Abstract
Objective
Mechanisms of air pollution-induced exacerbation of cardiovascular disease are currently unknown, thus we examined the roles of vascular endothelin-1 (ET-1) and reactive oxygen species (ROS) in regulating mediators of vascular remodeling, namely matrix metalloproteinases (MMPs), following exposure to vehicle engine emissions.
Methods and Results
ApoE-/- mice were exposed by inhalation to filtered air or gasoline engine exhaust (GEE, 1:12 dilution) 6 h/d for 1 or 7 days. Concurrently, mice were treated with either ETA receptor antagonist BQ-123 (100 ng/kg/day) via osmotic minipumps, Tempol (∼41 mg/kg/day, orally), or vehicle. GEE-exposure increased vascular MMP-2 and -9, endothelin-1 (ET-1), tissue inhibitor of metalloproteinases (TIMP)-2 mRNA and ROS levels. Aortic MMP protein and plasma MMP-9 were similarly upregulated. GEE-mediated increases in vascular ROS were attenuated by Tempol-treatment, as were MMP-2 and TIMP-2; whereas BQ-123 ameliorated GEE-induced vascular expression of MMP-9, MMP-2, ROS, and ET-1. In a parallel study, diesel exhaust exposure in volunteer human subjects induced significant increases in plasma ET-1 and MMP-9 expression and activity.
Conclusions
These findings demonstrate that acute exposure to vehicular source air pollutants results in upregulation of circulating and vascular factors associated with progression of atherosclerosis, mediated in part through activation of ET-1 - ETA receptor pathways.
Keywords: atherosclerosis, endothelin-1, matrix metalloproteinase, reactive oxygen species, air pollution
Atherosclerosis, a disease of the vasculature characterized by endothelial dysfunction and arterial plaque formation, has a multifactoral etiology that includes genetic, behavioral, and environmental influences. Numerous epidemiological studies indicate a positive correlation between exposure to common environmental air pollutants and increased risk of cardiovascular morbidity and mortality both chronically and acutely1-3. Furthermore, a clear relationship between exposure to air pollution of vehicular origin and cardiovascular events has been established2,4. Experimental findings have defined a role for components of environmental air pollution in the progression of atherosclerosis including: impaired vascular endothelial function5, increase in plaque cell turnover and lipids in aortic lesions6, and altered vasomotor tone and induced vascular inflammation7. While these studies describe a relationship between exposure to environmental air pollutants and factors associated with atherosclerosis, the underlying mechanisms have not been fully elucidated.
A hallmark of atherosclerosis is inappropriate vascular remodeling, mediated by extracellular matrix (ECM) degradation. The matrix metalloproteinase (MMP) family of endopeptidases represents the primary mediators of vascular ECM degradation8. While MMP activity is essential for vascular homeostasis, dysregulation of MMPs also underlies pathobiological alterations in the vasculature9, including progression of atherosclerosis and destabilization of advanced plaques10. Recent reports have shown that increasingly diverse stimuli, including reactive oxygen species (ROS), can upregulate most MMPs in the vasculature11,12.
Other vascular factors, including endothelin-1 (ET-1), are also mediate progression of atherosclerosis. ET-1, a constitutively secreted peptide, acts via the ETA and ETB receptors in the vasculature, where ETA predominantly mediates vasoconstriction and mitogenic pathways and ETB mediates vasodilation. ET-1 is significantly upregulated in atherosclerotic vessels13 and has been shown to increase MMP activity in cardiovascular pathologies14,15.
We have previously reported that subchronic inhalational exposure to the ubiquitous environmental air pollutant, gasoline engine emissions (GEE), results in increased expression of vascular factors associated with the progression of atherosclerosis, namely vascular ROS, ET-1, and MMPs in ApoE-/- mice16. However, it has not been determined (1) what mediates expression of MMPs, or (2) whether these pathways are activated during acute exposures, in a manner that is consistent with epidemiological findings. Thus, we tested the hypothesis that acute exposure to GEE results in an ET-1 - ETA mediated increase in vascular ROS and MMP expression and activity, in atherosclerotic ApoE-/- mice.
Materials and Methods
An expanded Methods section is available in the online data supplement available at http://atvb.ahajournals.org
Animals and Inhalation Exposure Protocol
Ten-week-old male ApoE-/- mice (Taconic, Oxnard, CA) were placed on a high fat diet (TD88137 Custom Research Diet, Harlan Teklad, Madison, WI; 21.2% fat by weight, 1.5g/kg cholesterol) beginning 30 days prior to exposure. ApoE-/- mice were inhalationally exposed to GEE (60 μg/m3 particulate matter whole exhaust) or filtered-air (controls) for 6 h/d for a period of 1 or 7 days (n=18 for each group/time point). The exposure concentration (Supplemental Table 1) was chosen to maximize the biological pathways relevant to exposures, without initiating lung injury or inflammation16. All procedures were approved by the Lovelace Respiratory Research Institute's Animal Care and Use Committee and conform to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
Gasoline emissions were generated as previously described16. The engines were fueled with conventional unleaded, non-oxygenated, non-reformulated gasoline (ChevronPhillips Specialty Fuels Division, The Woodlands, TX), and emissions diluted approximately 1:12 with filtered air. Components of GEE were characterized and are summarized in supplemental Table SI (available at http://atvb.ahajournals.org)
Tempol dosing groups
ApoE-/- mice assigned to filtered air or GEE, were randomly assigned to receive either 4-Hydroxy-TEMPO (Tempol, Sigma, St. Louis, MO), 1 mmol/L (approximately 41 mg/kg/day, sterile-filtered) in their drinking water, or vehicle (ddH2O), beginning 24 hrs prior to, and throughout exposure. Delivered dose was calculated from daily intake.
