SUMMARY
Live pentavalent human–bovine reassortant rotavirus vaccine is recommended in the United States for routine immunization of infants. We describe three infants, two with failure to thrive, who had dehydration and diarrhea within 1 month after their first or second rotavirus immunization and subsequently received a diagnosis of severe combined immunodeficiency. Rotavirus was detected, by means of reverse-transcriptase–polymerase-chain-reaction (RT-PCR) assay, in stool specimens obtained from all three infants, and gene-sequence analysis revealed the presence of vaccine rotavirus. These infections raise concerns regarding the safety of rotavirus vaccine in severely immunocompromised patients.
Rotavirus infection is a leading cause of childhood diarrhea worldwide.1 In 2006, a live oral pentavalent vaccine (RV5; marketed as Rota-Teq, Merck) that contains five human–bovine reassortant rotavirus strains was licensed and recommended for use in infants in the United States2 after a large, national multicenter trial showed its efficacy.3 The individual reassortant strains were generated with the use of a bovine rotavirus parent strain, Wistar calf 3 (WC3), and four human rotavirus strains.3 The reassortant strains are attenuated in their ability to replicate. Each oral vaccine dose contains at least 2.0×106 to 2.8×106 infectious units in a 2-ml solution.
Severe combined immunodeficiency (SCID) is a group of genetic disorders that results in a lack of T- and B-cell immunity. SCID is characterized by life-threatening infections during the first year of life and is fatal unless corrected, usually by means of stem-cell transplantation. Infections associated with the administration of live viral vaccines in infants with SCID have been described previously.4,5 Live viral vaccines are typically contraindicated in patients with known severe immunodeficiencies6; however, the rotavirus-vaccine series is recommended to be started at 2 months of age, which is before SCID is typically diagnosed in infants for whom there is no family history of immunodeficiency. We describe three infants with SCID in whom vaccine-associated disease developed after receipt of rotavirus vaccine.
CASE REPORTS
PATIENT 1
Patient 1 was a full-term female infant who presented to an outside hospital at 2 weeks of age with respiratory failure and pneumonia. At that time, the peripheral-blood white-cell count was 4300 cells per cubic millimeter with 11% lymphocytes (absolute lymphocyte count, 473 cells per cubic millimeter [normal range for age, 3400 to 7600]7). She was discharged home at 2 months of age and received the RV5 vaccine at 2 and 4 months of age at her pediatrician’s office. She did not attend day care. She was rehospitalized at 5 months of age with dehydration, severe diarrhea, metabolic acidosis, failure to thrive (weight at less than the third percentile), and pneumonia. The peripheral-blood white-cell count was 1120 cells per cubic millimeter with 3% lymphocytes (absolute lymphocyte count, 38 cells per cubic millimeter [normal range for age, 3900 to 9000]7). A bronchoscopy specimen grew Pseudomonas aeruginosa, and silver staining for Pneumocystis jiroveci and fungal organisms was negative. Stool specimens were positive for rotavirus by means of enzyme immunoassay. Adenosine deaminase was undetectable in white cells, and a diagnosis of SCID was made. There were multiple severe, persistent episodes of diarrhea until the age of 8 months, associated with intermittent fever, weight loss, dependence on total parenteral nutrition, and electrolyte disturbance, including marked metabolic acidosis requiring electrolyte replacement and periodic fluid resuscitation. The infant was discharged and received pegylated adenosine deaminase–replacement therapy, pending curative treatment through stem-cell transplantation. A stool specimen for rotavirus was positive at the age of 9 months, despite resolution of the diarrhea, and negative at the age of 10 months. The patient continues to receive pegylated adenosine deaminase–replacement therapy.
