Abstract
Objectives
To discuss the novel agents which are being developed for the treatment of advanced and recurrent endometrial carcinoma and to review other molecular targets that may be interesting in the treatment of this disease. While the majority of women with endometrial cancer enjoy a relatively good prognosis, the options for those women who suffer from a disease recurrence are limited and there is a need to identify novel agents.
Methods
A review of clinical trials of novel therapeutic agents and their molecular targets is provided. In addition, a review of the current literature on other potential molecular targets for endometrial cancer was performed.
Results
Several phase II trials of novel agents, both alone and in combination with traditional cytotoxic chemotherapy, have been completed or are nearing completion. It appears that the targeted agents may have the most efficacy in combination with cytotoxic chemotherapy or in a multi-targeted agent approach.
Conclusions
Chemotherapy offers the opportunity for a meaningful response rate in women with endometrial cancer, but the responses are often short lived and cure is uncommon in the setting of recurrent disease. The recent increase in molecular targets has led to the availability of many novel therapies. Determining how these agents are to be used, alone or in combination with “standard” therapies, needs to be defined and translational studies are needed to develop rational combinations of these novel agents before we can move into clinical trials.
Keywords: Endometrial cancer, Biologic therapy, Chemotherapy, Novel therapeutics, Molecular targets
Introduction
Endometrial carcinoma is the most common gynecologic malignancy in the United States with approximately 42,160 cases and 7780 deaths estimated for 2009 [1]. Although patients diagnosed with and treated for early stage-disease of the endometrioid histology enjoy relatively good survival rates, those women who are diagnosed with either advanced stage disease or who suffer a recurrence have a poor prognosis (Table 1) [2]. For those women with early stage disease, surgery with individualized use of volume directed radiotherapy is curative. For those women with advanced stage disease, there is no real standard of care and traditionally these women are treated with surgery, chemotherapy and radiation, in one or more combinations. In the setting of advanced or recurrent disease, particularly when it is not amenable to surgical resection, the hallmark of therapy has been chemotherapy. While we have enjoyed some success, there is considerable room for improvement. In the era of modern chemotherapy, the Gynecologic Oncology Group (GOG) has conducted 9 clinical trials in the setting of advanced or recurrent endometrial cancer [3–10]. The majority of these trials contained the more traditional chemo-therapeutic agents including platinum, taxanes and anthracyclines with response rates ranging from 20% to 35% for single agent regimens and up to 75% for combination regimens. While higher response rates with combination regimens appear promising, the duration of these responses (progression free survival—PFS) ranges from 5 to 7 months in the recurrent endometrial cancer trials and unfortunately, these regimens are associated with high morbidity and mortality. The GOG is opening a randomized phase III trial in women with surgical stage III or IVa (b2 cm residual disease) endometrial carcinoma. This trial (GOG 258) randomizes women to either cisplatin and tumor volume directed irradiation followed by paclitaxel and carboplatin versus paclitaxel and carboplatin alone. This trial was recently activated and should begin accruing patients in the near future.
Table 1.
Stage at presentation and overall survival by stage for type I and type II endometrial carcinomas.
| Endometrioid | USC/Clear cell | ||
|---|---|---|---|
| Present in earlier stage | Present with advanced stage disease | ||
| Stage I | 73% | Stage I | 54% |
| Stage II | 11% | Stage II | 8% |
| Stage III | 13% | Stage III | 22% |
| Stage IV | 3% | Stage IV | 16% |
| 5-year survival rates | 5-year survival rates | ||
| Stage I | 85-90% | Stage I | 60% |
| Stage II | 70% | Stage II | 50% |
| Stage III | 40-50% | Stage III | 20% |
| Stage IV | 15-20% | Stage IV | 5-10% |
While there have been several investigator initiated and pharmaceutical trials evaluating novel therapeutic agents in this patient population, the GOG, in recognizing the need to evaluate targeted agents in women with endometrial cancer, has also conducted a series of phase II trials in women with this disease (Table 2). These trials have included traditional chemotherapeutic agents as well as novel therapies, which are the focus of this review (Table 3). Several of these trials are currently open and accruing patients (GOG 129-Q [gemcitabine], GOG 238 [whole pelvic irradiation±concurrent cis platin], GOG 248 [temsirolimus]). Other trials are closed, however the data are not yet mature enough for presentation (GOG 188 [faslodex], GOG 229-F [VEGF-TRAP]). The phase II lapatinib trial (GOG 229-D) closed in 2005; however, the second stage of accrual was not indicated and final publication is pending. GOG 229-G [bevacizumab +temsirolimus], one of few multi-targeted therapy trials, evaluates combination bevacizumab and temsirolimus in women with persistent or recurrent endometrial cancer. This trial is open at a limited number of centers and is currently accruing. Several other trials have been presented in abstract form only (GOG 181-B [trastuzumab], GOG 229-C [gefitinib], GOG 229-E [bevacizumab], GOG 129-O [pemetrexed]); however, the trials evaluating trastuzumab, gefitinib and pemetrexed appear to have limited activity in endometrial carcinoma. Conversely, preliminarily ixabepilone and bevacizumab appear to have at least a modest activity in women with recurrent or persistent endometrial carcinoma. Several of these trials will be discussed in more detail throughout the review. Due to the American Cancer Society's estimates that the death from endometrial cancer has risen over 200% since the early 1990s, there has been interest in seeking other agents, which either alone or in combination may allow us to improve the progression free and overall survival of women with this disease. These novel targets and their associated biologic therapies will be the focus of this review. Fig. 1 depicts many of the pathways in endometrial carcinoma that currently have targeted therapy in development.
Table 2.
Phase II GOG studies for advanced or recurrent endometrial cancer.
| Author, year | Number of patients | Regimena | Molecular target | PFSb (median) | OSc (median) |
|---|---|---|---|---|---|
| GOG 181 B [11] | 67 | Trastuzumab | Her-2/neu | 2 | |
| GOG 188 | Faslodex | ||||
| GOG 238 | WPI ± concurrent | ||||
| CDDP | |||||
| GOG 248 | Temsirolimus | mTOR, PTEN | |||
| GOG 229 Series | |||||
| B [12] | 27 | Thalidomide | 1.9 | 8.3 | |
| C[13] | 29 | Gefitinib | EGFR | 4/26 pts PFS | N/A |
| >6 months | |||||
| D | 31 | Lapatinib | EGFR | ||
| E [14] | 56 | Bevacizumab | VEGF | 1.7 | 6.3 |
| F | 28 | VEGF-TRAP | |||
| G | 29 | Bevacizumab + | |||
| Temsirolimus | |||||
| GOG 129 Series | |||||
| N [15] | Docetaxel | 3 | 10.6 | ||
| O [16] | 27 | Pemetrexed | 2.7 | 9.4 | |
| P [17] | 52 | Ixabepilone | Epothilone B | 2.9 | 8.7 |
| Q | Gemcitabine |
WPI = whole pelvic irradiation.
Progression free survival.
Overall survival.
Table 3.
Molecular alterations in endometrial cancer: percent frequency of genetic mutations in Type I and Type II cancers.
| Genetic alteration | Type I carcinoma (%) | Type II carcinoma (%) |
|---|---|---|
| PTEN inactivation | 50–80 | 10 |
| K-ras mutation | 15–30 | 0–5 |
| B-catenin mutation | 20–40 | 0–3 |
| Microsatellite instability | 20–40 | 0–5 |
| p53 mutation | 10–20 | 80–90 |
| HER-2/neu | 10–30 | 40–80 |
| p16 inactivation | 10 | 40 |
| E-cadherin | 10–20 | 60–90 |
From Bansal N, Yendluri V, Wenham RM. The molecular biology of endometrial cancers and the implications for pathogenesis, classification, and targeted therapies. Cancer Control. 2009;16(1):8–13. Reprinted with permission © 2009.
