Abstract
Broccoli (Brassica oleracea var. italia) florets were extracted with 80% methanol and the extract was sequentially fractionated with n-hexane, ethyl acetate, n-butanol, and distilled water. The extract and the fractions were evaluated for total phenolic content, sulforaphane content, antioxidant activity, and anti-inflammatory activity in lipopolysaccharide (LPS)-stimulated RAW 264.7 cells. The total phenolic content and sulforaphane content of the ethyl acetate fraction (EF) were 35.5 mg gallic acid equivalents/g and 620.2 μg/g, respectively. These values were higher than those of the 80% methanol extract and organic solvent fractions. The oxygen radical absorbance capacity of the EF [1,588.7 μM Trolox equivalents (TE)/mg] was 11-fold higher than that of the distilled water fraction (143.7 μM TE/mg). The EF inhibited nitric oxide release from LPS-stimulated RAW 264.7 cells in a dose-dependent manner and inhibited IκB-α degradation and nuclear factor-κB activation in LPS-stimulated RAW 264.7 cells. In conclusion, the EF of broccoli florets exerted potent antioxidant and anti-inflammatory effects.
Keywords: broccoli florets, total phenolics, sulforaphane, anti-inflammatory activity, antioxidant activity
INTRODUCTION
A high intake of cruciferous vegetables such as broccoli, cauliflower, and cabbage is associated with a reduced risk of cancer because cruciferous vegetables are rich sources of glucosinolates and possess high levels of flavonoids, vitamins, and mineral nutrients (1). In particular, isothiocyanates and indole derivatives, including sulforaphane, phenylethyl isothiocyanate, and indole-3-carbinol, are reported to have antioxidant and anticancer activities (2). Sulforaphane is produced largely from glucoraphanin through the action of endogenous myrosinase during the chopping or crushing of broccoli (3). The concentrations and bioavailabilities of glucosinolates, sulforaphane, polyphenols, etc. depend on intrinsic and extrinsic factors such as plant cultivar, genetics, soil composition, growing conditions, postharvest conditions, and maturity stage (4).
During oxidative stress, large amounts of reactive oxygen species (ROS) such as hydrogen peroxide (H2O2), hydroxyl radical (•OH), and superoxide anion (O2−) are produced. DNA damage leads to mutations which in turn are associated with diseases such as cancer, coronary heart disease, arteriosclerosis, and inflammatory diseases (5).
Macrophages play important roles during a host’s immune response to infection and during disease development (6). Activation of macrophages by stimuli such as the bacterial endotoxin lipopolysaccharide (LPS) or viruses increases the production of numerous inflammatory mediators, including nitric oxide and various cytokines. Inflammation leads to the upregulation of a series of enzyme and signaling protein genes in affected cells and tissues (7). The nuclear transcription factor NF-κB is a major regulatory component of the inflammatory response induced by LPS or pro-inflammatory cytokines. Inducible nitric oxide synthase (iNOS) and cyclooxygenase (COX)-2 promoter regions contain NF-κB binding sites, which are necessary for inducing the expression of these genes (8). In unstimulated cells, NF-κB exists in the cytosol in a quiescent form bound to its inhibitory protein, I-κB, which is in a non-phosphorylated state (9,10). Upon stimulation with LPS, I-κB is phosphorylated and undergoes proteolytic degradation. Concomitantly, NF-κB becomes activated and translocates to the nucleus, where it activates target genes, such as those that encode iNOS, COX-2, and pro-inflammatory cytokines, by binding to a consensus sequence in the promoter region of the target genes. Phosphorylation of I-κBα may be directly inhibited by a corresponding I-κB kinase (IKK) (11,12). Inhibition of NF-κB activity has consistently been shown to be effective for controlling inflammatory diseases in animal models (13).
Sulforaphane, a major component of broccoli, is known to possess anti-inflammatory and anti-cancer properties by suppressing LPS-induced COX-2 genes expression via modulation of multiple core promoter elements (i.e., NF-κB, C/EBP, and CREB) that regulate COX-2 transcription (14). However, the anti-inflammatory effects of organic solvent fractions from broccoli have not been reported yet. Furthermore, the effects of organic solvent fractions from broccoli on pro-inflammatory mediators have not been investigated in LPS-induced RAW 264.7 cells.
Previous work indicates that organic solvent extracts from broccoli have antioxidant activities. Piao et al. (15) used methanol to extract dried broccoli, and then partitioned the extract with water, methylene chloride, and butanol, in succession. The butanol fraction, which contained high levels of hydroxycinnamic esters, was the most active fraction in terms of scavenging 2,2-diphenyl-1-picrylhydrazyl (DPPH) radicals. However, Piao et al. (15) didn’t analyze the levels of phenolic compounds, which are known to have potent antioxidant activity, in the fractions tested.
In the present study, broccoli florets were extracted with 80% methanol and then sequentially fractionated with n-hexane, ethyl acetate, n-butanol, and distilled water. The total phenolic contents, sulforaphane contents, antioxidant activities, and anti-inflammatory activities in LPS-stimulated RAW 264.7 cells were evaluated for the extract and all fractions.
MATERIALS AND METHODS
Chemicals
Folin-Ciocalteu phenol reagent, gallic acid, dehydroascorbic acid, 2,2′-azobis-2-methyl-propanimidamide, dihydrochloride (AAPH), fluorescein sodium salt (FL), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), and sulforaphane were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), and penicillin/streptomycin were purchased from Gibco BRL (Life Technologies Inc., Grand Island, NY, USA). LPS (Escherichia coli O111:B4) was obtained from Sigma-Aldrich Co. iNOS was purchased from Calbiochem (San Diego, CA, USA). IκB was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The acetonitrile was HPLC grade, and all other chemicals were analytical grade.
