Abstract
Background
Intensive statins are superior to moderate statins in reducing morbidity and mortality after an acute myocardial infarction (AMI). While studies have documented rates of statin prescription as a quality performance measure, variations in hospitals’ rates of initiating, intensifying and maximizing statin therapy after AMI are unknown.
Methods and Results
We assessed statins at admission and discharge among 4340 AMI patients from 24 US hospitals (2005–08). Hierarchical models estimated site variation in statin initiation in naïve patients, intensification in those on sub-maximal therapy, and discharge on maximal therapy (defined as a statin with expected LDL-C lowering ≥50%), after adjusting for patient factors including LDL-C. Site variation was explored with a median rate ratio (MRR), which estimates the relative difference in risk ratios of 2 hypothetically identical patients at 2 different hospitals. Among statin naïve patients, 87% without a contraindication were prescribed a statin, with no variability across sites (MRR 1.02). Among patients who arrived on sub-maximal statins, 26% had their statin therapy intensified with modest site variability (MRR 1.47). Among all patients without a contraindication, 23% were discharged on maximal statin therapy with substantial hospital variability (MRR 2.79).
Conclusions
In a large, multicenter AMI cohort, nearly 90% of patients were started on statins during hospitalization, with no variability across sites. However, rates of statin intensification and maximization were low and varied substantially across hospitals. Given that more intense statin therapy is associated with better outcomes, changing the existing performance measures to include the intensity of statin therapy may improve care.
Keywords: myocardial infarction, secondary prevention, lipids, statins
Statin therapy is a cornerstone of secondary prevention, with a wealth of data to support their use in nearly all patients after acute myocardial infarction (AMI), regardless of age, sex, or even baseline low-density lipoprotein cholesterol (LDL-C) levels.1–3 As such, prescription of statins at discharge is both a Class 1(A) recommendation in the AMI guidelines4–5 and a performance measure for quality AMI care.6 Importantly, clinical trials have shown that more potent statins reduce morbidity and mortality after AMI more effectively than less potent statins.7–11 This was reflected in the 2013 cholesterol guidelines, which recommended high-intensity statins to all patients with established atherosclerotic cardiovascular disease.12 Thus, while it is critical that all eligible patients be discharged on a statin after AMI, it is also important to discharge patients on high doses of potent statins to maximize their benefit in reducing recurrent ischemic events.
While the prescription of statins in any dose after AMI has been evaluated,13–14 studies have not examined rates of statin intensification and maximization. Understanding the prevalence and variations in these important strategies can highlight opportunities to optimize secondary prevention in this high-risk patient group. To address these gaps in knowledge, we examined the rates of statin initiation, intensification (among patients who arrived on a sub-maximal statin), and maximization among over 4000 post-AMI patients from 24 U.S. hospitals and evaluated patient- and site-level factors associated with more intensive statin use.
METHODS
Study population and protocol
Details regarding the study design, patient selection, site characteristics and follow-up assessments of the TRIUMPH study have been previously published.15 Briefly, 4340 patients from 24 U.S. hospitals were enrolled into the TRIUMPH registry between April 2005 and December 2008. Inclusion criteria included biomarker evidence of myocardial necrosis and additional clinical evidence supporting the diagnosis of an AMI, including prolonged ischemic signs/symptoms (≥20 minutes) or electrocardiographic ST changes during the initial 24 hours of admission. Patients were eligible only if presenting initially to an enrolling institution or if transferred to the enrolling hospital within 24 hours of presentation. Baseline sociodemographic and clinical data were obtained through chart abstraction and a detailed structured interview within 24 to 72 hours following admission. Lipid-lowering medications on admission and at discharge were recorded, as were any allergies or other contraindications to lipid-lowering therapy. Only patients who were discharged alive and had no documented contraindications to statin therapy were considered for our analyses (n=4271). Each participating hospital obtained Institutional Research Board approval, and all patients provided written informed consent.
Definition of statin initiation, intensification, and maximization
Initiation of statin therapy was defined as statin prescription on discharge in a patient who was not on a statin at admission. Among patients who arrived on a sub-maximal statin (i.e., a statin with expected LDL-C lowering of <50%), intensification of statin therapy was defined as either of the following: 1) increase in dose of current statin or 2) change in statin from a lower potency to a higher potency statin that was expected to result in an additional 10% LDL-C lowering (Table 1). Maximization of statin therapy was defined as discharge on a statin with a 50% or greater expected reduction in LDL-C12, 16 (i.e., atorvastatin 80mg or rosuvastatin 20–40mg daily).17
Table 1.
