Abstract
Four Korean kernel fruit (apple, pear, persimmon, and mandarin orange) juices were obtained by household processing techniques (i.e., blending, juicing). Whole and flesh fractions of each fruit were extracted by a blender or a juicer and then examined for phytochemical content (i.e., organic acids, polyphenol compounds). The antioxidant capacity of each juice was determined by ferric reducing antioxidant power (FRAP) and 2,2-diphenyl-1-picrylhydrazyl (DPPH) assays. Results revealed that juices that had been prepared by blending whole fruits had stronger antioxidant activities and contained larger amounts of phenolic compounds than juices that had been prepared by juicing the flesh fraction of the fruit. However, the concentration of ascorbic acid in apple, pear, and mandarin orange juices was significantly (P<0.05) higher in juice that had been processed by juicing, rather than blending. The juices with the highest ascorbic acid (233.9 mg/serving), total polyphenols (862.3 mg gallic acid equivalents/serving), and flavonoids (295.1 mg quercetin equivalents/serving) concentrations were blended persimmon juice, blended mandarin orange juice, and juiced apple juice, respectively. These results indicate that juice extraction techniques significantly (P<0.05) influences the phytochemical levels and antioxidant capacity of fruit juices.
Keywords: phenolic compounds, organic acids, antioxidant capacity, Korean kernel fruits, juice extraction
INTRODUCTION
Fruit juice is reported to be an excellent health-promoting beverage that is rich in antioxidant substances, such as phenolic compounds and vitamin C (1,2). For instance, consumption of fruit juice is associated with several health benefits, including increased antioxidant capacity (1), improved endothelial function (2), reduced low-density lipoprotein oxidation (3), and improved cardiovascular and neurocognitive function (4). Fruit is an important source of phytochemicals. Phytochemicals, which are also known as nutraceuticals or functional compounds, are secondary plant metabolites recognized for their antioxidant activities and other properties (5). Previous studies suggest that plant phytochemicals have anti-inflammatory, anti-proliferative, anti-carcinogenic, and anti-microbial properties (5,6). Thus, optimum dietary intake of these phytochemicals is essential for maintaining ideal health. The level of phytochemicals in fruit juice depends on various factors, namely, species, variety, growing conditions, seasonal variations, maturity index, processing methods, and storage conditions (7).
Organic acids are a useful index of fruit product authenticity (8). The nature and concentration of the organic acids found in fruits are of interest because of their important influence on the organoleptic properties and stability of fruit juices (7,8). The organic acids present in fruit exert an alkalizing effect on the human body, inhibit the growth of undesirable, and influence the course of metabolic processes (9). The main acids used to enhance beverages are citric acid and malic acid, which are acidulants, and ascorbic acid, which is an antioxidant (6,8).
Nowadays, consumers demand fruit and derivative products with high sensory, nutritional and health-related qualities. Determination of the effect of different household processing techniques on fruit phytochemical levels would be of interest to consumers seeking to obtain higher levels of these compounds (10,11). Previous studies report that juice extraction methods significantly influence chemical components levels and antioxidant activity of fruit (11,12). Rajasekar et al. (11) suggest that blending is more efficient than mechanical press juice extraction at preserving the overall physico-chemical characteristics of pomegranate juice.
The edible part of a fruit is the arils (i.e., pulp and flesh) which can be consumed fresh or as a processed product, such as juice. The outer rind of the fruit is frequently leathery and not consumable. Hence, when preparing fresh juice for consumption, most fruits are either peeled and blended or cut in half and the edible segments are juiced (12). However, recent studies suggest that use of the fruit peel can increase the amount of total polyphenols and flavonoids in the juice products (13,14). Thus, the removal of peels may result in a significant loss of constituents beneficial to human health. In recent years, many large-scale studies have been conducted on clarified fruit juice from a wide variety of sources (3,10,12).
Among different fruit cultivars, kernel fruits (e.g., apple, pear, persimmon, and mandarin orange) are one of the major types of bioactive components-rich fruits grown in Korea. These fruits are widely consumed in Korea year-round. However, there have been no reports comparing the bioactive compounds and antioxidant capacities of these fruits. Therefore, the purpose of this study was to evaluate and compare the antioxidant capacity, polyphenol compound content, and organic acid content of whole-fruit and flesh juices that had been extracted by blending and mechanical press juicing.
