Abstract

In this study, an anti-hapten antibody (single chain Fv, scFv) against a hapten probe was developed as a unique reporter system for molecular imaging or therapy. The hapten peptide (histamine-succinyl-GSYK, Him) was synthesized for phage displayed scFv affinity selection and for conjugation with cypate (Cy-Him) for in vivo near-infrared (NIR) optical imaging. Hapten-specific scFvs were affinity selected from the human single fold phage display scFv libraries (Tomlinson I + J) with high specificity and affinity. Utilizing HER2 targeting as a model system, the highest affinity scFv (clone J42) was recombinantly fused to an anti-HER2 affibody (scFv-L-Aff) with no loss of affinity of either protein. The functionality of the hapten-scFv reporter system was tested in vitro with a HER2-positive human breast cancer cell line, SK-BR3, and in vivo with SK-BR3 xenografts. ScFv-L-Aff mediated the binding of the hapten to HER2 on SK-BR3 cells and from tissue from the SK-BR3 xenograft; however, scFv-L-Aff did not mediate uptake of the hapten in the SK-BR3 xenografted tumors, presumably due to rapid internalization of the HER2/scFv-L-Aff complex. Our results suggest that this hapten-peptide and anti-hapten scFv can be a universal reporter system in a wide range of imaging and therapeutic applications.
Introduction
Direct targeting of monoclonal antibodies (mAbs) conjugated with radioisotopes or drugs to cell surface biomarkers is currently under development in preclinical animal models and under evaluation in clinical studies.1,2 Therefore, improving tumor-to-background ratio in targeted drug delivery still remains an important goal to obtain high tumor specific signals and therapeutic efficacy. The relatively large size (∼150 kDa) and long serum half-life of intact antibodies have been problematic in terms of deep tumor penetration and high radiation doses to radio-sensitive tissues, such as bone marrow. Tumor visualization with molecular imaging requires several days after the administration of the radiolabeled mAb due to the slow blood clearance of the antibody. Several strategies have been developed to take advantage of the high affinity and selectivity of mAbs and reduce the serum half-life, such as the utilization of mAb fragments. Pretargeting strategies have been employed to circumvent the shortcomings of antibody direct targeting; it allows localization of a bispecific protein that can simultaneously bind the targeted receptor and subsequently bind a labeled and rapidly clearing smaller molecule. Tumor pretargeting has solved the problems associated with slow clearing mAbs and has enabled high target tissue uptake with minimal nontarget accumulation.3,4
Pretargeting strategies have been developed utilizing receptor–ligand pairs with streptavidin (SA)/avidin–biotin or with bispecific antibodies.5−7 Streptavidin (SA)–biotin has been employed in in vivo systems, and multistep labeling using streptavidin or biotin-labeled proteins has been shown to increase target specificity.8,9 Because of the high binding affinity between SA and biotin (KD ∼10–15 M), their binding is considered to be equivalent to a covalent chemical bond in biological systems, showing high target specificity in tumor imaging and therapeutic efficacy.10 However, SA/avidin is immunogenic, and endogenous biotin levels (10–8–10–7 M) can affect the efficacy of SA binding, which is not favorable for clinical use.11,12 Therefore, employing bispecific antibodies consisting of humanized antibody fragments and a nonimmunogenic reporter probe instead is advantageous in preclinical and clinical applications, as they retain high specificity with subnanomolar affinity.13 A nonimmunogenic hapten is useful due to its specific recognition of the anti-hapten antibody in noninvasive imaging and drug delivery applications. Herein, we report on a novel binding pair based on a single chain fragment variable antibody, scFv, with nanomolar affinity to a hapten, where the scFv is fused to a tumor-targeting affibody.
