Abstract
Lactobacillus pentosus (L. pentosus) strain S-PT84, isolated from Kyoto pickles, enhances splenic natural killer (NK) cell activity, and has high T-helper1 (Th1) cytokine and type 1-IFN (IFN-α) inducing activity. In the present study, we investigated the influence of S-PT84 ingestion on the mucosal immunity of healthy and Salmonella Typhimurium (S. Typhimurium)-infected mice. In the S. Typhimurium infection model, numbers of S. Typhimurium in feces and the spleen were significantly decreased, and body weight loss and deterioration in the general health score of S. Typhimurium-infected mice were improved by S-PT84 ingestion. Oral administration of S-PT84 enhanced IL-5 and IL-6 production from Peyer’s patch cells in vitro, with a concomitant significant increase in IgA production from Peyer’s patch cells, which may explain the mechanism of enhanced IgA production in the small intestine in vivo. These results suggest that S-PT84 ingestion is useful for the maintenance of health or the improvement of certain symptoms during pathogen infection.
Keywords: Lactobacillus pentosus S-PT84, IgA, IL-5, IL-6, Salmonella Typhimurium, Peyer’s patch
INTRODUCTION
Lactic acid bacteria are widely used as a health food and are generally recognized as safe. These bacteria are useful in the treatment of several type of diarrhea, including antibiotics-associated, pathogen-induced or traveler’s diarrhea [1,2,3,4]. Several reports have indicated that lactic acid bacteria ingestion may inhibit pathogen invasion into mouse organs through immunomodulating effects in the intestine [5,6,7]. Other reports have indicated that heat-killed bacteria may show an anti-infective effect against S. Typhimurium [8, 9]. Furthermore, these bacteria were observed to improve inflammatory bowel disease or irritable bowel syndrome [10,11,12]. Therefore, it is thought that lactic acid bacteria in the intestine provide various beneficial effects to the host. In addition to the above reports, many reports have indicated that lactic acid bacteria [13, 14] or probiotics [15, 16] modulate systemic or mucosal immunity. However, while there is one report that a L. pentosus strain enhances salivary IgA production [17], it is not known whether L. pentosus strains affect mucosal immunity in the intestine.
L. pentosus strain S-PT84 is a lactic acid bacterium of plant origin which was isolated from Kyoto pickles (shibazuke). S-PT84 stimulates IL-12 and IFN-γ production through interactions between dendritic cells and natural killer (NK) cells [18]. S-PT84 enhances splenic NK cell activity and exhibits anti-allergic effects by modulating the T-helper 1 and T-helper 2 ratio and inducing regulatory T cells [19]. Furthermore, intranasal administration of S-PT84 enhances IL-12 and IFN-α production in the respiratory tract, promotes NK activity against influenza virus infection in the lung, improves weight loss, and reduces mortality associated with viral infection [20]. These results indicate that S-PT84 can stimulate the immune function in the spleen or in the respiratory tract and counteract viral infection. However, the effect of S-PT84 ingestion on mucosal immunity (as the first contact immune organ after lactic acid bacteria ingestion) has not yet been elucidated. In this study, we investigated the influence of S-PT84 ingestion on the mucosal immunity of healthy and S. Typhimurium-infected mice.
MATERIALS AND METHODS
Animals
BALB/c male and female mice, 4- to 10-weeks-old, were obtained from Japan SLC, Inc. (Hamamatsu, Japan). These animals were fed a commercial diet (AIN-93M, Oriental Yeast Co. Ltd., Tokyo, Japan) and tap water ad libitum, and they were housed for 1 week at 25 ± 1°C and 60 ± 5% humidity under a 12-hr light-dark cycle before experimentation. Experiments were approved by the Animal Care and Use Committee of Suntory Holdings Limited and performed according to the Guideline for Animal Care and Use of Suntory Holdings Limited (This guideline complies with the Law for the Humane Treatment and Management of Animals, Law No. 105, 1973, as revised on June 1, 2006).
Preparation of L. pentosus S-PT84
S-PT84 was cultured in MRS broth (Difco Laboratories, Detroit, MI, USA) at 37°C for 24 hr. Cultured S-PT84 were collected by centrifugation at 9190×g for 10 min, washed twice with sterile saline, washed with distilled water, and heat-killed at 95°C for 5 min. Heat-killed S-PT84 were freeze-dried and then added at 0.0075 wt% or 0.075 wt% to AIN-93M diet. Numbers of S-PT84 per unit weight were 500 million cells/mg.
Preparation of S. Typhimurium LT-2
S. Typhimurium LT-2 (ATCC15277) was cultured in Luria-Bertani (LB) broth (Difco Laboratories, Detroit, MI, USA). One hundred microliters of S. Typhimurium stock solution was added to 10 ml of LB broth, and cultured at 37°C for 2–6 hr until the OD550 reached 0.2– 0.3. Next, 2 ml of this culture solution was added to 1000 ml LB broth and cultured at 37°C for 18 hr. Cultured S. Typhimurium were collected by centrifugation at 9190×g for 10 min, washed with distilled water, and suspended at 2 × 109 colony forming unit (cfu)/ml in phosphate-buffered saline (PBS, Nissui Pharmaceutical Co., Tokyo, Japan).