BQ-123 dosing groups
ApoE-/- mice exposed to either filtered air or GEE, were randomly assigned to receive either BQ-123 (100 ng/kg/day, Sigma) or vehicle (sterile saline) via osmotic minipumps (model #1007D Alzet, Cupertino, CA). Animals were monitored daily for health status throughout the study.
Real time RT-PCR
Total RNA was isolated from the aorta (one-half, midsagittal cut from arch to common iliac bifurcation), n=6 per group, using RNeasy Fibrous Tissue Mini Kit (Qiagen, Valencia, CA). cDNA was synthesized, and real time PCR performed as previously described17. Control reactions (no RT or RNA) were run to verify the absence of contaminated DNA and primer-dimerization. ΔCT was calculated as previously described16. Results expressed as normalized gene expression as percentage of controls (18S).
TBARS Assay
Aortic thiobarbituric acid reactive substances (TBARs) levels were assessed using a TBARS assay kit (OXItek, ZeptoMetrix Corp Buffalo, NY) measuring TBARS levels in whole, uncentrifuged aorta homogenates per kit instructions, as previously described16.
Western blot analysis
Aortas were homogenized and protein isolated from cytosolic fractions. Aorta and plasma were concentrated on a Millipore column (YM-10, Fisher Scientific), and quantified using the Bradford assay (Biorad, Hercules, CA). 5 μg of protein was loaded (n = 3-5 per group) for SDS-PAGE electrophoresis under reducing conditions. Membranes were blocked overnight at 4°C in 5% blotto, incubated in either rabbit polyclonal anti-mouse MMP-9 (1:3000 dilution) (Abcam, Cambridge, MA), anti-mouse MMP-2 (1:3000, Abcam), or anti-mouse TIMP-2 (1:1000, Abcam), and β-actin (1:2000, Abcam) for 1 hr at RT, and then anti-rabbit -HRP was used for 1 hr at RT. Bands were visualized with chemiluminescence and densitometry performed utilizing Image J software (NIH).
In situ zymography
Aorta sections (6 μm) were incubated with 45 μl of 10μg/ml dye quenched (DQ)-gelatin (EnzChek, Molecular Probes, Invitrogen, Carlsbad, CA) and 1 μg/ml DAPI (Invitrogen) in 1% UltraPure™ LMP agarose (Invitrogen) cover-slipped, chilled for 5 minutes at 4 °C, and then incubated for 6 hours in a dark, humid chamber at 37°C. Some slides were pre-incubated with a gelatinase inhibitor (MMP -2, -9 inhibitor IV, Chemicon, Millipore, Temecula, CA).
Human plasma MMP-9, ET-1, and NOx assays
In conjunction with the Human Studies Division at the EPA, healthy subjects (n=10; 18-40 years old, 4 male/6 female) were exposed to a target concentration of 100 μg/m3 diesel (DE) whole exhaust or HEPA and charcoal filtered “clean” air (controls) for 2 hrs, on separate occasions. Subjects had 4 cycles of 15 min rest and 15 min exercise on a stationary bicycle at a target ventilation rate of 25 L oxygen/min/m3 body surface area. DE was generated from a Cummins engine (5.9L, 205 hp) operating at or near idle conditions using a certified commercial #2 fuel purchased from ChevronPhillips. Components of DE were measured and are summarized in supplemental Table I (available at http://atvb.ahajournals.org). Blood was collected pre-exposure, 30 min and 24 hrs post-exposure, and plasma stored at -80°C until analysis. MMP-9 ELISA (Biotrak #RPN2614, Amersham, Piscataway, NJ), and MMP-9 activity ELISA (#RPN263A, Amersham), were performed on pre- and post- exposure plasma samples (1:10 dilution in assay buffer) per manufacturer instructions. ET-1 levels were quantified by ELISA (#QET00B, R&D Systems, Minneapolis, MN) per manufacturer instructions. Plasma NOx levels were measured using a Nitrate/Nitrite colorimetric kit (#79001, Cayman Chemical, Ann Arbor, MI), per manufacturer instructions. One sample was eliminated due to lack of pre-exposure sample. All procedures were approved by the Lovelace Respiratory Research Institutional Review Board under exemption #4 (protocol #07-001) and all subjects provided informed consent.
Statistical analysis
Data expressed as mean ± SEM. One-way analysis of variance (ANOVA) with a post hoc Holm-Sidak test was used for analysis of multiple groups; human samples were analyzed with repeated measures ANOVA. A p < 0.05 was considered statistically significant.
Results
Vascular MMP-2 and -9 is activated by acute GEE-exposure in ApoE-/- mice
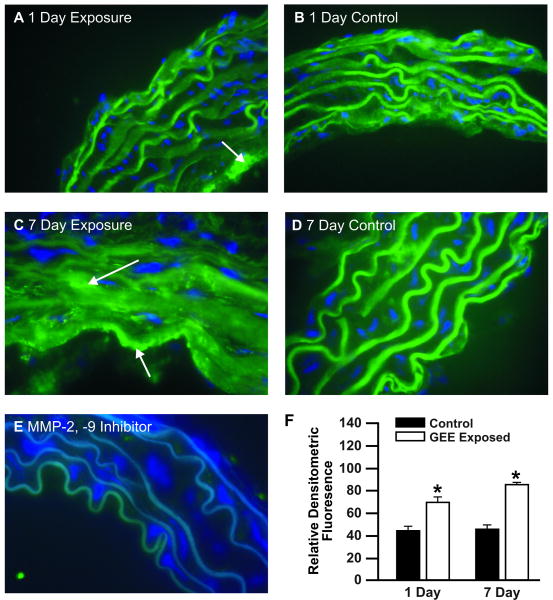
In situ zymography was used to quantify acute GEE-mediated effects in the aorta. Aorta gelatinase activity was increased at day 1 of exposure (Figure 1A), with further amplification at day 7 (Figure 1C), compared to controls (Figure 1B and Figure 1D, respectively). Localization of MMP-2/9 activity appears to be predominantly in the endothelial layer of the aorta at day 1 (Figure 1A, arrow); whereas by day 7 of exposure activity is found throughout the vasculature (Figure 1C, arrows). A MMP-2/9 -inhibitor confirms fluorescence is specific to gelatinase activity (Figure 1E). Densitometric quantification of relative fluorescence from all samples analyzed is shown in Fig 1F.