Preparation of extract and fractions
Broccoli (Brassica oleracea var. italia) was obtained from a local farm in Jeju, Korea in 2011 and washed with tap water. Broccoli florets were freeze-dried and extracted three times with 80% methanol at 25°C. The methanol extract was suspended in distilled water and then sequentially partitioned with equal volumes of n-hexane, ethyl acetate, n-butanol, and distilled water. The fractions were filtered through a 0.45-μm membrane filter (Millipore Co., Billerica, MA, USA) and then freeze-dried.
Analysis of total phenolic content
The total phenolic contents of the extract and fractions were estimated according to the Folin-Ciocalteu method (16). A 0.1-mL aliquot of a sample was added to 0.9 mL of distilled water and 0.1 mL of Folin-Ciocalteu phenol reagent. After 5 min, 0.3 mL of 20% NaCO3 was added and the solution was brought to a total volume of 2 mL with distilled water. The mixture was allowed to stand at 25°C for 2 h. Absorbance was measured at 760 nm using a microplate reader (μQuant-MQX 200, Bio-Tek Instruments, Inc., Winooski, VT, USA). The total phenolic content of each sample was assessed by plotting against a gallic acid calibration curve (0.0 mg/mL to 0.1 mg/mL). Total phenolic contents are expressed as mg gallic acid equivalents (GAE)/g dry weight of the sample.
Analysis of sulforaphane content
Chromatographic analysis of sulforaphane content was performed using an Agilent 1200 Series HPLC System (Agilent Technologies, Inc., Santa Clara, CA, USA) consisting of a solvent gradient delivery pump and a photodiode array detector. Separation was achieved using an XTerra RP18 reverse phase column (25 cm×4.6 mm; 5 μm; Waters, Milford, MA, USA). Acetonitrile (A) and water (B) were used as mobile phases and the gradient elution was programmed as follows: 0 min, 20% A; 10 min, 50% A; 15 min, 20% A. The flow rate was 0.8 mL/min and the detection wavelength was 240 nm. The injection volume was 20 μL.
Oxygen radical absorbance capacity assay
The antioxidant activities of the samples were measured as oxygen radical absorbance capacities (ORAC) (17) with a slight modification. The assay was carried out in black-walled 96-well plates (Fisher Scientific, Hanover Park, IL, USA). Reactants were added in the following order: 25 μL of 75 mM phosphate buffer, 25 μL of each sample, 100 μL of 78 nM FL (61.5:38.5 (v/v) of 0.075 M K2HPO4:0.075 M NaH2PO4), 50 μL AAPH (41.6 mM final concentration), and Trolox standard (200 μM final concentration). Immediately after the addition of AAPH, the plate was placed in a FLUOstar OPTIMA plate reader (BMG Labtech, Inc., Offenburg, Germany) with a 485/25-nm excitation filter and a 538/35-nm emission filter, and the absorbance of each well was read at 3-min intervals for 1 h at 37°C. The final fluorescence measurement was expressed relative to the initial reading. Results were calculated by comparing the net areas under the fluorescein decay curves of the blank and the samples. ORAC values were expressed in μmol Trolox equivalents (TE)/mg sample.
Cell culture
The murine macrophage RAW 264.7 (ATCC#TIB-71) cell line was obtained from the American Type Culture Collection (Manassas, VA, USA). The cells were cultivated in high-glucose DMEM supplemented with 100 units/mL penicillin, 100 μg/mL streptomycin, and 10% heat-inactivated FBS and maintained in a humidified atmosphere at 37°C and 5% CO2.
Cell viability assay
Cell viability was determined using an EZ-Cytox cell viability assay kit (Daeil Lab, Seoul, Korea). Cells were cultured in a 96-well plate at a concentration of 4×105 cells/well for 24 h and then treated with various concentrations of each sample for an additional 24 h. Subsequently, 10 μL of the kit solution was added to each well and incubated for 2 h at 37°C and 5% CO2. Cell viability, indicated by the production of formazan, was measured with an enzyme-linked immunosorbent assay (ELISA) reader set to detect absorbance at 480 nm.
Measurement of nitric oxide (NO) production
RAW 264.7 cells were cultured in 96-well plates in DMEM, pretreated with various concentrations of each sample for 1 h, and then stimulated with LPS for 24 h. Following stimulation, the nitrite concentration of the culture medium was used as an indicator of NO production (18). Briefly, the culture medium was mixed with an equal volume of Griess reagent, and the absorbance of the mixture was read at 540 nm with an ELISA microplate reader.
Total cellular RNA extraction
LPS-stimulated RAW 264.7 cells were incubated in the presence of various concentrations of each sample for 6 h. Then, total cellular RNA was isolated from the cells using TRI reagent (Molecular Research Center, Cincinnati, OH, USA).
RT-PCR analysis
One microgram of total RNA was reverse-transcribed from each sample using the ImProm-II™ Reverse Transcription System (Promega Corp., Madison, WI, USA). PCR was conducted on aliquots of the resulting cDNA preparation to detect iNOS, TNF-α, IL-1β, IL-6, and β-actin mRNA. The reactions were conducted in 25-μL volumes containing Taq DNA polymerase, dNTPs, reaction buffer, and primers (Table 1) (Bioneer, Daejeon, Korea). Relative band density was determined by densitometry using image acquisition and analysis software (LabWorks, UVP Inc., Upland, CA, USA).
