Abstract
The detailed mechanisms of emotional modulation in the nervous system by opioids remain to be elucidated, although the opioid system is well known to play important roles in the mechanisms of analgesia and drug dependence. In the present study, we conducted behavioral tests of anxiety and depression and measured corticosterone concentrations in both male and female μ-opioid receptor knockout (MOP-KO) mice to reveal the involvement of μ-opioid receptors in stress-induced emotional responses. MOP-KO mice entered more and spent more time in the open arms of the elevated plus maze compared with wild-type mice. MOP-KO mice also displayed significantly decreased immobility in a 15 min tail-suspension test compared with wild-type mice. Similarly, MOP-KO mice exhibited significantly decreased immobility on days 2, 3, and 4 in a 6 min forced swim test conducted for 5 consecutive days. The increase in plasma corticosterone concentration induced by tail-suspension, repeated forced swim, or restraint stress was reduced in MOP-KO mice compared with wild-type mice. Corticosterone levels were not different between wild-type and MOP-KO mice before stress exposure. In contrast, although female mice tended to exhibit fewer anxiety-like responses in the tail-suspension test in both genotypes, no significant gender differences were observed in stress-induced emotional responses. These results suggest that MOPs play an important facilitatory role in emotional responses to stress, including anxiety- and depression-like behavior and corticosterone levels.
Keywords: μ-opioid receptor, knockout mouse, corticosterone, stress, anxiety, depression
1. Introduction
Stress is hypothesized to be one of the triggering factors that causes mental illness, including anxiety and depression. Several brain areas are hypothesized to be involved in stress-induced emotional responses via corticosterone release by the hypothalamic-pituitary-adrenal (HPA) axis. Although several neurotransmitter systems, such as serotonin and catecholamines, have been hypothesized to be involved in these mechanisms, the precise molecular mechanisms are still unclear. Endogenous opioid peptides, such as endorphins, have been shown to modulate serotonergic and catecholaminergic neurotransmission (Chen et al., 2001; Hung et al., 2003; Ukai and Lin, 2002). Furthermore, pretreatment with naloxone, a nonselective opioid receptor antagonist, decreased immobility time in mice in a forced swim test (Amir, 1982). Chronic morphine facilitated immobility in a forced swim test (Molina et al., 1994). Opioids have also been reported to increase stress-related hormone levels (Mellon and Bayer, 1998). These previous reports indicate that the endogenous opioid system impacts behavioral responses to stress.
Opioid receptors have been classified into at least three subtypes, μ, δ, and κ (MOP, DOP, and KOP, respectively). Endomorphin-1 and -2, endogenous peptides that are selective for MOP, reportedly decreased immobility time in both the forced swim and tail-suspension tests (Fichna et al., 2007). A DOP selective agonist, SNC80, also decreased immobility time in a forced swim test (Broom et al., 2002). Furthermore, the KOP selective agonist U69593 increased, and the KOP selective antagonist nor-binaltorphimine decreased, immobility time in a forced swim test (Mague et al., 2003). Although three opioid receptor subtypes may be involved in stress-induced emotional responses, even the most selective ligands for a specific subtype (i.e., β-funaltrexamine for MOP, naltrindole for DOP, and nor-binaltorphimine for KOP) possess certain affinities for other subtypes (Newman et al., 2002) which may contribute to the discrepant findings about the role of opioid receptor subtypes in stress responses. Therefore, the precise molecular mechanisms underlying stress-induced emotional responses have not yet been clearly delineated by traditional pharmacological studies that use only selective ligands.
Recent success in developing knockout (KO) mice with MOP gene deletion has revealed the central role of MOPs, rather than other opioid receptor subtypes, in various opioid effects, including analgesia, reward, and tolerance (Ide et al., 2004; Kieffer, 1999; Loh et al., 1998; Sora et al., 2001; Sora et al., 1997). Although several compensatory changes might occur in KO animals, these animals have potential utility in investigating the in vivo roles of specific proteins. Opioid receptors have been shown to modulate responses to stress, including depression-like behavior (Filliol et al., 2000; McLaughlin et al., 2003). Thus, the use of MOP-KO mice has provided novel theories on the molecular mechanisms underlying stress-induced emotional responses. Both the forced swim test (Porsolt et al., 1977) and tail-suspension test (Steru et al., 1985) have been widely used to assess depression-like behavior, with several modifications. Many reports using these two tests have shown that the inescapable stress of swimming or suspending a mouse by its tail can provide valuable information about emotional responses in stressful situations. The present study investigated the contributory role of the MOP in emotional responses to height, tail-suspension, repeated forced swim, and restraint stress using MOP-KO mice.
2. Materials and methods
2.1. Animals
The present study used wild-type and homozygous MOP-KO mouse littermates on a C57BL/6J genetic background (backcrossed at least 10 generations) as previously described (Sora et al., 2001). The experimental procedures and housing conditions were approved by the Institutional Animal Care and Use Committee, and all animal care and treatment were in accordance with our institutional animal experimentation guidelines. Naive adult (>10 weeks old) male and female mice were group-housed in an animal facility maintained at 22 ± 2ºC and 55 ± 5% relative humidity under a 12 h/12 h light/dark cycle with lights on at 8:00 am and off at 8:00 pm. Food and water were available ad libitum. All behavioral tests and blood sample collections were conducted between 1:00 pm and 6:00 pm.
