Abstract
Much attention has been focused on the biological effects of equol, a metabolite of daidzein produced by intestinal microbiota. However, little is known about the role of isoflavone metabolizing bacteria in the intestinal microbiota. Recently, we isolated a dihydrodaidzein (DHD)-producing Clostridium-like bacterium, strain TM-40, from human feces. We investigated the effects of strain TM-40 on in vitro daidzein metabolism by human fecal microbiota from a male equol producer and two male equol non-producers. In the fecal suspension from the male equol non-producer and DHD producer, DHD was detected in the in vitro fecal incubation of daidzein after addition of TM-40. The DHD concentration increased as the concentration of strain TM-40 increased. In the fecal suspension from the equol producer, the fecal equol production was increased by the addition of strain TM-40. The occupation ratios of Bifidobacterium and Lactobacillales were higher in the equol non-producers than in the equol producer. Adding isoflavone-metabolizing bacteria to the fecal microbiota should facilitate the estimation of the metabolism of isoflavonoids by fecal microbiota. Studies on the interactions among equol-producing microbiota and DHD-producing bacteria might lead to clarification of some of the mechanisms regulating the production of equol by fecal microbiota.
Keywords: daidzein, equol, dihydrodaidzein, fecal microbiota
INTRODUCTION
Daidzein and daidzin are metabolized to equol by intestinal bacterial microbiota [1]. Equol is considerably more estrogenic than daidzein. It has been reported that equol is 100-fold more potent than daidzein in stimulating an estrogenic response. Equol is also stronger than daidzein at competing with 3H-estradiol for binding to the estrogen receptor (ER), suggesting that equol has a higher affinity for ER [2]. It has also been demonstrated to be a more effective antioxidant than daidzein or genistein [3, 4]. Equol or the ability to produce equol is related to anticarcinogenic characteristics in humans. A case-control study involving residents in Japan and Korea demonstrated that the ability to produce equol is closely related to a lower incidence of prostate cancer [5]. Thus, equol is an important bacterial metabolite in the gut. However, interindividual variations in equol production have been identified. Only 30% to 50% of humans are equol producers [6, 7]. Dihydrodaidzein (DHD), a bacterial metabolite of the widespread isoflavone, daidzein [8], is believed to be a precursor of equol [8]. An equol-producing bacterium, Slackia equolifaciens, which produces equol from daidzein, has been isolated from human feces [9], and an equol-producing bacterium, which produces equol from DHD, has also been isolated from human feces [10]. Although the latter bacterium cannot produce equol from daidzein, it can produce equol from DHD, which is a bacterial metabolite of daidzein, and seems to be an important bacterial metabolite related to equol production. We previously isolated the DHD-producing Clostridium-like bacterium, strain TM-40, from healthy human feces [11]. It seems important to clarify the role of the DHD-producing bacteria in equol production in the gut. However, few reports have addressed the effects of DHD-producing intestinal bacteria on in vitro daidzein metabolism by human fecal microbiota.
We tested our hypothesis that the DHD-producing Clostridium-like intestinal bacterium, strain TM-40, significantly affects the metabolism of isoflavonoids in a human male equol producer and two human male equol non-producers in vitro.
MATERIALS AND METHODS
Chemicals
Daidzein and equol were purchased from LC Laboratories, a division of PKC Pharmaceuticals, Inc. (Woburn, MA, USA). Dihydrodaidzein (DHD) was purchased from Toronto Research Chemicals, Inc. (North York, ON, Canada).
Sampling
We selected the feces of three adult men (from 21 to 45 years old) previously checked for equol production. The equol and DHD production of the three types of feces were as follows: equol non-producer and DHD non-producer, equol non-producer and DHD producer, and equol producer (equol production). Feces from the male equol producer and non-producers were collected and immediately processed by in vitro incubation of daidzein with the fecal microbiota. Feces from the male equol producer and non-producers were stored at –80 °C until T-RFLP analysis. This study was performed under following the principals of the Helsinki Declaration. The Human Investigations Review Board of the National Food Research Institute approved the study protocol, and informed consent was obtained from the subjects.