Figure 1. In situ zymography in aortas from ApoE-/- mice exposed to GEE for 1 or 7 days.
(A) 1 day GEE (60 μg/m3), (B) 1 day air-filtered control, (C) 7 day GEE (60 μg/m3); (D) 7 day control, (E) negative control pretreated for 30 minutes with MMP-2/9 specific inhibitor, and (F) densitometric quantification. Green fluorescence indicates gelatinase activity; blue fluorescence is DAPI nuclei staining. (n=3-4 sections per tissue, at least 3 sites of analysis per slide). Arrows indicate increased gelatinase activity. *p < 0.050 compared to control.
Acute GEE- exposure augments vascular MMP protein expression in ApoE-/- mice
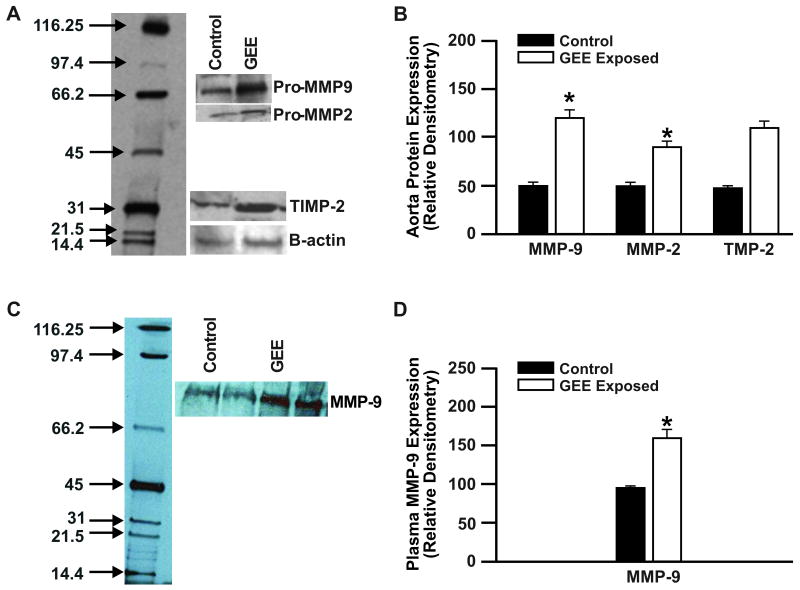
Because MMP expression is regulated through multiple mechanisms, we assessed aorta MMP-2, -9, and plasma MMP-9 protein levels from each study group by Western blot (Figure 2). In agreement with our transcriptional data, 7 day GEE-exposure resulted in a significant increase in aorta protein expression of MMP-9, MMP-2, and TIMP-2 (Figures 2A and 2B) and plasma MMP-9 (Figures 2C and D) in ApoE-/- mice.
Figure 2. Aorta and plasma MMP-9 protein expression from 7-day GEE exposures in Apo E -/- mice.
Aorta (A and B) and plasma (C and D) protein expression, determined by Western blot, in ApoE-/- mice exposed for 6 h/d for 7 days to: (A) Lane 1: control (filtered air); lane 2: GEE (60 μg/m3); (C) lane 1,2: control; lane 3,4: GEE. Densitometric analysis of aorta mRNA (B), and plasma MMP-9 (D). *p < 0.050 compared to controls.
Combustion source engine emissions increase plasma ET-, MMP-9, and NOx in human exposures
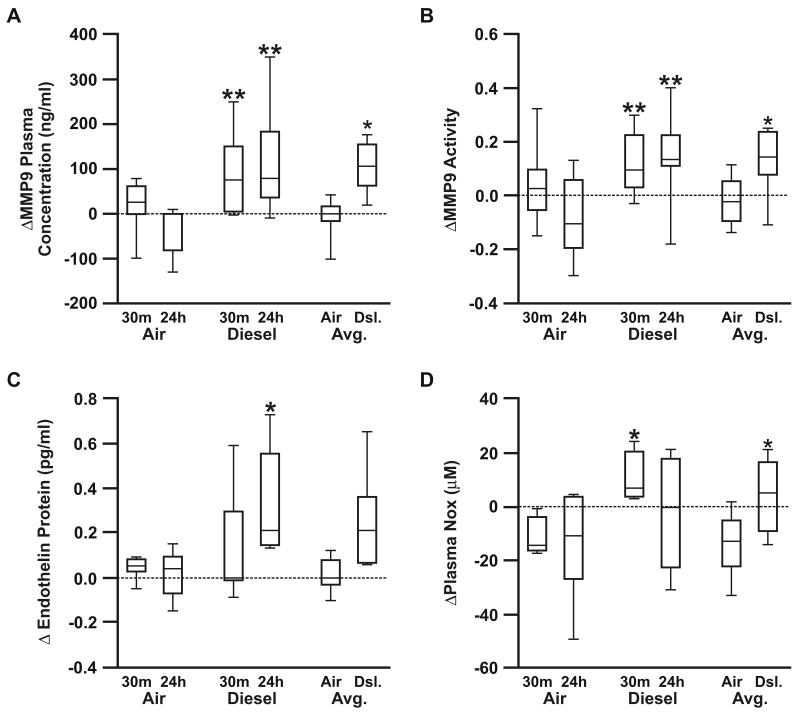
Plasma MMP-9 levels have been identified as a novel predictor of cardiovascular mortality in patients with CVD17. Since plasma MMP-9 expression and activity was found signficantly increased in GEE-exposed ApoE-/- mice, we tested whether plasma MMP-9 may serve as a useful translational biomarker for human models of exposure, as well. Plasma samples collected from humans exposed to diesel engine exhuast (DE), for only 2 hours, showed a signficant elevation in MMP-9 concentration (Figure 3A) and activity (Figure 3B). Intra-subject variation was apparent in baseline values of MMP-9, however the plasma MMP-9 concentration and activity was uniformly elevated post-DE exposure as compared to changes induced by filtered air exposure. Plasma ET-1 (Figure 3C) and NOx (Figure 3D) were also found to be signficantly upregulated as a result of DE (supplemental Table II available at http://atvb.ahajournals.org).