PATIENT 2
Patient 2 was a full-term male infant who was born without complications after an uneventful pregnancy. The patient had no adverse effects after the first dose of RV5 vaccine at 2 months of age, but 6 days after receipt of a second dose at 4 months of age, he presented with shock, dehydration, and watery diarrhea. The infant did not attend day care. The weight was in the 50th to 75th percentile. The peripheral-blood white-cell count on hospital admission was 9300 cells per cubic millimeter with 19% lymphocytes (absolute lymphocyte count, 1767 cells per cubic millimeter). Rotavirus was detected, with the use of enzyme immunoassay, in stool specimens, as was adenovirus and giardia species. Tests for red-cell adenosine deaminase and purine nucleoside phosphorylase levels were normal. A mutation was present in the common gamma chain of the interleukin-2 receptor, consistent with X-linked SCID. Stem-cell transplantation (without chemotherapy-induced bone marrow ablation beforehand or prophylaxis against graft-versus-host disease) was performed at the age of 5 months with the use of haploidentical peripheral-blood stem cells. The diarrhea had resolved by this time, but the patient remained dependent on total parenteral nutrition for an additional 3 weeks. A second transplantation was performed at the age of 8 months, because of post-transplantation neutropenia. After the engraftment of donor lymphocytes, the infant was discharged. Stool specimens remained positive for rotavirus at 8.5 months of age but were negative for rotavirus at 9 to 12 months of age.
PATIENT 3
Patient 3 was a full-term male infant who was born to consanguineous parents. He received the RV5 vaccine at 2 months of age in a pediatrician’s office, after which he presented with severe diarrhea, failure to thrive (weight at less than the 3rd percentile), and respiratory distress. He did not attend day care. Severe lymphopenia (absolute lymphocyte count, 135 cells per cubic millimeter) was present on admission to the hospital. Rotavirus was detected in stool specimens, by means of enzyme immunoassay. Bronchoscopy was performed, and silver staining of an aspirate specimen revealed P. jiroveci. The patient was treated with intravenous trimethoprim–sulfame-thoxazole. Respiratory syncytial virus was present in a nasal-wash specimen, as detected with the use of enzyme immunoassay. Tests for red-cell adenosine deaminase and purine nucleoside phosphorylase levels were normal. A homozygous mutation was present in recombination activating gene 1 (RAG1), consistent with a diagnosis of SCID. At 8 months of age, the patient underwent transplantation of bone marrow from a haploidentical, related donor (without chemotherapy-induced bone marrow ablation beforehand), and at 10 months of age, underwent a second transplantation (preceded by a reduced-intensity conditioning regimen) of bone marrow from a matched, unrelated donor, followed by boost transplantation with both CD34+ stem cells and CD3+ donor lymphocytes at 14 months of age, because of nonengraftment. He received methotrexate and cyclosporine as prophylaxis against graft-versus-host disease. At the most recent assessment, the diarrhea had improved, but the patient continued to require intravenous hyperalimentation. Stool specimens were positive for rotavirus before 14 months of age but were negative at 14 months of age (at the most recent evaluation).
METHODS
RT-PCR AND SEQUENCE ALIGNMENT
Viral genomic RNA was extracted from two different lots of RV5 (lot numbers 1194U and 0019X) and from stool samples obtained from each of the three case patients, as well as a serum specimen from Patient 1 only, with the use of a commercial assay (Qiagen). Stool samples were prepared as 10% suspensions in double-distilled water containing 0.5% zwitterionic sulfobetaine detergent (EMD Chemicals), which were vortexed and clarified by means of centrifugation for 8 minutes at 8000 rpm. RNA was extracted from the liquid supernatant.
After reverse transcription of RNA isolated from the sample from each patient and each lot of vaccine, sequences of 2 of the 11 rotavirus genes were amplified by means of PCR. Gene 6 was amplified with the use of a 5′-TTCGATTTCGGGTTACTTGG-3′ sense primer (spanning nucleotides 204 to 223) and a 5′-GACAAGAATACGCGATTCCC-3′ antisense primer (spanning nucleotides 1072 to 1091) based on the bovine rotavirus strain WC3.8 Gene 10 was amplified with the use of a 5′-GGCTTTTAAAAGTTCTGTTCCGAG-3′ sense primer (spanning nucleotides 1 to 24) and a 5′-GGTCACACTAAGACCATTCC-3′ antisense primer (spanning nucleotides 731 to 750) based on the sequence of human rotavirus A gene 10.9 DNA amplicons were isolated after electrophoresis on a 1% agarose gel and were sequenced using a Big Dye Terminator kit (version 3.1, Applied Biosystems). Sequence alignments were analyzed according to the European Bioinformatics Institute’s ClustalW2 sequence-alignment program (www.ebi.ac.uk/Tools/clustalw2/index.html).