| % LDL-C Reduction | Atorvastatin | Fluvastatin | Lovastatin | Pravastatin | Rosuvastatin | Simvastatin |
|---|---|---|---|---|---|---|
| 10–20% | — | ≤20 mg | ≤10 mg | ≤10 mg | — | ≤5 mg |
| 20–30% | — | 40 mg | 20 mg | 20 mg | — | 10 mg |
| 30–40% | ≤10 mg | 80 mg | 40 mg | 40 mg | ≤5 mg | 20 mg |
| 40–50% | 20–40 mg | — | 80 mg | 80 mg | 10 mg | 40–80 mg |
| 50–60% | 80 mg | — | — | — | 20–40 mg | — |
Statistical analysis
Hierarchical modified Poisson regression models with robust standard errors were used to explore site and patient characteristics associated with statin initiation (among patients without contraindications who did not arrive on a statin), statin intensification (among patients who arrived on sub-maximal statins), and statin maximization at discharge (among all patients without contraindications). Because the outcome (statin therapy) was >10%, we estimated relative rates directly by using Poisson regression to avoid overestimation of effect sizes. Covariates for the models included demographics, standard cardiac risk factors, severity of MI, absence of LDL-C assessment during hospitalization, and baseline LDL-C level (acquired during hospitalization). Site was entered as a random effect to account for clustering of patients within hospitals (with an exchangeable within-hospital correlation structure), and site-level variation was explored with a median rate ratio (MRR), which estimates the average relative difference in risk ratios of two hypothetical patients being started on a statin/intensified with therapy if enrolled at two different sites. Cubic splines were considered for all continuous variables to check for non-linear relationships between these covariates and the outcome. Additionally, as the data to intensify and maximize statin therapy among AMI patients with very low LDL-C levels are not as clear, we performed sensitivity analyses for intensification and maximization, excluding patients with LDL-C levels <70 mg/dL. For the maximization analysis, we also performed a sensitivity analysis by excluding 4 hospitals with formularies that favored simvastatin, as this may have affected the rates of prescription of maximally potent statins. Finally, to examine potential explanations for the differences in intensification and maximization rates, the site characteristics of the more-aggressive hospitals in regards to statin intensification and maximization (≥25% of the time for both) vs. less-aggressive hospitals (<25% of the time for either) were compared using t-tests for continuous variables and Chi-square test for categorical variables. All analyses were conducted using SAS v9.2 (SAS Institute, Inc., Cary, NC), and statistical significance was determined by a 2-sided p-value of <0.05.
RESULTS
Study population
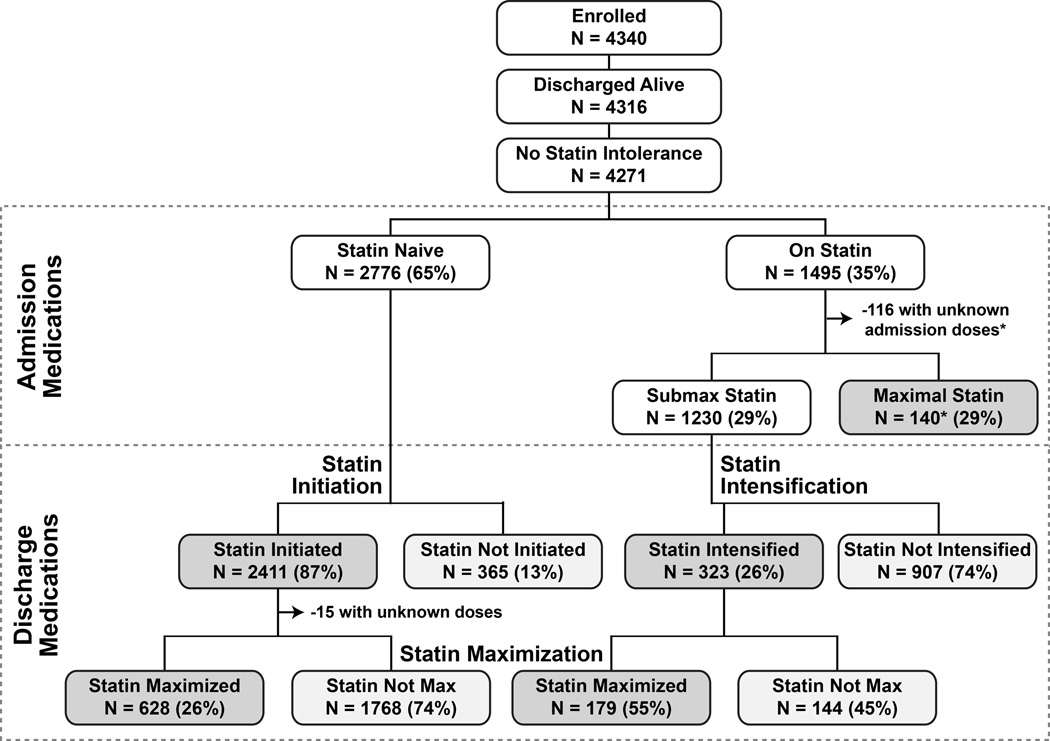
The patient characteristics of the 4271 patients enrolled in TRIUMPH who were discharged alive and had no documented contraindications to statin therapy are shown in Table 2. The mean age of patients was 59 years, 67% were male, and 31% had diabetes. Forty-three percent of patients presented with ST-elevations, and 92% underwent invasive management of their AMI. The analytic cohorts are outlined in Figure 1. Among the 4271 patients discharged alive without contraindications to statin therapy, 2776 reported that they were not taking a statin at admission (statin initiation analytic cohort). Of the 1495 patients (35%) who arrived on a statin, we did not have adequate drug or dose information on 125 patients at admission to be able to categorize the intensity of their statin therapy. An additional 140 patients were taking a maximal dose of statin at admission, leaving 1230 patients eligible for the statin intensification cohort. All patients discharged alive without contraindications for statin therapy were considered eligible for the statin maximization analysis.
