Abstract
Objective(s): We examined the effects of endurance exercise in the presence of Bunium persicum extract administration on lipid profile and cardiorespiratory capacity in hypercholesterolemic male mice.
Materials and Methods : Forty male hypercholesterolemic mice were divided into four groups: Vehicle, Endurance exercise (EE), Bunium persicum extract (BPE), and EE + BPE. The exercise protocol was performed at a speed of 18 m/min, 40 min/day, and 5 days/week for 6 weeks. The BPE was administered orally by a dose of 20 mg/Kg/day.
Results: The results indicated that the 6-week endurance training accompanied by Bunium Persicum extract administration increased cardiorespiratory capacity significantly (601±39 vs. 293±20 meters, p<0.001). Total cholesterol level was significantly reduced in EE + BPE compared with Vehicle and EE groups (p<0.05). LDL-c was lower in EE + BPE compared with the Vehicle (p<0.01). HDL-c in BPE and EE + BPE groups was significantly higher than Vehicle (p<0.001 and p<0.05, respectively). Serum triglyceride level was significantly (p<0.05) lower in BPE than the other three groups. Body weight changes were not significantly different between groups.
Conclusion: The results suggested that Bunium persicum extract is very useful in improvement of lipid profile in hypercholesterolemic animals. Supplementation of the extract to exercise significantly increased the cardiorespiratory capacity.
Key Words: Bunium persicum extract, Cardiorespiratory capacity, Endurance exercise, Hypercholesterolemia, Lipid profile
Introduction
Reports show that 17 million people die every year from cardiovascular diseases (CVD), of which about 7.6 million are due to coronary heart disease (Rosmand et al., 2008 ▶). An increase in blood lipids, especially cholesterol, plays an important role in aggravating this disease. Hypercholesterolemia is one of the major risk factors that is responsible for about 80% of coronary and cerebrovascular diseases. Contrary to a common belief that cardiovascular disease is a disease of rich men in developed nations, over 80% of CVD deaths take place in low and middle income countries (Asghary et al., 2008 ▶; Mendis et al., 2007). In the Middle East and Eastern Mediterranean countries including Iran, CVDs are considered as common health and social problem which are increasing dramatically. According to the reports from lipid and glucose study in Tehran, 8.8% of adult men and 12.7% of adult women were suffering from cardiovascular diseases in 2002 (Azizi et al., 2002 ▶).
Considering an approximately linear relationship between cholesterol level and mortality due to coronary disease, for each 20 mg/dl increase in total cholesterol level, related mortality rate will increase by 12% (Gaeini and Rajabi, 2005 ▶). Hence, adjusting lipid level is an important factor in health affairs. Undoubtedly, suitable exercise habits play an important role in this adjustment. Most of the researchers believe that physical activity (aerobic type) with moderate intensity, even of little frequency in a week, may lead to a decrease in beta lipoprotein and triglyceride, while an activity with a higher intensity, for at least two months, can result in a decrease in LDL and an increase in HDL (Dustine, 1994 ▶; Hawley, 1998; Rimmer, 1997 ▶; Stein, 2002 ▶).
On the other hand, taking herbal medicines in their traditional way has been issued a great deal in the treatment of a lot of illnesses and an improvement in sport-related abilities (strength and endurance) (Fallah, 2006 ▶; Kessler, 2001 ▶). A lot of researches have been conducted to study the effects of herbal medicine on lipid profile. For instance, Alhasan et al. (2006) reported that margarine herbal supplements and aerobic training resulted in changes in blood enzymes, lipoproteins, and lipids. In this regard, caraway (carum carvi) can decrease serum triglyceride, cholesterol, and body weight in normal and diabetic rats (Lemhadri, 2006) and prevent oxidative tissue injuries (Dadkhah and Fatemi, 2011 ▶). Therefore, it probably has a similar effect as physical activity on lipid profile. The effects of physical activity are similar to those of black caraway on lipid profile and body weight, in addition to an improvement in cardiorespiratory capacity. As there is a significant similarity between black caraway (called “Zireh Siah” in folk medicine) and Bunium persicum (B. persicum) (called “Zireh Koohi”) (Lemhadri et al., 2006), it is anticipated that having physical activity accompanied by B. persicum administration amplifies improvement in lipid profile and cardiovascular variables. Therefore, in the present study, we aimed to assess the effect of endurance exercise along with oral administration of aqueous extract of B. persicum on lipid profile and cardiorespiratory capacity in a model of hypercholesterolemic animals (male mice).