IMMUNOLOGIC AND GENETIC ANALYSES
Enumeration of selected peripheral-blood leukocytes, including CD3+ T cells, CD20+ B cells, and CD56+ natural killer cells, was performed by means of flow-cytometric analysis, involving a panel of standardized, commercially available reagents, according to previously published methods.7,10 In vitro proliferative responses of peripheral-blood mononuclear cells to phytohemagglutinin, concanavalin A, and pokeweed mitogen were measured by means of a standard tritiated-thymidine-incorporation assay. Humoral immune responses were measured by determination of the serum IgG, IgA, IgM, and IgE levels. Molecular analysis for SCID-gene defects (Correlagen) was performed with the use of blood samples obtained from the three patients. The adenosine deaminase level was also assessed, at the Cytogenetics Laboratory of Baylor College of Medicine.
RESULTS
RT-PCR, SEQUENCE ALIGNMENT, AND IMMUNOLOGIC ANALYSIS
RT-PCR that used the primer sets for rotavirus genes 6 and 10 resulted in isolation of DNA products for all specimens tested: both lots of RV5, stool samples obtained from all three case patients, and for a serum sample, obtained 30 days after the stool specimens were collected, from Patient 1 (see Table 1 in the Supplementary Appendix, available with the full text of this article at NEJM.org). Gene 6 amplicons isolated from the stool samples from each patient were similar in size to those from the vaccine (Fig. 1).
Figure 1. DNA Amplicons of Rotavirus Gene 6 Isolated from Stool Specimens Obtained from the Three Case Patients.
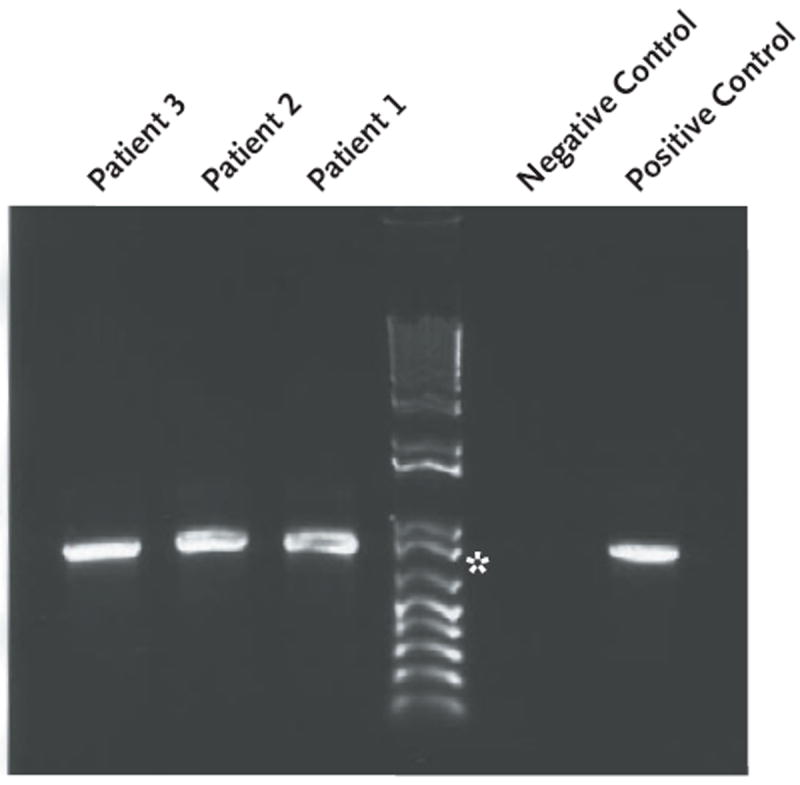
The amplicon size is 888 bp; for comparison, an 800-bp marker is shown (asterisk). The negative control was double-distilled water, and the positive control, rotavirus vaccine.