The character of strain TM-40
Strain TM-40 was isolated from a healthy boy’s feces. DHD was produced by in vitro incubation of daidzein with fecal microbiota from the boy’s feces. The cells of TM-40 were Gram-positive, rod-shaped, and anaerobic. Strain TM-40 produces DHD from both daidzein and daidzin. The sequence data were aligned, and the assembled partial 16S rRNA sequences were compared with those available in the GenBank database. The 16S rRNA partial sequence of strain TM-40 (Accession no. AB249652) that was isolated from human feces exhibited a 93% similarity to that of Coprobacillus catenaformis (Accession no. AB030218). This strain was identified to be a new species [11].
Effects of Strain TM-40 on in vitro incubation of daidzein with fecal microbiota from a human male equol producer and two non-producers
The following methods of in vitro fecal incubation of daidzein with fecal microbiota were employed. The anaerobic medium used in this experiment was prepared as follows. Brain heart infusion (37 g), agar (1 g), l-cysteine HCl (0.5 g), and Na2CO3 (4 g) were dissolved in 1000 ml distilled water. Aliquots of the broth (9 ml) were then distributed to test tubes, gassed with O2-free CO2, sealed with a butyl rubber stopper, and sterilized by autoclaving. Freshly voided feces (1 g) were collected in sterile glass homogenizers. Thirty milliliters of an anaerobic medium were added to the feces, and the mixture was homogenized. Daidzein (20 mg) was dissolved in 1 ml dimethyl sulphoxide (DMSO). The daidzein solution (1 μl) was transferred into 0.2 ml of homogenate. In the incubation mixture with strain TM-40, strain TM-40 previously incubated anaerobically for 24 h on an Eggerth-Gagnon (EG) agar plate was suspended in the anaerobic medium by inoculating loop-bearing bacteria isolated from the EG agar medium and adjusted to a concentration of 1010cells/ml (final concentration 2 × 108 cells/ml; high concentration) or 108 cells/ml (final concentration 2 × 106 cells/ml; low concentration). Four microliters of the anaerobic medium suspended with strain TM-40 were added to the incubation mixture (0.2 ml of fecal homogenate plus 1 μl daidzein solution). In the control incubation mixture, 4 μl of the anaerobic medium was added to the incubation mixture. The solution was incubated under an atmosphere of CO2 generated using the AnaeroPack system (Mitsubishi Gas Chemical Company, Inc., Tokyo, Japan) for 24 h at 37°C. After incubation, methanol-acetic acid (100:5, v/v) was added to the incubation mixture to make a total volume of 1.0 ml. The mixture was vortexed for 120 s and centrifuged at 5,000 × g at 4°C for 10 min. The supernatant was filtered through a 0.2-μm filter. The filtrate was subjected to high-performance liquid chromatography (HPLC) analysis.
HPLC analysis
For HPLC analysis, we injected 20 μl of each preparation into a 250 × 4.6 mm Capcell Pak C18 5-μm column (Shiseido, Tokyo, Japan). To detect isoflavonoids, a JASCO MD-1515 photodiode array detector (JASCO, Co., Ltd., Tokyo, Japan) was used to monitor the spectral data from 200 to 400 nm for each peak. We used standard samples of daidzein, DHD and equol to measure the isoflavonoids. We used the spectral data of 254 nm to quantify daidzein, 280 nm to quantify DHD, and 280 nm to quantify equol. The mobile phase consisted of methanol/acetic acid/water (35:5:60, v/v/v). The HPLC system was operated at a column temperature of 40°C and a flow rate of 1 ml/min.
DNA extraction from feces
DNA was extracted from feces according to Matsuki’s methods [12] with some modification. Feces (20 mg) were washed three times by suspending them in 1.0 ml of phosphate-buffered saline and centrifuging each preparation at 14,000 rpm in order to remove possible PCR inhibitors. The fecal pellets were resuspended in a solution containing 250 μl of extraction buffer (200 mM Tris-HCl, 80 mM EDTA; pH 9.0) and 50 μl of 10% sodium dodecyl sulphate. Three hundred milligrams of glass beads (0.1 mm diameter) and 500 μl of buffer-saturated phenol were added to the suspension, and the mixture was vortexed vigorously for 60 s in a mini-bead beater (BioSpec Products, Bartlesville, OK, USA) at a power level of 4800 rpm. After centrifugation at 14,000 rpm for 5 min, 400 μl of the supernatant was collected. Subsequently, phenol-chloroform-isoamyl alcohol extractions were performed, and 250 μl of the supernatant was subjected to isopropanol precipitation. Finally, the DNA was suspended in 1 ml of Tris-EDTA buffer. The DNA preparation was adjusted to a final concentration of 10 μg/ml in TE and checked by 1.5% agarose gel electrophoresis.