Table 1.
The primer sequences of the genes measured by RT-PCR analysis and the expected sizes of their PCR products
| Gene | Primer sequences (5′→3′) | Fragment size (bp) | |
|---|---|---|---|
| iNOS | Forward | CCC TTC CGA AGT TTC TGG CAG CAG C | 497 |
| Reverse | GGC TGT CAG AGC CTC GTG GCT TTG G | ||
| TNF-α | Forward | TTG ACC TCA GCG CTG AGT TG | 374 |
| Reverse | CCT GTA GCC CAC GTC GTA GC | ||
| IL-1β | Forward | CAG GAT GAC ATG AGC ACC | 447 |
| Reverse | CTC TGC AGA CTC AAA CTC CAC | ||
| IL-6 | Forward | GTA CTC CAG AAG ACC AGA GG | 308 |
| Reverse | TGC TGG TGA CAA CCA CGG CC | ||
| β-actin | Forward | AGG CTG TGC TGT CCC TGT ATG C | 395 |
| Reverse | ACC CAA GAA GGA AGG CTG GAA A |
Western blot analysis
Western bolt analysis was performed by lysing cells in RIPA buffer containing a protease inhibitor mixture. Bio-Rad protein assay reagent (Bio-Rad, Hercules, CA, USA) was used to determine the total protein concentration of each lysate. Proteins were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). The separated proteins were transferred to a polyvinylidene fluoride membrane (Millipore Co.). The membrane was blocked with 5% (w/v) nonfat dry milk dissolved in Tris-buffered saline with Tween 20 (TBST) [10 mM Tris-HCl (pH 7.5), 150 mM NaCl, 0.1% (v/v) Tween 20]. The blocked membrane was incubated with a specific primary antibody at 4°C overnight, washed four times with TBST, and then incubated for 30 min with a peroxidase-conjugated secondary antibody at room temperature. The WEST-ZOL Western Blot Detection System (iNtRON Biotechnology, Gyeonggi, Korea) was used to detect proteins of interest. Immunoreactive bands were visualized with an image analyzer (UVP Inc.).
Transfection and luciferase assay
FuGENE 6 transfection reagent (Roche, Indianapolis, IN, USA) was used to co-transfect RAW 264.7 cells with 1 mg of the NF-κB-promoter luciferase reporter gene plasmid pGL4.32[luc2P/NF-κB-RE/Hygro] (Promega) and 0.4 mg of the Renilla luciferase reporter plasmid pGL4.74 [hRluc/TK] (Promega), which served as the internal standard. At 24-h post-transfection, cells were pretreated with a sample (i.e., extract or fraction) for 1 h and then stimulated with LPS (100 ng/mL) for 24 h. Then, each well was washed with cold PBS, cells were lysed, and luciferase activity was determined with a Dual-Luciferase Reporter Assay Kit (Promega) (19).
Statistical analysis
Values are expressed as means±SD of three replicates. The statistical significance of differences between experimental values and their respective controls was tested by Duncan’s multiple range test (SPSS Inc., Chicago, IL, USA). A P<0.05 was considered statistically significant.
RESULTS AND DISCUSSION
Total phenolic content and antioxidant capacity
Polyphenols are secondary metabolites that defend plants against pathogens and ultraviolet radiation. Polyphenols act as potent antioxidants, as they protect cells against oxidative damage. They also exhibit free radical-scavenging and metal-chelating activities (20).
The total phenolic contents and antioxidant activities of an 80% methanol extract from freeze-dried broccoli florets and its subsequent organic solvent fractions are shown in Table 2. Total phenolic contents of the methanol extract and its fractions ranged from 9.4 mg GAE/g DW to 35.5 mg GAE/g DW. The total phenolic content was highest (35.5 mg GAE/g DW) in the ethyl acetate fraction (EF). The total phenol contents of the remaining samples were in decreasing order, 25.4 mg GAE/g DW in the n-butanol fraction (BF), 15.0 mg GAE/g DW in the n-hexane fraction (HF), 14.9 mg GAE/g DW in the 80% methanol extract (ME), and 9.4 mg GAE/g DW in the distilled water fraction (DF). Mrkic et al. (21) previously reported that the total phenolic content of broccoli was 10.2 mg GAE/g DW.
Table 2.
Total phenolic contents and antioxidant activities of an 80% methanol extract and organic solvent fractions of broccoli florets
| Extract and fractions | Total phenolic content (mg GAE/g) | ORAC value (μM TE/mg) |
|---|---|---|
| 80% Methanol extract | 14.9±0.1b1) | 391.1±8.1b |
| n-Hexane fraction | 15.0±0.1b | 1,219.7±3.4d |
| Ethyl acetate fraction | 35.5±0.5d | 1,588.7±14.8e |
| n-Butanol fraction | 25.4±0.1c | 915.4±6.5c |
| Distilled water fraction | 9.4±0.4a | 143.7±7.8a |
Mean±SD of three replicate determinations.
Within each column, values with different superscripts are significantly different at P<0.05 according to Duncan’s multiple range test.
The antioxidant capacities (i.e., ORAC) of the methanol extract and organic solvent fractions of broccoli florets are also shown in Table 2. The highest antioxidant activity was measured in the EF, with a value of 1,588 μM TE/mg DW; the antioxidant activities of the remaining samples were, in decreasing order, 1,219.7 μM TE/mg DW in the HF, 915.4 μM TE/mg DW in the BF, 391.1 μM TE/mg DW in the ME, and 143.7 μM TE/mg DW in the DF. ORAC correlated well with total phenolic content in the methanol extract and organic solvent fractions tested. Several studies have suggested that ORAC antioxidant activity could be attributed to the total phenolic content of medicinal plants (22).