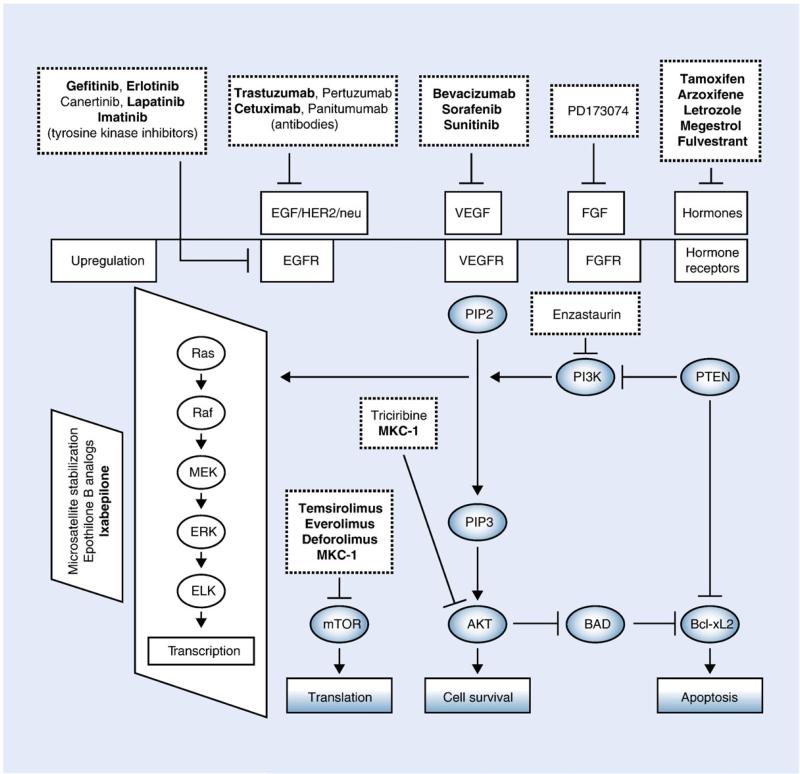
Fig. 1.
This figure illustrates many of the cellular pathways currently being targeted in the treatment of endometrial cancer. Drugs that are bold type are in trials that are either ongoing or have already been completed. Solid lines and arrows indicate stimulation or upregulation of target gene or receptor; dashed lines and arrows indicate downregulation or inhibition of the target gene or receptors. From Bansal N, Yendluri V, Wenham RM. The molecular biology of endometrial cancers and the implications for pathogenesis, classification, and targeted therapies. Cancer Control. 2009;16(1):8–13.
Genetic alterations in Type I and Type II endometrial carcinoma
Fortunately, there has been significant progress in the past few decades in understanding the molecular basis for the malignant transformation of normal cells and it is possible to take advantage of this increased understanding of tumorigenesis and develop novel therapies that target these molecular alterations (Table 3). The majority of endometrial carcinomas are sporadic, with approximately 10% being due to a hereditary predisposition [18]. There are two well-recognized subtypes of endometrial cancer based on the dualistic model of endometrial cancer tumorigenesis. They are referred to as Type I and Type II endometrial carcinoma based on the system described by Bokhman [19]. Type I endometrial cancers represent 70–80% of new cases [19]. These cancers tend to be of an endometrioid histology, are often estrogen-mediated and are associated with a high rate of PTEN (phosphatase and tensine homologue) tumor suppressor gene loss or mutation, as well as defects in mismatch repair that lead to microsatellite instability [20–24]. Women with Type I endometrial cancers typically have evidence of estrogen excess, either endogenous (obesity) or exo-genous. In addition, these women often have associated diabetes and hypertension.
Women with Type II endometrial cancer are older than those with Type I lesions and typically do not have evidence of estrogen excess. The Type II endometrial cancers typically show aneuploidy, p53 mutations and overexpression of HER-2/neu [18,25–30]. While these differences in tumorigenesis may be areas that can be further developed, one must not exclude women with Type II endometrial cancers from promising clinical trials as the molecular differences may or may not be clinically relevant. In a study designed to explore the association between histology and outcome in women with advanced or recurrent endometrial cancer, the authors did not find that response to doxorubicin, cisplatin or paclitaxel varied with regards to histologic subtype, and they did not recommend that women with uterine papillary serous carcinoma be excluded from future trials in women with endometrial carcinoma [31]. This is critically important as while Type II endometrial cancers represent approximately 10% of endometrial carcinomas, they are responsible for approximately 50% of all relapses. This is in part due to the more advanced stage at the time of diagnosis (Table 1).
Angiogenesis inhibitors
Angiogenesis, the formation of new blood vessels, is integral to the growth and metastasis of many malignancies, including endometrial cancer [32–34]. Vascular endothelial growth factor (VEGF) induces this new blood vessel formation and in many malignancies it is associated with a poor prognosis. VEGF expression is seen in the majority of endometrial cancer specimens (56–100%), and in many studies, it has been correlated with deep myometrial invasion, high histologic grade, lymphovascular space invasion, lymph node meta-stasis and poor prognosis [34–38]. However, there have been a series of studies that have not shown an association between VEGF and endometrial cancer prognosis and survival [39–41]. These apparent discrepancies may be explained by the variable expression of VEGF in different endometrial subtypes and with varying stages of disease. For example, VEGF is highly expressed in early stage and well-differentiated lesions, but has lower levels of expression in more advanced and poorly differentiated tumors [42].
Bevacizumab (Avastin®, Genentech) is a humanized mAb to VEGFA, and it has been studied as a single agent in women with recurrent endometrial carcinoma by the GOG (GOG 229-E). This study closed in 2008, and the findings were recently presented at the American Society of Clinical Oncology [14]. In this patient population, single agent bevacizumab (15 mg/kg IV every 3 weeks) provided an observed response rate of 15.1% with a 35.8% 6-month progression-free survival (PFS). Overall median PFS was 4.2 months, and median OS was 10.5 months. Based on the 6-month PFS, bevacizumab was deemed to show promising activity in this patient population. In addition, the translational correlation studies suggest that there is an association between plasma VEGF-A concentrations and tumor response and overall survival as well as an association between tumor VEGF-A staining intensity and overall survival.
The GOG has evaluated thalidomide in a phase II trial (GOG 229-B) and similarly correlated angiogenic biomarkers with survival [12]. Thalidomide is an anti-angiogenic, whose exact mechanism of action is unknown. This trial enrolled 27 women (of whom 24 were eligible) with recurrent or persistent endometrial cancer. Similar to GOG 229-E [bevacizumab], patients were required to have had one prior cytotoxic regimen and were allowed, but not required, to have had one additional cytotoxic regimen for the treatment of their disease. In this study, the median PFS and overall survival (OS) were 1.7 months and 6.3 months, respectively. Thalidomide was well tolerated in this patient population with no grade 4 toxicities. In this trial, patients started at a dose of 200 mg/day. For patients not experiencing grades 3–4 toxicities, thalidomide was increased by 200 mg/day at approximately 2-week intervals until a target dose of 1000 mg/day was reached. The set target dose was difficult to reach due to progressive disease as defined on the trial. The primary toxicities were gastrointestinal and neurologic (parasthesias). While no correlations were found between plasma VEGF levels and response to thalidomide, elevated plasma VEGF levels were associated with increased risk of progression and death. Due to its low response rate, thalidomide did not show sufficient activity to be considered as a single-agent therapy in women with recurrent or advanced endometrial cancer.
