Abstract
The extracts and pure saponins from the roots of Platycodon grandiflorum (PG) are reported to have a wide range of health benefits. Platycosides (saponins) from the roots of PG are characterized by a structure containing a triterpenoid aglycone and two sugar chains. Saponins are of commercial significance, and their applications are increasing with increasing evidence of their health benefits. The biological effects of saponins include cytotoxic effects against cancer cells, neuroprotective activity, antiviral activity, and cholesterol lowering effects. Saponins with commercial value range from crude plant extracts, which can be used for their foaming properties, to high purity saponins such as platycodin D, which can be used for its health applications (e.g., as a vaccine adjuvant). This review reveals that platycosides have many health benefits and have the potential to be used as a remedy against many of the major health hazards (e.g., cancer, obesity, alzheimer’s) faced by populations around the world. Methods of platycoside purification and analysis are also covered in this review.
Keywords: biological effect, health benefit, platycodon grandiflorum, platycoside, saponin
INTRODUCTION
Over the last decade, interest in platycodin saponins has been renewed due to the discovery of their novel pharmacological potential for treating adult diseases such as hyperlipidemia, hypertension, diabetes, and obesity (1,2). Recently, Platycodon grandiflorum (PG) extract and some of the major components of PG, such as platycodin D (PD) and platycodin D3, have been found to have diverse pharmacological activities, including anti-inflammatory activity (3,4), anti-allergy activity (5), the ability to augment immune responses (6), the ability to stimulate apoptosis in skin cells (7), antiobesity and hyperlipidemia effects (2,8), and a protective effect against oxidative hepatotoxicity (9).
PG roots (Platycodi Radix, PR) contain a mixture of different chemical compounds that may act individually, additively, or in synergy to improve human health. The main bioactive components of PR are platycodin saponins. PG [i.e., balloon flower (English), doraji (Korean), kikyo (Japanese), jiegeng (Chinese)], which belongs to the Campanulaceae family, is used as a herbal medicine and as a food in Asia. In Korea, the 2001 annual domestic consumption of the plant as a food material was estimated to be over 4,000 tonnes (10). PG roots are typically used when preparing Korean salad, cold soup, dried or fried vegetables, vegetables soaked in traditional Korean sauces, and mixed vegetables with spices. PG roots can also be pan fried and served alone.
In Korea, PG roots that have been cultivated for 4 years are used to treat bronchitis, asthma, pulmonary tuberculosis, diabetes, and inflammatory diseases (11,12). In traditional Chinese medicine, PG is used as an expectorant and antitussive to treat coughs, colds, sore throats, tonsillitis, and chest congestion. The applications of saponins include use as additives in the food and cosmetic industries, use as wetting agents in the agriculture and photography industries, and use as adjuvants in the pharmaceutical industry (13). The commercial significance, expanding applications, and increasing evidence of the health benefits of saponins have encouraged the development of processes that will allow for commercial-scale production of saponins from natural sources (14).
In many cases, the total triterpenoid saponins, rather than the pure components, are treated as the active ingredients in traditional herbal remedies. This may make the quality control of natural drugs difficult and complicate the search for potential new lead compounds (15) because modern allopathy is usually focused on developing a patentable single compound, or a magic bullet, that can treat specific conditions.
The antioxidant properties of plant extracts are of great interest due to their potential for use as natural replacements for synthetic additives. The antioxidant potential of functional foods attracts much attention because these foods are convenient and highly effective (16). Jeong et al. (17) confirmed that the butanol fraction of the aerial parts of PG has strong antioxidant activities that are correlated with its high concentration of phenolic compounds, particularly luteolin-7-O-glucoside and apigenin-7-O-glucoside. Ryu et al. (16) found that saponins isolated from PG roots have potent antioxidant activities that differ according to the structure of the aglycones and the number of attached sugar residues, suggesting that the antioxidant activity of PG accounts for its beneficial effects against oxidative stress. While there is little published information available regarding the antioxidant activity of single saponins isolated from PG, some triterpenoid saponins and their glycosides have been isolated from PG and are reported to have antiproliferative activities in human tumor cells and protective effects against ischemia/reperfusion injury (10). This paper reviews the biological activities of platycosides and methods for their purification and analysis.
CHEMICAL CONSTITUENTS OF P. grandiflorum
The roots of PG are reported to contain large amounts of carbohydrate (at least 90%), protein (2.4%), lipid (0.1%), ash (1.5%), and 24 kinds of triterpenoid saponins (e.g., platycodin A, platycodin C, platycodin D, platycodin D2, polygalacin D, and polygalacin D2) (about 2%) (18). The carbohydrates present in PG are monosaccharides (e.g., glucose, fructose), disaccharides (e.g., saccharose), trisaccharides (e.g., kestose), and some polysaccharides (e.g., inulin, platycodin). Our study established that PG roots also contain relatively small amounts of phenolics.
PLATYCOSIDES FROM ROOTS OF P. grandiflorum
Platycosides are saponins of PG. Saponins are a diverse group of compounds found in many plants, which are characterized by their structure containing a triterpene or steroid aglycone and one or more sugar chains. Glucose, galactose, glucuronic acid, xylose, and rhamnose are commonly bound monosaccharides. Saponins are polar in nature. Thus, they are freely soluble in water, but insoluble in nonpolar solvents. Saponins have a diverse range of physical, chemical, and biological properties because of their structural complexity. They are usually amorphous substances with high molecular weights. Due to the presence of a lipid-soluble aglycone and a water-soluble sugar chain in their structure, saponins are surface active compounds with detergent, wetting, emulsifying, and foaming properties. Saponins are classified as triterpenoid saponins or steroid saponins on the basis of their aglycone structure. Most triterpenoid saponins are derivatives of one of the triterpenes (e.g., oleanane, ursane, lupane), while steroid saponins generally possess a typical steroid skeleton with 2 extra rings: E, a furan structure and F, a pyran structure.
Many saponins have hemolytic properties and are toxic to cold-blooded animals, especially fish (19). The steroidal saponins are important precursors for steroid drugs, including anti-inflammatory agents, androgens, estrogens, and progestins. The triterpene saponins exhibit various pharmacological activities, including anti-inflammatory, molluscicidal, antitussive, expectorant, analgesic, and cytotoxic effects. In general, saponins have a variety of biological effects, including antioxidant, immunostimulant, antihepatotoxic, antibacterial, anticarcinogenic, antidiarrheal, antiulcerogenic, antioxytocic, hypocholesterolemic, anticoagulant, hepatoprotective, hypoglycemic, neuroprotective, and anti-inflammatory activities. In addition, saponins are useful for the treatment of diabetic retinopathy, the inhibition of dental caries, and the prevention of platelet aggregation (14,20). Notably, saponins can also activate the mammalian immune system, which has led to significant interest in their potential as vaccine adjuvants (21). As adjuvants, saponins stimulate the production of high levels of antibody against T-dependent and T-independent antigens; the induction of mouse IgG1, IgG2b, and IgG2a isotypes; and the induction of cytotoxic T lymphocyte responses (22).
