Summary
Objective
Secondary generalization of seizures has devastating consequences for patient safety and quality of life. The aim of this intracranial EEG (icEEG) study was to investigate the differences in onset and propagation patterns of temporal lobe seizures that remained focal vs. those with secondary generalization in order to better understand the mechanism of secondary generalization.
Methods
A total of 39 seizures were analyzed in 9 patients who met the following criteria: 1) icEEG-video monitoring with at least 1 secondarily generalized tonic clonic seizure (GTC), 2) pathologically proven hippocampal sclerosis, and 3) no seizures for at least 1 year after anteromedial temporal lobe resection. Seizures were classified as focal or secondary generalized by behavioral analysis of video. Onset and propagation patterns were compared by analysis of icEEG.
Results
We obtained data from 22 focal seizures without generalization (FS), and 17 GTC. Seizure onset patterns did not differ between FS and GTCs, but there were differences in later propagation. All seizures started with low voltage fast activity except 7 seizures in one patient (6 FS, 1 GTC), which started with sharply contoured theta activity. 15 of 39 seizures started from the hippocampus and 24 seizures (including 6 seizures in a patient without hippocampal contacts) started from other medial temporal lobe areas. We observed involvement or more prominent activation of the posterior-lateral temporal regions in GTCs prior to propagation to the other cortical regions, vs. FS which had no involvement or less prominent activation of the posterior lateral temporal cortex. Occipital contacts were not involved at the time of clinical secondary generalization.
Significance
The posterior-lateral temporal cortex may serve as an important “gateway” controlling propagation of medial temporal lobe seizures to other cortical regions. Identifying the mechanisms of secondary generalization of focal seizures may lead to improved treatments to confine seizure spread.
Keywords: temporal lobe seizure, secondary generalization, propagation, intracranial EEG, posterior lateral temporal cortex
Introduction
Generalized tonic-clonic seizures (GTCs), either primary generalized or secondarily generalized from focal seizures (FS), have devastating consequences for patient safety and quality of life. The occurrence and frequency of GTCs is the most important risk factor for sudden unexpected depth in epilepsy (SUDEP)1 and seizure-related serious injuries.2, 3 With considerable advance in our understanding about the clinical and physiological factors of SUDEP and its risk factors,4 increased attention has been drawn to understand the mechanism of secondary generalization of FS. This is particularly the case for temporal lobe epilepsy which is the most frequent drug resistant form of epilepsy.
Some studies investigated the clinical features associated with temporal lobe GTCs by comparing continuous video-electroencephalography (EEG) and magnetic resonance imaging (MRI) in patients with FS vs. GTCs,5 and by observing the occurrence of ictal dystonia during temporal lobe seizures to determine the role of basal ganglia in preventing secondary generalization.6 The ictal electrographic spread pattern of temporal lobe seizures have been studied,7-11 however so far no study has systematically compared the onset and propagation pattern of FS vs. GTCs.
There is growing evidence that GTCs are not truly generalized.12-16 The intracranial EEG (icEEG) provides a unique opportunity to study seizure propagation and areas of involvement in GTCs. Recent icEEG studies have demonstrated that the onset of secondary generalization does not involve the cortex globally.17, 18 We sought to study icEEG in a relatively homogeneous group of temporal lobe seizures to investigate the differences in onset and propagation patterns between seizures that remained focal vs. those with secondary generalization. Localizing the area that might be responsible in preventing or permitting secondary generalization may lead to the development of better treatment strategies and targets for medical devices or procedures in order to try to prevent seizure spread.
Methods
Patients
All procedures were in accordance with the institutional review board for human studies at Yale University School of Medicine. Informed consent was obtained from all subjects. Inclusion and exclusion criteria were chosen to identify a relatively homogenous group of patients with confirmed mesial temporal lobe epilepsy who had undergone intracranial EEG and video monitoring. Patients with the following inclusion criteria were used: (i) intracranial EEG monitoring performed between 1995 and 2010 at Yale, and where at least one GTC was recorded; (ii) pathology demonstrating hippocampal sclerosis; and (iii) no seizures during a minimum follow up period of 1 year after anteromedial temporal lobe resection. A total of 39 seizures from 9 patients were analyzed. Seven seizures that involved only the hippocampal depth or anterior medial temporal regions without spreading to other regions were excluded from analysis.