VEGF-trap (Afibercept®, Sanofi-Aventis) is a fusion protein of human VEGF receptor extracellular domains fused to the Fc portion of human immunoglobulin G that acts as a decoy receptor to bind VEGF-A and neutralize VEGF-A isoforms. In women with symptomatic ascites from ovarian cancer, it has been shown to decrease the frequency of paracentesis [43] and is now being studied in combination with docetaxel in women with recurrent ovarian cancer (Phase I/II). The GOG is conducting a Phase II trial of VEGF-TRAP (4 mg/kg IV every 14 days) in women with recurrent endometrial cancer with the primary objectives being PFS for at least 6 months. Similar to many other targeted therapeutic trials, translational correlative studies are being conducted. This study in particular is seeking to isolate, enumerate and phenotypically characterize circulating tumor cells and circulating endothelial cells recovered from patients before and during treatment with VEGF-Trap and to see if the counts and characteristics are associated with outcomes. In addition, the investigators seek to evaluate if tumor expression of proteins implicated in VEGF resistance (VEGF, VEGFR-1, VEGFR-2, FGF-1, FGF-2, FGF-7, etc.) will determine response to VEGF-Trap and whether they are associated with measures of clinical outcome. This study to date has accrued 28 women and based on the original study design is in its second stage of accrual. It is too early to draw any conclusions.
In addition to monoclonal antibodies, there are several small molecule inhibitors, which have been designed to target VEGF receptors (Fig. 1). Sorafenib (Nevaxar®, Bayer) is an oral multi-targeted kinase inhibitor that inhibits tumor growth by targeting the vascular endothelial cells and by inhibiting the VEGF receptor (VEGFR-1, VEGFR-2, VEGFR-3 and platelet derived growth factor receptor (PDGFR) [44]. Sorafenib has been evaluated as a single agent in women with recurrent endometrial cancer and carcinosarcomas. In this trial of 39 women, there was a 5% partial response and 50% had stable disease [45]. These findings are in line with the phase II trials of sorafenib either as a single agent or in combination with gemcitabine in women with recurrent ovarian cancer. In the single agent trial, 20/59 women had stable disease, and there were 2 partial responders [46]. In the combination trial with gemcitabine, there were few objective responses (5%), but 26% of women had stable disease. The median time PFS was 5.4 months and median overall survival was 13.3 [47]. Sunitinib (Sutent®, Pfizer) is an oral tyrosine kinase inhibitor of multiple VEGF receptors. It has recently been studied as a single-agent in phase II trial in women with recurrent or metastatic endometrial cancer. The first stage of the study, which consisted of 16 patients, was recently presented. Median time to progression was 2.5 months and OS was 6.2 months. Based on this activity, enrollment has begun to the second stage of accrual [48].
Other antiangiogenic agents that are being investigated in gynecologic malignancies include cediranib (Recentin®, AZD2171, AstraZeneca) an oral inhibitor of VEGFR-1, VEGFR-2, VEGFR-3, PDGFRB and c-kit [49]; volociximab (M200, PDL BioPharma and Biogen) a chimeric monoclonal antibody directed against α5β1 integrin on endothelial cells [50–52]; IMC-1121B (ImcClone) which is a fully humanized monoclonal antibody directed against VEGFR-2 (NCT00721162) [53]. It may be that these agents, while themselves primarily cytostatic, act synergistically when given in combination with more conventional cytotoxic therapy. The underlying mechanism of this synergistic relationship may be that antiangiogenics reduce the hyperpermeable nature of the vasculature and subsequently decrease the interstitial tumor pressures. This may in turn allow for an increased concentration of the cytotoxic agent to be delivered to the tumor [54].
Phosphoinositide 3 kinase/AKT/mammalian target of rapamycin inhibitors
PTEN is a common mutation in Type I endometrial carcinoma occurring in up to 40–80% of women [20–22,55]. The loss of PTEN results in activation of Akt which subsequently upregulates mTOR activity. Therefore, tumors that are deficient in PTEN may be perfect targets for mTOR inhibitors. MTOR inhibitors are known for their antiproliferative properties due their ability to modulate signal transduction pathways involved in cell cycle progression [56]. Three mTOR inhibitors have been or are currently being investigated in women with endometrial cancer: temsirolimus (CCI-779, Wyeth), everolimus (RAD001, Novartis) and deforolimus (AP23573, Merck). Temsirolimus is a water-soluble ester of rapamycin given by intravenous infusion. In a recently presented phase II trial of chemotherapy naïve women with advanced or recurrent endometrial cancer, single agent temsirolimus (25 mg IV weekly) was associated with a 26% partial response rate [57]. Response in this study did not correlate with PTEN status as evaluated by immunohistochemistry. This same group (NCIC) also conducted a phase II trial in women with heavily pretreated endometrial cancer. In this study, a 7% partial response rate was seen, and 44% of the patients experienced stable disease [58]. The GOG is currently conducting a phase II trial of temsirolimus as a single agent (25 mg IV weekly) versus temsirolimus (25 mg IV weekly) with megesterol acetate (80 mg orally BID) and tamoxifen (20 mg orally BID). Deforolimus, like temsirolimus, is an intravenous mTOR inhibitor. In a phase II trial of women with endometrial cancer and uterine carcinosarcoma, 28% of patients had either a complete or partial response [59]. Eighteen of 45 women discontinued therapy prior to 4 months, primarily due to progressive disease. While oral formulations of deforolimus are being developed, only one oral mTOR inhibitor has been studied to date in women with endometrial cancer. Everolimus is an oral bioavailable ester derivative of rapamycin and has been similarly evaluated in a phase II trial with biologic correlates. In this study, median PFS was 4.5 months. The authors reported that AKT and mTOR expression was not associated with response to therapy [60]. According to the results from the completed phase II trials to date, mTOR inhibitors do exhibit activity in women with advanced or recurrent endometrial cancer, and these agents merit continued evaluation.
Similar to the antiangiogenic agents, it may be that the mTOR inhibitors may potentiate the activity of and act synergistically with chemotherapeutic agents. In endometrial cancer cell lines, rapamycin has been shown to be synergistic with cisplatin (inhibition of cell growth, induction of apoptosis and increased expression of DNA mismatch repair proteins) and paclitaxel (inhibition of cellular proliferation, induction of apoptosis and increased polymerization of tubulin) [61,62]. Similar findings have also been seen in other tumor models including breast cancer [63]. These studies provide rationale for combination clinical trials of mTOR inhibitors and chemotherapeutic agents. Clinical trials evaluating mTOR inhibitors with other biologic agents (temsirolimus and bevacizumab-GOG 229G) and with cytotoxic agents (everolimus and topotecan NCT00703807) are underway or being planned.
Other agents that can affect the PI3K/PTEN/akt/mTOR pathway are also being developed and may warrant investigation in women with endometrial cancer. MKC-1 (EntreMed), an oral cell cycle inhibitor, reduces phospho-AKT has recently been evaluated in a phase II trial of women with recurrent ovarian and endometrial carcinoma [64]. In an in vitro model of endometrial stromal sarcoma, SAHA, a distone deacetylase inhibitor, treated cells exhibited decreased expression of mTOR and phospho-S6 ribosomal protein, which is a downstream target of mTOR [65]. There are two drugs in development, enzastaurin (LY 317615, Eli Lilly) and bryostatin-1, which can selectively inhibit specific serine/threonine kinase iso-forms. These drugs are currently being evaluated in clinical trials (breast, glioma, colorectal, renal cell, etc.) both as single agents and in combination with cytotoxic agents and other novel therapeutics.
