Abstract
Whether higher serum phosphorus levels are associated with a higher risk for death and/or progression of chronic kidney disease (CKD) is not well established, and whether the association is confounded by access and barriers to care is unknown. To answer these questions, data of 10,672 individuals identified to have CKD (estimated glomerular filtration rate <60 ml/min per 1.73 m2) from those participating in a community-based screening program were analyzed. Over a median follow-up of 2.3 years, there was no association between quartiles of serum phosphorus and all-cause mortality (adjusted hazards ratio for serum phosphorus over 3.3 to 3.7, over 3.7 to 4.1, and over 4.1 mg/dl, respectively: 1.22 (0.95–1.56), 1.00 (0.76–1.32), and 1.00 (0.75–1.33); reference, serum phosphorus of 3.3 mg/dl and below). Individuals in the highest quartile for serum phosphorus had a significantly higher risk for progression to end-stage renal disease (ESRD) (unadjusted hazards ratio, 6.72 (4.16–10.85)); however, the risk became nonsignificant on adjustment for potential confounders. There was no appreciable change in hazards ratio with inclusion of variables related to access and barriers to care. Additional analyses in subgroups based on 12 different variables yielded similar negative associations. Thus, in the largest cohort of individuals with early-stage CKD to date, we could not validate an independent association of serum phosphorus with risk for death or progression to ESRD.
Keywords: barrier to care, cardiovascular disease, chronic kidney disease, end-stage renal disease, mortality, phosphorus
There is consistent evidence that chronic kidney disease (CKD) is associated with a higher risk of death, particularly from cardiovascular causes.1 At every stage of CKD, the risk of death far exceeds the probability of progression to end-stage renal disease (ESRD).2 This has led to screening programs for early identification of CKD to implement interventions to ameliorate the increased cardiovascular risk. The components of effective cardiovascular risk reduction in CKD, however, remain unclear. For example, although statins reduces atherosclerotic cardiovascular events in CKD, they do not reduce all-cause or cardiovascular mortality.3 Similarly, the potential benefit with blood pressure reduction is smaller than in the general population.4 A variety of similar considerations suggest that the pathogenesis of vascular disease in CKD is substantially more complex and effective cardiovascular risk reduction would require us to also target nontraditional, renal-related risk factors.
A substantial body of evidence implicates abnormal phosphorus homeostasis associated with CKD as one such nontraditional risk factor.5 Cell culture and animal studies have demonstrated a central role of phosphorus in inducing vascular calcification.6–8 In humans, greater severity of vascular calcification is associated with a higher risk of death.9,10 Serum phosphorus is used as a biomarker for the abnormal phosphorus homeostasis in CKD even though it represents <1% of total body phosphorus and the serum levels are maintained within a tight range up until late in the course of the disease by compensatory changes in regulatory hormones. Its use as a biomarker has been bolstered by studies demonstrating an association between higher serum phosphorus and increased risk of death.11,12 Most of these studies have included individuals undergoing maintenance dialysis, a population with significant elevations of serum phosphorus. Some studies suggest that this association may also be present among individuals with earlier stages of CKD.13–15 Other studies have demonstrated an association between serum phosphorus and progression of kidney disease.16–18 However, in a post hoc analysis of the Modification of Diet in Renal Disease (MDRD), there was no demonstrable association between serum phosphorus and mortality.19 Moreover, residual confounding is an inherent concern with epidemiologic studies, and recent analyses have indicated significantly higher serum phosphorus in individuals with CKD and lower socioeconomic status, an important and independent determinant of access to care and health-related outcomes.20
Kidney Early Evaluation Program (KEEP) is a nationwide program to screen high-risk individuals for CKD.21 Measurement of serum phosphorus, calcium, and parathyroid hormone in individuals with estimated glomerular filtration rate (eGFR) <60 ml/min per 1.73 m2 has been included since November 2005. We analyzed the data from KEEP to test the hypothesis that the association between serum phosphorus and all-cause mortality and progression to ESRD in earlier-stage CKD is confounded by access and barriers to health care.
Results
Patient characteristics
Between November 2005 and December 2010, 85,992 individuals participated in the KEEP screening; of these, 11,992 participants were identified as having eGFR <60 ml/min per 1.73 m2 (13%). Serum phosphorus measurements were available for 11,197 eligible subjects (93%). Of these, data of 525 participants were excluded as the date of screening was determined to have been after the date of diagnosis of ESRD. This yielded the analytic cohort of 10,672 subjects for this analysis (number of subjects in consecutive years starting from 2005 to 2010: 393, 1979, 1917, 2043, 2636, and 1704).
The demographic, clinical, and laboratory characteristics of the cohort, stratified by quartiles of serum phosphorus, are summarized in Table 1. Subjects in the highest quartile of serum phosphorus were significantly younger, less likely to be men, non-Hispanic black, or have health insurance, but a higher proportion were aware of the presence of underlying CKD. Furthermore, they had significantly higher serum creatinine and lower eGFR and were more likely to have albuminuria. Individuals in the highest quartile of serum phosphorus were also more likely to report current tobacco use, dyslipidemia, and prevalent cardiovascular disease. Finally, these subjects had lower body mass index, blood glucose, and hemoglobin levels.
