Introduction
Transcranial MRI-guided focused ultrasound (FUS) can be utilized for non-invasive targeted brain therapies. For example, high-intensity FUS is being investigated in clinical trials as a thermal ablation method in essential tremor (1). In non-thermal applications, FUS at low intensities can be used to increase blood-brain barrier (BBB) permeability and facilitate the delivery of intravenous therapeutics from the blood to the brain (2–10).
FUS at low intensities has also demonstrated neuromodulatory properties (11–15) and the ability to increase growth factors (16,17), including brain-derived neurotrophic factor (BDNF), which are known to promote adult neurogenesis (18–20). Based on these findings, we hypothesized that FUS targeted to the hippocampus can stimulate neurogenesis.
Adult neurogenesis is a process involving the generation, development and integration of new neurons in the brain. Hippocampal neurogenesis, which occurs in the dentate gyrus (DG), contributes to learning and memory (21) and is impaired in neurological conditions such as Alzheimer's disease (22).
Methods and Materials
Animal Model
Adult C57Bl/6/C3H mice (136-137 days) were given food and water ad libitum. Experiments were approved by the Animal Care Committee at Sunnybrook Research Institute and performed in compliance with the Canadian Council on Animal Care and the Animals for Research Act.
MRI-guided FUS Treatment
Following isoflurane-medical air anaesthetization, mice heads were depilated and tails were fitted with a catheter. Mice were secured in a supine position to acquire T1- and T2- weighted scans in a 7.0-Tesla MRI (Bruker). FUS was generated using a custom-manufactured transducer (75 mm diameter, 60 mm radius of curvature) operating at 1.68 MHz, equipped with a wideband receiver (23). Signals recorded by the receiver were used with an acoustic emissions-based controller program to ensure safe exposure levels (24). FUS was delivered in 10 ms bursts at 1 Hz pulse repetition frequency for 120 s (6–8,25) and reached a peak pressure of 0.96 +/- 0.30MPa, with two 0.73 mm foci targeting the hippocampus in one hemisphere. The contralateral hippocampus remained an untreated control. At sonication start, mice received 20 μL/kg Definity microbubbles (Lantheus) to induce BBB disruption and 200 μL/kg Omniscan (GE) to visualize targeted foci in T1-weighted scans.
Starting 24 h post-treatment until day 4, mice received 50 mg/kg 5-bromo-2′-deoxyuridine (BrdU) intraperitoneally once daily to label proliferating cells.
Immunohistochemistry and Analysis
On Day 18, mice were deeply anaesthetized and perfused with 0.9% saline and 4% paraformaldehyde. Brains were harvested, post-fixed for 24 h, transferred to 30% sucrose and cut in 40 μm coronal sections.
Systematic series of 1 in 24 sections throughout the hippocampus (1.06 - 4.04 mm posterior to bregma) were immunostained. Sections were treated with 2N HCl (30 min, 37°C), neutralized with borate buffer (pH 8.5) and blocked with 1% bovine serum albumin, 2% donkey serum and 0.25% Triton X-100 in PBS. Sections were incubated in rat anti-BrdU (1:400) overnight (4°C). After 24 h, donkey anti-rat Cy3 (1:200) was applied for 1 h. Slices were incubated overnight (4°C) with mouse anti-neuronal nuclear protein (NeuN) biotin (1:200) labelling mature neurons and rabbit anti-S100β (1:500) labelling astrocytes. Sections were placed in streptavidin Cy5 (1:200) and donkey anti-rabbit dylight 488 (1:200) for 2 h and mounted.
BrdU co-localization with neuron or astrocyte markers was analyzed from confocal z-stacks throughout the DG (Zeiss LSM510). Counts were multiplied by the series interval for number estimation in the entire hippocampus. Paired t-tests were used to identify significance between hemispheres, defined as p<0.05. Pearson correlations were done to evaluate the dependence of cell proliferation and differentiation on the extent of BBB opening, as quantified by enhancement on T1- weighted post-treatment scans in MATLAB.