Epidermal growth factor receptor inhibitors (tyrosine kinase inhibitors, monoclonal antibodies inhibitors to EGFR)
The epidermal growth factor receptor (EGFR) family consists of 4 distinct tyrosine kinase cell-surface receptors [EGFR (ErbB-1), HER-2/ neu (ErbB-2), Her-3 (ErbB-3), and Her-4 (ErbB-4)]. Following binding to EGF-like growth factor, the intracellular tyrosine kinase domain is activated, leading to cellular proliferation and survival [66]. Both Type I and Type II endometrial cancer frequently overexpresses EGFR, and this overexpression has been correlated with tumor grade, deep myometrial invasion and poor survival [26–28,66–71]. Despite the excellent rationale, GOG 181-B, which evaluated trastuzumab (Herceptin®, Genentech) in women with advanced or recurrent endometrial cancer, failed to show significant activity, even in those women whose tumors overexpressed Her-2/neu [11]. Despite these results, there is merit in continuing to evaluate this class of drugs in women with endometrial carcinoma.
Small molecule tyrosine kinase inhibitors
There are several drugs in this class that have been studied in phase II studies, both within and outside the Gynecologic Oncology Group. The GOG has evaluated both lapatinib (GOG 229-D, Tykerb®, GSK) and gefitinib (GOG 229-C, ZD 1839 (Iressa®), Astra Zeneca) in phase II trials of women with persistent or recurrent endometrial cancer.
– Lapatinib is an oral dual kinase inhibitor, which targets both EGFR and Erb-B2 [72]. In vitro studies of lapatinib in endometrial cancer cell lines demonstrated anti-proliferative effects in all cell lines tested in a PTEN-independent manner [73]. GOG 229-D (lapatinib 1500 mg oral daily) enrolled 31 women, of whom 30 were evaluable. While the data have not been published, this study did not open to the second stage of accrual, so one can assume that there was not sufficient activity as a single agent to merit further investigation.
– Gefitinib is a specific inhibitor of EGFR tyrosine kinase activity and binds to the ATP binding site on the EGFR kinase domain [74]. Similarly, GOG 229-C (gefitinib 500 mg oral daily) enrolled 29 women of whom 26 were evaluable. In a preliminary analysis of this study, one patient experienced a complete response and others had stable disease after 6 months [75]. We await the final analysis of this study.
– Erlotinib (Tarceva®, Genentech), an oral EGFR tyrosine kinase inhibitor, has been evaluated outside of the GOG in phase II trials of women with advanced endometrial cancer. In one trial, partial responses were documented in 2 out of 27 women, and stable disease was demonstrated in 52% of patients with a median duration of response of 3.4 months [76]. In the other trial conducted by the NCIC, there was a 12.5% reported response rate [77].
– Type I and uterine papillary serous carcinomas express Abl and platelet derived growth factor receptor (PDGFR) by immunohistochemistry, in the setting of both primary and recurrent disease. [78]. Imatinib (Gleevac®, Novartis) inhibits several tyrosine kinases, including c-Kit and may be a reasonable therapeutic agent in endometrial cancer considering its targets. This rationale lead to a phase I dose escalation trial of imatinib with paclitaxel 175 mg/m2 trial in women with advanced or recurrent uterine papillary serous carcinoma. This trial accrued 11 patients with one of two patients with measurable disease having a partial response [79].
While we await the final results of these studies, the preliminary responses are not dramatic and in fact, the two GOG trials of these small molecules did not open to the second stage of accrual. It may be that endometrial cancer is resistant to this class of drugs and one potential mechanism may be related to downregulation of p53 and a related overexpression of MDM2, which is the principal factor that inhibits p53 function, leading to drug resistance [80].
Monoclonal antibody inhibitors to EGFR
There are several monoclonal antibodies directed at EGFR that are currently available, of which only one has been evaluated in women with endometrial cancer (trastuzumab). Matuzumab (EMD72000, Takeda), Pertuzumab (Omnitarg®, Genentech), and cetuximab (Erbitux®, Bristol-Myers Squibb) are all directed towards different targets on EGF (Fig. 1). While several of these agents are being investigated, to date, there are no published clinical trials in endometrial cancer. As previously discussed, trastuzumab has been evaluated in a phase II trial by the GOG. Unfortunately, despite several of the tumors overexpressing Her2/neu, there was minimal activity. However, it may be that the most appropriate endometrial cancer histology for treatment with trastuzumab was underrepresented in this phase II trial. Uterine papillary serous carcinoma (UPSC) is shown to overexpress ErbB-2 in up to 80% of cases (Table 3) [27]. Several authors have reported objective responses in women with UPSC who are treated with trastuzumab [81,82]. It may be that a prospective trial with trastuzumab should be conducted in women with UPSC. A phase II trial of cetuximab in women with recurrent endometrial cancer is ongoing. Overall the activity of monoclonal antibodies designed to target the EGFR pathway has been fairly limited in gynecologic malignancies, either as single agents or in combination with chemotherapy. It may be that patient selection has not been appropriate or that the right combination of agents has yet to be determined.
Miscellaneous agents
There are fortunately several other agents that may show promise in the treatment of endometrial cancer. Several experts have commented on the need for more clinical trials in endometrial cancer and in UPSC in particular [83,84]. Using microarray, investigators have been able to identify potential targets. Claudin-3 and claudin-4 are highly expressed in UPSC and may serve as novel targets for therapy using Clostridium perfringes enterotoxin (CPE) [85,86]. In an in vitro system, Santin et al. [86] were able to demonstrate a dose dependent cytotoxic effect of CPE in established primary and recurrent UPSC cell lines. In addition, in vivo experiments in UPSC xenografts exhibited promising results.
There is a clear association between obesity, estrogen excess and endometrial cancer. What is evolving is a better understanding of the relationship between insulin resistance and endometrial cancer. Adiponectin is secreted by adipose tissue and has been shown to be a surrogate marker for insulin resistance [87]. In addition, it is independently and inversely associated with endometrial cancer suggesting that insulin resistance is independently associated with endometrial cancer [88]. Metformin is a biguanide drug that is widely used as the first line treatment of type II diabetes. There is epidemiological evidence to suggest that metformin use lowers cancer risk and reduces the rate of cancer deaths among diabetic patients [89,90]. Recently metformin has been shown to inhibit cellular proliferation and induce apoptosis in endometrial cancer cell lines, and these effects were potentially mediated through the mTOR pathway [91]. Thus, metformin may behave like a novel mTOR inhibitor, with important chemotherapeutic implications for endometrial cancer treatment and even prevention. Additionally, a novel insulin-like growth factor 1 receptor (IGF-1R) inhibitor, AMG479 (Amgen), is gaining attention as a potential novel therapeutic [92–94]. There are several phase II trials in ovarian cancer, in combination with chemotherapy, in development. Preclinical data in endometrial cancer cell lines similarly show promise [94].
Ixabepilone (BMS-2474550, Bristol-Myers Squibb) is an epothi-lone B analog that induces microtubule stabilization and due to their different mechanism of action, can provide activity even in those tumors deemed taxane resistant [95]. GOG 129-P evaluated this drug in 52 women with recurrent or persistent endometrial cancer, the majority of whom (94%) had received prior paclitaxel therapy. In this patient population, single agent ixabepilone (40 mg/m2 IV q 21 days) resulted in a median PFS of 2.9 months and OS of 8.7 months. The authors concluded that in this paclitaxel-pretreated population, there was modest activity.
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL-Apo2L) is a member of the tumor necrosis factor ligand superfamily, and it is expressed at high levels in many tumor types [96,97]. Mapatumumab (Trm-1, HGS-ETR1), a monoclonal antibody with high affinity to TRAIL-R1, has shown activity amongst a broad range of tumor xenografts. As a single agent, in two phase I studies of heavily pretreated patients, the best response was stable disease [98,99]. In a more recent phase I study of mapatumumab with paclitaxel and carboplatin, 5/27 patients (one having primary peritoneal disease) exhibited a partial response. This drug may merit evaluation in endometrial cancer [97].
