Summary
While it is clear that a single hematopoietic stem cell (HSC) is capable of giving rise to all other hematopoietic cell types, the differentiation paths beyond HSC remain controversial. Contradictory reports on the lineage potential of progenitor populations have questioned the physiological contribution of progenitor populations to multilineage differentiation. Here, we established a lineage tracing mouse model that enabled direct assessment of differentiation pathways in vivo. We provide definitive evidence that differentiation into all hematopoietic lineages, including megakaryocyte/erythroid cell types, involves Flk2-expressing non-self-renewing progenitors. A Flk2-positive stage was used during steady-state hematopoiesis, after irradiation-induced stress and upon HSC transplantation. In contrast, HSC origin and maintenance do not include a Flk2-positive stage. These data demonstrate that HSC specification and maintenance are Flk2-independent, and that hematopoietic lineage separation occurs downstream of Flk2 upregulation.
Introduction
A core goal of stem cell biology is to understand how multipotent cells commit to specific cell fates. Comprehensive knowledge of differentiation pathways and the molecular regulation of fate decisions is necessary to efficiently guide uncommitted cells into specific fates; such directed differentiation serves as the basis for ESC differentiation into cell types of therapeutic value. Differentiation pathways must also be understood to enable rational drug design. Indeed, the concept of therapeutic targeting of cancer stem cells relies on understanding the cellular events underlying oncogenesis and distinguishing those from normal cell differentiation.
The well-studied hematopoietic system is at the forefront of lineage mapping during both normal and abnormal cell development. HSC have been isolated to high purity and single-cell transplantation has demonstrated that clonal HSC are capable of life-long reconstitution of all blood cell types (Ema et al., 2005; Kiel et al., 2005; Morita et al., 2010; Osawa et al., 1996; Wagers et al., 2002); reviewed in (Hock, 2010). Although the concept of hierarchical hematopoietic differentiation from HSC into lineage-restricted progenitor cells (Akashi et al., 2000; Kondo et al., 1997; Nakorn et al., 2003) is widely accepted, several recent reports have questioned the accuracy of schematic lineage maps (e.g., Figure 1A) (reviewed in (Akashi, 2009; Akashi et al., 2005; Hock and Orkin, 2005; Luc et al., 2008b)). Recent questions have focused on when the initial lineage decision is made, which lineage (or lineages) is specified or lost first, and whether differentiation follows linear, obligatory paths, or whether there are alternative pathways to a specific fate. In particular, pathways of megakaryocyte and erythroid (MegE) development from HSC are controversial (Adolfsson et al., 2005; Forsberg et al., 2006; Lai and Kondo, 2006; Lai et al., 2005; Nutt et al., 2005). Upregulation of Flk2 (Flt3), a tyrosine kinase receptor differentially expressed on functionally distinct hematopoietic subpopulations (Adolfsson et al., 2001; Christensen and Weissman, 2001; D'Amico and Wu, 2003; Karsunky et al., 2008; Karsunky et al., 2003) (Figure 1A), marks loss of self-renewal potential of hematopoietic cells; thus, HSC reside within the Flk2-negative c-kit+Lin-Sca1+ (KLS) fraction of adult bone marrow (BM) (Adolfsson et al., 2001; Christensen and Weissman, 2001). Flk2 itself and Flk2-positive lymphoid-committed populations have been shown to promote lymphoid development as genetic deletion of Flk2 leads to decreased numbers of B lineage cells (Mackarehtschian et al., 1995). In contrast, myeloid-committed progenitors do not express Flk2, and Flk2-deficient mice have normal numbers of mature myeloid cells. Flk2 expression on KLS cells has also been reported to correlate with loss of MegE potential and prime uncommitted cells for a lymphoid fate (Adolfsson et al., 2005). Extensive functional and molecular characterization of Flk2-positive KLS cells have demonstrated low MegE readout in vitro and increased expression of transcripts associated with lymphoid development, with retention of robust GM potential (Adolfsson et al., 2005; Luc et al., 2008a; Sitnicka et al., 2007). However, at least some cells within the Flk2-positive KLS fraction retain MegE potential in vivo (Forsberg et al., 2006; Lai and Kondo, 2006; Luc et al., 2008a). Quantitative assessment of the lineage potential of multiple cell populations in parallel showed that MegE contribution from Flk2-positive multipotent progenitors (MPPF) was more robust than that from progenitor populations with undisputed MegE potential (Forsberg et al., 2006). These seemingly contradictory findings raise the possibility that MPPF are capable of giving rise to MegE cells under conditions of acute need, but are normally dedicated to provide lymphoid cells. Transplantation assays, in combination with molecular characterization, have been unable to provide conclusive evidence for this model. Since no reports have ascertained the relative contribution of possible alternative pathways, the physiological relevance of different progenitor populations in development of distinct lineages remains uncertain (Figure 1A). Here, we have established a lineage tracing model that enabled us to determine the contribution of non-self-renewing multipotent progenitor cells, marked by Flk2 expression, to the distinct hematopoietic lineages during both steady-state and stress hematopoiesis in vivo.
Figure 1. Hematopoietic stem cell progeny switch from Tomato to GFP expression in FlkSwitch mice.