Anatomic localization of electrode positions
Depth electrodes were typically used to study the hippocampus, while strips were used to study the adjacent mesial temporal structures such as the entorhinal cortex and parahippocampal gyrus; in addition, grids and strips were commonly used to study other regions. Intracranial electrode locations were planned preoperatively based on clinical information in each case, therefore electrode positions were not standardized. MRI scans were performed on all patients after intracranial electrode implantation using 3D volume inversion recovery prepped fast spoiled gradient recalled echo (IR-FSPGR) imaging on a 1.5 T system. Surface reconstructions were then obtained with lateral, medial, anterior, posterior, and inferior views of both hemispheres to determine the positions of all electrode contacts. Boundaries between each lobe have been described previously,19 and within the temporal lobe, the areas were further divided into anterior, mid, and posterior regions. The brain surface was segmented into the following anatomical regions for both ipsilateral and contralateral hemispheres: medial temporal, anterior lateral temporal, middle lateral temporal, posterior lateral temporal, frontal, parietal and occipital. Electrode contacts identified on the MRI scans were assigned to these regions for icEEG analysis.
Intracranial electroencephalography recordings
Intracranial EEG signals were recorded continuously using Telefactor Beehive systems (Telefactor/Grass/Natus Medical Inc., San Carlos, CA, USA) or Bio-Logic Systems 128-channel clinical EEG and video monitoring equipment (Bio-Logic Systems Corp./Natus Medical Inc., San Carlos, CA, USA). Amplifier systems were either single-ended with ground and reference tied together, or with a separate ground and reference, with 128 EEG channels acquired at 12 bit (Telefactor/Grass) or 16 bit (Bio-logic) A/D conversion, 200Hz sampling (256Hz for Bio-Logic system), 90dB or better common-mode rejection ratio, with low-frequency filter setting of 0.1Hz and high-frequency filter setting of 70Hz.
Electroencephalography analysis
The video-icEEG recordings were independently reviewed by two neurologists specializing in epilepsy (J.Y. and P.F.) and any differences were then resolved by joint consensus. Only seizures which clearly progressed to bilateral tonic arm with or without tonic leg posturing that gradually transitioned to bilateral clonic activity were classified as GTCs. It has been described that the final phases of secondary generalized temporal lobe seizures are more stereotyped than the initial clinical signs of generalization.20 The onset of secondary generalization was defined based on video data demonstrating behavioral vocalization, facial clonus and tonic head/eye deviation. The spread pattern in each location was considered to be involved or activated if there were spike and slow or sharp and slow waves, spike or polyspike discharges, or low voltage fast activity. The regions were considered to be not involved or inactive if there was no change from the baseline, or showed theta-delta irregular slow waves, adopted and modified from the intracranial EEG rating scale developed by Blumenfeld et al.19 The onset was classified as hippocampal onset when the seizure activity started from the hippocampal depth. If there was no activity on the hippocampal depth and seizure activity started from the medial temporal electrode contacts, the seizure onset was classified as non-hippocampal onset. In one patient, hippocampal depth electrode was not placed and the seizures were included as non-hippocampal onset.
For statistical analysis of categorical variables, Fisher’s exact tests were carried out. Error probabilities of <0.05 were considered to be statistically significant.
Results
We obtained data from 22 focal seizures without generalization (FS), and 17 secondarily generalized seizures (GTC) in 9 patients (6 females, 3 males) (Table 1). The mean age at the time of recording was 36, range 20 to 51 years. Intracranial monitoring was done for these patients because the presurgical evaluation showed discordant data, as shown in Table 2.
Table 1.