S. Typhimurium infection model
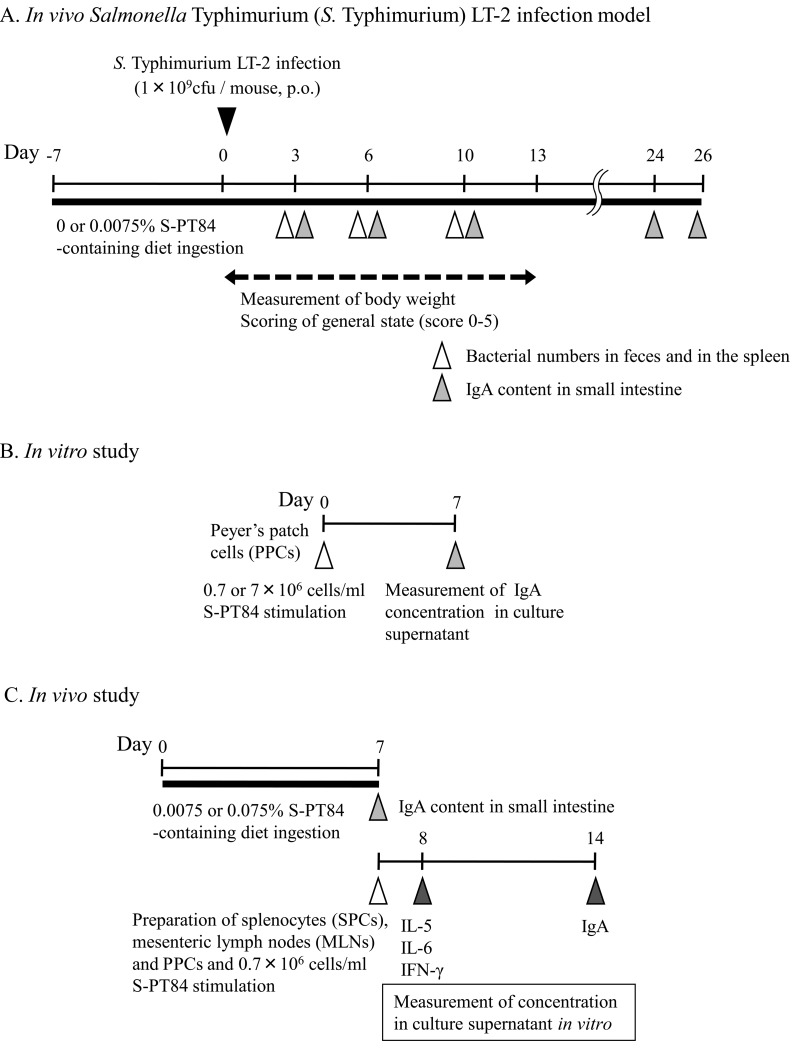
The experimental procedure is summarized in Fig. 1A. AIN-93M control diet or diet containing 0.0075% of S-PT84 was given for 7 days before S. Typhimurium infection. On the day following the 7-day ingestion period of each diet, mice were orally administered 0.5 ml of 5.6% NaHCO3 in Hank’s Balanced Salt Solution (HBSS, Invitrogen, California, USA) for neutralization of gastric acid. After 15 min, 0.5 ml of S. Typhimurium LT-2 (2 × 109 cfu/ml) was orally administered to mice. The mice were subsequently maintained on their respective diets until examination. The changes in body weight and general health scores of the mice were monitored for 13 days after infection. The general health score was scored following the description of Shu et al. (7). Score 5 : Mouse bright-eyed and alert, has a smooth coat with a sheen, responds to stimulus, shows interest in its environment. Score 4 : Fur slightly ruffled, a loss of sheen to the coat, mouse remains alert and active. Score 3 : Fur noticeably ruffled, parts of coat form clumps, mouse not as alert or active, less interested in environment outside of cage, signs of hyperventilating when handled. Score 2 : Mouse hunched over and sleepy, little interest shown in environment, fur clumped. Score 1 : Mouse not reactive to stimulus, fur has a “bottle brush” appearance, i.e., standing on end, mouse hunched over preferring to sleep than react to environment, mouse cold to touch, paws are cold to touch; Score 0 : death.
Fig. 1.
Experimental procedure of this study.
The feces and the spleen of each mouse were collected at 3, 6 and 10 days after infection, for determination of S. Typhimurium numbers. These samples were homogenized individually in 0.1% peptone water. One hundred microliters of the supernatants (serially diluted in 0.1% peptone water) were plated on LB agar (containing 1.5% agar and 20 g/l LB broth), and S. Typhimurium colonies were enumerated after incubation at 37°C for 24 h.
Mice were anesthetized with diethyl ether and sacrificed by exsanguinations at 3, 6, 10 or 24–26 days after infection to collect the small intestine, for determination of IgA concentration, and homogenized individually in PBS with 0.05% Tween 20. After centrifugation (9190×g, 10 min), supernatants were collected and stored at –80°C until further analysis.
Effect of S-PT84 on IgA production from Peyer’s patch cells in vitro
The experimental procedure is summarized in Fig. 1B. Mice were anesthetized with diethyl ether and sacrificed by exsanguination. Peyer’s patches in the small intestine were aseptically removed, and Peyer’s patch cells (PPCs) were prepared after removal of erythrocytes. PPCs were cultured in the absence or presence of S-PT84 (at 0.7×106 or 7×106 cells/ml) in 0.2 ml (approximately 2 × 106 cells/ ml) of RPMI-1640 (Nakalai Tesque, Kyoto, Japan) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Cambrex, Charles City, IA, USA), 100 U/ ml of penicillin, and 100 μg/ml of streptomycin in a 96-well culture plate (Corning, Corning, NY, USA) at 37°C for 7 days. Then, supernatants were collected for determination of IgA concentration and stored at –80°C until further analysis.