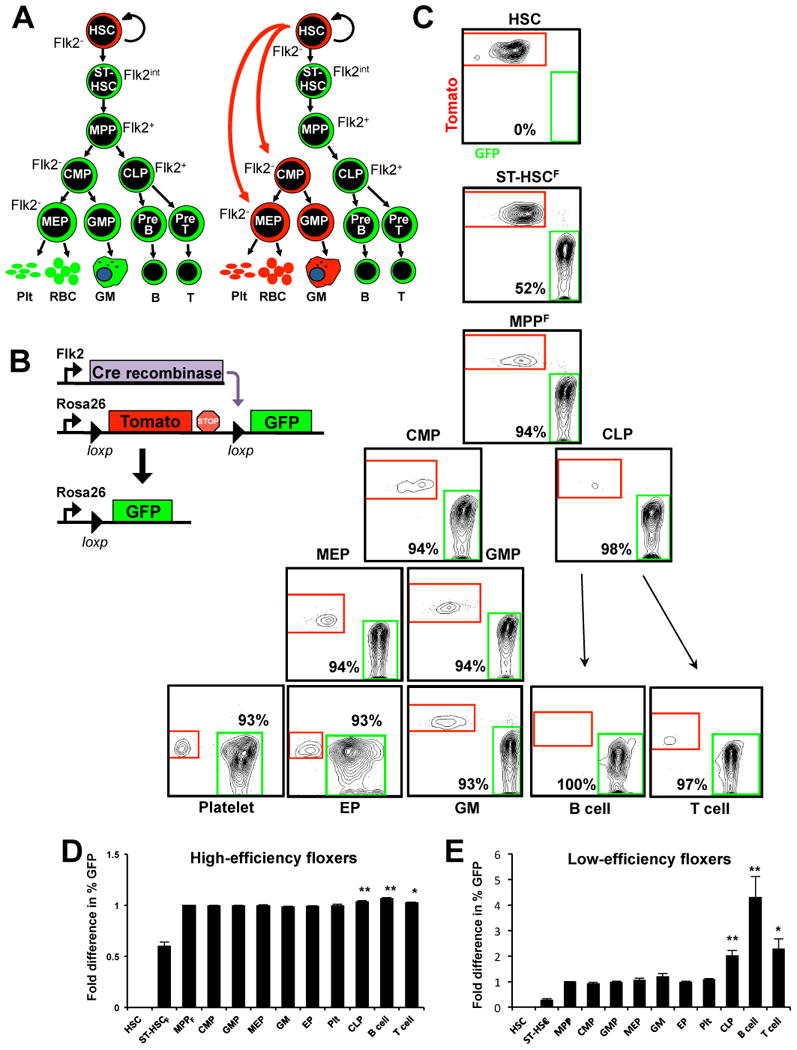
(A) Two simplified alternative models for HSC differentiation. Cell types that are Flk2-positive or derived from Flk2-positive cells should express GFP, while Flk2-negative cells that have no history of Flk2 expression should express Tomato. (B) Strategy for the generation of FlkSwitch reporter mice. (C) Representative flow cytometer data of Tomato and GFP expression in multiple cell populations from a FlkSwitch mouse with high floxing efficiency (mouse number 8 in Table 1). (D) Fold difference in percent GFP+ cells compared to MPPF in high-efficiency floxers (mice numbers 6-14 in Table 1; n=9), and (E) low-efficiency floxers (mice numbers 1-5 in Table 1; n=5). * p<0.01, ** p<0.001 by Wilcoxon Signed Rank test, calculated using the raw GFP percentage (Table 1). GFP percentages in HSC and ST-HSCF were significantly different from all other hematopoietic cell types. HSC, hematopoietic stem cells; ST-HSCF, short-term hematopoietic stem cells; MPPF, multipotent progenitors; CMP, common myeloid progenitors, GMP, granulocyte/macrophage progenitors; MEP, megakaryocyte/erythroid progenitors; GM, granulocyte/macrophage cells; EP, erythroid progenitors; RBC, red blood cells; Plt, platelets; CLP, common lymphoid progenitors; B, B cells; T, T cells. Error bars indicate standard error of the mean (SEM). See also Figure S1.
Results
Establishment of a dual-color reporter mouse model
To determine which hematopoietic lineages develop through a Flk2-positive stage, we generated “FlkSwitch” mice by crossing mice expressing Cre recombinase under the control of Flk2 regulatory elements (“Flk2-Cre” BAC transgenic mice) (Benz et al., 2008) to mice containing a dual-color fluorescent reporter in the Rosa26 locus (“mT/mG” mice) (Muzumdar et al., 2007) (Figure 1B). We expected that all cells in the resulting “FlkSwitch” mice would express the red-fluorescing protein Tomato, with the exception of cells expressing Cre because Cre-mediated excision of Tomato would result in induction of GFP expression. Importantly, because Tomato excision is an irreversible genetic event, all progeny of Cre-expressing cells would also express GFP regardless of whether these progeny themselves express Cre. Flow cytometry analysis revealed that >99% of platelets and nucleated BM and PB cells in both mT/mG and FlkSwitch mice express either the Tomato or GFP reporter gene.
We envisioned two main experimental outcomes: If Flk2-positive progenitors are significant physiological contributors to erythro- and megakaryopoiesis in vivo, MegE cells would inherit the floxed, GFP-expressing reporter allele and be GFP-positive, as indicated in the left schematic of Figure 1A. Alternatively, Flk2-positive progenitors may be capable of providing MegE cells when transplanted into irradiated animals due to the acute requirement for erythrocytes and platelets, but may not normally contribute to MegE generation. In this model, Flk2-negative multipotent progenitors or HSC would give rise to CMP or MEP without differentiation through a Flk2-positive stage, and mature MegE and/or GM cells, and their progenitors, would remain Tomato-positive (Figure 1A, right model).
All hematopoietic cell types except HSC switch from Tomato to GFP expression
To distinguish between these possibilities, we analyzed the color of distinct cell types in FlkSwitch mice by multicolor flow cytometry. HSC were defined as Flk2-negative BM cells that were also c-kit+, Lin-, Sca1+, CD150+, and CD48- (Figure S1A). This population contains all Flk2-negative KLS cells and is heterogeneous for CD34 and thus includes cells that have been referred to as “ST-HSC” in previous reports (Figure S1B). The entire remaining KLS fraction was divided into ST-HSCF and MPPF based on the level of Flk2 expression (intermediate for ST-HSCF and high for MPPF) and differential expression of CD150 and CD48 (Figure S1A).
Importantly, HSC expressed the Tomato transgene, but not GFP (Figure 1C, Table 1), demonstrating that excision of GFP does not occur aberrantly in Flk2-negative populations. Lack of recombination in HSC also demonstrated that all developmental precursors of HSC are Flk2-negative. Analysis of ST-HSCF and MPPF revealed a dramatic color change from Tomato to GFP as Flk2, and Cre, expression was turned on (Figure 1C, Figure S2A, Table 1). This striking color switch (up to 97%) from Flk2-negative HSC to Flk2-positive MPP established that the reporter model operated as expected.
Table 1. Percent GFP in hematopoietic populations from FlkSwitch mice.
| HSC | ST-HSCF | MPPF | CMP | GMP | MEP | GM | EP | Plt | CLP** | B cell** | T cell* | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0 | 1 | 6 | 7 | 7 | 7 | 10 | 7 | 6 | 15 | 38 | 23 |
| 2 | 0 | 2 | 7 | 6 | 6 | 9 | 10 | 6 | 8 | 14 | 32 | 20 |
| 3 | 0 | 5 | 12 | 12 | 12 | 13 | 11 | 12 | 13 | 27 | 47 | 23 |
| 4 | 0 | 5 | 12 | 10 | 12 | 12 | 13 | 12 | 13 | 27 | 50 | 28 |
| 5 | 0 | 4 | 20 | 18 | 20 | 19 | 21 | 18 | 21 | 25 | 44 | 19 |
| 6 | 0 | 63 | 89 | 91 | 90 | 89 | 86 | 86 | 92 | 91 | 97 | 94 |
| 7 | 0 | 51 | 90 | 88 | 89 | 91 | 90 | 88 | 93 | 91 | 99 | 92 |
| 8 | 0 | 44 | 90 | 88 | 89 | 90 | 89 | 90 | 92 | 97 | 98 | 96 |
| 9 | 0 | 61 | 92 | 92 | 92 | 93 | 92 | 92 | 91 | 96 | 100 | 95 |
| 10 | 0 | 37 | 94 | 94 | 94 | 94 | 93 | 93 | 93 | 98 | 100 | 97 |
| 11 | 0 | 68 | 95 | 94 | 93 | 92 | 92 | 93 | 86 | 98 | 99 | 94 |
| 12 | 0 | 45 | 95 | 95 | 95 | 97 | 94 | 97 | 98 | 100 | 100 | 96 |
| 13 | 0 | 74 | 96 | 94 | 94 | 93 | 94 | 94 | 93 | 99 | 100 | 94 |
| 14 | 0 | 60 | 97 | 98 | 99 | 99 | 98 | 98 | 96 | 99 | 100 | 100 |
The differences between MPPF versus CLP, B, and T cells were significant (p<0.001; p<0.001, and p<0.01, respectively), by Wilcoxon Signed Rank test. HSC and ST-HSCF were also significantly different from MPPF and all other populations. No other comparisons to MPPF were significantly different. Abbreviations as in Figure 1. See also Figure S2.
