Abstract
Autonomic nerves, consisting of both sympathetic and parasympathetic nerves, regulate various bodily functions such as blood pressure, body temperature, glucose metabolism, energy metabolism, and digestion. Our studies in rats and mice have demonstrated that food, flavor, and music affect physiological phenomena via changes in autonomic neurotransmissions. Intestinal injection of Lactobacillus johnsonii La1 (NCC533) suppressed sympathetic nerves that innervate the adrenal gland and kidney of urethane-anesthetized rats, lowering blood glucose and blood pressure levels, and excited the gastric parasympathetic nerve, elevating appetite and body weight. In contrast, intestinal injection of Lactobacillus paracasei ST11 (NCC2461) excited sympathetic nerves that innervate white and brown fat and the adrenal gland, increasing lipolysis and body temperature, and suppressed the gastric parasympathetic nerve, reducing appetite and body weight. Interestingly, we found that the hypothalamic suprachiasmatic nucleus (SCN), a master circadian clock, and histamine receptors in histaminergic neurons play important roles in peripheral autonomic control. To investigate the possible role of SCN and histamine receptors in lactobacilli-mediated pathology, we created an SCN-lesion model and experimented with histaminergic blocker injections. SCN lesion or injection of thioperamide, a histamine H3-receptor antagonist, eliminated the suppression of renal sympathetic nerve activity by NCC533, preventing blood pressure decline, and inhibited the enhancement of the gastric parasympathetic nerve induced by NCC533. In addition, diphenhydramine, a histamine H1-receptor antagonist, abolished the increases in renal sympathetic nerve activity and blood pressure caused by NCC2461. Infradiaphragmatic vagotomy eliminated the suppression of renal sympathetic nerve activity by NCC533, but did not affect the excitation of the renal sympathetic nerve by NCC2461. Collectively, these findings strongly suggest that SCN and histamine neurons are involved in the lactobacilli-mediated pathology of autonomic nerves and related physiological changes through abdominal afferent vagal pathway input to the central nervous system.
Keywords: sympathetic nerve, parasympathetic nerve, histaminergic nerve, lactobacilli, rats, afferent vagal nerve, dietary obesity, thermogenesis
INTRODUCTION
Autonomic nerves, consisting of sympathetic and parasympathetic nerves, primarily innervate the abdominal tissues and help regulate homeostatic functions such as blood pressure, blood glucose, body temperature, and energy metabolism. Our previous studies have indicated that some tissue-derived peptides, such as leptin, adiponectin, ghrelin, neuromedin-U, and orexin-A, and some sensory stimulations, such as odor exposure, light stimulation, and auditory stimulation, affect neural activities of autonomic nerves in urethane-anesthetized rats [1,2,3,4,5,6,7] and cause changes in blood pressure, feeding behavior, and body temperature in conscious animals [8,9,10]. These data suggest that autonomic nerves might play an important role in regulating homeostatic processes that modulate adaptations to changes in both the external and internal environment.
In general, studies have indicated that sympathetic nerves innervating the kidney and adrenal grand are involved in blood pressure regulation via the renin-angiotensin system [11] and that autonomic nerves innervating the liver or pancreas are involved in blood glucose regulation via glucose metabolism [12]. We have confirmed that L-carnosine (β-alanyl-L-histidine), a dipeptide produced by skeletal muscle, affects renal and adrenal sympathetic nerve activity and modulates blood pressure [13] as well as lowers blood glucose levels by affecting the activity of autonomic nerves innervating the pancreas [14]. These findings suggest that L-carnosine regulates cardiovascular functions and glucose metabolism in rats through the activity of autonomic nerves.
Ingestion of milk fermented with Lactobacillus helveticus is reported to decrease blood pressure and blood glucose levels in humans, rats and mice [15, 16]. Therefore, administration of other strains of Lactobacillus, such as Lactobacillus johnsonii La1 (NCC533) and Lactobacillus paracasei ST11 (NCC2461), might affect cardiovascular function and glucose metabolism. Whether these Lactobacillus strains affect autonomic nerves and regulate blood pressure and glucose metabolism has not been previously determined. Thus, we examined the effects of intraduodenal (ID) injection of NCC533 and NCC2461 on the neural activities of autonomic nerves and blood pressure in urethane-anesthetized rats and on hyperglycemia caused by 2-deoxy-D-glucose (2DG) in conscious rats. Here we discuss the possible role of Lactobacillus in the regulation of blood pressure, blood glucose, food intake, and energy metabolism via the autonomic nervous system and clarify the mechanisms of autonomic action of Lactobacillus by noting the interactions between the brain and peripheral tissue.
Effects of ID injection of Lactobacillus on autonomic nerve activity in urethane-anesthetized rats
We examined the effects of administering two strains of Lactobacillus into the small intestine on the activity of sympathetic nerves that innervate the kidney and found that NCC533 suppresses nerve activity in a dose-dependent manner while NCC2461 elevates nerve activity (Fig. 1) [17, 18]. Moreover, with respect to action on parasympathetic nerve activity, we examined the effects of administering NCC533 and NCC2461 into the small intestine on the activity of the parasympathetic nerves that innervate the stomach and confirmed that NCC533 increases nerve activity in a dose-dependent manner while NCC2461 decreases nerve activity (Fig. 1) [17, 18]. These findings suggest that intestinal Lactobacillus has biphasic effects on activities of autonomic nerves innervating the abdominal organs depending on the type of strain (i.e., NCC2461 versus NCC533).