Sulforaphane content
HPLC was used to quantitate the sulforaphane content of the methanol extract and organic solvent fractions from broccoli florets (Table 3). The sulforaphane contents in ME, EF, and BF were 60.6 μg/g, 620.2 μg/g, and 272.7 μg/g, respectively. However, sulforaphane was not detected in the HF and DF. The highest sulforaphane content was found in the EF. Previously, Liang et al. (23) reported a sulforaphane content of 32.9 μg/g in methylene chloride extracts of broccoli florets.
Table 3.
Sulforaphane contents of an 80% methanol extract and organic solvent fractions from broccoli florets and the ability of the extract and fractions to inhibit nitric oxide production by LPS (100 ng/mL)-induced RAW 264.7 cells
| Extract and fractions | Sulforaphane content (μg/g) | TC501) (μg/mL) | IC502) (μg/mL) | Selectivity index3) |
|---|---|---|---|---|
| 80% Methanol extract | 60.6±5.1 | >1,000 | 777.3±17.9 | 1.28 |
| n-Hexane fraction | ND4) | 81.3±10.0 | 24.3±1.1 | 3.34 |
| Ethyl acetate fraction | 620.2±14.1 | 192.3±6.7 | 46.6±0.5 | 4.12 |
| n-Butanol fraction | 272.7±21.7 | >1,000 | >1,000 | 1 |
| Distilled water fraction | ND | >1,000 | >1,000 | 1 |
TC50, the concentration of extract or fraction needed to reduce cell viability by 50%.
IC50, the concentration of extract or fraction needed to reduce nitric oxide production by 50%.
Selectivity index=TC50/IC50.
ND, not detected.
Inhibition of NO production and viability in LPS-induced RAW 264.7 cells
Excessive production of NO from up-regulated iNOS is involved in the formation of peroxy-nitrites. These peroxy-nitrites alter the functions of multiple proteins to induce cell proliferation and interfere with normal repair processes (7).
We investigated the ability of the methanol extract and organic solvent fractions of broccoli florets to inhibit NO production by LPS-stimulated RAW 264.7 cells (Fig. 1). In cells treated with 100 ng/mL LPS and HF (Fig. 1B) or EF (Fig. 1C), NO production decreased in a dose-dependent manner. At a sulforaphane concentration of 31.3 μg/mL (i.e., 0.22 μM), EF reduced NO production by 33.1%. Guo et al. (24) previously reported that extracts containing 0.24 μM, 0.48 μM, 0.72 μM, and 0.96 μM sulforaphane inhibited LPS-induced NO production by 18%, 33%, 44%, and 54%, respectively (i.e., in a dose dependent manner). In the present study, the inhibition of NO production at 0.22 μM was 1.8-fold higher than that reported by Guo et al. (24) for the same sulforaphane concentration.
Fig. 1.
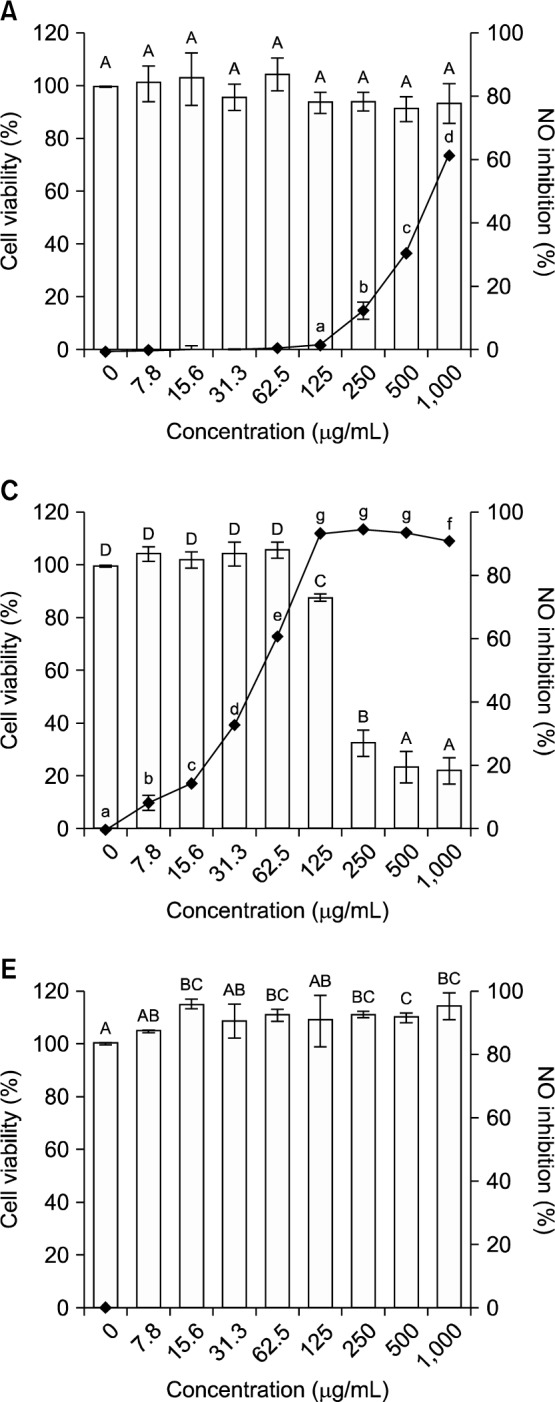
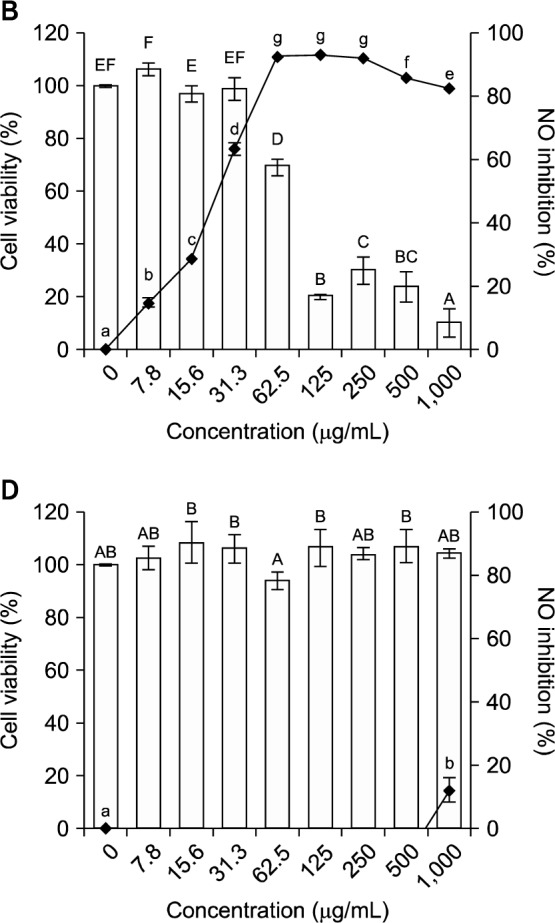
