 2007;164:778–788. doi: 10.1176/ajp.2007.164.5.778. [DOI] [PubMed] [Google Scholar]
- 11.Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, McGinnis S, Jerabek PA. Regional metabolic effects of fluoxetine in major depression: Serial changes and relationship to clinical response. Biol Psychiatry. 2000;48:830–843. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- 12.Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 13.Johansen-Berg H, Gutman DA, Behrens TEJ, Matthews PM, Rush-worth MFS, Katz E, et al. Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cereb Cortex. 2008;18:1374–1383. doi: 10.1093/cercor/bhm167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lozano AM, Mayberg HS, Giacobbe P, Hamani C, Craddock RC, Kennedy SH. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2008;64:461–467. doi: 10.1016/j.biopsych.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 15.Menon V. Large-scale brain networks and psychopathology: A unifying triple network model. Trends Cogn Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, et al. Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 18.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uddin LQ, Supekar KS, Ryali S, Menon V. Dynamic reconfiguration of structural and functional connectivity across core neuro-cognitive brain networks with development. J Neurosci. 2011;31:18578–18589. doi: 10.1523/JNEUROSCI.4465-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomason ME, Dennis EL, Joshi AA, Joshi SH, Dinov ID, Chang C, et al. Resting-state fMRI can reliably map neural networks in children. Neuroimage. 2011;55:165–175. doi: 10.1016/j.neuroimage.2010.11.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spreng RN, Mar RA, Kim ASN. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: A quantitative meta-analysis. J Cognitive Neurosci. 2009;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- 22.Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- 23.Rangel A, Camerer C, Montague PR. A framework for studying the neurobiology of value-based decision making. Nat Rev Neurosci. 2008;9:545–556. doi: 10.1038/nrn2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, et al. Resting-state functional connectivity in major depression: Abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamilton JP, Chen G, Thomason ME, Schwartz ME, Gotlib IH. Investigating neural primacy in major depressive disorder: Multi-variate Granger causality analysis of resting-state fMRI time-series data. Mol Psychiatr. 2011;16:763–772. doi: 10.1038/mp.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seminowicz DA, Mayberg HS, McIntosh AR, Goldapple K, Kennedy S, Segal Z, Rafi-Tari S. Limbic-frontal circuitry in major depression: A path modeling metanalysis. Neuroimage. 2004;22:409–418. doi: 10.1016/j.neuroimage.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 30.Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci U S A. 2010;107:11020–11025. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cullen KR, Gee DG, Klimes-Dougan B, Gabbay V, Hulvershorn L, Mueller BA, et al. A preliminary study of functional connectivity in comorbid adolescent depression. Neuroscience Lett. 2009;460:227–231. doi: 10.1016/j.neulet.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davey CG, Harrison BJ, Yücel M, Allen NB. Regionally specific alterations in functional connectivity of the anterior cingulate cortex in major depressive disorder. Psychol Med. 2012;42:2071–2081. doi: 10.1017/S0033291712000323. [DOI] [PubMed] [Google Scholar]
- 33.Gaffrey MS, Luby JL, Repovš G, Belden AC, Botteron KN, Luking KR, Barch DM. Subgenual cingulate connectivity in children with a history of preschool-depression. Neuroreport. 2010;21:1182–1188. doi: 10.1097/WNR.0b013e32834127eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fair DA, Cohen AL, Dosenbach NU, Church JA, Miezin FM, Barch DM, et al. The maturing architecture of the brain's default network. Proc Natl Acad Sci U S A. 2008;105:4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kelly AMC, Di Martino A, Uddin LQ, Shehzad Z, Gee DG, Reiss PT, et al. Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cereb Cortex. 2009;19:640–657. doi: 10.1093/cercor/bhn117. [DOI] [PubMed] [Google Scholar]
- 36.Mayberg H. CBT or Medication? Putative PET and fMRI biomarkers for optimizing treatment selection for MDD. Biol Psychiatry. 2013;73:139S. [Google Scholar]