Table 2.
Sociodemographic and clinical characteristics of patient population (N=4271)
| Sociodemographics | |
| Age (years) | 59.0 ± 12.3 |
| Male sex | 66.8% |
| Caucasian race | 67.4% |
| Married | 52.6% |
| Low social support | 17.1% |
| Lives alone | 24.6% |
| High school or greater education | 89.1% |
| Avoids care due to cost | 25.6% |
| Comorbidities | |
| Hypertension | 66.6% |
| Prior CABG | 11.2% |
| Diabetes mellitus | 30.7% |
| History of smoking | 59.6% |
| Chronic lung disease | 7.2% |
| Prior heart failure | 8.4% |
| Chronic kidney disease* | 21.7% |
| Body mass index (kg/m2) | 29.6 ± 6.5 |
| Prior Stroke | 4.9% |
| Depressive symptoms | 7.7% |
| Hospital procedures | |
| Cardiac catheterization | 92.3% |
| Percutaneous coronary intervention | 65.4% |
| Bypass graft surgery | 9.2% |
| Clinical presentation | |
| ST-elevations | 43.2% |
| Peak Troponin (ng/mL) | 28.5 ± 73.2 |
| GRACE risk score | 100.3 ± 30.0 |
| Ejection fraction (%) | 48.7 ± 13.1 |
| Initial heart rate (bpm) | 82.6 ± 22.1 |
| Initial systolic blood pressure (mmHg) | 143.4 ± 30.3 |
| Length of stay (days) | 5.4 ± 6.0 |
Estimated glomerular filtration rate <60mL/min/1.73m2
Figure 1.
Flowchart of patients in TRIUMPH from admission to discharge according to statin use. *Among the 116 patients who had missing statin dose at arrival, 44 were discharged on a maximal statin. Among the 140 patients who were on maximal statin on arrival, 114 were discharged on maximal statin.