PCR conditions and restriction enzyme digestion
The PCR mixture (25 μl) was composed of EX Taq buffer, 2 mM Mg2+, and each deoxynucleoside triphosphate at a concentration of 200 μmol/l, template DNA, and 0.625 U of TaKaRa EX Taq DNA polymerase (Takara Bio Inc., Otsu, Japan). The amount of fecal DNA was 10 ng. The primers used were 5’ FAM-labelled 516f (5’-TGCCAGCAGCCGCGGTA-3’) and 1510r (5’-GGTTACCTTGTTACGACTT-3’) at concentrations of 0.10 μmol/l. This process was carried out using the Dice PCR System (Takara Bio, Inc.). The amplification program consisted of one cycle at 95°C for 15 min, followed by 30 cycles at 95°C for 30 sec, 50°C for 30 sec, 72°C for 1 min, and finally one cycle at 72°C for 10 min. The amplification products were subjected to gel electrophoresis in 1.5% agarose followed by ethidium bromide staining. The PCR products were purified using QIAquick spin columns (Qiagen KK, Tokyo, Japan) according to the manufacturer’s instructions. The purified DNA was treated with 2U of BslI (New England Biolabs) for 3 h, at 55°C [13].
2.6. T-RFLP analysis
The fluorescently labelled T-RFs were analyzed by electrophoresis on an ABI PRISM 310 Genetic Analyzer automated sequence analyzer (Applied Biosystems) in GeneScan mode. Two microliters of the restriction enzyme digestion mixture was mixed with 0.5 μl of MapMarker 1000 size standard (BioVentures, Inc.) and 12 μl of deionized formamide, followed by denaturation at 96°C for 2 min and immediate chilling on ice. The injection time was 30 s for the analysis of T-RFs from the digestion with BslI. The run time was 40 min. The lengths and peak areas of T-RFs were determined with GeneMapper software. The predominant operational taxonomic units (OTUs, which correspond to either T-RFs or T-RF clusters) that were detected in the T-RFLP profiles from the fecal contents were applied to the phylogenetic groups of intestinal microbiota [13].
RESULTS
Effects of strain TM-40 on the in vitro incubation of daidzein with fecal microbiota from the male equol and DHD non-producer
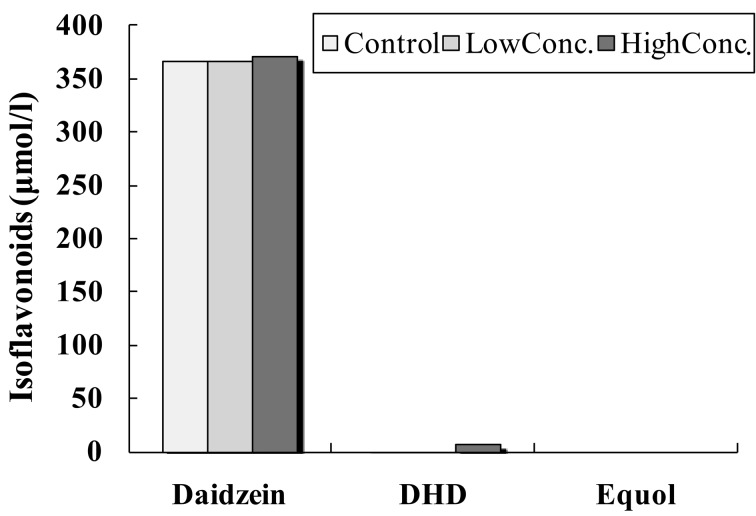
In the fecal suspension from the male equol non-producer and DHD non-producer (Fig. 1), DHD was not detected in the in vitro fecal incubation of daidzein in the control incubation solution. However, DHD was detected in the in vitro fecal incubation of daidzein by after the addition of the high concentration of the DHD-producing bacterium, TM-40. The DHD concentration was low in the in vitro fecal incubation of daidzein when the low concentration of strain TM-40 was added. Equol was not detected in any fecal suspension from the male equol and DHD non-producer.
