Abstract
The nicotinic receptor is a promising drug target currently being investigated for the treatment of cognitive symptoms in schizophrenia. A key step in this process is the development of noninvasive functional neuroimaging biomarkers that can be used to determine if nicotinic agents are eliciting their targeted biological effect, ideally through modulation of a fundamental aspect of neuronal function. To that end, neuroimaging researchers are beginning to understand how nicotinic modulation affects “intrinsic” brain networks to elicit potentially therapeutic effects. An intrinsic network is a functionally and (often) structurally connected network of brain areas whose activity reflects a fundamental neurobiological organizational principle of the brain. This review summarizes findings of the effects of nicotinic drugs on three topics related to intrinsic brain network activity: (1) the default mode network, a group of brain areas for which activity is maximal at rest and reduced during cognitive tasks, (2) the salience network, which integrates incoming sensory data with prior internal representations to guide future actions and change predictive values, and (3) multi-scale complex network dynamics, which describe these brain’s ability to efficiency integrate information while preserving local functional specialization. These early findings can be used to inform future neuroimaging studies that examine the network effects of nicotinic agents.
Keywords: Schizophrenia, Nicotinic, Salience network, Default network, Small-world network
1. Introduction: Schizophrenia, nicotinic receptors, and intrinsic brain networks
Despite decades of research into their causes and treatment, cognitive symptoms remain poorly managed in schizophrenia, exacting a heavy toll on patients and society. These symptoms include poor working and episodic memory, slow processing speed, executive dysfunction, and impaired sustained and selective attention. Cognitive dysfunction is associated with poor quality-of-life, i.e. chronic unemployment, social difficulties, depression, and high risk for suicide [1,2]. Clearly, new treatments are needed to improve functional outcomes for patients with this devastating illness. This review focuses on recent neuroimaging findings involving drugs that target the nicotinic receptor, an emerging therapeutic target of great interest in schizophrenia.
1.1. Neuropharmacology of nicotinic receptors
Nicotinic receptors are ionotropic, ligand-gated channels that are composed of various combinations of five α (α2–α10) and/or β (β2–β4) subunits [3]. Each subunit is coded by a separate gene. Nicotinic receptors can either be heteromeric (e.g. α4β2) or homomeric (e.g. α7), although the most prevalent combinations in the central nervous system are the α4β2 and α7 subtypes [3]. Upon activation, the primary function of nicotinic receptors is to depolarize the cell. This depolarizing current is carried by an influx of sodium through α4β2 receptors, and an influx of calcium through α7 receptors [4]. The influx of calcium through presynaptic α7 receptors also activates second-messenger systems that can induce the release of neurotransmitters into the synaptic cleft [5]. Nicotinic receptors are endogenously stimulated by release of the neurotransmitter acetylcholine from cholinergic neurons that originate from the basal forebrain [3]. Depending on the subunit composition, nicotinic receptors show different affinities for various exogenous ligands; e.g. nicotine, the direct agonist found in tobacco products, binds with high (nM) affinity to the α4β2 receptor and low (μM) affinity to the α7 receptor [6]. This agonist specificity suggests that receptor subtypes may be differentially targeted by pharmacologic compounds.
Nicotinic receptor subtypes display a variety of regional, cell-specific, and subcellular localizations that convey functional specialization. Regional areas of expression include the anterior and posterior cingulate, thalamus, basal ganglia, hippocampus, frontal and parietal cortex. The level of expression varies depending on subtype (reviewed by Ref. [7]). Nicotinic receptors are not only expressed in excitatory cells but also on inhibitory interneurons, where they exert localized control over activity and influence oscillatory firing patterns. Depending on the receptor subtype and the brain region and cell type in which they are expressed, receptors may be located on dendritic arbors, cell bodies, and/or pre/postsynaptic terminals. Nicotinic receptors are thus positioned to affect brain response on both a global and local level, and influence neuronal input and output [8].
1.2. The nicotinic receptor as a therapeutic target
Widespread interest in the pharmacology of nicotinic receptors is in part due to the well-studied, pro-cognitive effects of nicotine and other nicotinic receptor-targeting compounds. Concordantly, targeting these receptors is a major avenue of treatment development for cognitive symptoms in a number of neurologic, neurodevelopmental, and neuropsychiatric disorders, including Alzheimer’s disease [9], autism [10], attention deficit (hyperactivity) disorder [11], depression [12], and schizophrenia [13], as well as for cognitive enhancement in healthy individuals [14]. Interest in the receptor for the treatment of cognitive symptoms of schizophrenia also stems from the fact that over 60% of patients are chronic smokers [15], potentially as a form of “self-medication” to correct a fundamental deficit in receptor transmission [16]. In support of this hypothesis, single nucleotide polymorphisms (SNPs) in the 5′ upstream regulatory region of the α7 nicotinic receptor gene are associated with reduced expression of α7 receptors and increased risk for schizophrenia [17–21]. Nicotine improves measures of cognition in patients, including working memory, attention, and processing speed [22–24]. Furthermore, the sensory (P50) gating deficit, which may be related to the inability of patients to ignore extraneous stimuli, is transiently normalized by nicotine [25].
Nonetheless, nicotine, whether administered through cigarettes, a patch, or Nicorette gum, is not a suitable therapeutic. It rapidly desensitizes receptors and thus is only efficacious for a short period [26,27]. To compound this problem, nicotine cannot be degraded by endogenous enzymes; thus, receptor desensitization may persist for several hours after administration. Nicotine is also addictive, in part due to expression of α4β2 nicotinic receptors on dopaminergic neurons in the brain’s “reward” circuitry, the ventral tegmental area and nucleus accumbens [28]. The fact that the most readily available form of nicotine is cigarettes, a highly carcinogenic method of delivery [29], further makes it unsuitable for therapeutic use. Thus, although patients with schizophrenia may be attempting to “self-medicate” by smoking cigarettes, this “treatment” is largely ineffective and unsafe.
Investigators have therefore sought alternative methods to target nicotinic receptors, including partial (as opposed to full) agonists [30,31], allosteric modulators [32,33], and subtype-specific (e.g. α7 subunit) agonists [30]. The goal of this endeavor is to develop long-lasting, efficacious drugs without serious side effects. A Phase I proof-of-principle trial of the short-release, α7 partial agonist 3-(2,4-dimethoxybenzylidine) anabaseine (DMXB-A) showed improved P50 gating and improved selective attention in schizophrenia [34]. A Phase II double-blind three arm crossover trial in nonsmoking patients demonstrated efficacy of DMXB-A on attention/vigilance and working memory subscales of the Measurement and Treatment Research to Improve Cognition in Schizophrenia Consensus Cognitive Battery (MATRICS) in the first arm compared to baseline, but not in subsequent arms. The lack of effect in the second and third arm was attributed to practice effects of the MATRICS [35]. The drug had minimal side effects in both trials. A more recent trial in a mixed population of smokers and nonsmoking schizophrenia patients reported that the α7 partial agonist TC-5619 improved working memory in smokers and executive function in the entire patient group, relative to the atypical neuroleptics risperidone or quetiapine [36]. Due to these encouraging results, additional phase II as well as phase III clinical trials are currently in development.
1.3. An intrinsic network-based approach to studying nicotinic modulation in schizophrenia
Despite their therapeutic promise, little is understood about how nicotinic drugs affect the functional neurobiology of the brain in schizophrenia. To fill this knowledge gap, researchers are beginning to utilize functional magnetic resonance imaging (fMRI) to noninvasively understand the modulatory effects of nicotinic agents on neuronal activity relevant to disease-related processes. The addition of fMRI is advantageous to behavioral measurements alone in that it can be used to more directly examine the neurobiological effects of therapeutic candidates.
For neuroimaging to be a useful in drug development, it must report effects that reflect disease-relevant changes in neuronal function consistent with a drug’s described target(s). Traditionally, fMRI studies have been designed to compare brain activity during a task or stimulus-related condition compared to a “baseline” or comparison condition during which the task is not performed, varies, or is made easier along a dimension of interest. These designs have been highly informative in regards to understanding brain dysfunction across a wide range of sensory, cognitive, and emotional processing domains in schizophrenia (reviewed by Refs. [37,38]). A limitation, however, is that it is not always obvious that reversing a functional abnormality elicited by a specific cognitive task would have a therapeutic benefit in a patient. Indeed, neuronal response during a task may be specific to the conditions of that task and not indicative of a pathological state. A loss of prefrontal recruitment during a working memory task, for example, does not necessarily indicate that pathology is located specifically in this area; it may be a downstream consequence of abnormalities in another region that is elicited by specific task conditions. Related to this problem, some cognitive tasks may be too demanding for patient populations to adequately perform. In this instance, it may be impossible to separate disease-relevant neurobiological effects from the potential confounding effects of task non-compliance.