Effect of S-PT84 ingestion on IgA and cytokine production from Peyer’s patch cells (PPCs), mesenteric lymph nodes (MLNs) cells and splenocytes (SPCs)
The experimental procedure is summarized in Fig. 1C. AIN-93M control diet or S-PT84 diet (0.0075% or 0.075%) was given for 7 days before each tissue separation. The day following the 7-day ingestion period of each diet, mice were anesthetized with diethyl ether and sacrificed by exsanguination. The small intestine of each mouse was collected for determination of IgA content, and homogenized individually in PBS with 0.05% Tween 20. After centrifugation (9190 × g, 10 min), supernatants were collected and stored at –80°C until further analysis. SPCs, MLNs and PPCs were aseptically removed, and the cells from each tissue were prepared after depletion of erythrocytes. PPCs and MLNs were cultured in the presence of S-PT84 (at 0.7 × 106 cells/ml) in 0.2 ml (approximately 2 × 106 cells/ml), and SPCs, in 0.5 ml (approximately 5 × 106 cells/ml) of RPMI-1640 supplemented with 10% FBS, 100 U/ml of penicillin, and 100 microg/ml of streptomycin in a 96-well culture plate at 37°C for 24 hr (for determination of cytokine concentration) or 168 hr (for determination of IgA concentration). Supernatants were collected and stored at –80°C until further analysis.
ELISA for determination of cytokine or IgA concentration
IL-5, IL-6 or IFN-γ concentration was measured using BD OptEIATM Set Mouse IL-5, IL-6 or IFN-γ ELISA kits (BD Bioscience, NJ, USA), respectively, according to the manufacturer’s recommended protocol. Total and S. Typhimurium-specific IgA concentrations were also determined by ELISA. ELISA plates (96-well) were coated overnight with purified goat anti-mouse IgA (Southern Biotech, Alabama, USA) or 1× 107 cfu/well of UV-killed S. Typhimurium LT-2 in carbonate coating buffer. Each sample was added, and incubated for 1 hr. Plates were washed with PBS containing 0.05% Tween 20 and treated with biotin-conjugated anti-mouse IgA (Southern Biotech, Alabama, USA). Plates were labeled using streptavidin-horseradish peroxidase (HRP) (1/250, Millipore, Billerica, MA, USA), and developed using a tetramethylbenzidine and hydrogen peroxide substrate. Absorption at 450 nm was determined, and the IgA concentration was calculated as μg/g tissue or absorption/ g tissue.
Statistical analysis
Data are presented as the mean ± standard error (SE). Significant differences in values were determined using Student’s t-test or two-way ANOVA. p-values less than 0.05 were considered significant.
RESULTS
Effects of S-PT84 ingestion on S. Typhimurium LT-2-infected BALB/c mice
To determine whether several symptoms of pathogenic microbe infection are improved by S-PT84 ingestion, we examined the effects of S-PT84 ingestion on body weight change, general health score and mucosal immunity in S. Typhimurium LT-2-infected mice.
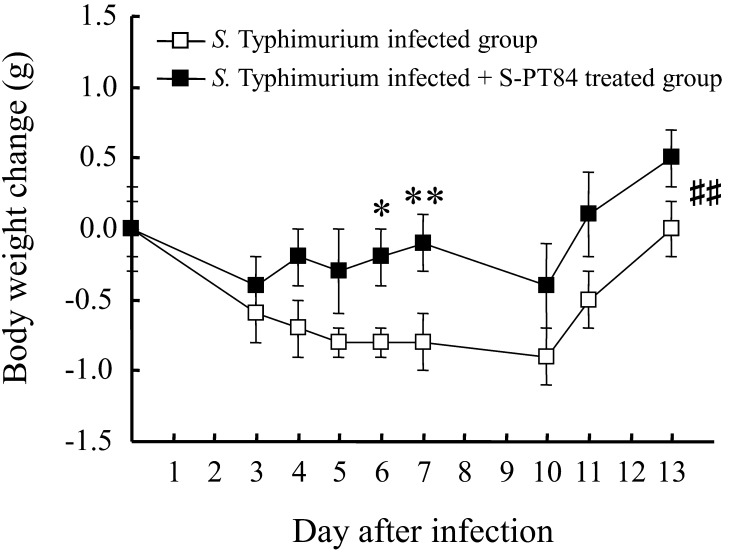
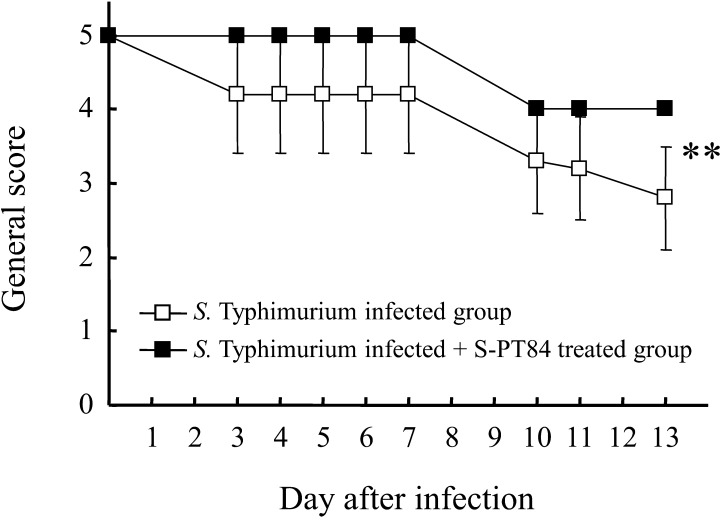
As shown in Fig. 2, the transient weight reduction that peaks 10 days after infection was significantly improved by S-PT84 ingestion. Furthermore, the deterioration in general health score (fur noticeably ruffled, mouse not as alert or active, signs of hyperventilating when handled) was significantly improved by S-PT84 ingestion (Fig. 3). The amount of S-PT84 intake, calculated from the average intake of food, was about 110 million cells per mouse (data not shown).