Patient demographics and seizures.
| Patient # | Gender | Age | #GTC | #FS | #Seizures | Hemispherea |
|---|---|---|---|---|---|---|
| 1 | F | 39 | 1 | 2 | 3 | L |
| 2 | F | 38 | 1 | 4 | 5 | FS-R, GTC-L |
| 3 | M | 20 | 2 | 5 | 7 | L |
| 4 | F | 32 | 1 | 6 | 7 | R |
| 5 | F | 34 | 2 | 0 | 2 | R |
| 6 | F | 49 | 3 | 3 | 6 | R |
| 7 | F | 26 | 3 | 0 | 3 | R |
| 8 | M | 38 | 1 | 1 | 2 | L |
| 9 | M | 51 | 3 | 1 | 4 | L |
| Total: | 17 | 22 | 39 |
Values (in columns 4, 5, and 6) represent the number of seizures in each group
Seizure onset in left versus right hemisphere regardless of language dominance
GTC=secondarily generalized tonic clonic seizures; FS=focal seizures
Table 2.
Presurgical evaluation
| Pt # | Scalp EEG | MRI lesions | PET hypo-metabolism | Neuropsych Localization | Ictal SPECT Hyperperfusion | Site of temporal lobe resection |
|---|---|---|---|---|---|---|
| 1 | 2 L T, 3 L FT, 1 L hemi, 1 R hemi, 2 NL | L HA | L med T | R T | L T | L |
| 2 | 4 L T, 2 Bilat T, 2 Bilat F | R HA | R T | NA | L T | R |
| 3 | 6 L T, 1 L F | Bilat HA | L T | L T | L med T | L |
| 4 | 5 R T, 1 L T | R HA | R TP | Bilat T, R worse | NA | R |
| 5 | 7 R FT, 1 R F | R HA | R T | NL | NA | R |
| 6 | 6 L T, 4 NL | NL | NL | NL | L F, R T | R |
| 7 | 2 R T | NL | R T | NL | NA | R |
| 8 | 6 L FT | R F, L T | R T | NL | NA | L |
| 9 | 2 L T, 2 NL | L HA | L F | Bilat T, R worse | NA | L |
Pt #: patient number, Abbreviations: R=right, L=left, Bilat=bilateral, med=medial, HA=hippocampal atrophy, T=temporal, F=frontal, O=occipital, P=parietal, FT=frontotemporal, TP=temporoparietal, hemi=hemisphere, NA=not available, NL=non-localized
All patients had coverage over the ipsilateral temporal region, which was divided into medial, anterior lateral, mid lateral and posterior lateral temporal areas. All patients had ipsilateral hippocampal depth electrodes except one. All patients but one had ipsilateral frontal coverage. Seven patients had coverage over the ipsilateral parietal region and six of them also had coverage over the occipital region. Five patients had electrodes over the contralateral temporal region, and four of them had electrodes over the contralateral frontal lobe as well (Table 3).
Table 3.
Electrode placement in each patient
| Patient | Ipsilaterala | Contralaterala | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HC | Ant med T | Ant lat T | Mid lat T | Post lat T | O | F | P | HC | Ant med T | Ant lat T | Mid lat T | Post lat T | O | F | P | |
| 1 | + | + | + | + | + | − | − | + | + | + | + | + | + | − | − | + |
| 2 | + | + | + | + | + | + | + | + | + | + | + | − | + | + | + | + |
| 3 | + | + | + | + | + | + | + | + | − | − | − | − | − | − | − | − |
| 4 | + | + | + | + | + | + | + | + | − | − | − | − | + | − | + | − |
| 5 | + | + | + | + | + | + | + | + | − | − | − | − | − | − | − | − |
| 6 | − | + | + | + | + | + | + | + | − | + | + | + | + | + | + | + |
| 7 | + | + | + | + | + | + | + | + | − | − | − | − | − | − | − | − |
| 8 | + | + | + | + | + | − | + | − | + | + | + | + | + | − | + | − |
| 9 | + | + | + | + | + | − | + | + | − | + | + | + | + | − | − | − |
Symbols represent whether the electrodes in each regions are present(+) or absent(−).