Materials and Methods
Experiments were performed on 40 adult male mice, weighing 30-40 g. The animals were housed under standard environmental conditions (231 C, and a 12 h light/12 h dark cycle) with free access to water and standard diet. Study protocol was approved by the Ethic Committee of the Kerman University of Medical Sciences, Iran (Ethic code No. 85/86 KA).
Induction of hypercholesterolemia
In order to induce hypercholesterolemia, animals were given a high cholesterol diet including standard chow supplemented with 2% pure cholesterol and 0.5% cholic acid for 4 weeks. Cholic acid was used to dissolve cholesterol before being added to the chow. This was necessary as adding cholesterol without cholic acid did not induce hypercholesterolemia in mice (Luo, 2008 ▶). Blood samples were taken from the tail vein at the beginning and at the end of the period of four weeks to assure induction of hypercholesterolemia.
Preparation and oral administration of Bunium persicum aqueous extract
B. persicum fruit was purchased from Bazar in Kerman and confirmed by a Pharmacogenosist in Department of Pharmacogenosy, Faculty of Pharmacy, Kerman University of Medical Sciences, Iran (herbarium number kf 1141). Ten grams of powdered fruit of B. persicum was mixed with 1000 ml of distilled water, boiled for 10 minutes, and then cooled. Thereafter, the solution was filtered using a Millipore filter to remove particulate material. The filtrate was then freeze-dried (Freeze drier, Eyela, Japan). The dried sample was kept away from moisture in -20 C. For administration, 8 mg of B. persicum extract was reconstituted in 4 ml of distilled water (making 2 mg/ml solution) and then given daily using an intergasteric tube (gavage) (1 ml/100 gr BW equal to 20 mg/kg body weight) (Lemhadri, 2006). The extract solution was prepared daily, just before administration.
Endurance exercise protocol
The exercise protocol in two exercised groups was similar to that of Al-Jarrah and colleagues (Al-Jarrah et al., 2007 ▶). A six-lane motorized rodent treadmill (Tecmachine, France) was used for exercise training. The exercise groups of animals were introduced to treadmill slowly over the course of a week with initial orientation and walking on the moving treadmill. The 6-week exercise protocol did not begin until induction of hypercholesterolemia and mice could run at a speed of 18 m/min. The exercise protocol was individualized for each animal, with an aim of running for 40 min/day for 5 days/week at a speed of 18 m/min.
Experimental design
After acclimatization for a week, animals were put on hypercholesterolemia regimen for four weeks (see above) and then randomly assigned to four groups of 10 including: Control (vehicle, received distilled water), Endurance exercise (EE), B. persicum extract administered (BPE), and EE + BPE groups. All groups underwent their special therapeutic protocol for 6 weeks while hypercholesterolemia regimen was continued during this period.
The BPE group received the extract for 5 days/week and training groups were put on animal treadmill for endurance exercise. The training in related groups lasted for 6 weeks. The EE + BPE received the B. persicum extract 2 hours before exercise each day while EE group received the same volume of distilled water. The control groups received a daily dose of 1 ml/100 gr body weight distilled water during this period and were also put on non-running treadmill (Al-Jarrah, 2007 ▶). This means that the control and BPE groups did not exercise but were transported daily to the training room were exposed to the same environment as the exercised groups of animals.
Weight measurement
The animal’s body weight was measured at the beginning and end of experiments (24 hours after the last session of weekly training) using a digital scale with 0.1 gram precision (Grampresicion digital scale, Canada)
Assessing cardiorespiratory capacity
Before starting the 6-week training program, all mice underwent a test to assess their capacity for endurance running in five consecutive days. The test was performed between 8 and 12 am. Assessing exercise capacity was according to speed-ramped treadmill to exhaustion (Luo, 2008 ▶). The test was performed as follow: at first, the mouse ran at a speed of 10 m/min and 0 degree slope, then, 2 m/min was added to the speed every two minutes until the mouse became exhausted. Exhaustion was referred to the time that the animal was not able to keep up with the speed of the treadmill for three times and preferred to bear the machine's shock. The machine would go off at the time of exhaustion and the exact time was registered to calculate total running distance (in meters). The best performance during 5 days before and the day after 6-week training program (as pre-test and post-test values, respectively) was considered as cardiorespiratory capacity (Al-Jarrah et al., 2007 ▶).