Fig. 2.
Body weight change after S. Typhimurium LT-2 infection. Each group consists of 9–10 mice. *, **, ##Statistically significant differences between S. Typhimurium infected group and S. Typhimurium infected + S-PT84 treated group (*p<0.05, **p<0.01 : Student’s t-test, ##p<0.01 : two-way ANOVA).
Fig. 3.
General health score change after S. Typhimurium LT-2 infection. Each group consists of 6 mice. **Statistically significant difference between S. Typhimurium infected group and S. Typhimurium infected + S-PT84 treated group (**p<0.01: two-way ANOVA).
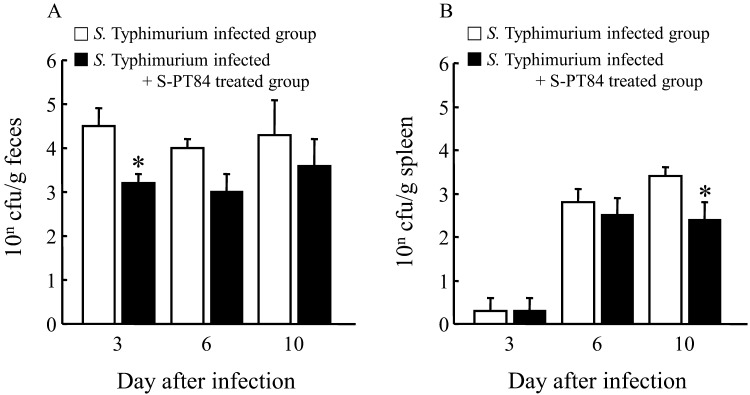
To determine why several symptoms were improved, we examined numbers of S. Typhimurium in feces and the spleen at days 3, 6 and 10. As shown in Fig. 4, numbers of S. Typhimurium in feces in the S-PT84 treated group were decreased on each observation day, and were significantly decreased on day 3 (Fig. 4A). Although remarkable changes were not observed on day 3 and day 6, numbers of S. Typhimurium were significantly decreased on day 10 in the spleens of S-PT84 treated mice (Fig. 4B).
Fig. 4.
Numbers of S. Typhimurium in feces (A) and in the spleen (B) after S. Typhimurium. LT-2 infection. Each group consists of 6–12 mice. *Statistically significant difference between S. Typhimurium infected group and S. Typhimurium infected + S-PT84 treated group (p<0.05 : Student’s t-test).
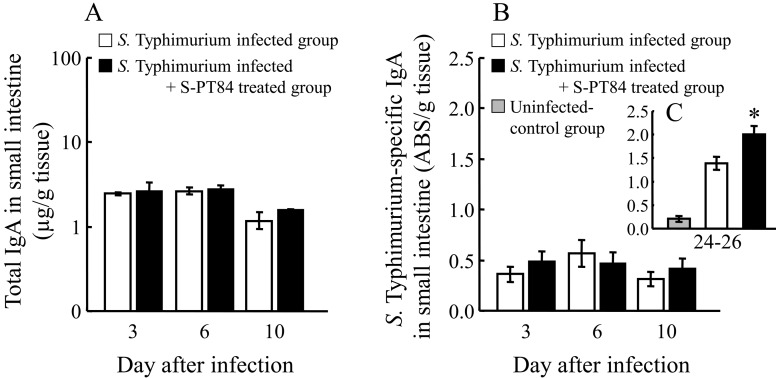
To understand why bacterial numbers decreased, we examined the total- and S. Typhimurium-specific IgA content in the small intestine according to the protocol described in Fig. 1A (Fig. 5). Total IgA was slightly increased by S-PT84 ingestion on day 10, but without statistical significance (Fig. 5A). S. Typhimurium-specific IgA was slightly increased by S-PT84 ingestion on day 3 or 10, but without statistical significance (Fig. 5B), on the other hand, it was significantly increased on day 24–26 (Fig. 5C).
Fig. 5.
Effects of S-PT84 treatment on the amounts of IgA in small intestine. Total (A) and S. Typhimurium-specific (B) IgA in small intestine of BALB/c mice at day 3–10, and S. Typhimurium-specific (C) IgA in small intestine at day 24–26. Each group consists of 3–9 mice. *Statistically significant difference S. Typhimurium infected group and S. Typhimurium infected + S-PT84 treated group (p<0.05).
Effects of S-PT84 on the mucosal immunity of normal BALB/c mice
To investigate other mechanisms involved in the beneficial effect of S-PT84 ingestion in the pathogen infection model, we examined the effect of S-PT84 ingestion on mucosal immunity before infection.