MATERIALS AND METHODS
Chemicals and reagents
Folin-Ciocalteu reagent, 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,4,6-tripyridyl-s-triazine (TPTZ), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), gallic acid, quercetin, and organic acid standards (citric acid, malic acid, and ascorbic acid) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). All other reagents and solvents were analytical grade.
Fruit materials
The apple (Malus pumila Miller, Fuji), pear (Pyrus pyrifolia Nakai), persimmon (Diospyros kaki, ‘Fuyu’ cultivar), and mandarin orange (Citrus unshiu) used in this research was purchased from a local supermarket in Seoul, Korea. These fruit samples were selected because they are important sources of dietary antioxidants and are consumed year-round in Korea. The fruits were stored at 5°C before processing.
Household processing of fruit juice
The fruits were washed with water and wiped completely dry. Each individual fruit was divided into equal portions. Half of the portions were left intact (i.e., whole) and half of the portions were peeled to isolate the arils from the peel (i.e., flesh-only). Care was taken to avoid the inclusion of seeds in the samples. For all fruits, flesh-only (F) and whole fruit (W) samples were processed by blending or juicing, yielding a total of four types of fruit juices for each fruit cultivar. Each replicate of the blended samples was processed with a blender (Hanil HH M-640, Seoul, Korea) for 3 min at a speed greater than approximately ≥20,000 rpm. Juiced samples were processed with a mechanical press juice extractor (Hurom HH-SBF11, HUROM Group Co., Kyungnam, Korea) fitted with a strainer to minimize the presence of whole juice vesicles. All juice samples were immediately stored at −4°C in the dark until further use.
Determination of organic acids
Malic acid, ascorbic acid, and citric acid determination was carried out according the method described by Scherer et al. (15). An HP 1100 series liquid chromatograph (Agilent Technologies, Santa Clara, CA, USA) equipped with a degasser, a quaternary pump, an autosampler adjusted to deliver 20 μL injections, an RP-C18 column (5 μm particle size, 250×4.6 mm I.D., kept at 25°C), and a UV-visible diode array detector. The mobile phase consisted of a 25 mM KH2PO4 buffer solution (pH=2.60 adjusted with o-phosphoric acid). An isocratic elution procedure with a flow rate of 0.8 mL/min was used. Malic acid and citric acid were detected at 210 nm, and ascorbic acid was detected at 250 nm. Peaks of interest were identified by their retention times. Peaks observed on the UV-visible spectra of each sample were compared to peaks observed on the UV-visible spectra of known standards. A five-point external standard curve was used to quantify each analyte. The concentrations of the standard curves ranged from the quantification limits and 0.25 mg/mL, 0.5 mg/mL, 0.01 mg/mL, and 0.8 mg/mL for malic, ascorbic, and citric acids, respectively. The linearity range of each analyte was evaluated by plotting the peak area, as a function of the known concentration of each standard.
Determination of total polyphenols
The total polyphenol content (TPC) of each juice was determined by the Folin-Ciocalteu method (16). Biefly, 50 μL of each diluted juice sample was mixed with 50 μL of 50% Folin-Ciocalteu reagent and 1 mL of 2% of sodium carbonate. The samples were then mixed well and allowed to stand for 30 min in the dark at room temperature. A Shimadzu 300 UV-vis spectrophotometer (Perkin-Elmer Inc., Waltham, MA, USA) was used to read the absorbance of each mixture at 760 nm. Quantification was based on a standard curve generated with 1~15 mg/L of gallic acid. Average results from triplicate determinations are expressed as mg of gallic acid equivalents (GAE)/mL of juice sample.
Determination of total flavonoids
The total flavonoid content (TFC) was analyzed according to the method described by Jia et al. (17). Briefly, 20 μL of 5% NaNO2 was mixed with 1 mL of each diluted juice sample and allowed to react at room temperature for 5 min. Then, 20 μL of 10% AlCl3·6H2O was added and the samples were incubated for 5 min before the addition of 150 μL of 1 M NaOH. The absorbance at 510 nm was measured after 15 min of incubation. TFC (mg/mL) is expressed as quercetin equivalents (QE).