Table 1. Patient characteristics stratified by quartile of serum phosphorus.
| n | Quartile 1 2979 | Quartile 2 2947 | Quartile 3 2577 | Quartile 4 2169 | P-value |
|---|---|---|---|---|---|
| Serum phosphorus range, mg/dl | ≤3.3 | >3.3 to 3.7 | >3.7 to 4.1 | >4.1 | |
| Mean serum phosphorus, mg/dl | 3.1 ± 0.3 | 3.6 ± 0.1 | 3.9 ± 0.1 | 4.6 ± 0.5 | |
| Demographics | |||||
| Age, years | 71 ± 11 | 71 ± 10 | 71 ± 11 | 69 ± 12 | <0.0001 |
| Gender, % men | 47 | 33 | 25 | 21 | <0.0001 |
| Race/ethnicity, % | <0.0001 | ||||
| Non-Hispanic white | 66 | 66 | 68 | 65 | |
| Non-Hispanic black | 23 | 22 | 21 | 19 | |
| Hispanic, white, or black | 2 | 2 | 2 | 3 | |
| Asian | 3 | 4 | 3 | 5 | |
| Others | 5 | 6 | 6 | 7 | |
| Highest education, % | 0.49 | ||||
| Grade school or less | 6 | 6 | 6 | 6 | |
| Some high school | 11 | 12 | 11 | 11 | |
| High school graduate | 32 | 30 | 32 | 33 | |
| Some college | 23 | 24 | 24 | 24 | |
| College graduate | 16 | 15 | 15 | 15 | |
| Postgraduate or professional | 13 | 13 | 11 | 12 | |
| Barriers/access to care | |||||
| Difficulty in getting medical care, % | 0.02 | ||||
| Extremely difficult | 3 | 3 | 3 | 4 | |
| Moderately difficult | 4 | 4 | 4 | 4 | |
| Somewhat difficult | 7 | 7 | 7 | 9 | |
| Not very difficult | 21 | 21 | 20 | 22 | |
| Not difficult | 66 | 66 | 66 | 61 | |
| Health insurance available, % | 92 | 92 | 91 | 89 | <0.0001 |
| Last time made visit to doctor | 0.85 | ||||
| Within past year | 95 | 95 | 95 | 95 | |
| 1–2 years ago | 4 | 3 | 4 | 4 | |
| More than 2 years ago | 1 | 2 | 2 | 1 | |
| Awareness of CKD, % | 16 | 15 | 16 | 19 | 0.002 |
| CKD | |||||
| Serum creatinine, mg/dla | 1.3 (1.1, 1.5) | 1.3 (1.1, 1.5) | 1.2 (1.1, 1.4) | 1.3 (1.1, 1.6) | <0.0001 |
| eGFR, ml/min per 1.73m2 | 49 ± 9 | 48 ± 9 | 47 ± 10 | 44 ± 13 | <0.0001 |
| <15.0 | 0.03 | 0.07 | 0.35 | 3 | |
| 15.0–29.9 | 3 | 5 | 6 | 11 | |
| 30.0–44.9 | 25 | 26 | 28 | 29 | |
| 45.0–59.9 | 71 | 69 | 66 | 57 | |
| Albuminuria, % | |||||
| <30 mg/g | 76 | 78 | 78 | 72 | <0.001 |
| 30–300 mg/g | 21 | 19 | 18 | 20 | |
| >300 mg/g | 3 | 4 | 4 | 8 | |
| Comorbidities, % | |||||
| Diabetes mellitus | 47 | 47 | 47 | 48 | 0.67 |
| Hypertension | 94 | 94 | 93 | 94 | 0.23 |
| Current tobacco use | 4 | 5 | 6 | 7 | 0.0001 |
| Dyslipidemia | 84 | 86 | 86 | 88 | 0.0004 |
| Prevalent CVD | 44 | 44 | 42 | 46 | 0.04 |
| Body mass index, kg/m2 | 30.4 ± 6.3 | 30.2 ± 6.3 | 29.9 ± 6.4 | 29.8 ± 6.6 | 0.002 |
| Laboratory parameters | |||||
| Plasma glucose, mg/dla | 112 (97, 144) | 108 (95, 133) | 107 (95, 131) | 107 (95, 133) | <0.0001 |
| Serum calcium, mg/dl | 9.6 ± 0.5 | 9.7 ± 0.5 | 9.7 ± 0.5 | 9.7 ± 0.5 | 0.001 |
| Serum PTH, pg/mla | 73 (49, 109) | 68 (45, 100) | 67 (46, 99) | 64 (42, 100) | <0.0001 |
| Hemoglobin, g/dl | 13.5 ± 1.5 | 13.3 ± 1.5 | 13.1 ± 1.4 | 12.9± 1.6 | <0.0001 |
Abbreviations: CKD, chronic kidney disease; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; PTH, parathyroid hormone.
Data presented as median with 25th and 75th percentiles.
Serum phosphorus and prevalent cardiovascular disease
Individuals in the highest quartile of serum phosphorus had a higher prevalence of prevalent cardiovascular disease, an association that persisted upon adjustment of data for potential confounders that included demographic variables, cardiovascular risk factors, year of screening, severity of CKD, coexisting diseases, laboratory data, CKD awareness, and health insurance (Table 2). There was no meaningful change in the odds ratio with additional adjustments for self-reported difficulty in getting care, or for a language other than English as the preferred language for communication.
Table 2. Odds ratio for the association between quartiles of serum phosphorus and prevalent cardiovascular disease.
| Group (sample size) | Serum phosphorus range, mg/dl | Number of subjects with prevalent cardiovascular disease (%) | Unadjusted | Model 1 | Model 2 | Model 3 |
|---|---|---|---|---|---|---|
| Quartile 1 (n=2979) | ≤3.3 | 1314 (44) | Ref | Ref | Ref | Ref |
| Quartile 2 (n=2947) | >3.3 to 3.7 | 1285 (44) | 0.98 (0.89–1.09) | 1.03 (0.92–1.15) | 1.02 (0.91–1.14) | 1.05 (0.93–1.18) |
| Quartile 3 (n=2577) | >3.7 to 4.1 | 1069 (42) | 0.90 (0.81–1.00) | 0.96 (0.86–1.08) | 0.98 (0.87–1.11) | 0.99 (0.87–1.12) |
| Quartile 4 (n=2169) | >4.1 | 989 (46) | 1.06 (0.95–1.19) | 1.19 (1.05–1.35) | 1.19 (1.05–1.36) | 1.23 (1.08–1.41) |
Model 1: adjusted for demographics (age, gender, race/ethnicity, highest education), year of screening, cardiovascular risk factors (diabetes, hypertension, dyslipidemia, current tobacco use, body mass index), and severity of chronic kidney disease (estimated glomerular filtration rate and albuminuria).
Model 2: adjusted model 1 plus laboratory parameters (plasma glucose, calcium, parathyroid hormone, and hemoglobin).