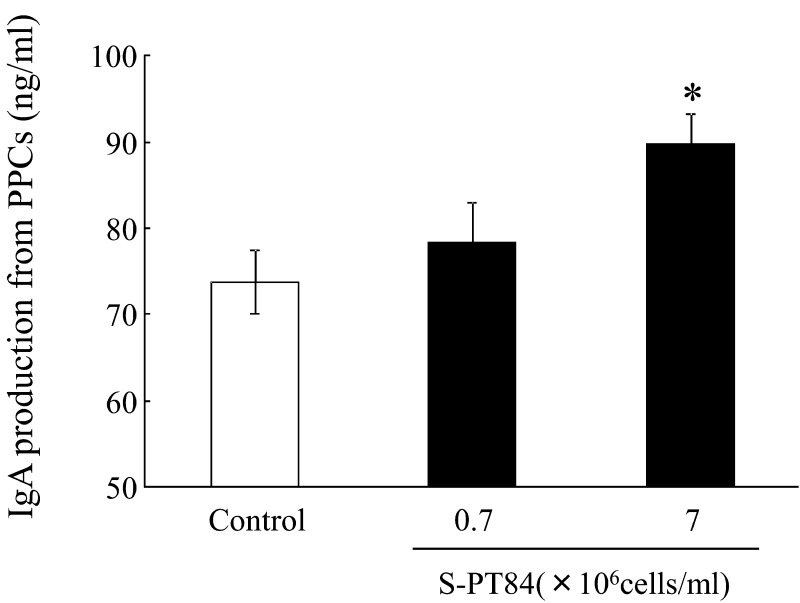
First, we examined the effect of S-PT84 on IgA production from PPCs in vitro. As shown in Fig. 6, PPCs treated with S-PT84 showed a dose-dependent increase in IgA production and produced significantly higher amounts of IgA than the control when treated with 7 × 106 S-PT84 cells/mL.
Fig. 6.
IgA production from Peyer’s patch cells (PPCs) of BALB/c mice treated with S-PT84. Each group consists of 3 mice. *Statistically significant difference between control group and 7 × 106 cells/ml S-PT84 group (p<0.05).
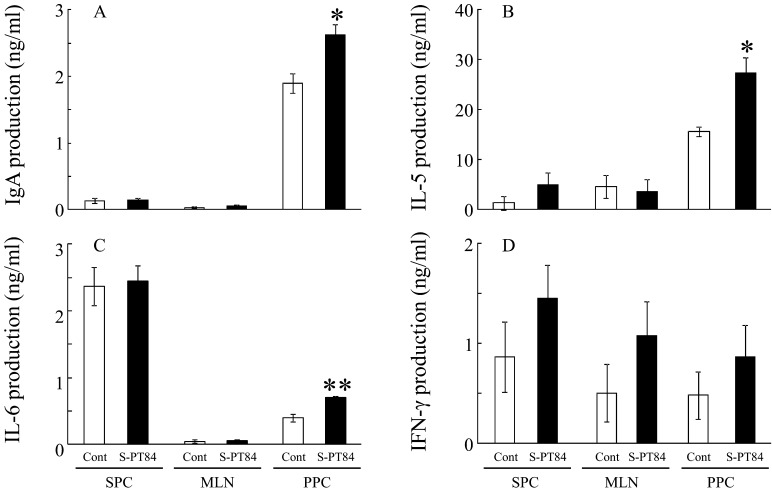
Next, we examined whether the immune response of several organs in mice was affected by 0.0075% S-PT84-diet ingestion. The amount of S-PT84 intake, calculated from the average intake of food, was about 100 million cells per mouse over 7 days (data not shown). In splenocytes and mesenteric lymph nodes, IgA, IL-5 and IL-6 production was not affected by S-PT84 ingestion (Figs. 7A, B, and C). IFN-γ production was increased in each tissue, but without statistical significance (Fig. 7D). On the other hand, IgA, IL-5 and IL-6 production in PPCs were significantly increased by S-PT84 ingestion (Figs. 7A, B and C).
Fig. 7.
IgA (A), IL-5 (B), IL-6 (C) and IFN-γ (D) production from splenocytes (SPCs), mesenteric lymph node cells (MLNs) or Peyer’s patch cells (PPCs) of BALB/c mice treated with S-PT84. Each group consists of 3 mice. *, **Statistically significant differences between control group and S-PT84 group (p<0.05, p<0.01 : Student’s t-test).