Strikingly, all cell populations of the myeloid lineage, including CMP, GMP, MEP, erythroid progenitors (EPs) and platelets (Plts), exhibited the same GFP percentage as MPPF (Figure 1C-E, Figure S2A, Table 1). Subfractionation of myeloid populations using alternative markers (Pronk et al., 2007) revealed similar results (Figure S2B). The almost complete color switch (up to 98%) in erythroid cells and platelets was particularly surprising given the uncertainty of Flk2-positive cell contribution to MegE lineages. Even in mice with low floxing efficiency in Flk2-positive populations (mice #1-5 in Table 1; Figure S2A), the GFP-positive percentage in all myeloid and erythroid cell populations closely followed the GFP-positive percentage in MPPF (Figure 1D and E, Table 1).
To determine whether myeloid cells switch color due to differentiation through a Flk2-positive progenitor stage or due to aberrant recombination, we investigated Cre expression and activity. Cre mRNA was readily detectable in MPPF, but not in HSC or myeloid progenitors, demonstrating a Flk2-dependent Cre expression pattern (Figure 2A). We then tested whether myeloid progenitors exhibit Cre recombinase activity. Unfloxed (Tom+) and floxed (GFP+) CMP and MPPF were isolated from FlkSwitch mice and differentiated in vitro or transplanted into recipient mice. As expected, all progeny of GFP-positive CMP and MPPF in vitro and in the PB and spleen of recipient mice were GFP-positive (Figures 2B-D). In contrast, Tomato-positive MPPF gave rise to both Tomato-positive and GFP-positive progeny in all three assays (Figures 2B-D). Importantly, we never detected GFP-positive progeny from Tomato-positive CMP, as they exclusively gave rise to Tomato-positive progeny in vitro and in vivo. The lack of detectable Cre mRNA and recombinase activity in myeloid progenitors and their progeny demonstrated that Cre is expressed and functional in a Flk2-dependent pattern, and support a model where myeloid cells are derived from Flk2-expressing progenitors.
Figure 2. Lack of Cre expression and activity in myeloid progenitors at steady-state and upon in vivo and in vitro differentiation.
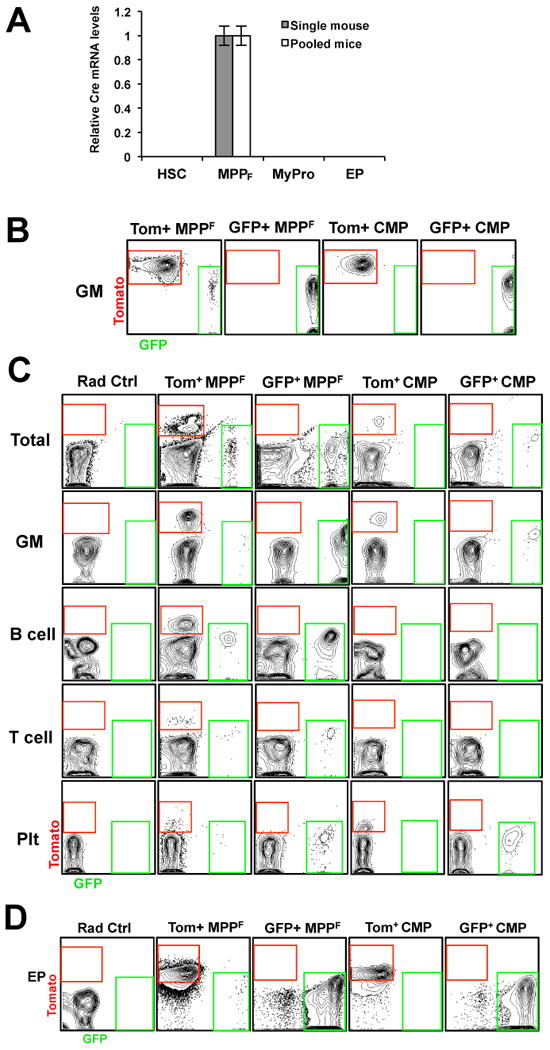
(A) Quantitative RT-PCR analyses of Cre recombinase mRNA levels in Flk2+ (MPPF) and Flk2- (HSC; myeloid progenitors (MyPro; Lin-c-kit+Sca1- cells), and erythroid progenitors (EP; Ter119-Mac1-Gr1-B220-CD3-CD71+)) cell populations from FlkSwitch mice revealed that only MPPF express Cre. Bar graph indicates the relative levels of Cre mRNA in cell populations sorted from individual (grey bar) or multiple (white bar; n=3) FlkSwitch mice. β-actin was used as a positive control for all populations. Error bars indicate SEM. (B-D) Tom+ CMP remain unfloxed during myeloid development. (B) Flow cytometry analysis of CMP progeny after 10 days of in vitro methylcellulose culture (n=6 in 2 independent experiments). (C) Tom and GFP analysis of donor-derived nucleated cells (total), GM, B-cells, T-cells, and Plts in PB of sublethally irradiated mice transplanted with Tomato+ or GFP+ MPPF (800 per mouse) or CMP (10,000 per mouse) (n=5-7 in 2 independent experiments). (D) Erythroid progenitor (EP) readout in spleens of lethally irradiated mice 9 days post-transplantation of Tom+ or GFP+ MPPF or CMP (n=10 in 2 independent experiments).
Tomato-expressing and GFP-expressing cells are functionally equivalent
The highly variable floxing efficiency between different mice (from 6% to 97% in MPPF) makes it unlikely that Tomato-positive and GFP-positive cells within a phenotypic fraction are fundamentally different. Transcriptional analysis revealed a trend towards increased levels of Flk2 and Cre mRNA in GFP-positive compared to Tom-positive MPPF (Figure 3A), consistent with floxing efficiency increasing with Flk2 levels. To directly test whether floxed and unfloxed cells are functionally equivalent, we compared the in vivo CFU-S and PB reconstitution abilities of purified Tomato-positive and GFP-positive MPPF and CMP. We did not detect significant differences in PB reconstitution potential (Figure 3B-C) or CFU-S frequencies (Figure 3D-E) between Tomato-positive and GFP-positive MPPF and between Tom-positive and GFP-positive CMP. We then analyzed the relative number of Cre transgenes between different mice by performing qPCR of genomic DNA. Indeed, mice with low floxing efficiency had fewer copies of the Flk2-Cre construct compared to high-floxing mice (Figure 3F). These data are consistent with the increase in floxing efficiency observed upon selective backcrossing of FlkSwitch mice. Collectively, these results led us to conclude that Tom-positive and GFP-positive cells within a phenotypic population are functionally equivalent and that the differential floxing efficiency between different mice is due to varying copy numbers of the Cre transgene.