Statin prescription patterns
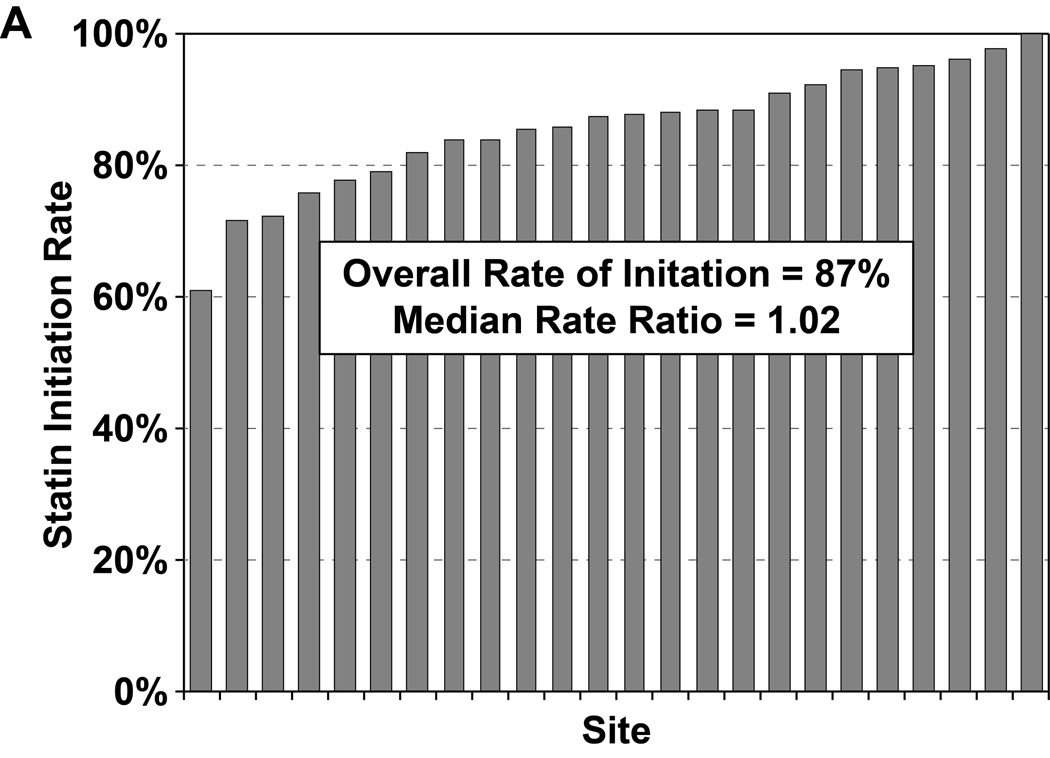
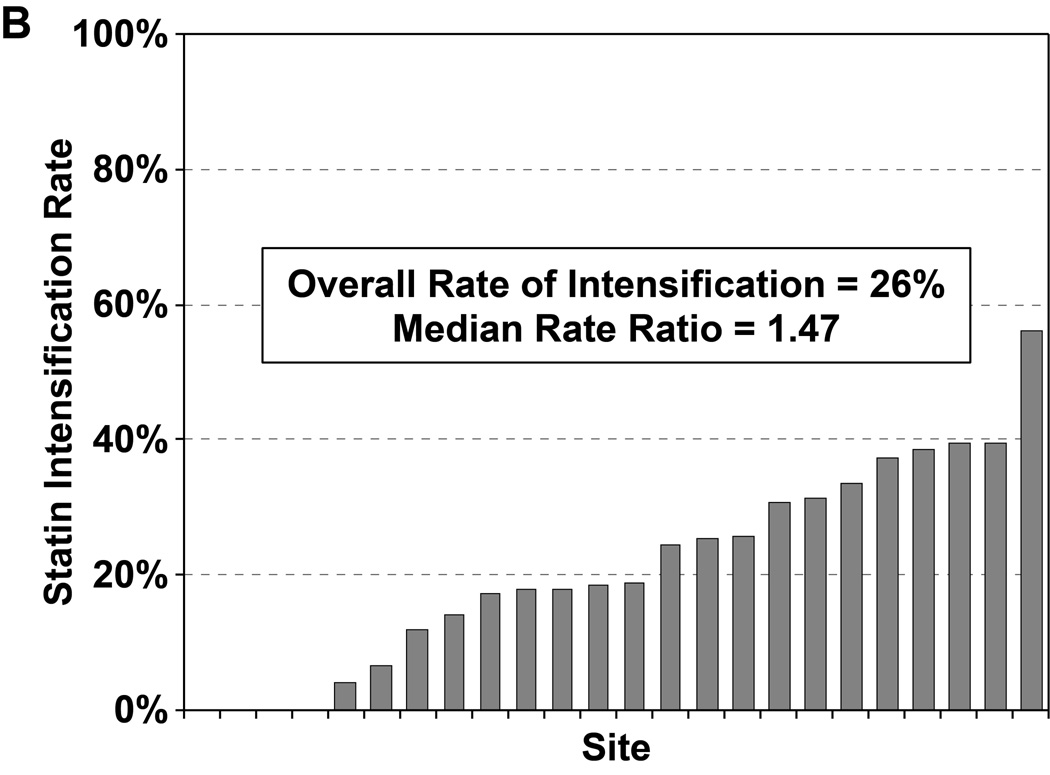
Of the 2776 patients who were statin naïve on presentation for their AMI and had no contraindications to statin therapy, 2411 patients (87%) were started on a statin during hospitalization, most commonly atorvastatin (47%) or simvastatin (39%). In a hierarchical, multivariable model adjusted for patients’ sociodemographic and clinical characteristics, there was virtually no site-level variability in statin initiation, with an MRR of 1.02 (95% CI 1.01–∞), indicating that the likelihood of 2 identical patients being started on a statin at 2 different sites was nearly identical (Figure 2a). Of the 1230 patients on sub-maximal statins at admission, 323 (26.3%) had their statin therapy intensified during hospitalization (195 patients [60%] had their dose increased and 128 patients [40%] were switched to a more potent statin). In a hierarchical, multivariable model adjusted for patient characteristics, there was modest site level variability observed in statin intensification, with an MRR of 1.47 (95% CI 1.27– 2.58, Figure 2b). After excluding patients with LDL-C levels <70 mg/dL, the rate of statin intensification increased slightly to 29.4%, but there continued to be modest site level variability (MRR 1.40, 95% CI 1.23–2.42).
Figure 2.
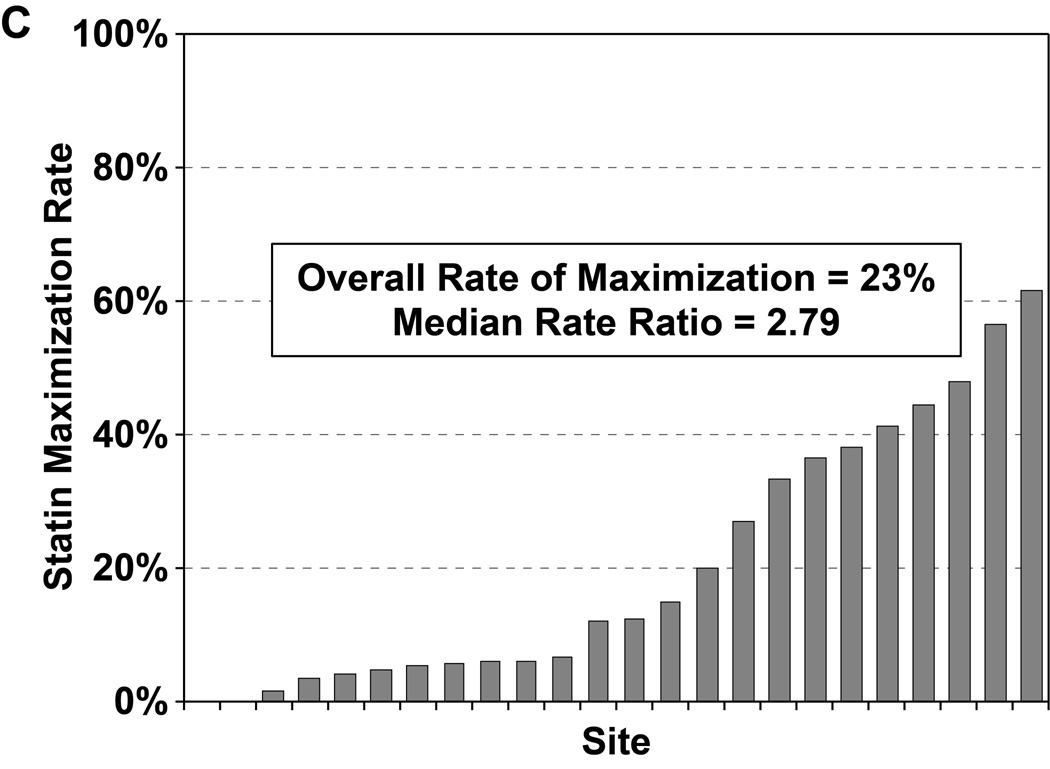
Rates of statin initiation (a), intensification (b), and maximization (c) by hospital.