2.2. Elevated plus maze
The testing apparatus was a white plastic plus-shaped maze, elevated 80 cm from the floor. The maze consisted of two open arms (50 × 10 cm) and two closed arms (50 × 10 × 50 cm) without a roof. During testing, the time spent in the open arms and the number of entries into the open arms were recorded for 5 min. A mouse was considered to have entered an arm only if all four paws entered that arm.
2.3. Locomotor activity
Locomotor activity was assessed with an animal activity-monitoring apparatus equipped with an infrared detector (SUPERMEX, CompACT FSS, Muromachi Kikai Co., Tokyo, Japan). Mice were placed individually in 30 × 45 × 30 cm plastic cages, to which they had not been previously exposed, under dim light and sound-attenuated conditions. Locomotor activity was monitored for 3 h.
2.4. Tail-suspension test
For tail-suspension testing, mice were suspended by their tail which was taped on a metal hook in test chambers (20 × 20 × 25 cm) constructed of white plastic walls and floor. Each hook was connected to a computerized strain gauge that was adjusted to detect animal movements (Tail-suspension System, Neuroscience Inc., Osaka, Japan). The total duration of immobility was measured for 15 min per day for 2 consecutive days.
2.5. Forced swim test
For forced swim testing, animals were forced to swim in a cylindrical Plexiglas tank (30 cm height × 30 cm diameter) containing 20 cm deep water for 6 min per day for 5 consecutive days. The water temperature was maintained at approximately 25°C. Immobility time was recorded with an animal activity-monitoring apparatus equipped with an infrared detector (SUPERMEX, CompACT FSS, Muromachi Kikai Co., Tokyo, Japan). After each session, the mice were immediately removed from the cylinder, dried with a towel, and kept under a heating lamp until completely dry, before being returned to their home cages.
2.6. Stress procedures and corticosterone enzyme immunoassay
After the 2 day tail-suspension test or 5 day forced swim test, blood samples (50 μl) were obtained from the tail vein. For restraint stress, mice were placed in a 50 ml conical centrifuge tube with multiple ventilation holes. Mice were restrained vertically in the tube for 12 h, followed by a 12 h rest with food and water available ad libitum. Mice were restrained again for 12 h, and then blood samples were obtained. All blood samples were immediately centrifuged for 20 min at 1,000 × g. Plasma samples were stored at -80°C until analysis. Plasma corticosterone levels were determined with a Corticosterone Enzyme Immunoassay Kit (Assay Design Inc., Ann Arbor, MI, USA).
2.7. Statistical analysis
Entry counts and time spent on the open arms of the elevated plus maze and stress-induced changes in plasma corticosterone concentrations were analyzed with Student's t-test. The results of other analyses were statistically evaluated with analysis of variance (ANOVA) followed by the Tukey-Kramer test. Values of p < 0.05 were considered statistically significant.
3. Results
We first assessed basal anxiety-like behavior of both mouse genotypes in the elevated plus maze (Fig. 1). Compared with wild-type mice, MOP-KO mice had significantly higher entry counts (p < 0.05, Student's t-test) and a longer time spent on the open arms (p < 0.01, Student's t-test) in both male and female mice. Although female mice tended to have more entry counts and more time spent in the open arms than male mice in both genotypes, no significant differences were observed.
Figure 1.
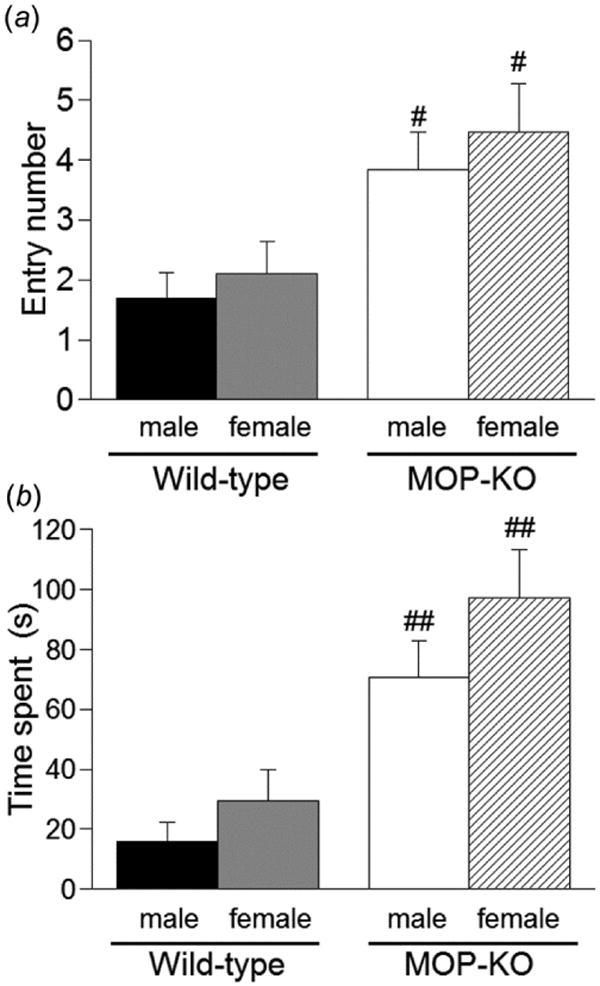
Anxiety-like behavior in wild-type and MOP-KO mice in the elevated plus maze. The (a) number of entries and (b) time spent in the open arms of the elevated plus maze were measured for 5 min in wild-type mice (male, n = 10; female, n = 9) and MOP-KO mice (male, n = 12; female, n = 13). #p < 0.05, ##p < 0.01, significant difference from corresponding value in wild-type mice. Data are expressed as mean ± SEM.