Ipsilateral/Contralateral to the seizure onset hemisphere
HC = hippocampus; Ant med T=anterior medial temporal; Ant lat T=anterior lateral temporal; Mid lat T= middle lateral temporal; Post lat T=posterior lateral temporal; O=occipital; F=frontal; P=parietal
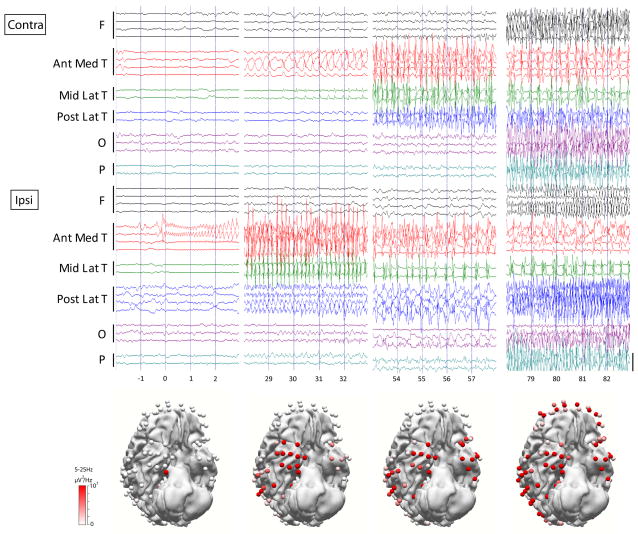
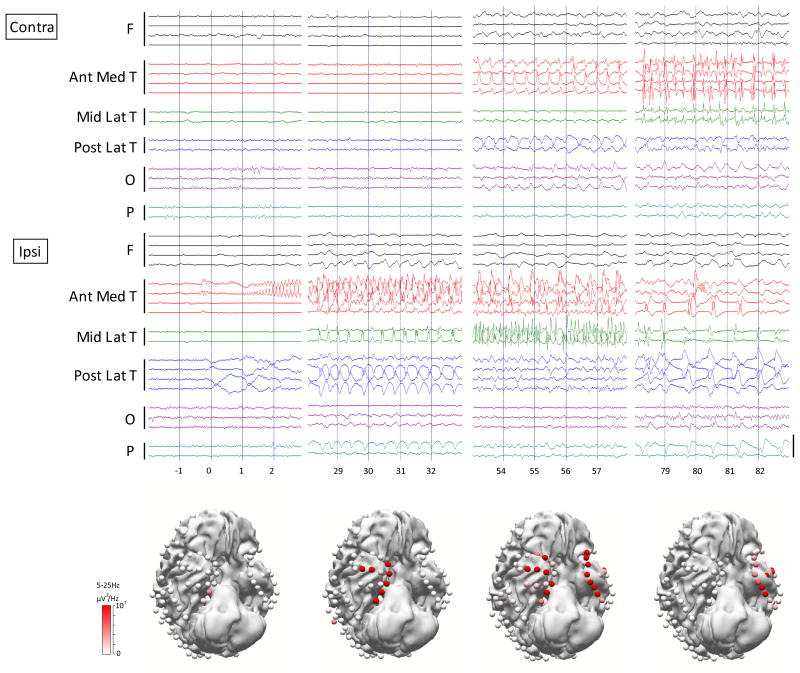
The propagation pattern differed between patients, but followed a consistent pattern within each patient in all but one (patient #2) whose seizures had slight differences in initial propagation. In all patients, we observed prominent ictal discharges in the posterior-lateral temporal regions in secondary generalized seizures prior to propagation to the other cortical regions. There was no involvement or less prominent activation of the posterior lateral temporal cortex in ictal epileptiform discharges in seizures which did not secondarily generalize (Figure 1, Figure 2). Twenty four out of 39 seizures had posterior lateral temporal lobe propagation, and 17 out of these 24 seizures generalized. None of the seizures generalized if there was no posterior lateral temporal involvement (p=<0.001) (Table 4). Interestingly, we also observed that in all 6 patients who had contacts placed on the occipital lobes, none of the 11 secondary generalized seizures involved the occipital lobes at the onset of clinical generalization, in agreement with prior work suggesting that the entire brain is not involved in tonic-clonic seizures.17
Figure 1.