Of the 4271 patients who were discharged alive and had no documented contraindications to statin therapy, 965 patients (22.7%) were discharged on a maximally potent statin (i.e., atorvastatin 80mg or rosuvastatin 20–40mg daily). In a hierarchical, multivariable model adjusted for patient characteristics, there was substantial site-level variability in regards to statin maximization (Figure 2c), with an MRR of 2.79 (95% CI 2.16–4.65), indicating that 2 hypothetically identical patients would have an almost 3-fold greater likelihood of being discharged on maximal statin therapy at one random hospital as compared with another. After excluding patients with LDL-C levels <70 mg/dL, the rate of statin maximization slightly increased to 24.3%, but there continued to be substantial site-level variability (MRR 2.77, 95% CI 2.14–4.64). In a second sensitivity analysis, after excluding 4 hospitals with formularies that favored simvastatin, the rate of statin maximization increased to 26.6% but the site-level variability remained substantial (MRR 2.33, 95% CI 1.85–3.81).
In regard to rates of aggressive lipid management across hospitals, the rates of intensification and maximization were highly correlated among hospitals (r=0.81, p<0.001), indicating that these two practice patterns often occur in parallel. The site-characteristics of the more aggressive hospitals in terms of intensification and maximization compared with the less aggressive hospitals are shown in Table 3 and the patient characteristics of those enrolled from those hospitals are shown in Supplemental Table 1. While there were no statistically significant differences due to small sample size, the 8 more aggressive hospitals tended to be larger, more urban, and more likely to be academic than the 16 less aggressive hospitals.
Table 3.
Site Characteristics of More- and Less-Aggressive Hospitals
| More Aggressive Hospitals N=8 |
Less Aggressive Hospitals N=16 |
p-value | |
|---|---|---|---|
| Practice Location | 0.298 | ||
| Urban | 87.5% | 56.3% | |
| Suburban | 12.5% | 37.5% | |
| Rural | 0% | 6.3% | |
| Hospital Type | 0.424 | ||
| Not for Profit | 100% | 81.3% | |
| For Profit | 0% | 6.3% | |
| VA/Governmental | 0% | 12.6% | |
| Teaching Status | |||
| University or Affiliated | 87.5% | 81.3% | 0.699 |
| Cardiology Fellows | 87.5% | 56.3% | 0.126 |
| Residents | 87.5% | 68.8% | 0.317 |
| Total Beds | 615 ± 272 | 590 ± 325 | 0.856 |
| Intensive Care Beds | 74 ± 35 | 45 ± 43 | 0.139 |
| PCIs | 87.5% | 87.5% | |
| PCI Volume | 1253 ± 608 | 831 ± 819 | 0.345 |
PCI, percutaneous coronary intervention
Patient factors associated with statin prescribing patterns
In the hierarchical multivariable models, there were few patient factors that were associated with a greater likelihood of statin initiation, intensification, or maximization (Supplemental Table 2). Patients with lower LDL-C levels were less likely to be started on a statin, were less likely to have their statin dose intensified, and were less likely to be discharged on a maximal statin (Supplemental Figure 1a–c). In addition, while there was no difference in the rate of statin initiation between patients with non-ST-elevation and ST-elevation AMIs, patients who presented with ST-elevations were nearly 50% more likely to have their statin intensified (RR 1.47, 95% CI 1.12–1.92) and maximized (RR 1.49, 95% CI 1.25–1.77) than those who did not have ST-elevation AMIs (Supplemental Table 2).