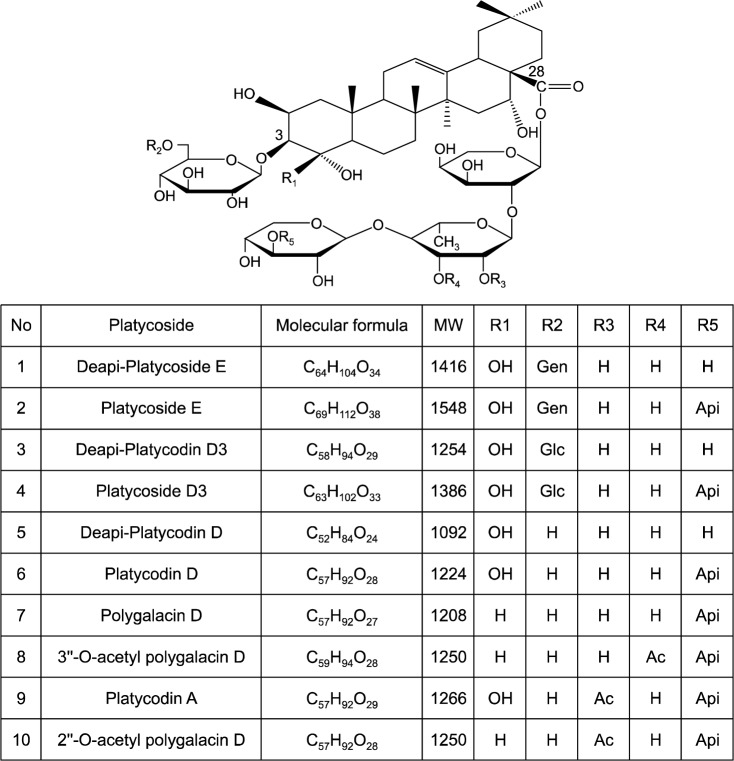
Platycosides are typically composed of an oleanane backbone with two side chains: a glucose unit attached by an ether linkage at the C-3 position of the triterpene and an ester linkage between C-28 and arabinose (23). Hydrolysis of PG saponins yields an aglycone known as “sapogenin”. Several substituents (i.e., methyl groups, carboxyl groups, and hydroxymethyl groups) have been identified at the C-4 position of the platycoside backbone (24).
To date, more than 30 saponins, including deapi-platycodin D (dPD) and PD, have been discovered and identified in PG (25,26). In addition, ten major triterpenoidal saponins have been identified in PR, including deapi-platycoside E, platycoside E, deapi-platycodin D3, platycodin D3, dPD, PD, polygalacin D, 3″-O-acetyl polygalacin D, platycodin A, and 2″-O-acetyl polygalacin D (27) (Fig. 1).
Fig. 1.
Structure of platycosides. Gen, glucose-glucose; Glc, glucose; Api, apiose; Ac, acetyl. Ha et al. (15).
The contents and types of platycosides isolated during extraction depend on the method used and the mode of PR cultivation (23). For example, the major saponins reported by Ha et al. (15) were platycoside E, PD, and polygalacin D, whereas the major saponins found by Saeki et al. (28) in commercial, botanical, and wild samples were platycodin A, platycodin C, and PD. Yan et al. (29) determined the composition of platycosides in 11 specimens of PG harvested from different locations in Korea and China. Deapio-platycoside E, platycoside E, platycodin D3, platyconic, platycodin D2 and platycodin were found in all PG specimens, but the content of each platycoside varied depending on petal color (i.e., white or blue) and geographical location of the PG plant. Saponin concentrations were higher in blue flowered PG than in white flowered PG (Table 1).
Table 1.
Saponin content of blue and white balloon flowers Yan et al. (29)
| No1) | Flower color | Saponin (μg/g)
|
|||||
|---|---|---|---|---|---|---|---|
| Deapio-Platycoside E | Platycoside E | Platycodin D3 | Platyconic acid | Platycodin D2 | Platycodin D | ||
| 1 | Blue | 73.1±5.2 | 309.8±32.6 | 67.2±6.3 | 182.4±20.3 | 96.8±11.3 | 441.5±39.5 |
| 2 | 71.7±8.1 | 249.8±21.8 | 65.1±5.3 | 272.7±24.8 | 104.9±12.5 | 555.5±62.2 | |
| 3 | 91.3±8.3 | 138.4±11.6 | 56.7±6.2 | 240.1±27.1 | 76.7±8.4 | 457.5±50.8 | |
| 4 | 31.5±2.5 | 115.2±10.3 | 43.1±3.6 | 174.2±15.8 | 80.9±6.9 | 371.4±34.6 | |
| 5 | 38.4±3.2 | 144.8±15.2 | 40.2±3.9 | 197.7±21.8 | 117.9±12.6 | 533.2±47.3 | |
| 6 | 40.2±3.7 | 148.4±15.6 | 46.9±4.7 | 190.9±17.5 | 93.1±10.4 | 401.6±37.1 | |
| 7 | White | 36.4±4.4 | 139.2±12.9 | 37.0±3.4 | 140.8±12.4 | 56.0±8.2 | 255.8±31.6 |
| 8 | 35.7±4.2 | 103.7±9.4 | 44.8±5.1 | 169.6±17.8 | 58.2±5.6 | 323.1±36.2 | |
| 9 | 30.8±2.8 | 110.2±10.7 | 39.9±4.3 | 152.2±13.6 | 50.1±4.8 | 258.7±23.5 | |
| 10 | 41.1±3.7 | 116.5±12.5 | 42.4±3.8 | 204.2±18.5 | 68.7±7.4 | 410.6±38.4 | |
| 11 | 27.0±2.3 | 134.8±11.3 | 37.8±3.5 | 140.5±13.6 | 61.2±5.7 | 335.1±30.9 | |
1~5 and 7~9 are PGs produced in china; 6, 10, and 11 are PGs produced in Korea.
Values presented are the mean±standard deviation of triplicates.
BIOLOGICAL ACTIVITIES OF PLATYCOSIDES
Studies demonstrating the physiological, immunological, and pharmacological properties of platycosides have generated considerable clinical interest in these substances. Platycodin saponins are responsible for a variety of biological effects, including anti-inflammation, anti-allergy, and anti-tumor activities. Platycodin saponins can also augment the immune response and stimulate apoptosis in skin cells (30–33).