- 37.Perlman G, Simmons AN, Wu J, Hahn KS, Tapert SF, Max JE, et al. Amygdala response and functional connectivity during emotion regulation: A study of 14 depressed adolescents. J Affect Disord. 2012;139:75–84. doi: 10.1016/j.jad.2012.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, et al. Reciprocal limbic-cortical function and negative mood: Converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- 39.Drevets WC. Neuroimaging studies of mood disorders. Biol Psychiatry. 2000;48:813–829. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- 40.Hamilton JPJ, Furman DJD, Chang CC, Thomason MEM, Dennis EE, Gotlib IHI. Default-mode and task-positive network activity in major depressive disorder: Implications for adaptive and maladaptive rumination. Biol Psychiatry. 2011;70:327–333. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berman MG, Peltier S, Nee DE, Kross E, Deldin PJ, Jonides J. Depression, rumination and the default network. Soc Cogn Affect Neurosci. 2011;6:548–555. doi: 10.1093/scan/nsq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berman MG, Nee DE, Casement M, Kim HS, Deldin P, Kross E, et al. Neural and behavioral effects of interference resolution in depression and rumination. Cogn Affective Behav Neurosci. 2011;11:85–96. doi: 10.3758/s13415-010-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ray RD, Ochsner KN, Cooper JC, Robertson ER, Gabrieli JDE, Gross JJ. Individual differences in trait rumination and the neural systems supporting cognitive reappraisal. Cogn Affective Behav Neurosci. 2005;5:156–168. doi: 10.3758/cabn.5.2.156. [DOI] [PubMed] [Google Scholar]
- 44.Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can't shake that feeling: Event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biol Psychiatry. 2002;51:693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- 45.Shaffer D, Fisher P, Lucas CP, Dulcan MK. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- 46.Lucas CP, Zhang H, Fisher PW, Shaffer D, Regier DA, Narrow WE, et al. The DISC Predictive Scales (DPS): Efficiently screening for diagnoses. J Am Acad Child Adolesc Psychiatry. 2001;40:443–449. doi: 10.1097/00004583-200104000-00013. [DOI] [PubMed] [Google Scholar]
- 47.Kaufman J, Birmaher B, Brent DA, Ryan ND, Rao U. K-SADS-PL. J Am Acad Child Adolesc Psychiatry. 2000;39:1208. doi: 10.1097/00004583-200010000-00002. [DOI] [PubMed] [Google Scholar]
- 48.Poznanski EO. Children's Depression Rating Scale-Revised (CDRS-R) Los Angeles: Western Psychological Services; 1996. [Google Scholar]
- 49.Beck AT, Steer RA, Brown GK. Beck Depression Inventory-Second Edition Manual. San Antonio, Texas: The Psychological Corporation; 1996. [Google Scholar]
- 50.Nolen-Hoeksema S, Morrow J. A prospective study of depression and posttraumatic stress symptoms after a natural disaster: the 1989 Loma Prieta Earthquake. J Pers Soc Psychol. 1991;61:115–121. doi: 10.1037//0022-3514.61.1.115. [DOI] [PubMed] [Google Scholar]
- 51.Dyrborg J, Larsen FW, Nielsen S, Byman J, Nielsen BB, Gautre-Delay F. The Children's Global Assessment Scale (CGAS) and Global Assessment of Psychosocial Disability (GAPD) in clinical practice—substance and reliability as judged by intraclass correlations. Eur Child Adolesc Psychiatry. 2000;9:195–201. doi: 10.1007/s007870070043. [DOI] [PubMed] [Google Scholar]
- 52.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 53.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 54.Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- 55.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 56.Andersson JLR, Jenkinson M, Smith S. Non-Linear Registration, aka Spatial Normalisation. Oxford, UK: FMRIB, University of Oxford; 2007. [Accessed May 17, 2012]. Available at: http://www.fmrib.ox.ac.uk/analysis/techrep/tr07ja2/tr07ja2.pdf. [Google Scholar]
- 57.Andersson JLR, Jenkinson M, Smith S. Non-Linear Optimisation. Oxford, UK: FMRIB, University of Oxford; 2007. [Accessed May 17, 2012]. Available at www.fmrib.ox.ac.uk/analysis/techrep/tr07ja1/tr07ja1.pdf. [Google Scholar]