Fig. 1.
Equol, daidzein, and DHD concentrations of the fecal suspension from the male equol non-producer and DHD non-producer. The fecal incubation mixture was supplemented with a high or low concentration of strain TM-40. The control fecal incubation mixture was supplemented with an anaerobic medium.
Effects of strain TM-40 on the in vitro incubation of daidzein with fecal microbiota from the male equol non-producer and DHD producer
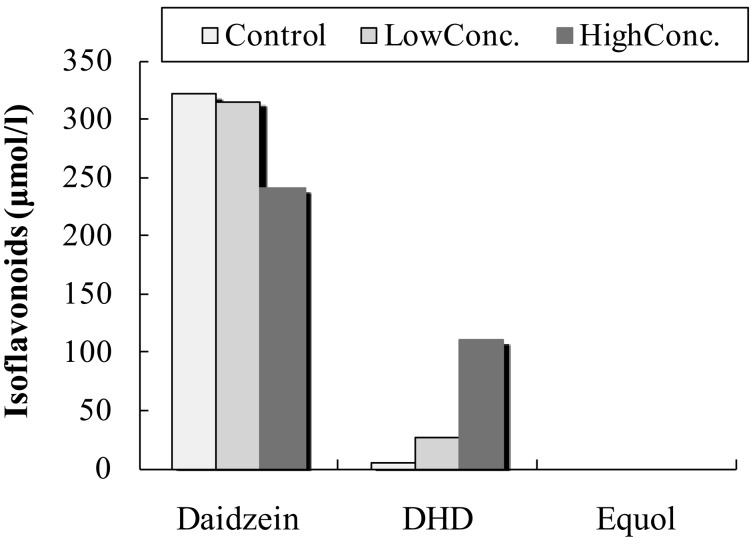
In the fecal suspension from the male equol non-producer and DHD producer (Fig. 2), DHD was detected in the in vitro fecal incubation of daidzein in the presence of either a low or high concentration of the DHD-producing bacterium, TM-40. DHD was also detected in the control incubation solution. However, the DHD concentration was the highest in the fecal suspension supplemented with the high concentration of strain TM-40, moderate in the presence of the low concentration of TM-40, and the lowest in the control fecal suspension. The DHD concentration increased as the concentration of strain TM-40 increased. Adding strain TM-40 to the fecal suspension greatly increased DHD production in the male equol non-producer and DHD producer, compared to the male equol and DHD non-producer.
Fig. 2.
Equol, daidzein, and DHD concentrations of the fecal suspension from the male equol non-producer and DHD producer. The fecal incubation mixture was supplemented with a high or low concentration of strain TM-40. The control fecal incubation mixture was supplemented with an anaerobic medium.
Effects of strain TM-40 on in vitro incubation of daidzein with fecal microbiota from the male equol producer
In the fecal suspension from the male equol producer (Fig. 3), DHD and equol were detected in the in vitro fecal incubation of daidzein by adding a low or high concentration of strain TM-40. DHD and equol were also detected in the control incubation solution. The equol concentration was the highest in the fecal suspension supplemented with the high concentration of strain TM-40, moderate in the presence of the low concentration of strain TM-40, and the lowest in the control fecal suspension. The equol concentration in the fecal suspensions increased as the concentration of strain TM-40 increased. In contrast, the DHD concentration was the highest in the fecal suspension supplemented with the low concentration of strain TM-40, moderate in the control fecal suspension, and lowest in the fecal suspension supplemented with the high concentration of strain TM-40. Strain TM-40 affected both DHD and equol production in the in vitro incubation of daidzein with fecal microbiota from the male equol producer. In fecal suspensions from the equol producers, the equol/daidzein ratio increased as the concentration of strain TM-40 increased.
Fig. 3.
Equol, daidzein, and DHD concentrations of the fecal suspension from the male equol producer. The fecal incubation mixture was supplemented with a high or low concentration of strain TM-40. The control fecal incubation mixture was supplemented with an anaerobic medium.