The single chain variable fragment (scFv) is the smallest functional binding unit (220 amino acids) of an antibody retaining high molecular specificity and consists of the variable heavy (VH) and variable light (VL) chains ligated with a short peptide linker.14 Because of its low molecular weight (∼25 kDa), an scFv is amenable to fusion with other proteins for a variety of purposes.15 In this study, we hypothesized that an scFv isolated by affinity selection from a phage library can be effectively isolated with high affinity toward a hapten. Second, we hypothesized that an scFv can be fused to a protein with specific binding toward a cell surface marker to generate a heterobivalent molecule such that the binding of both is retained. In this way, the scFv and hapten, labeled with fluorescence optical dyes, radio-metals, or therapeutic molecules, could serve as a robust imaging reporter system as well as an effective drug delivery system. The hapten consists of histamine conjugated to succinic acid (Him-Suc), which is in turn coupled to the backbone peptide sequence GSYK. The hapten-peptide, Him-Suc-GSYK, can incorporate chelators for radiometals or near-infrared dyes through conjugation to Lys or radiohalogenation on the Tyr residue to generate a multimodal imaging probe for optical, SPECT, or PET imaging. Here, we report the novel Him-Suc-GSYK specific anti-hapten scFv and its fusion with an anti-HER2 affibody as a model heterobivalent fusion protein for pretargeted imaging of HER2-expressed tumors using modular hapten probes.
The human epidermal growth factor receptor type 2 (HER2) is a transmembrane tyrosine kinase receptor and is a member of the endothelial growth factor receptor (EGFR) family, which is expressed in a wide range of tumors.16 Particularly, HER2 overexpression is found in ∼30% of human breast cancer, and increased HER2 expression is known to correlate with a poor prognosis and resistance to a number of cancer therapies.17 Currently, anti-HER2 mAb (trastuzumab, herceptin) labeled with PET tracers, such as 64Cu, 89Zr, etc., has been used for HER2 immunoPET/CT scanning in clinical applications.18,19 In this study, we designed and evaluated an HER2-targeted bispecific fusion protein as a model for HER2 imaging to demonstrate the functionality and specificity of the novel hapten and anti-hapten antibody reporter system.
Results
Synthesis of Hapten Peptide for Biopanning and NIR Dye Labeling
Biotinylated hapten (Him-Suc-GSYK-Bt) was synthesized by solid phase peptide synthesis (Figures S1–3, Supporting Information). The peptide without the histamine-succinyl group, GSYK-Bt, was obtained separately for background subtraction of phage libraries. For fluorescence optical imaging, the hapten peptide without biotin (Him-Suc-GSYK, Him) was synthesized and conjugated to the near-infrared (NIR) fluorescent dye, cypate, and sulfo-Cy5-NHS via an amide coupling reaction.20 Monoconjugated cypate-hapten (Cy-Him) and sulfo-Cy5-coupled hapten (Cy5-Him) were isolated by HPLC purification (Figures S4 and S5, Supporting Information). The stock solutions of Cy-Him and Cy5-Him were prepared in dimethyl sulfoxide (DMSO) and diluted with aqueous media for in vitro and in vivo evaluations.
Phage Library Screening
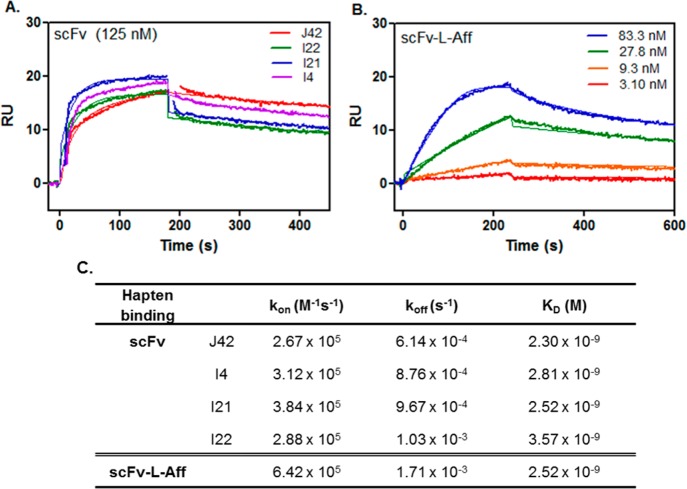
The high hapten binders were selected from phage libraries, specifically the human single fold scFv libraries I + J (Tomlinson I + J). To deplete the library phages that bind nonspecifically, the library was negatively selected with GSYK-Bt. Then, selections for antibodies that bind the hapten were performed with the biotinylated hapten peptide, Him-Suc-GSYK-Bt (Figure S2, Supporting Information). The decline of phage titers confirmed that most of the hapten binding phages with low affinities were removed during the initial selection steps (Table S1, Supporting Information). After each round of panning, the extensive course of washing excluded fast off-rate phage antibodies. Thus, phages with strong affinities and slow off-rates could remain on the magnetic bead surface during the washing process. Hapten-specific scFvs with high affinity (KD < 4 nM) with slow off-rate (∼10–4 s–1) were successfully obtained (Table 1 and Figure 1).