An alternative approach is to use fMRI to understand how intrinsic neuronal networks, which are always present regardless of cognitive state, can be modulated. An intrinsic network is a functionally and (often, but not necessarily) structurally connected network of brain areas whose activity reflects a fundamental neurobiological organizational principle of the brain. By definition, the network is active both during and in the absence of goal-directed or stimulus-evoked cognitive activities, although its overall level of activity and connectivity may change depending on these factors. Intrinsic networks make up the vast majority (80%) of the brain’s metabolic activity [39], and task-related increases in energy consumption are small (5%) compared to the brain’s resting state energy consumption [40]. Due to their relative contribution to total neuronal activity, intrinsic networks are logical targets by which to measure the neurobiological effects of nicotinic agents. Due to recently increased interest in these topics, this review will examine two intrinsic networks, the default mode network and salience network, as well complex network approaches to understanding whole brain network dynamics (e.g. efficiency of communication, integration of information, and the extent of local specialization). These studies have revealed a number of abnormalities in schizophrenia for which the effects of nicotinic drugs have only begun to be examined. As the existing literature on this topic is limited, a major goal of this review is to promote future research into the effects of these compounds on the neural mechanisms of mental illness.
1.4. Comment: “Activity” vs “connectivity”
The terms “activity” and “connectivity” are used in these review to describe intrinsic network dynamics. Although these terms are related, they are actually statistically distinct characteristics. “Activity” refers to the average amplitude of oscillatory signal fluctuations in a network, and in fMRI studies is quantified by the relative percent blood oxygen level-dependent (BOLD) signal. “Hyperactivity” thus implies greater BOLD signal for a given network compared to another group or neuronal state. “Connectivity,” on the other hand, refers to the degree to which two or more regions are synchronously active or inactive. “Hyperconnectivity” between two regions thus implies that the BOLD signal for one region modulates in time with the signal for the second region. Hyperconnectivity does not imply that the amplitude of this change is similar for both regions; neither does it imply that the overall level of network activity is increased. Network activity can change independently of connectivity, although some studies have reported relationships between the two (e.g. [41]). Similarly, a drug may have a therapeutic effect by modulating network activity, connectivity, or both.
2. The default mode network
2.1. Background and phenotype in schizophrenia
fMRI studies have traditionally focused on “task related” activity, i.e. how local brain areas are recruited during sensory stimulation and during specific cognitive functions such as working memory, selective and sustained attention and executive control. These studies observed that a number of brain areas were consistently deactivated, reflecting suppression during the more cognitively demanding “task” condition compared to the baseline or low-load condition. It was later discovered that these regions were synchronously active and therefore constituted a functionally connected network [42]. Due to its tendency to be down-modulated during many tasks, and therefore be active as a “default”, the network was coined the default mode network (DMN).
The primary hubs of the DMN are (1) the precuneus/posterior cingulate cortex (PCC), (2) the medial prefrontal cortex (mPFC), and (3) the bilateral inferior parietal cortex (IPC) (Fig. 1). The bilateral hippocampus/medial temporal lobe is considered an accessory hub of the network due its being less functionally connected to the other three DMN hubs, on average, relative to the primary hubs [43]. The relative contribution of each of these hubs to the overall activity of the network is not static, however, and likely depends on the cognitive state of the individual. The DMN is readily and reproducibly detectable regardless of the analytic technique used, and irrespective of the cognitive state of the individual, be it during an effortful task, rest, or even during sleep [44]. The functions of the DMN are not completely understood. The network is particularly active during actions that are self-referential: e.g. reflecting on the past, planning for the future, or monitoring internal state [43]. Because the network also shows activity while under anesthesia [45] and during the early stages of sleep [46–48], however, its activity does not necessarily imply self-referential thinking. DMN activity is inversely proportional to activity in the central executive network (CEN), another intrinsic network that includes the dorsolateral prefrontal cortex (DLPFC) and posterior parietal cortex. Concordantly, the CEN is more active and the DMN is less active during challenging tasks; in the absence of task (i.e. at “rest”), the DMN is more active and the CEN is less active [49]. Abnormally high DMN activity during tasks is associated with poor performance, likely due to competing resource allocation toward task-irrelevant thoughts [50].
Fig. 1.
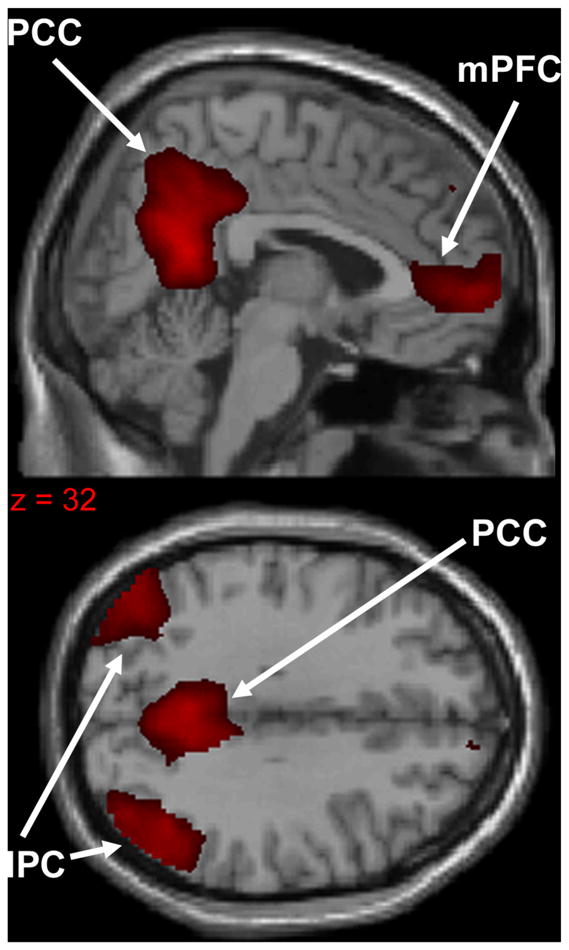
Primary hubs of the default mode network: (1) precuneus/posterior cingulate cortex (PCC), (2) medial prefrontal cortex (mPFC), (3) bilateral inferior parietal cortex (IPC). Network extracted using 20-component ICA from a sample of 36 healthy subjects.
Soon after the discovery of the DMN, abnormalities in its function were observed in schizophrenia. Patients inappropriately recruit the DMN, as evidenced by hyperactivity of the network during an auditory oddball task [51], working memory tasks [41,52,53], and language (semantic priming) tasks [54]. Patients are similarly impaired in their ability to deactivate the DMN as task difficulty is increased [41,52,53]. Hyperactivity persists when controlling for the effect of performance, suggesting that impaired DMN suppression is not solely a downstream consequence of behavior [41]. DMN abnormalities extend to task-free (resting) states, during which DMN hyperactivity and hyperconnectivity have been observed [55–57], although loss of connectivity has been observed as well, albeit less consistently [58]. DMN dysfunction is correlated with the severity of positive symptoms [51], negative symptoms [59], and impaired social cognition [60]. DMN hyperactivity and hyperconnectivity have been reported in unaffected first-degree relatives of schizophrenia patients, albeit to a lesser extent [41,56]. These findings suggest that DMN pathology has both state-dependent and independent characteristics.
2.2. Nicotinic modulation of the DMN
It is possible to speculate that because DMN activity and cognitive performance are inversely related, a drug that reduces DMN activity during cognitive tasks may show clinical efficacy for improving cognition in schizophrenia. A plausible mechanism for nicotinic effects on the DMN is via activation of inhibitory interneurons that express α7 receptors, as has been described previously in the hippocampus [61,62]. The pattern of receptor expression in DMN regions suggests it is readily amenable to nicotinic modulation, as the PCC, mPFC, and hippocampus express α7 nicotinic receptor mRNA and protein [7,63]. Although it is unclear if schizophrenia is associated with deficits in receptor expression throughout the DMN, reduced α7 expression in the hippocampus has been observed [64,65]. Several studies have therefore examined the effect of nicotinic modulation on DMN activity.
A 2007 study by Hahn and colleagues was the first to report nicotinic modulation of the DMN [66]. The investigators used a crossover design, with separate (scheduled 2–14 days apart) scans for nicotine and placebo. 17 healthy smokers who were minimally (~3 h) deprived of cigarette smoking were given either a 21 mg nicotine or placebo patch, and 2–2.5 h later were scanned while performing a cued spatial attention task. As anticipated, nicotine decreased reaction time and errors of omission on the task. Neurobiologically, using event-related fMRI, nicotine was associated with significant deactivation of two DMN hubs: the PCC and the mPFC. Deactivation of these areas was associated with improved performance, i.e. lower reaction time. Nicotine’s effects were magnified by specific task conditions, e.g. valid vs. non-valid cues, suggesting that they were not caused by changes in neurovascular coupling or basal cerebral blood flow (CBF). These results support the hypothesis that nicotine enhances cognitive performance, in part, by suppressing DMN activity.
Nicotinic modulation of DMN activity has also been examined in the resting state by Tanabe et al. [67]. This study used a crossover design, in which 19 healthy nonsmoking subjects received either a 7 mg nicotine or placebo patch in two scanning sessions separated by approximately 1 week. Resting fMRI scans were acquired before and 90 m after patch application and analyzed using spatial Independent Components Analysis (ICA) followed by selection of the DMN component using an atlas-based mask [68]. The investigators found that compared to baseline (pre-patch), nicotine decreased DMN activity in the PCC, precuneus, and mPFC hubs. Significant drug (nicotine or placebo) X scanning session (pre- or post-patch) interactions were also observed, as nicotine, but not placebo, decreased activity in the precuneus, and nicotine reduced DMN activity in the PCC to a greater extent than placebo as well. These results remained significant when physiological noise was removed as an ICA component, suggesting that the effects were not due to changes in blood pressure or heart rate.