Serum lipids and lipoproteins measurement
Animals' tail blood samples were taken after a 12-hour fasting at the beginning and 6 weeks after initiation of experimental protocol at a similar condition. Serum samples were analyzed enzymatically for the level of total cholesterol (TC), triglyceride (TG), and high density lipoprotein (HDL-c) using commercially available diagnostic kits. Low density lipoprotein was calculated according to the formula: LDL-c = TC- (HDL-c + TG/5).
Statistical analysis
Data in the text and figures are expressed as meanSEM. One way ANOVA was used to compare between groups using the SPSS15 computer package for windows. Post-hoc test was preformed for intergroup comparisons using HSD test. Differences were considered to be significant when p<0.05.
Results
Body weight
There was no significant change in body weight during the course of experiment between groups. Body weight changed in Vehicle from 34±2.5 to 32.8±1.6, in EE from 35.4±1.8 to 33.8±1.9, in BPE from 33.8±1.1 to 31.5±1.5, and in EE + BPE from 32.3±1.4 to 28.9±1.2 grams.
Cardiorespiratory capacity
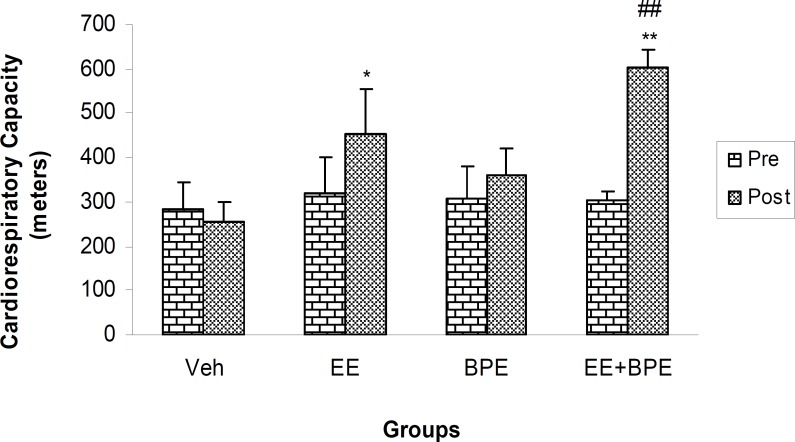
Figure 1 shows the initial and final cardiorespiratory capacity values in control (Vehicle) and experimental groups. No significant difference was found between the initial values in all groups. The difference existed between post-test values of EE with control (p<0.05), EE + BPE with both EE and BPE groups (p<0.05), and EE + BPE with control (p<0.01), with highest increase in capacity in EE + BPE group.
Figure 1.
Pre- and post-intervention values for cardiorespiratory capacity in control (Vehicle) and experimental groups. There was no significant difference between the initial (pretest) values in all groups. *=p<0.05, **=p<0.01, compared with related (post-test) control group. ##=p<0.01 compared with endurance exercise (EE) and Bunium persicum extract (BPE) groups
Lipid profile
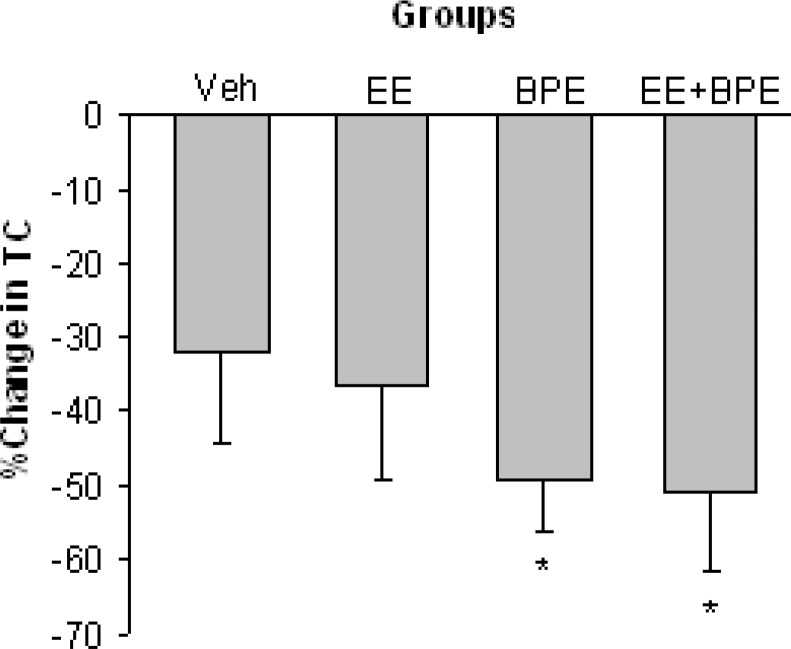
Figure 2 shows changes in total cholesterol (TC) level in experimental groups. TC was significantly reduced in BPE and EE + BPE groups compared with control group (p<0.05). The basal (end of hypercholesterolemia induction period) values of TC were 213.1±12.3 in Vehicle, 220±12.5 in EE, 242.1±6.7 in BPE, and 297.7±10.8 mg/dl in EE + BPE groups (The normal value of TC in mice is around 100 mg/dl). The cholesterol value of the EE+BPE group was significantly higher compared with the vehicle group (p<0.05).