There is great interest in the bioactivities and commercial use of platycosides, including dPD and PD (24,34). PD is the major contributor to the biological activity and content of PG saponins. Several previous studies have reported that the bioactivities of PD, the major component of platycosides, are superior to those of other saponins (8,23,35–37). PD is abundant in PR and has been used as an indicator in quality evaluations and in pharmacological research.
PD consists of an aglycone with one unbranched sugar chain (one monosaccharide residue) attached to C-3, one unbranched sugar chain (four monosaccharide residues) attached to C-28, and no acetyl domain (Fig. 1). This saponin is well known as a potent triterpenoid saponin with various pharmacological activities.
The most widely used saponin-based adjuvants are Quil A and its derivative, QS-21. Quil A and QS-21 are isolated from the bark of Quillaja saponaria Molina. Both adjuvants have been evaluated in numerous clinical trials (21). The unique capacity of Quil A and QS-21 to stimulate both the Th1 immune response and the production of cytotoxic T-lymphocytes against exogenous antigens makes them ideal for use in subunit vaccines, vaccines directed against intracellular pathogens, and therapeutic cancer vaccines. However, Quillaja saponins have serious disadvantages, including high toxicity, an undesirable hemolytic effect, and instability in an aqueous phase, which limits their use as vaccine adjuvants (21). These disadvantages have driven the search for other natural sources of saponin-based adjuvants. The use of PD as an adjuvant has several advantages over the use of QS-21. For example, PD is less hemolytic and is very stable in aqueous solutions. Furthermore, plant resources from which PG are obtained are readily available.
Antioxidant activity
In the body, excessive accumulation of reactive oxygen species, such as superoxide anions, hydrogen peroxide, hydroxyl radicals, and peroxynitrite radicals, can lead to cumulative protein, lipid, and DNA damage, resulting in oxidative stress. Oxidative stress has been implicated in a wide variety of pathological processes, including cancer, diabetes mellitus, steatohepatitis, atherosclerosis, neurological degeneration, and inflammatory diseases.
Ryu et al. (16) used the total oxidant-scavenging capacity (TOSC) assay to investigate the antioxidant activity of 5 platycosides. The results of their study indicate that PG saponins have potent antioxidant activities that differ according to the structure of the aglycones and the number of attached sugar residues. Specific TOSC (sTOSC) values were obtained from the slope of the linear regression lines for the TOSC curves. PD exhibited the highest antioxidant activity against peroxyl radicals (324.4±31.4 TOSC/mM), followed by polygalacic acid (aglycone form, Fig. 2) (229.0±30.2 TOSC/mM), platycodigenin (aglycone) (199.1±43.2 TOSC/mM), deapioplatycosides E (162.3±24.5 TOSC/mM), and platycoside E (117.3±43.9 TOSC/mM). The sTOSC values of all of the platycosides tested were lower than that of the glutathione (GSH) control (378.0±66.9 TOSC/mM).
Fig. 2.
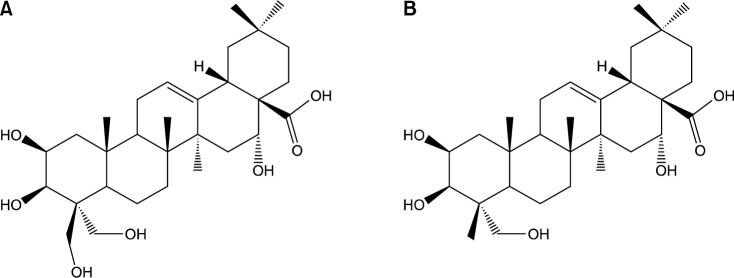
Structures of platycodigenin (A) and polygalacic acid (B) isolated from PG. Ryu et al. (16).
TOSC values of platycodigenin, deapioplatycoside E, PD, and platycoside E against peroxynitrite were 2.35-, 1.27-, 1.02-, and 0.75-fold that of GSH, respectively. Polygalacic acid did not exhibit any scavenging capacity against peroxynitrites. All platycosides studied, except for platycodigenin, exhibited weak peroxynitrite-scavenging capacities, compared with their peroxyl radical-scavenging capacity. Despite the fact that the antioxidant capacities of pure platycosides against peroxyl radicals are lower than that of GSH, platycosides do have beneficial effects against oxidative stress.
Antiproliferative effect on human tumor cells
Platycosides can be used as anticancer agents. Anticancer agents play a curative role in a damaged system by triggering the apoptosis signaling system in cancer cells and inhibiting the proliferation of the cancer cells. Choi et al. (10) reported that saponins with a platycodigenin unit or a polygalacic acid unit (Fig. 2) as a sapogenin demonstrated significant inhibitory effects on the proliferation of a small panel of cultured human tumor cells. Zhang et al. (38) reported that two new platycosides (i.e., 3-O-β-D-glucopyranosyl-2β,12α,16α,23,24-pentahydroxyoleanane-28(13)-lactone, 3-O-β-D-glucopyranosyl-(1→3)-β-D-glucopyranosyl-2β,12α,16α,23α-tetrahydroxyoleanane-28(13)-lactone) have cytotoxic activity against human ECA-109 cells. These two new platycosides had IC50 values of 0.649 μg/mL and 0.503 μg/mL, respectively. Lee et al. (39) reported that a 7:3 petroleum ether and ethyl ether mixture fraction of platycosides was highly cytotoxic against multiple human cancer cell lines (i.e., HT-29, HRT-18, and HepG2).
Antiobese and hypolipidemic effect
Obesity is associated with complications such as coronary heart disease, diabetes, hyperlipidemia, and hypertension. PG is known to improve the insulin resistance and lipid profile of rats with diet-induced obesity (40). The saponins found in PG are reported to inhibit intestinal reuptake of bile acids and increase fecal excretion of bile acids, resulting in decreased serum cholesterol and blood lipid concentrations (1). Park et al. (41) reported that a PG extract significantly inhibited pancreatic lipase activity in a dose dependent manner. In addition, a 10 mg/mL dose of PG extract decreased pancreatic lipase activity by approximately 50%. Inhibition of pancreatic lipase activity by PG extracts results in decreased lipolysis and decreased absorption of dietary fat by the small intestine. Previous reports indicate that PD has a regulatory effect on cholesterol metabolism and an antioxidant effect on hyperlipidemic emulsion-reduced rats (42). These findings reveal that PD has potential for use as a therapeutic agent for the treatment of hyperlipidemia. Zhao et al. (43) found that platycosides had profound effects on the obesity control and lipid metabolism, resulting in a reduction of LDL-cholesterol in diet-induced obese rats. These findings suggest that platycosides have greater anti-obesity, hypolipidemic, and liver protective effects than previously thought. Both the in vivo and in vitro results from Zhao et al. (37) demonstrate the potential value of PD as a novel cholesterol-lowering and anti-atherogenic candidate.