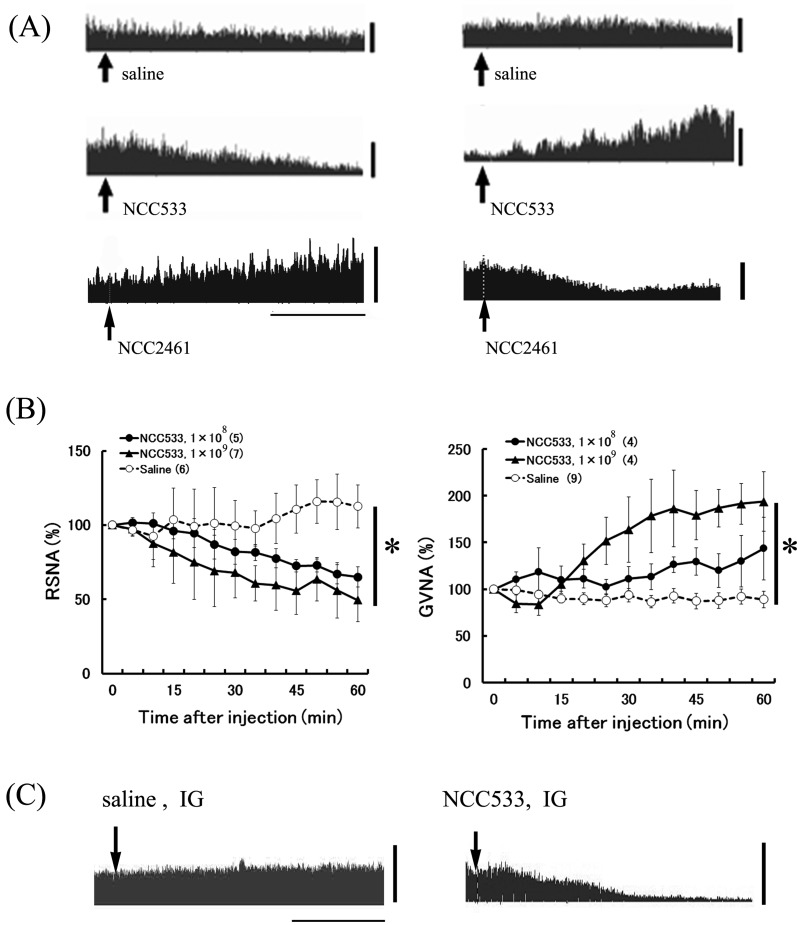
Fig. 1.
Effects of gastro-intestinal injection of NCC533 or NCC2461 on autonomic nerve activities in anesthetized rats.
Raw data of RSNA before and after intraduodenal (ID) injection of saline, NCC533 or NCC2461 (A). The arrows indicate the time of injection. The horizontal bars represent 20 min, and the vertical scale bars to the right of the recordings represent neural discharge rates of 100 spikes/5 sec. Time course data of RSNA and GVNA responses to ID injection of two doses of NCC533 are expressed as means ± SEM of the percentage of their values at 0 min (B). The numbers of animals used are shown in parentheses. The significance of the difference between values after saline and NCC533 from 5 to 60 min were analyzed as a group by ANOVA (*P<0.05). Raw data of RSNA before and after intragastric (IG) injection of saline or NCC533 (A).
Possible role of changes in autonomic nerve activity induced by NCC533 in hypotensive, hypoglycemic, and temperature-lowering actions
NCC533 has an autonomic action that decreases sympathetic nerve activity, such as renal sympathetic nerve activity (RSNA) or adrenal sympathetic nerve activity (ASNA), and increases parasympathetic nerve activity in anesthetized rats. With regard to the relationship between the sympathetic nervous system and cardiovascular function, it was reported that the renal sympathetic nerve is involved in blood pressure regulation via the renin-angiotensin system in the kidneys [11]. We examined the effect of acute ID injection of NCC533 on blood pressure in anesthetized rats and found that NCC533 lowered the mean arterial pressure in a dose-dependent manner (Fig. 2) [17]. This data suggests that NCC533 might lower blood pressure via the renal sympathetic nervous system.
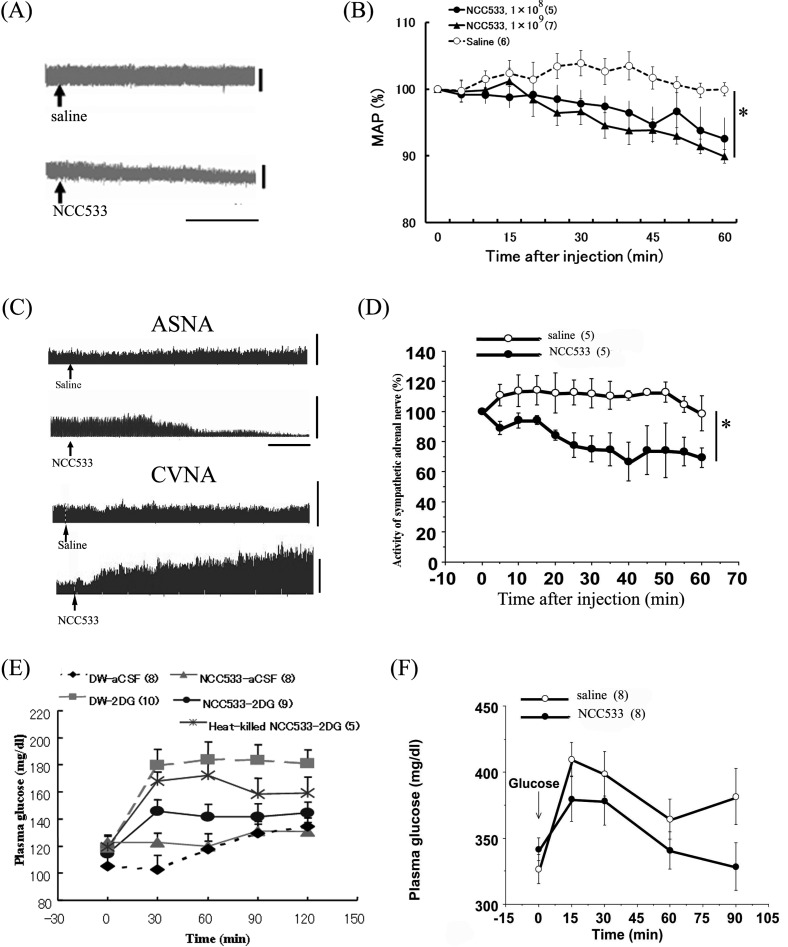
Fig. 2.