DPPH radical scavenging assay
The free radical scavenging activity of fruit juice samples, based on the scavenging activity of the stable DPPH free radical, was determined using the method described by Brand-Williams et al. (18) with slight modifications. Briefly, 100 μL of each diluted juice sample was added to 900 μL of 35 μM DPPH dissolved in methanol. After incubating the solution at room temperature in the dark for 10 min, the absorbance of the solution was measured at 517 nm. Trolox calibration solutions (100 μM, 200 μM, 400 μM, 500 μM, and 750 μM) were used to generate the standard curve. The radical scavenging activity of each juice is expressed as mM Trolox equivalents (TE)/mL.
Ferric reducing antioxidant capacity (FRAP) assay
The FRAP assay was performed according to the method of Benzie and Strain (19) with minor modifications. Stock solutions of 300 mM acetate buffer, 10 mM TPTZ in 40 mM HCl, and 20 mM FeCl3·6H2O were prepared. The FRAP reagent was prepared by mixing the stock solutions in a 10:1:1 ratio (pH 3.6) and maintained at 37°C. Then, 100 μL of the sample was added to 900 μL of FRAP reagent and incubated at room temperature in the dark for 5 min. The absorbance of the solution was measured at 593 nm. The standard curve was linear between 0 μM and 700 μM Trolox. Results are expressed as mM TE/mL of juice sample.
Statistical analysis
All samples were analyzed in triplicate and the results were expressed as the mean±standard deviation. Unpaired Student’s t-tests were used for statistical analysis of differences between the juices obtained by juicing and juices obtained by blending. Significance was set at P<0.05 at the 95% confidence level. The Microsoft Excel software package (Microsoft Corp., Redmond, WA, USA) was used to perform correlation studies and to assess the significance of correlations.
RESULTS AND DISCUSSION
Variation of organic acid content in fruit juice
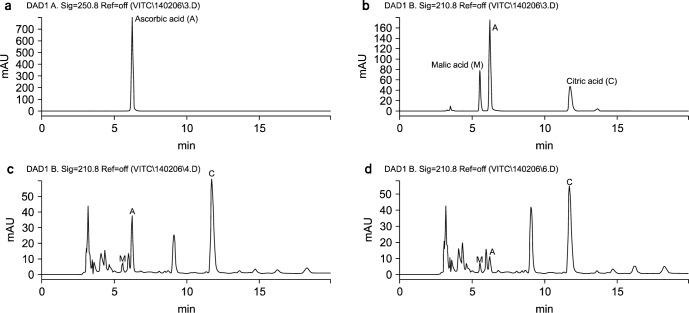
The organic acid profile and concentration in fruits depends on factors such as species, soil condition, and stress conditions (8). In this study, ascorbic, malic, and citric acids were separated and identified in 4 Korean kernel fruit juices. Fig. 1 shows the chromatograms obtained for a standard solution at 250 nm (a) and 210 nm (b) and the chromatograms (210 nm) obtained mandarin orange juice that had been prepared by juicing (c) and by blending (d). Citric acid and malic acids were monitored at 210 nm, while ascorbic acid was monitored at 250 nm due to a higher absorption. As shown in Fig. 1, the method used in the current study presented good selectivity and resolution.
Fig. 1.
Chromatogram of the standard solution of the organic acids (a: 250 nm, b: 210 nm) and mandarin orange juice by juicing (c) and blending (d) at 210 nm.
There was significant variation in the organic acid profiles of the juices tested in this study (Table 1). Among the analyzed fruit juices, the highest concentrations of ascorbic acid, citric acid, and malic acids were detected in blended persimmon juice (1,144.9 μg/mL), blended mandarin orange juice (5,777.7 μg/mL), and blended apple juice (4,091.6 μg/mL), respectively. With the exception of the persimmon juices, the concentration of ascorbic acid was significantly lower in the juice of blended fruits than the juice of juiced fruits. Moreover, ascorbic acid was not found in blended pear juice. In contrast, blended juices had significantly higher concentrations of citric and malic acids than juices obtained by juicing. Thus, the content of organic acids in different fruit juices was significantly affected by the juice extraction method. This is consistent with previously reported data. Uckoo et al. (12) reported that the concentration of ascorbic was significantly higher in grapefruit juice that had been processed by juicing, while the concentration of citric acid was significantly higher in grapefruit juice that had been processed by blending. As shown in Table 1, the most abundant organic acid present in the apple juices was malic acid (3,744.5∼4,091.6 μg/mL), and the least abundant organic acid present in the apple juices was ascorbic acid (38.2∼112.2 μg/mL). Fernandez-Fernandez et al. (9) reported that the concentration of malic acid in five commercial apple juices was between 100 mg/100 g and 500 mg/100 g.