Results
Qualitatively, confocal z-stacks throughout the DG showed more BrdU-positive cells in the FUS-treated hippocampus (Figure 1A). Quantification of BrdU-positive cell counts confirmed that FUS significantly increased proliferation (Figure 1B, 174%, **p=0.008). Quantification of cells expressing BrdU and NeuN showed that FUS significantly increased neurogenesis in the treated hemisphere (Figure 1C, 228%, *p=0.013). No significant difference in astrogenesis was observed (Figure 1D, p=0.12). This indicates that proliferating hippocampal cells stimulated by FUS survive to 18 days and contribute to neurogenesis. No significant differences were found in the percentage of BrdU-positive cells that differentiated into neurons, astrocytes or other cell types following FUS treatment (Figure 1E). Proliferation, neurogenesis and astrogenesis were not correlated with the extent of BBB opening (data not shown).
Figure 1.
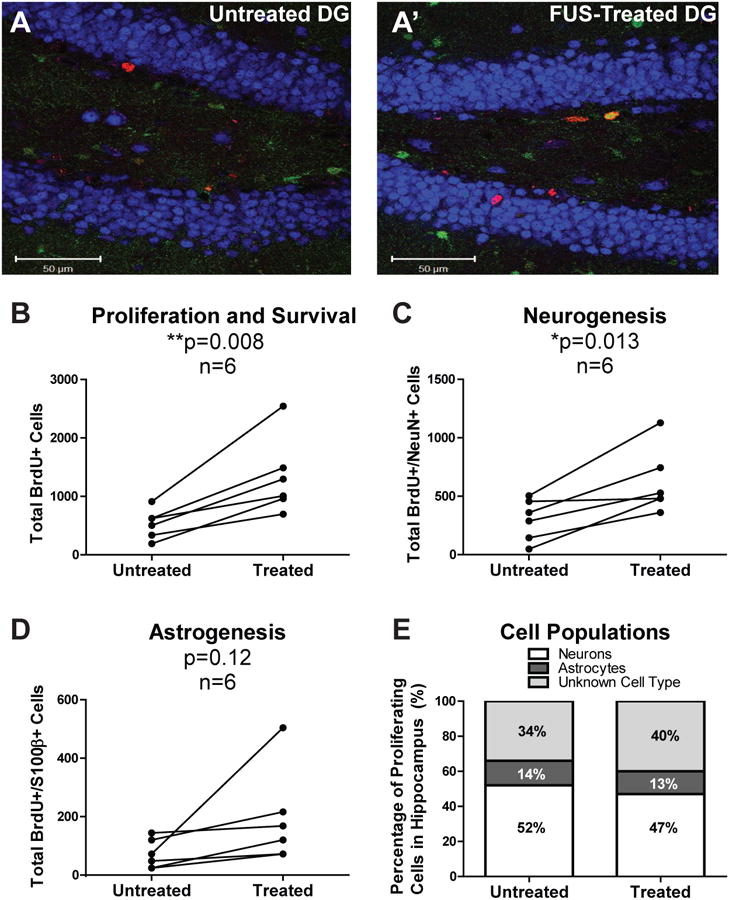
Hippocampal cell proliferation, neurogenesis and astrogenesis in adult mice 18 days following MRIgFUS treatment. Confocal z-stacks imaging the subgranular zone (SGZ) and granular cell layer (GCL) were acquired for both treated and untreated hemispheres (A) and utilized for analysis. Proliferating cells are labeled with BrdU (red), mature neurons with NeuN (blue) and astrocytes with S100β (green). From these images, the total number of BrdU-positive cells in the treated and untreated hemispheres were counted, extrapolated for the entire hippocampus and compared (B). Co-localization with the mature neuronal marker NeuN (C) and the astrocyte marker S100β (D) were determined, indicative of neurogenesis and astrogenesis, respectively. Overall, the proportion of hippocampal cell populations in the untreated and treated hemispheres (E) were examined to elucidate MRIgFUS effects on the hippocampus as a whole. Significant differences were defined as *p<0.05 and **p<0.01 (n=6).