Figure 3. Human plasma MMP-9, ET-1, and NOx levels in response to DE exposure.
Human plasma MMP-9 (A), MMP-9 activity (B), ET-1 (C), and NOx (D), in response to 2 hours of DE exposure, determined by ELISA (MMP-9, ET-1) and colorimetric assay (NOx). Data presented as the difference from pre-exposure to each post-exposure values (30 min-post and 24 h-post) for pro-MMP9, MMP9 activity, ET-1, and NOx. To further identify whether individuals had a response to DE at either time point, compared to air exposure, the difference between the pre-exposure and the average of the two post-exposure values is also presented. *p < 0.050 compared to controls.
BQ-123-treatment normalizes vascular ROS, ET-1 and MMP-9; whereas Tempol-treatment attenuates only vascular ROS
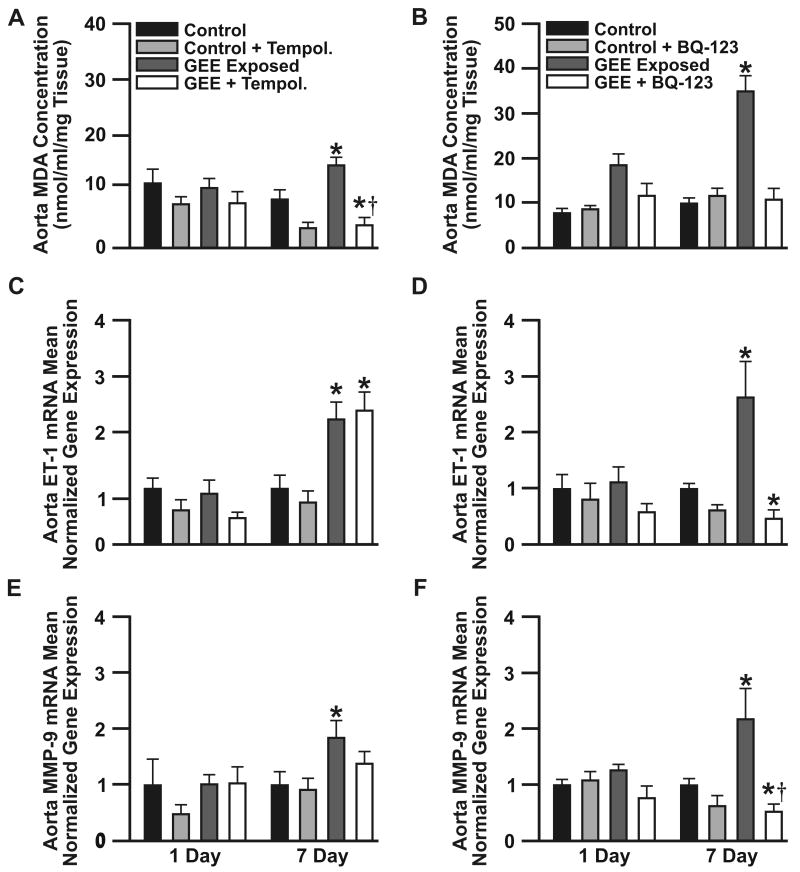
It has been previously reported that exposure to vehicular emissions induces vascular ROS16, which was confirmed in the present acute exposures (supplemental Figure IA and Figure IC; available at http://atvb.ahajournals.org), as well as increases vascular16 and circulating18 levels of ET-1. To elucidate the role of ROS vs. ET-1 in mediating expression of vascular MMP-9, ApoE-/- mice were treated orally with either 1 mmol/L of Tempol (avg. 41 mg/kg/day) or vehicle, beginning 24 h prior to exposure; in a separate study ApoE-/- mice were treated with either BQ-123 (100 ng/kg/day, osmotic minimpump) or vehicle, throughout the study. Tempol-treatment resulted in reduced TBARs levels, in both control and GEE-exposed mice at day1, with further decreases at day7 (Fig 4A). Tempol resulted in modest reductions in GEE-mediated vascular ET-1 expression at day1, with no effects observed by day7 (Figure 4B), as well a decrease in MMP-9 expression (Fig 4C), which remained elevated compared to controls. Conversely, BQ-123-treatement effectively attenuated expression of vascular ROS (Figure 4D), as well as reduced ET-1 (Figure 4E) and MMP-9 (Figure 4F) mRNA to below control levels. Such findings indicate that while Tempol was successful in reducing vascular ROS, it was unable to normalize vascular ET-1 or MMP-9; whereas ET-receptor blockade ameliorated both ET-1 and MMP-9 mRNA expression in the vasculature of GEE-exposed ApoE-/- mice. Similarly, GEE-exposure also results in significant elevations in aorta MMP-2 and TIMP-2 mRNA by day 7 of exposure (supplemental Figure II, available at http://atvb.ahajournals.org). Only TIMP-2 expression was significantly upregulated by day 1 of exposure (supplemental Figure IIB). While Tempol-treatment normalized vascular expression of MMP-2 (supplemental Figure IIA) and TIMP-2 (supplemental Figure IIB); BQ-123-treatment normalized MMP-2 mRNA (supplemental Figure IIC) and reduced TIMP-2 at day 1; however, it was elevated above controls by day 7 (supplemental Figure IID). Such findings suggest TIMP-2 expression is likely mediated through additional pathways than ET-1 - ETA. GEE-exposure had no effect on vascular TIMP-1 expression (data not shown).