Cell viability (□) and inhibitory activity of nitric oxide production (-•-) by LPS (100 ng/mL)-stimulated RAW 264.7 cells treated with an 80% methanol extract and organic solvent fractions of broccoli florets. (A) 80% methanol extract, (B) n-hexane fraction, (C) ethyl acetate fraction, (D) n-butanol fraction, (E) distilled water fraction. Values with different letters (A–F, a–g) are significantly different at P<0.05 according to Duncan’s multiple range test.
EF also had the highest selectivity index (4.12), which is the ratio of the extract or fraction concentration needed to reduce LPS-induced RAW 264.7 cell viability by 50% (TC50) to the extract or fraction concentration needed to induce a 50% reduction of nitric oxide production (IC50) by LPS-induced RAW 264.7 macrophage cells (Table 3).
To assess whether the methanol extract and organic solvent fractions of broccoli florets affected cell viability, RAW 264.7 cells were incubated with LPS (100 ng/mL) in the presence of the extract and each fraction (1,000 μg/mL). Only the HF and EF were cytotoxic, with TC50 values of 81.3 μg/mL and 192.3 μg/mL, respectively. The EF was half as toxic as the HF. Therefore, we used EF doses of <100 μg/mL to investigate inhibition of iNOS, TNF-α, IL-1β, and IL-6 gene and protein expression in LPS-induced RAW 264.7 cells.
Inhibition of iNOS, TNF-α, IL-1β, and IL-6 gene and protein expression in LPS-induced RAW 264.7 cells
Macrophage expression of iNOS is a prerequisite for high NO output, which mediates many bactericidal and tumoricidal actions of immune cells. The expression of iNOS in mammalian cells is governed predominantly by the transcription factor NF-κB, which regulates the expression of many host defense proteins. Proinflammatory cytokine production is a sign of inflammation. NF-κB activation is necessary for the expression of several cytokines, including TNF-α, IL-1β, and IL-6 (25).
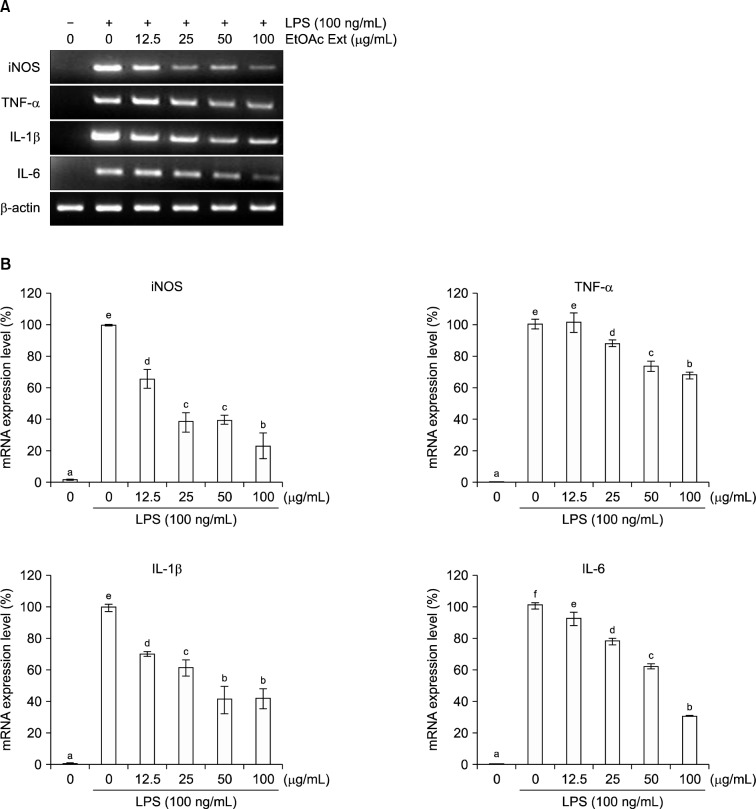
We investigated the inhibitory effects of the EF (0~100 μg/mL) on a variety of inflammatory parameters (Fig. 2). The expression of iNOS in LPS-induced RAW 264.7 cells was inhibited in a dose-dependent manner by the EF. At dose of 100 μg/mL, the iNOS mRNA level was reduced by 76.9%. Production of the pro-inflammatory cytokines TNF-α, IL-1β, and IL-6 was also inhibited by the EF in a dose-dependent manner. At dose of 100 μg/mL, TNF-α, IL-1β, and IL-6 mRNA levels were reduced by 32.2%, 58.3%, and 69.1%, respectively. Furthermore, a 100 μg/mL dose of the EF reduced iNOS protein expression by 94.3% (Fig. 3). Therefore, the EF inhibited NO production by LPS-stimulated RAW 264.7 cells via the downregulation of iNOS mRNA and protein levels.