Taken together, these results demonstrate that FUS promotes proliferation and differentiation of neurons without altering the proportion of cell populations intrinsic to the DG.
Discussion
This investigation demonstrated that transcranial FUS stimulates hippocampal neurogenesis in adult mice. FUS stimulation was accompanied by a significant increase in proliferation of newborn cells and in their differentiation and survival as mature neurons. Treatment did not significantly alter the proportion of different cell types within the hippocampus, maintaining the innate cellular environment. Neurogenesis was induced at FUS parameters typical for therapeutic delivery (6–8,25); however, these effects were not dependent on the extent of BBB opening.
The mechanisms through which FUS induces hippocampal neurogenesis have not been elucidated. However, transcranial pulsed ultrasound applied to the hippocampus in mice has been shown to significantly increase BDNF (16), a vital protein involved in synaptic plasticity and neuron generation (18,26). Two separate studies demonstrated that ultrasound treatment in vitro and in vivo significantly up-regulated vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) (17,27), two trophic factors that promote hippocampal neurogenesis (19,20). In addition to these direct effects on neurogenesis, other mechanisms may include VEGF-induced angiogenesis in hippocampal neurogenic niches (28).
These proposed mechanisms of FUS-induced neurogenesis are speculative and warrant further investigation. Recent data from our group indicates that successive FUS treatments targeted to the hippocampus increase dendritic branching and the number of immature neurons, while improving behaviour in a mouse model of Alzheimer's disease (29). Here, we provide evidence that one FUS treatment stimulates the birth, differentiation, maturation and survival of neurons in adult mice. Since neurogenesis is involved in learning and memory (21), FUS may present as a multifactorial treatment for neurological diseases, including Alzheimer's disease.
Acknowledgments
The authors would like to thank Kelly Markham-Coultes and Ying-Qi Weng for their technical assistance with animal care and immunohistochemistry protocols. We would also like to extend our gratitude to Kristiana Xhima for analytical assistance and Shawna Rideout-Gros and Alex Garces for animal preparation on experiment days.
Financial Disclosures: Research funding was provided by the Government of Ontario (TS), Alzheimer Society of Canada (IA) and National Institutes of Health (KH).
Footnotes
- Scarcelli T, Jordão JF, Ellens N, O'Reilly M, McLaurin J, Hynynen K, Aubert I. Neuronal and astrocytic differentiation following transcranial focused ultrasound. Society for Neuroscience Meeting, San Diego, CA (Nov 2013).