The two lots of RV5 yielded identical sequences for each of the two genes tested (gene 6 and gene 10). On the basis of the alignment of 542 bp of DNA (nucleotides 148 to 689), gene-10 sequences from stool specimens obtained from all three patients with SCID were identical to the RV5 gene-10 sequence (Fig. 1 in the Supplementary Appendix). The gene-10 sequence from the serum sample from Patient 1 was also identical to the vaccine gene-10 sequence (on the basis of alignment of nucleotides 157 to 641) (Fig. 1 in the Supplementary Appendix). On the basis of the alignment of 806 bp of DNA (nucleotides 263 to 1068), the gene-6 sequences from all stool specimens obtained from the three patients and from the serum sample from Patient 1 were identical to the RV5 gene-6 sequence, with the exception of a single nucleotide difference (C→T) at residue 974 in the specimens obtained from Patients 1 and 2 (Fig. 2). This nucleotide change was present in both the serum and stool specimens from Patient 1 and in all five stool specimens obtained from Patient 2 over a 5-day period (Fig. 2 in the Supplementary Appendix).
Figure 2. Sequences of Rotavirus Gene 6 in DNA Isolated from Stool Specimens Obtained from the Three Case Patients.
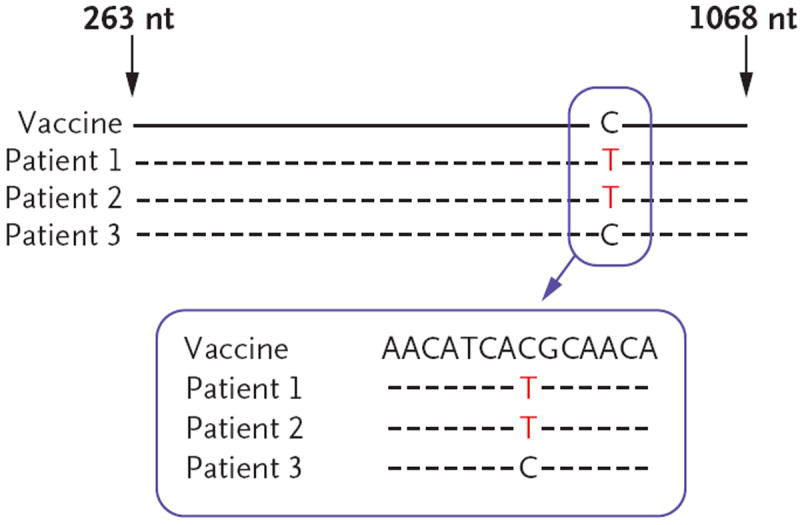
Representative sequences are shown for the rotavirus vaccine RV5 and for specimens obtained from Patients 1, 2, and 3. The sequences were identical between the two lots of vaccine, among each patient’s multiple stool specimens, and between the vaccine and the specimens from each patient, with the exception of the single nucleotide difference shown for Patients 1 and 2. Amplicons were isolated with the use of a reverse-transcriptase–polymerase-chain-reaction assay. The “nt” denotes nucleotides.
All three case patients had markedly depressed levels of IgG, IgA, and IgM, CD3+ T cells, and proliferation in response to mitogens (Table 1).
Table 1.