Figure 4. Lipid peroxidation and transcriptional changes in aorta ET-1 and MMP-9, from ApoE-/- mice exposed to GEE and co-treated with either Tempol or BQ-123.
Expression of aortic TBARS levels (A), and ET-1 (B) and MMP-9 (C) mRNA in ApoE-/- mice exposed for 6 h/d for 1 or 7 days to either filtered air (controls), tempol (10mmol/L in drinking water), GEE (60 μg/m3), or tempol + GEE (n=6 each). Expression of aortic TBARS levels (D), and ET-1 (E) and MMP-9 (F) mRNA in ApoE-/- mice exposed for 6 h/d for 1 or 7 days to either filtered air (controls), BQ-123 (100 ng/kg/day, osmotic minipump), GEE (60 μg/m3), or BQ-123 + GEE (n=6 each). *p≤0.050 compared to controls, †p≤ 0.050 compared to GEE-exposed.
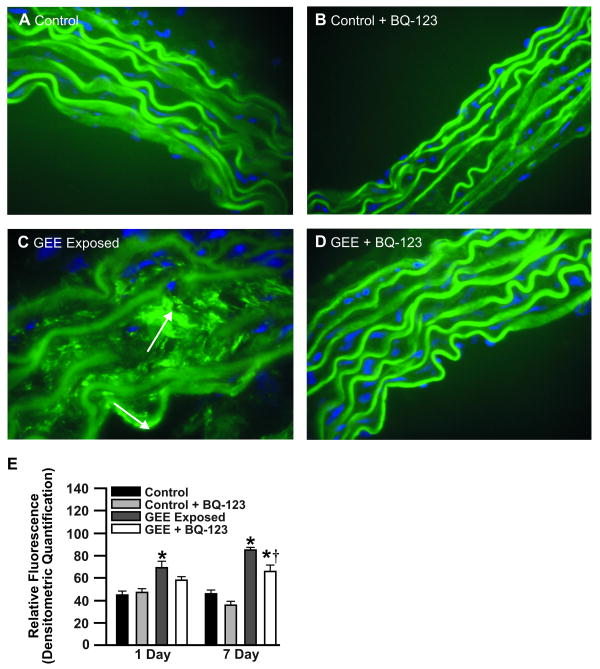
BQ-123 – treatment attenuates GEE-mediated vascular MMP-2 and -9 activity
To determine whether MMP-2/9 activity are similarly affected by GEE-induced ET-1, as that observed at the transcript level, aortas were analyzed by in situ zymography. Compared to controls (Figure 5A and 5B), GEE-mediated gelatinase activity was significantly increased (Figure 5C) in ApoE-/- mice, which was attenuated with BQ-123 treatment (Figure 5D). BQ-123-treatment also resulted in significant reductions in MMP-9 protein levels in GEE exposed animals (supplemental Figure III, available at http://atvb.ahajournals.org). Interestingly, BQ-123 did not normalize gelatinase activity, as observed with mRNA and protein levels, suggesting alternative mechanisms of MMP-2 and -9 activation (vs. expression) in addition to ET-1 - ETA cell signaling pathways.
Figure 5. In situ zymography in aortas from GEE-exposed ApoE-/- treated with BQ-123 for 7 days.
(A) filtered air control (B) control + BQ-123 (100 nmol/mg/day, osmotic minipump), (C) GEE (60 μg/m3); (D) GEE + BQ-123. Green fluorescence indicates gelatinase activity; blue fluorescence is nuclei staining. (n=3-4 sections per tissue, at least 3 sites of analysis per slide). Arrows indicate increased gelatinase activity. *p < 0.050 compared to control.
Discussion
The present study demonstrates that the ETA receptor pathway plays a central role in GEE-induced effects on the vasculature, including expression and activity of MMP-2 and -9, ET-1, and oxidative stress. In controlled human exposures to DE, we report a pattern of plasma MMP-9 expression and activity induction that is remarkably consistent to that observed in the GEE-mouse model exposures. While typically considered to be highly different exhausts in terms of chemical compositions, the biological findings of the present study suggest that the similar constituents in DE and GEE (e.g. CO, NO, NO2, hydrocarbons) may be critical to vascular toxicity. It is important to note that the CO levels in these exposures did not result in apparent hypoxemic conditions, as carboxyhemoglobin levels were not elevated in the human DE exposures (unpublished data, 2008) and vascular endothelial growth factor (VEGF), which contains a hypoxia response element (HRE) in its promoter, was not altered in GEE exposures (supplemental Figure IV, available at http://atvb.ahajournals.org). Considering the relationship between MMP expression and vascular event outcomes19,20, these results represent a very promising link to recent epidemiological findings that identify vehicular emissions as risk factors for acute cardiovascular events4.