DISCUSSION
In a large, multicenter, contemporary prospective cohort of AMI patients, we found that statin initiation is very common, with 87% of statin naïve patients being started on a statin during hospitalization. Furthermore, there was no site-level variation in statin initiation, indicating that practice patterns for starting patients on statins during AMI were uniform across all hospitals studied. However, among patients who were already taking a statin at the time of AMI, intensification of the statin occurred infrequently, in only 26% of patients, with modest site-level variability. Moreover, statin maximization occurred in only 23% of patients with substantial site-level variability, including 2 hospitals maximizing therapy in none of their patients, while 2 hospitals maximized statins in more than half of their patients. The low rates and substantial variation in the practice of intensifying and maximizing statin therapy may be a target for quality improvement, particularly given the evidence of greater benefit with more potent statin therapy after AMI7–11 and the updated cholesterol guidelines that recommend high-intensity statins in all AMI patients.12
A key finding of our study is that higher LDL-C levels and ST-elevations on admission were each associated with a substantially higher likelihood of intensive statin therapy (i.e., more intensification and maximization). This likely reflects a belief that these patients are at greatest risk of recurrent ischemic events and, thus, most likely to benefit from more intense statin therapy. While targeting intensive secondary prevention strategies to the patients most likely to benefit is reasonable and appropriate, there is no compelling evidence that there is a differential effect of statin therapy according to either the type of MI (i.e. STEMI vs. NSTEMI) or baseline LDL levels.1–3 Furthermore, while high baseline LDL-C levels are associated with higher risk of adverse events among patients with stable coronary disease and patients without known disease, this relationship has not been demonstrated among patients with an AMI and may actually be reversed in the acute setting.18 This likely results from a combination of factors relating to acute changes in LDL-C levels as an acute phase reactant and lead-time bias (i.e., patients with low LDL-C levels who present with an AMI despite this favorable lipid profile carry some unmeasured risk factors that makes them high-risk, despite a low LDL-C). This finding suggests that educating clinicians about the benefits of intensive statin therapy and constructing performance measures that emphasize statin maximization in all eligible AMI patients may improve patient care and outcomes.
Our findings both support and extend the prior literature on patterns of statin use after AMI. Several prior studies evaluated lipid-lowering therapy in patients after AMI but focused on absolute rates of treatment with any statin dose, rather than the intensity of statin therapy13–14 In an analysis of patients with acute coronary syndromes discharged from 344 hospitals participating in Get With The Guidelines registry from 2005–2009, 89% of eligible patients were discharged on some statin, but only 38% of statin prescriptions at discharge were for intensive statin therapy, as defined by an expected LDL-C lowering of >50%.16 In a small sub-study of 788 patients from 41 hospitals within the Get With The Guidelines registry, 84% of statin naïve patients were started on statin therapy during their hospitalization, with diabetes mellitus, peripheral vascular disease, prior stroke, prior heart failure, prior revascularization, and a lower LDL-C being associated with a lower likelihood of statin initiation. Of patients with LDL-C >100 mg/dL and not on intensive statin therapy at admission, only 37% had their statin intensified during hospitalization.19 Our study supports these prior analyses by demonstrating high rates of statin initiation but low rates of statin intensification and maximization. Our study substantially extends the existing literature by examining patient- and site-level factors associated with intensification and maximization. Finding large variability in the use of intensive statin therapy, even after adjusting for patient factors and LDL-C, supports the creation of system-level interventions to improve the use of the more effective secondary prevention strategies with intensive statin therapy, including the potential use of maximal statin therapy as a quality metric in quality improvement registries, such as the ACTION-Get with the Guidelines program.
There are several potential reasons for the low rates of statin intensification and maximization among AMI patients. As performance measures only judge providers on the prescription of statins in any dose, it is possible that clinicians do not fully recognize the importance of prescribing intensive statins. While initiating a statin in any dose is critical for patients after AMI, the intensification of therapy can further reduce the risk of recurrent ischemic events. The recently published cholesterol guidelines state “high-intensity statin therapy should be initiated for adults ≤75 years of age with clinical atherosclerotic cardiovascular disease and who are not receiving statin therapy or the intensity should be increased in those receiving a low- or moderate-intensity statin.”12 As such, there should be increasing awareness of the importance of intensifying and maximizing statins in AMI patients. Second, physicians may be choosing to start a lower dose of statin and expect this to be titrated up in the outpatient setting. However, given the well-documented challenges of health care transition and outpatient up-titration of statins,19 there seem to be few valid reasons not to start a patient on intensive statin therapy prior to discharge. Finally, there may be cost or formulary reasons for lower rates of intensification or maximization, as we found lower rates of statin maximization at the 4 VA or county hospitals with formularies that favored simvastatin use. However, after excluding the 4 hospitals with more restricted formularies, the rate of maximization only increased to 27%, indicating that formulary was not the most important driver of a lack of intensification or maximization. Furthermore, we found that 45% of patients were discharged on atorvastatin or rosuvastatin, which were branded drugs at the time of the study, and an additional 35% of patients were discharged on simvastatin, which became generic half-way through TRIUMPH. There is typically little cost difference between a high dose and a low dose of the same statin, and given that most patients were already on potent statins, the suboptimal intensification and maximization of statins highlights an important potential opportunity to improve care. Our study should be interpreted in the context of the following potential limitations. First, while our analysis included 24 hospitals from rural, suburban, and urban sites across the U.S., and the study participants had a broad range of socioeconomic and demographic characteristics, this represents only a small subset of U.S. practice. Replicating these analyses in a larger sample of hospitals would further ensure the generalizability of our findings and could provide further insight into the characteristics of hospitals that influence choice of statin at discharge. Second, we categorized statin potency based on expected LDL-C reduction and defined intensification as a movement to a higher category of statin potency (or an increase in dose). While this strategy was necessary to capture intensification of statin therapy when patients were changed to different medications, it is not explicitly known that a higher category of expected LDL-C reduction would correspond to improved outcomes. While the effect of statins on LDL-C and on reducing recurrent ischemic events are congruent, it is possible that other aspects of statins’ efficacy in reducing ischemic events may vary among different statins and are not directly related to their ability to lower LDL-C. Finally, although studies have demonstrated that intensive statin therapy is superior to moderate statin therapy in reducing morbidity and mortality after AMI,7–11 an explicit strategy of statin intensification or maximization has not been evaluated.