When spontaneous locomotor activity of both wild-type and MOP-KO mice was analyzed (Fig. 2), MOP-KO mice displayed normal locomotor activity, similar to wild-types, during the 3 h test. A three-way, mixed-design ANOVA of spontaneous locomotor activity with two within-subjects factors (genotype and gender) showed no significant interactions (genotype: F1,30 = 1.56, p = 0.221; gender: F1,30 = 0.08, p = 0.784).
Figure 2.
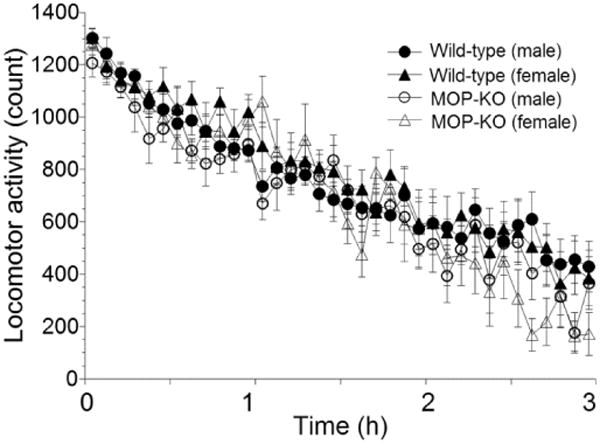
Spontaneous locomotion in wild-type and MOP-KO mice. Spontaneous locomotion during 3 h habituation to a novel environment in wild-type mice (male, n = 12; female, n = 9) and MOP-KO mice (male, n = 6; female, n = 7). Each point represents the sum of 5 min locomotor activity. Data are expressed as mean ± SEM.
To test the influence of MOP-KO in stress-induced responses, immobility time in a 15 min tail-suspension test was analyzed every minute in wild-type and MOP-KO mice (Fig. 3). A three-way, mixed-design ANOVA of immobility time with two within-subjects factors (genotype and gender) revealed that immobility time was significantly different between genotypes in the tail-suspension test (F1,22 = 6.92, p < 0.05), although both genotypes showed time-dependent increases (Fig. 3a). The ANOVA also revealed that immobility time was not significantly different between male and female mice (F1,22 = 3.01, p = 0.097), although female mice tended to show less immobility than males. When the data of male and female mice were combined (Fig. 3b), significant differences were found in immobility time between genotypes (F1,24 = 5.45, p < 0.05, two-way, repeated-measures ANOVA). Post hoc tests revealed that MOP-KO mice had significantly less immobility time compared with wild-type mice from 7 to 9, 12 and 13 min after the tail-suspension test commenced. These differences in immobility time between wild-type and MOP-KO mice were not found during the second trial of the tail-suspension test on the next day (data not shown).
Figure 3.
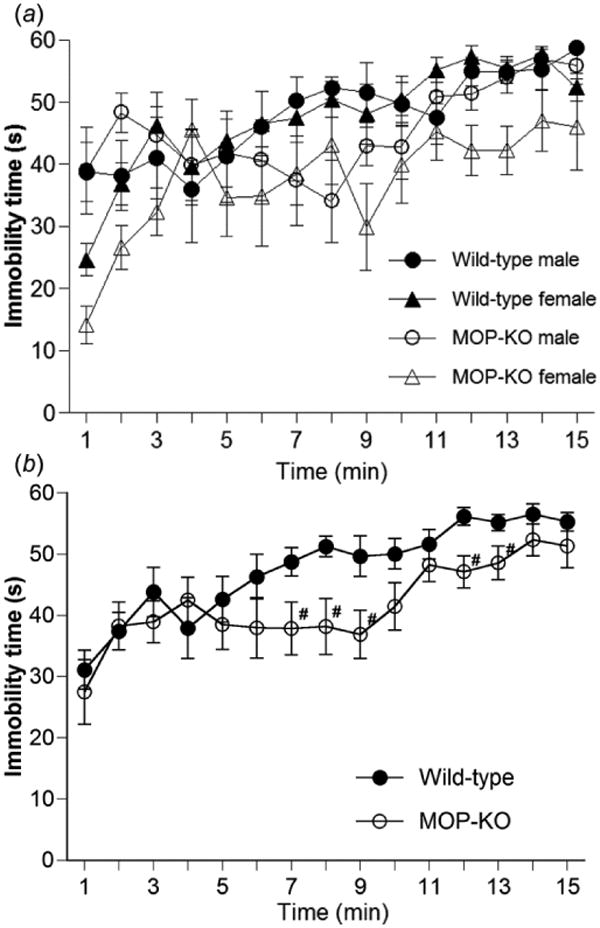
Immobility in wild-type and MOP-KO mice in the 15 min tail-suspension test. (a) Immobility time was measured in wild-type mice (male, n = 6; female, n = 7) and MOP-KO mice (male, n = 7; female, n = 6). (b) Combined data of male and female mice in the 15 min tail-suspension test. #p < 0.05, significant difference from corresponding value in wild-type mice. Data are expressed as mean ± SEM.