Table 1.
Organic acid contents of blended and juiced Korean kernel fruit juices
| Organic acid (μg/mL)
|
Ascorbic acid
|
Citric acid
|
Malic acid
|
||||
|---|---|---|---|---|---|---|---|
| Fruit juice | Juiced | Blended | Juiced | Blended | Juiced | Blended | |
| Apple | Flesh | 90.8±0.4* | 38.2±0.6 | 93.5±1.4* | 173.6±1.4 | 3,744.5±15.3 | 3,790.1±17.1 |
| Whole | 112.2±1.8* | 52.6±1.1 | 84.1±2.2* | 168.2±1.7 | 3,919.2±13.2 | 4,091.6±13.3 | |
| Persimmon | Flesh | 335.3±2.4* | 726.4±9.3 | 208.5±2.7* | 304.8±1.5 | 2,035.7±16.1 | 2,124.1±11.5 |
| Whole | 395.8±1.6* | 1,144.9±11.2 | 114.9±1.6* | 202.4±1.2 | 2,302.9±14.7* | 2,551.4±14.4 | |
| Pear | Flesh | 18.6±0.3 | ND1) | 944.1±11.3 | 975.3±2.2 | 1,485.9±11.2 | 1,528.5±12.1 |
| Whole | 19.1±0.2 | ND | 813.1±4.4* | 931.5±4.4 | 1,564.5±17.4* | 1,740.5±13.6 | |
| Mandarin orange | Flesh | 283.5±2.5* | 177.4±2.8 | 5,495.6±12.5 | 5,777.7±15.8 | 338.6±7.7 | 356.8±1.8 |
| Whole | 386.3±3.4* | 278.9±2.1 | 5,548.5±15.4 | 5,687.4±14.2 | 353.4±1.9* | 455.4±2.5 | |
Data are expressed as mean±SD of triplicate experiments.
Significantly different from blended juice at P <0.05.
ND: not detected.
It is well known that citric acid and ascorbic acid are the major organic acid components of citrus fruits (9,15). In the present study, mandarin orange juice had significantly higher levels of citric acid (5,495.6∼5,777.7 μg/mL) than the other fruit juices (83.2∼975.3 μg/mL). Citric acid is a weak acid, but in high concentrations, it prevents the oxidation of ascorbic acid (9). Thus, citric acid has an indirect, but important effect on the antioxidant activity of fruit juice.
Interestingly, blended whole persimmon juice (1,144.9 μg/mL) had significantly higher levels of ascorbic acid than any of the other juices tested (18.6∼726.4 μg/mL). For instance, the ascorbic acid content in persimmon juice was about 34.5-fold higher than the ascorbic acid content in pear juice, which contained the lowest concentration of ascorbic acid. In general, ascorbic acid is a natural and potent antioxidant with several health benefits that is mainly present in fruits and vegetables (20). Due to the health maintaining properties of ascorbic acid, consumers are very interested in consuming food with high ascorbic acid contents. In the present study, whole fruit juices from the four fruits tested had higher concentrations of ascorbic acid and malic acids than the flesh juices, while flesh juices contained larger amounts of citric acid. This difference may be because the citric acid content of the flesh is higher than the citric acid content of the peel. The flavor of fruit flesh is highly dependent on the balance between soluble sugars and organic acids. For example, citric acid imparts the tart flavor of fruit which makes it an important component of sensory quality (8). As a result, juices made with the peel had higher concentrations of ascorbic acid and malic acids, but lower concentrations of citric acid than the flesh-only juices tested in this study.