Secondary generalized temporal lobe seizure shows propagation to the posterior lateral temporal cortex. Seizure begins with low voltage fast activity from the mesial temporal contact. Representative time samples of raw EEG are shown. Bars along left margin indicate electrode contacts in the indicated brain regions. A subset of representative electrodes in bipolar montage is shown of the 128 electrodes in this patient (patient #6). Calibration bar on right is 1.5 mV. The color of the 3D electrodes displayed reflects the average spectral power over the frequency range of 5Hz–25Hz corresponding to each time course; this frequency range was chosen to represent dominant ictal frequencies, so as to capture the propagation of seizure activity. 3D images were made in Curry (Compumedics, Charlotte, NC, USA). F= frontal; Ant Med T = anterior medial temporal; Mid Lat T= middle lateral temporal; Post Lat T = posterior lateral temporal; O=occipital; P=parietal.
Figure 2.
Focal temporal lobe seizure does not propagate to the posterior lateral temporal cortex. Seizure begins with low voltage fast activity from the medial temporal contact, spreading to the temporal contacts but fails to generalize. Posterior lateral temporal contacts show slow activity but no polyspike discharges. Representative time samples of raw EEG in the same patient at the same time as Figure 1 are shown. The color of the 3D electrodes displayed reflects the average power over the frequency range of 5Hz–25Hz. 3D images were made in Curry 7 (Compumedics, Charlotte, NC, USA).
Table 4.
Intracranial EEG patterns
| Seizure description | GTC | FS | p-value |
|---|---|---|---|
| Post lat temp propagation | 17/17 | 7/22 | <0.001* |
| No post lat temp propagation | 0/17 | 15/22 | |
| Hippocampal onset | 4/17 | 11/22 | 0.11 |
| Non-hippocampal onset | 13/17 | 11/22 | |
| Low voltage fast onset | 16/17 | 16/22 | 0.11 |
| Sharply contoured theta onset | 1/17 | 6/22 |
Values represent total number of seizures in each group/total number of seizures
p ≤ 0.05, Fisher’s exact test
The seizure onset pattern was similar between FS and GTC. Most of the seizures started with low voltage fast activity except 7 seizures in one patient (patient #4, 6 FSs, 1 GTC) which started with sharply contoured activity in the theta-frequency band (Table 4). In this patient, the location of seizure onset was from the mid-medial temporal contact, which was different from the other non-hippocampal onset seizures which were from the anterior-medial temporal regions. Fifteen of 39 seizures started from the hippocampus and 24 seizures (including 6 seizures recorded in the patient without hippocampal depth electrode, patient #6, 3 GTCs and 3 FSs, which started from the anterior medial temporal area with low voltage fast activity) started from the non-hippocampal medial temporal lobe contacts, but did not differ between FS and GTC. The non-hippocampal onset seizures tended to generalize more frequently compared to the hippocampal onset seizures, however this was not statistically significant (p=0.11) (Table 4).
Discussion
In the present study, we compared the onset and propagation patterns of temporal lobe seizures which remained focal vs. those which secondarily generalized. We observed that the onset pattern was similar between FS vs. GTCs. The initial propagation pattern differed between patients, but followed a consistent pattern in a given patient in all but one. The non-hippocampal onset seizures (i.e., onset in medial temporal areas other than the hippocampus) tended to generalize more frequently compared to the hippocampal onset seizures, but this finding was not statistically significant. There was more commonly involvement of posterior lateral temporal region in secondary generalized seizures, prior to spreading to other cortical regions. It is interesting to note that no seizures generalized without involvement of the posterior lateral temporal region. This suggests that the posterior lateral temporal region may serve as an important “gateway” controlling propagation of temporal lobe seizures to other cortical regions.