Figure 2.
Changes in serum total cholesterol (TC) level in experimental groups. TC was significantly (p<0.05) reduced in BPE and EE + BPE groups compared with Vehicle. *=p<0.05 compared with Vehicle group
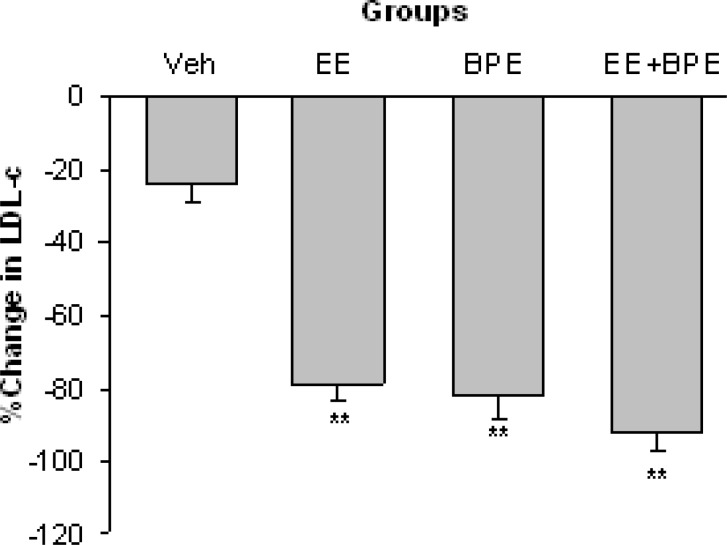
The changes in LDL-c are shown in Figure 3. The changes in this variable follow more or less the same trend of changes in TC. LDL-c was also significantly reduced in EE, BPE, and EE + BPE groups compared with control group (p<0.01). The basal (end of hypercholesterolemia induction period) values of LDL-c were 104.1±7.6 in Vehicle, 112.3±4.1 in EE, 143.2±6.7 in BPE, and 151.8± 5.1 mg/dl in EE + BPE groups (The normal value of LDL-c in mice is around 55 mg/dl).
Figure 3.
Changes in serum low density lipoprotein cholesterol (LDL-c) level in experimental groups. All three interventions reduced LDL-c significantly. **=p<0.01 compared with vehicle group
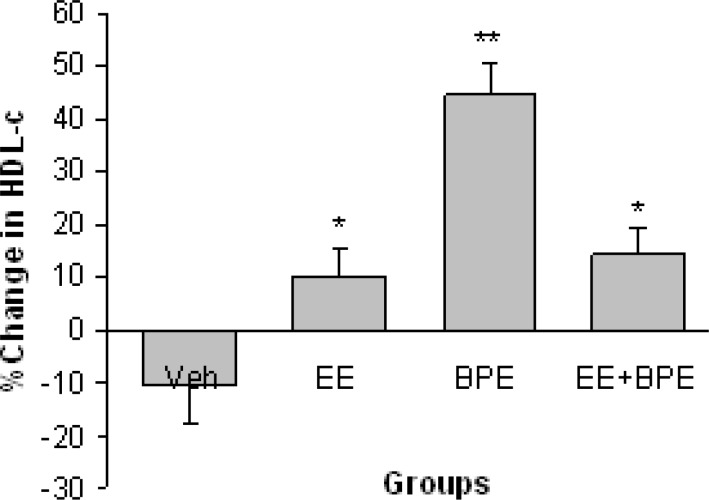
Figure 4 shows changes in HDL-c level in experimental groups. All three interventions increased HDL-c significantly (p<0.05 for EE and EE + BPE groups, p<0.01 for BPE group). The basal (end of hypercholesterolemia induction period) values of HDL-c were 92.4 ± 5.6 in Vehicle, 81.5 ± 5.9 in EE, 75.9±6.1 in BPE and 101.4±5.1 mg/dl in EE + BPE groups (The normal value of HDL-c in mice is around 30 mg/dl).