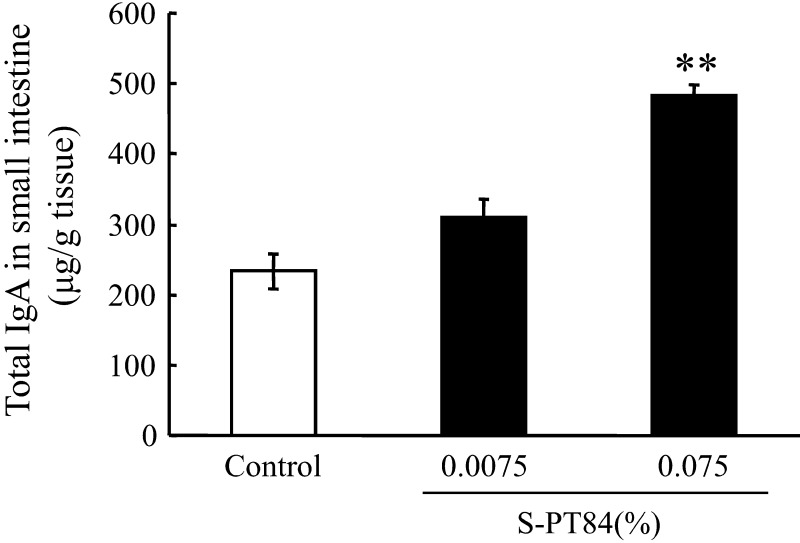
Finally, we examined whether IgA content in the small intestine was increased by 0.0075 or 0.075% S-PT84-diet ingestion, and whether this effect was dose-dependant. The amount of ingested S-PT84, calculated from the average intake of food, was about 0.1 billion and 1 billion cells per mouse over 7 days, respectively (data not shown). As shown in Fig. 8, IgA content in the small intestine showed a dose-dependent increase, and became significantly, about 2-fold, higher than the control after administration of the high dose of S-PT84.
Fig. 8.
IgA content in small intestine of BALB/c mice untreated or treated with S-PT84. BALB/c mice were fed an AIN-93M diet or AIN-93M diet containing 0.0075 or 0.075% S-PT84 for 7 days ad libitum. Each group consists of 5–9 mice. **Statistically significant. difference between control group and 0.075% S-PT84 group (p<0.01).
DISCUSSION
Lactic acid bacteria have various beneficial effects on human health [21, 22]. Especially, many reports have indicated that lactic acid bacteria promote immunomodulatory effects, anti-infectious activity, or epithelial barrier function in the gut [23,24,25]. S. Typhimurium is a notorious diarrhea-inducing pathogen, and several reports have demonstrated that lactic acid bacteria ingestion decreases the number of S. Typhimurium in feces, liver or the spleen via immunological or non-immunological functions (5,6,7,8,9). However, only a few reports have shown that deterioration in the general health score of S. Typhimurium-infected mice was improved by lactic acid bacteria ingestion [8, 26].
In the present study, we evaluated the effects of S-PT84 ingestion on S. Typhimurium-induced body weight loss and general health score deterioration. S-PT84 ingestion provided a significant protective effect against S. Typhimurium-induced body weight loss and general health score deterioration of the mice (Figs. 2 and 3). The number of S. Typhimurium in feces and the spleen were observed to be low in the S-PT84 group compared to the control. We consider that S. Typhimurium translocation to the spleen was significantly reduced (Fig. 4B), probably because the growth of S. Typhimurium in mice was inhibited, as shown by the decrease in the bacterial number in feces (Fig. 4A). These results suggest that S-PT84 ingestion was effective for maintenance of health or for improvement of several symptoms during pathogen infection. Furthermore, these efficacies may have been the result of inhibition of S. Typhimurium growth and reduction of organ invasion in the mice.
To determine why the number of S. Typhimurium decreased, we examined changes in the mucosal immunity of the small intestine. In the S. Typhimurium infection model, total and S. Typhimurium-specific IgA were not changed by S-PT84 ingestion from 3 to 10 days after infection, although S. Typhimurium-specific IgA was significantly enhanced on days 24-26 (Fig. 5). On the other hand, total IgA production from PPCs was enhanced by S-PT84 stimulation in vitro (Fig. 6), and IgA content in the small intestine was increased by 7 days of S-PT84 ingestion in vivo (Fig. 8). Cerutti and Rescigno reported that secretory IgA provides protection against several pathogens (bacteria and virus) [27]. Revolledo et al. reported that the ingestion of several probiotic-containing products was effective in the S. Typhimurium infection model and that potent reduction in S. Typhimurium colonization and inhibition of organ invasion were indicated in groups possessing high intestinal total IgA content [28]. These reports and the present data indicate the possibility that IgA content in the small intestine is enhanced by S-PT84 ingestion before S. Typhimurium infection, and that the alteration in IgA content results in inhibition of body weight loss and general health score deterioration resulting from Salmonella infection.
Peyer’s patches play an important role in the homeostasis or activation of mucosal immunity, and these tissue are closely associated with IgA production [29]. In the present study, we demonstrated that IgA content in the small intestine was significantly increased by the high dose of S-PT84 ingestion, and IgA production from PPCs in the S-PT84 group was enhanced by S-PT84 re-stimulation compared to the control. IL-5 and IL-6 production from PPCs was enhanced at the same time. Both IL-5 and IL-6 are known as potent IgA-producing cytokines in the mucosal immune system [30, 31]. Several reports have demonstrated that both IL-5 and IL-6 synergistically enhance IgA production from PPCs in vitro [32], or increase in IgA content in vivo [33]. Furthermore, Harata et al. reported that Lactobacillus GG and Lactobacillus gasseri exhibit distinct strain-dependant immunomodulatory effects in mice PP, and that Lactobacillus GG has a high IL-6-producing capacity that indicates high IgA production in vivo [34]. Therefore, we consider that the enhancement of IgA production from PPCs by S-PT84 was caused by the induction of IL-5 and IL-6. In other tissues (SPCs and MLNs), IL-5 and IL-6 production was unchanged in both the control and S-PT84 groups, probably resulting in unaltered IgA production. These results suggest that S-PT84 affects cytokine production in PP only in the intestine, and where it also enhance IgA production.. On the other hand, IFN-γ production was induced in all tissues (though without statisticall significance), therefore we think that S-PT84 induces Th1 immunity in both the systemic and mucosal immune systems.