To test another type of stress stimulus, immobility time during the 6 min, 5-consecutive-day forced swim test was also analyzed in wild-type and MOP-KO mice (Fig. 4). Both genotypes and both male and female mice showed time-dependent increases in immobility time (Fig. 4a-d). Furthermore, immobility time during the 6 min forced swim test significantly increased, or tended to increase, in a day-dependent manner (wild-type male mice: F4,45 = 8.07, p < 0.001; wild-type female mice: F4,40 = 11.9, p < 0.001; MOP-KO male mice: F4,30 = 2.35, p = 0.077; MOP-KO female mice: F4,30 = 7.00, p < 0.001; two-way, repeated-measures ANOVA). Post hoc comparisons revealed that immobility time on days 2-5 significantly increased compared with day 1 in both wild-type male and female mice (p < 0.05). Immobility time significantly increased on day 5 compared with day 1 in MOP-KO male mice and on days 4 and 5 compared with day 1 in MOP-KO female mice (p < 0.05). A three-way, mixed-design ANOVA of total immobility time during the 6 min tests on each of the 5 days with two within-subjects factors (genotype and gender) revealed that immobility time was significantly different between genotypes (F1,29 = 10.9, p < 0.005) but was not significantly different between genders (F1,29 = 1.39, p = 0.248) (Fig. 4e). Thus, when the male and female data were combined (Fig. 4f), MOP-KO mice showed significantly less immobility time compared with wild-type mice on days 2, 3, and 4.
Figure 4.
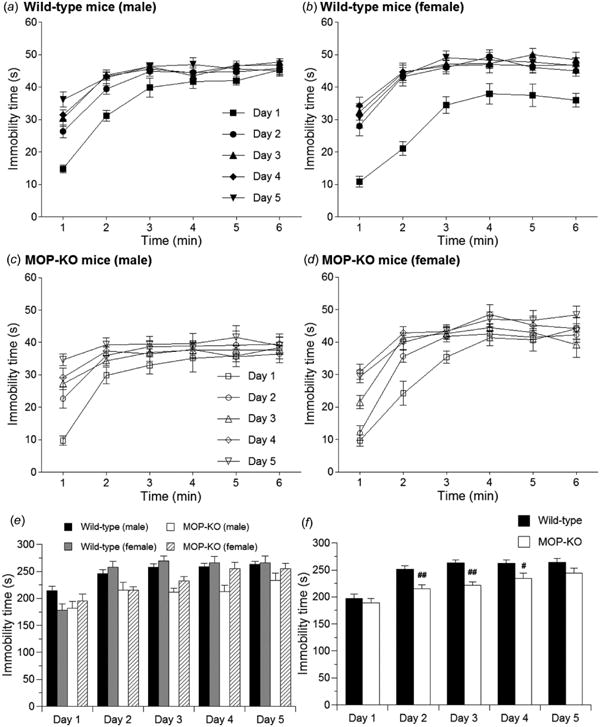
Immobility in wild-type and MOP-KO mice in the 6 min, 5-consecutive-day forced swim test. Immobility time was measured in (a) wild-type male mice (n = 10), (b) wild-type female mice (n = 9), (c) MOP-KO male mice (n = 7), and (d) MOP-KO female mice (n = 7). (e) Sum of 6 min immobility time over 5 days. (f) Combined data of male and female mice. #p < 0.05, ##p < 0.01, significant difference from corresponding value in wild-type mice. Data are expressed as mean ± SEM.
We then analyzed stress-induced changes in plasma corticosterone concentrations in wild-type and MOP-KO mice (Fig. 5). The three types of stress significantly increased plasma corticosterone concentrations in both genotypes and in both male and female mice (p < 0.05, Student's t-test). Although no significant differences were observed in basal plasma corticosterone concentrations in naive mice, the stress-induced increases in plasma corticosterone concentrations were significantly different (p < 0.05, Student's t-test), or tended to be significantly different (restraint stress in female mice: p = 0.065, Student's t-test), between genotypes in both male and female mice. Both male and female MOP-KO mice had significantly lower plasma corticosterone concentrations compared with wild-type mice after the stress procedures. Although female mice tended to have slightly higher corticosterone concentrations than male mice (i.e., naive or after tail-suspension or restraint stress), no significant differences were observed (Student's t-test). Contrary to these findings, female mice tended to exhibit lower corticosterone concentrations than male mice after forced swim stress in both genotypes, although no significant differences were observed (Student's t-test).
Figure 5.