Variation of total polyphenols and flavonoids in fruit juice
The main active substances in fruit are polyphenols, which have antioxidant, anti-mutagen, anti-inflammatory, and antimicrobial abilities (1,7). Polyphenols are present in different parts of each fruits (1). Fig. 2 shows that there were significant differences in the TPC and the TFC of the 4-blended and juiced fruit juice products. Among the analyzed fruit juices, the highest TPC and TFC were detected in blended mandarin orange juice (4.64 mg GAE/mL) and juiced apple juice (1.29 mg QE/mL), respectively, while the lowest TPC and TFC were detected in the blended pear juice (0.68 mg GAE/mL and 0.05 mg QE/mL, respectively). Therefore, the TPC of mandarin orange juice was about 6.8-fold higher than that of pear juice and the TFC of apple juice was remarkably higher than that of pear juice.
Fig. 2.
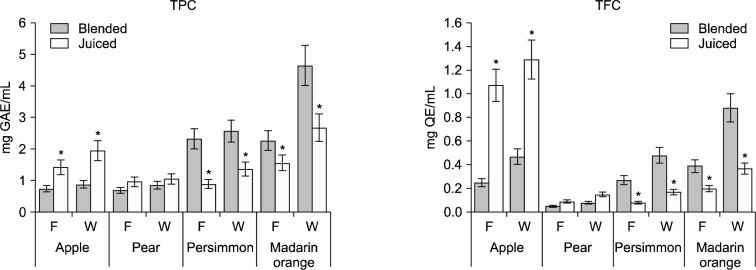
Contents of total polyphenols (TPC) and total flavonoids (TFC) of flesh (F) and whole (W) juices by blending and juicing from Korean kernel fruits. Data are expressed as mean±SD of triplicate experiments. *Significantly differences from blended juice at P <0.05.
The results of the present study indicate that the polyphenol content of different fruit juices is significantly affected by juice extraction method. TPC and TFC were significantly higher in blended persimmon and mandarin orange juices than in their juiced counterparts. Similar results have been reported by other investigators. According to Uckoo et al. (12), blended grapefruit juice has a higher pulp content, which is correlated to TPC, than juiced grapefruit juice. Rajasekar et al. (11) determined that blending was more efficient than mechanical press juicing when producing pomegranate juice. In contrast, the TPC and TFC of a single serving of juiced apple juice were significantly higher than that of blended apple juice (417.5 mg GAE vs. 195.3 mg GAE and 295.1 mg QE vs. 90.1 mg QE, respectively; Table 2). Similarly, the TPC and TFC of juiced pear juice were also significantly higher than that of blended pear juice (250.2 mg GAE vs. 190.1 mg GAE and 30.2 mg QE vs. 17.5 mg QE, respectively). Therefore, juicing, a common household processing method, could be a better strategy than blending when significantly higher levels of apple and pear flavonoids are desired. These results suggest that the effect of household juice extraction technique on juice polyphenol levels varies by fruit cultivar. It is very important to understand that TPC and TFC are affected by different processing techniques.
Table 2.
Phytochemicalcontent and antioxidant capacity of single serving of blended and juiced Korean kernel fruit juices
| Fruit juice | Total phenolics (mg GAE) 1)
|
Flavonoids (mg QE) 2)
|
Ascorbic acids (mg)
|
TEAC (mM)3)
|
||||
|---|---|---|---|---|---|---|---|---|
| Juiced | Blended | Juiced | Blended | Juiced | Blended | Juiced | Blended | |
| Apple | 417.5±1.4* | 195.3±0.9 | 295.1±1.7* | 90.1±1.6 | 25.4±0.2* | 11.4±0.1 | 307.3±2.4* | 121.9±0.8 |
| Persimmon | 217.5±2.2* | 610.1±2.6 | 17.6±0.2* | 42.5±0.1 | 91.5±1.1* | 233.9±2.8 | 380.3±2.6* | 1001.3±5.1 |
| Pear | 250.2±1.7* | 190.1±1.1 | 30.2±0.4* | 17.5±0.1 | 4.7±0.1 | ND4) | 90.3±0.4* | 59.5±0.3 |
| Mandarin orange | 527.5±2.8* | 862.3±3.1 | 72.5±0.6* | 160.1±1.2 | 83.7±0.5* | 57.1±0.5 | 620.5±3.8* | 391.1±1.7 |
Data are expressed as mean±SD of triplicate experiments.