Table 1. Amino Acid Sequences of Anti-hapten scFvs from Phage Libraries (Tomlinson Library I + J).
| sequence |
||||||||
|---|---|---|---|---|---|---|---|---|
| H-CDR1 | H-CDR2 | H-CDR3 | L-CDR1 | L-CDR2 | L-CDR3 | |||
| scFv | 1 | 31 | 50 | 95 | 162 | 184 | 225 | 239 |
| J42 (32/48) | MAE | ··SYAMS | ··GISGGGSDTNYADSVKG | ··YAASFDY | ··SISSYLN | ··GASYLQ | ··TATSPD | ··KR |
| I22 (2/48) | MAE | ··SYAMS | ··GIGAVGYYTAYADSVKG | ···YTNTFDY | ··SISSYLN | ··DASNLQ | ··SSSGPD | ··KR |
| I21 (3/48) | MAE | ··SYAMS | ··GISNYGDTTS YADSVKG | ··YYSTF DY | ··SISSYLN | ··AASSLQ | ··DTTSPY | ··KR |
| I4 (3/48) | MAE | ··SYAMS | ··GISSYGGTTG YADSVKG | ··YSTAFDY | ··SISSYLN | ··DASSLQ | ··SASSPD | ··KR |
Figure 1.
Surface plasmon resonance (SPR) sensorgrams of (A) four anti-hapten scFvs and (B) fusion protein (scFv-L-Aff) to the hapten-immobilized sensor chip. (C) Binding kinetic information (kon and koff) and equilibrium constant (KD) from the Langmuir 1:1 binding model.
The monomeric phage antibodies were isolated efficiently using biotinylated hapten in solution with subsequent capture on the streptavidin magnetic beads. To obtain high binders on the basis of affinity, the concentration of soluble hapten was gradually decreased each round of stringent selection. That is, for the initial rounds of selection, high micromolar concentrations of hapten were used to capture poorly expressed phage antibodies that could evolve into high affinity binders. In later rounds of panning, the antigen concentration was reduced to increase stringency and select for the highest affinity antibodies. In round 3 panning using 100 nM of hapten, a significantly higher ratio of output to input phage titers was observed compared to that in the earlier panning, presenting the highest enrichment (Table S1, Supporting Information). Additional panning was conducted using 50 nM hapten in round 4, showing slightly lower phage antibody recovery than round 3 with reduced enrichment, which was more distinctively shown in Library I.
Discovery of Anti-Hapten scFv
Phage-infected clones were randomly picked from Library I and J for sequencing after the fourth round of panning, and 56 phagemids were isolated from TG1 bacteria. From DNA plasmid sequencing, 48/56 phagemids were successfully sequenced, and 7 unique sequences were discovered from the 48 clones. Eight of the clones had two UAG amber stop codons, which are not suitable for antibody production in HB2151, and these were not further examined. The four remaining sequences that produce full length scFv were found in 21 and 19 phagemids from Library J and I, respectively. Clone J42 dominated the population of recovered clones (32/48 clones). The other three sequences appeared at lower frequencies of 3/48, 3/48, and 2/48, for I4, I21, and I22, respectively (Table 1).
The four scFvs corresponding to clones J42, I4, I21, and I22 were produced and purified from HB2151 bacteria. The binding affinities of the four anti-hapten scFvs were evaluated by SPR against the biotinylated hapten-captured surface (Figure 1). The KD values of the four scFvs showed high binding affinities in the nanomolar range (KD < 4 nM). Among them, the slowest off-rate was observed in the scFv from clone J42, and this unique sequence was used for fusion protein plasmid construction with anti-HER2 affibody (ZHER2:477) for HER2 membrane receptor target imaging using a hapten imaging reporter.