Anti-allergic activity
Allergy is a disorder of the immune system. Allergic reactions occur in response to normally harmless environmental substances known as allergens. An allergy is characterized by excessive activation of mast cells and basophils by a type of antibody known as IgE, resulting in an extreme inflammatory response (44). There are several allergic mediators, including interleukin-6 (IL-6), prostaglandin D2 (PGD2), leukotriene C4 (LTC4), β-Hexosaminidase (β-Hex), and cyclooxygenase-2 (COX-2). Oh et al. (45) reported that inhibition of phorbol 12-myristate 13-acetate (PMA)+A23187 by a PR ethanol extract induced the production of IL-6, PGD2, LTC4, β-Hex, and COX-2. These results indicate that platycosides have the potential to be used for allergy treatment.
Antimicrobial activities of Platycodon grandiflorum
Lee et al. (46) investigated the antioxidant and antimicrobial activities of hot-water and methanol extracts of raw and black PG. The antimicrobial activities of PG extracts were studied against bronchus disease bacteria (i.e., Mycobacterium sp., Staphylococcus aureus, Klebsiella pneumoniae, Corynebacterium diphtheriae, and Streptococcus pyogenes) and food poisoning bacteria (i.e., Escherichia coli O157:H7 and Bacillus cereus). Black PG was prepared by steaming raw PG at 60°C for 15 days, followed by drying at 30°C for 3 h. The methanol extract from the black PG contained higher levels of total polyphenols than the methanol extract of the raw PG. The black PG hot-water extract had the highest total flavonoid concentration (7.94 mg QE/g). The black PG methanol extract had the highest radical scavenging activity. This was attributed to the high total polyphenol concentration of the black PG methanol extract. The hot-water extract of black PG exhibited higher antimicrobial activities than the methanol extract. This was attributed to the high total flavonoid concentrations in the processed PG. Zhang et al. (47) also reported a clear correlation between antimicrobial activities and the flavonoid contents of 30 ethanol plant extracts, a finding that is in agreement with the literature. To our knowledge, the role of saponins in the antimicrobial activities of PG extracts has not been fully studied.
Kim et al. (18) developed a pharmaceutical composition from PG root extract and/or PG saponin components. This product was useful as an antiviral agent for the prevention or treatment of hepatitis C. Because components of this invention are harmless to humans and inhibit the proliferation of the hepatitis C virus, this product can be used effectively as a preventive or a therapeutic agent for hepatitis C (18).
Neuroprotective activity
Alzheimer’s disease (AD), a progressive neurodegenerative disease, is a major health problem in developed countries. AD accounts for approximately 50~70% of all types of dementia, followed by vascular dementia (30~40%), mixed dementia (10±15%), and other dementing illnesses (10~15%) (48). AD is associated with the accumulation of L-glutamate deposits in senile plaques and neurofibrillary tangle lesions in specific areas of the brain (49). Platycosides have the potential to be used as neuroprotective agents. Previously, fresh roots of PG were eaten as pickles to prevent NF-kappaB activity (35). NF-kappaB is a protein complex that controls the transcription of DNA and is understood to be responsible for cytokine production and cell survival. Ahn et al. (35) explored the effect of PD on the apoptosis of immortalized keratinocytes (HaCaT). The results of their study demonstrate that NF-kappaB activation plays a crucial role in the PD-induced apoptosis of human HaCaT cells. Son et al. (49) reported that an aqueous extract of PR had a neuroprotective effect against glutamate-induced toxicity in primary cultured rat cortical cells. Further investigations by the same group revealed that platycodin A had significant neuroprotective activities against glutamate-induced toxicity, exhibiting a cell viability of approximately 50% at concentrations ranging from 0.1 μM to 10 μM.
Metabolism and absorption of PG metabolites in the gastrointestinal tract
Platycosides can be converted into smaller and more active compounds that are easily absorbed by the human body. Despite the potent biological effects of platycosides, only a few studies characterizing human intestinal metabolism of platycosides have been conducted. These studies show that the metabolism of platycosides by intestinal bacteria may affect their bioavailability, their overall absorption in the gastrointestinal tract, and their biological activities in vivo.
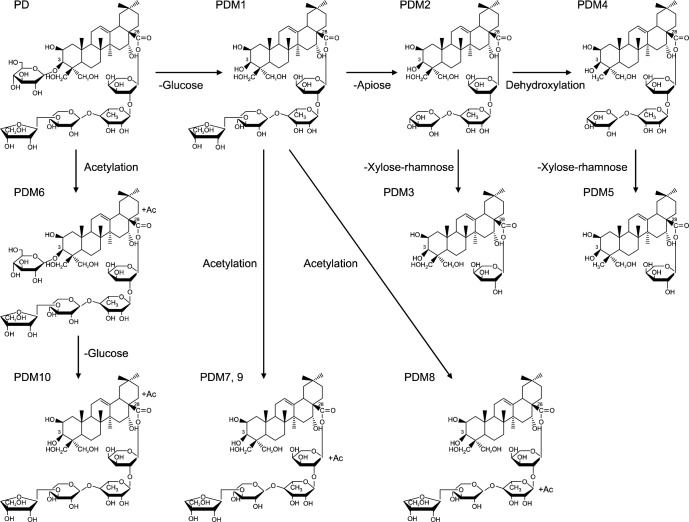
Ha et al. (24) applied an HPLC electrospray ionizationtandem mass spectrometry (LC/ESI-MSn) approach to reveal the complex platycoside metabolites produced by human intestinal bacteria (bacteroides JY-6). Their findings indicate that the fermentation of platycosides using intestinal bacteria yielded 11 metabolites (Fig. 3) that were small and relatively non polar. Fig. 4 shows the proposed metabolism pathway of platycosides in the human intestine. As shown in Fig. 4, the transformation of platycosides in the intestinal tract involves the sequential loss of sugars linked to the aglycone at the C-3 and C-28 positions. Bacteroides transform PD into various metabolites by cleavage of the glucose attached to the C-3 position of the aglycone, followed by the sequential hydrolysis of the apiosyl, xylosyl, rhamnosyl, and arabinosyl sugars attached to C-28 of the aglycone. Acetylation of PD and its metabolites can occur at the aglycone, arabione, or rhamnose.
Fig. 3.