Conclusions
As our knowledge of the pathogenesis of endometrial cancer has evolved, we have had the opportunity to identify potential novel targets for treating a disease that has seen a dramatic increase in cancer-related mortality in the recent decades. The current challenge is how to best study these agents in an effort to maximize patient response and minimize the cost and time of the traditional clinical trial mechanisms. In addition to posing unique challenges in clinical trial design as single-agents, emerging data from other tumor types suggest that these novel agents may be most effective when given in combination with existing cytotoxic agents or with other novel therapeutic agents. Developing pre-clinical models and expanding the understanding of the mechanisms behind endometrial cancer pathogenesis and metastases will be vitally important as we advance our knowledge in the treatment of this disease.
The authors receive research support (study drug) from Amgen.
Footnotes
Conflict of interest statement
No conflict of interest.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Dunton CJ, Balsara G, Mcfarland M, Hernandez E. Uterine papillary serous carcinoma: a review. Obstet Gynecol Surv. 1991;46:97–102. doi: 10.1097/00006254-199102000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Homesley HD, Filiaci V, Gibbons SK, Long HJ, Cella D, Spirtos NM, et al. A randomized phase III trial in advanced endometrial carcinoma of surgery and volume directed radiation followed by cisplatin and doxorubicin with our without paclitaxel: A Gynecologic Oncology Group study. Gynecol Oncol. 2009;112:543–52. doi: 10.1016/j.ygyno.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Randall ME, Filiaci VL, Muss H, Spirtos NM, Mannel RS, Fowler J, et al. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2006;24:36–44. doi: 10.1200/JCO.2004.00.7617. [DOI] [PubMed] [Google Scholar]
- 5.Fleming GF, Brunetto VL, Cella D, Look KY, Reid GC, Munkarah AR, et al. Phase III trial of doxorubicin plus cisplatin with or without paclitaxel plus filgrastin in advanced endometrial carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2004;22:2159–66. doi: 10.1200/JCO.2004.07.184. [DOI] [PubMed] [Google Scholar]
- 6.Fleming GF, Filiaci VL, Bentley RC, Herzog T, Sorosky J, Vaccarello L, et al. Phase III randomized trial of doxorubicin + cisplatin versus doxorubicin + 24-h paclitaxel + filgrastim in endometrial carcinoma: a Gynecologic Oncology Group study. Ann Oncol. 2004;15:1173–8. doi: 10.1093/annonc/mdh316. [DOI] [PubMed] [Google Scholar]
- 7.Thigpen JT, Brady MF, Homesley HD, Malfetano J, DuBeshter B, Burger RA, et al. Phase III trial of doxorubicin with or without cisplatin in advanced endometrial carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2004;22:3902–8. doi: 10.1200/JCO.2004.02.088. [DOI] [PubMed] [Google Scholar]
- 8.Gallion HH, Brunetto VL, Cibull M, Lentz SS, Reid G, Soper JT, et al. Randomized phase III trial of standard timed doxorubicin plus cisplatin versus circadian timed doxorubicin plus cisplatin in stage III and IV or recurrent endometrial carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21:3808–13. doi: 10.1200/JCO.2003.10.083. [DOI] [PubMed] [Google Scholar]
- 9.Thigpen JT, Blessing JA, DiSaia PJ, Yordan E, Carson LF, Evers CA. randomized comparison of doxorubicin alone versus doxorubicin plus cyclophosphamide in the management of advanced or recurrent endometrial carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 1994;12:1408–14. doi: 10.1200/JCO.1994.12.7.1408. [DOI] [PubMed] [Google Scholar]
- 10.Cohen CJ, Bruckner HW, Deppe G, Blessing JA, Homesley H, Lee JH. Multidrug treatment of advanced and recurrent endometrial carcinoma: a Gynecologic Oncology Group Study. Obstet Gynecol. 1984;63:719–26. [PubMed] [Google Scholar]
- 11.Fleming GF, Sill MA, Thigpen JT, et al. Phase II evaluation of trastuzumab in patients with advanced or recurrent endometrial carcinoma: a report on GOG 181B. Proc Am Soc Clin Oncol. 2003;22:A–1821. 453. [Google Scholar]
- 12.McMeekin DS, Sill MW, Benbrook D, Darcy KM, Stearns-Kurosawa DJ, Eaton L, et al. A phase II trial of thalidomide in patients with refractory endometrial cancer and correlation with angiogenesis biomarkers: a Gynecologic Oncology Group study. Gynecol Oncol. 2007;105:508–16. doi: 10.1016/j.ygyno.2007.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leslie KK, Sill MW, Darcy KM, Baron AT, Wilken JA, Godwin AK, et al. Efficacy and safety of gefitinib and potential prognostic value of soluble EGFR, EGFR mutations, and tumor markers in a Gynecologic Oncology Group phase II trial of persistent or recurrent endometrial cancer. J Clin Oncol. 2009:27. abstr e16542. [Google Scholar]
- 14.Aghajanian C, Sill MW, Darcy K, Greer B, McMeekin DS, Rose PG, et al. A phase II evaluation of bevacizumab in the treatment of recurrent or persistent endometrial cancer. A Gynecologic Oncology Group study. J Clin Oncol. 2009;27:5531. doi: 10.1200/JCO.2010.32.6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia AA, Blessing JA, Nolte S, Mannel RS. A phase II evaluation of weekly docetaxel in the treatment of recurrent or persistent endometrial carcinoma: a study by the Gynecologic Oncology Group. Gynecol Oncol. 2008;111:22–6. doi: 10.1016/j.ygyno.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 16.Miller DS, Blessing JA, Kranser CN, Drake RD, Higgins R, McMeekin DS, et al. A phase II evaluation of pemetrexed (Alimta, LY231514, INC#40061) in the treatment of recurrent or persistent endometrial carcinoma. A phase II study of the Gynecologic Oncology Group. J Clin Oncol. 2009:27. doi: 10.1016/j.ygyno.2009.09.004. abstract e16507. [DOI] [PubMed] [Google Scholar]
- 17.Dizon DS, Blessing JA, McMeekin DS, Sharma Sk, Disilvestro P, Alvarez R. Phase II trial of Ixabepilone as second-line treatment in advanced endometrial cancer: gynecologic Oncology Group study. J Clin Oncol. 2009;27:3104–8. doi: 10.1200/JCO.2008.20.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doll A, Abal M, Rigau M, Monge M, Gonzalez M, Demajo S, et al. Novel molecular profiles of endometrial cancer-new light through old windows. J Steroid Biochem Mol Biol. 2008;108:221–9. doi: 10.1016/j.jsbmb.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 19.Bokhman JV. Two pathogenic types of endometrial carcinoma. Gynecol Oncol. 1983;15:10–7. doi: 10.1016/0090-8258(83)90111-7. [DOI] [PubMed] [Google Scholar]
- 20.Hecht J, Mutter GL. Molecular and pathologic aspects of endometrial carcinogen-esis. J Clin Oncol. 24:4783–91. doi: 10.1200/JCO.2006.06.7173. 206. [DOI] [PubMed] [Google Scholar]