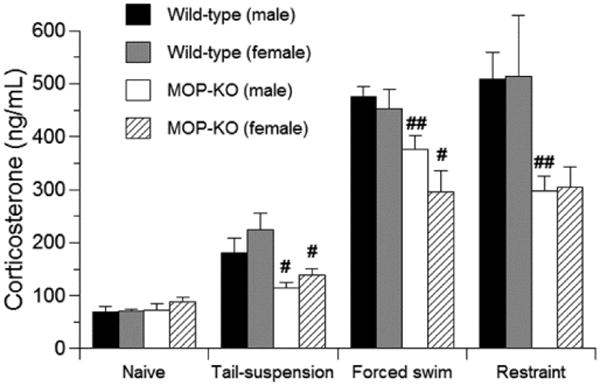
Stress-induced increase in plasma corticosterone concentrations in wild-type and MOP-KO mice. Plasma corticosterone levels were analyzed (i) in naive wild-type mice (male, n = 6; female, n = 5) and MOP-KO mice (male, n = 9; female, n = 8), (ii) after the 2 day tail-suspension test in wild-type mice (male, n = 6; female, n = 5) and MOP-KO mice (male, n = 9; female, n = 8), (iii) after the 5 day forced swim test in wild-type mice (male, n = 10; female, n = 8) and MOP-KO mice (male, n = 7; female, n = 7), and (iv) after restraint stress in wild-type mice (male, n = 6; female, n = 5) and MOP-KO mice (male, n = 9; female, n = 8). #p < 0.05, ##p < 0.01, significant difference from corresponding value in wild-type mice. Data are expressed as mean ± SEM.
4. Discussion
In the present study, MOP-KO mice displayed significantly decreased immobility time in both the tail-suspension and repeated forced swim tests and significantly reduced stress-induced increases in plasma corticosterone concentrations compared with wild-type mice. Moreover, MOP-KO mice also entered more, and spent more time in, the open arms of the elevated plus maze. These results suggest that MOP-KO mice are resistant to stress exposure and exhibit fewer stress-induced emotional responses (i.e., anxiety- and depression-like behaviors) compared with wild-type mice, although the influences of other factors (e.g., response to novelty) should be considered in future studies.
No significant differences were observed in locomotor activity between wild-type and MOP-KO mice, although MOP-KO mice exhibited a slight tendency toward decreased locomotion. These results indicate that the present behavioral effects in MOP-KO mice were not attributable to variations in locomotor activity. MOP-KO mice entered more, and spent more time in, the open arms of the elevated plus maze in the present study. Similar results have been reported with another MOP-KO mouse strain in both the elevated plus maze test and light-dark box test (Filliol et al., 2000). This anxiolytic-like state of MOP-KO mice is consistent with a previous report in which the MOP-selective agonist DAMGO ([D-Ala2, N-MePhe4, Gly-ol]-enkephalin) induced anxiogenic-like activity in the elevated plus maze (Calenco-Choukroun et al., 1991). In contrast, several contradictory studies have reported an anxiolytic-like effect of morphine and MOP agonists (Asakawa et al., 1998; Koks et al., 1999). One of the reasons for this discrepancy using MOP-selective ligands might involve other opioid receptor subtypes. The most selective ligands for a specific opioid receptor subtype possess certain affinities for other subtypes (Newman et al., 2002). Although further studies using our and other MOP-KO mouse strains in various paradigms to assess anxiety-like responses (e.g., open field test) might be needed, the present results suggest that MOPs are involved in anxiety-like responses to height stress.
The decrease in immobility time in MOP-KO mice compared with wild-type mice in both the tail-suspension and repeated forced swim tests is consistent with previous reports. The decrease in immobility time in the forced swim test has been reported using another MOP-KO mouse strain (Filliol et al., 2000). These results suggest that MOP activation facilitates stress-induced, depression-like behavioral responses. Additionally, Fichna et al. (2007) reported contradictory findings in which intracerebroventricular treatment with endomorphin-1 and -2, endogenous MOP-selective peptides, decreased immobility time in both the forced swim and tail-suspension tests. Codeine, a relatively weak MOP agonist, also decreased immobility time in tail-suspension tests in mice (Berrocoso and Mico, 2009). Although these reports might suggest that the MOP modulates depression-like behavior in contrast to our present results, other reports are consistent with our results. Chronic morphine facilitated immobility time in a rat forced swim test (Molina et al., 1994). Pretreatment with naloxone, a nonselective opioid receptor antagonist, decreased immobility time in a forced swim test in mice (Amir, 1982). Furthermore, intraperitoneal treatment of morphine enhanced immobility time in rats in a naloxone-sensitive manner (Zurita and Molina, 1999). These discrepant results might be attributable to differences in animals, mouse strains, time course, injection route, or other experimental conditions. Notably, different mouse strains have exhibited differential responses in forced swim tests (David et al., 2003). Further studies may reveal the reasons for these discrepant results.
To study the involvement of the MOP in emotional responses to repeated stress, the present study used both the 6 min forced swim test conducted for 5 consecutive days and the 15 min tail-suspension test conducted for 2 consecutive days, two regimens which were modified from typically used procedures in mice (Porsolt et al., 1977; Steru et al., 1985). When we analyzed immobility time from day 1 at 3-6 min in the forced swim test (excluding the data from the first 2 min), no significant differences were found between wild-type and MOP-KO mice. Additionally, no significant differences in immobility time were observed from day 1 for the first 6 min between wild-type and MOP-KO mice in the tail-suspension test. Although standard procedures for the analysis of depression-like behavior did not reveal significant differences, MOP-KO mice showed significant differences in depression-like behavior after repeated or longer stress exposure in the forced swim and tail-suspension tests. Our present results might suggest that MOPs facilitate emotional responses to repeated or longer stress exposure. In the present procedures, MOP-KO mice exhibited significantly decreased immobility time in the repeated forced swim test only on days 2, 3, and 4, and they only showed a tendency toward decreased immobility on day 5. In the tail-suspension test, MOP-KO mice had significantly decreased immobility time only after the first 5 min from the beginning of the test during the first trial, and no significant differences were observed during the second trial. Interestingly, the increase in plasma corticosterone concentrations in MOP-KO mice was still significantly lower than wild-type mice after the differences in behaviors between wild-type and MOP-KO mice in both tests disappeared. MOPs may facilitate the early behavioral responses to stress but are not necessary to fully express the behavioral responses after chronic stress procedures. Other neuronal systems might regulate the expression of stress-induced behavioral responses, and MOPs might facilitate this regulation.