Significantly different from blended juice at P <0.05.
Expressed as mg gallic acid equivalents (GAE) per 250 mL serving.
Expressed as mg quercetin equivalents (QE) per 250 mL serving.
Expressed as mM Trolox equivalent antioxidant capacity (TEAC) per 250 mL serving as measured by FRAP and DPPH assays.
ND, not detected.
Generally, the arils of fruit contain large amounts of organic acids, sugars, minerals, and vitamins (12), but the peels contain higher amount of phenolic compounds than the flesh (13). As shown in Fig. 1, the polyphenol content of whole fruit juice from Korean kernel fruits was higher than juice of the flesh fraction of the fruit. Our results revealed that juicing whole fruits, rather than the flesh alone, was the main factor contributing to high TPC and TFC in the fruit juices analyzed. Tzulker et al. (10) reported that the content of phenolics, flavonoids, and their constituents in peel extracts of different fruit juices was much higher complex than the pulp extracts of the same fruits. The TPC of whole fruit juices from 29 different pomegranates was between 1,875 mg/L and 11,250 mg/L, which is about 6.5-fold higher than juices prepared from the arils (10). Likewise, the results of the present study indicate that whole-fruit squeezing tends to be superior to aril juicing. The descending rank order of the mean of the TPC values obtained for a single serving (250 mL) of the juiced and blended juices of each fruit tested in this study is: mandarin orange> persimmon> apple> pear (Table 2). The descending rank order of the mean of the TFC values obtained for a single serving (250 mL) of the juiced and blended juices of each fruit tested in this study is: apple> mandarin orange> persimmon> pear.
Variation of antioxidant activity in fruit juice
Determination of antioxidant capacity of fruit juice provides information about the biological activities of the major components responsible for improving human health and nutrition (21). Two widely used antioxidant assays (DPPH and FRAP) were used to evaluate the effect of household juice extraction techniques on the antioxidant capacities of four fruit juices. There was a high positive correlation (r=0.9224) between FRAP and DPPH values for juiced and blended fruit juices. This suggests that both methods are suitable for the determination of the antioxidant capacity of the fruit juices selected in this study. FRAP and DPPH are electron transfer mechanism based assays (22).
The results of both assays confirmed that the fruit juices tested had significant antioxidant capacities. As shown in Fig. 3, the antioxidant properties of blended persimmon juice were the strongest (4.36 mM Trolox/mL for DPPH and 5.12 mM Trolox/mL for FRAP), while the antioxidant capacity of blended pear juice was the lowest (0.14 mM Trolox/mL for DPPH and 0.23 mM Trolox/mL for FRAP). This can be explained by the fact that blended persimmon juice had the highest pulp content, which corresponds to antioxidants levels. These results are consistent with the fact that blended persimmon juice contains a higher TPC, TFC, and ascorbic acid content than juiced persimmon juice. Previous reports indicate that juicing is associated with the elimination of free radicals and the inhibition of oxidation (21). However, the antioxidant capacities of mandarin orange, apple, and pear juices obtained using a juicer were higher than those of the juices obtained by blending. Thus, the effect of juice extraction techniques on antioxidant capacity depended on the fruit cultivar.
Fig. 3.
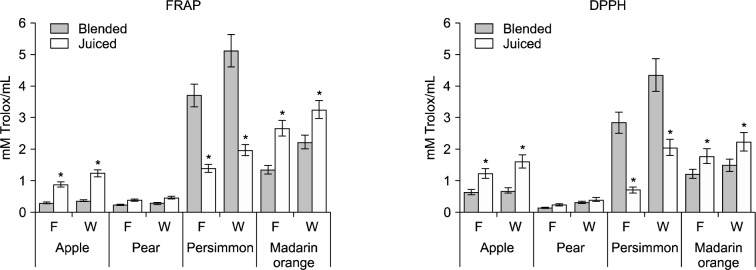
Antioxidant capacities of flesh (f) and whole (W) juices by blending and juicing from Korean kernel fruits. Data are expressed as mean±SD of triplicate experiments. *Significantly different from blended juice at P <0.05.