Peters et al.4 reported an increased risk of acute myocardial infarction (AMI) within 4-24 hours of traffic exposure. Other studies have also identified acute air pollution exposure, related to vehicular sources, as a significant risk factor for clinical events2,21,22. It is plausible to suspect activation of plaque-degrading enzymes, such as MMP-2 and -9, may play an important role in mediating air pollution-induced cardiovascular events such as AMI. This premise is further supported by recent animal studies that show conditional overexpression of MMP-9 results in substantive enhancement of atherosclerotic plaque instability23. While the present study did not focus on plaque disruption, per se, the biological dynamics of vascular MMP-2/9 regulation and activity, in response to exposure to vehicular emissions, provides a plausible link to epidemiological findings.
The results of the present study are temporally congruous with the epidemiological findings of acute induction of cardiovascular events associated with air pollution exposures, in that we demonstrate activation of vascular MMP-2 and -9 within 24 hours of exposure to GEE. Subsequently, transcription of MMP-2 and -9 is upregulated over the following week, leading to de novo synthesis of additional proteins and prolonged maintenance of vascular MMP response. The observed increase in activation of gelatinases may be due to a decreased interaction between these MMPs and their respective inhibitor (TIMP). The balance between MMPs and TIMPs is crucial in regulating vascular ECM remodeling; MMP-9 is preferentally inhibitied by TIMP-1, while MMP-2 is preferentially inhibited by TIMP-224. Interestingly, we observe no change in expression of vascular TIMP-1 mRNA in ApoE-/- mice exposed to GEE; however TIMP-2 mRNA is signficantly elevated by day 1 of exposure to GEE (supplemental Figure II, available at http://atvb.ahajournals.org). The implications of these results are two-fold: (1) the increased gelatinase activity observed in GEE-exposed ApoE-/- mice aortas are likely predominantly MMP-9 driven (due to the imbalance of expression of vascular MMP-9 to TIMP-1, and thus a decrease in MMP-9:TIMP-1 binding), and (2) TIMP-1 and TIMP-2 are regulated differently in the vasculature in response to both GEE-exposure and MMP activation.
Vascular ROS are significantly upregulated by GEE, as evidenced by increased aortic TBARs levels and DHE fluorescence (supplemental Figures IA and IC available at http://atvb.ahajournals.org). Importantly, lung ROS levels are not increased as a result of GEE exposure (supplemental Figure ID), which is consistent with previous studies16, thereby indicating that the observed vascular effects of GEE may be independent of pulmonary oxidative stress pathways. Vascular NAD(P)H oxidase activation appears to be one source of GEE-induced ROS (supplemental Figure V, available at http://atvb.ahajournals.org), although other sources (e.g. xanthine oxidase, uncoupled eNOS, mitochondria) cannot be ruled out. We show that Tempol-treatment ameliorates vascular oxidative stress, and attenuates acute increases in vascular MMP-2 and TIMP-2 in GEE-exposed ApoE-/- mice, although MMP-9 and ET-1 levels remain elevated above control. The observed partial antagonism of MMP-9, by Tempol, suggests regulation through additional signaling pathways, which based on our findings includes those mediated through the ETA receptor. Interestingly, we observe an increase in vascular TBARs in the BQ-123 study by day 1 of exposure (Figure 4D), not seen in the Tempol study (Figure 4A). Several factors may account for this observation including (but not limited to): variability in vascular pathology amongst animal groups (note the difference in day1 control groups between Figures 4A and 4D), surgical procedures required for osmotic minipumps in the BQ-123 study, differences in compensatory antioxidant pathway responses, as well as overall assay limitations.
A key feature of atherosclerosis is the imbalance of vasoactive factors produced by the endothelium, including ET-1, which may aggravate atherosclerosis through its ability to stimulate ROS via NADPH oxidases25, and MMPs14. While studies have confirmed the association between air pollution and elevated plasma ET-1 levels in humans26, the mechanisms which mediate ET-1 expression, the respective contributions of environmental air pollutants, and the ultimate cardiovascular sequelae have not been elucidated. Our findings suggest that ET-1 may mediate expression of MMP-9 in this model, as BQ-123-treatment normalizes expression and activity of MMP-9. One plausible explanation for ET-1 regulation of MMP-9 may be through mitogen activated protein kinase (MAPK) signaling pathways, via the ETA receptor, since MMP-9 is known to contain AP-1 sites in its gene promoter region27. Nevertheless, we cannot discount the potential role of other factors whose expression is mediated or co-regulated through ET-1 / ET-receptor pathways, which are also known to regulate expression of MMPs (e.g. osteopontin, angiotensin II), nor can we exclude signaling pathways downstream of the ETB receptor. It is possible that enhanced endothelial ETB receptor activation after selective ETA receptor blockade may lead to increased ETB –mediated signaling; however one might expect increased levels of oxidative stress due to increased nitric oxide and subsequent peroxynitrite generation, in this scenario. Further studies are required to definitively identify responsible signaling pathways.
Elevated plasma MMP-9 has been identified as a novel predictor of cardiovascular mortality17. Our results show an abrupt increase in MMP-9, concomitant with elevated ET-1 and NOx, in plasma from humans exposed to DE, as well as plasma from ApoE-/- mice exposed to GEE. The ubiquitous nature of both GEE and DE as components of air pollution has been well defined. While there are differences in the chemical fingerprint of each emission, their chemical species including CO, NOx, and volatile/semi volatile organic compounds are similar16,28. Perhaps more relevant to our findings is that epidemiological reports of cardiovascular events associated with exposure to traffic-related air pollutants are assessments of overall traffic levels (not just one type of engine emission). Thus, it is reasonable to propose that the public health risk from vehicular emissions is due to the cumulative impact of these common chemical species present in the ambient air.