At the hormonal level, one of the major responses to stress is an increase in corticosterone secretion caused by stimulation of the HPA axis. In the present study, plasma corticosterone concentration significantly increased after stress exposure in both wild-type and MOP-KO mice. The increased corticosterone levels after both forced swim and restraint stress were higher than after the tail-suspension test. This finding might be attributable to differences in the intensity of the stressors, although variations in the duration and frequency of these stressors might modify these levels. Additionally, the stress-induced increases in plasma corticosterone concentration were less in MOP-KO mice compared with wild-type mice. Our present results are consistent with previous reports. Endogenous opioids have been reported to have facilitatory effects on the HPA axis (Douglas et al., 1998). The increase in plasma corticosterone levels by morphine indicated activation of the HPA axis by MOP (Coventry et al., 2001; Ignar and Kuhn, 1990). In a different MOP-KO mouse strain, morphine- and restraint stress-induced increases in plasma corticosterone levels were also reduced (Roy et al., 2001; Wang et al., 2002). Stress is well known to activate the HPA axis and increase norepinephrine release in the locus coeruleus. Moreover, stress-induced norepinephrine release in the locus coeruleus is partially regulated by both opioid and noradrenergic mechanisms (Nakai et al., 2002; Nestler et al., 1999; Valentino and Van Bockstaele, 2001), suggesting that MOPs may be involved in the activation of the HPA axis and locus coeruleus.
Knockout animals may be hypothesized to have potential utility in investigating the in vivo roles of specific proteins. Previous reports using gene mutant mice suggest that MOPs play an important role in various effects of opioids, such as antinociception, tolerance, reward, and locomotion (Ide et al., 2004; Matthes et al., 1996; Sora et al., 2001; Sora et al., 1997). Our present results also demonstrated the involvement of MOPs in stress-induced emotional responses. However, although no differences in DOP and KOP expression were evident in MOP-KO mice in the present study (Sora et al., 1997), several compensatory changes might occur in MOP-KO mice. These possible compensatory changes, especially with regard to neurotransmitter release and hormonal valence, could elicit changes in stress-induced emotional responses. Future studies, such as behavioral analyses using MOP-KO mice with viral expression of MOPs, may reveal the influences of compensatory changes in stress-induced emotional responses.
Gender differences in emotional responses may also exist (Toufexis, 2007; Toufexis et al., 2006). In the present study, several differences were found between male and female mice in stress-induced emotional responses, although these differences were not significant. In the elevated plus maze, female mice showed less anxiety-like behavior than male mice of both genotypes. These results are consistent with previous reports using rodents (Fernandes et al., 1999; Steenbergen et al., 1990) and suggest the presence of gender differences in anxiety-like behavior. However, no differences in immobility time were found between male and female wild-type mice in either the tail-suspension or forced swim tests. A previous report found that male and female C57BL/6J mice, the genetic background strain used in the present study, exhibited no differences in immobility time in either the tail-suspension or forced swim tests (Caldarone et al., 2003). Interestingly, female MOP-KO mice tended to exhibit less immobility in the tail-suspension test and more immobility in the forced swim test compared with male MOP-KO mice. Although the present study found no significant differences between genders, and additional studies may be required, MOPs may differentially modulate depression-like responses in both tests, especially in female mice.
In conclusion, we found decreased anxiety-like behavior in the elevated plus maze, decreased immobility in both the tail-suspension and forced swim tests, and reduced stress-induced plasma corticosterone concentrations in MOP-KO mice compared with wild-type mice. These results suggest that MOPs play an important facilitatory role in stress sensitivity and/or stress-induced emotional responses, including anxiety- and depression-like responses.
Acknowledgments
We thank M. Arends for editing the language of the manuscript. This study was supported by the Naito Foundation, the Suzuken Memorial Foundation, and the NIDA-IRP, NIH, DHSS.