The persimmon used in this study was a non-astringent variety of persimmon fruit, which can be consumed in a raw state or a processed product. Lee et al. (23) reported that the strong antioxidant activities of non-astringent persimmons correlate with high concentrations of phenolic acids, catechins, gallic acid, and epicatechin gallate. Among the fruit juices tested in our study, the antioxidant capacities measured by the FRAP and DPPH assays corresponded with TPC and ascorbic acid content. For all fruit juices, a positive and significant linear correlation was confirmed between ascorbic acid contents and the antioxidant capacities determined by the FRAP (r=0.8901) and DPPH (r=0.9115) assays. Thus, ascorbic acid was a main contributor to the total antioxidant capacities of the fruit juices analyzed, which may be due to the variation in their chemical structure (22). This result was consistent with the results of Gardner et al. (1), who report that vitamin C is a major antioxidant in citrus juice that contributes to 65∼100% of the total antioxidant capacity of citrus juice.
As can be expected, the juice products containing fruit peels (i.e., whole fruit juices) had higher total antioxidant capacities than the juice products that did not contain peels (i.e., flesh-only juices). For instance, the antioxidant levels of whole persimmon juices was an average of 1.6 times higher than the antioxidant levels of persimmon flesh juices. As shown in Table 2, the TPC, TFC, and ascorbic acid content was lower in blended pear juice than in other juices. These results suggest that the antioxidant capacity of fruit juices corresponds to antioxidants content. In the present study, the fruit juices that contained peels had stronger antioxidant activities and contained larger amounts of phenolic compounds and ascorbic acid than flesh-only juices. The juices with the highest ascorbic acid (233.9 mg/serving), total polyphenol (862.3 mg GAE/serving), and flavonoid (295.1 mg QE/serving) contents were blended persimmon juice, blended mandarin orange juice, and juiced apple juice, respectively (Table 2). Thus, blended persimmon and blended mandarin orange juices may provide higher levels of beneficial components such as ascorbic acid and total polyphenol compounds, while juiced apple juice may provide higher levels of flavonoids. The descending rank order of the mean of the antioxidant capacity values obtained for a single serving (250 mL) of the juiced and blended juices of each fruit tested in this study is: persimmon> mandarin orange> apple> pear.
In conclusion, the phytochemical content and antioxidant capacity of Korean kernel fruit (apple, pear, persimmon, and mandarin orange) juices were significantly affected by the juice extraction method. For the four fruits tested, blending the edible parts of whole fruits yielded a juice that contained higher levels of total polyphenols and flavonoids but lower levels of ascorbic acid than juicing the flesh fraction of the fruits. The blended persimmon juice had the highest antioxidant capacity (1,001.3 mM Trolox equivalent antioxidant capacity/serving size) of all of the fruit juices tested. The results of this study could help consumer’s select fruit juices that contain higher levels of health-maintaining phytochemicals.