In conclusion, in the S. Typhimurium infection model, the numbers of S. Typhimurium in feces and the spleen were significantly decreased by S-PT84 ingestion, and these efficacies might be due to enhanced mucosal immunity. Several symptoms observed in S. Typhimurium-infected mice were improved by S-PT84 ingestion, and these improvements were the result of inhibited S. Typhimurium growth and invasion. These results suggest that S-PT84 ingestion is useful for the maintenance of health or for the improvement of certain symptoms occurring during pathogen infection. However, we could not confirm in this study whether S-PT84 ingestion induces S. Typhimurium-specific IgA in the early stage of infection. Therefore, we need to examine further the potency of S-PT84-induced antibody production in response to Salmonella or other pathogens.
Acknowledgments
We thank M. Ide for technical help with this study. We also thank Dr. S. Hamada, Dr. M. Ida and T. Maekawa for their valuable discussions.
REFERENCES
- 1.Arvola T, Laiho K, Torkkeli S, Mykkänen H, Salminen S, Maunula L, Isolauri E. 1999. Prophylactic Lactobacillus GG reduces antibiotic-associated diarrhea in children with respiratory infections: a randomized study. Pediatrics 104: e64. [DOI] [PubMed] [Google Scholar]
- 2.Szajewska H, Mrukowicz JZ. 2001. Probiotics in the treatment and prevention of acute infectious diarrhea in infants and children: a systematic review of published randomized, double-blind, placebo-controlled trials. J Pediatr Gastroenterol Nutr 33: S17–S25 [DOI] [PubMed] [Google Scholar]
- 3.Hilton E, Kolakowski P, Singer C, Smith M. 1997. Efficacy of Lactobacillus GG as a diarrheal preventive in travelers. J Travel Med 4: 41–43 [DOI] [PubMed] [Google Scholar]
- 4.McFarland LV. 2009. Evidence-based review of probiotics for antibiotic-associated diarrhea and Clostridium difficile infections. Anaerobe 15: 274–280 [DOI] [PubMed] [Google Scholar]
- 5.Vinderola G, Matar C, Perdigón G. 2007. Milk fermented by Lactobacillus helveticus R389 and its non-bacterial fraction confer enhanced protection against Salmonella enteritidis serovar Typhimurium infection in mice. Immunobiology 212: 107–118 [DOI] [PubMed] [Google Scholar]
- 6.Jain S, Yadav H, Sinha PR. 2009. Probiotic dahi containing Lactobacillus casei protects against Salmonella enteritidis infection and modulates immune response in mice. J Med Food 12: 576–583 [DOI] [PubMed] [Google Scholar]
- 7.Shu Q, Lin H, Rutherfurd KJ, Fenwick SG, Prasad J, Gopal PK, Gill HS. 2000. Dietary Bifidobacterium lactis (HN019) enhances resistance to oral Salmonella typhimurium infection in mice. Microbiol Immunol 44: 213–222 [DOI] [PubMed] [Google Scholar]
- 8.Lin WH, Yu B, Lin CK, Hwang WZ, Tsen HY. 2007. Immune effect of heat-killed multistrain of Lactobacillus acidophilus against Salmonella typhimurium invasion to mice. J Appl Microbiol 102: 22–31 [DOI] [PubMed] [Google Scholar]
- 9.Ishikawa H, Kutsukake E, Fukui T, Sato I, Shirai T, Kurihara T, Okada N, Danbara H, Toba M, Kohda N, Maeda Y, Matsumoto T. 2010. Oral administration of heat-killed Lactobacillus plantarum strain b240 protected mice against Salmonella enterica serovar Typhimurium. Biosci Biotechnol Biochem 74: 1338–1342 [DOI] [PubMed] [Google Scholar]
- 10.Guslandi M, Mezzi G, Sorghi M, Testoni PA. 2000. Saccharomyces boulardii in maintenance treatment of Crohn’s disease. Dig Dis Sci 45: 1462–1464 [DOI] [PubMed] [Google Scholar]
- 11.Brigidi P, Vitali B, Swennen E, Bazzocchi G, Matteuzzi D. 2001. Effects of probiotic administration upon the composition and enzymatic activity of human fecal microbiota in patients with irritable bowel syndrome or functional diarrhea. Res Microbiol 152: 735–741 [DOI] [PubMed] [Google Scholar]
- 12.Cary VA, Boullata J. 2010. What is the evidence for the use of probiotics in the treatment of inflammatory bowel disease? J Clin Nurs 19: 904–916 [DOI] [PubMed] [Google Scholar]
- 13.Christensen HR, Frøkiaer H, Pestka JJ. 2002. Lactobacilli differentially modulate expression of cytokines and maturation surface markers in murine dendritic cells. J Immunol 168: 171–178 [DOI] [PubMed] [Google Scholar]
- 14.Galdeano CM, Perdigón G. 2006. The probiotic bacterium Lactobacillus casei induces activation of the gut mucosal immune system through innate immunity. Clin Vaccine Immunol 13: 219–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heczko PB, Strus M, Kochan P. 2006. Critical evaluation of probiotic activity of lactic acid bacteria and their effects. J Physiol Pharmacol 57: 5–12 [PubMed] [Google Scholar]