Model 3: adjusted model 2 plus chronic kidney disease (CKD) awareness and health insurance.
Serum phosphorus and all-cause mortality
Over a median follow-up of 2.3 years, 578 of the 10,672 CKD subjects died with an overall death rate of 21.5 per 1000 patient-years; the mortality rate by quartiles of serum phosphorus is summarized in Table 3. There was no demonstrable relationship between the quartile of baseline serum phosphorus and all-cause mortality in any of the models examined (Table 3 and Figure 1). There was no meaningful change in the hazards ratio for death with additional adjustments for self-reported difficulty in getting care, or for a language other than English as the preferred language for communication. Furthermore, there was no demonstrable interaction of any of the 12 covariates examined with the relationship of serum phosphorus with the risk of death (Figure 2). Similar results were obtained in additional analyses by dividing the cohort into tertiles or deciles of serum phosphorus or by using serum phosphorus as a continuous variable (data not shown).
Table 3. Association between serum phosphorus and all-cause mortality.
| Serum phosphorus range, mg/dl | Number of subjects | Mortality per 1000 person-years | Unadjusted | Model 1 | Model 2 | Model 3 | |
|---|---|---|---|---|---|---|---|
| Quartile 1 | ≤3.3 | 2979 | 19.8 | Ref | Ref | Ref | Ref |
| Quartile 2 | >3.3 to 3.7 | 2947 | 23.2 | 1.18 (0.94–1.47) | 1.12 (0.88–1.43) | 1.20 (0.94–1.54) | 1.22 (0.95–1.56) |
| Quartile 3 | >3.7 to 4.1 | 2577 | 20.3 | 1.01 (0.80–1.29) | 0.96 (0.75–1.25) | 1.01 (0.78–1.32) | 1.00 (0.76–1.32) |
| Quartile 4 | >4.1 | 2169 | 22.7 | 1.12 (0.88–1.42) | 1.01 (0.77–1.33) | 1.02 (0.77–1.36) | 1.00 (0.75–1.33) |
Model 1: adjusted for demographics (age, gender, race/ethnicity, highest education), year of screening, cardiovascular risk factors (diabetes, hypertension, dyslipidemia, current tobacco use, body mass index), and severity of chronic kidney disease (CKD; estimated glomerular filtration rate (eGFR) and albuminuria).
Model 2: adjusted model 1 plus laboratory parameters (plasma glucose, calcium, parathyroid hormone, and hemoglobin).
Model 3: adjusted model 2 plus CKD awareness and health insurance.
Figure 1.
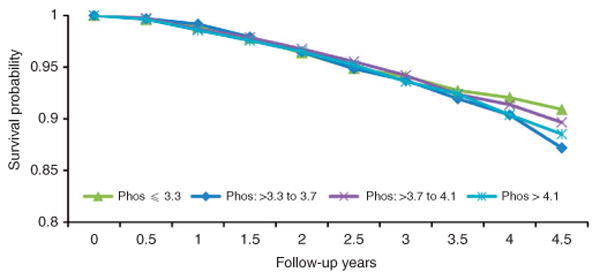
Kaplan–Meier survival analysis of time to death in individuals identified to have chronic kidney disease by quartiles of baseline serum phosphorus (Phos).
Figure 2. Forest plot showing hazards ratio with 95% confidence interval for the association between quartiles of serum phosphorus and all-cause mortality in subgroups based on 12 variables and in the entire study population.
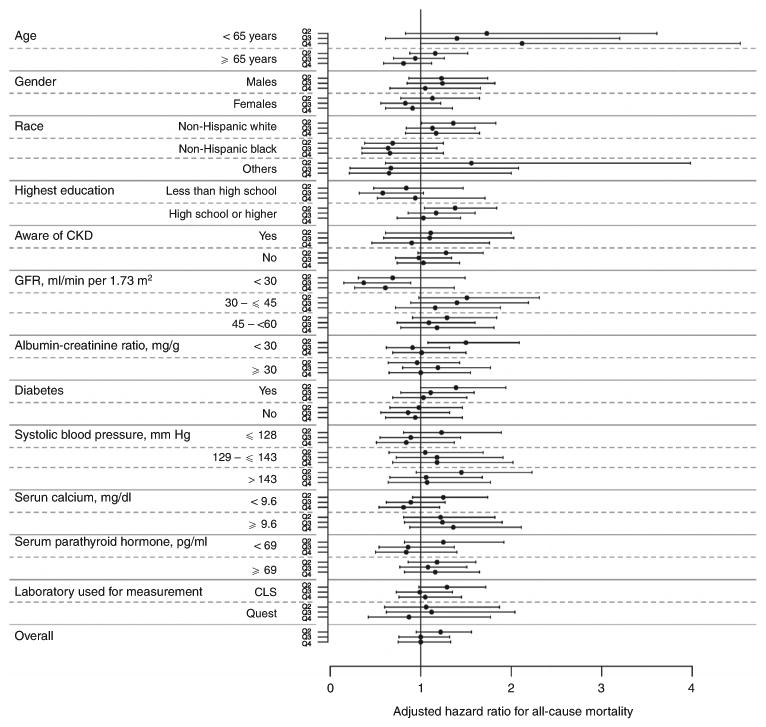
The range of serum phosphorus for each of the four quartiles were: quartile 1, <3.3 mg/dl (reference); quartile 2, >3.3 to 3.7 mg/dl; quartile 3, >3.7 to 4.1 mg/dl; and quartile 4, >4.1 mg/dl. All analyses are adjusted for the following covariates (except for the variable used to define the subgroup in each case): age, gender, race/ethnicity, year of screening, diabetes, hypertension, dyslipidemia, current tobacco use, body mass index, estimated glomerular filtration rate (eGFR), albuminuria, plasma glucose, calcium, parathyroid hormone, hemoglobin, chronic kidney disease (CKD) awareness, and health insurance.