Figure 3. Tomato-positive MPPF and CMP exhibit similar in vivo reconstitution potential as their GFP-positive counterparts.
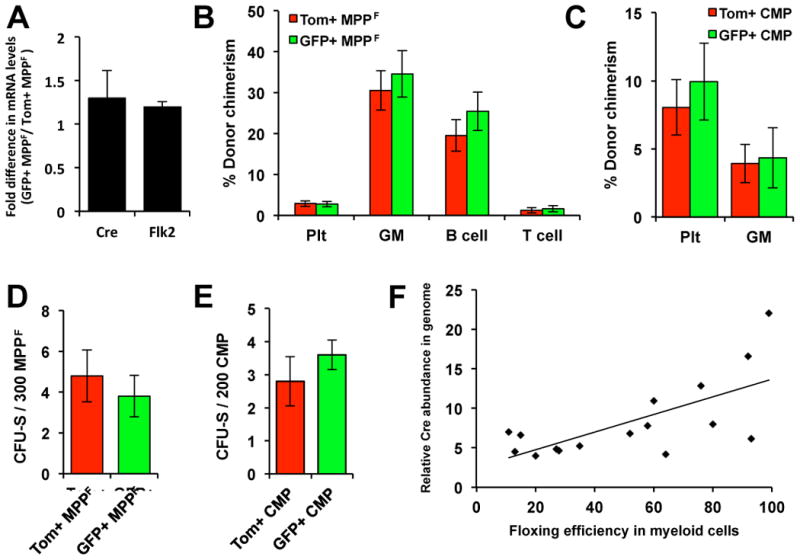
(A) Quantitative RT-PCR analyses of Cre recombinase and Flk2 mRNA levels in Tom+ and GFP+ MPPF isolated from individual FlkSwitch mice (n=4). (B-E) Tom+ and GFP+ MPPF (B, D) and CMP (C, E) give rise to similar numbers of Plts, GM, B and T cells (B-C) and CFU-S (D-E). Purified Tom+ and GFP+ MPPF (800 per mouse, n=7 and 8) or Tom+ and GFP+ CMP (10,000 per recipient, n=5) were transplanted into sublethally irradiated hosts and the PB readout was analyzed weekly for 30 days posttransplantation (B-C). For CFU-S analysis, 300 Tom+ or GFP+ MPPF (n=10) or 200 Tom+ and GFP+ CMP (n=10 in 2 independent experiments) per recipient were transplanted into lethally irradiated hosts. CFU-S were enumerated 11 or 9 days posttransplantation (D-E). (E) The percentage of GFP+ cells in PB myeloid cells correlates with Cre transgene copy number. Error bars in A-E indicate SEM; no comparisons were statistically significantly different.
Analysis of low-efficiency floxers revealed increased reporter switching in lymphoid, but not myeloid, cells
In contrast to MegE development, there is considerable agreement that MPPF are capable of both GM and lymphoid differentiation (Adolfsson et al., 2001; Adolfsson et al., 2005; Christensen and Weissman, 2001; Forsberg et al., 2006; Lai and Kondo, 2006; Lai et al., 2005). In addition, several populations with lymphoid-restricted potential, including CLP and fractions of ProB and ProT cells, express Flk2 (Karsunky et al., 2008; Karsunky et al., 2003). It is therefore less surprising that cells of the lymphoid lineage also switch to GFP expression in the FlkSwitch mice (Figure 1C, Figure S1, Table 1). Indeed, statistical analysis revealed that Tomato excision increased during lymphoid development (Table 1). Because it is not possible to substantially increase the percentage of floxed cells when already very high in MPPF, this effect is most apparent in low-efficiency floxers (mice numbers 1-5 in Table 1; Figure 1E). The increased reporter switch is likely a result of sustained Cre expression during development through additional Flk2-positive progenitor stages, such as CLP, ProB and ProT cells (Karsunky et al., 2008; Karsunky et al., 2003). In contrast, myeloid cells did not display a detectable increase in percent GFP-positive cells compared to MPPF, consistent with Flk2-positive cells not contributing significantly to myelopoiesis beyond the MPPF stage.
Hematopoietic differentiation occurs through a Flk2-positive stage under stress conditions
To test whether alternative differentiation pathways can be used when there is an acute requirement for MegE cells, we analyzed the color composition of hematopoietic cells under stress conditions. First, we subjected FlkSwitch mice to sublethal irradiation and analyzed the color composition of mature cells in the PB over time. Intriguingly, the percentage of GFP-positive Plts, EPs, and GM cells, but not B and T cells, decreased for the first three weeks after irradiation (Figure 4A). Three weeks post-irradiation, the percentage of GFP-positive myeloid cells started to rebound. A second sublethal dose of irradiation at week 5 elicited a similar response, with GFP percentages in Plts, EPs, and GM cells decreasing for 3 weeks to then increase over the next several weeks (Figure 4A). The magnitude of the changes in GFP percentages was similar for Plts, EPs and GM cells, whereas neither B nor T cells displayed significant changes in the percent of GFP-positive cells.
Figure 4. Hematopoietic differentiation under irradiation-induced stress progresses through a Flk2-positive stage.
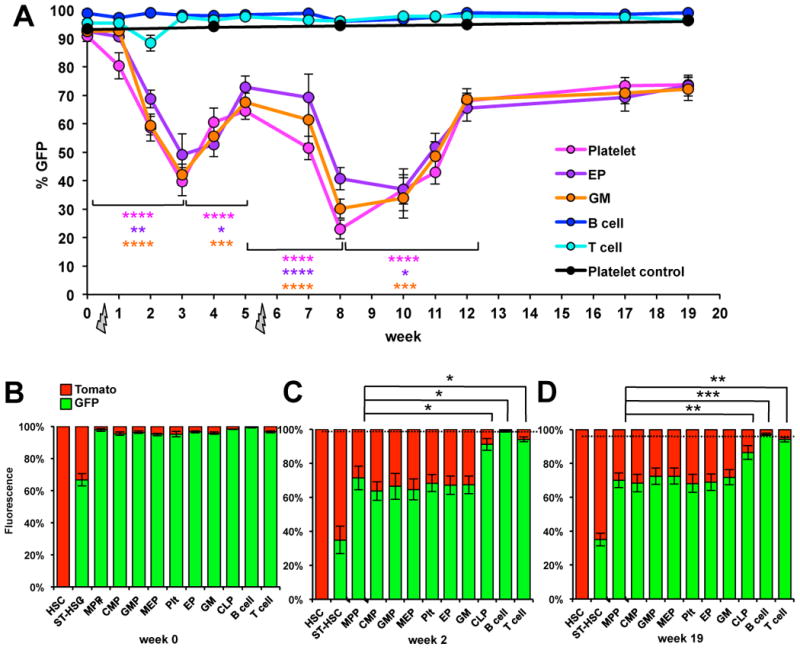
(A) Analysis of PB cells of FlkSwitch mice before and after irradiation-induced stress revealed a myeloid-specific decrease in the percentage of cells expressing the GFP reporter gene, with a concomitant increase in the percentage of cells expressing Tomato. GFP percentages in Plts (black line), GM, B and T cells (not shown) remain unchanged over time in unirradiated mice. (B-D) Ratio of GFP to Tomato expression in BM cell populations without irradiation (B), 2 weeks after one sublethal irradiation dose (C), and 19 weeks after two sublethal doses administered at wks 0 and 5 (D), demonstrating that the decrease in MPPF floxing efficiency is reflected in myeloid populations. The black dotted line indicates average GFP percentages of myeloid PB cells of the same mice prior to irradiation. Error bars indicate SEM. P-values were determined using a paired two-tailed t-test. * p<0.05, ** p<0.005, ***, p<0.0005, **** p<0.00005. GFP percentages in HSC and ST-HSCF were significantly different from all other hematopoietic cell types (B-D).