Immunologic Evaluation and Characteristics of the Three Case Patients with Severe Combined Immunodeficiency (SCID).*
| Patient No. |
Age at Receipt of Rotavirus Vaccine |
Absolute Lymphocyte Count before SCID Diagnosis |
CD3+ T-Cell Count |
CD19+ B-Cell Count |
CD16+56+ Natural Killer Cell Count |
Cell Proliferation | Immunoglobulin | Molecular Diagnosis |
Outcome | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| per μl | Phyto- hemagglutinin |
Concanavalin A |
Pokeweed Mitogen |
IgG | IgA | IgM | IgE | |||||||
|
|
|
|
||||||||||||
| per ml | % of normal value | mg/dl | IU/ml | |||||||||||
| 1 | 2 and 4 mo | 473 at 0.5 mo of age, 38 at 5 mo of age | 0 | 0 | 0 | <1 | <1 | <1 | 58 | <6 | <5 | ND | ADA deficiency | Alive; ADA-replacement therapy started at 7 mo of age |
|
| ||||||||||||||
| 2 | 2 and 4 mo | 1767 at 4 mo of age | 4 | 1711 (within normal range) | 29 | 2 | 4 | 11 | <150 | <6 | 24 | <1 | IL2RG mutation | Alive; underwent transplantation at 5 and 8 mo of age |
|
| ||||||||||||||
| 3 | 2 mo | 135 at 2 mo of age | 4 | 3 | 74 | 0 | 2 | 1 | <270 | <6 | <5 | <1 | RAG1 mutation | Alive; underwent transplantation at 8 and 10 mo of age (boost at 14 mo of age) |
Levels were abnormal unless otherwise specified. Normal values were as follows7: for the absolute lymphocyte count at 0 to 3 months of age, 3400 to 7600 per cubic millimeter, and at 3 to 6 months of age, 3900 to 9000 per cubic millimeter; for CD3+ T cells, 2500 to 6500 cells per milliliter; for CD19+ B cells, 430 to 3000 cells per milliliter; for CD16+56+ natural killer cells, 170 to 830 cells per milliliter; for IgG, 231 to 659 mg per deciliter; for IgA, 7 to 59 mg per deciliter; and for IgM, 27 to 212 mg per deciliter. In infants, values for IgE can be less than 1 IU per milliliter but should not be 0. The limit of detection of the IgE assay used is 1 IU per milliliter. To convert values for IgE to micrograms per liter, multiply by 2.40. ADA denotes adenosine deaminase, IL2RG common gamma chain of the interleukin-2 receptor gene, ND not done, and RAG1 recombination activating gene 1.
DISCUSSION
RV5 is currently recommended for routine childhood immunization, and prelicensure and post-licensure data indicate that the vaccine is efficacious and has a low risk of associated adverse events.3,11 Although administering live rotavirus vaccine is not absolutely contraindicated in persons with compromised immune systems, some live viral vaccines are contraindicated in persons with severe immunodeficiencies.6 Cases of disease acquired from the receipt of vaccines such as the oral poliovirus vaccine have been described in patients with congenital immunodeficiencies.12-14
RT-PCR analysis of DNA isolated from stool samples from all three infants revealed sequences of WC3 bovine rotavirus genes 6 and 10, the same sequences contained in the vaccine. WC3-like bovine rotaviruses are not known to circulate among humans, and the gene sequences of the WC3 strain are distinct from those of human rotaviruses, indicating that the infections in our patients were caused by the vaccine strain.
Genes 6 and 10 were also detected by means of RT-PCR in a serum sample from one infant, indicating that systemic spread of the vaccine virus may have occurred. Extraintestinal manifestations of rotavirus infection, including viremia, have been described.15-17 Confirmation (by sequence-alignment analysis) of vaccine-associated infection detected a single nucleotide difference in gene 6 in two of the three infants with rotavirus infection. However, a T nucleotide is present in one of the five reassortant strains of RV5 (Ciarlet M, Merck: personal communication), so this finding could represent the preferential growth of one reassortant strain in two of the three patients with SCID.
Viral shedding after the administration of rotavirus vaccine has been detected in stool specimens from 1 to 15 days after vaccination.18 In our study, enzyme immunoassay revealed the presence of rotavirus at 157, 197, and 362 days after the last dose of vaccine in Patients 1, 2, and 3, respectively, indicating a longer duration of shedding than has been previously reported in immunocompetent children.18 Rotavirus was detected in multiple stool samples, obtained several days apart, from each of the three case patients, suggesting viral replication. Chemotherapy administered before the second transplantation in Patient 3, followed by post-transplantation prophylaxis against graft-versus-host disease, may also have contributed to the viral persistence observed. Prolonged shedding has also been reported in immunocompromised patients with naturally acquired rotavirus disease.19-21
In the absence of a suggestive family history, immunization with live viral vaccines may be performed before the diagnosis of a severe immunodeficiency. The absolute lymphocyte count was severely low in all three case patients before diagnosis of SCID, and a complete blood count with differential may be a clue of immunodeficiency before the rotavirus vaccine is delivered. In the United States, archived neonatal blood spots (Guthrie cards) have been used successfully to identify common genetic disorders; they also offer the clinician an opportunity to perform necessary medical interventions in the first weeks of life. Several U.S. states are pursuing the use of these cards to measure markers of immune deficiency, such as circulating levels of mature T cells or presence of T-cell–receptor excision circles found in normal hosts but not in patients with profound T-cell deficiencies.22 The detection of such immune deficiencies in the first weeks of life would allow clinicians the opportunity to select infants who can benefit the most from this efficacious vaccine and to diminish potential vaccine-associated morbidity.