- 16.Mileti E, Matteoli G, Iliev ID, Rescigno M. 2009. Comparison of the immunomodulatory properties of three probiotic strains of lactobacilli using complex culture systems: prediction for in vivo efficacy. PLoS ONE 4: e7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kotani Y, Shinkai S, Okamatsu H, Toba M, Ogawa K, Yoshida H, Fukaya T, Fujiwara Y, Chaves PH, Kakumoto K, Kohda N. 2010. Oral intake of Lactobacillus pentosus strain b240 accelerates salivary immunoglobulin A secretion in the elderly: a randomized, placebo-controlled, double-blind trial. Immun Ageing 7: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koizumi S, Wakita D, Sato T, Mitamura R, Izumo T, Shibata H, Kiso Y, Chamoto K, Togashi Y, Kitamura H, Nishimura T. 2008. Essential role of Toll-like receptors for dendritic cell and NK1.1(+) cell-dependent activation of type 1 immunity by Lactobacillus pentosus strain S-PT84. Immunol Lett 120: 14–19 [DOI] [PubMed] [Google Scholar]
- 19.Nonaka Y, Izumo T, Izumi F, Maekawa T, Shibata H, Nakano A, Kishi A, Akatani K, Kiso Y. 2008. Antiallergic effects of Lactobacillus pentosus strain S-PT84 mediated by modulation of Th1/Th2 immunobalance and induction of IL-10 production. Int Arch Allergy Immunol 145: 249–257 [DOI] [PubMed] [Google Scholar]
- 20.Izumo T, Maekawa T, Ida M, Noguchi A, Kitagawa Y, Shibata H, Yasui H, Kiso Y. 2010. Effect of intranasal administration of Lactobacillus pentosus S-PT84 on influenza virus infection in mice. Int Immunopharmacol 10: 1101–1106 [DOI] [PubMed] [Google Scholar]
- 21.Goldin BR, Gorbach SL. 2008. Clinical indications for probiotics: an overview. Clin Infect Dis 46: S96–S100 [DOI] [PubMed] [Google Scholar]
- 22.Erickson KL, Hubbard NE. 2000. Probiotic immunomodulation in health and disease. J Nutr 130: 403S–409S [DOI] [PubMed] [Google Scholar]
- 23.Delcenserie V, Martel D, Lamoureux M, Amiot J, Boutin Y, Roy D. 2008. Immunomodulatory effects of probiotics in the intestinal tract. Curr Issues Mol Biol 10: 37–54 [PubMed] [Google Scholar]
- 24.Maragkoudakis PA, Chingwaru W, Gradisnik L, Tsakalidou E, Cencic A. 2010. Lactic acid bacteria efficiently protect human and animal intestinal epithelial and immune cells from enteric virus infection. Int J Food Microbiol 141: S91–S97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohland CL, Macnaughton WK. 2010. Probiotic bacteria and intestinal epithelial barrier function. Am J Physiol Gastrointest Liver Physiol 298: G807–G819> [DOI] [PubMed] [Google Scholar]
- 26.Gill HS, Shu Q, Lin H, Rutherfurd KJ, Cross ML. 2001. Protection against translocating Salmonella typhimurium infection in mice by feeding the immuno-enhancing probiotic Lactobacillus rhamnosus strain HN001. Med Microbiol Immunol (Berl) 190: 97–104 [DOI] [PubMed] [Google Scholar]
- 27.Cerutti A, Rescigno M. 2008. The biology of intestinal immunoglobulin A responses. Immunity 28: 740–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Revolledo L, Ferreira CS, Ferreira AJ. 2009. Prevention of Salmonella Typhimurium colonization and organ invasion by combination treatment in broiler chicks. Poult Sci 88: 734–743 [DOI] [PubMed] [Google Scholar]
- 29.Macpherson AJ, McCoy KD, Johansen FE, Brandtzaeg P. 2008. The immune geography of IgA induction and function. Mucosal Immunol 1: 11–22 [DOI] [PubMed] [Google Scholar]
- 30.Beagley KW, Eldridge JH, Lee F, Kiyono H, Everson MP, Koopman WJ, Hirano T, Kishimoto T, McGhee JR. 1989. Interleukins and IgA synthesis. Human and murine interleukin 6 induce high rate IgA secretion in IgA-committed B cells. J Exp Med 169: 2133–2148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramsay AJ, Kohonen-Corish M. 1993. Interleukin-5 expressed by a recombinant virus vector enhances specific mucosal IgA responses in vivo. Eur J Immunol 23: 3141–3145 [DOI] [PubMed] [Google Scholar]
- 32.Kunimoto DY, Nordan RP, Strober W. 1989. IL-6 is a potent cofactor of IL-1 in IgM synthesis and of IL-5 in IgA synthesis. J Immunol 143: 2230–2235 [PubMed] [Google Scholar]
- 33.Pockley AG, Montgomery PC. 1991. In vivo adjuvant effect of interleukins 5 and 6 on rat tear IgA antibody responses. Immunology 73: 19–23 [PMC free article] [PubMed] [Google Scholar]
- 34.Harata G, He F, Kawase M, Hosono A, Takahashi K, Kaminogawa S. 2009. Differentiated implication of Lactobacillus GG and L. gasseri TMC0356 to immune responses of murine Peyer’s patch. Microbiol Immunol 53: 475–480 [DOI] [PubMed] [Google Scholar]