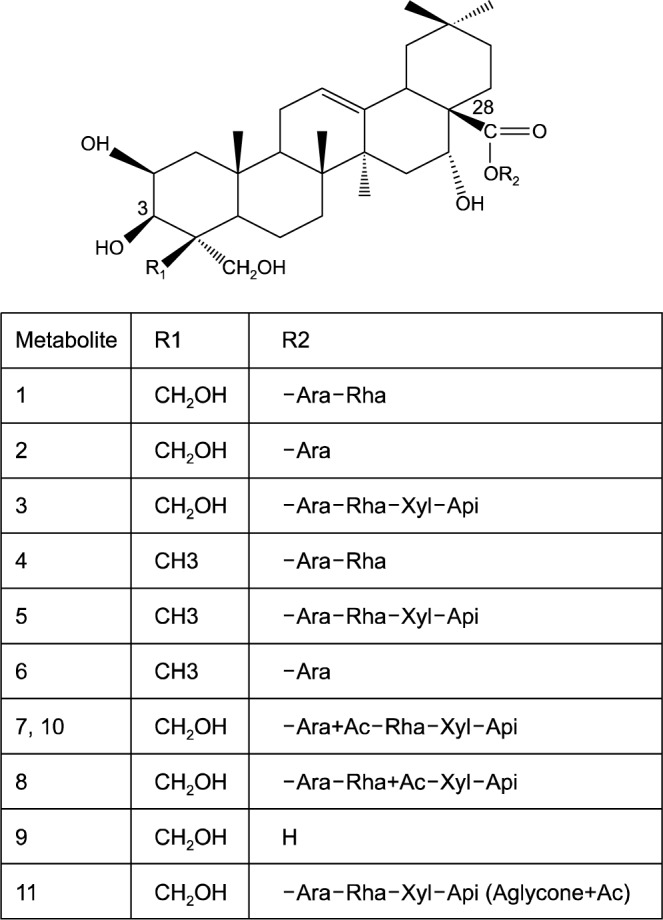
The proposed structure of platycoside metabolites transformed by human intestinal bacteria. Rha, α-L-rhamnose (pyra-nose); Ara, α-L-arabinose (pyranose); Xyl, β-D-xylose; Api, β-D-apiose (furanose); Ac, acetyl. Ha et al. (24).
Fig. 4.
Proposed metabolic pathways of platycodin D by human intestinal microorganisms. PD, platycodin D; PDM, platycodin D metabolite. Ha et al. (24).
BIOTRANSFORMATION OF PLATYCOSIDES
Active platycosides are of great significance to the pharmaceutical industry. The aglycone moiety linked only with a 3-O-glycosyl chain (i.e., a platycoside without the 28-O-glycosyl chain) is called prosapogenin (PRS). Zhao et al. (43) reported that, in obese rats, PRS-D (i.e., PD without C-28 sugar residues) was associated with a 30% increase in pancreatic lipase inhibition, while C-28 sugar residues alone did not inhibit lipase. In addition, PD with short 28-O-sugar chains (e.g., dPD; dexyl-platycodin D) is reported to have increased biological activity.
The large-scale biotransformation of platycosides to more biologically active compounds is necessary to carry out pharmacological studies and to discover new medicinal drugs. Recently, a variety of methods, including mild acid hydrolysis, alkali treatment, and microbial conversion, have been applied to the preparation of platycosides (50). However, chemical methods inevitably produce side-reactions and environmental pollution (51). There is little published information on the use of microorganisms to produce PG metabolites by modifying PG saponins.
Wie et al. (52) used Aspergillus niger to modify platycosides, but these preparation processes were time-consuming and had a low selectivity and a low conversion rate. Enzymatic preparation methods have been proposed as an alternative to the above preparation methods. Because of their high specificity, yield, and productivity, enzymatic preparation methods have been implicated as the most promising method for the preparation of active constituents via the selective hydrolysis of the sugar moieties (53–55). Wie et al. (52) used an A. niger crude enzyme extract to transform PD to many partially degraded glycosides. Transformed PDs were isolated and subjected to various tests. One of the transformed PDs had a shorter C28 side chain and exhibited considerably reduced V79-4 cell (Chinese hamster lung fibroblasts) cytotoxicity, considerably reduced erythrocyte hemolytic toxicity, and increased nitrite-scavenging activity. In addition, the transformed PD sensory scores for pungency, bitterness, and aftertaste were better than those of PD. This confirms that biotransformation can yield metabolites with improved bioactivities, improved sensory values, and reduced toxicity. Ha et al. (24) reported that successful enzymatic transformation of platycosides (e.g., platycodin E and platycodin D3) can enrich PD in a complicated PG medicinal plant extract.
PURIFICATION AND ANALYSIS OF PLATYCOSIDES
The separation of individual saponins is still complicated and time consuming (56). In most plant species, saponins occur as a multi-component mixture of compounds with similar polarities. For qualitative and quantitative analysis, crude extracts of PR must be purified. The isolated individual platycosides can be identified and characterized by nuclear magnetic resonance (NMR) and mass spectrometry (MS).
A common method for the preliminary purification of saponins following extraction involves partitioning saponins between aqueous extracts and a water immiscible solvent such as n-butanol (57). Partitioning is one of the simplest separation methods and is widely used as an initial extract purification step. Combinations of miscible and immiscible solvents are used to separate the phytochemicals making up the extract. This method relies on the ability of the components to dissolve in water or in an organic phase. Ethyl acetate and butanol are organic solvents that are commonly used in the purification of platycosides. First, ethyl acetate is used to extract relatively non-polar compounds present in the solution. Then, the water-saturated butanol solvent is used to remove the polar platycosides from the water solution. A rotary evaporator with a temperature of less than 40°C is commonly used to evaporate off the butanol solvent. The saponins are then dissolved in the appropriate solvents for further purification by open column chromatography and high performance liquid chromatography (HPLC).
Chromatography techniques have been instrumental in the separation of natural products. Thin layer chromatography (TLC) is one of the fastest and most widely used chromatographic techniques. TLC is used as a supporting technique in the analysis of saponin fractions obtained from column chromatography. TLC can also be used to confirm purity and to identify isolated compounds. This method employs glass or aluminum plates pre-coated with a sorbent (e.g. silica gel) of a thickness that can be modified to accommodate the amount of sample to be loaded. The compound mixture is loaded on the preparative or analytical plates about 1 cm from the bottom of the plate. Loaded plates are then lowered into a tank containing the solvent. The solvent migrates up the plates and separates the compound mixture according to the polarity of the components. Several reagents are available for visualization of the separated materials. Because this technique allows a large number of samples be analyzed or separated simultaneously, TLC has the advantage of being a highly cost-effective qualitative technique. Its drawbacks include poor detection, poor control, and poor elution.
The HPLC method of separation is very popular and is widely used for the analysis and isolation of bioactive natural products. The analytical sensitivity of this method can be enhanced by the type of detector used. UV detectors, such as photodiode array (PDA) detectors, enable the acquisition of the UV spectra of eluting peaks between 190 nm to 800 nm. The use of PDA UV detection is advantageous because it allows one to detect compounds with poor UV characteristics. This is particularly useful in the analysis of natural products such as terpenoids or polyketides, which may not necessarily have chromophores that will rise to a characteristic UV signature.