To our knowledge, this is the first study to compare the onset and propagation pattern of focal vs. secondary generalized seizures using intracranial EEG. The similarity and stereotyped manner of intracranial ictal onset patterns for recurrent seizures from a single focus in a given patient has been demonstrated with time-frequency analysis using matching pursuit and Gabor atom density (GAD) based measures. 21,22
Recent studies have indicated that all brain areas may not be involved in secondary generalized seizures, and this has been investigated using icEEG in GTCs of temporal origin by Rektor et al.18 In their study, ictal icEEG at the onset of secondary generalization showed highest intracranial rating scale within the temporolateral neocortex and varying degree in other cortical regions, which agrees with our observation. No comparison, however, was made between FS vs. GTCs in that study. The occipital lobe was not included in their study. We have observed that contacts placed on the occipital lobes were often not involved at the time of clinical generalization.
It has been shown that the propagation times of FS with hippocampal onset were significantly longer than those of seizures originating in neocortical tissue.23 Latencies of spread may be related to the degree of integrity of inhibition in these areas, with early spread as a possible indicator of secondary epileptogenesis.24 Ictal propagation areas out of cortico-cortical evoked potential (CCEP) -positive areas were found to be significantly broader in patients with history of generalized seizures vs. no history of generalized seizures,25 suggesting that secondary generalization is more commonly caused by indirect propagation from outside the ictal onset zone rather than direct connection. Trevelyan et al. suggested that feed forward inhibition was the determinant of the speed of epileptiform propagation and that the absence of this inhibitory restraint may be associated with seizure generalization.26 There is growing evidence that basal ganglia may inhibit propagation to the contralateral hemisphere and prevent secondary generalization in focal seizures,6, 27 and the basal ganglia were observed to produce no epileptiform activity during temporal lobe seizures, suggesting an inhibitory role.28
Based on our observation, the temporal neocortex, especially the posterior lateral temporal region may serve as the site of a “functional hub” which is important for propagating ictal activity to other brain regions, leading to the clinical phenomenon of secondary generalization. Measures looking at the epileptic networks such as graph analysis of epileptogenic networks29 or functional connectivity mapping30 may provide further information in identifying the networks responsible for secondary generalization in patients with focal epilepsy. Further quantitative analysis, such as spectral analysis or information theoretical measures may provide additional insight into the possible role of cortical slow or fast activity, representing inhibitory or excitatory drives, in gating seizure propagation beyond the temporal lobe.
This study has several limitations. First, the intracranial study can never explore the whole areas of brain, thus under sampling bias is inevitable. Second, patients’ electrode placements were not standardized, so the electrographic signal is not representative of exactly the same anatomical regions in each patient. Third, the number of patients and seizures are relatively small in this study, in part because most patients with mesial temporal onset seizures do not require icEEG monitoring. In addition, in patients who undergo icEEG monitoring, not many had generalized seizures. This is not unexpected given the careful taper of AEDs during their hospital stay. However, additional studies in a larger sample and with more quantitative analysis methods will be essential to more firmly establish these findings.
Our current very preliminary observations, when combined with more objective measures and further studies as noted above, may lead to a better understanding of the mechanisms of secondary generalization, and may potentially lead to a novel treatment strategy to detect and intervene early in seizure propagation.
Acknowledgments
This work was supported by NIH R01 NS055829 and by the Betsy and Jonathan Blattmachr Family.