Fig. 2.
Effects of the ethyl acetate fraction of broccoli florets on iNOS, TNF-α, IL-1β, and IL-6 mRNA levels in LPS (100 ng/mL)-induced RAW 264.7 cells. (A) RT-PCR analysis of mRNA transcripts, (B) relative levels of mRNA expression (normalized to β-actin). Values with different letters (a–f) are significantly different at P<0.05 according to Duncan’s multiple range test.
Fig. 3.
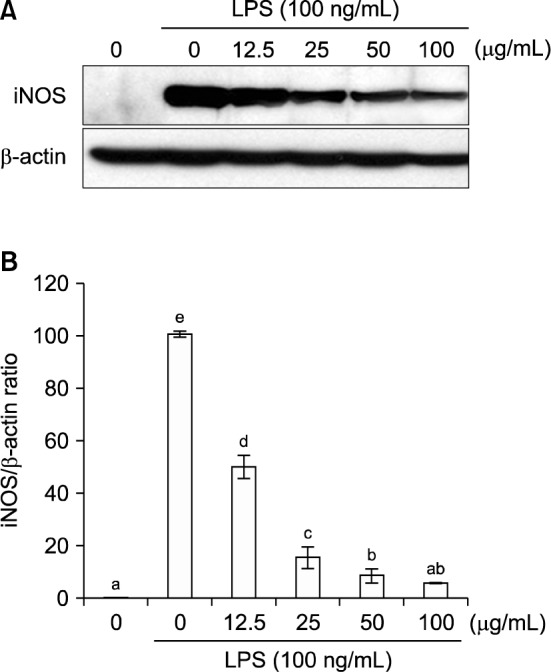
Effects of the ethyl acetate fraction of broccoli florets on iNOS protein levels in LPS (100 ng/mL)-induced RAW 264.7 cells. (A) Immunoblotting analysis of iNOS protein, (B) relative levels of iNOS protein (normalized to β-actin). Values with different letters (a–e) are significantly different at P<0.05 according to Duncan’s multiple range test.
Inhibition of NF-κB activity in LPS-induced RAW 264.7 cells
Regulation of iNOS and IL-1β occurs predominantly at the transcriptional level, with the transcription factor NF-κB playing a crucial role (26). A previous report indicates that activation of NF-κB induces the expression of several inflammatory mediators, such as iNOS, IL-1β, COX-2, and many other genes (27). NF-κB is important for the regulation of IL-1β gene transcription. The significant inhibition of this cytokine via NF-κB inhibition may result in the blockade of several inflammatory cascades that are stimulated by IL-1β (28). Accordingly, NF-κB inhibition has been shown to effectively control inflammatory diseases in several animal models (29). NF-κB activation requires the classical signaling cascade initiated by Toll-like receptor-4, which leads to I-κB phosphorylation and degradation (30). Blocking NF-κB activity by the overexpression of I-κBα has been reported to inhibit the inflammatory response and tissue destruction in rheumatoid synovium (8). In a colitis model, the inhibition of NF-κB by an IKK inhibitor ameliorated colonic inflammatory injury by down-regulating the production of NF-κB-mediated pro-inflammatory cytokines (e.g., TNF-α, IL-1β, and IL-6) (31).
To determine whether iNOS mRNA and protein levels are reduced when NF-κB activity is inhibited in LPS-treated RAW 264.7 cells, we used a pNF-κB-Luc plasmid that had been generated by inserting four NF-κB binding sites into the pLuc-promoter vector to investigate the effect of the EF on NF-κB activity inhibition (Fig. 4). RAW 264.7 cells were transiently transfected with the pNF-κB-Luc plasmid, pretreated with various concentrations of the EF, and subsequently stimulated with LPS (100 ng/mL). LPS-mediated NF-κB-dependent luciferase activity was significantly decreased in a dose-dependent manner by the EF. In addition, a 100 μg/mL dose of the EF inhibited LPS-mediated NF-κB-dependent luciferase activity by 81.3%.
Fig. 4.