Based on these findings, Tregellas and colleagues [69] hypothesized that nicotinic agonists might also modulate the DMN in schizophrenia. The α7 receptor was a particularly attractive target due to its reduced expression and proposed role in neuronal hyperactivity in the illness. To test their hypothesis, Tregellas et al. examined DMN activity in 16 nonsmoking patients after 1 month each of (1) a 75 mg dose of the nicotinic α7 receptor partial agonist DMXB-A, (2) a 150 mg dose of DMXB-A, or (3) placebo. Patients were scanned after treatment while performing a smooth pursuit eye movement (SPEM) task. The DMN was extracted by group ICA followed by identification of the network with an a priori anatomical DMN mask [51]. The investigators found that relative to placebo, both doses of DMXB-A reduced DMN activity, specifically in the PCC, bilateral IPC, and mPFC hubs. No effect of dose was observed. Decreased PCC activity was correlated with decreased Brief Psychiatric Rating Scale total score (BPRS; a brief measure of general psychiatric symptoms) [70]. Interestingly, the magnitude of decreased PCC activity was dependent on genotype: patients who carried the minor allele (C/C or C/A based on an allelic model) on a SNP (rs3087454) located on the 5′ upstream regulatory region of the α7 receptor gene promoter were less responsive to DMXB-A than patients who carried the major allele (A/A). Although the rs3087454 SNP has no known function, it is located near other SNPs that reduce expression of α7 receptors, and along with these SNPs increases schizophrenia risk [21]. Patients with this allele may thus be less responsive to DMXB-A due to reduced expression of nicotinic α7 receptors. Overall, these findings suggest that α7 receptor activation may normalize DMN hyperactivity in schizophrenia and have clinical benefit.
Given the number of previous studies that have associated symptom severity with DMN dysfunction (reviewed by Ref. [44]), nicotinic modulation of the DMN is a promising area of research that is a prime candidate for further study. Experiments in the near future may include (1) examination of the effect of nicotinic drugs in smoking patients, in which receptor desensitization is a potential concern, (2) further exploration of nicotinic modulation during multiple cognitive states, including during high- and low-cognitive load, (3) investigation into how nicotinic modulation affects DMN connectivity (in addition to activity). An investigation into the latter question would be particularly informative given that no study has examined how nicotinic agents affect DMN connectivity either within the network or between the DMN and other networks in schizophrenia. Normalization of DMN abnormalities by DMXB-A or a similar nicotinic agent and an associated cognitive performance enhancement would support the measurement of the DMN as a clinically relevant indicator of brain function.
3. The salience network
3.1. Background and phenotype in schizophrenia
The brain is constantly bombarded by information. In order to process what is important, ignore what is irrelevant, and update its internal model of the external world – in other words, process saliency – it must have a way of shifting focus from self-monitoring to monitoring external information (and vice-versa) as needed. In this manner, a stimulus that is pleasurable, threatening, or important to a task is given precedence. The dynamics of this process must also be plastic, with the ability to change depending on preexisting expectations. A bite from an obviously rotten apple elicits a different brain response than a mouthful of freshly picked yet foul-tasting fruit, an event called an “expectancy violation” [71]. The remainder of that batch of fruit may then be thrown away as internal expectations are updated. A functionally and structurally interconnected set of cortical and subcortical areas called the salience network (SN) is ideally situated to perform all of the above processes.
The SN is anchored by the anterior cingulate cortex (ACC) and bilateral insula (Fig. 2). These hubs in turn are connected to limbic structures (e.g. hippocampus, amygdala) as well as to the PCC (part of the DMN) and DLPFC (part of the CEN). Inputs into the insula provide sensory and emotional information, including subjective feelings of taste, touch, hearing, vision, pain, temperature, and pleasure [72]. Inputs into the anterior cingulate from the DLPFC provide information regarding goals, expectations, and internal representations [49]. As a result, the SN effectively integrates external and internal information so that a course of action (or inaction) can be chosen. Indeed, one of the functions of the SN is to coordinate the “switch” between a task-positive state, where the CEN dominates, and a task-negative state, where the DMN dominates, depending on the result of integrative computations [49,73]. The integrative nature of SN processing may be why the network is hypothesized to be important for distinguishing “self” from “non-self” as well as establishing a strong sense of identity, purpose, and self-worth [72,74].
Fig. 2.
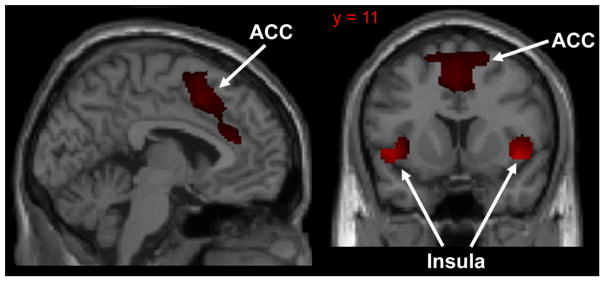
Primary hubs of the salience network: (1) anterior cingulate cortex (ACC), (2) bilateral insula. Network extracted using 20-component ICA from a sample of 36 healthy subjects.
The functions of the SN suggest that pathology of the network could be important for symptom etiology in schizophrenia. Hallucinations, for example, occur when patients have difficulty distinguishing external stimuli from internal thoughts. Given that separating self from non-self is an important function of the SN, dysfunction of the network may thus contribute to this positive symptom. Loss of affect and motivation may arise in part due to pathology of the network; accordingly, loss of GM volume in the insula is associated with severity of negative symptoms in schizophrenia [75]. Cognitive symptoms, including deficits in selective attention, may be partially explained by a relative inability of the SN to modulate activity in sensory areas according to perceptual expectations [76]. Patients may thus become overly reliant on sensory evidence to interpret their surroundings. As a result, the salience of external stimuli is inappropriately enhanced, leading to perceptual abnormalities and distractibility [71].
A wealth of structural and functional neuroimaging evidence suggests that the SN is indeed dysfunctional in schizophrenia. Loss of gray matter volume in the insula and ACC are among the most striking and consistently replicated structural brain abnormalities in the illness (reviewed by Ref. [72]). Gray matter loss of the insula and ACC is present at the first episode of psychosis, but also may be progressive in chronic schizophrenia [77,78]. Insula and ACC structural deficits have been linked to reality distortion, suggesting that SN dysfunction is associated with abnormal stimulus processing and positive symptoms [79]. Functionally, abnormalities in SN activation across a variety of paradigms have been observed in schizophrenia, including working memory [80], social and affective processing [81–83], and error processing [84,85]. Network analyses have found a loss of connectivity between nodes of the SN and other brain areas both at rest [86,87] and during tasks [88–90] in patients. This loss of connectivity has been proposed to underlie the relative inability of patients to adjust DMN activity according to task demands, resulting in performance deficits [49].
3.2. Nicotinic modulation of the SN
Like the DMN, immunohistochemical evidence suggests that the SN would be responsive to nicotinic modulation. The insula and ACC receive cholinergic input from the basal forebrain [91], and show enriched nicotinic receptor expression [7,63]. One study has suggested that, relative to nonsmoking and smoking controls, α7 receptor expression is reduced and α4β2 receptor is increased in the ACC in patient postmortem brain [92]. It is unclear, however, if these results were driven by smokers, as these investigators did not segregate the patient group by smoking history. In addition, nicotine administration increases basal CBF in the ACC and insula [93].
Using connectivity analyses, Hong and colleagues [87,94] have investigated how nicotine affects communication between hubs of the SN as well as with other networks during the resting state. The first of these studies [94] examined resting state activity in a cohort of 19 healthy smokers. The study used a double-blind, randomized crossover design to compare the effect of a nicotine patch and placebo patch. The nicotine dose was 21 mg for subjects who smoked 10–15 cigarettes/day and 35 mg for individuals who smoked more than 15 cigarettes/day. Subjects were given either the nicotine or placebo patch 2.5 h after their last cigarette and underwent a subsequent 2 h scan. Functional connectivity was analyzed between regions of interest (ROIs) drawn from ACC subregions, including the dorsal ACC (dACC), rostral ACC (rACC), and subcallosal ACC (sACC), as well as other cortical and subcortical brain areas. The investigators observed that nicotine withdrawal was negatively correlated with connectivity between the dACC and the striatum. Although nicotine did not affect connectivity of this circuit, the patch did increase connectivity between the left dACC and the contralateral superior parietal lobule and postcentral gyrus, the left sACC and bilateral medial frontal gyrus, and between the bilateral dACC and right PCC. These results suggest that nicotine can affect communication between the ACC and other regions, most notably areas associated with the DMN. Enhanced connectivity between these networks may facilitate the hypothesized ability of the SN to coordinate activity between the DMN and the CEN as needed.