Serum phosphorus and progression to ESRD
Over a median follow-up of 2.0 years, 194 subjects with CKD progressed to ESRD with an overall rate of 8.1 per 1000 patient-years (Table 4). In the unadjusted analyses, individuals in the highest quartile of serum phosphorus had a 6.72-fold higher risk of progression to ESRD; however, the hazards ratio was attenuated to a nonsignificant level on adjustment for demographic data and clinical variables (Table 4 and Figure 3). There was no meaningful change in the hazards ratio for progression to ESRD with additional adjustments for self-reported difficulty in getting care, or for a language other than English as the preferred language for communication. Similar results were obtained when the data were reanalyzed mortality as a competing risk for ESRD as an outcome (adjusted hazards ratio with quartile 1 as reference: quartile 2, 1.60 (0.84–3.03); quartile 3, 1.20 (0.61–2.35); and quartile 4, 1.82 (0.96–3.44)). There was no demonstrable interaction of 10 of the 12 covariates examined with the relationship of serum phosphorus and progression to ESRD (Figure 4). Additional analyses by dividing the cohort into tertiles or deciles of serum phosphorus or by using serum phosphorus as a continuous variable did not demonstrate any increase in the risk of progression to ESRD with higher serum phosphorus levels (data not shown).
Table 4. Association between serum phosphorus and progression to end-stage renal disease (ESRD).
| Serum phosphorus range, mg/dl | Number of subjects | ESRD per 1000 person-years | Unadjusted | Model 1 | Model 2 | Model 3 | |
|---|---|---|---|---|---|---|---|
| Quartile 1 | ≤3.3 | 2979 | 3.1 | Ref | Ref | Ref | Ref |
| Quartile 2 | >3.3 to 3.7 | 2947 | 5.3 | 1.71 (0.98–2.97) | 1.37 (0.77–2.43) | 1.42 (0.77–2.60) | 1.60 (0.84–3.04) |
| Quartile 3 | >3.7 to 4.1 | 2577 | 5.4 | 1.75 (1.00–3.06) | 1.15 (0.63–2.09) | 1.15 (0.61–2.18) | 1.19 (0.60–2.35) |
| Quartile 4 | >4.1 | 2169 | 20.5 | 6.72 (4.16–10.85) | 1.67 (0.95–2.94) | 1.65 (0.89–3.03) | 1.83 (0.97–3.48) |
Model 1: adjusted for demographics (age, gender, race/ethnicity, highest education), year of screening, cardiovascular risk factors (diabetes, hypertension, dyslipidemia, current tobacco use, body mass index), and severity of chronic kidney disease (CKD; estimated glomerular filtration rate (eGFR) and albuminuria).
Model 2: adjusted model 1 plus laboratory parameters (plasma glucose, calcium, parathyroid hormone, and hemoglobin).
Model 3: adjusted model 2 plus CKD awareness and health insurance.
Figure 3.
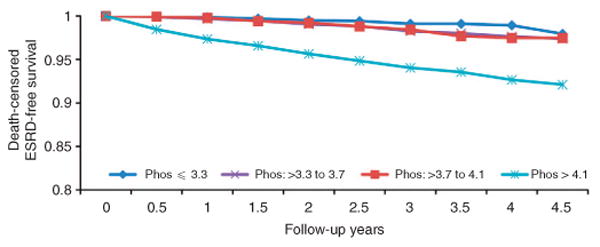
Kaplan–Meier survival analysis of time to end-stage renal disease (ESRD) in individuals identified to have chronic kidney disease by quartiles of baseline serum phosphorus (Phos).
Figure 4. Forest plot showing hazards ratio with 95% confidence interval for the association between quartiles of serum phosphorus and progression to end-stage renal disease (ESRD) in subgroups based on 12 variables and in the entire study population.
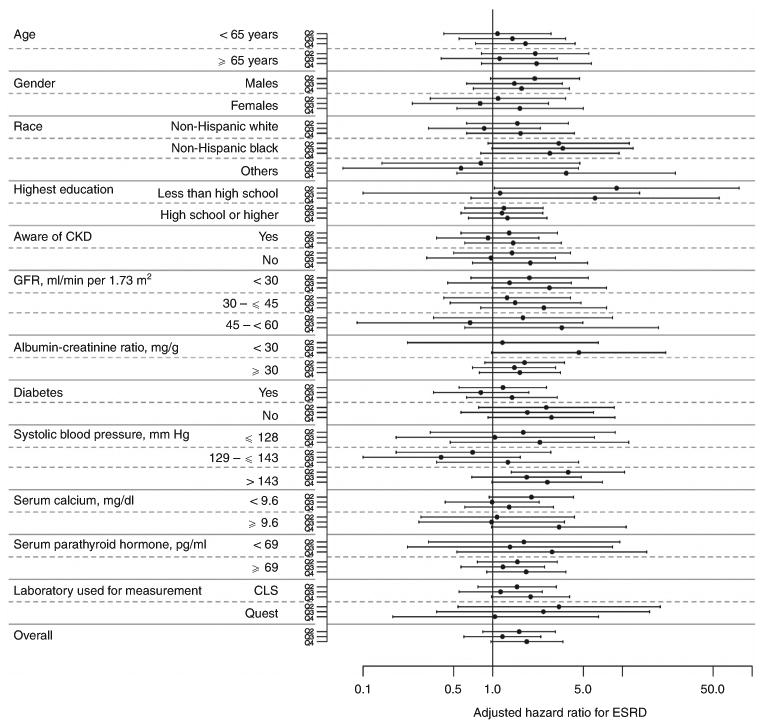
The range of serum phosphorus for each of the four quartiles were: quartile 1, <3.3 mg/dl (reference); quartile 2, >3.3 to 3.7 mg/dl; quartile 3, >3.7 to 4.1 mg/dl; and quartile 4, >4.1 mg/dl. All analyses are adjusted for the following covariates (except for the variable used to define the subgroup in each case): age, gender, race/ethnicity, year of screening, diabetes, hypertension, dyslipidemia, current tobacco use, body mass index, estimated glomerular filtration rate (eGFR), albuminuria, plasma glucose, calcium, parathyroid hormone, hemoglobin, chronic kidney disease (CKD) awareness, and health insurance. Please note that there was only one event of end-stage renal disease in individuals with albumin–creatinine ratio of <30 in quartile 3 of serum phosphorus.