- 21.Mutter GL, Lin MC, Fitzgerald JT, Kum JB, Baak JP, Lees JA, et al. Altered PTEN expression as a diagnostic marker for the earliest endometrial precancers. J Natl Cancer Inst. 2000;92:924–30. doi: 10.1093/jnci/92.11.924. [DOI] [PubMed] [Google Scholar]
- 22.Maxwell GL, Risinger JI, Gumbs C, Shaw H, Bentley RC, Barrett JC, et al. Mutation of the PTEN tumor suppressor gene in endometrial hyperplasias. Cancer Res. 1998;58:2500–3. [PubMed] [Google Scholar]
- 23.Basil JB, Goodfellow PJ, Rader JS, Mutch DG, Herzog TJ. Clinical significance of microsatellite instability in endometrial carcinoma. Cancer. 2000;89:1758–64. doi: 10.1002/1097-0142(20001015)89:8<1758::aid-cncr16>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 24.Bilbao C, Rodriguez G, Ramirez R, Falcon O, Leon L, Chirino R, et al. The relationship between microsatellite instability and PTEN gene mutations in endometrial cancer. Int J Cancer. 2006;119:563–70. doi: 10.1002/ijc.21862. [DOI] [PubMed] [Google Scholar]
- 25.Lax S, Kendall B, Tashiro H, Slebos RJ, Hedrick L. The frequency of p53,K-ras mutations and microsatellite instability differs in uterine endometrioid and serous carcinoma: evidence of distinct molecular genetic pathways. Cancer. 2000;88:814–24. [PubMed] [Google Scholar]
- 26.Zheng W, Cao P, Zheng M, Kramer EE, Godwin TA. p53 overexpression and bcl-2 persistence in endometrial carcinoma: comparison of papillary serous and endometrioid subtypes. Gynecol Oncol. 1996;61:167–72. doi: 10.1006/gyno.1996.0120. [DOI] [PubMed] [Google Scholar]
- 27.Santin AD, Bellone S, Gokden M, Palmieri M, Dunn D, Agha J, et al. Overexpression of Her-2/neu in uterine serous papillary cancer. Clin Cancer Res. 2002;8:1271–9. [PubMed] [Google Scholar]
- 28.Slomovitz BM, Broaddus RR, Burke TW, Sneige N, Soliman PT, We W, et al. Her-2/ neu overexpression and amplification in uterine papillary serous carcinoma. J Clin Oncol. 2004;22:3126–32. doi: 10.1200/JCO.2004.11.154. [DOI] [PubMed] [Google Scholar]
- 29.Risinger JL, Maxwell GL, Chandramouli GV, Jazaeir A, Aprelikova O, Patterson T, et al. Microarray analysis reveals distinct gene expression profiles among different histologic types of endometrial cancer. Cancer Res. 2003;63:6–11. [PubMed] [Google Scholar]
- 30.Zorn KK, Bonome T, Gangi L, Chandramouli GV, Awtrey CS, Gardner GJ, et al. Gene expression profiles of serous, endometrioid and clear cell subtypes of ovarian and endometrial cancer. Clin Cancer Res. 2005;11:6422–30. doi: 10.1158/1078-0432.CCR-05-0508. [DOI] [PubMed] [Google Scholar]
- 31.McMeekin DS, Filiaci VL, Thigpen JT, Gallion HH, Fleming GF, Rodgers WH. The relationship between histology and outcome in advanced and recurrent endometrial cancer patients participating in first-line chemotherapy trials: a Gynecologic Oncology Group study. Gynecol Oncol. 2007;106:16–22. doi: 10.1016/j.ygyno.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 32.Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumor activity. Nat Rev, Cancer. 2008;8:579–91. doi: 10.1038/nrc2403. [DOI] [PubMed] [Google Scholar]
- 33.Salvesen HB, Iversen OE, Akslen LA. Independent prognostic importance of microvessel density in endometrial cancer. Br J Cancer. 1998;77:1140–4. doi: 10.1038/bjc.1998.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mazurek A, Telego M, Pierzynski P, Lapuc G, Niklinska W, Juczewska M, et al. Angiogenesis in endometrial cancer. Neoplasma. 1998;45:360–4. [PubMed] [Google Scholar]
- 35.Hirai M, Nakagawara A, Oosaki T, Hayashi, Hirono M, Yoshihara T. Expression of vascular endothelial growth factors (VEGF-A/VEGF-1 and VEGF-C/VEGF-2) in postmenopausal uterine endometrial carcinoma. Gynecol Oncol. 2001;80:181–8. doi: 10.1006/gyno.2000.6056. [DOI] [PubMed] [Google Scholar]
- 36.Holland CM, Day K, Evans A, Smith SK. Expression of the VEGF and angiopoietin genes in endometrial atypical hyperplasia and endometrial cancer. Br J Cancer. 2003;89:891–8. doi: 10.1038/sj.bjc.6601194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kamat AA, Merritt WM, Coffey D, Lin YG, Patel PR, Broaddus R, et al. Clinical and biological significance of vascular endothelial growth factor in endometrial cancer. Clin Cancer Res. 2007;13:7487–95. doi: 10.1158/1078-0432.CCR-07-1017. [DOI] [PubMed] [Google Scholar]
- 38.Lee CN, Cheng WF, Chen CA, Chu JS, Hsieh DY, Hsieh FJ. Angiogenesis of endometrial carcinomas assessed by measurement of intratumoral blood flow, microvessel density, and vascular endothelial growth factor levels. Obstet Gynecol. 2000;96:615–21. doi: 10.1016/s0029-7844(00)00976-5. [DOI] [PubMed] [Google Scholar]
- 39.Yokoyama Y, Sato S, Futagami M, Fukushi Y, Sakamoto T, Umemoto M, et al. Prognostic significance of vascular endothelial growth factor and its receptors in endometrial carcinoma. Gynecol Oncol. 2000;77:413–8. doi: 10.1006/gyno.2000.5802. [DOI] [PubMed] [Google Scholar]
- 40.Fine BA, Valente PT, Feinstein GI, Dey T. VEGF, flt-1 and KDR/flk-1 as prognostic indicators in endometrial carcinoma. Gynecol Oncol. 2000;76:33–9. doi: 10.1006/gyno.1999.5658. [DOI] [PubMed] [Google Scholar]
- 41.Talvensaari-Mattila A, Soini Y, Santala M. VEGF and its receptors (flt-1 and KDR/ flk-1) as prognostic indicators in endometrial carcinoma. Tumour Biol. 2005;26:81–7. doi: 10.1159/000085589. [DOI] [PubMed] [Google Scholar]
- 42.Fujimoto J, Toyoki H, Jahan I, Alam SM, Sakaguchi H, Sato E, et al. Sex-steroid-dependent angiogenesis in uterine endometrial cancers. Ster Biochem Mol Biol. 2005;93:161–5. doi: 10.1016/j.jsbmb.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 43.Columbo N, Mangilia G, Mammoliti S, Kalling M, Tholander B, Sternas L, et al. Afibercept (VEGF trap) for advanced epithelial ovarian cancer (EOC) patients with symptomatic malignant ascites: preliminary results of a pilot study. J Clin Oncol. 2008;26:14598. [Google Scholar]
- 44.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathways and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 45.Nimeiri H, Oza AM, Morgan RJ, Friberg G, Kasza K, Faora L, et al. Sorafenib in patients with advanced/recurrent uterine carcinoma or carcinosarcoma: a Phase II trial of the Chicago, PMH and California Phase II consortia. J Clin Oncol. 2008;26:5585. [2008 ASCO Annu Meet Proc] [Google Scholar]
- 46.Matei D, Sill MW, DeGeest K, Bristow RE. Phase II trial of sorafenib in persistent or recurrent epithelial ovarian cancer (EOC) or primary peritoneal cancer (PPC): a Gynecologic Oncology Group study. Proc Am Soc Clin Oncol. 2008;26:5537. [Google Scholar]
- 47.Welch S, Hirte H, Schilder RJ, Elit L, Townsley C, Tinker L, et al. Phase II study of sorafenib (BAY 43-9006) in combination with gemcitabine in recurrent epithelial ovarian cancer: a PMH phase II consortium trial. J Clin Oncol. 2006;24:5084. [Google Scholar]