In summary, vaccine-acquired rotavirus disease was detected after the administration of RV5 in three infants with SCID. In two of the three, rotavirus disease developed after receipt of the second rotavirus immunization, not the first, which may reflect early protection in infancy by transplacentally acquired maternal antibodies.23,24 Rotavirus clearance was accomplished only after successful immune reconstitution. These cases of rotavirus infection raise concerns regarding the safety of rotavirus vaccines in severely immunocompromised patients and the need to prevent vaccine-acquired disease, as with other live viral vaccines.
Acknowledgments
Supported by grants from the National Institutes of Health (General Clinical Research Centers grant M01 RR-00188, K12 HD41648 to Dr. Hertel, and an unrestricted grant to Dr. Estes).
Dr. Estes reports receiving consulting fees and stock options from LigoCyte Pharmaceuticals and lecture fees from Merck and being an inventor on a patent for a rotavirus viruslike-particle vaccine that is licensed to GlobalVaccines.
We thank Drs. Wendy A. Keitel and Robert L. Atmar for helpful comments on an earlier version of this article.
Footnotes
No other potential conflict of interest relevant to this article was reported.
References
- 1.Parashar UD, Gibson CJ, Bresse JS, Glass RI. Rotavirus and severe childhood diarrhea. Emerg Infect Dis. 2006;12:304–6. doi: 10.3201/eid1202.050006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cortese MM, Parashar UD. Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2009;58(RR-2):1–25. [PubMed] [Google Scholar]
- 3.Vesikari T, Matson DO, Dennehy P, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354:23–33. doi: 10.1056/NEJMoa052664. [DOI] [PubMed] [Google Scholar]
- 4.Pariyaprasert W, Pacharn P, Visitsunthorn N, et al. Successful treatment of disseminated BCG infection in a SCID patient with granulocyte colony stimulating factor. Asian Pac J Allergy Immunol. 2008;26:71–5. [PubMed] [Google Scholar]
- 5.Culic S, Kuzmic I, Culic V, et al. Disseminated BCG infection resembling Langerhans cell histiocytosis in an infant with severe combined immunodeficiency: a case report. Pediatr Hematol Oncol. 2004;21:563–72. doi: 10.1080/08880010490477257. [DOI] [PubMed] [Google Scholar]
- 6.The Pink Book: epidemiology and prevention of vaccine preventable diseases. 10. Atlanta: Centers for Disease Control and Prevention; 2008. [Google Scholar]
- 7.Shearer WT, Rosenblatt HM, Gelman RS, et al. Lymphocyte subsets in healthy children from birth through 18 years of age: the Pediatric AIDS Clinical Trials Group P1009 study. J Allergy Clin Immunol. 2003;112:973–80. doi: 10.1016/j.jaci.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Ciarlet M, Hyser JM, Estes MK. Sequence analysis of the VP4, VP6, VP7, and NSP4 gene products of the bovine rotavirus WC3. Virus Genes. 2002;24:107–18. doi: 10.1023/a:1014512314545. [DOI] [PubMed] [Google Scholar]
- 9.Sahoo GC, Nayak MK, Battacharya R, et al. Human group A rotavirus NSP4 gene. [January 4, 2010]; at http://www.ncbi.nlm.nih.gov/nuccore/AB196958.1?ordinalpos=6&itool=EntrezSystem2.PEntrez.Sequence.Sequence_ResultsPanel.Sequence_RVDocSum.)