Footnotes
Disclosure of Conflicts of Interest
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Walczak TS, Leppik IE, D’Amelio M, et al. Incidence and risk factors in sudden unexpected death in epilepsy: a prospective cohort study. Neurology. 2001;56:519–25. doi: 10.1212/wnl.56.4.519. [DOI] [PubMed] [Google Scholar]
- 2.Lawn ND, Bamlet WR, Radhakrishnan K, et al. Injuries due to seizures in persons with epilepsy: a population-based study. Neurology. 2004;63:1565–70. doi: 10.1212/01.wnl.0000142991.14507.b5. [DOI] [PubMed] [Google Scholar]
- 3.Tiamkao S, Shorvon SD. Seizure-related injury in an adult tertiary epilepsy clinic. Hong Kong medical journal = Xianggang yi xue za zhi/Hong Kong Academy of Medicine. 2006;12:260–3. [PubMed] [Google Scholar]
- 4.Hirsch LJ, Donner EJ, So EL, et al. Abbreviated report of the NIH/NINDS workshop on sudden unexpected death in epilepsy. Neurology. 2011;76:1932–8. doi: 10.1212/WNL.0b013e31821de7de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bone B, Fogarasi A, Schulz R, et al. Secondarily generalized seizures in temporal lobe epilepsy. Epilepsia. 2012;53:817–24. doi: 10.1111/j.1528-1167.2012.03435.x. [DOI] [PubMed] [Google Scholar]
- 6.Popovic L, Vojvodic N, Ristic AJ, et al. Ictal dystonia and secondary generalization in temporal lobe seizures: a video-EEG study. Epilepsy & behavior : E&B. 2012;25:501–4. doi: 10.1016/j.yebeh.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 7.Gotman J. Interhemispheric interactions in seizures of focal onset: data from human intracranial recordings. Electroencephalography and clinical neurophysiology. 1987;67:120–33. doi: 10.1016/0013-4694(87)90034-4. [DOI] [PubMed] [Google Scholar]
- 8.Lieb JP, Hoque K, Skomer CE, et al. Inter-hemispheric propagation of human mesial temporal lobe seizures: a coherence/phase analysis. Electroencephalography and clinical neurophysiology. 1987;67:101–19. doi: 10.1016/0013-4694(87)90033-2. [DOI] [PubMed] [Google Scholar]
- 9.Lieb JP, Dasheiff RM, Engel J., Jr Role of the frontal lobes in the propagation of mesial temporal lobe seizures. Epilepsia. 1991;32:822–37. doi: 10.1111/j.1528-1157.1991.tb05539.x. [DOI] [PubMed] [Google Scholar]
- 10.Bertashius KM. Propagation of human complex-partial seizures: a correlation analysis. Electroencephalography and clinical neurophysiology. 1991;78:333–40. doi: 10.1016/0013-4694(91)90095-l. [DOI] [PubMed] [Google Scholar]
- 11.Napolitano CE, Orriols MA. Graduated and sequential propagation in mesial temporal epilepsy: analysis with scalp ictal EEG. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2010;27:285–91. doi: 10.1097/WNP.0b013e3181eaaa0e. [DOI] [PubMed] [Google Scholar]
- 12.Blumenfeld H, Westerveld M, Ostroff RB, et al. Selective frontal, parietal, and temporal networks in generalized seizures. NeuroImage. 2003;19:1556–66. doi: 10.1016/s1053-8119(03)00204-0. [DOI] [PubMed] [Google Scholar]
- 13.Holmes MD, Brown M, Tucker DM. Are “generalized” seizures truly generalized? Evidence of localized mesial frontal and frontopolar discharges in absence. Epilepsia. 2004;45:1568–79. doi: 10.1111/j.0013-9580.2004.23204.x. [DOI] [PubMed] [Google Scholar]