The myeloid-specific decrease in GFP frequencies upon irradiation suggested that a proportion of the myeloid cells may be derived directly from Flk2-negative progenitors, “skipping” the Flk2-positive intermediate stage used during steady-state hematopoiesis. Alternatively, overall floxing efficiency could be decreased in Flk2-positive progenitors. To distinguish between these possibilities, we analyzed the color composition of stem and progenitor cells in the BM at 2 weeks (one sublethal dose) and 19 weeks (two sublethal doses) post-irradiation. As shown in Figures 4C-D, the percentage of GFP-positive MPPF was the same as that of mature myeloid cells, and of all myeloid progenitor populations. These data are inconsistent with a bypass pathway and instead support an irradiation-induced decrease in floxing efficiency. Indeed, comparing GFP percentages of cells from unirradiated mice (Figure 4B) to cells from irradiated mice (Figures 4C-D) with similar floxing efficiencies in the PB prior to irradiation (∼95%) indicated that irradiation caused a reduction in floxing efficiency in MPPF. This effect was not detectable in B and T cells, presumably due to the multiple Flk2-positive progenitor stages during lymphoid differentiation.
HSC differentiate through a Flk2-positive stage upon transplantation
We then tested differentiation pathways upon transplantation. HSC isolated from high-floxing (∼95% GFP+ myeloid PB cells) FlkSwitch mice were transplanted into sublethally irradiated wt mice, followed by PB analysis of donor-derived progeny. Figure 5A shows the robust, long-term reconstitution of nucleated blood cells and Plts in recipient mice. Analysis of the color composition of PB progeny revealed that the percentages of GFP-positive Plts, GM and B cells increased with time after transplantation (Figure 5B). B cells quickly reached >90% GFP-positive cells, whereas the increase in the GFP-positive percentage of Plts and GM cells plateaued at ∼50% eight weeks after transplantation. Robust T cell readout was first detected at 5 weeks post-transplantation, with T cells displaying high GFP percentages throughout the timecourse. Analysis of the BM cell populations from the same mice 16-18 weeks after transplantation revealed that the GFP percentages were similar in MPPF, myeloid progenitors, Plts and mature GM cells (Figure 5C). These results are similar to those from the sublethal irradiation experiments and prompted us to conclude that myeloid cells, including MegE cells, are derived through a Flk2-positive progenitor stage even under hematopoietic stress conditions.
Figure 5. HSC differentiate into all lineages through a Flk2-positive stage upon transplantation.
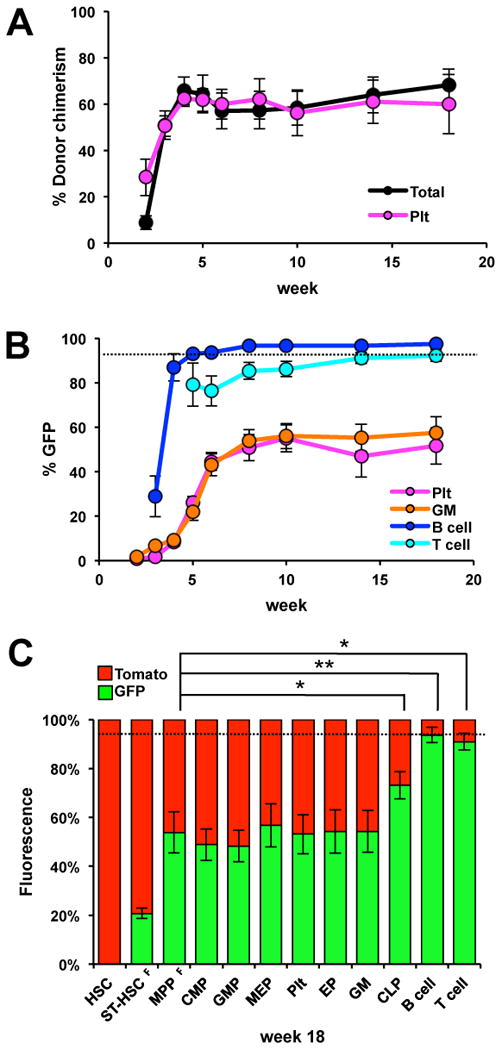
(A) Donor chimerism in recipient mice transplanted with 100 HSC from FlkSwitch mice demonstrate robust long-term engraftment of nucleated (GM, B and T cells) PB cells, as well as Plts. (B) GFP percentages of donor-derived B cells, Plts and GM cells increase for the first few weeks after transplantation and then remain stable. (C) BM analysis of recipient mice >16 wks posttransplantation revealed that MPPF displayed similar GFP percentages as myeloid progenitors, Plts and GM cells. The black dotted line indicates the GFP percentage of MPP of the same mice prior to transplantation. (n=7 in 2 independent experiments). Error bars indicate SEM. P-values were determined using a two-tailed paired t-test. * p< 0.005, ** p<0.001. GFP percentages in HSC and ST-HSCF were significantly different from all other hematopoietic cell types.
Discussion
All hematopoietic cells differentiate through a Flk2-positive stage
Our data clearly demonstrate that upregulation of Flk2 is a shared feature of HSC differentiation into all hematopoietic cell types. Thus, the FlkSwitch lineage tracing model definitively rules out differentiation of HSC directly into Flk2-negative myeloid-committed progenitors or mature myeloid cells. The model also provides definitive evidence that HSC specification and maintenance do not involve Flk2-positive developmental stages. During steady-state, irradiation-induced stress, and upon HSC transplantation, all myeloid subpopulations displayed reporter recombination, with up to 98% of Plts and EPs being GFP-labeled. Although we did not observe a 100% color switch in myeloid populations, we showed that this is the result of imperfect floxing efficiency as opposed to bypass of the Flk2-positive stage (Figures 1, 2, 4 and 5). MPPF, by definition, do not bypass the Flk2-positive stage and displayed <100% floxing efficiency. If it was favorable to “escape” the Flk2-positive stage during myeloid differentiation, a proportion of myeloid progeny would be generated through the bypass pathway and remain Tomato-positive. This scenario may be more readily detectable in low-efficiency floxers and mice under hematopoietic stress, but was not observed under any condition examined. Furthermore, we ruled out Cre misexpression or activity as significant contributors to reporter switching in myeloid cells (Figure 2). This allowed us to conclude that all hematopoietic cells, including cells of the MegE lineage, are derived from HSC through a Flk2-positive progenitor. Boiers et al recently made similar conclusions for granulocyte differentiation when analyzing granulocytic and MegE development; however, they did not report their findings on erythroid cell or platelet origins (Boiers et al., 2010).