In conclusion, our findings show a significant increase in factors that are involved in mediating vascular remodeling and the progression of atherosclerosis, namely ET-1, MMP-2 and -9, and ROS, in response to acute exposure to the environmental air pollutant, GEE. Furthermore, our data suggests that ET-1 signaling is regulating expression and/or activity of vascular MMP-9, as well as plays a role in ROS generation, in response to GEE exposure. Furthermore, the similarity in vascular response to engine emissions in both mice and humans lends credence to the translatable relevance of this model, as well as identifies potential pathologically-relevant biomarkers that can assist in future mechanistic and risk assessment research. Considering the impact of cardiovascular disease on health care today, it is imperative to gain insight into pollution-induced alterations in pathways which mediate atherosclerotic plaque development and destabilization, leading to events such as AMI and stroke.
Supplementary Material
Acknowledgments
This project was supported by the National Environmental Respiratory Center, a United States Environmental Protection Agency (EPA) Star Award (R830839-010) and Grants F32ES015404 and ES014639 from the National Institute of Environmental Health Sciences (NIEHS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIEHS, the NIH, or the EPA.
Footnotes
Disclosures: None
References
- 1.Pope CA, III, Burnett RT, Thruston GD, Thun MJ, Calle EE, Krewski D, Godleski JJ. Cardiovascular mortality and long-term exposure to particulate air pollution: Epidemiological evidence of general pathophysiology pathways of disease. Circulation. 2004;109:71–77. doi: 10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- 2.Pope CA, III, Muhlestein JB, May HT, Renlund DG, Anderson JL, Horne BD. Ischemic heart disease events triggered by short-term exposure to fine particulate air pollution. Circulation. 2006;114:2443–2448. doi: 10.1161/CIRCULATIONAHA.106.636977. [DOI] [PubMed] [Google Scholar]
- 3.Hoffmann B, Moebus S, Möhlenkamp S, Stang A, Lehmann N, Dragano N, Schmermund A, Memmesheimer M, Mann K, Erbel R, Jöckel KH, Heinz Nixdorf Recall Study Investigative Group Residential Exposure to Traffic Is Associated With Coronary Atherosclerosis. Circulation. 2007;116:489–496. doi: 10.1161/CIRCULATIONAHA.107.693622. [DOI] [PubMed] [Google Scholar]
- 4.Peters A, von Klot S, Heier M, Trentinaglia I, Hörmann A, Wixhmann HE, Löwel H. Cooperative health research in the region of Augsburg study group. Exposure to traffic and the onset of myocardial infarction. N Engl J Med. 2004;351:1721–1730. doi: 10.1056/NEJMoa040203. [DOI] [PubMed] [Google Scholar]
- 5.Knuckles TL, Lund AK, Lucas SN, Campen MJ. Diesel exhaust exposure enhances venoconstriction via uncoupling of eNOS. Toxicol Appl Pharmacol. 2008;230:346–51. doi: 10.1016/j.taap.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Suwa T, Hogg JC, Quinlan KB, Ohgami A, Vincent R, van Eeden SF. Particulate air pollution induces progression of atherosclerosis. J Am Coll Cardiol. 2002;39:935–942. doi: 10.1016/s0735-1097(02)01715-1. [DOI] [PubMed] [Google Scholar]
- 7.Sun Q, Wang A, Jin X, Natanzon A, Duquaine D, Brook RD, Aguinaldo JG, Fayad ZA, Fuster V, Lippmann M, Chen LC, Rajagopalan S. Long-term air pollution exposure and acceleration of atherosclerosis and vascular inflammation in an animal model. JAMA. 2005;21:3003–3010. doi: 10.1001/jama.294.23.3003. [DOI] [PubMed] [Google Scholar]
- 8.Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherosclerosis: The good, the bad and the ugly. Circ Res. 2002;90:251–262. [PubMed] [Google Scholar]
- 9.McMillan WD, Patterson BK, Keen RR, Shively VP, Cipollone M, Pearce WH. In situ localization and quantification of mRNA for 92-kd type IV collagenase and its inhibitor in aneurismal, occlusive, and normal aorta. Arterioscler Thromb Vasc Biol. 1995;15:1139–1144. doi: 10.1161/01.atv.15.8.1139. [DOI] [PubMed] [Google Scholar]
- 10.Newby AC. Dual role of matrix metalloproteinases (matrixins) in intimal thickening and atherosclerotic plaque rupture. Physiol Rev. 2005;85:1–31. doi: 10.1152/physrev.00048.2003. [DOI] [PubMed] [Google Scholar]
- 11.Rajagopalan S, Meng XP, Ramasamy S, Harrison DG, Galis ZS. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro: implications for atherosclerotic plaque stability. J Clin Invest. 1996;98:2572–2579. doi: 10.1172/JCI119076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zalba G, Fortuño A, Orbe J, San José G, Moreno MU, Belzunce M, Rodríguez JA, Beloqui O, Páramo JA, Díez J. Phagocytic NADPH oxidase-dependent superoxide production stimulates matrix metalloproteinase-9. Implications for human atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:587–593. doi: 10.1161/01.ATV.0000256467.25384.c6. [DOI] [PubMed] [Google Scholar]