Production and Binding Study of Fusion Protein scFv-L-Aff
scFv-L-Aff, a novel heterobivalent fusion protein, was recombinantly constructed with anti-HER2 affibody (ZHER2:477) and anti-hapten scFv (clone J42) to generate a hapten reporter modular system. The two proteins were assembled via a 15 amino acid linker, (G4S)3, for a flexible ligation of the two proteins (Figure S6, Supporting Information).21,22 In the HER2 affibody (ZHER2:477), a His6-tag was attached at the C-terminus for affinity protein purification and immunofluorescence microscopy. The plasmid sequence of scFv-L-Aff was confirmed by DNA sequencing and transformed into BL21 E. coli for protein expression. After IPTG-induced expression and His-tag affinity purification, a scFv-L-Aff protein band appeared at a molecular weight of ∼35 kDa (calculated 37 kDa), which was confirmed by sodium dodecyl sulfate (SDS)-gel and Western blotting (Figure 2).
Figure 2.
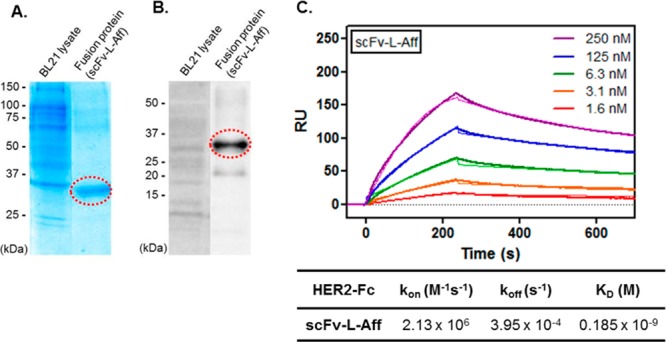
Characterization of the fusion protein (scFv-L-Aff) by (A) SDS-gel and (B) Western blotting using anti-His tag mAb. (C) SPR binding study.
The bispecific binding kinetics of the purified fusion protein scFv-L-Aff was measured by SPR. Five concentrations were independently injected over the hapten-captured and HER2-immobilized chips, and this was duplicated with a different set of concentrations. The heterobivalent fusion protein bound to the HER2 and to hapten with KD values of 185 pM and 2.52 nM, respectively (Figures 1 and 2). The KD of the parent HER2 affibody (ZHER2:477) is 32 pM with kon = 4.3 × 106 M–1 s–1 and koff = 1.4 × 10–4 s–1 against the extracellular domain of HER2.23 The slower association (kon = 2.13 × 106 M–1 s–1) and faster dissociation (koff = 3.95 × 10–4 s–1) rates of the fusion protein to the HER2-Fc resulted in a 5.8-fold decreased binding affinity (Figure 2). The equilibrium dissociation constant to hapten (KD = 2.30 nM, J42) was preserved in the fusion protein; the faster off-rate was offset by a faster on-rate (Figure 1).
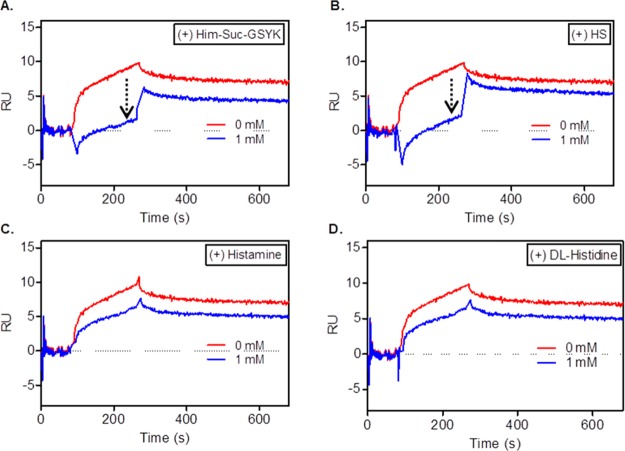
The hapten specificity of scFv-L-Aff was further verified by an inhibition SPR assay. Four small probes including Him-Suc-GSYK were preincubated with the fusion protein and injected over the hapten-captured surface in SPR (Figure 3). Free histamine and dl-histidine showed similar weak inhibitory effects (∼20%) in hapten binding at the highest concentration (1 mM). The HS pretreated fusion protein significantly reduced hapten binding by 47% at a concentration of 1 mM compared to the untreated fusion protein.24 This Him-Suc-mediated inhibition was greatly increased in the hapten-treated sample by 84% (Figure 3A). These results indicate that the epitope for J42 comprised largely the histamine-succinyl headgroup.