- 48.Fleming GF, Morgan R, Wang L, Welch S, Mackay HJ, Hirte H, et al. A phase II study of sunitinib in recurrent or metastatic endometrial carcinoma: a trial of the PMH Phase II consortium. J Clin Oncol. 2009;27:5576. 2009 ASCO Annu Meet Proc. [Google Scholar]
- 49.Hirte HW, Vidal L, Fleming GF, Sugimoto AK, Morgan RJ, Biagi JJ, et al. A phase II study of cediranib (AZD2171) in recurrent or persistent ovarian, peritoneal, or fallopian tube cancer;final results of PMH, Chicago and California consortia trial. Proc Am Soc Clin Oncol. 2008;26:5521. [Google Scholar]
- 50.Bhaskar V, Fox M, Breinberg D, Wong MH, Wales PE, Rhodes S, et al. Volociximab, a chimeric integrin α5β1 antibody inhibits the growth of VX2 tumors in rabbits. Invest New Drugs. 2008;26:7–12. doi: 10.1007/s10637-007-9078-z. [DOI] [PubMed] [Google Scholar]
- 51.Delmonte A, Del Conte G, Sessa C, Perotti A, Fasolo A, Williams E, et al. Results from a phase 1/2 study of volociximab in combination with liposomal doxorubicin in relapsed advanced epithelial ovarian and primary peritoneal carcinoma. Proc Am Soc Clin Oncol. 2008;26:16527. [Google Scholar]
- 52.Vergote IB, Colombo N, Kutarska E, Del Camp J, Pippitt C, Casado A, et al. Phase II study of comparing volociximab (an antiangiogenic antibody) and peglylated liposomal doxorubicin (PLD) with PLD alone in recurrent ovarian or primary peritoneal cancer. J Clin Oncol. 2009;27:5560. [Google Scholar]
- 53.Camidge DR, Eckhardt SG, Diab S, Gore L, Chow L, O'Bryant C, et al. A phase I dose-escalation study of weekly IMC-1121B, a fully human anti-vascular endothelial growth factor receptor 2 (VEGFR2) IgG1 monoclonal antibody in patients with advanced cancer. Proc Am Soc Clin Oncol. 2008;26:16527. [Google Scholar]
- 54.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 55.Wolf J, Slomovitz BM. Novel biologic therapies for the treatment of endometrial cancer. Int J Gynecol Cancer. 2005;15:411. [Google Scholar]
- 56.Abraham RT. Mammalian target of rapamycin: immunosuppressive drugs uncover a novel pathway of cytokine receptor signaling. Curr Opin Immunol. 1998;10:330–6. doi: 10.1016/s0952-7915(98)80172-6. [DOI] [PubMed] [Google Scholar]
- 57.Oza AM, Elit L, Biagni J, Chapman W, Tsao M, Hedley D, et al. Molecular correlates associated with a phase II study of temsirolimus (CCI-779) in patients with metastatic or recurrent endometrial cancer. J Clin Oncol. 2006;24:3003. [Google Scholar]
- 58.Oza AM, Elit L, Provencher D, et al. A Phase II study of temsirilimus (CCI-779) in patients with metastatic and/or locally advanced recurrent endometrial cancer previously treated with chemotherapy. J Clin Oncol. 2008;26:5516. [Google Scholar]
- 59.Colombo N, McMeekin DS, Schwartz P, Kostka Sessa C, Gehrig PA, et al. A phase II trial of the mTOR inhibitor AP23573 as a single agent in advanced endometrial cancer. Proc Am Soc Clin Oncol. 2007;25:5516. [Google Scholar]
- 60.Slomovitz BM, Burke T, Lu KH, Wolf J, Johnston T, Wu W, et al. Loss of PTEN expression associated with response to RAD001 (mTOR inhibitor) in patients with recurrent endometrial cancer. Translational evaluation from a phase II study. 2007;104:S30. abstract 70. [Google Scholar]
- 61.Bae-Jump VL, Zhou C, Boggess JF, Gehrig PA. Synergistic effect of rapamycin and cisplatin in endometrial cancer cells. Cancer. 2009;115:3887–96. doi: 10.1002/cncr.24431. [DOI] [PubMed] [Google Scholar]
- 62.Shafer A, Zhou C, Gehrig PA, Boggess JF, Bae-Jump VL. Rapamycin potentiates the effects of paclitaxel in endometrial cancer cells through inhibition of cell proliferation and induction of apoptosis. AACR. 2008 doi: 10.1002/ijc.24837. Accepted Int J Cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mondesire WH, Jian W, Zhang H, Ensor J, Hung MC, Mills GB, et al. Targeting mammalian target of rapamycin synergistically enhances chemotherapy-induced cytotoxicity in breast cancer cells. Clin Cancer Res. 2004;10:7031–42. doi: 10.1158/1078-0432.CCR-04-0361. [DOI] [PubMed] [Google Scholar]
- 64.Elser D, Hirte H, Kaizer L, MacKay H, Bindra S, Tinker L, et al. Phase II study of MKC-1 in patients with metastatic or resistant epithelial ovarian cancer or advanced endometrial cancer. J Clin Oncol. 2009;27:5577. [Google Scholar]
- 65.Hrzenjak A, Kremser ML, Storhmeier B, Moinfar F, Zatloukal K, Denk H. SAHA induces caspase-independent, autophagic cell death of endometrial stromal sarcoma cells by influencing the mTOR pathway. J Pathol. 2008;216:495–504. doi: 10.1002/path.2434. [DOI] [PubMed] [Google Scholar]
- 66.De Luca A, Carotenuto A, Rachiglio A, Gallo M, Maiello MR, Aldinucci D, et al. The role of EGFR signaling in tumor microenvironment. J Cell Physiol. 2008;214:559–67. doi: 10.1002/jcp.21260. [DOI] [PubMed] [Google Scholar]
- 67.Niikura H, Sasano H, Kaga K, Sato S, Yajima A. Expression of epidermal growth factor family proteins and epidermal growth factor receptor in human endometrium. Hum Pathol. 1996;27:282–9. doi: 10.1016/s0046-8177(96)90070-2. [DOI] [PubMed] [Google Scholar]
- 68.Brys M, Semczuk A, Rechberger T, Krajewska WM. Expression of erbB-1 and erbB-2 genes in normal and pathological human endometrium. Oncol Rep. 2007;18:261–5. [PubMed] [Google Scholar]
- 69.Khalifa MA, Mannel RS, Haraway SD, Walker J, Min KW. Expression of EGFR, HER2/neu, p53 and PCNA in endometrioid, serous papillary, and clear cell endometrial carcinomas. Gynecol Oncol. 1994;53:84–92. doi: 10.1006/gyno.1994.1092. [DOI] [PubMed] [Google Scholar]
- 70.Reinartz JJ, George G, Lindren BR, Neihas GA. Expression of p53, transforming growth factor α, epidermal growth factor receptor, and c-erbB-2 in endometrial carcinoma and correlation with survival and known predictors of survival. Hum Pathol. 1994;25:1075–83. doi: 10.1016/0046-8177(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 71.Grushko TA, Filiaci VL, Mundt AJ, Riddersrale K, Olopade OI, Fleming GF. An exploratory analysis of HER-2 amplification and overexpression in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. Gynecol Oncol. 2008;108:3–9. doi: 10.1016/j.ygyno.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moy B, Goss PE. Lapatinib associated toxicity and practical management recommendations. Oncologist. 2007;12:756–65. doi: 10.1634/theoncologist.12-7-756. [DOI] [PubMed] [Google Scholar]