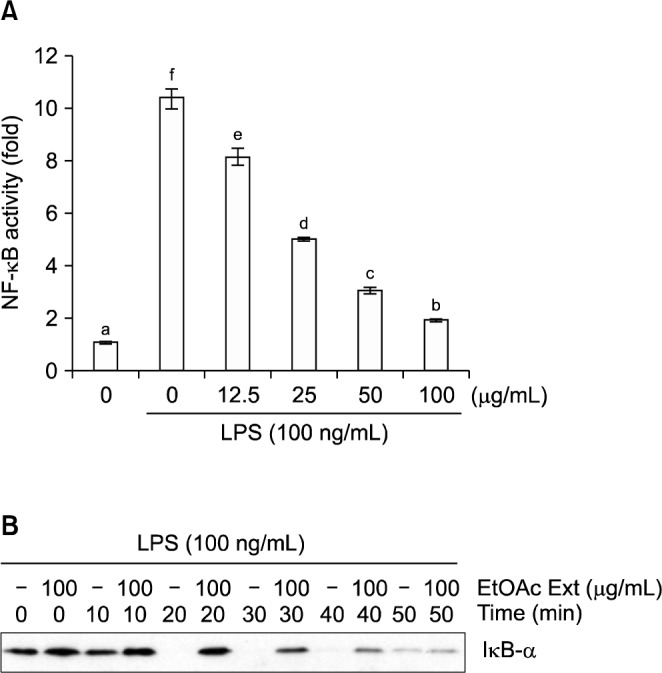
Effects of the ethyl acetate fraction of broccoli florets on the inhibition of (A) NF-κB-luciferase activity and (B) I-κBα degradation in LPS (100 ng/mL)-induced in RAW 264.7 cells (Western blot analysis). Values with different letters (a–f) are significantly different at P<0.05 according to Duncan’s multiple range test.
We also examined the effect of the EF (100 ng/mL) on I-κBα degradation. The EF caused significant degradation of I-κBα in LPS (100 ng/mL)-induced cells after more than 20-min of treatment with the fraction. Thus, we concluded that inhibition of the expression of pro-inflammatory mediators, such as iNOS and cytokines, by the EF of broccoli florets might be mediated by suppression of NF-κB activation.
In conclusion, the EF from broccoli florets had the highest total phenolic content, the highest sulforaphane content, and high antioxidant and anti-inflammatory activities, as demonstrated by its ability to inhibit NO production, IκB-α degradation, and NF-κB activation in LPS-stimulated RAW 264.7 macrophages. However, prior to the use of the EF from broccoli florets as a dietary supplement to improve human nutrition and for the treatment of chronic inflammatory pathologies, further research should be done in vivo.
ACKNOWLEDGEMENTS
This research was supported by the Ministry of Trade, Industry and Energy (MOTIE) and Korea Institute for Advancement of Technology (KIAT) through the Research and Development for Regional Industry.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Moreno DA, Carvajal M, López-Berenguer C, García-Viguera Chemical and biological characterisation of nutraceutical compounds of broccoli. J Pharm Biomed Anal. 2006;41:1508–1522. doi: 10.1016/j.jpba.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Stoewsand GS. Bioactive organosulfur phytochemicals in Brassica oleracea vegetables–a review. Food Chem Toxicol. 1995;33:537–543. doi: 10.1016/0278-6915(95)00017-v. [DOI] [PubMed] [Google Scholar]
- 3.Kushad MM, Brown AF, Kurilich AC, Juvik JA, Klein BP, Wallig MA, Jeffery EH. Variation of glucosinolates in vegetable crops of Brassica oleracea. J Agric Food Chem. 1999;47:1541–1548. doi: 10.1021/jf980985s. [DOI] [PubMed] [Google Scholar]
- 4.Mahn A, Reyes A. An overview of health-promoting compounds of broccoli (Brassica oleracea var. italica) and the effect of processing. Food Sci Technol Int. 2012;18:503–514. doi: 10.1177/1082013211433073. [DOI] [PubMed] [Google Scholar]
- 5.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Schluger NW, Rom WN. The host immune response to tuberculosis. Am J Respir Crit Care Med. 1998;157:679–691. doi: 10.1164/ajrccm.157.3.9708002. [DOI] [PubMed] [Google Scholar]
- 7.Guzik TJ, Korbut R, Adamek-Guzik T. Nitric oxide and superoxide in inflammation and immune regulation. J Physiol Pharmacol. 2003;54:469–487. [PubMed] [Google Scholar]
- 8.Li Q, Verma IM. NF-κB regulation in the immune system. Nat Rev Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 9.Tsoyi K, Park HB, Kim YM, Chung JI, Shin SC, Lee WS, Seo HG, Lee JH, Chang KC, Kim HJ. Anthocyanins from black soybean seed coats inhibit UVB-induced inflammatory cylooxygenase-2 gene expression and PGE2 production through regulation of the nuclear factor-κB and phosphatidylinositol 3-kinase/Akt pathway. J Agric Food Chem. 2008;56:8969–8974. doi: 10.1021/jf801345c. [DOI] [PubMed] [Google Scholar]
- 10.Shin JS, Noh YS, Lee YS, Cho YW, Baek NI, Choi MS, Jeong TS, Kang E, Chung HG, Lee KT. Arvelexin from Brassica rapa suppresses NF-κB-regulated pro-inflammatory gene expression by inhibiting activation of IκB kinase. Br J Pharmacol. 2011;164:145–158. doi: 10.1111/j.1476-5381.2011.01351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raghav SK, Gupta B, Shrivastava A, Das HR. Inhibition of lipopolysaccharide-inducible nitric oxide synthase and IL-1β through suppression of NF-κB activation by 3-(1’-1’-dimethyl-allyl)-6-hydroxy-7-methoxy-coumarin isolated from Ruta graveolens L. Eur J Pharmacol. 2007;560:69–80. doi: 10.1016/j.ejphar.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Moro C, Palacios I, Lozano M, D’Arrigo M, Guillamón E, Villares A, Martínez JA, García-Lafuente A. Anti-inflammatory activity of methanolic extracts from edible mushrooms in LPS activated RAW 264.7 macrophages. Food Chem. 2012;130:350–355. [Google Scholar]