Serum phosphorus and the risk of either death or progression to ESRD
Over the follow-up period, 732 subjects reached the composite outcome of either death or progression to ESRD (Figure 5). There was no demonstrable relationship between the quartile of serum phosphorus and reaching the composite outcome (adjusted hazards ratio, quartile 2, 1.20 (0.95–1.52); quartile 3, 1.01 (0.79–1.30); and quartile 4, 1.17 (0.90–1.51)).
Figure 5.
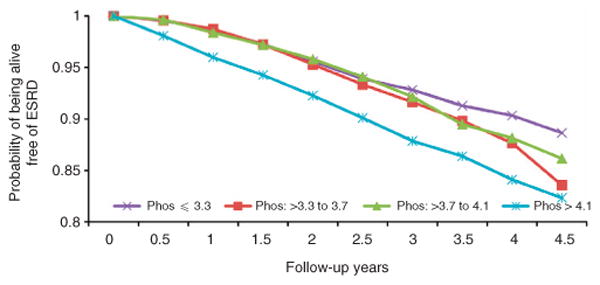
Kaplan–Meier survival analysis of time to death or end-stage renal disease (ESRD) in individuals identified to have chronic kidney disease by quartiles of baseline serum phosphorus (Phos).
Discussion
This study examined the association of serum phosphorus with the risk of death and progression to ESRD in the largest cohort of individuals with earlier-stage CKD to date. Despite an association of serum phosphorus with self-reported history of prevalent cardiovascular disease, unlike some of the previous studies in individuals with earlier-stage CKD, the association with all-cause mortality, or progression to ESRD, or the composite outcome of death or progression to ESRD in this cohort was robustly null.
There are compelling laboratory data that support the notion that disordered phosphorus homeostasis is an important contributor to the vascular disease seen with CKD.22,23 Addition of inorganic phosphorus to culture media leads to the expression of osteoblast lineage transcription factors in vascular smooth cells and the mineralization via the secretion of matrix vesicles and apoptotic bodies.6,7 In animal models (CKD in low-density lipoprotein receptor knockout mice fed a high-fat diet, and adenine-induced CKD in rats treated with active vitamin D3), reducing systemic phosphorus burden with phosphate binders ameliorates the development of vascular calcification.8,24,25 The importance of these findings is underscored by the increased prevalence and severity of vascular calcification in CKD, which in turn, is a predictor of patient survival.9,10,26,27 In addition to contribution to vascular calcification, higher dietary phosphorus intakes in humans are associated with reduced flow-mediated dilation of brachial artery, a measure of endothelial dysfunction.28 Thus, phosphorus participates in several mechanistic pathways to potentially induce/worsen the vascular disease in CKD.
Notwithstanding the biologic plausibility linking disordered phosphorus homeostasis with vascular disease in CKD, one potential interpretation of this study could lead us to question the validity of using serum phosphorus as a biomarker for such abnormalities in earlier-stage CKD. Serum phosphorus represents <1% of the total body phosphorus and the serum phosphorus levels in patients with earlier-stage CKD are significantly lower than the concentrations that induce mineralization in cell cultures. Most of the studies linking serum phosphorus with risk of death have been performed in patients undergoing maintenance dialysis, a population with significantly higher serum phosphorus levels than are typically observed in patients with earlier-stage CKD.12 At least five studies have previously examined the association of serum phosphorus with the risk of death in individuals with CKD not undergoing renal replacement therapies.13–16,19 Although four of these five studies demonstrated a higher risk of death with higher serum phosphorus levels, but within the reference range, post hoc analyses of the MDRD was unable to validate this association.13–16 Moreover, each of these studies included a selected patient population that potentially may limit the external validity of their findings—two were post hoc analyses of randomized controlled clinical trials (Cholesterol and Recurrent Events, CARE, which included a subgroup analyses of individuals with CKD, and MDRD),13,19 two were nearly or completely exclusively limited to male veterans,14,15 and one was a single-center study of patients with advanced CKD (mean eGFR, 13 ml/min per 1.73 m2) with significantly higher serum phosphorus than generally seen in earlier stages of CKD.16 In contrast, our study cohort of individuals with CKD was derived from community-based screening of high-risk individuals and, thus differs from the CKD populations included in the studies to date. Indeed, the overall mortality rate in our study cohort (21.5 per 1000 patient-years) was considerably lower than in the two studies from the Veterans Administration (141 and 119 per 1000 patient-years, respectively).14,15 This consideration may allow an alternative interpretation of our findings that serum phosphorus is not an independent predictor of mortality in a lower-risk CKD population like the one identified from community screening efforts such as the KEEP. Thus, our findings should inform future decision making about interventions designed to mitigate the risk of death in CKD populations identified through community-based screening.
There is some laboratory evidence that raises the possibility that disordered phosphorus homeostasis may also contribute to the progression of CKD. In animal models, CKD is associated with intrarenal calcification that is ameliorated with dietary phosphorus restriction.29–32 Furthermore, individuals with proteinuric diabetic kidney disease with renal artery calcification are more likely to progress to ESRD.33 At least three previous studies have demonstrated an association between serum phosphorus and CKD progression—one was a post hoc analysis of a randomized controlled clinical trial (African American Study of Kidney Disease), the second was limited to male veterans, and the third enrolled individuals with advanced CKD with significantly higher serum phosphorus levels.16–18 In contrast, our study could not validate the findings from these previous publications even with the use of models that accounted for competing risk of mortality for the ESRD outcome. Caution must be exercised in interpreting the modest statistical interaction of the outcome with albuminuria and serum calcium levels as our analyses were not adjusted for multiple comparisons. Like for mortality, there are two potential interpretation of our findings—either serum phosphorus is not a predictor of progression to ESRD in early-stage CKD or is not an appropriate biomarker for this outcome in relatively low-risk populations identified by community-wide screening. Indeed, the rate of progression to ESRD (8.5/1000 patient years) was considerably lower than the rate of 89/1000 patient-years in the study by Schwarz et al.17 and 67% of the study population reaching ESRD over 0.9 years in the study by Voormolen et al.16 Nevertheless, the association of serum phosphorus with progression of CKD should be examined in other cohorts with differing levels of risk of progression to ESRD to adequately guide risk assessment in clinical practice.