- 13.Ihling C, Szombathy T, Bohrmann B, Brockhaus M, Schaefer HE, Loeffler BM. Coexpression of endothelin-converting enzyme-1 and endothelin-1 in different stages of human atherosclerosis. Circulation. 2001;104:864–869. doi: 10.1161/hc3301.094742. [DOI] [PubMed] [Google Scholar]
- 14.Ergul A, Portik-Dobos V, Giulumian AD, Molero MM, Fuchs LC. Stress upregulates arterial matrix metalloproteinase expression and activity via endothelin A receptor activation. Am J Physiol Heart Circ Physiol. 2003;285:H2225–H2232. doi: 10.1152/ajpheart.00133.2003. [DOI] [PubMed] [Google Scholar]
- 15.Spiers JP, Kelso EJ, Siah WF, Edge G, Song G, McDermott BJ, Hennessy M. Alterations in vascular matrix metalloproteinase due to ageing and chronic hypertension: effects of endothelin receptor blockade. J Hypertens. 2005;23:1717–1724. doi: 10.1097/01.hjh.0000176787.04753.ee. [DOI] [PubMed] [Google Scholar]
- 16.Lund AK, Knuckles TL, Obat Akata C, Shohet R, McDonald JD, Seagrave JC, Campen MJ. Exposure to Gasoline Exhaust Results in Alterations of Pathways Involved in Atherosclerosis. Toxicol Sci. 2007;95:485–494. doi: 10.1093/toxsci/kfl145. [DOI] [PubMed] [Google Scholar]
- 17.Blankenberg S, Rupprecht HJ, Poirier O, Bickel C, Smieja M, Hafner G, Meyer J, Cambein F, Tiret L. Plasma concentrations and genetic variation of matrix metalloproteinase 9 and prognosis of patients with cardiovascular disease. Circulation. 2003;107:1579–1585. doi: 10.1161/01.CIR.0000058700.41738.12. [DOI] [PubMed] [Google Scholar]
- 18.Campen MJ, McDonald JD, Reed MD, Seagrave J. Fresh Gasoline Emissions, Not Paved Road Dust, Trigger Alterations in Cardiac Repolarization in ApoE-/- Mice. Cardiovasc Toxicol. 2006;6:199–210. doi: 10.1385/ct:6:3:199. [DOI] [PubMed] [Google Scholar]
- 19.Kai H, Ikeda H, Yusukawa H, Kai M, Seki Y, Kuwahara F, Ueno T, Sugi K, Imaizumi T. Peripheral blood levels of matrix metalloproteinases-2 and -9 are elevated in patients with acute coronary syndromes. J Am Coll Cardiol. 1998;32:368–372. doi: 10.1016/s0735-1097(98)00250-2. [DOI] [PubMed] [Google Scholar]
- 20.Abilleira S, Montaner J, Molina CA, Monasterio J, Castillo J, Avarez-Sabin J. Matrix metalloproteinase-9 concentration after spontaneous intracerebral hemorrhage. J Neurosurg. 2003;99:65–70. doi: 10.3171/jns.2003.99.1.0065. [DOI] [PubMed] [Google Scholar]
- 21.Ruidavets JB, Cournot M, Cassadou S, Giroux M, Meybeck M, Ferrières J. Ozone air pollution is associated with acute myocardial infarction. Circulation. 2005;111:563–569. doi: 10.1161/01.CIR.0000154546.32135.6E. [DOI] [PubMed] [Google Scholar]
- 22.Mills NL, Törnqvist H, Gonzalez MC, Vink E, Robinson SD, Söderberg S, Boon NA, Donaldson K, Sandström T, Blomberg A, Newby DE. Ischemic and thrombotic effects of dilute diesel-exhaust inhalation in men with coronary heart disease. N Engl J Med. 2007;357:1075–1082. doi: 10.1056/NEJMoa066314. [DOI] [PubMed] [Google Scholar]
- 23.de Nooijer R, Verkleij CJ, von der Thusen JH, Jukema JW, van der Wall EE, van Berkel TJ, Baker AH, Biessen EA. Lesional overexpression of MMP-9 promotes intraplaque hemorrhage in advanced lesions, but not earlier stages of atherogenesis. Arterioscler Thromb Vasc Biol. 2006;26:340–346. doi: 10.1161/01.ATV.0000197795.56960.64. [DOI] [PubMed] [Google Scholar]
- 24.Olson MW, Gervasi DC, Mobashery S, Fridman R. Kinetic analysis of the binding of human matrix metalloproteinase-2 and -9 to tissue inhibitors of metalloproteinase (TIMP)-1 and TIMP-1. J Biol Chem. 1997;272:29975–29983. doi: 10.1074/jbc.272.47.29975. [DOI] [PubMed] [Google Scholar]
- 25.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 26.Calderon-Garciduenas L, Vincent R, Mora-Tiscareno A, Franco-Lira M, Henriquez-Roldan C, Barragan-Mejia G, Garrido-Garcia L, Camacho-Reyes L, Valencia-Salazar G, Paredes R, Romero L, Osnaya H, Villarreal-Calderon R, Torres-Jardon R, Hazucha MJ, Reed W. Respiratory damage in children exposed to urban pollution. Pediatr Pulmonol. 2003;36:148–161. doi: 10.1002/ppul.10338. [DOI] [PubMed] [Google Scholar]
- 27.Fini ME, Cook JR, Mohan R, Brinckerhoff CE. Regulation of matrix metalloproteinase gene expression. In: Parks WC, Mecam RP, editors. Matrix Metalloproteinases. San Diego, Ca: Academic; 1998. pp. 300–356. [Google Scholar]
- 28.McDonald JD, Barr EB, White RK, Chow JC, Schauer JJ, Zielinska B, Grosjean E. Generation and characterization of four dilutions of diesel engine exhaust for a subchronic inhalation study. Environ Sci Technol. 2004;38:2513–2522. doi: 10.1021/es035024v. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.