Figure 4.
Changes in serum level of high density lipoprotein cholesterol (HDL-c) in experimental groups. All three interventions increased HDL-c significantly but B. persicum extract was more potent than the other two. *=p<0.05, **=p<0.01, compared with vehicle group
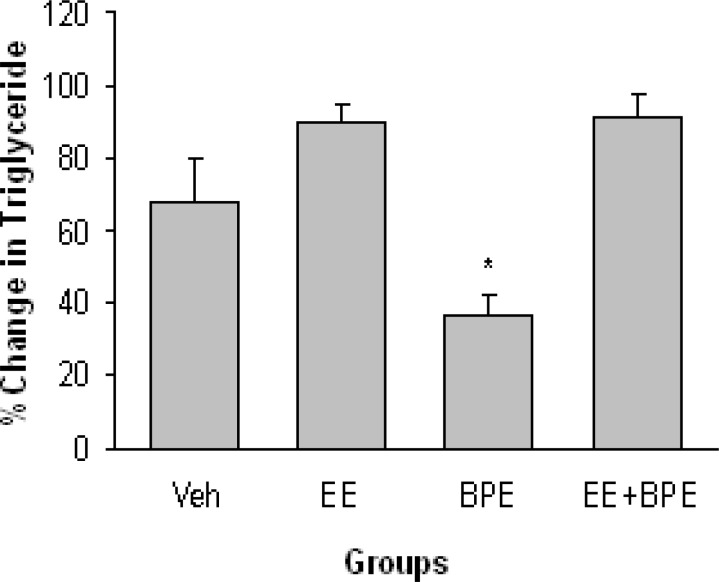
The changes in serum triglyceride (TG) level are shown in Figure 5. TG was increased in all groups, but this increment was significantly (p<0.05) lower in BPE compared with the other three groups. The basal (end of hypercholesterolemia induction period) values of TG were 80.4±12 in Vehicle, 75.8±5.4 in EE, 113.5±5.7 in BPE, and 93.1±6.9 mg/dl in EE + BPE groups (The normal value of TG in mice is around 75 mg/dl).
Figure 5.
Changes in serum triglyceride (TG) level in experimental groups. TG was increased in all groups, but this increment was significantly less in animals receiving Bunium persicum extract. *=p<0.05 compared with the other three groups
Discussion
The present study was designed to investigate the influence of oral administration of aqueous extract of B. persicum fruit for 6 weeks, alone and in combination with endurance exercise, on cardiorespiratory capacity and serum lipids, as well as on body weight in hypercholesterolemic mice.
The results showed a significant increase in cardiorespiratory capacity in the group taking extract together with endurance exercise. Despite improvement in serum lipid conditions, the group taking B. persicum alone did not set a significant higher record (Figure 1). This is true for sport nutrition supplements as well, as they cannot improve body ability in the absence of body activity. However, B. persicum in combination with exercise potentiatively increased cardiorespiratory capacity (Figure 1) that is a positive finding in regard to cardiovascular health improving strategies.
The aqueous extract of B. persicum per se caused significant decrease in TC, LDL-c, and TG concentrations along with significant increase in HDL-c (Figures 2-5). These show that B. persicum may be very effective for improving lipid profile in hyperlipidemia. B. persicum was even more effective than exercise, as it had a better effect on increasing HDL-c and on reducing TG along with an equal potency in decrement of LDL-c. Dhandapani and colleagues assessed the effect of green caraway (Cuminum cyminum L.) on lipid profile of diabetic rats (Dhandapani et al., 2002 ▶). They gave rats a diet containing green caraway for 6 weeks and concluded that this plant causes significant decrease in TG and TC in diabetic rats. The probable reason for change in lipid profile in the present study is the anti-oxidative effect of Bunium persicum. These results could be addressed to B. persicum effect considering its structural similarity with green caraway (Haidari and Seyed-Sadjadi, 2011 ▶). Lemhardi and coworkers also evaluated the effect of black caraway extract on two groups of healthy and diabetic rats and reported significant reduction in TC and TG in both groups (Lemhardi et al., 2006). HDL-c and LDL-c were not measured in their study. They suggested four possible mechanisms for lipoprotein reducing effect of black caraway including binding of cholesterol to bile acids in small bowel, increase in bile acids secretion, reduction in activity of 3-hydroxy-3-methylglutaryl coenzyme A reductase (the key enzyme for cholesterol regeneration) and decrement of NADPH needed for cholesterol and fatty acid synthesis (Sharma et al., 2003 ▶).