- Scarcelli T, Jordão JF, Ellens N, O'Reilly M, McLaurin J, Hynynen K, Aubert I. Quantification of amyloid-beta load and cell differentiation following transcranial focused ultrasound in a mouse model of Alzheimer's disease. Canadian Neuroscience Meeting, Toronto, ON (May 2013).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lipsman N, Schwartz ML, Huang Y, et al. MR-guided Focused Ultrasound Thalamotomy For Essential Tremor: A Proof-of-Concept Study. Lancet Neurology. 2013;12(5):462–8. doi: 10.1016/S1474-4422(13)70048-6. [DOI] [PubMed] [Google Scholar]
- 2.Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA. Noninvasive MR Imaging-Guided Focal Opening of the Blood-Brain Barrier in Rabbits. Radiology. 2001;220:640–6. doi: 10.1148/radiol.2202001804. [DOI] [PubMed] [Google Scholar]
- 3.Kinoshita M, McDannold N, Jolesz FA, Hynynen K. Targeted Delivery of Antibodies Through the Blood-Brain Barrier by MRI-Guided Focused Ultrasound. Biochemical and Biophysical Research Communications. 2006;340:1085–90. doi: 10.1016/j.bbrc.2005.12.112. [DOI] [PubMed] [Google Scholar]
- 4.Kinoshita M, McDannold N, Jolesz FA, Hynynen K. Noninvasive Localized Delivery of Herceptin to the Mouse Brain by MRI-Guided Focused Ultrasound-Induced Blood-Brain Barrier Disruption. PNAS. 2006;103(31):11719–23. doi: 10.1073/pnas.0604318103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raymond SB, Treat LH, Dewey JD, McDannold NJ, Hynynen K, Bacskai BJ. Ultrasound Enhanced Delivery of Molecular Imaging and Therapeutic Agents in Alzheimer's Disease Mouse Models. PloS one. 2008;3(5):e2175. doi: 10.1371/journal.pone.0002175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burgess A, Ayala-Grosso CA, Ganguly M, Jordão JF, Aubert I, Hynynen K. Targeted Delivery of Neural Stem Cells to the Brain Using MRI-Guided Focused Ultrasound to Disrupt the Blood-Brain Barrier. PloS one. 2011;6(11):e27877. doi: 10.1371/journal.pone.0027877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thévenot E, Jordão JF, O'Reilly MA, et al. Targeted Delivery of Self-Complementary Adeno-Associated Virus Serotype 9 to the Brain, Using Magnetic Resonance Imaging-Guided Focused Ultrasound. Human Gene Therapy. 2012;23:1144–55. doi: 10.1089/hum.2012.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jordão JF, Ayala-Grosso CA, Markham K, et al. Antibodies Targeted to the Brain with Image-Guided Focused Ultrasound Reduces Amyloid-β Plaque Load in the TgCRND8 Mouse Model of Alzheimer's Disease. PloS one. 2010;5(5):e10549. doi: 10.1371/journal.pone.0010549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baseri B, Choi JJ, Deffieux T, et al. Activation of Signaling Pathways Following Localized Delivery of Systemically Administered Neurotrophic Factors Across the Blood-Brain Barrier using Focused Ultrasound and Microbubbles. Physics in Medicine and Biology. 2012;57:N65–81. doi: 10.1088/0031-9155/57/7/N65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ting CY, Fan CH, Liu HL, et al. Concurrent Blood-Brain Barrier Opening and Local Drug Delivery Using Drug-Carrying Microbubbles and Focused Ultrasound for Brain Glioma Treatment. Biomaterials. 2012;33:704–12. doi: 10.1016/j.biomaterials.2011.09.096. [DOI] [PubMed] [Google Scholar]
- 11.Min BK, Yang PS, Bohlke M, et al. Focused Ultrasound Modulates the Level of Cortical Neurotransmitters: Potential as a New Functional Brain Mapping Technique. International Journal of Imaging Systems and Technology. 2011;21:232–40. [Google Scholar]
- 12.Kim H, Taghados SJ, Fischer K, Maeng LS, Park S, Yoo SS. Noninvasive Transcranial Stimulation of Rat Abducens Nerve by Focused Ultrasound. Ultrasound in Medicine and Biology. 2012;38(9):1568–75. doi: 10.1016/j.ultrasmedbio.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang PS, Kim H, Lee W, et al. Transcranial Focused Ultrasound to the Thalamus is Associated with Reduced Extracellular GABA Levels in Rats. Neuropsychobiology. 2012;65:153–60. doi: 10.1159/000336001. [DOI] [PubMed] [Google Scholar]