Figure 3.
Hapten inhibitory effect in scFv-L-Aff binding using SPR. scFv-L-Aff (100 nM) was incubated with (A) Him-Suc-GSYK, (B) HS, (C) histamine, and (D) dl-histidine and injected over the hapten-immobilized sensor chip.
Activity of scFv-L-Aff on Whole Cells
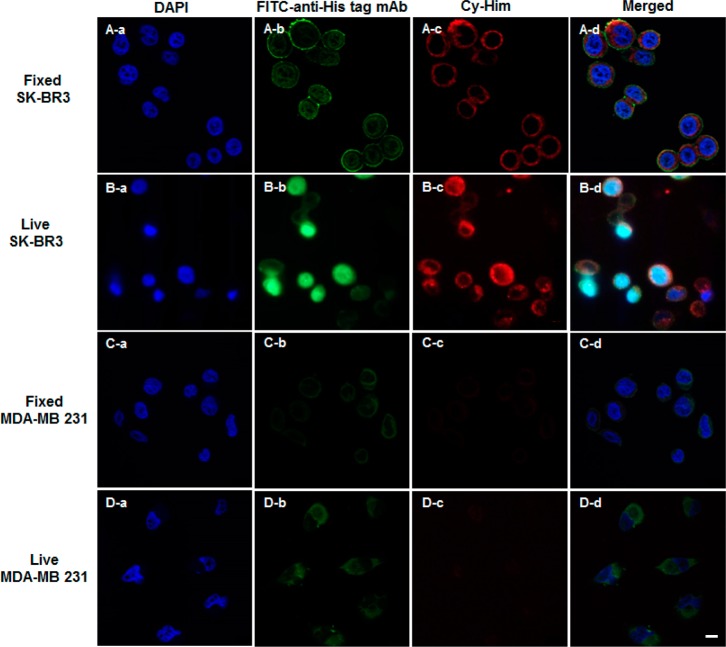
The functionality of the HER2-targeted heterobivalent fusion protein scFv-L-Aff and the hapten imaging reporter Cy-Him was validated by confocal fluorescence microscopy with breast carcinoma cells, which overexpress HER2.25,26 SK-BR3 cells were fixed and incubated with the fusion protein scFv-L-Aff. The HER2 localization of the fusion protein was visualized using an fluorescein isothiocyanate (FITC)-labeled anti-His6 tag antibody (FITC-anti-His tag mAb). The FITC fluorescence signal was detected at the cell surface from the His-tag FITC labeling of the fusion protein (Figure 4A-b), confirming successful membrane HER2 localization of the fusion protein. Consecutive Cy-Him treatment showed colocalized NIR fluorescence merging with the green FITC signal on the cellular membrane (Figure 4A-c and A-d). The specificity of scFv-L-Aff to the labeled hapten was tested with hapten blocking while the fusion protein was bound to SK-BR3 cells. In fixed cells, the hapten binding sites were blocked using preincubation with unlabeled hapten on the fusion-protein-treated cells prior to Cy-Him labeling, producing decreased NIR fluorescence (Figure S8, Supporting Information). Moreover, weak NIR fluorescence from nonspecific binding was observed from Cy-Him only incubation from both fixed and live cells, reflecting the specificity of the hapten probe to the fusion protein (Figure S10, Supporting Information).
Figure 4.