- 14.Varghese GI, Purcaro MJ, Motelow JE, et al. Clinical use of ictal SPECT in secondarily generalized tonic-clonic seizures. Brain : a journal of neurology. 2009;132:2102–13. doi: 10.1093/brain/awp027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blumenfeld H, Varghese GI, Purcaro MJ, et al. Cortical and subcortical networks in human secondarily generalized tonic-clonic seizures. Brain : a journal of neurology. 2009;132:999–1012. doi: 10.1093/brain/awp028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeSalvo MN, Schridde U, Mishra AM, et al. Focal BOLD fMRI changes in bicuculline-induced tonic-clonic seizures in the rat. NeuroImage. 2010;50:902–9. doi: 10.1016/j.neuroimage.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schindler K, Leung H, Lehnertz K, et al. How generalised are secondarily “generalised” tonic clonic seizures? Journal of neurology, neurosurgery, and psychiatry. 2007;78:993–6. doi: 10.1136/jnnp.2006.108753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rektor I, Zakopcan J, Tyrlikova I, et al. Secondary generalization in seizures of temporal lobe origin: Ictal EEG pattern in a stereo-EEG study. Epilepsy & behavior : E&B. 2009;15:235–9. doi: 10.1016/j.yebeh.2009.03.032. [DOI] [PubMed] [Google Scholar]
- 19.Blumenfeld H, Rivera M, McNally KA, et al. Ictal neocortical slowing in temporal lobe epilepsy. Neurology. 2004;63:1015–21. doi: 10.1212/01.wnl.0000141086.91077.cd. [DOI] [PubMed] [Google Scholar]
- 20.Jobst BC, Williamson PD, Neuschwander TB, et al. Secondarily generalized seizures in mesial temporal epilepsy: clinical characteristics, lateralizing signs, and association with sleep-wake cycle. Epilepsia. 2001;42:1279–87. doi: 10.1046/j.1528-1157.2001.09701.x. [DOI] [PubMed] [Google Scholar]
- 21.Franaszczuk PJ, Bergey GK, Durka PJ, et al. Time-frequency analysis using the matching pursuit algorithm applied to seizures originating from the mesial temporal lobe. Electroencephalography and clinical neurophysiology. 1998;106:513–21. doi: 10.1016/s0013-4694(98)00024-8. [DOI] [PubMed] [Google Scholar]
- 22.Jouny CC, Adamolekun B, Franaszczuk PJ, et al. Intrinsic ictal dynamics at the seizure focus: effects of secondary generalization revealed by complexity measures. Epilepsia. 2007;48:297–304. doi: 10.1111/j.1528-1167.2006.00963.x. [DOI] [PubMed] [Google Scholar]
- 23.Gotz-Trabert K, Hauck C, Wagner K, et al. Spread of ictal activity in focal epilepsy. Epilepsia. 2008;49:1594–601. doi: 10.1111/j.1528-1167.2008.01627.x. [DOI] [PubMed] [Google Scholar]
- 24.Lieb JP, Engel J, Jr, Babb TL. Interhemispheric propagation time of human hippocampal seizures. I. Relationship to surgical outcome. Epilepsia. 1986;27:286–93. doi: 10.1111/j.1528-1157.1986.tb03541.x. [DOI] [PubMed] [Google Scholar]
- 25.Enatsu R, Jin K, Elwan S, et al. Correlations between ictal propagation and response to electrical cortical stimulation: a cortico-cortical evoked potential study. Epilepsy research. 2012;101:76–87. doi: 10.1016/j.eplepsyres.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Trevelyan AJ, Sussillo D, Yuste R. Feedforward inhibition contributes to the control of epileptiform propagation speed. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:3383–7. doi: 10.1523/JNEUROSCI.0145-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feddersen B, Remi J, Kilian M, et al. Is ictal dystonia associated with an inhibitory effect on seizure propagation in focal epilepsies? Epilepsy research. 2012;99:274–80. doi: 10.1016/j.eplepsyres.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 28.Rektor I, Kuba R, Brazdil M, et al. Ictal and peri-ictal oscillations in the human basal ganglia in temporal lobe epilepsy. Epilepsy & behavior : E&B. 2011;20:512–7. doi: 10.1016/j.yebeh.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Wilke C, Worrell G, He B. Graph analysis of epileptogenic networks in human partial epilepsy. Epilepsia. 2011;52:84–93. doi: 10.1111/j.1528-1167.2010.02785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Constable RT, Scheinost D, Finn ES, et al. Potential use and challenges of functional connectivity mapping in intractable epilepsy. Frontiers in neurology. 2013;4:39. doi: 10.3389/fneur.2013.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]