- 58.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE T Med Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- 59.Saad ZS, Glen DR, Chen G, Beauchamp MS, Desai R, Cox RW. A new method for improving functional-to-structural MRI alignment using local Pearson correlation. Neuroimage. 2009;44:839–848. doi: 10.1016/j.neuroimage.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Margulies DS, Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Mapping the functional connectivity of anterior cingulate cortex. Neuroimage. 2007;37:579–588. doi: 10.1016/j.neuroimage.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 61.Fisher RA. On the “probable error” of a coefficient of correlation deduced from a small sample. Metron. 1921;1:3–32. [Google Scholar]
- 62.R Development Core Team. R: A Language and Environment for Statistical Computing. 2nd. Vienna, Austria: R Foundation for Statistical Computing; 2012. [Accessed May 17, 2012]. Available at: http://cran.r-project.org/doc/manuals/fullrefman.pdf. [Google Scholar]
- 63.Hedges LV, Olkin I. Statistical Methods for Meta-Analysis. New York: Academic Press; 1985. [Google Scholar]
- 64.Erceg-Hurn DM, Mirosevich VM. Modern robust statistical methods: An easy way to maximize the accuracy and power of your research. Am Psychol. 2008;63:591–601. doi: 10.1037/0003-066X.63.7.591. [DOI] [PubMed] [Google Scholar]
- 65.Botteron KN, Raichle ME, Drevets WC, Heath AC, Todd RD. Volumetric reduction in left subgenual prefrontal cortex in early onset depression. Biol Psychiatry. 2002;51:342–344. doi: 10.1016/s0006-3223(01)01280-x. [DOI] [PubMed] [Google Scholar]
- 66.Drevets W. Functional anatomical correlates of antidepressant drug treatment assessed using PET measures of regional glucose metabolism. Eur Neuropsychopharmacol. 2002;12:527–544. doi: 10.1016/s0924-977x(02)00102-5. [DOI] [PubMed] [Google Scholar]
- 67.Drevets WC, Price JL, Simpson JR, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 68.Hirayasu Y, Shenton ME, Salisbury DF, Kwon JS, Wible CG, Fischer IA, et al. Subgenual cingulate cortex volume in first-episode psychosis. Am J Psychiatry. 1999;156:1091–1093. doi: 10.1176/ajp.156.7.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kennedy SH, Evans KR, Krüger S, Mayberg HS, Meyer JH, McCann S, et al. Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. Am J Psychiatry. 2001;158:899–905. doi: 10.1176/appi.ajp.158.6.899. [DOI] [PubMed] [Google Scholar]
- 70.Öngür D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci U S A. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Craig ADB. How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 72.Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kay BP, Meng X, DiFrancesco MW, Holland SK, Szaflarski JP. Moderating effects of music on resting state networks. Brain Res. 2012;1447:53–64. doi: 10.1016/j.brainres.2012.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fales CL, Barch DM, Rundle MM, Mintun MA, Snyder AZ, Cohen JD, et al. Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biol Psychiatry. 2008;63:377–384. doi: 10.1016/j.biopsych.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Matthews SC, Strigo IA, Simmons AN, Yang TT, Paulus MP. Decreased functional coupling of the amygdala and supragenual cingulate is related to increased depression in unmedicated individuals with current major depressive disorder. J Affect Disord. 2008;111:13–20. doi: 10.1016/j.jad.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 76.Anand A, Li Y, Wang Y, Wu J, Gao S, Bukhari L, et al. Activity and connectivity of brain mood regulating circuit in depression: A functional magnetic resonance study. Biol Psychiatry. 2005;57:1079–1088. doi: 10.1016/j.biopsych.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 77.Yang TT, Simmons AN, Matthews SC, Tapert SF, Frank GK, Max JE, et al. Adolescents with major depression demonstrate increased amygdala activation. J Am Acad Child Adolesc Psychiatry. 2010;49:42–51. doi: 10.1097/00004583-201001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Forbes EE, Christopher May J, Siegle GJ, Ladouceur CD, Ryan ND, Carter CS, et al. Reward-related decision-making in pediatric major depressive disorder: An fMRI study. J Child Psychol Psychiatry. 2006;47:1031–1040. doi: 10.1111/j.1469-7610.2006.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lau JYF, Goldman D, Buzas B, Fromm SJ, Guyer AE, Hodgkinson C, et al. Amygdala function and 5-HTT gene variants in adolescent anxiety and major depressive disorder. Biol Psychiatry. 2009;65:349–355. doi: 10.1016/j.biopsych.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Roberson-Nay R, McClure EB, Monk CS, Nelson EE, Guyer AE, Fromm SJ, et al. Increased amygdala activity during successful memory encoding in adolescent major depressive disorder: An FMRI study. Biol Psychiatry. 