The primary goal of this analysis was to determine whether access to care confounds the relationship between serum phosphorus and risk of death and/or progression to ESRD in individuals with early-stage CKD. To date, study of three independent cohorts has provided consistent evidence for higher serum phosphorus levels in individuals with lower socioeconomic status.20,34,35 Consistent with these findings, in our study cohort, individuals in the highest quartile of serum phosphorus were somewhat less likely to have health insurance. However, the lack of any significant association between serum phosphorus and risk of death and/or progression to ESRD made testing our primary hypothesis moot.
Despite the strengths of a large sample size with substantial racial diversity and significant external validity from a CKD cohort derived from community-based screening of high-risk individuals, our findings have to be considered in light of the potential limitations of the study. First, serum phosphorus was measured only once at the time of screening and no data on interval measurements or treatments were available. However, this is unlikely to be the reason for the difference between our findings from the previous publications as five of the seven preceding studies also used data from a single baseline visit.13,15,17–19 Second, the serum phosphorus levels are affected by dietary intakes and vary during the course of the day. There was no standardization of collection of blood samples vis-à-vis meals. However, measurements of serum phosphorus in all but one of the preceding studies also reflected random values.14–17,19 Third, similar to the previously published observational studies examining this issue, the finding of the association between serum phosphorus and prevalent cardiovascular disease is also subject to residual confounding. Fourth, the follow-up period of 2.1–2.3 years was relatively short. However, only two studies—post hoc analyses of the MDRD and the AASK clinical trials—had longer follow-up period (10.3 and 4.0 years, respectively);18,19 for each of the other studies, the follow-up period ranged from 0.9 to 2.1 years.14–17 Fifth, the number of ESRD events was relatively modest and our study may not have been adequately powered to detect an association with this outcome. Nevertheless, the sample size of our study is the largest population with early-stage CKD in which this association has been examined thus far. Sixth, the study population was identified from a self-referred community-based population of high-risk individuals who participated in a CKD screening program. Seventh, data on other relevant measures like fibroblast growth factor-23 and urinary phosphorus excretion were not available. Finally, by linking data from KEEP with national registries, we were able to assess the association of serum phosphorus with ESRD and mortality, but not cardiovascular events, which may be an equally relevant clinical outcome.
In conclusion, in this large cohort of individuals with early-stage CKD identified from community-based screening of a high-risk population, there was no demonstrable association between serum phosphorus levels and the risk of either mortality or progression to ESRD. Thus, care must be exercised in using serum phosphorus levels as a biomarker for disordered phosphorus homeostasis in individuals with early-stage CKD identified during community-based screening. Future studies should determine whether alternative measures such as urinary phosphorus excretion better capture the putative risk with phosphorus in early-stage CKD and should be the target for cardiovascular risk reduction in this patient population.
Materials and Methods
Study participants, procedures, and definitions
KEEP, a nationwide program to screen individuals at high risk for CKD (individuals ⩾18 years of age with a history of diabetes or hypertension, or first-degree relatives with diabetes, hypertension, or kidney disease), was launched by the National Kidney Foundation on 1 January 2000. Measurement of serum calcium, phosphorus, and parathyroid hormone for individuals identified to have an eGFR <60 ml/min per 1.73 m2 was included in the screening procedures starting in November 2005. Demographic data (age, gender, race, and highest educational level), clinical conditions, and barriers to access to care (subject report of difficulty in getting care, availability of health insurance, and timing of last visit to a doctor) were determined through a questionnaire administered at the screening visit. Subjects were deemed to be aware of their CKD if they answered ‘yes’ to the question: have you ever been told by a doctor or a health-care professional that you have kidney disease. Blood glucose concentration and semiquantitative ascertainment of albuminuria were ascertained by point-of-care testing on the day of screening; venipuncture was performed for additional testing as indicated.
Diabetes was defined as history of diabetes (self-report or retinopathy), use of medications to treat diabetes, or fasting blood glucose level ⩾126 mg/dl or nonfasting blood glucose level ⩾200 mg/dl in the absence of self-report or medication use. Hypertension was defined as self-report, use of antihypertensive medications, or systolic blood pressure ⩾130 mm Hg or diastolic blood pressure ⩾80 mm Hg. History of cardiovascular disease was defined as self-reported history of heart attack, heart angioplasty, bypass surgery, heart failure, abnormal heart rhythm, or stroke. Dyslipidemia was defined as a positive history (self-report or taking lipid-lowering medications), or total cholesterol >200 mg/dl or triglycerides >150 mg/dl.
Laboratory measurements
Blood samples collected at the time of screening were shipped to a central laboratory for additional testing. For the period from November 2005 to 15 June 2008, testing was performed by Central Laboratory Services (Van Nuys, CA; n=5234; mean serum phosphorus, 3.78 ± 0.65 mg/dl); all subsequent testing was performed at Quest Diagnostics (Wood Dale, IL; n=5438; mean serum phosphorus, 3.63 ± 0.56 mg/dl). Hemoglobin, lipid panel, and serum creatinine were measured for all participants. Serum creatinine measurements in each of the two laboratories were calibrated to the Cleveland Clinic Research Laboratory. eGFR was estimated using the CKD Epidemiology Collaboration (CKD-Epi) equation.36 Serum calcium, phosphorus, and parathyroid hormone were measured for participants with eGFR <60 ml/min per 1.73 m2. Serum phosphorus was measured with an auto analyzer using the molybedate reaction and the coefficient of variation ranged from 1 to 3%. Serum parathyroid hormone was measured using a two-site chemiluminescent enzyme-labeled immunometric assay with a coefficient of variation of 4.0–6.5%. Glycosylated hemoglobin was measured for participants with self-reported diabetes or with point-of-care measurement of blood glucose consistent with diabetes using an immunoturbidometric method.