Robustly Flk2-positive cells are the likely immediate progenitors of myeloid-committed cells
Our observation that all myeloid populations exhibit the same floxing efficiency as MPPF in FlkSwitch mice under both steady-state and dynamic conditions, and the lack of identification of a downstream Flk2-positive cell type with MegE capability, points to MPPF as the likely intermediate Flk2-positive cell population (Figure 1A, left). Importantly, multipotent progenitor cells with previously demonstrated MegE potential (Flk2-CD34+ KLS cells) and our ST-HSCF (here defined by intermediate Flk2 cell surface expression) lack (Figure S1B) or display significantly lower (Figure 1C-E, Figure 4B-D, Figure 5C, Table 1) floxing efficiency compared to myeloid progenitors and mature cells. Because CMP lacked any detectable recombinase expression or activity, we find it unlikely that these ST-HSC populations are the immediate progenitors of myeloid-committed cells. In contrast, MPPF and all myeloid populations exhibited the same proportion of floxed cells in every mouse examined (Figures 1C-E, Figures 4B-D, Figure 5C, Table 1). This concordance in GFP percentages between MPPF and myeloid populations was remarkably conserved even during the highly dynamic conditions of hematopoietic stress (Figures 4 and 5). Known marker heterogeneity within Flk2-positive populations (as well as HSC; see below) raises the possibility that lineage biases occur during or prior to Flk2 upregulation. Thus, while it is possible that the cells within the Flk2-positive fraction that give rise to MegE lineages are different from the cells that give rise to lymphoid cells, loss of MegE ability before the onset of Flk2 expression (Figure 1A, right) is incompatible with the data we present here. Instead, our results support a model where all hematopoietic lineages develop through a robustly Flk2-positive intermediate.
Comparison of steady-state hematopoietic differentiation to pathways in transplantation models
Given the relatively poor MegE readout in vitro and low CFU-S frequency in vivo from Flk2-positive progenitors (Adolfsson et al., 2005; Forsberg et al., 2006), one might have expected that at least a fraction of MegE cells would be derived from a Flk2-independent pathway. In contrast, our data revealed that all MegE cells are derived via a Flk2-positive stage. Flk2 clearly plays a role in lymphoid development (Mackarehtschian et al., 1995). However, it is important to distinguish between the function of Flk2 itself and the role of cell populations defined by Flk2 expression. Our data show that upregulation of Flk2, and other lymphoid-characteristic genes, does not exclude MegE development in vivo, and suggest that in vitro assays may underestimate MegE potential.
Hematopoietic differentiation pathways have been modeled primarily on hematopoietic reconstitution upon transplantation, and we therefore considered the possibility that steady-state hematopoiesis may differ from transplantation hematopoiesis. The FlkSwitch model is uniquely suited to assess differences between unmanipulated, steady-state hematopoiesis and differentiation that occurs under high-stress conditions. However, our lineage tracing results showed that hematopoietic pathways are similar during steady-state and transplantation, and consistent with the hierarchical differentiation model inferred from transplantation assays (Figure 1A, left). The increased floxing efficiency observed in the lymphoid lineage with GFP percentages increasing from MPPF to CLP and again from CLP to B and T cells (e.g., Figure 1E) is consistent with MPPF giving rise to B and T cells via a CLP intermediate. In contrast to lymphoid cells, GM cells did not display a detectable increase in percent GFP-positive cells compared to MPPF, possibly indicating that any physiological contribution of Flk2-positive myeloid progenitors to GM cells (Boiers et al., 2010; Nutt et al., 2005) is relatively low compared to the contribution by Flk2-negative CMP. In addition, neither high- nor low-efficiency floxers showed an increase in GFP percentages in CMP, GMP, MEP, EP or platelets compared to MPPF, consistent with Flk2-positive cells not contributing significantly to those lineages beyond the MPPF stage. These observations justify a focus exclusively on Flk2-negative cell types when further investigating myeloid-committed progenitor cells. Intriguingly, the dynamics and magnitude of the changes in GFP percentages were strikingly similar for Plts, EPs, and GM cells during irradiation-induced stress and recovery. These data are consistent with the existence of a shared myeloid progenitor cell, as concluded upon the identification of CMP (Akashi et al., 2000). By establishing the physiological contribution of specific progenitors to distinct hematopoietic lineages, our lineage tracing results eliminate several question marks from pathway models based on transplantation assays.
HSC specification and maintenance do not include Flk2-positive stages
Our data also provides important clues on the developmental origin and maintenance of HSC themselves. First, because adult HSC are Tomato-positive in FlkSwitch mice, all developmental precursors to BM HSC must be Flk2-negative. Second, because HSC remain Tomato-positive in adulthood, we conclude that dedifferentiation of Flk2-positive progenitor cells back to the stem cell stage is extremely low or non-existent. The recently reported heterogeneity of cells that fits the classical definition of HSC (reviewed by (Hock, 2010; Schroeder, 2010)) has put into question the hierarchy of different “HSC” populations. Importantly, previous studies have shown that HSC separated by cell cycle status show differential reconstitution ability (Fleming et al., 1993; Passegue et al., 2005). Because these cells are arguably HSC even though their reconstitution ability (and likely at least some markers) is altered by cell cycling, some degree of heterogeneity must be allowed. This transient difference in reconstitution ability also reminds us that transplantation models are dependent on the engraftability of cells at any given time, and that the behavior of transplanted cells may differ from unmanipulated resident cells with the same marker phenotype. It is not yet clear whether HSC cycle back and forth between different marker phenotypes. Here we show that such possible HSC differentiation/dedifferentiation does not include cells beyond the Flk2-positive stage, establishing a clear hierarchy between HSC and non-self-renewing progenitors.
Stem cell fate decisions
Do stem cells, themselves, make fate decisions? While we addressed this question in a specific system (hematopoiesis) with a specific tool (Flk2-driven Cre expression), the concept of when cell fate decisions are made is relevant for all stem cell types and developmental systems. Even in the intensely investigated hematopoietic system, it is clear from the many proposed versions of hematopoietic lineage maps (Akashi, 2009; Ceredig et al., 2009; Hock and Orkin, 2005; Laiosa et al., 2006; Luc et al., 2008b) that fundamentally important questions remain unresolved. Our results clearly demonstrate that “multipotent” progenitors are not only capable of providing all hematopoietic lineages upon transplantation under acute stress conditions, but that they do contribute to all lineages under both steady-state and stress conditions in vivo. This definitive demonstration of the physiological significance of the multilineage capability of a progenitor subpopulation justifies further efforts into targeting MPP, and analogous populations in other stem cell systems, to enable efficient manipulation of cell fate decisions.