Moran et al. [87] expanded upon these findings with a double-blind, crossover investigation of resting state functional connectivity in 20 smoking patients with schizophrenia and 24 smoking controls. These investigators placed seed ROIs in the primary nodes of the SN—the anterior insula, posterior insula, and dACC. Either a 21 mg nicotine, 35 mg nicotine, or placebo patch was administered; nicotine dose depended on nicotine dependence as in the Hong et al. [94] experiment. The study found that, across all subjects, the severity of nicotine withdrawal (i.e. during the placebo patch) was negatively correlated with connectivity between the posterior insula and left striatum, dACC, inferior frontal gyrus, and DLPFC. In addition, withdrawal was negatively correlated with connectivity between the left dACC and left anterior insula and bilateral putamen, as well as between the right dACC and bilateral putamen. Nicotine, however, increased connectivity between the dACC and somatosensory-parietal regions and between the left anterior insula and occipital cortex. Comparing the patient and control groups, patients had a greater increase in withdrawal symptoms on placebo. A main effect of group was also observed in connectivity between the right posterior insula and bilateral dACC and the left dACC and left anterior insula, indicating lower connectivity in patients. Connectivity-withdrawal correlations did not differ between groups. These results suggest that both nicotine withdrawal and a diagnosis of schizophrenia (independent of withdrawal) are associated with reduced connectivity within the SN as well as between the SN and other areas. The effect of nicotine on connectivity between the dACC and PCC in patients was not reported; ergo, it is unclear if nicotine increased connectivity between these regions as it did in healthy smokers in the previous study.
These two studies are important first steps that pave the way for future research into nicotinic modulation of the SN. Particularly intriguing in regards to potential therapeutic effects is the observed increase in connectivity between the SN and DMN after nicotine administration. The SN has been proposed to coordinate the relative level of activity of the DMN and the CEN, orchestrating a “switch” between the networks if incoming information is sufficiently salient to warrant a change from rest to task-state, or vice-versa [49,73]. The reduction in DMN and SN connectivity observed in patients thus may in part explain why the DMN is inappropriately engaged during cognitive tasks, resulting in impaired performance. A nicotinic drug that increases connectivity between these networks may help normalize this abnormality. Although such a potentially therapeutic effect was not reported with nicotine in patients, other nicotinic receptor targeting drugs, such as α7 agonists or allosteric modulators, are candidates for future study.
This review focused on two intrinsic networks, the DMN and SN, as previous investigations into the effects of nicotinic agents have thus far been restricted to these networks in schizophrenia. Other intrinsic networks, however, have been also been identified in the human brain [114]. Most notably, the central executive network (CEN), which is anchored by dorsolateral prefrontal cortex and posterior parietal cortex, shows functional abnormalities in schizophrenia patients [115–118]. This network is essential for working memory, problem solving, and goal-directed behavior [49]. Dysfunction of the network may be associated with positive and cognitive symptoms [49,117]. These findings suggest that pharmacologic targeting of the CEN may have clinical efficacy and should be a focus of future studies as well.
4. Complex network dynamics
4.1. Background and phenotype in schizophrenia
The brain is an extraordinarily efficient and yet fully integrated network. It is designed to exchange information across structurally and functionally disparate areas while minimizing “wiring” cost –i.e. connection length – and preserving local functional specialization. Characterizing these features across the entire brain network is the goal of complex network analysis, a technique that uses connectivity data from fMRI, electroencephalography (EEG), or magnetoencephalography (MEG) to analyze network dynamics using mathematical concepts from graph theory—i.e. the study of the dynamic, functional, and/or structural relationships between components of a group of objects. Complex network analysis provides a quantitative measure of the efficiency of global and local network processing that has previously eluded neuroimaging researchers.
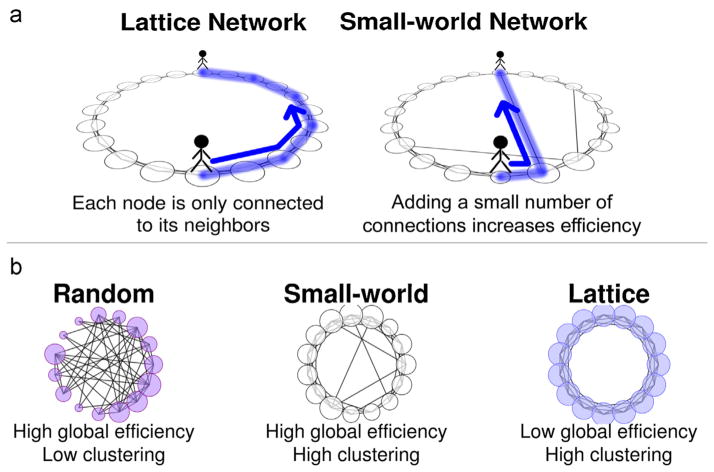
To understand the metrics of complex network analysis in regards to brain function, it may be helpful to draw upon an analogy (Fig. 3a). Imagine a group of 30,000 people standing in a large circle. Let each adjacent person be separated by 3 ft, resulting in a circle diameter of ~5.5 mile. Assume the only way a person can travel is by walking. Now draw a line (an edge) between any two people (or nodes) that are friends. Under these circumstances, it is far more likely that people who are close by are friends rather than people who are at opposite sides of the circle. It is much easier to turn and talk to a neighbor, or walk 6 ft, than to walk 5.5 mile across the circle. This phenomenon is called clustering, and is quantified in a value called the clustering coefficient—i.e. the extent that a person’s friends are also friends with each other. From a neuroscience perspective, clustering, and a related measure called local efficiency, describes the extent to which a brain region, e.g. primary visual cortex, communicates with its neighbors, e.g. accessory visual cortex, to locally process information.
Fig. 3.
(a and b) Network topology examples. Lattice networks, with only local connections, have low global efficiency, as shown by the lack of connections across the center of the graph, and high local efficiency, as shown by the high number of connections between nearby nodes. Random networks show the reverse pattern. Small-world networks are intermediate, with properties of both. Adapted from Wylie et al. [113].
Assuming that only friends talk to each other and that there are no long-distance friendships, communicating across the entirety of the network would be difficult and time-consuming. A message would have to be passed through 15,000 people before it reached the other side of the circle. Now, let’s imagine that occasionally a person is in need of information his neighbors cannot provide. Thus, he takes the 5.5 mile trek across the circle to meet someone new, and his footprints leave a path through which future communication might occur. Suddenly the situation has changed. Although the network is still highly clustered, the new connection has made it easier to communicate across the network. For the relatively low cost of one long-distance pathway, the average number of people it would require to deliver a message to any other person has dramatically decreased, as that new path could be used to cross the entire circle through only one connection. The global efficiency of information transfer has increased. Because the cost of this increase is relatively low (only one long walk), the network is now said to have increased its small world characteristics, or small-worldness. A small-world network is defined as having high global efficiency but low wiring cost—i.e. the total length of all paths.
The human brain has small-world properties in its functional and structural architecture. The majority of information is only processed locally—e.g. the visual cortex; however, when necessary, a small number of well-connected hubs – e.g. the posterior cingulate and inferior parietal cortex – are recruited to transfer information between distal sites. The extent to which a brain displays these characteristics is associated with cognitive function and intelligence [95–97]. Evidence also exists that the brain is able to flexibly change its configuration, increasing global efficiency during challenging tasks, and adopting a more clustered organization during easier tasks [98]. A network with increased global efficiency at the expense of low clustering and high average wiring cost, called a “random” network, or decreased average wiring cost and increased clustering at the expense of global efficiency, called a “lattice” network (akin to a network having no long-distance friendships) is considered less optimal (Fig. 3b).
Using complex network analysis, researchers have consistently observed abnormal network architectures in schizophrenia. In a resting state study using 31 schizophrenia patients and 31 matched controls, Liu et al. [99] observed a decrease in clustering coefficient, increased path length, and reduced local and global efficiency in patients. These effects were correlated with disease duration, suggesting they may progressively worsen over time. However, Rubinov et al. [100] also reported a decreased clustering coefficient and diminished small-worldness in first-episode patients. Remarkably, a study comparing human neonates at high genetic risk for schizophrenia with matched controls also observed reduced global efficiency and increased path length in the at-risk group [101]. Although these findings are preliminary, network abnormalities are a promising early stage biomarker for schizophrenia as well as a possible measure of disease progression.
In addition to functional connectivity, complex network dynamics can also be informed by structural tractography—i.e the visualization and mapping of axonal connections in the brain. For example, Wang et al. [102] used diffusion tensor tractography –a type of tractography that allows the investigator to determine the direction of information flow – to structurally and functionally map axon pathways in 79 schizophrenia patients and 96 controls. Similar to previous functionally informed findings, these researchers discovered reduced global efficiency and decreased clustering in frontal associative and limbic/paralimbic nodes in patients. The severity of these deficits was negatively correlated the severity of positive and negative symptoms.
Complex network dynamics can also be observed during performance of a task. Using an auditory oddball task, Yu et al. [103] analyzed connectivity of a temporal cortical network recruited during the task in patients with schizophrenia and healthy controls, and found that efficiency and small-worldness within the network was reduced in patients. Based on these and other findings, it is apparent that network abnormalities in schizophrenia are manifested during both task-related and task-free states.
4.2. Nicotinic modulation of brain network efficiency
Complex network dynamics have revealed persistent abnormalities in brain organization in schizophrenia during both resting state and task performance. This suggests that it may be a useful tool to measure disease-relevant neurobiological effects of pharmacologic treatment. Nicotinic agents may be particularly promising candidates to modulate network architecture based on the functional and structural characteristics of the cholinergic nervous system.