Conclusions
We found that nearly 90% of AMI patients are started on statins during hospitalization, with essentially no variability across sites. However, rates of statin intensification and maximization were much lower and varied substantially across hospitals. Given that there are few contraindications to acutely initiating intensive statin therapy, novel strategies, such as modification of existing performance measures, are needed to support more consistent, evidence-based practice strategies across hospitals to improve care.
Supplementary Material
Clinical Summary.
As trials have shown that intensive statin therapy after an acute myocardial infarction (AMI) is superior to moderate statins in reducing morbidity and mortality, we sought to examine variations in hospitals’ rates of initiating, intensifying and maximizing statin therapy after AMI. Among 4340 AMI patients from 24 US hospitals, we found nearly 90% of patients were started on statins during hospitalization, with no variability across sites. However, only 26% of patients on sub-maximal statins had their statin therapy intensified, with modest site variability. Furthermore, only 23% of patients were discharged on maximal statin therapy with substantial hospital variability. We also found that higher LDL-C levels and ST-elevations on admission were each associated with a substantially higher likelihood of intensification and maximization. This likely reflects a belief that these patients are most likely to benefit from more intense statin therapy. However, there is no compelling evidence that there is a differential effect of statin therapy according to either the type of AMI or baseline LDL levels, which suggests that educating clinicians about the benefits of intensive statin therapy may improve patient care and outcomes. In addition, the creation of system-level interventions, such as modification of existing performance measures, could be used to support more consistent, evidence-based practice strategies across hospitals to improve care.
Acknowledgments
Funding Sources: TRIUMPH was sponsored by a grant from the National Institutes of Health (National Heart, Lung, Blood Institute): Washington University School of Medicine SCCOR Grant #P50HL077113-01. The funding organization did not play a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript. This research project was funded through a research grant from Eli Lilly. Dr. Maddox is supported by a Career Development Grant Award from the Veterans Affairs Health Services Research and Development Service.
Footnotes
Conflict of Interest Disclosures: SVA and JAS received research grant support from Eli Lilly. ZZ, PLM, and JB are employees of Eli Lilly.
References
- 1.Mrc/bhf heart protection study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: A randomised placebo-controlled trial. Lancet. 2002;360:7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 2.Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, Collins R. Efficacy and safety of more intensive lowering of ldl cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.LaRosa JC, He J, Vupputuri S. Effect of statins on risk of coronary disease: A meta-analysis of randomized controlled trials. JAMA. 1999;282:2340–2346. doi: 10.1001/jama.282.24.2340. [DOI] [PubMed] [Google Scholar]
- 4.Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Jr, Chavey WE, 2nd, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS, Smith SC, Jr, Jacobs AK, Halperin JL, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura R, Ornato JP, Page RL, Riegel B. Acc/aha 2007 guidelines for the management of patients with unstable angina/non st-elevation myocardial infarction: A report of the american college of cardiology/american heart association task force on practice guidelines (writing committee to revise the 2002 guidelines for the management of patients with unstable angina/non st-elevation myocardial infarction): Developed in collaboration with the american college of emergency physicians, the society for cardiovascular angiography and interventions, and the society of thoracic surgeons: Endorsed by the american association of cardiovascular and pulmonary rehabilitation and the society for academic emergency medicine. Circulation. 2007;116:e148–e304. doi: 10.1161/CIRCULATIONAHA.107.181940. [DOI] [PubMed] [Google Scholar]
- 5.Kushner FG, Hand M, Smith SC, Jr, King SB, 3rd, Anderson JL, Antman EM, Bailey SR, Bates ER, Blankenship JC, Casey DE, Jr, Green LA, Hochman JS, Jacobs AK, Krumholz HM, Morrison DA, Ornato JP, Pearle DL, Peterson ED, Sloan MA, Whitlow PL, Williams DO. 2009 focused updates: Acc/aha guidelines for the management of patients with st-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and acc/aha/scai guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update): A report of the american college of cardiology foundation/american heart association task force on practice guidelines. Circulation. 2009;120:2271–2306. doi: 10.1161/CIRCULATIONAHA.109.192663. [DOI] [PubMed] [Google Scholar]