- 73.Konecny GE, Venkatesan N, Yan G, Dering J, Ginther C, Finn R, et al. Activity of lapatanib a novel HER2 and EGFR dual kinase inhibitor in human endometrial cancer cells. Br J Cancer. 2008;98:1076–84. doi: 10.1038/sj.bjc.6604278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cohen MH, Williams GA, Sridhara R, Chen G, McGuinn WE, Morse D. United States Food and Drug Administration Approval summary: fefitinig (ZD 1839) tablets. Clin Cancer Res. 2004;10:1212–8. doi: 10.1158/1078-0432.ccr-03-0564. [DOI] [PubMed] [Google Scholar]
- 75.Leslie KK, Laidler L, Albitar L, et al. Tyrosine kinase inhibitors in endometrial cancer. Int J Gynecol Cancer. 2005;15:409–11. [Google Scholar]
- 76.Jasa KV, Fyles A Elit L, Hoskins PJ, Biagi J, Dubuc-Lissoir J, et al. Phase II study of erlotinib (OSI 774) in women with recurrent or metastatic endometrial cancer. J Clin Oncol. 2004;22:5019. [Google Scholar]
- 77.Oza AM, Eisenhauer EA, Elit L, Cutz JC, Sakurada A, Tsao MS, et al. Phase II study of erlotinib in recurrent or metastatic endometrial cancer: NCIC IND-148. J Clin Oncol. 2008;26:4319–25. doi: 10.1200/JCO.2007.15.8808. [DOI] [PubMed] [Google Scholar]
- 78.Slomovitz BM, Broaddus RR, Schmandt R, Wu W, Oh JC, Ramondetta L, et al. Expression of imatinib mesylate-targeted kinases in endometrial carcinoma. Gynecol Oncol. 2004;95:32–6. doi: 10.1016/j.ygyno.2004.06.052. [DOI] [PubMed] [Google Scholar]
- 79.Slomovitz BM, Ramondetta L, Johnston T, Lu KH, Broaddus RR, Muller P, et al. A phase I study of imatinib mesylate and paclitaxel in patients with advanced or recurrent uterine papillary serous carcinoma. J Clin Oncol. 2007;25:p16025. [Google Scholar]
- 80.Albitar L, Carter MB, Davies S, Leslie KK. Consequences of the loss of p53, RB1, and PTEN: Relationship to gefitinib resistance in endometrial cancer. Gynecol Oncol. 2007;106:94–104. doi: 10.1016/j.ygyno.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 81.Villella JA, Cohen S, Smith DH, Hibshoosh H, Hershman D. HER-2/neu over-expression in uterine papillary serous cancers and its possible therapeutic implications. Int J Gynecol Cancer. 2006;16:1897–902. doi: 10.1111/j.1525-1438.2006.00664.x. [DOI] [PubMed] [Google Scholar]
- 82.Jewell E, Secord AA, Brotherton T, Berchuck A. Trastuzumab in the treatment of metastatic endometrial cancer. Int J Gynecol Cancer. 2006;16:1370–3. doi: 10.1111/j.1525-1438.2006.00543.x. [DOI] [PubMed] [Google Scholar]
- 83.Goff BA. Uterine papillary serous carcinoma: what have we learned over the past quarter century? Gynecol Oncol. 2005;98:341–3. doi: 10.1016/j.ygyno.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 84.Podratz KC, Mariani A. Uterine papillary serous carcinomas: the exigency for clinical trials. Gynecol Oncol. 2003;91:461–2. doi: 10.1016/j.ygyno.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 85.Santin AD, Zhan F, Cane S, Bellone S, Palmieri M, Thomas M, et al. Gene expression fingerprint of uterine serous papillary carcinoma: identification of novel molecular markers for uterine serous cancer diagnosis and therapy. Br J Cancer. 2005;92:1561–73. doi: 10.1038/sj.bjc.6602480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Santin AD, Bellone S, Marizzoni M, Palmieri M, Siegel ER, McKenney JK, et al. Overexpression of claudin-3 and claudin-4 receptors in uterine serous papillary carcinoma: novel targets for a type-specific therapy using Clostridium perfringes enterotoxin (CPE0. Cancer. 2007;109:1312–22. doi: 10.1002/cncr.22536. [DOI] [PubMed] [Google Scholar]
- 87.Weyer D, Funahashi T, Tanaka S, et al. Hypoadiponectiemia in obesity and Type 2 diabetes: close association with insulin resistance and hyperinsuliemia. J Clin Endocrinol Metab. 86:1930–5. doi: 10.1210/jcem.86.5.7463. 201. [DOI] [PubMed] [Google Scholar]
- 88.Soliman PT, Wu D, Tortolero-Luna G, Schmeler KM, Slomovitz BM, Bray MS, et al. Association between adiponectin, insulin resistance and endometrial cancer. Cancer. 2006;106:2376–81. doi: 10.1002/cncr.21866. [DOI] [PubMed] [Google Scholar]
- 89.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330(7503):1304–5. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care. 2006;29(2):254–8. doi: 10.2337/diacare.29.02.06.dc05-1558. [DOI] [PubMed] [Google Scholar]
- 91.Cantrell LA, Zhou C, Mendivil A, Gehrig PA, Bae-Jump V. Metformin is a potent inhibitor of endometrial cancer cell proliferation: implications for a novel treatment strategy. Gynecol Oncol. 2009;112:S79. doi: 10.1016/j.ygyno.2009.09.024. Accepted Gynecol Oncol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Beltran PJ, Mitchell P, Chung YA, Cajeulis E, Lu J, Belmontes B, et al. AMG 479, a fully human anti-insulin-like growth factor receptor type I monoclonal antibody, inhibits the growth and survival of pancreatic carcinoma cells. Mol Cancer Ther. 2009;8:1095–105. doi: 10.1158/1535-7163.MCT-08-1171. [DOI] [PubMed] [Google Scholar]
- 93.Hewish M, Chau I, Cunningham D. Insulin-like growth factor 1 receptor targeted therapeutics: novel compounds and novel treatment strategies for cancer medicine. Recent Pat Anticancer Drug Discov. 2009;4:54–72. doi: 10.2174/157489209787002515. [DOI] [PubMed] [Google Scholar]
- 94.Mendivil A, Zhou C, Cantrell L, Gehrig PA, Bae-Jump V. AMG 479, a novel IGF-1R antibody, inhibits endometrial cancer proliferation through disruption of the P13K/Akt and MAPK pathways. Proceedings of the American Association for Cancer Research. Abstract 2804. [Google Scholar]
- 95.Chon HS, Hu W, Kavanagh JJ. Targeted therapies in gynecologic cancers. Curr Cancer Drug Targets. 2006;6:333–6. doi: 10.2174/156800906777441799. [DOI] [PubMed] [Google Scholar]
- 96.Younes A, Kadin ME. Emerging applications of the tumor necrosis family of ligands and receptors in cancer therapy. J Clin Oncol. 2003;21:3526–34. doi: 10.1200/JCO.2003.09.037. [DOI] [PubMed] [Google Scholar]
- 97.Leong S, Cohen RB, Gustafson DL, Langer CJ, Camidge DR, Padavic K, et al. Mapatumumab, an antibody targeting TRAIL-R1 in combination with paclitaxel and carboplatin in patients with advanced solid malignancies: results of a phase I and pharmacokinetic study. J Clin Oncol. 2009;27:4413–21. doi: 10.1200/JCO.2008.21.7422. [DOI] [PubMed] [Google Scholar]
- 98.Hotte SJ, Hirte HW, Chen EX, Siu LL, Le LH, Corey A, et al. A phase I study of mapatumumab (fully human monoclonal antibody to TRAIL-R1) in patients with advanced solid malignancies. Clin Cancer Res. 2008;14:3450–5. doi: 10.1158/1078-0432.CCR-07-1416. [DOI] [PubMed] [Google Scholar]
- 99.Tolcher AW, Mita M, Meropol NJ, von Mehren M, Patnaik A, Padavic K, et al. Phase I pharmacokinetic and biologic correlative study of mamatumumab, a fully human monoclonal antibody with agonist activity to tumor necrosis factor-related apoptosis-inducing ligand receptor. J Clin Oncol. 2007;25:1390–5. doi: 10.1200/JCO.2006.08.8898. [DOI] [PubMed] [Google Scholar]