Experimental Procedures
Mice
Mice were maintained in the UCSC vivarium according to IACUC approved protocols. mT/mG reporter mice containing a loxP-flanked Tomato transgene followed by enhanced GFP in the Rosa26 locus, and Flk2-Cre mice generated by Cre recombinase expression from a Flk2 BAC transgene were described previously (Benz et al., 2008; Muzumdar et al., 2007). “FlkSwitch” mice were generated by breeding of mT/mG reporter mice and Flk2-Cre mice. Early generations displayed low floxing efficiency, while subsequent backcrossed generations displayed increasing floxing efficiencies nearing 100%.
Cell isolation and analysis
BM and PB cells were isolated and processed as described previously (Forsberg et al., 2005; Forsberg et al., 2006; Smith-Berdan et al., 2011) using a 4-laser FACSAria or LSRII (BD Biosciences, San Jose, CA). Flowjo Software (Ashland, OR) was used for data analysis and display. Cell populations were defined by the following cell surface phenotypes: HSC (Lin-Sca1+cKit+CD48-Slamf1+Flk2-), ST-HSCF (Lin-Sca1+cKit+Flk2intermediate), MPPF (Lin-Sca1+cKit+CD48+Slamf1-Flk2+), CMP (Lin-Flk2-Sca1-cKit+FcgRmidCD34mid), GMP (Lin-Flk2-Sca1-cKit+FcgRhiCD34hi), MEP (Lin-Flk2-Sca1-cKit+FcgRloCD34lo), CLP (Lin-Sca1midcKitmidFlk2+IL7Ra+), GM (Ter119-Mac1+Gr1+CD3-B220-), EP (Mac1-Gr1-CD3-B220-Ter119+CD71+), platelets (FSCloTer119-CD61+), B cell (Ter119-Mac1-Gr1-CD3-B220+), T cell (Ter119-Mac1-Gr1-CD3+B220-). The lineage cocktail consisted of CD3, CD4, CD5, CD8, B220, Gr1, Mac1 and Ter119.
Statistics
A Wilcoxon Signed Rank Test, appropriate for application to data with non-parametric distribution, was used to calculate the significance of the differences in percent GFP-positive cells between cell types at steady-state (Table 1). Paired two-tailed t-tests were used to calculate the significance of the difference in percent GFP-positive cells between hematopoietic cell types after sublethal irradiation and HSC transplantation. Unpaired two-tailed t-test were used for all other statistical analyses.
Transplantation Assays
Double-sorted HSC (100 per mouse), Tomato- or GFP-positive MPPF (800 per mouse) and CMP (10,000 per mouse) were injected into sublethally irradiated mice (770 rads). The BM and PB of recipients were analyzed by flow cytometry for GFP and Tomato fluorescence posttransplantation. The data in Figures 2 and 3 display PB reconstitution of Plts and GM at 9 days posttransplantation; at 9 days for total nucleated cells (CMP only); 17 days for for total nucleated cells (MPP only); and at 30 days posttransplantation for B and T cells (MPP only). For CFU-S analysis, Tomato- or GFP-positive MPPF or CMP were injected into lethally irradiated mice (1036 rads). Spleens were dissected, colonies were enumerated, and cells were analyzed by flow cytometry for GFP and Tomato fluorescence 9 (CMP) and 11 (MPPF) days posttransplantation.
Sublethal Irradiation Assays
Mice with high floxing efficiency (>95%) were given a half-lethal dose (518 rads) of irradiation. Consecutive doses were given 5 weeks apart. PB and BM cells were analyzed by flow cytometry for GFP and Tomato fluorescence.
In vitro culture
25-50 Tomato- or GFP-positive MPPF and CMP from FlkSwitch mice with high floxing efficiency (>90% GFP in mature myeloid cells) were plated in Methocult M3231 (Stem Cell Technologies®) supplemented with SCF (25 ng/mL), IL-11 (25 ng/mL), IL-3 (10ng/mL), Tpo (25ng/mL), Epo (2.5U/mL), and GM-CSF (10ng/mL). Cells were harvested and analyzed by flow cytometry for GFP and Tomato fluorescence after 10 days of culture.
Supplementary Material
Acknowledgments
We thank Drs. Bleul and Boehm for providing the Flk2-Cre mice. We thank Daniel Carlin, Joshua Stuart and Abel Rodriguez for expert advice on statistical analyses, Holger Karsunky and Hanno Hock for comments on the manuscript, and Matthew Zimmer for animal care. This work was supported by a California Institute for Regenerative Medicine (CIRM) New Faculty Award to E.C.F and a CIRM Shared Stem Cell Facility award to UCSC.
Footnotes
The authors declare no conflicts of interest.
References
- Adolfsson J, Borge OJ, Bryder D, Theilgaard-Monch K, Astrand-Grundstrom I, Sitnicka E, Sasaki Y, Jacobsen SE. Upregulation of Flt3 expression within the bone marrow Lin(-)Sca1(+)c-kit(+) stem cell compartment is accompanied by loss of self-renewal capacity. Immunity. 2001;15:659–669. doi: 10.1016/s1074-7613(01)00220-5. [DOI] [PubMed] [Google Scholar]
- Adolfsson J, Mansson R, Buza-Vidas N, Hultquist A, Liuba K, Jensen CT, Bryder D, Yang L, Borge OJ, Thoren LA, Anderson K, Sitnicka E, Sasaki Y, Sigvardsson M, Jacobsen SE. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell. 2005;121:295–306. doi: 10.1016/j.cell.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Akashi K. Lymphoid lineage fate decision of hematopoietic stem cells. Ann N Y Acad Sci. 2009;1176:18–25. doi: 10.1111/j.1749-6632.2009.04570.x. [DOI] [PubMed] [Google Scholar]
- Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–197. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- Akashi K, Traver D, Zon LI. The complex cartography of stem cell commitment. Cell. 2005;121:160–162. doi: 10.1016/j.cell.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Benz C, Martins VC, Radtke F, Bleul CC. The stream of precursors that colonizes the thymus proceeds selectively through the early T lineage precursor stage of T cell development. J Exp Med. 2008;205:1187–1199. doi: 10.1084/jem.20072168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boiers C, Buza-Vidas N, Jensen CT, Pronk CJ, Kharazi S, Wittmann L, Sitnicka E, Hultquist A, Jacobsen SE. Expression and role of FLT3 in regulation of the earliest stage of normal granulocyte-monocyte progenitor development. Blood. 2010;115:5061–5068. doi: 10.1182/blood-2009-12-258756. [DOI] [PubMed] [Google Scholar]
- Ceredig R, Rolink AG, Brown G. Models of haematopoiesis: seeing the wood for the trees. Nat Rev Immunol. 2009;9:293–300. doi: 10.1038/nri2525. [DOI] [PubMed] [Google Scholar]