In another study, Haidari and colleagues reported reduction of TC and LDL-c but no change in TG level due to caraway administration in diabetic rats (Haidari et al., 2011 ▶) (. It has also been reported that black caraway can improve hypercholesterolemia by balancing lipoprotein metabolism which means more LDL-c usage via increase in its receptors and/or increase in lecithin cholesterol acyltransferase (LCAT) (Khanna et al., 2002 ▶). LCAT plays a key role in the combination of free cholesterol with HDL-c and its reverse transfer to VLDL-c or LDL-c in order to return to hepatic cells (Rajlakshmi et al., 2004 ▶). Black Caraway can also facilitate LDL-c catabolism. According to the structural similarity between B. persicum and black caraway, these mechanisms probably could also be attributed to the effects of Bunium persicum. Although the basal (pre-intervention) value of TC was higher in BPE and BPE + EE groups (Result section), the maximum reduction in TC also belonged to these groups (more than 50%, Figure 2) confirming the effectiveness of B. persicum extract as a good cholesterol-lowering agent. If this finding is reproduced in clinical studies, it may be recommended as a therapeutic strategy for cholesterol lowering in hypercholesterolemic patients.
Exercise group results are consistent with the results of similar studies conducted on animals (Ensign et al., 2002; Venditti and Dimeo, 1996 ▶). These are also similar to the finding of a study conducted by Ravikiran and coworkers in which they showed a decrease in the level of TC, TG, LDL-c, and an increase in HDL-c in rats having a 4 week swimming training (6 days a week), with the exception that in the present study, TG increased in all groups (Ravikiran et al,. 2006 ▶). In that study, a 40-minute practice a day (the same as training duration in our study) led to decrease in TG. The reason that TG level in the present study was changed reverse to the anticipated direction is not known at present but it may be due to the colic acid added to the regimen (method section above) to induce hypercholesterolemia in mice as cholic acid is one of the components of the bile that facilitates fat digestion and absorption. In this regard, higher increases in TG in the exercised groups (EE and EE + BPE) compared with the other two groups (Figure 5) may be due to the higher food consumption (more cholic acid ingestion) in these groups to compensate their higher energy expenditure.
Results from another part of the study have shown that endurance training and B. persicum did not change body weight. Our results were in contrast to the results of Asha Devi and colleagues in which mice's weight increased after 2 months of training (Asha Devi et al., 2003) and in contrast with the findings of Lemhadri and colleagues in which they showed significant body weight loss (Lemhadri et al., 2006). In addition, these results are different from those of Dhandapani and colleagues in which they reported weight gain after using green caraway (Dhandapani et al., 2002 ▶). Intensity of training program in the present study has helped to keep animals' body weight stable during training period in some ways. Not changing body weight could be an advantage for Bunium persicum, as overweighting and obesity are predisposing factors for coronary artery diseases.
Overall, the results of the present study show that B. persicum extract have a very beneficial effect on lipid profile. When its administration is accompanied by endurance training leads to significant increase in cardiorespiratory capacity. These along with keeping body weight stable which are all beneficial for prevention of cardiovascular diseases, candidate this plant for conducting more researches about its potential therapeutic application. At present, complementary studies are needed to determine the exact mechanism of B. persicum actions on cardiorespiratory capacity.
Acknowledgement:
This research was financially supported by School of Physical Education in Kerman Bahonar University and Physiology Research Center of Kerman University of Medical Sciences, Iran. We also express our terms of gratitude to Dr S. Joukar, M. Gohargazi, Ghasemzade, Sedaghat, Samadi, and Arjomandi K. for their help in the laboratory.
Conflict of interest
There is not any conflict of interest in this study.