Effects of NCC533 on cardiovascular function and glucose metabolism through autonomic nerves.
Raw data of blood pressure before and after ID injection of saline or NCC533 (A). The arrows indicate the time of injection. The horizontal bars represent 20 min, and the vertical scale bars to the right of the recordings represent values of 100 mmHg. Time course data of mean arterial pressure (MAP) responses to ID injection of two dose of NCC533 are expressed as means ± SEM of the percentage of their values at 0 min (B). Raw data of ASNA and celiac vagal nerve activity (CVNA) before and after ID injection of saline or NCC533 (C). The arrows indicate the time of injection. The horizontal bars represent 20 min, and the vertical scale bars to the right of the recordings represent neural discharge rates of 200 spikes/5 sec. Time course data of ASNA and the response to ID injection of NCC533 are expressed as means ± SEM of the percentage of their values at 0 min (D). Effect of NCC533 on hyperglycemia induced by intracranial injection of 2DG is presented as means ± SEM. (E). Sterile distilled water with or without NCC533 (7.56 × 107 cfu/ml) was given as the only source of drinking water for 2 weeks. Changes in plasma glucose after intracranial injection of 2DG or aCSF are shown. Numbers of animals used are shown in parentheses. Effects of NCC533 on oral glucose tolerance in streptozotocin-diabetic rats are expressed as means±SEM (F). Sterile distilled water with or without NCC533 (7.56 × 107 cfu/ml) was given as the sole source of drinking water for 2 weeks. Changes in plasma glucose concentrations after oral glucose load (1.7 g/1.5 ml/kg) are shown. Numbers of animals used are shown in parentheses. The significance of the differences between values after saline and NCC533 from 5 to 60 min were analyzed as a group by ANOVA (*P<0.05).
On the other hand, with respect to the relationship between autonomic nerves and blood glucose regulation, it is generally recognized that sympathetic activation of the adrenal gland accelerates adrenalin release into the bloodstream and causes hyperglycemia because of glycogen degradation in the liver, while parasympathetic activation in the pancreas stimulates insulin release and causes hypoglycemia. Next, we examined the effect of acute ID injection of NCC533 on ASNA and neural activity of the celiac parasympathetic nerve innervating the pancreas in anesthetized rats and found that NCC533 reduced ASNA and activated celiac parasympathetic nerve activity (Fig. 2) [19]. Furthermore, to determine if NCC533 might affect glucose metabolism, we evaluated the acute effect of orally administered NCC533 on hyperglycemia by central injection of 2DG in conscious rats and observed that NCC533 inhibited hyperglycemia (Fig. 2) [19]. More specifically, we recently found that heat-treated NCC533 does not lower high blood glucose levels caused by 2DG injection (Fig. 2), suggesting that in the case of NCC533-containing culture, the activity of the Lactobacillus and not the culture medium might play a crucial role in suppressing the effect of 2DG-induced hyperglycemia via the autonomic nervous system.
The above findings raised the possibility that NCC533 might increase glucose tolerance. Therefore, we examined the effect of NCC533 on oral glucose tolerance using streptozotocin-diabetic rats and found that an NCC533-containing solution given for 2 weeks as the sole source of drinking water lowered the increase in plasma glucose (Fig. 2) and glucagon levels following an oral glucose load [19]. Thus, NCC533 might enhance oral glucose tolerance in diabetic rats, at least partially by suppressing the increase in the plasma glucagon level.
Possible role of changes in autonomic nerve activity induced by NCC2461 in anti-obese, thermogenic, and appetite-reducing actions
Obesity is closely linked with metabolic syndromes such as hypertension, diabetes, and arteriosclerosis, all of which are leading global public health concerns. A recent study reported a close relationship between intestinal bacteria and obesity [20], and obese animals often have abnormalities of the autonomic nervous system [21]. In addition, a close relationship exists between sympathetic excitation and body weight reduction [22]. For example, we observed that sympathetic excitation of neural outflow to white adipose tissue after exposure to the odor of grapefruits causes acceleration of lipolysis and decrease in body weight and food intake in rats [8]. Thus, to determine whether NCC2461, one of the sympathetic activating Lactobacillus strains, might be effective as an anti-obesity agent, we investigated the effects of ID injection of NCC2461 on neural activities of the sympathetic nerves innervating white adipose tissue (WAT-SNA) and brown adipose tissue (BAT-SNA). We found that NCC2461 stimulated neural activities of both white and brown adipose tissues and reduced the activity of the parasympathetic nerve that innervates the stomach (Fig. 3); this finding suggests that NCC2461 might exert a weight-reducing action via activation of WAT-SNA and BAT-SNA [23]. In addition, blood levels of FFA, a lipocatabolic marker, which is stimulated by excitation of WAT-SNA, were significantly elevated by intraoral injection of NCC2461 in conscious rats (Fig. 5) [23]. On the other hand, heat production in BAT via activation of the sympathetic nerves is mediated by uncoupling protein 1, which enhances thermogenesis and energy consumption. Moreover, we confirmed via a telemetry system that oral injection of NCC2461 increases the temperature in the brown adipose tissue in conscious rats (Fig. 3) [23]. Taken together, these findings indicate that NCC2461 might increase energy expenditure and reduce body weight by causing acceleration of BAT thermogenesis and WAT lipolysis.