References
- Amir S. Involvement of endogenous opioids with forced swimming-induced immobility in mice. Physiol Behav. 1982;28:249–251. doi: 10.1016/0031-9384(82)90070-1. [DOI] [PubMed] [Google Scholar]
- Asakawa A, Inui A, Momose K, Ueno N, Fujino MA, Kasuga M. Endomorphins have orexigenic and anxiolytic activities in mice. Neuroreport. 1998;9:2265–2267. doi: 10.1097/00001756-199807130-00022. [DOI] [PubMed] [Google Scholar]
- Berrocoso E, Mico JA. Cooperative opioid and serotonergic mechanisms generate superior antidepressant-like effects in a mice model of depression. Int J Neuropsychopharmacol. 2009 doi: 10.1017/S1461145709000236. in press. [DOI] [PubMed] [Google Scholar]
- Broom DC, Jutkiewicz EM, Folk JE, Traynor JR, Rice KC, Woods JH. Nonpeptidic δ-opioid receptor agonists reduce immobility in the forced swim assay in rats. Neuropsychopharmacology. 2002;26:744–755. doi: 10.1016/S0893-133X(01)00413-4. [DOI] [PubMed] [Google Scholar]
- Caldarone BJ, Karthigeyan K, Harrist A, Hunsberger JG, Wittmack E, King SL, Jatlow P, Picciotto MR. Sex differences in response to oral amitriptyline in three animal models of depression in C57BL/6J mice. Psychopharmacology (Berl) 2003;170:94–101. doi: 10.1007/s00213-003-1518-7. [DOI] [PubMed] [Google Scholar]
- Calenco-Choukroun G, Dauge V, Gacel G, Feger J, Roques BP. Opioid δ agonists and endogenous enkephalins induce different emotional reactivity than μ agonists after injection in the rat ventral tegmental area. Psychopharmacology (Berl) 1991;103:493–502. doi: 10.1007/BF02244249. [DOI] [PubMed] [Google Scholar]
- Chen JC, Liang KW, Huang EY. Differential effects of endomorphin-1 and -2 on amphetamine sensitization: neurochemical and behavioral aspects. Synapse. 2001;39:239–248. doi: 10.1002/1098-2396(20010301)39:3<239::AID-SYN1005>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Coventry TL, Jessop DS, Finn DP, Crabb MD, Kinoshita H, Harbuz MS. Endomorphins and activation of the hypothalamo-pituitary-adrenal axis. J Endocrinol. 2001;169:185–193. doi: 10.1677/joe.0.1690185. [DOI] [PubMed] [Google Scholar]
- David DJ, Renard CE, Jolliet P, Hascoet M, Bourin M. Antidepressant-like effects in various mice strains in the forced swimming test. Psychopharmacology (Berl) 2003;166:373–382. doi: 10.1007/s00213-002-1335-4. [DOI] [PubMed] [Google Scholar]
- Douglas AJ, Johnstone HA, Wigger A, Landgraf R, Russell JA, Neumann ID. The role of endogenous opioids in neurohypophysial and hypothalamo-pituitary-adrenal axis hormone secretory responses to stress in pregnant rats. J Endocrinol. 1998;158:285–293. doi: 10.1677/joe.0.1580285. [DOI] [PubMed] [Google Scholar]
- Fernandes C, Gonzalez MI, Wilson CA, File SE. Factor analysis shows that female rat behaviour is characterized primarily by activity, male rats are driven by sex and anxiety. Pharmacol Biochem Behav. 1999;64:731–738. doi: 10.1016/s0091-3057(99)00139-2. [DOI] [PubMed] [Google Scholar]
- Fichna J, Janecka A, Piestrzeniewicz M, Costentin J, do Rego JC. Antidepressant-like effect of endomorphin-1 and endomorphin-2 in mice. Neuropsychopharmacology. 2007;32:813–821. doi: 10.1038/sj.npp.1301149. [DOI] [PubMed] [Google Scholar]
- Filliol D, Ghozland S, Chluba J, Martin M, Matthes HW, Simonin F, Befort K, Gaveriaux-Ruff C, Dierich A, LeMeur M, Valverde O, Maldonado R, Kieffer BL. Mice deficient for δ- and μ-opioid receptors exhibit opposing alterations of emotional responses. Nat Genet. 2000;25:195–200. doi: 10.1038/76061. [DOI] [PubMed] [Google Scholar]
- Hung KC, Wu HE, Mizoguchi H, Leitermann R, Tseng LF. Intrathecal treatment with 6-hydroxydopamine or 5,7-dihydroxytryptamine blocks the antinociception induced by endomorphin-1 and endomorphin-2 given intracerebroventricularly in the mouse. J Pharmacol Sci. 2003;93:299–306. doi: 10.1254/jphs.93.299. [DOI] [PubMed] [Google Scholar]
- Ide S, Minami M, Satoh M, Uhl GR, Sora I, Ikeda K. Buprenorphine antinociception is abolished, but naloxone-sensitive reward is retained, in μ-opioid receptor knockout mice. Neuropsychopharmacology. 2004;29:1656–1663. doi: 10.1038/sj.npp.1300463. [DOI] [PubMed] [Google Scholar]
- Ignar DM, Kuhn CM. Effects of specific mu and kappa opiate tolerance and abstinence on hypothalamo-pituitary-adrenal axis secretion in the rat. J Pharmacol Exp Ther. 1990;255:1287–1295. [PubMed] [Google Scholar]
- Kieffer BL. Opioids: first lessons from knockout mice. Trends Pharmacol Sci. 1999;20:19–26. doi: 10.1016/s0165-6147(98)01279-6. [DOI] [PubMed] [Google Scholar]
- Koks S, Soosaar A, Voikar V, Bourin M, Vasar E. BOC-CCK-4, CCKB receptor agonist, antagonizes anxiolytic-like action of morphine in elevated plus-maze. Neuropeptides. 1999;33:63–69. doi: 10.1054/npep.1999.0015. [DOI] [PubMed] [Google Scholar]