Determination of outcomes
The KEEP data were linked to the Social Security Administration Master Death File at the National Death Index to determine all-cause mortality. The occurrence of ESRD was determined by linking the data to that from the United States Renal Data System. All outcomes were ascertained through December 2010.
Statistical analyses
Participant characteristics were described by dividing the study population into quartiles of serum phosphorus (≤3.3, >3.3 to 3.7, >3.7 to 4.1, and >4.1 mg/dl). For categorical variables, the differences in proportions across phosphorus groups were tested using the χ2 test of independence. Continuous data are presented as mean ± s.d. or median with interquartile range, and differences between groups were tested by using one-way analysis of variance, or Kruskall–Wallis test, respectively. Logistic regression analysis was used to test the association of quartile of serum phosphorus with prevalent cardiovascular disease. Time-to-event survival analysis using Cox proportional hazards was used to determine the association of quartile of baseline serum phosphorus with all-cause mortality, or progression to ESRD, or a composite outcome of death or progression to ESRD. Additional analyses were done for ESRD outcome using a competing risk model, with mortality as a competing risk.
Three nested sets of covariates were examined for all statistical models to adjust for potential confounding variables—(1) demographics (age, gender, race/ethnicity, highest education), year of screening, traditional cardiovascular risk factors (diabetes, hypertension, dyslipidemia, current tobacco use, body mass index), and severity of chronic kidney disease (estimated glomerular filtration rate and albuminuria); (2) variables in model 1 plus laboratory parameters (plasma glucose, calcium, parathyroid hormone, and hemoglobin) for additional adjustment for severity of CKD; and (3) variables in model 2 plus CKD awareness and medical insurance.
To test the robustness of the results, post hoc survival analyses for each of the two outcomes (death and ESRD progression) were performed in subgroups based on 12 variables (age, gender, race, highest education, CKD awareness, eGFR, albuminuria, diabetes, systolic blood pressure, serum calcium, parathyroid hormone, and laboratory used for measurement). Furthermore, results were re-examined dividing the entire population based upon tertiles and deciles of serum phosphorus.
All analyses were performed using SAS 9.1 (Cary, NC; www.sas.com).
Acknowledgments
RM is supported by RO1DK95668. KN is supported in part by NIH grants U54MD007598, UL1TR000124, P30AG021684, and P20MD000182.
Disclosure: RM has received grant support and/or honoraria from Amgen, Mitsubishi Tanabe, Shire Pharmaceuticals, and Vifor. AS has received grant support from Genzyme.
Appendix
The KEEP investigators are: Peter A McCullough (Chair), Adam Whaley-Connell (Co-Chair), Andrew Bomback, Kerri Cavanaugh, Linda Fried, Claudine Jurkovitz, Mikhail Kosiborod, Samy McFarlane, Rajnish Mehrotra, Keith Norris, Rulan Savita Parekh, Carmen A Peralta, Georges Saab, Stephen Seliger, Michael Shlipak, Lesley Stevens, Manjula Kurella Tamura, and John Wang; ex officio: Bryan Becker, Allan Collins, Nilka Rios Burrows, Lynda A Szczech, and Joseph Vasssalotti; advisory group: George Bakris and Wendy Brown; data coordinating center: Shu-Cheng Chen.
Footnotes
These KEEP investigators are listed in the Appendix.
All the other authors declared no competing interests.
References
- 1.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 2.Keith DS, Nichols GA, Gullion CM, et al. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med. 2004;164:659–663. doi: 10.1001/archinte.164.6.659. [DOI] [PubMed] [Google Scholar]
- 3.Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377:2181–2192. doi: 10.1016/S0140-6736(11)60739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holtkamp FA, de Zeeuw D, de Graeff PA, et al. Albuminuria and blood pressure, independent targets for cardioprotective therapy in patients with diabetes and nephropathy: a post hoc analysis of the combined RENAAL and IDNT trials. Eur Heart J. 2011;32:1493–1499. doi: 10.1093/eurheartj/ehr017. [DOI] [PubMed] [Google Scholar]
- 5.Block GA. Therapeutic interventions for chronic kidney disease-mineral and bone disorders: focus on mortality. Curr Opin Nephrol Hypertens. 2011;20:376–381. doi: 10.1097/MNH.0b013e328346f93f. [DOI] [PubMed] [Google Scholar]
- 6.Jono S, McKee MD, Murry CE, et al. Phosphate regulation of vascular smooth muscle cell calcification. Circ Res. 2000;87:E10–E17. doi: 10.1161/01.res.87.7.e10. [DOI] [PubMed] [Google Scholar]
- 7.Reynolds JL, Joannides AJ, Skepper JN, et al. Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: a potential mechanism for accelerated vascular calcification in ESRD. J Am Soc Nephrol. 2004;15:2857–2867. doi: 10.1097/01.ASN.0000141960.01035.28. [DOI] [PubMed] [Google Scholar]
- 8.Mathew S, Tustison KS, Sugatani T, et al. The mechanism of phosphorus as a cardiovascular risk factor in CKD. J Am Soc Nephrol. 2008;19:1092–1105. doi: 10.1681/ASN.2007070760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiu YW, Adler SG, Budoff MJ, et al. Coronary artery calcification and mortality in diabetic patients with proteinuria. Kidney Int. 2010;77:1107–1114. doi: 10.1038/ki.2010.70. [DOI] [PubMed] [Google Scholar]
- 10.Block GA, Raggi P, Bellasi A, et al. Mortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patients. Kidney Int. 2007;71:438–441. doi: 10.1038/sj.ki.5002059. [DOI] [PubMed] [Google Scholar]
- 11.Kalpakian MA, Mehrotra R. Vascular calcification and disordered mineral metabolism in dialysis patients. Semin Dial. 2007;20:139–143. doi: 10.1111/j.1525-139X.2007.00261.x. [DOI] [PubMed] [Google Scholar]