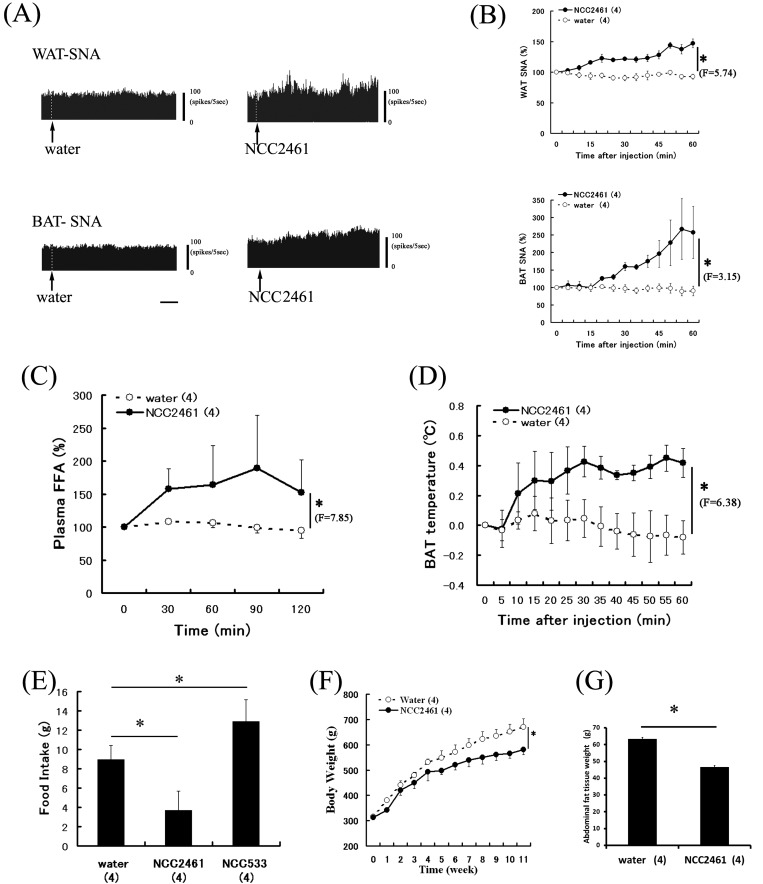
Fig. 3.
Effects of NCC2461 on feeding behavior, thermogenesis and lipolysis through autonomic nerves.
Raw data of WAT-SNA and BAT-SNA before and after ID injection of saline NCC2461 (A). The arrows indicate the time of injection. The horizontal bars represent 10 min. Time course data of WAT-SNA and BAT-SNA responses to ID injection of NCC2461 are expressed as means ± SEM of the percentage of their values at 0 min (B). Plasma FFA level (C) and BAT-T (D) after intraoral injection of NCC2461 are expressed as means ± SEM of percentages or difference of values at 0 min. Effects of ingestion of water, NCC2461 or NCC533 on cumulative (24 hrs) food intake are expressed as means ± SEM (E). Effects of NCC2461 on body weight (F) and abdominal fat weight (G) of rats fed a high-fat diet for 11 weeks are expressed as means ± SEM in each group. The numbers of animals used are shown in parentheses. The significance of the difference between values after saline and NCC2461 from 5 to 60 min were analyzed as a group by ANOVA (*P<0.05).
Fig. 5.
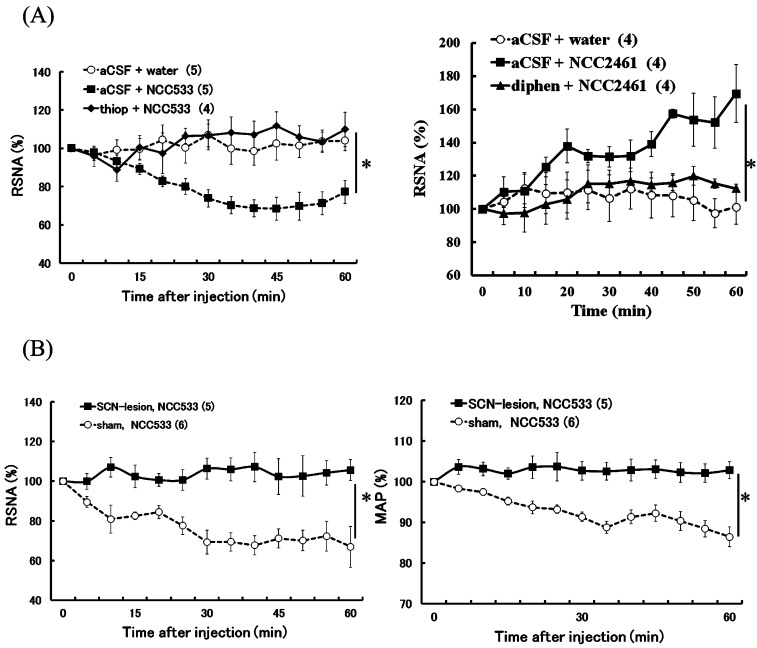
Possible role of histaminergic nervous system or the suprachiasmatic nucleus (SCN) in changes in sympathetic nerve activity induced by NCC533.
RSNA after ID injection of NCC533 or NCC2461 are expressed as means ± SEM of percentage of their values at 0 min. An ICV injection of aCSF, thioperamide (thiop) or diphenhydramine (diphen) was given 30 min before NCC533 injection (A). Effects of bilateral lesions of the SCN on changes in RSNA after ID injection of NCC533 are expressed as means ± SEM of percentages of their values at 0 min. RSNA and MAP data from sham-operated (SCN-sham) and SCN-lesioned (SCN-lesion) rats are shown (B). The numbers of animals used are shown in parentheses. The significance of the differences between the values after saline and NCC533 from 5 to 60 min were analyzed as a group by ANOVA.
It is well known that appetite control is a factor in the regulation of body weight. Therefore, we investigated the effect of NCC2461 on gastric vagal nerve activity (GVNA), one of the appetite-related nerves of the abdominal tissue, and food intake. We found that acute ID injection of NCC2461 suppressed GVNA in anesthetized rats (Fig. 3) [18]. Parasympathetic suppression is related to food intake inhibition [8], and as expected, we expectedly observed that food intake in rats administered NCC2461 was significantly decreased for 24 h (Fig. 3) [18]. Thus, NCC2461 might function as a suppressor of food intake. However, in our previous study, long-term ingestion of NCC2461 did not affect total food intake [23]. One possible explanation for this discrepancy was that chronic intake of NCC2461 might regulate gastro-intestinal functions, resulting in overall stabilization of appetite.