HPLC is the pharmaceutical industry’s method of choice for analyzing most natural products. However, the high cost of this machine and its consumables are the major drawback of this method. Normal HPLC and reversed-phase HPLC (RP-HPLC) are frequently used for the separation, identification, and purification of saponins. However, RP-HPLC provides the best separation of saponins. This technique is rapid, selective, and highly sensitive. A variety of stationary and mobile phases can be used to separate saponins by HPLC. PR saponins have been separated and quantified by HPLC with a evaporative light scattering detector (ELSD), a C18 column, and an aqueous acetonitrile gradient mobile phase (15). Most studies concerned with triterpene saponins prefer HPLC-ultraviolet (UV) chromatographic conditions with a C18 column and an aqueous acetonitrile gradient mobile phase for the separation of PD (56).
Saponins are usually difficult to detect by HPLC-UV because most lack a strong UV chromophore (58). Generally, after separation by a reversed-phase C18 column, saponins are monitored at a lower UV wavelength (from 200 to 210 nm). Because saponins require lower UV wavelengths for detection and acetonitrile has a lower absorbance at these wavelengths than methanol, acetonitirile-water gradients are the mobile phase of choice for HPLC-UV detection of saponins (59).
POTENTIAL RISKS OF PHYTOMEDICINE ON HEALTH
Recent publications have highlighted severe negative side effects of certain herbal products (60,61). These side effects may occur through several different mechanisms, including direct toxic effects of the herbs, effects of contaminants, and interactions with drugs or other herbs. Side effects may also occur due to contamination of herbal products by heavy metals, including lead, mercury, and arsenic, or other undeclared pharmaceuticals that are purposefully and illegally added to the herbs to produce a desired effect (62). There is limited information available regarding the safety of PG medicinal plants and platycosides for clinical use. In a single oral dose toxicity test of PD in mice, Lee et al. (63) reported that up to 2,000 mg of PD/kg could be administered to female and male mice without causing treatment related mortality, abnormal clinical signs, significant changes in body or organ weights, or meaningful histopathological changes in 14 principle organs. They concluded that the LD50 (50% lethal dose) and the approximate LD of a single oral treatment of playtcodin D in female and male mice was over 2,000 mg/kg. The Korean Food and Drug Administration (KFDA) (64) guidelines indicate that the maximum platycodin D dosage recommendation is 2,000 mg/kg.
CONCLUSION
This review reveals that platycosides have numerous health benefits and the potential to be used as a remedy against a number of the major health hazards (e.g., cancer, obesity, and alzheimer’s) faced by many countries. However, a lot of work still needs to be done to explore and confirm some of the claimed health benefits of PG saponins and to develop commercially feasible procedures that can address the processing challenges posed by the complex nature of PG saponins.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Han LK, Xu BJ, Kimura Y, Zheng YN, Okuda H. Platycodi radix affects lipid metabolism in mice with high fat diet-induced obesity. J Nutr. 2000;130:2760–2764. doi: 10.1093/jn/130.11.2760. [DOI] [PubMed] [Google Scholar]
- 2.Han LK, Zheng YN, Xu BJ, Okuda H, Kimura Y. Saponins from Platycodi radix ameliorate high fat diet induced obesity in mice. J Nutr. 2002;132:2241–2245. doi: 10.1093/jn/132.8.2241. [DOI] [PubMed] [Google Scholar]
- 3.Ashok BT, Ali R. The aging paradox: free radical theory of aging. Exp Gerontol. 1999;34:293–303. doi: 10.1016/s0531-5565(99)00005-4. [DOI] [PubMed] [Google Scholar]
- 4.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 5.Halliwell B. Oxidative stress and neurodegeneration: where are we now? J Neurochem. 2006;97:1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- 6.Halliwell B. Oxidative stress and cancer: have we moved forward? Biochem J. 2007;401:1–11. doi: 10.1042/BJ20061131. [DOI] [PubMed] [Google Scholar]
- 7.Tiwari AK. Imbalance in antioxidant defense and human diseases: multiple approach of natural antioxidant therapy. Curr Sci. 2001;81:1179–1187. [Google Scholar]
- 8.Zhao HL, Harding SV, Marinangeli CP, Kim YS, Jones PJ. Hypocholesterolemic and anti-obesity effects of saponins from Platycodon grandiflorum in hamsters fed atherogenic diets. J Food Sci. 2008;73:H195–H200. doi: 10.1111/j.1750-3841.2008.00915.x. [DOI] [PubMed] [Google Scholar]
- 9.Evans P, Halliwell B. Micronutrients: oxidant/antioxidant status. Br J Nutr. 2001;85:S67–S74. [PubMed] [Google Scholar]
- 10.Choi YH, Yoo DS, Cha MR, Choi CW, Kim YS, Choi SU, Lee KR, Ryu SY. Antiproliferative effects of saponins from the roots of Platycodon grandiflorum on cultured human tumor cells. J Nat Prod. 2010;73:1863–1867. doi: 10.1021/np100496p. [DOI] [PubMed] [Google Scholar]
- 11.Takagi K, Lee EB. Pharmacological studies on Platycodon grandiflorum A. DC. 3. Activities of crude platycodin on respiratory and circulatory systems and its other pharmacological activities. Yakugaku Zasshi. 1972;92:969–973. doi: 10.1248/yakushi1947.92.8_969. [DOI] [PubMed] [Google Scholar]
- 12.Lee EB. Pharmacological studies on Platycodon grandiflorum A. DC. IV. A comparison of experimental pharmacological effects of crude platycodin with clinical indications of Platycodi Radix. Yakugaku Zasshi. 1973;93:1188–1194. doi: 10.1248/yakushi1947.93.9_1188. [DOI] [PubMed] [Google Scholar]
- 13.San Martin R, Briones R. Industrial uses and sustainable supply of Quillaja saponaria (Rosaceae) saponins. Econ Bot. 1999;53:302–311. [Google Scholar]
- 14.Güçlü-Ustündağ O, Mazza G. Saponins: properties, applications and processing. Crit Rev Food Sci Nutr. 2007;47:231–258. doi: 10.1080/10408390600698197. [DOI] [PubMed] [Google Scholar]
- 15.Ha YW, Na YC, Seo JJ, Kim SN, Linhardt RJ, Kim YS. Qualitative and quantitative determination of ten major saponins in Platycodi Radix by high performance liquid chromatography with evaporative light scattering detection and mass spectrometry. J Chromatogr A. 2006;1135:27–35. doi: 10.1016/j.chroma.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryu CS, Kim CH, Lee SY, Lee KS, Choung KJ, Song GY, Kim BH, Ryu SY, Lee HS, Kim SK. Evaluation of the total oxidant scavenging capacity of saponins isolated from Platycodon grandiflorum. Food Chem. 2012;132:333–337. doi: 10.1016/j.foodchem.2011.10.086. [DOI] [PubMed] [Google Scholar]
- 17.Jeong CH, Choi GN, Kim JH, Kwak JH, Kim DO, Kim YJ, Hoe HJ. Antioxidant activities of the aerial parts of Platycodon grandiflorum. Food Chem. 2010;118:278–282. [Google Scholar]
- 18.Kim JW, Lee SW, Park SJ, Shin JC, Yang JW, Lim JH. Pharmaceutical composition for preventing or treating hepatitis C, comprising the root extracts of Platycodon grandiflorum or Platycodon grandiflorum saponin components. 2011. US Patent Application 2011/0274656 A1.