Fecal microbiota of male equol producer and non-producers
It has been confirmed that human intestinal microbiota predominantly consist of members of approximately 10 phylogenetic bacterial groups and that these bacterial groups can be distinguished by the T-RFLP system developed by Nagashima et al. [13, 14]. Figure 4 depicts the compositions of the fecal microbiota, which differed between the equol producer and the equol non-producers. The occupation ratios of Bifidobacterium and Lactobacillales were higher in the equol non-producers than in the equol producer. The occupation ratio of Bifidobacterium was highest in the DHD producer and equol non-producer.
Fig. 4.
Composition of fecal intestinal microbiota of the male equol producer and non-producers. OTUs (operational taxonomic units), which correspond to either T-RFs (terminal restriction fragments) or T-RF clusters were identified by T-RFLP analysis. The alphabetical key to Fig. 4 refers to the following phylogenetic bacterial groups. A: Bacteroides, Clostridium cluster IV (OTUs 370). B: Clostridium cluster IV (OTUs 168, 749). C: Clostridium cluster IX, Megamonas (OTUs 110). D: Clostridium cluster XI (OTUs 338). E: Clostridium subcluster XIVa (OTUs 106, 494, 505, 517, 754, 955, 990)., F: Clostridium cluster XI, Clostridium subcluster XIVa (OTUs 919). G: Clostridium subcluster XIVa, Enterobacteriales (OTUs 940). H: Clostridium cluster XVIII (OTUs 423, 650). I: Bacteroides (OTUs 469, 853). J: Bifidobacterium (OTUs 124). K: Lactobacillales (OTUs 332, 520, 657). L: Prevotella (OTUs 137, 317). M: Others
DISCUSSION
Much attention has been focused on the biological effects of equol (4’,7-dihydroxyisoflavan), a metabolite of daidzein produced by intestinal microbiota. DHD is also a bacterial metabolite of daidzein and is a proposed precursor of equol [8], and seems to be an important substance in bacterial equol production in vivo. Recently, we isolated the DHD-producing bacterium TM-40 from the feces of a healthy human. The TM-40 strain produced DHD from daidzein [11]. Estimating the effects of DHD-producing bacteria on equol production of intestinal microbiota is important for clarifying the role of DHD-producing bacteria in equol production in the gut. We examined the effects of DHD-producing intestinal bacteria on in vitro daidzein metabolism by human fecal microbiota from a male equol producer and two male equol non-producers. Despite the limited number of samples, we could examine the effects of the DHD-producing human intestinal bacterium, TM-40, on in vitro fecal incubation with daidzein from both the equol producer and the equol non-producers. Our results reveal that the DHD-producing bacterium, TM-40, affected DHD production by fecal microbiota in both the equol producer and the equol non-producers.
In the equol non-producer and DHD producer, DHD production was greatly increased by adding the low concentration of TM-40 to the in vitro fecal incubation solution with daidzein. In contrast, in the equol and DHD non-producer, DHD production was slightly increased by adding the low concentration of TM-40 to the in vitro fecal incubation solution with daidzein. The most important difference between these two male subjects was that one was able to produce DHD from daidzein, while the other was not. Adding DHD-producing bacteria to the fecal suspension did not produce a significant amount of DHD in the equol and DHD non-producer and one possible explanation for this is that DHD production from daidzein was suppressed by some inhibitory substances and/or some inhibitory bacteria that exist in the gut. It has been reported that several bacteria may be involved in daidzein metabolism, and that they may differ among subjects [15]. Our experiment revealed three types of daidzein metabolism. The results suggest that the DHD-producing bacterium, strain TM-40, assists equol production via the fecal microbiota even in the presence of different kinds of fecal microbiota. It has been suggested that DHD is an intermediate substance bacterial equol production from daidzein [8]. Equol production might be increased or stimulated by increased DHD production induced by strain TM-40. In general, many kinds of bacteria exist in the gut, and intestinal bacteria seem to affect the environment of the gut by producing many kinds of metabolites, such as short chain fatty acids (SCFAs) [16, 17], bacteriocin [18], and gas [19,20,21,22]. The gut environment of the DHD and equol producer appears to be suitable for the production of DHD by DHD-producing bacteria.