- 12.Palmer SC, Hayen A, Macaskill P, et al. Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: a systematic review and meta-analysis. JAMA. 2011;305:1119–1127. doi: 10.1001/jama.2011.308. [DOI] [PubMed] [Google Scholar]
- 13.Tonelli M, Sacks F, Pfeffer M, et al. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation. 2005;112:2627–2633. doi: 10.1161/CIRCULATIONAHA.105.553198. [DOI] [PubMed] [Google Scholar]
- 14.Kestenbaum B, Sampson JN, Rudser KD, et al. Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol. 2005;16:520–528. doi: 10.1681/ASN.2004070602. [DOI] [PubMed] [Google Scholar]
- 15.Kovesdy CP, Anderson JE, Kalantar-Zadeh K. Outcomes associated with serum phosphorus level in males with non-dialysis dependent chronic kidney disease. Clin Nephrol. 2010;73:268–275. doi: 10.5414/cnp73268. [DOI] [PubMed] [Google Scholar]
- 16.Voormolen N, Noordzij M, Grootendorst DC, et al. High plasma phosphate as a risk factor for decline in renal function and mortality in pre-dialysis patients. Nephrol Dial Transplant. 2007;22:2909–2916. doi: 10.1093/ndt/gfm286. [DOI] [PubMed] [Google Scholar]
- 17.Schwarz S, Trivedi BK, Kalantar-Zadeh K, et al. Association of disorders in mineral metabolism with progression of chronic kidney disease. Clin J Am Soc Nephrol. 2006;1:825–831. doi: 10.2215/CJN.02101205. [DOI] [PubMed] [Google Scholar]
- 18.Norris KC, Greene T, Kopple J, et al. Baseline predictors of renal disease progression in the African American Study of Hypertension and Kidney Disease. J Am Soc Nephrol. 2006;17:2928–2936. doi: 10.1681/ASN.2005101101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Menon V, Greene T, Pereira AA, et al. Relationship of phosphorus and calcium-phosphorus product with mortality in CKD. Am J Kidney Dis. 2005;46:455–463. doi: 10.1053/j.ajkd.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 20.Gutierrez OM, Anderson C, Isakova T, et al. Low socioeconomic status associates with higher serum phosphate irrespective of race. J Am Soc Nephrol. 2010;21:1953–1960. doi: 10.1681/ASN.2010020221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown WW, Peters RM, Ohmit SE, et al. Early detection of kidney disease in community settings: the Kidney Early Evaluation Program (KEEP) Am J Kidney Dis. 2003;42:22–35. doi: 10.1016/s0272-6386(03)00405-0. [DOI] [PubMed] [Google Scholar]
- 22.Moe SM, Chen NX. Mechanisms of vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2008;19:213–216. doi: 10.1681/ASN.2007080854. [DOI] [PubMed] [Google Scholar]
- 23.Hruska KA, Mathew S, Lund R, et al. Hyperphosphatemia of chronic kidney disease. Kidney Int. 2008;74:148–157. doi: 10.1038/ki.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davies MR, Lund RJ, Hruska KA. BMP-7 is an efficacious treatment of vascular calcification in a murine model of atherosclerosis and chronic renal failure. J Am Soc Nephrol. 2003;14:1559–1567. doi: 10.1097/01.asn.0000068404.57780.dd. [DOI] [PubMed] [Google Scholar]
- 25.Terai K, Nara H, Takakura K, et al. Vascular calcification and secondary hyperparathyroidism of severe chronic kidney disease and its relation to serum phosphate and calcium levels. Br J Pharmacol. 2009;156:1267–1278. doi: 10.1111/j.1476-5381.2008.00108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehrotra R, Budoff M, Christenson P, et al. Determinants of coronary artery calcification in diabetics with and without nephropathy. Kidney Int. 2004;66:2022–2031. doi: 10.1111/j.1523-1755.2004.00974.x. [DOI] [PubMed] [Google Scholar]
- 27.Russo D, Palmiero G, De Blasio AP, et al. Coronary artery calcification in patients with CRF not undergoing dialysis. Am J Kidney Dis. 2004;44:1024–1030. doi: 10.1053/j.ajkd.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 28.Shuto E, Taketani Y, Tanaka R, et al. Dietary phosphorus acutely impairs endothelial function. J Am Soc Nephrol. 2009;20:1504–1512. doi: 10.1681/ASN.2008101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ibels LS, Alfrey AC, Haut L, et al. Preservation of function in experimental renal disease by dietary restriction of phosphate. N Engl J Med. 1978;298:122–126. doi: 10.1056/NEJM197801192980302. [DOI] [PubMed] [Google Scholar]
- 30.Ibels LS, Alfrey AC, Huffer WE, et al. Calcification in end-stage kidneys. Am J Med. 1981;71:33–37. doi: 10.1016/0002-9343(81)90255-2. [DOI] [PubMed] [Google Scholar]
- 31.Haut LL, Alfrey AC, Guggenheim S, et al. Renal toxicity of phosphate in rats. Kidney Int. 1980;17:722–731. doi: 10.1038/ki.1980.85. [DOI] [PubMed] [Google Scholar]
- 32.Brown SA, Crowell WA, Barsanti JA, et al. Beneficial effects of dietary mineral restriction in dogs with marked reduction of functional renal mass. J Am Soc Nephrol. 1991;1:1169–1179. doi: 10.1681/ASN.V1101169. [DOI] [PubMed] [Google Scholar]
- 33.Chiu YW, Adler S, Budoff M, et al. Prevalence and prognostic significance of renal artery calcification in patients with diabetes and proteinuria. Clin J Am Soc Nephrol. 2010;5:2093–2100. doi: 10.2215/CJN.03730410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gutierrez OM, Isakova T, Enfield G, et al. Impact of poverty on serum phosphate concentrations in the Third National Health and Nutrition Examination Survey. J Ren Nutr. 2011;21:140–148. doi: 10.1053/j.jrn.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gutierrez OM, Katz R, Peralta CA, et al. Associations of socioeconomic status and processed food intake with serum phosphorus concentration in community-living adults: the Multi-Ethnic Study of Atherosclerosis (MESA) J Ren Nutr. 2012;22:480–489. doi: 10.1053/j.jrn.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]