References
- Al-Jarrah M, Pothakos K, Novikova L, Smirnova IV, Kurz MJ, Stehno-Bittel L, Lau YS. Endurance exercise promotes cardiorespiratory rehabilitation without neurorestoration in the chronic mouse model of parkinsonism with severe neurodegeneration. Neuroscience. 2007;149:28–37. doi: 10.1016/j.neuroscience.2007.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LemhadriL , HajjiJ , MichelB , EddouksM Cholesterol and triglycerides lowering activities of caraway fruits in normal and streptozotocin diabetic rats. J Ethnopharmacol. 2006;106:321–326. doi: 10.1016/j.jep.2006.01.033. [DOI] [PubMed] [Google Scholar]
- Al Hassan S, Reese KA, Mahurin J, Plaisance EP, Hilson BD, Garner JC, Wee SO, Grandjean PW. Blood lipid responses to plant stanol ester supplementation and aerobic exercise training. Metabolism. 2006;55:541–549. doi: 10.1016/j.metabol.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Asgary S, Sarrafzadegan N, Naderi GA, Rozbehani R. Effect of opium addiction on new and traditional cardiovascular risk factors: do duration of addiction and route of administration matter? . Lipids Health Dis. 2008;7:42–48. doi: 10.1186/1476-511X-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizi F, Rahmani M, Emami H, Mirmiran P, et al. Tehran lipid and glucose study, Methodology and summarized findings. 1st Ed. Tehran, Iran: Tehran Endocrine and Metabolism Research Center; 2002. pp. 40–65. [Google Scholar]
- Asha Devi S, Prathima S, Subramanyam MV. Dietary vitamin E and physical exercise: In: A. Lemhadri, L. Hajji, J.-B. Michel,, M. Eddouks. 2006. Cholesterol and triglycerides lowering activities of caraway fruits in normal and streptozotocin diabetic rats. J Ethnopharmacol. 2003;106:321–326. doi: 10.1016/j.jep.2006.01.033. [DOI] [PubMed] [Google Scholar]
- Chandra M, Rajkumar M, Debidas G. Comparative Study on Antihyperglycemic and Antihyperlipidemic Effects of Separate and Composite Extract of Seed of Eugenia jambolana and Root of Musa paradisiaca in Streptozotocin-Induced Diabetic Male Albino Rat. Ir J Pharmacol Therapeut. 2006;5(1):27–33. [Google Scholar]
- Dadkhah A, Fatemi F. Heart and kidney oxidative stress status in septic rats treated with caraway extracts. 2011;49:679–686. doi: 10.3109/13880209.2010.539618. [DOI] [PubMed] [Google Scholar]
- Dhandapani S, Subramanian VR, Rajagopal S, Namasivayam N. Hypolipidemic effect of Cuminum cyminum L on alloxan-induced diabetic rats. Pharmacol Res. 2002;46:251–255. doi: 10.1016/s1043-6618(02)00131-7. [DOI] [PubMed] [Google Scholar]
- Durstine JL, Grandjean PW, Davis PG, Ferguson MA, Alderson NL, DuBose KD. Blood lipid and lipoprotein adaptations to exercise: a quantitative analysis. Sports Med. 2001;31:1033–1062. doi: 10.2165/00007256-200131150-00002. [DOI] [PubMed] [Google Scholar]
- Durstine JL, Haskell W L. Effects of exercise training on plasma lipids and lipoproteins. Exerc Sport Sci Rev. 1994;22:477–521. [PubMed] [Google Scholar]
- Eisenberg DM, Davis RB, Ettner SL, Appel S, Wilkey S, Van Rompay M, Kessler RC. Trends in alternative medicine use in the United States, 1990-1997: results of a follow-up national survey. JAMA. 1998;280:1569–1575. doi: 10.1001/jama.280.18.1569. [DOI] [PubMed] [Google Scholar]
- Ensign WY, McNamara DJ, Fernandez ML. Exercise improves plasma lipid profiles and modifies lipoprotein composition in guinea pigs. J Nutr Biochem. 2002;13:747–753. doi: 10.1016/s0955-2863(02)00219-x. [DOI] [PubMed] [Google Scholar]
- Fallah-Hosseini H, Fakhr-zadeh H. Review of anti-hyperlipidemic herbal medicine. J Med Plants. 2006;5:9–20. [Google Scholar]
- Gaeini AA, Rajabi H. Physical fitness. Samt publication. Tehran, Iran: 2005. pp. 36–8. [Google Scholar]
- Haidari F, Seyed-Sadjadi N, Taha-Jalali M, Mohammed-Shahi M. The effect of oral administration of Carum carvi on weight, serum glucose, and lipid profile in streptozotocin-induced diabetic rats. Saudi Med J. 2011;32:695–700. [PubMed] [Google Scholar]