Cholinergic projections originating from the basal forebrain extend throughout numerous cortical and subcortical areas. As such, the release of acetylcholine can activate receptors located in both excitatory and inhibitory cell populations and can influence firing patterns on a global and local level [104]. From a network perspective, cost-efficient integration and local specialization requires local populations of cells to fire in synchrony across a range of frequencies, and then to communicate changes in local activity over long distances to other communities of neurons. Although the cholinergic system has many functions, one of its key roles is to facilitate synchronous firing of local neuronal populations [105–108]. This may be accomplished via activation of receptors on inhibitory interneurons [61], leading to the release of the inhibitory neurotransmitter gamma-aminobutyric acid (GABA) and subsequent hyperpolarization of excitatory cells. After hyperpolarization has decayed, these cells enter an excitable state that facilitates synchronous firing [109–111]. The simultaneous discharge of a large population of neurons may thus become sufficient to activate network hubs that enable long-distance communication within the brain—the so-called avalanche effect [112]. Thus, activation of the cholinergic system, including nicotinic receptors, may facilitate long-range information transfer using relatively few connections, thereby increasing efficiency and small-worldness.
To test this hypothesis, Wylie and colleagues [113] compared the effect of a low dose (7 mg patch) of nicotine to placebo on network topology in a cohort of 15 nonsmoking healthy adults, using a single-blind crossover design. Resting state data was acquired 90 m after patch application. Wylie et al. found that relative to placebo, nicotine significantly increased local efficiency and showed a trend toward increasing global efficiency. Examination of groups of nodes (i.e. prefrontal, sensorimotor, association, limbic/paralimbic, subcortical) revealed significantly increased efficiency of limbic/paralimbic areas (orbitofrontal cortex, amygdala, insula, olfactory cortex, anterior cingulate, temporal pole, hippocampus, parahippocampal gyrus). These results suggest that acute nicotine administration affects brain network topology and information transfer.
To our knowledge, at the time of writing this review, no study has examined the effect of nicotine on network topology in schizophrenia. Given previous findings that schizophrenia is associated with reduced global and local efficiency and that nicotine improves these measures in healthy subjects, it is possible to speculate that nicotine or other nicotinic drugs may have a similarly beneficial effect on these metrics in patients. Complex network analysis of nicotinic modulation of network dynamics in schizophrenia and its relationship to symptomatology is an area worthy of future research.
5. Conclusion
Ultimately, a complete understanding of the neurobiological effects of nicotinic drugs in schizophrenia and other disorders will require the incorporation of multivariate, network-level imaging techniques that can measure the function and complex dynamics of intrinsic brain networks. The examination of intrinsic networks goes beyond simple task-based mapping of brain function in that they are not specific to task conditions, are not confounded by performance and/or non-compliance with a task, and are likely more closely related to underlying brain pathology. The utility of these approaches is beginning to be realized, as researchers have identified robust effects of nicotinic agents on the default mode network and (to a lesser extent) the salience network. Along with the central executive network, for which the effects of nicotinic drugs have yet to be examined, these networks form a triumvirate of intrinsic networks that constitute a significant proportion of brain activity and may provide a framework by which to understand neurocognitive dysfunction across a multitude of disorders [49]. In addition, new research into topographical, complex network dynamics has revealed that schizophrenia is associated with reduced ability of the brain to efficiently process information both globally and locally. The effects of nicotinic modulation on network topology are just beginning to be understood and should remain a priority for future neuroimaging research.
Although intrinsic networks have been the focus of this review, a complete understanding of nicotinic modulation of the brain in schizophrenia requires additional task-based fMRI research to examine activity that is more directly relevant to cognitive processing. Indeed, only a handful of studies have examined the effects of nicotinic modulation on cognitive domains (e.g. attention) in schizophrenia (e.g. [119]), despite the potential of these drugs for the treatment of cognitive dysfunction in the illness (reviewed by Refs. [120–123]. The collaborative Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia (CNTRICS) initiative has outlined several tasks that may be important for understanding how nicotinic modulation affects cognitive processing in the disease [124].
In conclusion, given the tremendous surge in interest in network-based approaches to studying the brain in schizophrenia and the potentially therapeutic benefit of nicotinic modulation in the illness, it is surprising that a very limited number of studies have examined how intrinsic networks can be targeted by nicotinic drugs in schizophrenia. The early studies described here suggest that nicotinic agents may be an effective means to modify function of these networks. The therapeutic ramifications of these effects in clinical populations should increasingly be examined in the next decade.
Acknowledgments
The authors thank Korey Wylie, M.D. and Kristina McFadden, Ph.D. for assistance with manuscript preparation.
References
- 1.Fenton WS, McGlashan TH, Victor BJ, Blyler CR. Symptoms, subtype, and suicidality in patients with schizophrenia spectrum disorders. Am J Psychiatry. 1997;154(2):199–204. doi: 10.1176/ajp.154.2.199. [DOI] [PubMed] [Google Scholar]
- 2.Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153(3):321–30. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- 3.Iversen LL. Introduction to neuropsychopharmacology. New York: Oxford University Press; 2009. [Google Scholar]
- 4.Role LW. Diversity in primary structure and function of neuronal nicotinic acetylcholine receptor channels. Curr Opin Neurobiol. 1992;2(3):254–62. doi: 10.1016/0959-4388(92)90112-x. [DOI] [PubMed] [Google Scholar]
- 5.Wonnacott S, Barik J, Dickinson J, Jones IW. Nicotinic receptors modulate transmitter cross talk in the CNS: nicotinic modulation of transmitters. J Mol Neurosci. 2006;30(1–2):137–40. doi: 10.1385/JMN:30:1:137. [DOI] [PubMed] [Google Scholar]
- 6.Le Houezec J. Nicotine: abused substance and therapeutic agent. J Psychiatry Neurosci. 1998;23(2):95–108. [PMC free article] [PubMed] [Google Scholar]
- 7.Paterson D, Nordberg A. Neuronal nicotinic receptors in the human brain. Prog Neurobiol. 2000;61(1):75–111. doi: 10.1016/s0301-0082(99)00045-3. [DOI] [PubMed] [Google Scholar]
- 8.Picciotto MR, Higley MJ, Mineur YS. Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron. 2012;76(1):116–29. doi: 10.1016/j.neuron.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wallace TL, Porter RH. Targeting the nicotinic alpha7 acetylcholine receptor to enhance cognition in disease. Biochem Pharmacol. 2011;82(8):891–903. doi: 10.1016/j.bcp.2011.06.034. [DOI] [PubMed] [Google Scholar]
- 10.Deutsch SI, Urbano MR, Neumann SA, Burket JA, Katz E. Cholinergic abnormalities in autism: is there a rationale for selective nicotinic agonist interventions. Clin Neuropharmacol. 2010;33(3):114–20. doi: 10.1097/WNF.0b013e3181d6f7ad. [DOI] [PubMed] [Google Scholar]
- 11.Wilens TE, Decker MW. Neuronal nicotinic receptor agonists for the treatment of attention-deficit/hyperactivity disorder: focus on cognition. Biochem Pharmacol. 2007;74(8):1212–23. doi: 10.1016/j.bcp.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Philip NS, Carpenter LL, Tyrka AR, Price LH. The nicotinic acetylcholine receptor as a target for antidepressant drug development. Sci World J. 2012;2012:104105. doi: 10.1100/2012/104105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones CK, Byun N, Bubser M. Muscarinic and nicotinic acetylcholine receptor agonists and allosteric modulators for the treatment of schizophrenia. Neuropsychopharmacology. 2012;37(1):16–42. doi: 10.1038/npp.2011.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lynch G, Palmer LC, Gall CM. The likelihood of cognitive enhancement. Pharmacol Biochem Behav. 2011;99(2):116–29. doi: 10.1016/j.pbb.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dickerson F, Stallings CR, Origoni AE, Vaughan C, Khushalani S, Schroeder J, et al. Cigarette smoking among persons with schizophrenia or bipolar disorder in routine clinical settings, 1999–2011. Psychiatry Serv. 2013;64(1):44–50. doi: 10.1176/appi.ps.201200143. [DOI] [PubMed] [Google Scholar]