- Loh HH, Liu HC, Cavalli A, Yang W, Chen YF, Wei LN. μ Opioid receptor knockout in mice: effects on ligand-induced analgesia and morphine lethality. Brain Res Mol Brain Res. 1998;54:321–326. doi: 10.1016/s0169-328x(97)00353-7. [DOI] [PubMed] [Google Scholar]
- Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WC, Jr, Jones RM, Portoghese PS, Carlezon WA., Jr Antidepressant-like effects of κ-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Ther. 2003;305:323–330. doi: 10.1124/jpet.102.046433. [DOI] [PubMed] [Google Scholar]
- Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dolle P, Tzavara E, Hanoune J, Roques BP, Kieffer BL. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the μ-opioid-receptor gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- McLaughlin JP, Marton-Popovici M, Chavkin C. κ Opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J Neurosci. 2003;23:5674–5683. doi: 10.1523/JNEUROSCI.23-13-05674.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon RD, Bayer BM. Evidence for central opioid receptors in the immunomodulatory effects of morphine: review of potential mechanism(s) of action. J Neuroimmunol. 1998;83:19–28. doi: 10.1016/s0165-5728(97)00217-8. [DOI] [PubMed] [Google Scholar]
- Molina VA, Heyser CJ, Spear LP. Chronic variable stress or chronic morphine facilitates immobility in a forced swim test: reversal by naloxone. Psychopharmacology (Berl) 1994;114:433–440. doi: 10.1007/BF02249333. [DOI] [PubMed] [Google Scholar]
- Nakai T, Hayashi M, Ichihara K, Wakabayashi H, Hoshi K. Noradrenaline release in rat locus coeruleus is regulated by both opioid and α2-adrenoceptors. Pharmacol Res. 2002;45:407–412. doi: 10.1006/phrs.2002.0962. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Alreja M, Aghajanian GK. Molecular control of locus coeruleus neurotransmission. Biol Psychiatry. 1999;46:1131–1139. doi: 10.1016/s0006-3223(99)00158-4. [DOI] [PubMed] [Google Scholar]
- Newman LC, Sands SS, Wallace DR, Stevens CW. Characterization of μ, κ, and δ opioid binding in amphibian whole brain tissue homogenates. J Pharmacol Exp Ther. 2002;301:364–370. doi: 10.1124/jpet.301.1.364. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Roy S, Wang JH, Balasubramanian S, Sumandeep, Charboneau R, Barke R, Loh HH. Role of hypothalamic-pituitary axis in morphine-induced alteration in thymic cell distribution using mu-opioid receptor knockout mice. J Neuroimmunol. 2001;116:147–155. doi: 10.1016/s0165-5728(01)00299-5. [DOI] [PubMed] [Google Scholar]
- Sora I, Elmer G, Funada M, Pieper J, Li XF, Hall FS, Uhl GR. μ Opiate receptor gene dose effects on different morphine actions: evidence for differential in vivo μ receptor reserve. Neuropsychopharmacology. 2001;25:41–54. doi: 10.1016/S0893-133X(00)00252-9. [DOI] [PubMed] [Google Scholar]
- Sora I, Takahashi N, Funada M, Ujike H, Revay RS, Donovan DM, Miner LL, Uhl GR. Opiate receptor knockout mice define μ receptor roles in endogenous nociceptive responses and morphine-induced analgesia. Proc Natl Acad Sci U S A. 1997;94:1544–1549. doi: 10.1073/pnas.94.4.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenbergen HL, Heinsbroek RP, Van Hest A, Van de Poll NE. Sex-dependent effects of inescapable shock administration on shuttlebox-escape performance and elevated plus-maze behavior. Physiol Behav. 1990;48:571–576. doi: 10.1016/0031-9384(90)90302-k. [DOI] [PubMed] [Google Scholar]
- Steru L, Chermat R, Thierry B, Simon P. The tail-suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- Toufexis D. Region- and sex-specific modulation of anxiety behaviours in the rat. J Neuroendocrinol. 2007;19:461–473. doi: 10.1111/j.1365-2826.2007.01552.x. [DOI] [PubMed] [Google Scholar]
- Toufexis DJ, Myers KM, Davis M. The effect of gonadal hormones and gender on anxiety and emotional learning. Horm Behav. 2006;50:539–549. doi: 10.1016/j.yhbeh.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Ukai M, Lin HP. Endomorphins 1 and 2 induce amnesia via selective modulation of dopamine receptors in mice. Eur J Pharmacol. 2002;446:97–101. doi: 10.1016/s0014-2999(02)01760-0. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Van Bockstaele E. Opposing regulation of the locus coeruleus by corticotropin-releasing factor and opioids: potential for reciprocal interactions between stress and opioid sensitivity. Psychopharmacology (Berl) 2001;158:331–342. doi: 10.1007/s002130000673. [DOI] [PubMed] [Google Scholar]
- Wang J, Charboneau R, Barke RA, Loh HH, Roy S. μ-Opioid receptor mediates chronic restraint stress-induced lymphocyte apoptosis. J Immunol. 2002;169:3630–3636. doi: 10.4049/jimmunol.169.7.3630. [DOI] [PubMed] [Google Scholar]
- Zurita A, Molina V. Prior morphine facilitates the occurrence of immobility and anhedonia following stress. Physiol Behav. 1999;65:833–837. doi: 10.1016/s0031-9384(98)00247-9. [DOI] [PubMed] [Google Scholar]