In our results, the occupation ratios of Bifidobacterium and Lactobacillales were higher in the equol non-producers than in the equol producer. It has previously been reported that many equol producing bacteria belong to the genus Slackia, Adlercreutzia or Eggerthella. Intestinal bacteria of Bifidobacterium and Lactobacillales produce short chain fatty acids in the gut and affect the microecology of the gut. Many kinds of intestinal bacteria live in the gut and impact on each other by competing for nutrition for their survival. It has been reported that a combination of dietary fructooligosaccharides and isoflavone conjugates increases equol production in ovariectomized mice [23]. Dietary fructooligosaccharides increased the population of bifidobacteria in feces and lower the stool pH [24]. However, some intestinal bacteria other than bifidobacteria also utilize the fructooligosaccharides. Further studies must be undertaken to understand the relationship between Bifidobacterium, Lactobacillales and equol-producing bacterium.
Our investigation revealed that adding isoflavone-metabolizing bacterium to fecal microbiota is useful for stimulating the metabolism of isoflavonoids by fecal microbiota. Studies of the interactions among equol-producing microbiota and DHD-producing bacteria might lead to the identification of mechanisms regulating equol production by fecal microbiota. Our study was limited by the fact that we could not measure the composition of the intestinal microbiota from several human equol producers and non-producers. Further studies should be conducted to examine the relationship between the composition of the intestinal microbiota and equol production in large scale volunteer studies.
Acknowledgments
This study was financially supported by the Ministry of Agriculture, Forestry and Fisheries of Japan.
REFERENCES
- 1.Bowey E, Adlercreutz H, Rowland I. 2003. Metabolism of isoflavones and lignans by the gut microflora: a study in germ-free and human flora associated rats. Food Chem Toxicol 41: 631–636 [DOI] [PubMed] [Google Scholar]
- 2.Sathyamoorthy N, Wang TT. 1997. Differential effects of dietary phyto-oestrogens daidzein and equol on human breast cancer MCF-7 cells. Eur J Cancer 33: 2384–2389 [DOI] [PubMed] [Google Scholar]
- 3.Mitchell JH, Gardner PT, McPhail DB, Morrice PC, Collins AR, Duthie GG. 1998. Antioxidant efficacy of phytoestrogens in chemical and biological model systems. Arch Biochem Biophys 360: 142–148 [DOI] [PubMed] [Google Scholar]
- 4.Vedavanam K, Srijayanta S, O’Reilly J, Raman A, Wiseman H. 1999. Antioxidant action and potential antidiabetic properties of an isoflavonoid-containing soyabean phytochemical extract (SPE). Phytother Res 13: 601–608 [DOI] [PubMed] [Google Scholar]
- 5.Akaza H, Miyanaga N, Takashima N, Naito S, Hirao Y, Tsukamoto T, Fujioka T, Mori M, Kim WJ, Song JM, Pantuck AJ. 2004. Comparisons of percent equol producers between prostate cancer patients and controls: case-controlled studies of isoflavones in Japanese, Korean and American residents. Jpn J Clin Oncol 34: 86–89 [DOI] [PubMed] [Google Scholar]
- 6.Cassidy A, Brown JE, Hawdon A, Faughnan MS, King LJ, Millward J, Zimmer-Nechemias L, Wolfe B, Setchell KD. 2006. Factors affecting the bioavailability of soy isoflavones in humans after ingestion of physiologically relevant levels from different soy foods. J Nutr 136: 45–51 [DOI] [PubMed] [Google Scholar]
- 7.Song KB, Atkinson C, Frankenfeld CL, Jokela T, Wähälä K, Thomas WK, Lampe JW. 2006. Prevalence of daidzein-metabolizing phenotypes differs between Caucasian and Korean American women and girls. J Nutr 136: 1347–1351 [DOI] [PubMed] [Google Scholar]