- 16.Winterer G. Why do patients with schizophrenia smoke? Curr Opin Psychiatry. 2010;23(2):112–9. doi: 10.1097/YCO.0b013e3283366643. [DOI] [PubMed] [Google Scholar]
- 17.Freedman R, Leonard S, Gault JM, Hopkins J, Cloninger CR, Kaufmann CA, et al. Linkage disequilibrium for schizophrenia at the chromosome 15q13–14 locus of the alpha7-nicotinic acetylcholine receptor subunit gene (CHRNA7) Am J Med Genet. 2001;105(1):20–2. [PubMed] [Google Scholar]
- 18.Gault J, Hopkins J, Berger R, Drebing C, Logel J, Walton C, et al. Comparison of polymorphisms in the alpha7 nicotinic receptor gene and its partial duplication in schizophrenic and control subjects. Am J Med Genet B Neuropsychiatry Genet. 2003;123B(1):39–49. doi: 10.1002/ajmg.b.20061. [DOI] [PubMed] [Google Scholar]
- 19.Leonard S, Freedman R. Genetics of chromosome 15q13–q14 in schizophrenia. Biol Psychiatry. 2006;60(2):115–22. doi: 10.1016/j.biopsych.2006.03.054. [DOI] [PubMed] [Google Scholar]
- 20.Sinkus ML, Lee MJ, Gault J, Logel J, Short M, Freedman R, et al. A 2-base pair deletion polymorphism in the partial duplication of the alpha7 nicotinic acetylcholine gene (CHRFAM7A) on chromosome 15q14 is associated with schizophrenia. Brain Res. 2009;1291:1–11. doi: 10.1016/j.brainres.2009.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stephens SH, Logel J, Barton A, Franks A, Schultz J, Short M, et al. Association of the 5′-upstream regulatory region of the alpha7 nicotinic acetylcholine receptor subunit gene (CHRNA7) with schizophrenia. Schizophr Res. 2009;109(1–3):102–12. doi: 10.1016/j.schres.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levin ED, Wilson W, Rose JE, McEvoy J. Nicotine-haloperidol interactions and cognitive performance in schizophrenics. Neuropsychopharmacology. 1996;15(5):429–36. doi: 10.1016/S0893-133X(96)00018-8. [DOI] [PubMed] [Google Scholar]
- 23.Smith RC, Singh A, Infante M, Khandat A, Kloos A. Effects of cigarette smoking and nicotine nasal spray on psychiatric symptoms and cognition in schizophrenia. Neuropsychopharmacology. 2002;27(3):479–97. doi: 10.1016/S0893-133X(02)00324-X. [DOI] [PubMed] [Google Scholar]
- 24.Smith RC, Warner-Cohen J, Matute M, Butler E, Kelly E, Vaidhyanathaswamy S, et al. Effects of nicotine nasal spray on cognitive function in schizophrenia. Neuropsychopharmacology. 2006;31(3):637–43. doi: 10.1038/sj.npp.1300881. [DOI] [PubMed] [Google Scholar]
- 25.Adler LE, Hoffer LJ, Griffith J, Waldo MC, Freedman R. Normalization by nicotine of deficient auditory sensory gating in the relatives of schizophrenics. Biol Psychiatry. 1992;32(7):607–16. doi: 10.1016/0006-3223(92)90073-9. [DOI] [PubMed] [Google Scholar]
- 26.Lester RA. Activation and desensitization of heteromeric neuronal nicotinic receptors: implications for non-synaptic transmission. Bioorg Med Chem Lett. 2004;14(8):1897–900. doi: 10.1016/j.bmcl.2004.02.081. [DOI] [PubMed] [Google Scholar]
- 27.Quick MW, Lester RA. Desensitization of neuronal nicotinic receptors. J Neurobiol. 2002;53(4):457–78. doi: 10.1002/neu.10109. [DOI] [PubMed] [Google Scholar]
- 28.Laviolette SR, van der Kooy D. The neurobiology of nicotine addiction: bridging the gap from molecules to behaviour. Nat Rev Neurosci. 2004;5(1):55–65. doi: 10.1038/nrn1298. [DOI] [PubMed] [Google Scholar]
- 29.Office of the Surgeon General. How tobacco smoke causes disease: the biology and behavioral basis for smoking-attributable disease, a report of the surgeon general. Atlanta: Centers for Disease Control and Prevention, Office on Smoking and Health; 2010. [cited 2013 February 21] [PubMed] [Google Scholar]
- 30.Olincy A, Freedman R. Nicotinic mechanisms in the treatment of psychotic disorders: a focus on the alpha7 nicotinic receptor. Handb Exp Pharmacol. 2012;213:211–32. doi: 10.1007/978-3-642-25758-2_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hong LE, Thaker GK, McMahon RP, Summerfelt A, Rachbeisel J, Fuller RL, et al. Effects of moderate-dose treatment with varenicline on neurobiological and cognitive biomarkers in smokers and nonsmokers with schizophrenia or schizoaffective disorder. Arch Gen Psychiatry. 2011;68(12):1195–206. doi: 10.1001/archgenpsychiatry.2011.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams DK, Wang J, Papke RL. Positive allosteric modulators as an approach to nicotinic acetylcholine receptor-targeted therapeutics: advantages and limitations. Biochem Pharmacol. 2011;82(8):915–30. doi: 10.1016/j.bcp.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grupe M, Jensen AA, Ahring PK, Christensen JK, Grunnet M. Unraveling the mechanism of action of NS9283, a positive allosteric modulator of (alpha4)(3) (beta2)(2) nicotinic acetylcholine receptors. Br J Pharmacol. 2012 doi: 10.1111/bph.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olincy A, Harris JG, Johnson LL, Pender V, Kongs S, Allensworth D, et al. Proof-of-concept trial of an alpha7 nicotinic agonist in schizophrenia. Arch Gen Psychiatry. 2006;63(6):630–8. doi: 10.1001/archpsyc.63.6.630. [DOI] [PubMed] [Google Scholar]
- 35.Freedman R, Olincy A, Buchanan RW, Harris JG, Gold JM, Johnson L, et al. Initial phase 2 trial of a nicotinic agonist in schizophrenia. Am J Psychiatry. 2008;165(8):1040–7. doi: 10.1176/appi.ajp.2008.07071135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lieberman JA, Dunbar G, Segreti AC, Girgis RR, Seoane F, Beaver JS, et al. A randomized exploratory trial of an alpha-7 nicotinic receptor agonist (TC-5619) for cognitive enhancement in schizophrenia. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2012.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown GG, Thompson WK. Functional brain imaging in schizophrenia: selected results and methods. Curr Top Behav Neurosci. 2010;4:181–214. doi: 10.1007/7854_2010_54. [DOI] [PubMed] [Google Scholar]
- 38.Gur RE, Gur RC. Functional magnetic resonance imaging in schizophrenia. Dialogues Clin Neurosci. 2010;12(3):333–43. doi: 10.31887/DCNS.2010.12.3/rgur. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8(9):700–11. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 40.Raichle ME, Mintun MA. Brain work and brain imaging. Annu Rev Neurosci. 2006;29:449–76. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- 41.Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. PNAS. 2009;106(4):1279–84. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. PNAS. 2003;100(1):253–8. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann NY Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 44.Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- 45.Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, et al. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447(7140):83–6. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- 46.Fukunaga M, Horovitz SG, van Gelderen P, de Zwart JA, Jansma JM, Ikonomidou VN, et al. Large-amplitude, spatially correlated fluctuations in BOLD fMRI signals during extended rest and early sleep stages. Magn Reson Imaging. 2006;24(8):979–92. doi: 10.1016/j.mri.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 47.Horovitz SG, Fukunaga M, de Zwart JA, van Gelderen P, Fulton SC, Balkin TJ, et al. Low frequency BOLD fluctuations during resting wakefulness and light sleep: a simultaneous EEG-fMRI study. Hum Brain Mapp. 2008;29(6):671–82. doi: 10.1002/hbm.20428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Picchioni D, Fukunaga M, Carr WS, Braun AR, Balkin TJ, Duyn JH, et al. fMRI differences between early and late stage −1 sleep. Neurosci Lett. 2008;441(1):81–5. doi: 10.1016/j.neulet.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15(10):483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 50.Gordon EM, Stollstorff M, Vaidya CJ. Using spatial multiple regression to identify intrinsic connectivity networks involved in working memory performance. Hum Brain Mapp. 2012;33(7):1536–52. doi: 10.1002/hbm.21306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant default mode functional connectivity in schizophrenia. Am J Psychiatry. 2007;164(3):450–7. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- 52.Meyer-Lindenberg AS, Olsen RK, Kohn PD, Brown T, Egan MF, Weinberger DR, et al. Regionally specific disturbance of dorsolateral prefrontal–hippocampal functional connectivity in schizophrenia. Arch Gen Psychiatry. 2005;62(4):379–86. doi: 10.1001/archpsyc.62.4.379. [DOI] [PubMed] [Google Scholar]
- 53.Pomarol-Clotet E, Salvador R, Sarro S, Gomar J, Vila F, Martinez A, et al. Failure to deactivate in the prefrontal cortex in schizophrenia: dysfunction of the default mode network. Psychol Med. 2008;38(8):1185–93. doi: 10.1017/S0033291708003565. [DOI] [PubMed] [Google Scholar]