Because body weight reduction is strongly linked with increased energy expenditure and appetite suppression, the above evidence suggested that NCC2461 would suppress body weight. Therefore, to investigate the possible role of NCC2461 in body weight regulation, we examined the effect of NCC2461 on body weight changes in rats fed a high-fat diet and found that administration of NCC2461-containing drinking water for 11 weeks suppressed the gain in body weight and abdominal fat weight (total weight of epididymal, perirenal, and mesenteric adipose tissues) (Fig. 3) [23]. To the best of our knowledge, this was the first report to demonstrate that NCC2461 directly suppresses dietary obesity by regulating autonomic nerve activities.
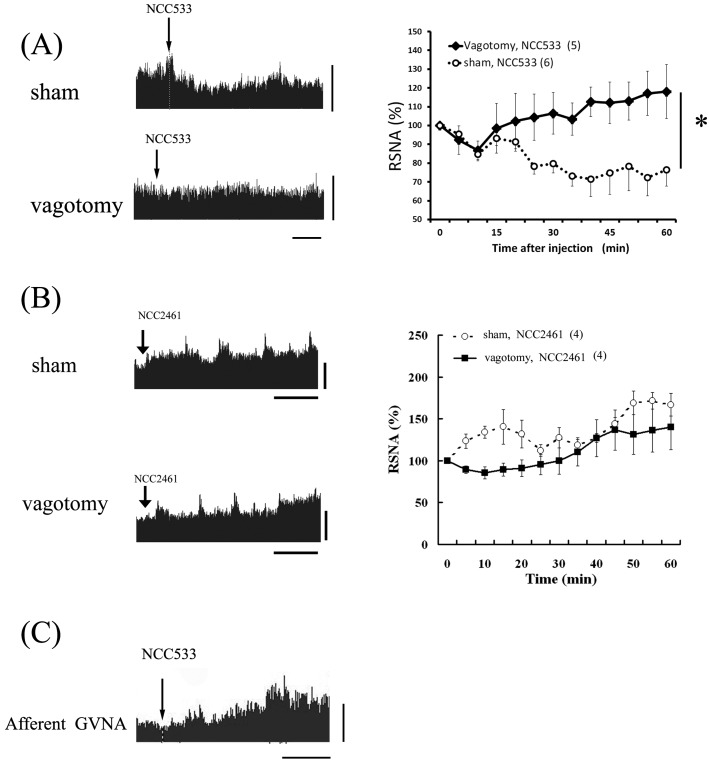
Involvement of afferent nerves of the abdominal tissue in changes in autonomic nerve activity induced by Lactobacillus
There are two types of nerves in the autonomic nervous system that control some abdominal tissues, efferent nerves and afferent nerves. The abdominal afferent nerves are involved in signal transduction from internal organs to the brain [24,25,26]. Specific examples include intestinal stimulation by some agents, the hyperthermic response to intestinal osmotic stimulation [26], the sympatho-inhibited response to ID injection of oolong tea [10], and the sympatho-excited response to intragastric injection of glutamate [27], all of which are attenuated in vagotomized rats (in which some afferent nerves including gastric, hepatic, and celiac branches are removed). Therefore, to determine if the afferent nerves were implicated in the autonomic effects of intestinal stimulation induced by Lactobacillus, the acute effects of ID injection of NCC533 or NCC2461 on RSNA were examined. RSNA suppression by NCC533 was blocked in vagotomized rats, while the elevating effects of NCC2461 on RSNA were detected in vagotomized rats (Fig. 4) [18]. Thus, we concluded that NCC533, but not NCC2461, relies on afferent nerves that reach the intestine and stimulate abdominal afferent vagal nerves for transmission of information to the brain. The NCC2461 pathway may be independent of the afferent nerve pathways, suggesting that hormonal factors including interleukin or TGF-β mediate the effects of NCC2461. Following this line of inquiry, a previous study found that NCC2461 elevated interleukin-10 levels and induced TGF-β production [28]. In addition, peripheral administration of interleukin changed autonomic nerve activities in urethane-anesthetized rats [29]. These findings support our above-mentioned notion that some cytokines mediate autonomic changes by lactobalilli, but measurements of the levels of these factors in the blood after ID injection of NCC2461 will be required to determine the detailed mechanism.
Fig. 4.
Effects of denervation of vagus nerves on changes in RSNA after ID injection of NCC533 or NCC2461.
Data of typical recordings of the RSNA of a sham-operated rat (sham) and a vagotomized rat (vagotomy) injected with NCC533 (A) or NCC2461 (B). RSNA after ID injection of NCC533 or NCC2461 are expressed as means ± SEM of the percentage of the value at 0 min (RSNA). Data from sham-operated (sham) and vagotomized (vagotomy) rats are shown. Injection points are indicated by arrows. Horizontal bars represent 10 min, vertical scale bars to the right of the recordings represent neural discharge rates of 100 spikes/5 sec. Significant differences (*P<0.05) between values from 5–60 min after intraduodenal injection of NCC2461 were analyzed as groups by ANOVA. Effect of intragastric injection of NCC533 on afferent gastric vagal nerve activity (GVNA) is presented as raw trace data (C).
In our vagotomy experiment, the gastric, hepatic and celiac branches of the vagus nerve were ablated. Previous studies have shown that selective excision of the gastric branch of the vagus nerve, but not the celiac or hepatic branches, significantly inhibits the ability to drink a monosodium glutamate solution (1%, w/v) [27], suggesting the importance of the role of the afferent pathway from the stomach. In addition, our preliminary study confirmed that an intragastric injection of NCC533 increases afferent neural activity of the gastric branch (Fig. 4), but suppresses neural activity of efferent sympathetic nerves innervating the kidneys (Fig. 1). Thus, these data suggest that gastric afferent signals may be involved in the efferent autonomic nervous actions by gastro-intestinal stimulation with NCC533. Taken together, these findings indicate that afferent neural signals are transmitted to the hypothalamic autonomic center via the nucleus of the solitary tract in the medulla [30]. It was recently shown that the nucleus of the solitary tract, where noradrenergic transmission takes place, functions as an important junction area in the afferent gastric neural pathway of feeding behavior following gastric hormone administration [30]. Because this neural pathway from the stomach to the central autonomic center plays an important role in maintaining homeostasis via the autonomic nerves, we consider that the suppression of RSNA by intragastric NCC533 may be mediated by a signaling mechanism from the afferent gastric vagal nerve to the hypothalamus via the nucleus of the solitary tract.