- 19.Francis G, Kerem Z, Makkar HP, Becker K. The biological action of saponins in animal systems: a review. Br J Nutr. 2002;88:587–605. doi: 10.1079/BJN2002725. [DOI] [PubMed] [Google Scholar]
- 20.Rao AV, Gurfinkel DM. The bioactivity of saponins: triterpenoid and steroidal glycosides. Drug Metabol Drug Interact. 2000;17:211–235. doi: 10.1515/dmdi.2000.17.1-4.211. [DOI] [PubMed] [Google Scholar]
- 21.Sun HX, Xie Y, Ye YP. Advances in saponin-based adjuvants. Vaccine. 2009;27:1787–1796. doi: 10.1016/j.vaccine.2009.01.091. [DOI] [PubMed] [Google Scholar]
- 22.Kensil CR. Saponins as vaccine adjuvants. Crit Rev Ther Drug Carrier Syst. 1996;13:1–55. [PubMed] [Google Scholar]
- 23.Na YC, Ha YW, Kim YS, Kim KJ. Structural analysis of platycosides in Platycodi Radix by liquid chromatography/electrospray ionization-tandem mass spectrometry. J Chromatogr A. 2008;1189:467–475. doi: 10.1016/j.chroma.2007.11.085. [DOI] [PubMed] [Google Scholar]
- 24.Ha IJ, Ha YW, Kang MS, Lee JS, Park DH, Kim YS. Enzymatic transformation of platycosides and one-step separation of platycodin D by high-speed countercurrent chromatography. J Sep Sci. 2010;33:1916–1922. doi: 10.1002/jssc.200900842. [DOI] [PubMed] [Google Scholar]
- 25.Li W, Zhang W, Xiang L, Wang Z, Zheng YN, Wang YP, Zhang J, Chen L. Platycoside N: a new oleanane-type triterpenoid saponin from the roots of Platycodon grandiflorum. Molecules. 2010;15:8702–8708. doi: 10.3390/molecules15128702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie Y, Sun HX, Li D. Platycodin D is a potent adjuvant of specific cellular and humoral immune responses against recombinant hepatitis B antigen. Vaccine. 2009;27:757–764. doi: 10.1016/j.vaccine.2008.11.029. [DOI] [PubMed] [Google Scholar]
- 27.Bensky D, Gamble A. Chinese Herbal Medicine: Materia Medica. 3rd ed. Eastland Press; Seattle, WA, USA: 1993. pp. 429–432. [Google Scholar]
- 28.Saeki T, Koike K, Nikaido T. A comparative study on commercial, botanical gardens and wild samples of the roots of Platycodon grandiflorum by HPLC analysis. Planta Med. 1999;65:428–431. doi: 10.1055/s-1999-14021. [DOI] [PubMed] [Google Scholar]
- 29.Yan YZ, Xue JC, Wu JR, Yoo DS, Lee SY, Kim YK, Uddin MR, Park SU. Variation of triterpenoid saponin content in Platycodon grandiflorum (Jacq.) A.D.C. Asian J Chem. 2012;24:1268–1270. [Google Scholar]
- 30.Ishii H, Tori K, Tozyo T, Yoshimura Y. Saponins from roots of Platycodon grandiflorum. Part 2. Isolation and structure of new triterpene glycosides. J Chem Soc Perkin Trans 1. 1984:661–668. [Google Scholar]
- 31.Choi CY, Kim JY, Kim YS, Chung YC, Hahm KS, Jeong HG. Augmentation of macrophage functions by an aqueous extract isolated from Platycodon grandiflorum. Cancer Lett. 2001;166:17–25. doi: 10.1016/s0304-3835(01)00440-2. [DOI] [PubMed] [Google Scholar]
- 32.Choi CY, Kim JY, Kim YS, Chung YC, Seo JK, Jeong HG. Aqueous extract isolated from Platycodon grandiflorum elicits the release of nitric oxide and tumor necrosis factor-alpha from murine macrophages. Int Immunopharmacol. 2001;1:1141–1151. doi: 10.1016/s1567-5769(01)00047-9. [DOI] [PubMed] [Google Scholar]
- 33.Kim YP, Lee EB, Kim SY, Li DW, Ban HS, Lim SS, Shin KH, Ohuchi K. Inhibition of prostaglandin E2 production by platycodin D isolated from the root of Platycodon grandiflorum. Planta Med. 2001;67:362–364. doi: 10.1055/s-2001-14317. [DOI] [PubMed] [Google Scholar]
- 34.Jeong EK, Cha HJ, Ha YW, Kim YS, Ha IJ, Na YC. Development and optimization of a method for the separation of platycosides in Platycodi Radix by comprehensive two-dimensional liquid chromatography with mass spectrometric detection. J Chromatogr A. 2010;1217:4375–4382. doi: 10.1016/j.chroma.2010.04.053. [DOI] [PubMed] [Google Scholar]
- 35.Ahn KS, Hahn BS, Kwack K, Lee EB, Kim YS. Platycodin D-induced apoptosis through nuclear factor-κB activation in immortalized keratinocytes. Eur J Pharmacol. 2006;537:1–11. doi: 10.1016/j.ejphar.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 36.Zhao HL, Cho KH, Ha YW, Jeong TS, Lee WS, Kim YS. Cholesterol-lowering effect of platycodin D in hypercholesterolemic ICR mice. Eur J Pharmacol. 2006;537:166–173. doi: 10.1016/j.ejphar.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 37.Zhao HL, Kim YS. Determination of the kinetic properties of platycodin D for the inhibition of pancreatic lipase using a 1,2-diglyceride-based colorimetric assay. Arch Pharm Res. 2004;27:968–972. doi: 10.1007/BF02975852. [DOI] [PubMed] [Google Scholar]
- 38.Zhang L, Liu ZJ, Tian JK. Cytotoxic triterpenoid saponins from the roots of Platycodon grandiflorum. Molecules. 2007;12:832–841. doi: 10.3390/12040832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee JY, Hwang WI, Lim ST. Antioxidant and anticancer activities of organic extracts from Platycodon grandiflorum A. De Candolle roots. J Ethnopharmacol. 2004;93:409–415. doi: 10.1016/j.jep.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 40.Tada A, Kaneiwa Y, Shoji J, Shibata S. Studies on the saponins of the root of Platycodon grandiflorum A. De Candolle. I. Isolation and the structure of platycodin-D. Chem Pharm Bull (Tokyo) 1975;23:2965–2972. doi: 10.1248/cpb.23.2965. [DOI] [PubMed] [Google Scholar]