- 8.Rowland I, Faughnan M, Hoey L, Wähälä K, Williamson G, Cassidy A. 2003. Bioavailability of phyto-oestrogens. Br J Nutr 89:(Suppl 1): S45–S58 [DOI] [PubMed] [Google Scholar]
- 9.Jin JS, Kitahara M, Sakamoto M, Hattori M, Benno Y. 2010. Slackia equolifaciens sp. nov., a human intestinal bacterium capable of producing equol. Int J Syst Evol Microbiol 60: 1721–1724 [DOI] [PubMed] [Google Scholar]
- 10.Wang XL, Hur HG, Lee JH, Kim KT, Kim SI. 2005. Enantioselective synthesis of S-equol from dihydrodaidzein by a newly isolated anaerobic human intestinal bacterium. Appl Environ Microbiol 71: 214–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tamura M, Tsushida T, Shinohara K. 2007. Isolation of an isoflavone-metabolizing, Clostridium-like bacterium, strain TM-40, from human faeces. Anaerobe 13: 32–35 [DOI] [PubMed] [Google Scholar]
- 12.Matsuki T. 2006. Procedure of DNA Extraction from Fecal Sample for the Analysis of Intestinal Microflora. J Intestinal Microbiol 20: 259–262 [Google Scholar]
- 13.Nagashima K, Hisada T, Sato M, Mochizuki J. 2003. Application of new primer-enzyme combinations to terminal restriction fragment length polymorphism profiling of bacterial populations in human feces. Appl Environ Microbiol 69: 1251–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagashima K, Mochizuki J, Hisada T, Suzuki S, Shimomura K. 2006. Phylogenetic analysis of 16S ribosomal RNA gene sequences from human fecal microbiota and improved utility of terminal restriction fragment length polymorphism profiling. Biosci Microflora 25: 99–107 [Google Scholar]
- 15.Atkinson C, Berman S, Humbert O, Lampe JW. 2004. In vitro incubation of human feces with daidzein and antibiotics suggests interindividual differences in the bacteria responsible for equol production. J Nutr 134: 596–599 [DOI] [PubMed] [Google Scholar]
- 16.McBurney MI, Thompson LU. 1989. Effect of human faecal donor on in vitro fermentation variables. Scand J Gastroenterol 24: 359–367 [DOI] [PubMed] [Google Scholar]
- 17.Khan KM, Edwards CA. 2005. In vitro fermentation characteristics of a mixture of Raftilose and guar gum by human faecal bacteria. Eur J Nutr 44: 371–376 [DOI] [PubMed] [Google Scholar]
- 18.Zhu WM, Liu W, Wu DQ. 2000. Isolation and characterization of a new bacteriocin from Lactobacillus gasseri KT7. J Appl Microbiol 88: 877–886 [DOI] [PubMed] [Google Scholar]
- 19.Strocchi A, Furne J, Ellis C, Levitt MD. 1994. Methanogens outcompete sulphate reducing bacteria for H2 in the human colon. Gut 35: 1098–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pouteau E, Vahedi K, Messing B, Flourié B, Nguyen P, Darmaun D, Krempf M. 1998. Production rate of acetate during colonic fermentation of lactulose: a stable-isotope study in humans. Am J Clin Nutr 68: 1276–1283 [DOI] [PubMed] [Google Scholar]
- 21.Chassard C, Bernalier-Donadille A. 2006. H2 and acetate transfers during xylan fermentation between a butyrate-producing xylanolytic species and hydrogenotrophic microorganisms from the human gut. FEMS Microbiol Lett 254: 116–122 [DOI] [PubMed] [Google Scholar]
- 22.Levitt MD, Furne JK, Kuskowski M, Ruddy J. 2006. Stability of human methanogenic flora over 35 years and a review of insights obtained from breath methane measurements. Clin Gastroenterol Hepatol 4: 123–129 [DOI] [PubMed] [Google Scholar]
- 23.Ohta A, Uehara M, Sakai K, Takasaki M, Adlercreutz H, Morohashi T, Ishimi Y. 2002. A combination of dietary fructooligosaccharides and isoflavone conjugates increases femoral bone mineral density and equol production in ovariectomized mice. J Nutr 132: 2048–2054 [DOI] [PubMed] [Google Scholar]
- 24.Mitsuoka T, Hidaka H, Eida T. 1987. Effect of fructo-oligosaccharides on intestinal microflora. Nahrung 31: 427–436 [DOI] [PubMed] [Google Scholar]