Confocal fluorescence microscope images of HER2 labeling in HER2-positive SK-BR3 and HER2-negative MDA-MB-231 cells. Fixed cells were incubated with scFv-L-Aff (7 μg/mL) for 1 h at room temperature, followed by consecutive incubations with FITC–anti-His tag mAb and Cy-Him (1 μM) for 30 min each at room temperature. Live cells were treated with scFv-L-Aff and Cy-Him consecutively. After fixation, scFv-L-Aff was stained with FITC-anti-His tag mAb. The nucleus was counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (scale bar = 10 μm).
To investigate the possible internalization of the fusion protein, live cells were tested. Membrane and cytosolic localization of Cy-Him was observed after 30 min of incubation, presumably by HER2-mediated endocytosis (Figure 4B-b and B-c). Internalization of HER2 ligands such as trastuzumab and other affibody conjugates has been previously observed.23,27,28 Excess amounts of anti-HER2 affibody inhibited binding of the fusion protein, indicating that binding of the fusion protein is mediated by the specific interactions with HER2 receptors (Figure S9, Supporting Information). HER2-negative breast cancer cell line (MDA-MB-231) was tested using the same labeling conditions. No binding of scFv-L-Aff and Cy-Him was detected in both live and fixed cell incubation (Figure 4C and D). These confocal microscopy data indicate that scFv-L-Aff was fully functional and mediated the visualization of HER2 receptors by specific affibody binding of HER2 and scFv binding of Cy-Him.
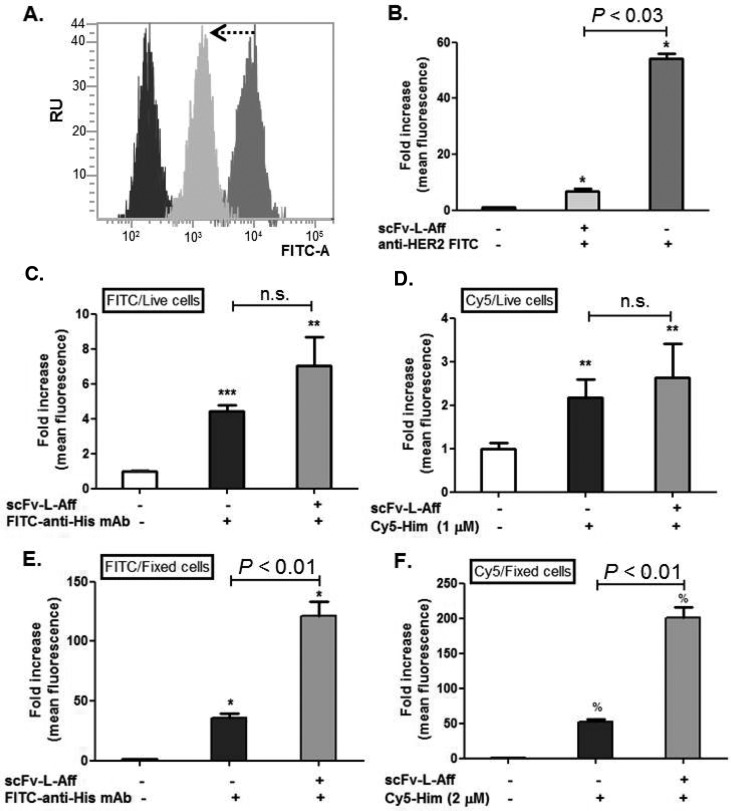
The binding specificity of the fusion protein and hapten probe was further tested using quantitative flow cytometry analysis. Fusion protein binding was evaluated by FITC-anti-His-mAb labeling, and hapten binding was quantified from Cy5-Him. The fold increase was calculated from the mean fluorescence intensity of the untreated cells in FITC and Cy5 channels. Preincubating cells with the fusion protein blocked the FITC-labeled anti-HER2 affibody binding, resulting in 88% suppression of fluorescent intensity in comparison to the unblocked cells (Figure 5A and B). HER2 inhibition and decreased FITC intensity indicate the binding specificity of the fusion protein to the HER2 membrane receptors. In live cell incubation, the fusion-protein-bound cells showed a 1.6-fold increase in FITC mean fluorescence (P < 0.01 to the untreated cells, Figure 5C), while Cy5 fluorescence was enhanced 1.2- fold compared to that of the controls (P < 0.03 to the untreated cells; Figure 5D). Live cell incubation did not now show statistical difference between the control and stepwise labeling. However, fixed cell labeling showed 3.8-fold and 3.4-fold increases in FITC and Cy5 fluorescence, respectively, compared to those of the nonspecific binding controls (P < 0.01, Figure 5E and F). The decreased fluorescent signals from the live cell incubation reflect that the fusion proteins most likely internalized resulting in reduced binding sites for both FITC-anti-His tag mAb and Cy5-Him.