- 13.Majumdar S, Aggarwal BB. Methotrexate suppresses NF-κB activation through inhibition of IκBα phosphorylation and degradation. J Immunol. 2001;167:2911–2920. doi: 10.4049/jimmunol.167.5.2911. [DOI] [PubMed] [Google Scholar]
- 14.Woo KJ, Kwon TK. Sulforaphane suppresses lipopolysaccharide-induced cyclooxygenase-2 (COX-2) expression through the modulation of multiple targets in COX-2 gene promoter. Int Immunopharmacol. 2007;7:1776–1783. doi: 10.1016/j.intimp.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 15.Piao XL, Kim HY, Yokozawa T, Lee YA, Piao XS, Cho EJ. Protective effects of broccoli (Brassica oleracea) and its active components against radical-induced oxidative damage. J Nutr Sci Vitaminol (Tokyo) 2005;51:142–147. doi: 10.3177/jnsv.51.142. [DOI] [PubMed] [Google Scholar]
- 16.Singleton VL, Rossi JA., Jr Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- 17.Ou B, Hampsch-Woodill M, Prior RL. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescence as the fluorescent prove. J Agric Food Chem. 2001;49:4619–4626. doi: 10.1021/jf010586o. [DOI] [PubMed] [Google Scholar]
- 18.Snell JC, Colton CA, Chernyshev ON, Gilbert DL. Location-dependent artifact for no measurement using multiwell plates. Free Radic Biol Med. 1996;20:361–363. doi: 10.1016/0891-5849(96)02083-7. [DOI] [PubMed] [Google Scholar]
- 19.Nagahama Y, Obama T, Usui M, Kanazawa Y, Iwamoto S, Suzuki K, Miyazaki A, Yamaguchi T, Yamamoto M, Itabe H. Oxidized low-density lipoprotein-induced periodontal inflammation is associated with the up-regulation of cyclooxygenase-2 and microsomal prostaglandin synthase 1 in human gingival epithelial cells. Biochem Biophys Res Commun. 2011;413:566–571. doi: 10.1016/j.bbrc.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 21.Mrkic V, Cocci E, Dalla Rosa M, Sacchetti G. Effect of drying conditions on bioactive compounds and antioxidant activity of broccoli (Brassica oleracea L.) J Sci Food Agric. 2006;86:1559–1566. [Google Scholar]
- 22.Kratchanova M, Denev P, Ciz M, Lojek A, Mihailov A. Evaluation of antioxidant activity of medicinal plants containing polyphenol compounds. Comparison of two extraction systems. Acta Biochim Pol. 2010;57:229–234. [PubMed] [Google Scholar]
- 23.Liang H, Yuan QP, Dong HR, Liu YM. Determination of sulforaphane in broccoli and cabbage by high-performance liquid chromatography. J Food Compos Anal. 2006;19:473–476. [Google Scholar]
- 24.Guo S, Qiu P, Xu G, Wu X, Dong P, Yang G, Zheng J, McClements DJ, Xiao H. Synergistic anti-inflammatory effects of nobiletin and sulforaphane in lipopolysaccharide-stimulated RAW 264.7 cells. J Agric Food Chem. 2012;60:2157–2164. doi: 10.1021/jf300129t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Connelly L, Palacios-Callender M, Ameixa C, Moncada S, Hobbs AJ. Biphasic regulation of NF-κB activity underlies the pro- and anti-inflammatory actions of nitric oxide. J Immunol. 2001;166:3873–3881. doi: 10.4049/jimmunol.166.6.3873. [DOI] [PubMed] [Google Scholar]
- 26.Heiss E, Herhaus C, Klimo K, Bartsch H, Gerhäuser C. Nuclear factor kappa B is a molecular target for sulforaphane-mediated anti-inflammatory mechanisms. J Biol Chem. 2001;276:32008–32015. doi: 10.1074/jbc.M104794200. [DOI] [PubMed] [Google Scholar]
- 27.Lee AK, Sung SH, Kim YC, Kim SG. Inhibition of lipopolysaccharide-inducible nitric oxide synthase, TNF-α and COX-2 expression by sauchinone effects on I-κBα phosphorylation, C/EBP and AP-1 activation. Br J Pharmacol. 2003;139:11–20. doi: 10.1038/sj.bjp.0705231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–3732. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tak PP, Firestein GS. NF-κB: a key role in inflammatory diseases. J Clin Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Basak C, Pathak SK, Bhattacharyya A, Mandal D, Pathak S, Kundu M. NF-κB- and C/EBPβ-driven interleukin-1β gene expression and PAK1-mediated caspase-1 activation play essential roles in interleukin-1β release from Helicobacter pylori lipopolysaccharide-stimulated macrophages. J Biol Chem. 2005;280:4279–4288. doi: 10.1074/jbc.M412820200. [DOI] [PubMed] [Google Scholar]
- 31.Shibata W, Maeda S, Hikiba Y, Yanai A, Ohmae T, Sakamoto K, Nakagawa H, Ogura K, Omara M. Cutting edge: the IκB kinase (IKK) inhibitor, NEMO-binding domain peptide, blocks inflammatory injury in murine colitis. J Immunol. 2007;179:2681–2685. doi: 10.4049/jimmunol.179.5.2681. [DOI] [PubMed] [Google Scholar]