- 54.Jeong B, Kubicki M. Reduced task-related suppression during semantic repetition priming in schizophrenia. Psychiatry Res. 2010;181(2):114–20. doi: 10.1016/j.pscychresns.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jafri MJ, Pearlson GD, Stevens M, Calhoun VD. A method for functional network connectivity among spatially independent resting-state components in schizophrenia. Neuroimage. 2008;39(4):1666–81. doi: 10.1016/j.neuroimage.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu H, Kaneko Y, Ouyang X, Li L, Hao Y, Chen EY, et al. Schizophrenic patients and their unaffected siblings share increased resting-state connectivity in the task-negative network but not its anticorrelated task-positive network. Schizophr Bull. 2012;38(2):285–94. doi: 10.1093/schbul/sbq074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Skudlarski P, Jagannathan K, Anderson K, Stevens MC, Calhoun VD, Skudlarska BA, et al. Brain connectivity is not only lower but different in schizophrenia: a combined anatomical and functional approach. Biol Psychiatry. 2010;68(1):61–9. doi: 10.1016/j.biopsych.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liang M, Zhou Y, Jiang T, Liu Z, Tian L, Liu H, et al. Widespread functional disconnectivity in schizophrenia with resting-state functional magnetic resonance imaging. Neuroreport. 2006;17(2):209–13. doi: 10.1097/01.wnr.0000198434.06518.b8. [DOI] [PubMed] [Google Scholar]
- 59.Bluhm RL, Miller J, Lanius RA, Osuch EA, Boksman K, Neufeld RW, et al. Spontaneous low-frequency fluctuations in the BOLD signal in schizophrenic patients: anomalies in the default network. Schizophr Bull. 2007;33(4):1004–12. doi: 10.1093/schbul/sbm052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Holt DJ, Cassidy BS, Andrews-Hanna JR, Lee SM, Coombs G, Goff DC, et al. An anterior-to-posterior shift in midline cortical activity in schizophrenia during self-reflection. Biol Psychiatry. 2011;69(5):415–23. doi: 10.1016/j.biopsych.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Frazier CJ, Rollins YD, Breese CR, Leonard S, Freedman R, Dunwiddie TV. Acetylcholine activates an alpha-bungarotoxin-sensitive nicotinic current in rat hippocampal interneurons, but not pyramidal cells. J Neurosci. 1998;18(4):1187–95. doi: 10.1523/JNEUROSCI.18-04-01187.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miwa JM, Freedman R, Lester HA. Neural systems governed by nicotinic acetylcholine receptors: emerging hypotheses. Neuron. 2011;70(1):20–33. doi: 10.1016/j.neuron.2011.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Breese CR, Adams C, Logel J, Drebing C, Rollins Y, Barnhart M, et al. Comparison of the regional expression of nicotinic acetylcholine receptor alpha7 mRNA and [125I]-alpha-bungarotoxin binding in human postmortem brain. J Comp Neurol. 1997;387(3):385–98. doi: 10.1002/(sici)1096-9861(19971027)387:3<385::aid-cne5>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 64.Breese CR, Lee MJ, Adams CE, Sullivan B, Logel J, Gillen KM, et al. Abnormal regulation of high affinity nicotinic receptors in subjects with schizophrenia. Neuropsychopharmacology. 2000;23(4):351–64. doi: 10.1016/S0893-133X(00)00121-4. [DOI] [PubMed] [Google Scholar]
- 65.Freedman R, Hall M, Adler LE, Leonard S. Evidence in postmortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Biol Psychiatry. 1995;38(1):22–33. doi: 10.1016/0006-3223(94)00252-X. [DOI] [PubMed] [Google Scholar]
- 66.Hahn B, Ross TJ, Yang Y, Kim I, Huestis MA, Stein EA. Nicotine enhances visuospatial attention by deactivating areas of the resting brain default network. J Neurosci. 2007;27(13):3477–89. doi: 10.1523/JNEUROSCI.5129-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tanabe J, Nyberg E, Martin LF, Martin J, Cordes D, Kronberg E, et al. Nicotine effects on default mode network during resting state. Psychopharmacology (Berl) 2011;216(2):287–95. doi: 10.1007/s00213-011-2221-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Correa N, Adali T, Calhoun VD. Performance of blind source separation algorithms for fMRI analysis using a group ICA method. Magn Reson Imaging. 2007;25(5):684–94. doi: 10.1016/j.mri.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tregellas JR, Tanabe J, Rojas DC, Shatti S, Olincy A, Johnson L, et al. Effects of an alpha 7-nicotinic agonist on default network activity in schizophrenia. Biol Psychiatry. 2011;69(1):7–11. doi: 10.1016/j.biopsych.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mortimer AM. Symptom rating scales and outcome in schizophrenia. Br J Psychiatry Suppl. 2007;50:s7–14. doi: 10.1192/bjp.191.50.s7. [DOI] [PubMed] [Google Scholar]
- 71.Palaniyappan L, White TP, Liddle PF. The concept of salience network dysfunction in schizophrenia: from neuroimaging observations to therapeutic opportunities. Curr Top Med Chem. 2012 doi: 10.2174/156802612805289881. [DOI] [PubMed] [Google Scholar]
- 72.Wylie KP, Tregellas JR. The role of the insula in schizophrenia. Schizophr Res. 2010;123(2–3):93–104. doi: 10.1016/j.schres.2010.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214(5–6):655–67. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Palaniyappan L, Liddle PF. Does the salience network play a cardinal role in psychosis? An emerging hypothesis of insular dysfunction. J Psychiatry Neurosci. 2012;37(1):17–27. doi: 10.1503/jpn.100176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Koutsouleris N, Gaser C, Jager M, Bottlender R, Frodl T, Holzinger S, et al. Structural correlates of psychopathological symptom dimensions in schizophrenia: a voxel-based morphometric study. Neuroimage. 2008;39(4):1600–12. doi: 10.1016/j.neuroimage.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 76.Chambon V, Pacherie E, Barbalat G, Jacquet P, Franck N, Farrer C. Mentalizing under influence: abnormal dependence on prior expectations in patients with schizophrenia. Brain. 2011;134(Pt 12):3728–41. doi: 10.1093/brain/awr306. [DOI] [PubMed] [Google Scholar]
- 77.Chan RC, Di X, McAlonan GM, Gong QY. Brain anatomical abnormalities in high-risk individuals, first-episode, and chronic schizophrenia: an activation likelihood estimation meta-analysis of illness progression. Schizophr Bull. 2011;37(1):177–88. doi: 10.1093/schbul/sbp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ellison-Wright I, Glahn DC, Laird AR, Thelen SM, Bullmore E. The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. Am J Psychiatry. 2008;165(8):1015–23. doi: 10.1176/appi.ajp.2008.07101562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Palaniyappan L, Mallikarjun P, Joseph V, White TP, Liddle PF. Reality distortion is related to the structure of the salience network in schizophrenia. Psychol Med. 2011;41(8):1701–8. doi: 10.1017/S0033291710002205. [DOI] [PubMed] [Google Scholar]
- 80.Repovs G, Barch DM. Working memory related brain network connectivity in individuals with schizophrenia and their siblings. Front Hum Neurosci. 2012;6:137. doi: 10.3389/fnhum.2012.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mitchell RL, Elliott R, Barry M, Cruttenden A, Woodruff PW. Neural response to emotional prosody in schizophrenia and in bipolar affective disorder. Br J Psychiatry. 2004;184:223–30. doi: 10.1192/bjp.184.3.223. [DOI] [PubMed] [Google Scholar]
- 82.Phillips ML, Williams L, Senior C, Bullmore ET, Brammer MJ, Andrew C, et al. A differential neural response to threatening and non-threatening negative facial expressions in paranoid and non-paranoid schizophrenics. Psychiatry Res. 1999;92(1):11–31. doi: 10.1016/s0925-4927(99)00031-1. [DOI] [PubMed] [Google Scholar]
- 83.Seiferth NY, Pauly K, Kellermann T, Shah NJ, Ott G, Herpertz-Dahlmann B, et al. Neuronal correlates of facial emotion discrimination in early onset schizophrenia. Neuropsychopharmacology. 2009;34(2):477–87. doi: 10.1038/npp.2008.93. [DOI] [PubMed] [Google Scholar]
- 84.Laurens KR, Ngan ET, Bates AT, Kiehl KA, Liddle PF. Rostral anterior cingulate cortex dysfunction during error processing in schizophrenia. Brain. 2003;126 (Pt 3):610–22. doi: 10.1093/brain/awg056. [DOI] [PubMed] [Google Scholar]
- 85.Polli FE, Barton JJ, Thakkar KN, Greve DN, Goff DC, Rauch SL, et al. Reduced error-related activation in two anterior cingulate circuits is related to impaired performance in schizophrenia. Brain. 2008;131(Pt 4):971–86. doi: 10.1093/brain/awm307. [DOI] [PubMed] [Google Scholar]
- 86.Tu PC, Hsieh JC, Li CT, Bai YM, Su TP. Cortico-striatal disconnection within the cingulo-opercular network in schizophrenia revealed by intrinsic functional connectivity analysis: a resting fMRI study. Neuroimage. 2012;59(1):238–47. doi: 10.1016/j.neuroimage.2011.07.086. [DOI] [PubMed] [Google Scholar]
- 87.Moran LV, Sampath H, Stein EA, Hong LE. Insular and anterior cingulate circuits in smokers with schizophrenia. Schizophr Res. 2012;142(1–3):223–9. doi: 10.1016/j.schres.2012.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gradin VB, Waiter G, O’Connor A, Romaniuk L, Stickle C, Matthews K, et al. Salience network-midbrain dysconnectivity and blunted reward signals in schizophrenia. Psychiatry Res. 2012 doi: 10.1016/j.pscychresns.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 89.Tu P, Buckner RL, Zollei L, Dyckman KA, Goff DC, Manoach DS. Reduced functional connectivity in a right-hemisphere network for volitional ocular motor control in schizophrenia. Brain. 2010;133(Pt 2):625–37. doi: 10.1093/brain/awp317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.White TP, Joseph V, Francis ST, Liddle PF. Aberrant salience network (bilateral insula and anterior cingulate cortex) connectivity during information processing in schizophrenia. Schizophr Res. 2010;123(2–3):105–15. doi: 10.1016/j.schres.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 91.Selden NR, Gitelman DR, Salamon-Murayama N, Parrish TB, Mesulam MM. Trajectories of cholinergic pathways within the cerebral hemispheres of the human brain. Brain. 1998;121(Pt 12):2249–57. doi: 10.1093/brain/121.12.2249. [DOI] [PubMed] [Google Scholar]