Central mechanism of changes in autonomic nerve activity induced by Lactobacillus: the possible role of the circadian clock and histaminergic nervous system in the brain
The hypothalamus is the primary regulation site of the autonomic nervous system. We recently confirmed the possible role of two hypothalamic nuclei, the histaminergic tuberomamillary nucleus (TMN) and the suprachiasmatic nucleus (SCN), as autonomic centers [1, 2, 4,5,6]. In the TMN of the histaminergic nervous system, the presynaptic H3 receptor mediates auto-inhibition of histamine release from the histaminergic neurons into synaptic clefts, and the affinity of the H3 receptor for histamine is much higher than the affinities of the postsynaptic histaminergic H1 and H2 receptors [31]. Therefore, a small amount of histamine in the synaptic cleft is thought to suppress histamine release from the presynaptic histaminergic neurons via the H3 receptor, while a large amount of histamine in the synaptic cleft functions to transmit histaminergic neural signals. In fact, our previous study demonstrated that a central injection of a small dose of histamine lowers RSNA, while a high dose of histamine elevates RSNA [5]. In addition, we found that suppression of RSNA by a small dose of histamine was blocked by thioperamide, an H3 receptor blocker, and activation of RSNA by a high dose of histamine was blocked by diphenhydramine, an H1 receptor blocker [5], further confirming the suggested mechanism. Therefore, we investigated the possible role of histaminergic neurotransmission in autonomic changes caused by NCC533 and NCC2461 and revealed that the suppressing effects of NCC533 on RSNA were eliminated by pretreatment with thioperamide, and that the elevating effects of NCC2461 on RSNA were abolished by pretreatment with diphenhydramine (Fig. 5) [17, 18]. These findings suggest that the hypothalamic histaminergic nerves might be involved in regulation of autonomic nerves by Lactobacillus.
Next, with respect to SCN, our previous study demonstrated that SCN, a master circadian oscillator, plays an important role in the control of glucose metabolism via regulation of the autonomic nervous system [32]. Moreover, bilateral lesions of SCN eliminated changes in autonomic nerve activities induced by illumination [2]. Therefore, to elucidate the role of SCN in the autonomic changes induced by NCC533, we examined the effect of SCN bilateral lesions on changes in RSNA and blood pressure after ID injection of NCC533. Suppression effects induced by NCC533 on RSNA and blood pressure disappeared in SCN-lesioned rats (Fig. 5) [17]. These findings suggest that SCN is involved in the mechanism of NCC533 action on RSNA and blood pressure. Using a pseudorabies virus to investigate the neural connection between SCN and the tissues related to the control of glucose metabolism, we found evidence indicating that SCN transmits multisynaptic neural signals to the pancreas and liver and that separate SCN neurons transmit signals to the peripheral sympathetic and parasympathetic neurons [33, 34]. These multisynaptic efferent projections extending from SCN to the spinal cord-containing group of neurons in the sympathetic pathway modulate blood pressure [33, 34]. With respect to the kidneys, Sly et al. [35] verified the existence of an efferent neural pathway from SCN to the kidneys using the pseudorabies virus. Although multisynaptic efferent projections extending from SCN to the spinal cord-containing sympathetic preganglionic cells and to the medulla oblongata-containing group of neurons in the sympathetic pathway modulate blood pressure [33, 34], the exact descending pathway responsible for the cardiovascular effect of intestinal NCC533 remains unclear. All these pathways may be associated with the suppression and elevation of RSNA and blood pressure.
It was recently demonstrated that there are many circadian clock molecules in SCN [36]. One such molecule is Clock, and Clock mutant (CM) mice exhibit blood pressure elevation during the light period and a decrease in body temperature during the dark period [37, 38], these mutants have an abnormal circadian rhythm in their locomotor activity under constant dark conditions [39]. Moreover, we found that the elevating effect of odor stimulation on RSNA is not observed in CM mice [40], suggesting that Clock in SCN might be a regulator of the sympathetic nervous system. However, it is unclear whether Clock in SCN is involved in changes in autonomic nerve activity induced by NCC533 or NCC2461. Hence, further investigation is required to evaluate this.
CONCLUSION
In conclusion, to determine possible role of lactobacillus in homeostasis regulation in vivo, we examined the effects of two strains of Lactobacilli, NCC533 or NCC2461, on the autonomic nerve activities which contribute to the regulation of cardiovascular function, glucose metabolism, thermogenesis and feeding behavior in rats. Our results show that ID injection of NCC533 lowered RSNA, ASNA and blood pressure, and elevated GVNA in urethane-anesthetized rats, and suppressed hyperglycemic responses to 2-DG injection in normal conscious rats or oral glucose load in STZ-diabetic rats. In addition, both abdominal vagotomy and SCN lesion abolished autonomic and cardiovascular changes due to NCC533. On the other hand, NCC2461 administration into the small intestine stimulated BAT-SNA, WAT-SNA, ASNA and RSNA, and suppressed GVNA in urethane-anesthetized rats, and elevated temperatures of brown adipose tissue and lipolysis, reducing appetite and body weight. To clarify the possible role of histaminergic system in the brain, we next investigated the effects of histaminergic blockers, and found that ICV injection of thioperamide, a histamine H3-receptor antagonist, eliminated the suppression of RSNA, preventing blood pressure decline, and suppressed the enhancement of the gastric parasympathetic nerve induced by NCC533. In addition, diphenhydramine, a histamine H1-receptor antagonist, abolished the increases in RSNA and blood pressure induced by NCC2461. The present evidence strongly suggests that lactobacilli reaching the intestine send some signals to the brain, via the afferent nerve pathway or blood cytokines pathway, and affect autonomic nerve activities through the SCN or histaminergic neurons regulating cardiovascular, metabolic, glycemic and feeding functions.