- 10.Breard J, Reinherz EL, Kung PC, Goldstein G, Schlossman SF. A monoclonal antibody reactive with human peripheral blood monocytes. J Immunol. 1980;124:1943–8. [PubMed] [Google Scholar]
- 11.Delayed onset and diminished magnitude of rotavirus activity — United States, November 2007–May 2008. MMWR Morb Mortal Wkly Rep. 2008;57:697–700. [PubMed] [Google Scholar]
- 12.Khetsuriani N, Prevots DR, Quick L, et al. Persistence of vaccine-derived polioviruses among immunodeficient persons with vaccine-associated paralytic poliomyelitis. J Infect Dis. 2003;188:1845–52. doi: 10.1086/379791. [DOI] [PubMed] [Google Scholar]
- 13.Monafo WJ, Haslam DB, Roberts RL, Zaki SR, Bellini WJ, Coffin CM. Disseminated measles infection after vaccination in a child with a congenital immunodeficiency. J Pediatr. 1994;124:273–6. doi: 10.1016/s0022-3476(94)70318-3. [DOI] [PubMed] [Google Scholar]
- 14.Jean-Philippe P, Freedman A, Chang MW, et al. Severe varicella caused by varicella-vaccine strain in a child with significant T-cell dysfunction. Pediatrics. 2007;120(5):e1345–e1349. doi: 10.1542/peds.2004-1681. [DOI] [PubMed] [Google Scholar]
- 15.Blutt SE, Kirkwood CD, Parreño V, et al. Rotavirus antigenaemia and viraemia: a common event? Lancet. 2003;362:1445–9. doi: 10.1016/S0140-6736(03)14687-9. [DOI] [PubMed] [Google Scholar]
- 16.Chiappini E, Galli L, de Martino M. Viremia and clinical manifestations in children with rotavirus infection. J Infect Dis. 2006;193:1333. doi: 10.1086/501374. [DOI] [PubMed] [Google Scholar]
- 17.Gilger MA, Matson DO, Connor ME, Rosenblatt HM, Finegold MJ, Estes MK. Extraintestinal rotavirus infections in children with immunodeficiency. J Pediatr. 1992;120:912–7. doi: 10.1016/s0022-3476(05)81959-6. [DOI] [PubMed] [Google Scholar]
- 18.Anderson EJ. Rotavirus vaccines: viral shedding and risk of transmission. Lancet Infect Dis. 2008;8:642–9. doi: 10.1016/S1473-3099(08)70231-7. [DOI] [PubMed] [Google Scholar]
- 19.Rayani A, Bode U, Habas E, et al. Rotavirus infections in paediatric oncology patients: a matched-pairs analysis. Scand J Gastroenterol. 2007;42:81–7. doi: 10.1080/00365520600842179. [DOI] [PubMed] [Google Scholar]
- 20.Losonsky GA, Johnson JP, Winkelstein JA, Yolken RH. Oral administration of human serum immunoglobulin in immunodeficient patients with viral gastroenteritis: a pharmacokinetic and functional analysis. J Clin Invest. 1985;76:2362–7. doi: 10.1172/JCI112248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saulsbury FT, Winkelstein JA, Yolken RH. Chronic rotavirus infection in immunodeficiency. J Pediatr. 1980;97:61–5. doi: 10.1016/s0022-3476(80)80131-4. [DOI] [PubMed] [Google Scholar]
- 22.Puck JM. Population-based newborn screening for severe combined immunodeficiency: steps toward implementation. J Allergy Clin Immunol. 2007;120:760–8. doi: 10.1016/j.jaci.2007.08.043. [DOI] [PubMed] [Google Scholar]
- 23.Bishop RF, Barnes GL, Cipriani E, Lund JS. Clinical immunity after neonatal rotavirus infection: a prospective longitudinal study in young children. N Engl J Med. 1983;309:72–6. doi: 10.1056/NEJM198307143090203. [DOI] [PubMed] [Google Scholar]
- 24.Newman RD, Grupp-Phelan J, Shay DK, Davis RL. Perinatal risk factors for infant hospitalization with viral gastroenteritis. Pediatrics. 1999;103(1):E3. doi: 10.1542/peds.103.1.e3. [DOI] [PubMed] [Google Scholar]