- 41.Park YS, Yoon YS, Ahn HS. Platycodon grandiflorum extract represses up-regulated adipocyte fatty acid binding protein triggered by a high fat feeding in obese rats. World J Gastroenterol. 2007;13:3493–3499. doi: 10.3748/wjg.v13.i25.3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu J, Yang G, Wen W, Zhang F, Yuan J, An L. Cholesterol metabolism regulation and antioxidant effect of platycodin D on hyperlipidemic emulsion-reduced rats. Afr J Pharm Pharmacol. 2011;5:2444–2453. [Google Scholar]
- 43.Zhao HL, Sim JS, Shim SH, Ha YW, Kang SS, Kim YS. Antiobese and hypolipidemic effects of platycodin saponins in diet-induced obese rats: evidences for lipase inhibition and calorie intake restriction. Int J Obes (Lond) 2005;29:983–990. doi: 10.1038/sj.ijo.0802948. [DOI] [PubMed] [Google Scholar]
- 44.Kay AB. Overview of ‘allergy and allergic diseases: with a view to the future’. Br Med Bull. 2000;56:843–864. doi: 10.1258/0007142001903481. [DOI] [PubMed] [Google Scholar]
- 45.Oh YC, Kang OH, Choi JG, Lee YS, Brice OO, Jung HJ, Hong SH, Lee YM, Shin DW, Kim YS, Kwon DY. Antiallergic activity of a platycodon root ethanol extract. Int J Mol Sci. 2010;11:2746–2758. doi: 10.3390/ijms11072746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee SJ, Bang WS, Hong JS, Kwon OJ, Shin SR, Yoon KY. Antioxidant and antimicrobial activities of black Doraji (Platycodon grandiflorum) Korean J Food Preserv. 2013;20:510–517. [Google Scholar]
- 47.Zhang L, Ravipati AS, Koyyalamudi SR, Jeong SC, Reddy N, Bartlett J, Smith PT, de la Cruz M, Monteiro MC, Melguizo A, Jiménez E, Vicente F. Anti-fungal and anti-bacterial activities of ethanol extracts of selected traditional Chinese medicinal herbs. Asian Pac J Trop Med. 2013;6:673–681. doi: 10.1016/S1995-7645(13)60117-0. [DOI] [PubMed] [Google Scholar]
- 48.Cacabelos R. Alzheimer disease. Rev Med Pract Clin. 1997;2:124–142. [Google Scholar]
- 49.Son IH, Park YH, Lee SI, Yang HD, Moon HI. Neuroprotective activity of triterpenoid saponins from Platycodi Radix against glutamate-induced toxicity in primary cultured rat cortical cells. Molecules. 2007;12:1147–1152. doi: 10.3390/12051147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ha YW, Kim YS. Preparative isolation of six major saponins from Platycodi Radix by high-speed counter-current chromatography. Phytochem Anal. 2009;20:207–213. doi: 10.1002/pca.1116. [DOI] [PubMed] [Google Scholar]
- 51.Li W, Zhao LC, Wang Z, Zheng YN, Liang J, Wang H. Response surface methodology to optimize enzymatic preparation of deapio-platycodin d and platycodin d from radix platycodi. Int J Mol Sci. 2012;13:4089–4100. doi: 10.3390/ijms13044089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wie HJ, Zhao HL, Chang JH, Kim YS, Hwang IK, Ji GE. Enzymatic modification of saponins from Platycodon grandiflorum with Aspergillus niger. J Agric Food Chem. 2007;55:8908–8913. doi: 10.1021/jf0716937. [DOI] [PubMed] [Google Scholar]
- 53.Park JS, Rho HS, Kim DH, Chang IS. Enzymatic preparation of kaempferol from green tea seed and its antioxidant activity. J Agric Food Chem. 2006;54:2951–2956. doi: 10.1021/jf052900a. [DOI] [PubMed] [Google Scholar]
- 54.Ko SR, Choi KJ, Suzuki K, Suzuki Y. Enzymatic preparation of ginsenosides Rg2, Rh1 and F1. Chem Pharm Bull (Tokyo) 2003;51:404–408. doi: 10.1248/cpb.51.404. [DOI] [PubMed] [Google Scholar]
- 55.Ko SR, Suzuki Y, Choi KJ, Kim YH. Enzymatic preparation of genuine prosapogenin, 20(S)-ginsenoside Rh1, from ginsenosides Re and Rg1. Biosci Biotechnol Biochem. 2000;64:2739–2743. doi: 10.1271/bbb.64.2739. [DOI] [PubMed] [Google Scholar]
- 56.Oleszek W, Bialy Z. Chromatographic determination of plant saponins—an update (2002–2005) J Chromatogr A. 2006;1112:78–91. doi: 10.1016/j.chroma.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 57.Kitagawa I. Method of isolating soyasaponins. 1986. US Patent US4594412A.
- 58.Oleszek WA. Chromatographic determination of plant saponins. J Chromatogr A. 2002;967:147–162. doi: 10.1016/s0021-9673(01)01556-4. [DOI] [PubMed] [Google Scholar]
- 59.Kite GC, Porter EA, Simmonds MS. Chromatographic behavior of steroidal saponins studied by high-performance liquid chromatography-mass spectrometry. J Chromatogr A. 2007;1148:177–183. doi: 10.1016/j.chroma.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 60.Gurib-Fakim A. Medicinal plants: traditions of yesterday and drugs of tomorrow. Mol Aspects Med. 2006;27:1–93. doi: 10.1016/j.mam.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 61.Bush TM, Rayburn KS, Holloway SW, Sanchez-Yamamoto DS, Allen BL, Lam T, So BK, Tran de H, Greyber ER, Kantor S, Roth LW. Adverse interactions between herbal and dietary substances and prescription medications: a clinical survey. Altern Ther Health Med. 2007;13:30–35. [PubMed] [Google Scholar]
- 62.Gagnier JJ, DeMelo J, Boon H, Rochon P, Bombardier C. Quality of reporting of randomized controlled trials of herbal medicine interventions. Am J Med. 2006;119:e1–e11. doi: 10.1016/j.amjmed.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 63.Lee WH, Gam CO, Ku SK, Choi SH. Single oral dose toxicity test of platycodin D, a saponin from Platycodin Radix in mice. Toxicol Res. 2011;27:217–224. doi: 10.5487/TR.2011.27.4.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.KFDA. Testing Guidelines for Safety Evaluation of Drugs. Korea Food and Drug Administration; Sejong, Korea: 2009. Notification 2009-116. [Google Scholar]