- 14.Min BK, Bystritsky A, Jung KI, et al. Focused Ultrasound-Mediated Suppression of Chemically-Induced Acute Epileptic EEG Activity. BMC Neuroscience. 2011 Jan;12:23–35. doi: 10.1186/1471-2202-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoo SS, Bystritsky A, Lee JH, et al. Focused Ultrasound Modulates Region-Specific Brain Activity. NeuroImage. 2011;56:1267–75. doi: 10.1016/j.neuroimage.2011.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tufail Y, Matyushov A, Baldwin N, et al. Transcranial Pulsed Ultrasound Stimulates Intact Brain Circuits. Neuron. 2010;66:681–94. doi: 10.1016/j.neuron.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Ziadloo A, Burks SR, Gold EM, et al. Enhanced Homing Permeability and Retention of Bone Marrow Stromal Cells (BMSC) by Non-Invasive Pulsed Focused Ultrasound. Stem Cells. 2012;30(6):1216–27. doi: 10.1002/stem.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C, Croll S. Increased Neurogenesis and The Ectopic Granule Cells After Intrahippocampal BDNF Infusion in Adult Rats. Experimental Neurology. 2005;192:348–56. doi: 10.1016/j.expneurol.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 19.Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular Endothelial Growth Factor (VEGF) Stimulates Neurogenesis In Vitro and In Vivo. PNAS. 2002;99(18):11946–50. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin K, Sun Y, Xie L, et al. Neurogenesis and Aging: FGF-2 and HB-EGF Restore Neurogenesis in Hippocampus and Subventricular Zone of Aged Mice. Aging Cell. 2003;2:175–83. doi: 10.1046/j.1474-9728.2003.00046.x. [DOI] [PubMed] [Google Scholar]
- 21.Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. A Role for Adult Neurogenesis in Spatial Long-Term Memory. Neuroscience. 2005;130(4):843–52. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Li B, Yamamori H, Tatebayashi Y, et al. Failure of Neuronal Maturation in Alzheimer Disease Dentate Gyrus. Journal of Neuropathology and Experimental Neurology. 2008;67(1):78–84. doi: 10.1097/nen.0b013e318160c5db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Reilly MA, Hynynen K. A PVDF Receiver for Ultrasound Monitoring of Transcranial Focused Ultrasound Therapy. IEEE Transactions on Bio-Medical Engineering. 2010;57(9):2286–94. doi: 10.1109/TBME.2010.2050483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Reilly MA, Hynynen K. Blood-Brain Barrier: Real-Time Feedback-Controlled Focused Ultrasound Disruption by Using an Acoustic Emissions - Based Controller. Radiology. 2012;263(1):96–106. doi: 10.1148/radiol.11111417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jordão JF, Thévenot E, Markham-Coultes K, et al. Amyloid-β Plaque Reduction, Endogenous Antibody Delivery and Glial Activation by Brain-Targeted, Transcranial Focused Ultrasound. Experimental Neurology. 2013;248:16–29. doi: 10.1016/j.expneurol.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu B, Nagappan G, Guan X, Nathan PJ, Wren P. BDNF-Based Synaptic Repair as a Disease-Modifying Strategy for Neurodegenerative Diseases. Nature Reviews Neuroscience. 2013;14:401–16. doi: 10.1038/nrn3505. [DOI] [PubMed] [Google Scholar]
- 27.Reher P, Doan N, Bradnock B, Meghji S, Harris M. Effect of Ultrasound on the Production of IL-8, Basic FGF and VEGF. Cytokine. 1999;11(6):416–23. doi: 10.1006/cyto.1998.0444. [DOI] [PubMed] [Google Scholar]
- 28.Cao L, Jiao X, Zuzga DS, et al. VEGF Links Hippocampal Activity with Neurogenesis, Learning and Memory. Nature Genetics. 2004;36(8):827–35. doi: 10.1038/ng1395. [DOI] [PubMed] [Google Scholar]
- 29.Burgess A, Dubey S, Yeung S, et al. Society for Neuroscience. San Diego, CA, USA: Nov, 2013. (2013) Safety and Efficacy of Blood-Brain Barrier Disruption with Focused Ultrasound in a Mouse Model of Alzheimer's Disease. oral presentation, nanosymposium. [Google Scholar]