ACKNOWLEDGEMENTS
This research was supported by a Sungshin Women’s University research grant.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Gardner PT, White TAC, McPhail DB, Duthie GG. The relative contributions of vitamin C, carotenoids and phenolics to the antioxidant potential of fruit juices. Food Chem. 2000;68:471–474. [Google Scholar]
- 2.Sun J, Chu YF, Wu X, Liu RH. Antioxidant and antiproliferative activities of common fruits. J Agric Food Chem. 2002;50:7449–7454. doi: 10.1021/jf0207530. [DOI] [PubMed] [Google Scholar]
- 3.Ruel G, Pomerleau S, Couture P, Lemieux S, Lamarche B, Couillard C. Favourable impact of low-calorie cranberry juice consumption on plasma HDL-cholesterol concentrations in men. Br J Nutr. 2006;96:357–364. doi: 10.1079/bjn20061814. [DOI] [PubMed] [Google Scholar]
- 4.Gunathilake KDPP. MSc Thesis. Dalhousie University; Halifax, NS, Canada: 2012. A fruit-based functional beverage designed to reduce the risk of cardiovascular disease. [Google Scholar]
- 5.Dillard CJ, German JB. Phytochemicals: nutraceuticals and human health. J Sci Food Agric. 2000;80:1744–1756. [Google Scholar]
- 6.Prior RL, Cao G. Antioxidant phytochemicals in fruits and vegetables: diet and health implications. HortScience. 2000;35:588–592. [Google Scholar]
- 7.Bravo L. Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutr Rev. 1998;56:317–333. doi: 10.1111/j.1753-4887.1998.tb01670.x. [DOI] [PubMed] [Google Scholar]
- 8.Kader AA. Flavor quality of fruits and vegetables. J Sci Food Agric. 2008;88:1863–1868. [Google Scholar]
- 9.Fernandez-Fernandez R, Lopez-Martinez JC, Romero-Gonzalez R, Martinez-Vidal JL, Flores MIA, Frenich AG. Simple LC–MS determination of citric and malic acids in fruits and vegetables. Chromatographia. 2010;72:55–62. [Google Scholar]
- 10.Tzulker R, Glazer I, Holland D, Aviram M, Amir R. Antioxidant activity, polyphenol content, and related compounds in different fruit juices and homogenates prepared from 29 different pomegranate accessions. J Agric Food Chem. 2007;55:9559–9570. doi: 10.1021/jf071413n. [DOI] [PubMed] [Google Scholar]
- 11.Rajasekar D, Akoh CC, Martino KG, MacLean DD. Physico-chemical characteristics of juice extracted by blender and mechanical press from pomegranate cultivars grown in Georgia. Food Chem. 2012;133:1383–1393. [Google Scholar]
- 12.Uckoo RM, Jayaprakasha GK, Balasubramaniam VM, Patil BS. Grapefruit (Citrus paradisi Macfad) phytochemicals composition is modulated by household processing techniques. J Food Sci. 2012;77:C921–C926. doi: 10.1111/j.1750-3841.2012.02865.x. [DOI] [PubMed] [Google Scholar]
- 13.Ma JN, Wang SL, Zhang K, Wu ZG, Hattori M, Chen GL, Ma CM. Chemical components and antioxidant activity of the peels of commercial apple-shaped pear (fruit of Pyrus pyrifolia cv. pingguoli) J Food Sci. 2012;77:C1097–C1102. doi: 10.1111/j.1750-3841.2012.02899.x. [DOI] [PubMed] [Google Scholar]
- 14.Wasila H, Li X, Liu L, Ahmad I, Ahmad S. Peel effects on phenolic composition, antioxidant activity, and making of pomegranate juice and wine. J Food Sci. 2013;78:C1166–C1172. doi: 10.1111/1750-3841.12204. [DOI] [PubMed] [Google Scholar]
- 15.Scherer R, Rybka ACP, Ballus CA, Meinhart AD, Filho JT, Godoy HT. Validation of a HPLC method for simultaneous determination of main organic acids in fruits and juices. Food Chem. 2012;135:150–154. [Google Scholar]
- 16.Singleton VL, Rossi JA., Jr Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- 17.Jia Z, Tang M, Wu J. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–559. [Google Scholar]
- 18.Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol. 1995;28:25–30. [Google Scholar]
- 19.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 20.Davey MW, Van Montagu M, Inze D, Sanmartin M, Kanellis A, Smirnoff N, Benzie IJJ, Strain JJ, Favell D, Fletcher J. Plant L-ascorbic acid: chemistry, function, metabolism, bioavailability and effects of processing. J Sci Food Agric. 2000;80:825–860. [Google Scholar]
- 21.Burda S, Oleszek W. Antioxidant and antiradical activities of flavonoids. J Agric Food Chem. 2001;49:2774–2779. doi: 10.1021/jf001413m. [DOI] [PubMed] [Google Scholar]
- 22.Huang D, Ou B, Prior RL. The chemistry behind antioxidant capacity assays. J Agric Food Chem. 2005;53:1841–1856. doi: 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- 23.Lee JH, Lee YB, Seo WD, Kang ST, Lim JW, Cho KM. Comparative studies of antioxidant activities and nutritional constituents of persimmon juice (Diospyros kaki L. cv. Gapjubaekmok) Prev Nutr Food Sci. 2012;17:141–151. doi: 10.3746/pnf.2012.17.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]