Acknowledgments
We are grateful to Dr. Yoichi Fukushima (Nestle Japan Ltd. Nutrition Business Group) for the donation of experimental samples and helpful advice.
REFERENCE
- 1.Nakamura T, Tanida M, Niijima A, Hibino H, Shen J, Nagai K. 2007. Auditory stimulation affects renal sympathetic nerve activity and blood pressure in rats. Neurosci Lett 416: 107–112 [DOI] [PubMed] [Google Scholar]
- 2.Niijima A, Nagai K, Nagai N, Akagawa H. 1993. Effects of light stimulation on the activity of the autonomic nerves in anesthetized rats. Physiol Behav 54: 555–561 [DOI] [PubMed] [Google Scholar]
- 3.Tanida M, Niijima A, Shen J, Nakamura T, Nagai K. 2005. Olfactory stimulation with scent of essential oil of grapefruit affects autonomic neurotransmission and blood pressure. Brain Res 1058: 44–55 [DOI] [PubMed] [Google Scholar]
- 4.Tanida M, Niijima A, Shen J, Yamada S, Sawai H, Fukuda Y, Nagai K. 2006. Dose-different effects of orexin-A on the renal sympathetic nerve and blood pressure in urethane-anesthetized rats. Exp Biol Med (Maywood) 231: 1616–1625 [DOI] [PubMed] [Google Scholar]
- 5.Tanida M, Kaneko H, Shen J, Nagai K. 2007. Involvement of the histaminergic system in renal sympathetic and cardiovascular responses to leptin and ghrelin. Neurosci Lett 413: 88–92 [DOI] [PubMed] [Google Scholar]
- 6.Tanida M, Shen J, Horii Y, Matsuda M, Kihara S, Funahashi T, Shimomura I, Sawai H, Fukuda Y, Matsuzawa Y, Nagai K. 2007. Effects of adiponectin on the renal sympathetic nerve activity and blood pressure in rats. Exp Biol Med (Maywood) 232: 390–397 [PubMed] [Google Scholar]
- 7.Tanida M, Satomi J, Nagai K. 2009. Autonomic and cardiovascular effects of central neuromedin U in rats. Physiol Behav 96: 282–288 [DOI] [PubMed] [Google Scholar]
- 8.Shen J, Niijima A, Tanida M, Horii Y, Maeda K, Nagai K. 2005. Olfactory stimulation with scent of grapefruit oil affects autonomic nerves, lipolysis and appetite in rats. Neurosci Lett 380: 289–294 [DOI] [PubMed] [Google Scholar]
- 9.Tanida M, Shen J, Nakamura T, Niijima A, Nagai K. 2008. Day-night difference in thermoregulatory responses to olfactory stimulation. Neurosci Lett 439: 192–197 [DOI] [PubMed] [Google Scholar]
- 10.Tanida M, Tsuruoka N, Shen J, Kiso Y, Nagai K. 2008. Effects of oolong tea on renal sympathetic nerve activity and spontaneous hypertension in rats. Metabolism 57: 526–534 [DOI] [PubMed] [Google Scholar]
- 11.Nakamura A, Johns EJ. 1995. Renal nerves, renin, and angiotensinogen gene expression in spontaneously hypertensive rats. Hypertension 25: 581–586 [DOI] [PubMed] [Google Scholar]
- 12.Kalsbeek A, Foppen E, Schalij I, Van Heijningen C, van der Vliet J, Fliers E, Buijs RM. 2008. Circadian control of the daily plasma glucose rhythm: an interplay of GABA and glutamate. PLoS ONE 3: e3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanida M, Niijima A, Fukuda Y, Sawai H, Tsuruoka N, Shen J, Yamada S, Kiso Y, Nagai K. 2005. Dose-dependent effects of L-carnosine on the renal sympathetic nerve and blood pressure in urethane-anesthetized rats. Am J Physiol Regul Integr Comp Physiol 288: R447–R455 [DOI] [PubMed] [Google Scholar]
- 14.Yamano T, Niijima A, Iimori S, Tsuruoka N, Kiso Y, Nagai K. 2001. Effect of L-carnosine on the hyperglycemia caused by intracranial injection of 2-deoxy-D-glucose in rats. Neurosci Lett 313: 78–82 [DOI] [PubMed] [Google Scholar]
- 15.Seppo L, Jauhiainen T, Poussa T, Korpela R. 2003. A fermented milk high in bioactive peptides has a blood pressure-lowering effect in hypertensive subjects. Am J Clin Nutr 77: 326–330 [DOI] [PubMed] [Google Scholar]
- 16.Sipola M, Finckenberg P, Santisteban J, Korpela R, Vapaatalo H, Nurminen ML. 2001. Long-term intake of milk peptides attenuates development of hypertension in spontaneously hypertensive rats. J Physiol Pharmacol 52: 745–754 [PubMed] [Google Scholar]
- 17.Tanida M, Yamano T, Maeda K, Okumura N, Fukushima Y, Nagai K. 2005. Effects of intraduodenal injection of Lactobacillus johnsonii La1 on renal sympathetic nerve activity and blood pressure in urethane-anesthetized rats. Neurosci Lett 389: 109–114 [DOI] [PubMed] [Google Scholar]
- 18.Tanida M, Fukushima Y, Yamano T, Maeda K, Horii Y, Shen J, Nagai K. 2007. Effects of probiotic strain Lactobacillus paracasei ST11 (NCC2461) on autonomic nerve activities, blood pressure and appetite in rats. Curr Top Nutraceutical Res 4: 157–164 [Google Scholar]
- 19.Yamano T, Tanida M, Niijima A, Maeda K, Okumura N, Fukushima Y, Nagai K. 2006. Effects of the probiotic strain Lactobacillus johnsonii strain La1 on autonomic nerves and blood glucose in rats. Life Sci 79: 1963–1967 [DOI] [PubMed] [Google Scholar]