- Hoerger TJ, Bala MV, Bray JW, Wilcosky TC, LaRosa J. Treatment patterns and distribution of low-density lipoprotein cholesterol levels in treatment-eligible United States adults. Am J Cardiol. 1998;82:61–65. doi: 10.1016/s0002-9149(98)00227-6. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Davis RB, Foster DF, Van Rompay MI, Walters EE, Wilkey SA, Kaptchuk TJ, Eisenberg DM. Long-term trends in the use of complementary and alternative medical therapies in the United States. Ann Intern Med. 2001;135:262–268. doi: 10.7326/0003-4819-135-4-200108210-00011. [DOI] [PubMed] [Google Scholar]
- Khabazian BM, Ghanbari-Niaki A, Safarzadeh-Golpordesari A, Ebrahimi M R, ahbarizadeh F. Eur J Appl Physiol. 2009;107:351–358. doi: 10.1007/s00421-009-1133-3. [DOI] [PubMed] [Google Scholar]
- Khanna K, Rizvi F, Chander R. Lipid lowering activity of Phylanthus niruri in hyperlipidemic rats. J Ethnopharmacol. 2002;82:19–22. doi: 10.1016/s0378-8741(02)00136-8. [DOI] [PubMed] [Google Scholar]
- Kist WB, Thomas TR, Horner KE, Laughlin MH. Effects of Aerobic training and gender on HDL-c and LDL-c subfractions in yucatan miniature swine . J Exerc Physiol Online. 1999;2:7–15. [Google Scholar]
- Luo QF, Sun L Si JY, Chen DH. Hypocholesterolemic effect of stilbenes containing extract-fraction from Cajanus cajan L. on diet-induced hypercholesterolemia in mice. Phytomedicine. 2008;11:932–939. doi: 10.1016/j.phymed.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Moathar F, Shams-Ardakani MR. Guide in plant therapy. Tehran, Iran : Academic Publication of Medical Sciences; 2000. p. 39. [Google Scholar]
- Rajlakshmi D, Sharma DK. Hypolipidemic effect of different extracts of clerodendron colebrookranum in normal and high-fat diet fed rats. J Ethnopharmacol. 2004;90:63–68. doi: 10.1016/j.jep.2003.09.022. [DOI] [PubMed] [Google Scholar]
- Ravi Kiran T, Subramanyam MVV, Prathima S, Asha Devi S. Blood lipid profile and myocardial superoxide dismutase in swim-trained young and middle-aged rats: comparison between left and right ventricular adaptations to oxidative stress. J Comp Physiol. 2006;176:749–762. doi: 10.1007/s00360-006-0096-5. [DOI] [PubMed] [Google Scholar]
- Roberts S, Robergs R. Fundamental principles of exercise physiology for fitness, Performance and Health: Essentials of Strength Training and Conditioning. 3rd Edition. McGraw-Hill; 2000. [Google Scholar]
- Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y. Heart disease and stroke statistics- 2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- Rimmer JH, Looney MA. Effects of an aerobic activity program on the cholesterol levels of adolescents. Res Q Exerc Sport. 1997;68:74–79. doi: 10.1080/02701367.1997.10608868. [DOI] [PubMed] [Google Scholar]
- Stein RA, Michielli DW, Glantz , MD , Sardy H. Effects of different exercise training intensities on lipoproteins cholesterol fractions in health middle aged men. Am Heart J. 2002;119:277–283. doi: 10.1016/s0002-8703(05)80017-1. [DOI] [PubMed] [Google Scholar]
- Sharma SB, Nasir A, Prabhu KM, Murthy PS, Dev G. Hypoglycemic and hypolipidemic effect of ethanol extract of seeds of Eugenia Jambolana in alloxan-induced diabetic rabbits. J Ethnopharmacol. 2003;85:201–206. doi: 10.1016/s0378-8741(02)00366-5. [DOI] [PubMed] [Google Scholar]
- van Oort G, Gross DR, Spiekerman AM. Effects of eight weeks of physical conditioning on atherosclerotic plaque in swine. Am J Vet Res. 1987;48:51–55. [PubMed] [Google Scholar]
- Venditti P, Dimeo SD. Antioxidant Tissue damages and endurance in trained and untrained young male rats. Arch. Biochem Biophys. 1996;331:63–66. doi: 10.1006/abbi.1996.0283. [DOI] [PubMed] [Google Scholar]
- Volaklis KA, Spassis AT, Tokmakidis SP. Land versus water exercise in patients with coronary artery disease: effects on body composition, blood lipids, and physical fitness. Am Heart J. 2007;154:e561–56. doi: 10.1016/j.ahj.2007.06.029. [DOI] [PubMed] [Google Scholar]
- Von Duvillard SP, Foxall TL, Davis WP, Terpstra AH. Effects of exercise on plasma high-density lipoprotein cholesteryl ester metabolism in male and female miniature swine. Metabolism. 2000;49:826–832. doi: 10.1053/meta.2000.6746. [DOI] [PubMed] [Google Scholar]