- 6.Krumholz HM, Anderson JL, Bachelder BL, Fesmire FM, Fihn SD, Foody JM, Ho PM, Kosiborod MN, Masoudi FA, Nallamothu BK. Acc/aha 2008 performance measures for adults with st-elevation and non-st-elevation myocardial infarction: A report of the american college of cardiology/american heart association task force on performance measures (writing committee to develop performance measures for st-elevation and non-st-elevation myocardial infarction): Developed in collaboration with the american academy of family physicians and the american college of emergency physicians: Endorsed by the american association of cardiovascular and pulmonary rehabilitation, society for cardiovascular angiography and interventions, and society of hospital medicine. Circulation. 2008;118:2596–2648. doi: 10.1161/CIRCULATIONAHA.108.191099. [DOI] [PubMed] [Google Scholar]
- 7.Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, Joyal SV, Hill KA, Pfeffer MA, Skene AM. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 8.de Lemos JA, Blazing MA, Wiviott SD, Lewis EF, Fox KA, White HD, Rouleau JL, Pedersen TR, Gardner LH, Mukherjee R, Ramsey KE, Palmisano J, Bilheimer DW, Pfeffer MA, Califf RM, Braunwald E. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: Phase z of the a to z trial. JAMA. 2004;292:1307–1316. doi: 10.1001/jama.292.11.1307. [DOI] [PubMed] [Google Scholar]
- 9.Murphy SA, Cannon CP, Wiviott SD, de Lemos JA, Blazing MA, McCabe CH, Califf RM, Braunwald E. Effect of intensive lipid-lowering therapy on mortality after acute coronary syndrome (a patient-level analysis of the aggrastat to zocor and pravastatin or atorvastatin evaluation and infection therapy-thrombolysis in myocardial infarction 22 trials) Am J Cardiol. 2007;100:1047–1051. doi: 10.1016/j.amjcard.2007.04.053. [DOI] [PubMed] [Google Scholar]
- 10.Ribeiro RA, Ziegelmann PK, Duncan BB, Stella SF, da Costa Vieira JL, Restelatto LM, Moriguchi EH, Polanczyk CA. Impact of statin dose on major cardiovascular events: A mixed treatment comparison meta-analysis involving more than 175,000 patients. Int J Cardiol. 2013;166:431–439. doi: 10.1016/j.ijcard.2011.10.128. Epub 2011 Dec 20. [DOI] [PubMed] [Google Scholar]
- 11.Josan K, Majumdar SR, McAlister FA. The efficacy and safety of intensive statin therapy: A meta-analysis of randomized trials. CMAJ. 2008;178:576–584. doi: 10.1503/cmaj.070675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stone NJ, Robinson J, Lichtenstein AH, Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Jr, Watson K, Wilson PW. 2013 acc/aha guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the american college of cardiology/american heart association task force on practice guidelines. Circulation. 2013 Nov 12; doi: 10.1016/j.jacc.2013.11.002. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 13.Ho PM, Spertus JA, Masoudi FA, Reid KJ, Peterson ED, Magid DJ, Krumholz HM, Rumsfeld JS. Impact of medication therapy discontinuation on mortality after myocardial infarction. Arch Intern Med. 2006;166:1842–1847. doi: 10.1001/archinte.166.17.1842. [DOI] [PubMed] [Google Scholar]
- 14.Arnold SV, Spertus JA, Tang F, Krumholz HM, Borden WB, Farmer SA, Ting HH, Chan PS. Statin use in outpatients with obstructive coronary artery disease. Circulation. 2011;124:2405–2410. doi: 10.1161/CIRCULATIONAHA.111.038265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arnold SV, Chan PS, Jones PG, Decker C, Buchanan DM, Krumholz HM, Ho PM, Spertus JA. Translational research investigating underlying disparities in acute myocardial infarction patients' health status (triumph): Design and rationale of a prospective multicenter registry. Circ Cardiovasc Qual Outcomes. 2011;4:467–476. doi: 10.1161/CIRCOUTCOMES.110.960468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Javed U, Deedwania PC, Bhatt DL, Cannon CP, Dai D, Hernandez AF, Peterson ED, Fonarow GC. Use of intensive lipid-lowering therapy in patients hospitalized with acute coronary syndrome: An analysis of 65,396 hospitalizations from 344 hospitals participating in get with the guidelines (gwtg) Am Heart J. 2010;160:1130–1136. doi: 10.1016/j.ahj.2010.08.041. 1136 e1131–1133. [DOI] [PubMed] [Google Scholar]
- 17.Chong PH. Lack of therapeutic interchangeability of hmg-coa reductase inhibitors. Ann Pharmacother. 2002;36:1907–1917. doi: 10.1345/aph.1C116. [DOI] [PubMed] [Google Scholar]
- 18.Cho KH, Jeong MH, Ahn Y, Kim YJ, Chae SC, Hong TJ, Seong IW, Chae JK, Kim CJ, Cho MC, Seung KB, Park SJ. Low-density lipoprotein cholesterol level in patients with acute myocardial infarction having percutaneous coronary intervention (the cholesterol paradox) Am J Cardiol. 2010;106:1061–1068. doi: 10.1016/j.amjcard.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 19.Melloni C, Shah BR, Ou FS, Roe MT, Smith SC, Jr, Pollack CV, Jr, Ohman M, Gibler WB, Peterson ED, Alexander KP. Lipid-lowering intensification and low-density lipoprotein cholesterol achievement from hospital admission to 1-year follow-up after an acute coronary syndrome event: Results from the medications applied and sustained over time (maintain) registry. Am Heart J. 2010;160:1121–1129. doi: 10.1016/j.ahj.2010.09.008. 1129 e1121. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.