- 20.Ding S, Chi MM, Scull BP, Rigby R, Schwerbrock NM, Magness S, Jobin C, Lund PK. 2010. High-fat diet: bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PLoS ONE 5: e12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iwashita S, Tanida M, Terui N, Ootsuka Y, Shu M, Kang D, Suzuki M. 2002. Direct measurement of renal sympathetic nervous activity in high-fat diet-related hypertensive rats. Life Sci 71: 537–546 [DOI] [PubMed] [Google Scholar]
- 22.Fischer J, Koch L, Emmerling C, Vierkotten J, Peters T, Brüning JC, Rüther U. 2009. Inactivation of the Fto gene protects from obesity. Nature 458: 894–898 [DOI] [PubMed] [Google Scholar]
- 23.Tanida M, Shen J, Hori Y, Maeda K, Yamano T, Fukushima Y, Nagai K. 2008. High fat diet-induced obesity is attenuated by probiotic strain Lactobacillus paracasei ST11 (NCC2461) in rats. Obes Res Clin Pract 2: 159–169 [DOI] [PubMed] [Google Scholar]
- 24.Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H, Kangawa K, Nakazato M. 2002. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology 123: 1120–1128 [DOI] [PubMed] [Google Scholar]
- 25.Niijima A. 1998. Afferent signals from leptin sensors in the white adipose tissue of the epididymis, and their reflex effect in the rat. J Auton Nerv Syst 73: 19–25 [DOI] [PubMed] [Google Scholar]
- 26.Osaka T, Kobayashi A, Inoue S. 2002. Vago-sympathoadrenal reflex in thermogenesis induced by osmotic stimulation of the intestines in the rat. J Physiol 540: 665–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanida M, Satomi J. 2011. Effects of intragastric injection of glutamate on efferent sympathetic nerve activity in rats. Neurosci Lett 491: 211–215 [DOI] [PubMed] [Google Scholar]
- 28.von der Weid T, Bulliard C, Schiffrin EJ. 2001. Induction by a lactic acid bacterium of a population of CD4(+) T cells with low proliferative capacity that produce transforming growth factor beta and interleukin-10. Clin Diagn Lab Immunol 8: 695–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niijima A, Hori T, Katafuchi T, Ichijo T. 1995. The effect of interleukin-1 beta on the efferent activity of the vagus nerve to the thymus. J Auton Nerv Syst 54: 137–144 [DOI] [PubMed] [Google Scholar]
- 30.Date Y, Shimbara T, Koda S, Toshinai K, Ida T, Murakami N, Miyazato M, Kokame K, Ishizuka Y, Ishida Y, Kageyama H, Shioda S, Kangawa K, Nakazato M. 2006. Peripheral ghrelin transmits orexigenic signals through the noradrenergic pathway from the hindbrain to the hypothalamus. Cell Metab 4: 323–331 [DOI] [PubMed] [Google Scholar]
- 31.Arrang JM, Garbarg M, Schwartz JC. 1983. Autoinhibition of brain histamine release mediated by a novel class (H3) of histamine receptor. Nature 302: 832–837 [DOI] [PubMed] [Google Scholar]
- 32.Nagai K, Nagai N, Shimizu K, Chun S, Nakagawa H, Niijima A. 1996. SCN output drives the autonomic nervous system: with special reference to the autonomic function related to the regulation of glucose metabolism. Prog Brain Res 111: 253–272 [DOI] [PubMed] [Google Scholar]
- 33.Buijs RM, Chun S, Niijima A, Romijn HJ, Nagai K. 2001. Parasympathetic and sympathetic control of the pancreas: a role for the suprachiasmatic nucleus and other hypothalamic centers that are involved in the regulation of food intake. J Comp Neurol 431: 405–423 [DOI] [PubMed] [Google Scholar]
- 34.Buijs RM, Fleur Sl Wortel J, Heynigen CV, Zuiddam L, Mettenleiter TC, Kalsbeek A, Nagai K, Niijima A. 2003. The suprachiasmatic nucleus balances sympathetic and parasympathetic output to peripheral organs through separate preautonomic neurons. J Comp Neurol 464: 36–48 [DOI] [PubMed] [Google Scholar]
- 35.Sly JD, Colvill L, McKinley JM, Oldfield JB. 1999. Identification of neural projections from the forebrain to the kidney using the virus pseudorabies. J Auton Nerv Syst 77: 73–82 [PubMed] [Google Scholar]
- 36.Lowrey PL, Takahashi JS. 2004. Mammalian circadian biology: elucidating genome-wide levels of temporal organization. Annu Rev Genomics Hum Genet 5: 407–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanai S, Masuo Y, Shirai H, Oishi K, Saida K, Ishida N. 2005. Differential circadian expression of endothelin-1 mRNA in the rat suprachiasmatic nucleus and peripheral tissues. Neurosci Lett 377: 65–68 [DOI] [PubMed] [Google Scholar]
- 38.Sei H, Oishi K, Ishida N, Morita Y. 2005. Diurnal profiles of blood pressure and heart rate in Clock mutant mice on Jcl: ICR background. Jpn J Physiol 55: S26 [Google Scholar]
- 39.Oishi K, Miyazaki K, Ishida N. 2002. Functional CLOCK is not involved in the entrainment of peripheral clocks to the restricted feeding: entrainable expression of mPer2 and BMAL1 mRNAs in the heart of Clock mutant mice on Jcl: ICR background. Biochem Biophys Res Commun 298: 198–202 [DOI] [PubMed] [Google Scholar]
- 40.Tanida M, Shen J, Niijima A, Yamatodani A, Oishi K, Ishida N, Nagai K. 2008. Effects of olfactory stimulations with scents of grapefruit and lavender oils on renal sympathetic nerve and blood pressure in Clock mutant mice. Auton Neurosci 139: 1–8 [DOI] [PubMed] [Google Scholar]