- Christensen JL, Weissman IL. Flk-2 is a marker in hematopoietic stem cell differentiation: a simple method to isolate long-term stem cells. Proc Natl Acad Sci U S A. 2001;98:14541–14546. doi: 10.1073/pnas.261562798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amico A, Wu L. The early progenitors of mouse dendritic cells and plasmacytoid predendritic cells are within the bone marrow hemopoietic precursors expressing Flt3. J Exp Med. 2003;198:293–303. doi: 10.1084/jem.20030107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ema H, Sudo K, Seita J, Matsubara A, Morita Y, Osawa M, Takatsu K, Takaki S, Nakauchi H. Quantification of self-renewal capacity in single hematopoietic stem cells from normal and Lnk-deficient mice. Dev Cell. 2005;8:907–914. doi: 10.1016/j.devcel.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Fleming WH, Alpern EJ, Uchida N, Ikuta K, Spangrude GJ, Weissman IL. Functional heterogeneity is associated with the cell cycle status of murine hematopoietic stem cells. J Cell Biol. 1993;122:897–902. doi: 10.1083/jcb.122.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg EC, Prohaska SS, Katzman S, Heffner GC, Stuart JM, Weissman IL. Differential Expression of Novel Potential Regulators in Hematopoietic Stem Cells. PLoS Genet. 2005;1:e28. doi: 10.1371/journal.pgen.0010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg EC, Serwold T, Kogan S, Weissman IL, Passegue E. New evidence supporting megakaryocyte-erythrocyte potential of flk2/flt3+ multipotent hematopoietic progenitors. Cell. 2006;126:415–426. doi: 10.1016/j.cell.2006.06.037. [DOI] [PubMed] [Google Scholar]
- Hock H. Some hematopoietic stem cells are more equal than others. J Exp Med. 2010;207:1127–1130. doi: 10.1084/jem.20100950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hock H, Orkin SH. Stem cells: the road not taken. Nature. 2005;435:573–575. doi: 10.1038/435573a. [DOI] [PubMed] [Google Scholar]
- Karsunky H, Inlay MA, Serwold T, Bhattacharya D, Weissman IL. Flk2+ common lymphoid progenitors possess equivalent differentiation potential for the B and T lineages. Blood. 2008;111:5562–5570. doi: 10.1182/blood-2007-11-126219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsunky H, Merad M, Cozzio A, Weissman IL, Manz MG. Flt3 ligand regulates dendritic cell development from Flt3+ lymphoid and myeloid-committed progenitors to Flt3+ dendritic cells in vivo. J Exp Med. 2003;198:305–313. doi: 10.1084/jem.20030323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–672. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- Lai AY, Kondo M. Asymmetrical lymphoid and myeloid lineage commitment in multipotent hematopoietic progenitors. J Exp Med. 2006;203:1867–1873. doi: 10.1084/jem.20060697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai AY, Lin SM, Kondo M. Heterogeneity of Flt3-expressing multipotent progenitors in mouse bone marrow. J Immunol. 2005;175:5016–5023. doi: 10.4049/jimmunol.175.8.5016. [DOI] [PubMed] [Google Scholar]
- Laiosa CV, Stadtfeld M, Graf T. Determinants of lymphoid-myeloid lineage diversification. Annu Rev Immunol. 2006;24:705–738. doi: 10.1146/annurev.immunol.24.021605.090742. [DOI] [PubMed] [Google Scholar]
- Luc S, Anderson K, Kharazi S, Buza-Vidas N, Boiers C, Jensen CT, Ma Z, Wittmann L, Jacobsen SE. Down-regulation of Mpl marks the transition to lymphoid-primed multipotent progenitors with gradual loss of granulocyte-monocyte potential. Blood. 2008a;111:3424–3434. doi: 10.1182/blood-2007-08-108324. [DOI] [PubMed] [Google Scholar]
- Luc S, Buza-Vidas N, Jacobsen SE. Delineating the cellular pathways of hematopoietic lineage commitment. Semin Immunol. 2008b;20:213–220. doi: 10.1016/j.smim.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Mackarehtschian K, Hardin JD, Moore KA, Boast S, Goff SP, Lemischka IR. Targeted disruption of the flk2/flt3 gene leads to deficiencies in primitive hematopoietic progenitors. Immunity. 1995;3:147–161. doi: 10.1016/1074-7613(95)90167-1. [DOI] [PubMed] [Google Scholar]
- Morita Y, Ema H, Nakauchi H. Heterogeneity and hierarchy within the most primitive hematopoietic stem cell compartment. J Exp Med. 2010;207:1173–1182. doi: 10.1084/jem.20091318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- Nakorn TN, Miyamoto T, Weissman IL. Characterization of mouse clonogenic megakaryocyte progenitors. Proc Natl Acad Sci U S A. 2003;100:205–210. doi: 10.1073/pnas.262655099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt SL, Metcalf D, D'Amico A, Polli M, Wu L. Dynamic regulation of PU.1 expression in multipotent hematopoietic progenitors. J Exp Med. 2005;201:221–231. doi: 10.1084/jem.20041535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa M, Hanada K, Hamada H, Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273:242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- Passegue E, Wagers AJ, Giuriato S, Anderson WC, Weissman IL. Global analysis of proliferation and cell cycle gene expression in the regulation of hematopoietic stem and progenitor cell fates. J Exp Med. 2005;202:1599–1611. doi: 10.1084/jem.20050967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pronk CJ, Rossi DJ, Mansson R, Attema JL, Norddahl GL, Chan CK, Sigvardsson M, Weissman IL, Bryder D. Elucidation of the phenotypic, functional, and molecular topography of a myeloerythroid progenitor cell hierarchy. Cell Stem Cell. 2007;1:428–442. doi: 10.1016/j.stem.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Schroeder T. Hematopoietic stem cell heterogeneity: subtypes, not unpredictable behavior. Cell Stem Cell. 2010;6:203–207. doi: 10.1016/j.stem.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Sitnicka E, Buza-Vidas N, Ahlenius H, Cilio CM, Gekas C, Nygren JM, Mansson R, Cheng M, Jensen CT, Svensson M, Leandersson K, Agace WW, Sigvardsson M, Jacobsen SE. Critical role of FLT3 ligand in IL-7 receptor independent T lymphopoiesis and regulation of lymphoid-primed multipotent progenitors. Blood. 2007;110:2955–2964. doi: 10.1182/blood-2006-10-054726. [DOI] [PubMed] [Google Scholar]
- Smith-Berdan S, Nguyen A, Hassanein D, Zimmer M, Ugarte F, Ciriza J, Li D, Garcia-Ojeda ME, Hinck L, Forsberg EC. Robo4 cooperates with CXCR4 to specify hematopoietic stem cell localization to bone marrow niches. Cell Stem Cell. 2011;8:72–83. doi: 10.1016/j.stem.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagers AJ, Sherwood RI, Christensen JL, Weissman IL. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002;297:2256–2259. doi: 10.1126/science.1074807. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