2006;60:966–973. doi: 10.1016/j.biopsych.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 81.Beesdo K, Lau JYF, Guyer AE, McClure-Tone EB, Monk CS, Nelson EE, et al. Common and distinct amygdala-function perturbations in depressed vs anxious adolescents. Arch Gen Psychiatry. 2009;66:275–285. doi: 10.1001/archgenpsychiatry.2008.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Canli T, Cooney RE, Goldin P, Shah M, Sivers H, Thomason ME, et al. Amygdala reactivity to emotional faces predicts improvement in major depression. Neuroreport. 2005;16:1267–1270. doi: 10.1097/01.wnr.0000174407.09515.cc. [DOI] [PubMed] [Google Scholar]
- 83.Yang TT, Simmons AN, Matthews SC, Tapert SF, Frank GK, Bischoff-Grethe A, et al. Depressed adolescents demonstrate greater subgenual anterior cingulate activity. Neuroreport. 2009;20:440–444. doi: 10.1097/WNR.0b013e3283262e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJS. Default-mode brain dysfunction in mental disorders: A systematic review. Neurosci Biobehav R. 2009;33:279–296. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 85.Kier EL, Staib LH, Davis LM, Bronen RA. MR imaging of the temporal stem: Anatomic dissection tractography of the uncinate fasciculus, inferior occipitofrontal fasciculus, and Meyer's loop of the optic radiation. Am J Neuroradiol. 2004;25:677–691. [PMC free article] [PubMed] [Google Scholar]
- 86.Cullen KR, Klimes-Dougan B, Muetzel R, Mueller BA, Camchong J, Houri A, et al. Altered white matter microstructure in adolescents with major depression: A preliminary study. J Am Acad Child Adolesc Psychiatry. 2010;49:173.e1–183.e1. doi: 10.1097/00004583-201002000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: Evidence from a partial correlation network analysis. Neuroimage. 2008;42:1178–1184. doi: 10.1016/j.neuroimage.2008.05.059. [DOI] [PubMed] [Google Scholar]
- 88.Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- 89.Chambers CD, Bellgrove MA, Stokes MG, Henderson TR, Garavan H, Robertson IH, et al. Executive “brake failure” following deactivation of human frontal lobe. J Cognitive Neurosci. 2006;18:444–455. doi: 10.1162/089892906775990606. [DOI] [PubMed] [Google Scholar]
- 90.Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 91.Buchsbaum BR, Greer S, Chang WL, Berman KF. Meta-analysis of neuroimaging studies of the Wisconsin card-sorting task and component processes. Hum Brain Mapp. 2005;25:35–45. doi: 10.1002/hbm.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Menon V, Adleman NE, White CD, Glover GH, Reiss AL. Error-related brain activation during a Go/NoGo response inhibition task. Hum Brain Mapp. 2001;12:131–143. doi: 10.1002/1097-0193(200103)12:3<131::AID-HBM1010>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: Reappraisal and suppression of negative emotion. Biol Psychiatry. 2008;63:577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JDE, Gross JJ. For better or for worse: Neural systems supporting the cognitive down-and up-regulation of negative emotion. Neuroimage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 95.Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: Counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cooney RE, Joormann J, Eugène F, Dennis EL, Gotlib IH. Neural correlates of rumination in depression. Cogn Affective Behav Neurosci. 2010;10:470–478. doi: 10.3758/CABN.10.4.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Arch Gen Psychiatry. 2003;60:837–844. doi: 10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- 98.Kilpatrick DG, Ruggiero KJ, Acierno R, Saunders BE, Resnick HS, Best CL. Violence and risk of PTSD, major depression, substance abuse/dependence, and comorbidity: Results from the National Survey of Adolescents. J Consult Clin Psych. 2003;71:692–700. doi: 10.1037/0022-006x.71.4.692. [DOI] [PubMed] [Google Scholar]
- 99.Choy Y, Fyer AJ, Goodwin RD. Specific phobia and comorbid depression: A closer look at the National Comorbidity Survey data. Compr Psychiatry. 2007;48:132–136. doi: 10.1016/j.comppsych.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 100.Wade TJ, Cairney J, Pevalin DJ. Emergence of gender differences in depression during adolescence: National panel results from three countries. J Am Acad Child Adolesc Psychiatry. 2002;41:190–198. doi: 10.1097/00004583-200202000-00013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