- 92.Marutle A, Zhang X, Court J, Piggott M, Johnson M, Perry R, et al. Laminar distribution of nicotinic receptor subtypes in cortical regions in schizophrenia. J Chem Neuroanat. 2001;22(1–2):115–26. doi: 10.1016/s0891-0618(01)00117-x. [DOI] [PubMed] [Google Scholar]
- 93.Stein EA, Pankiewicz J, Harsch HH, Cho JK, Fuller SA, Hoffmann RG, et al. Nicotine-induced limbic cortical activation in the human brain: a functional MRI study. Am J Psychiatry. 1998;155(8):1009–15. doi: 10.1176/ajp.155.8.1009. [DOI] [PubMed] [Google Scholar]
- 94.Hong LE, Gu H, Yang Y, Ross TJ, Salmeron BJ, Buchholz B, et al. Association of nicotine addiction and nicotine’s actions with separate cingulate cortex functional circuits. Arch Gen Psychiatry. 2009;66(4):431–41. doi: 10.1001/archgenpsychiatry.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Langer N, Pedroni A, Gianotti LR, Hanggi J, Knoch D, Jancke L. Functional brain network efficiency predicts intelligence. Hum Brain Mapp. 2012;33(6):1393–406. doi: 10.1002/hbm.21297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.van den Heuvel MP, Stam CJ, Kahn RS, Hulshoff Pol HE. Efficiency of functional brain networks and intellectual performance. J Neurosci. 2009;29(23):7619–24. doi: 10.1523/JNEUROSCI.1443-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wen W, Zhu W, He Y, Kochan NA, Reppermund S, Slavin MJ, et al. Discrete neuroanatomical networks are associated with specific cognitive abilities in old age. J Neurosci. 2011;31(4):1204–12. doi: 10.1523/JNEUROSCI.4085-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kitzbichler MG, Henson RN, Smith ML, Nathan PJ, Bullmore ET. Cognitive effort drives workspace configuration of human brain functional networks. J Neurosci. 2011;31(22):8259–70. doi: 10.1523/JNEUROSCI.0440-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu Y, Liang M, Zhou Y, He Y, Hao Y, Song M, et al. Disrupted small-world networks in schizophrenia. Brain. 2008;131(Pt 4):945–61. doi: 10.1093/brain/awn018. [DOI] [PubMed] [Google Scholar]
- 100.Rubinov M, Knock SA, Stam CJ, Micheloyannis S, Harris AW, Williams LM, et al. Small-world properties of nonlinear brain activity in schizophrenia. Hum Brain Mapp. 2009;30(2):403–16. doi: 10.1002/hbm.20517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shi F, Yap PT, Gao W, Lin W, Gilmore JH, Shen D. Altered structural connectivity in neonates at genetic risk for schizophrenia: a combined study using morphological and white matter networks. Neuroimage. 2012;62(3):1622–33. doi: 10.1016/j.neuroimage.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang Q, Su TP, Zhou Y, Chou KH, Chen IY, Jiang T, et al. Anatomical insights into disrupted small-world networks in schizophrenia. Neuroimage. 2012;59(2):1085–93. doi: 10.1016/j.neuroimage.2011.09.035. [DOI] [PubMed] [Google Scholar]
- 103.Yu Q, Sui J, Rachakonda S, He H, Pearlson G, Calhoun VD. Altered small-world brain networks in temporal lobe in patients with schizophrenia performing an auditory oddball task. Front Syst Neurosci. 2011;5:7. doi: 10.3389/fnsys.2011.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mesulam MM, Geula C. Nucleus basalis (Ch4) and cortical cholinergic innervation in the human brain: observations based on the distribution of acetyl-cholinesterase and choline acetyltransferase. J Comp Neurol. 1988;275(2):216–40. doi: 10.1002/cne.902750205. [DOI] [PubMed] [Google Scholar]
- 105.Lee MG, Chrobak JJ, Sik A, Wiley RG, Buzsaki G. Hippocampal theta activity following selective lesion of the septal cholinergic system. Neuroscience. 1994;62(4):1033–47. doi: 10.1016/0306-4522(94)90341-7. [DOI] [PubMed] [Google Scholar]
- 106.Buhl EH, Tamas G, Fisahn A. Cholinergic activation and tonic excitation induce persistent gamma oscillations in mouse somatosensory cortex in vitro. J Physiol. 1998;513(Pt 1):117–26. doi: 10.1111/j.1469-7793.1998.117by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fellous JM, Sejnowski TJ. Cholinergic induction of oscillations in the hippocampal slice in the slow (0. 5–2 Hz), theta (5–12 Hz), and gamma (35–70 Hz) bands. Hippocampus. 2000;10(2):187–97. doi: 10.1002/(SICI)1098-1063(2000)10:2<187::AID-HIPO8>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 108.Siok CJ, Rogers JA, Kocsis B, Hajos M. Activation of alpha7 acetylcholine receptors augments stimulation-induced hippocampal theta oscillation. Eur J Neurosci. 2006;23(2):570–4. doi: 10.1111/j.1460-9568.2005.04560.x. [DOI] [PubMed] [Google Scholar]
- 109.Penttonen M, Kamondi A, Acsady L, Buzsaki G. Gamma frequency oscillation in the hippocampus of the rat: intracellular analysis in vivo. Eur J Neurosci. 1998;10(2):718–28. doi: 10.1046/j.1460-9568.1998.00096.x. [DOI] [PubMed] [Google Scholar]
- 110.Ford JM, Mathalon DH. Neural synchrony in schizophrenia. Schizophr Bull. 2008;34(5):904–6. doi: 10.1093/schbul/sbn090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11(2):100–13. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- 112.Pasquale V, Massobrio P, Bologna LL, Chiappalone M, Martinoia S. Self-organization and neuronal avalanches in networks of dissociated cortical neurons. Neuroscience. 2008;153(4):1354–69. doi: 10.1016/j.neuroscience.2008.03.050. [DOI] [PubMed] [Google Scholar]
- 113.Wylie KP, Rojas DC, Tanabe J, Martin LF, Tregellas JR. Nicotine increases brain functional network efficiency. Neuroimage. 2012;63(1):73–80. doi: 10.1016/j.neuroimage.2012.06.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, et al. Correspondence of the brain’s functional architecture during activation and rest. PNAS. 2009;106(31):13040–45. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Woodward ND, Rogers B, Heckers S. Functional resting-state networks are differentially affected in schizophrenia. Schizophr Res. 2011;130(1–3):86–93. doi: 10.1016/j.schres.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wolf ND, Sambataro F, Vasic N, Frasch K, Schmid M, Schonfeldt-Lecuona C, et al. Dysconnectivity of multiple resting-state networks in patients with schizophrenia who have persistent auditory verbal hallucinations. J Psychiatry Neurosci. 2011;36(6):366–74. doi: 10.1503/jpn.110008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Manoliu A, Riedl V, Zherdin A, Muhlau M, Schwerthoffer D, Scherr M, et al. Aberrant dependence of default mode/central executive network interactions on anterior insular salience network activity in schizophrenia. Schizophr Bull. 2013 doi: 10.1093/schbul/sbt037. http://dx.doi.org/10.1093/schbul/sbt037. [DOI] [PMC free article] [PubMed]
- 118.Rotarska-Jagiela A, van de Ven V, Oertel-Knochel V, Uhlhaas PJ, Vogeley K, Linden DE. Resting-state functional network correlates of psychotic symptoms in schizophrenia. Schizophr Res. 2010;117(1):21–30. doi: 10.1016/j.schres.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 119.Hong LE, Schroeder M, Ross TJ, Buchholz B, Salmeron BJ, Wonodi I, et al. Nicotine enhances but does not normalize visual sustained attention and the associated brain network in schizophrenia. Schizophr Bull. 2011;37(2):416–25. doi: 10.1093/schbul/sbp089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wallace TL, Ballard TM, Pouzet B, Riedel WJ, Wettstein JG. Drug targets for cognitive enhancement in neuropsychiatric disorders. Pharmacol Biochem Behav. 2011;99(2):130–45. doi: 10.1016/j.pbb.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 121.Kucinski AJ, Stachowiak MK, Wersinger SR, Lippiello PM, Bencherif M. Alpha7 neuronal nicotinic receptors as targets for novel therapies to treat multiple domains of schizophrenia. Curr Pharm Biotechnol. 2011;12(3):437–48. doi: 10.2174/138920111794480589. [DOI] [PubMed] [Google Scholar]
- 122.Radek RJ, Kohlhaas KL, Rueter LE, Mohler EG. Treating the cognitive deficits of schizophrenia with alpha4beta2 neuronal nicotinic receptor agonists. Curr Pharm Des. 2010;16(3):309–22. doi: 10.2174/138161210790170166. [DOI] [PubMed] [Google Scholar]
- 123.AhnAllen CG. The role of the alpha7 nicotinic receptor in cognitive processing of persons with schizophrenia. Curr Opin Psychiatry. 2012;25(2):103–8. doi: 10.1097/YCO.0b013e3283503637. [DOI] [PubMed] [Google Scholar]
- 124.Carter CS, Barch DM, Bullmore E, Breiling J, Buchanan RW, Butler P, et al. Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia II: developing imaging biomarkers to enhance treatment development for schizophrenia and related disorders. Biol Psychiatry. 2011;70(1):7–12. doi: 10.1016/j.biopsych.2011.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]