Figure 5.
Flow cytometry analysis with SK-BR3 cells. (A and B) Live cells were pretreated with scFv-L-Aff and subsequently incubated with the FITC-anti-HER2 affibody. Live and fixed cells were preincubated with scFv-L-Aff followed by (C and E) FITC-anti-His tag mAb and (D and F) Cy5-Him, independently. Each bar represents the mean ± SEM of three separate experiments with triplicates (n.s.; no significant difference was observed. * P < 0.03; ** P < 0.01; *** P < 0.03; % P < 0.05 to the untreated cells).
In Vivo NIR Fluorescence Tomography and Ex Vivo Tissue Staining
Pretargeting of the fusion protein scFv-L-Aff to HER2 expressed tumors and subsequent hapten labeling were investigated in SK-BR3 tumor-bearing mice using in vivo NIR fluorescence molecular tomographic imaging (FMT). As a control group, Cy-Him (3 nmol, i.v.) without the fusion protein was injected intravenously to mice via tail vein injections. The other group of mice was treated with the fusion protein (2.5 nmol, i.p.) followed by intravenous Cy-Him (3 nmol, i.v.) injection ∼5 h after the fusion protein. The NIR signals from tumors appeared at ∼5 h and disappeared at 24 h post-Cy-Him injection in FMT. The Cy-Him tumor uptake was measured from both treatments at 5 h post-injection (Figure 6A). There was no significant enhancement of Cy-Him tumor uptake observed in the fusion protein pretargeted group versus the Cy-Him only control group. Early time points imaging at 30 min, 60 min, 1.5 h, and 3 h were tried but did not show significant differences (data not shown). Besides 3 nmol, 1.5 and 2 nmol of Cy-Him were tested. However, no significant differences were observed from the tested concentrations. In order to deliver more scFv-L-Aff for enhancing the efficiency of pretargeting, the fusion protein was concentrated using centrifugal concentration. However, the fusion protein was prone to aggregate during concentration, which limited the amount that could be administered.
Figure 6.
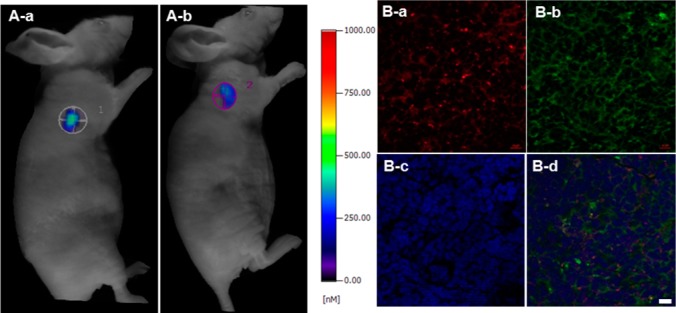
(A) In vivo FMT images of SK-BR3 tumor-bearing mice (n = 3). (A-a) scFv-L-Aff (2.5 nmol) was administered i.p. to mice, and Cy-Him (3 nmol) was injected i.v. ∼5 h post-injection of scFv-L-Aff. (A-b) Cy-Him (3 nmol) only was administered. NIR fluorescent tomographic signals were measured at 5 h post-Cy-Him administration. (B) Immunofluorescent ex vivo tumor tissue staining. The tissues were first incubated with scFv-L-Aff followed by (a) Cy-Him and (b) FITC-anti-His tag mAb consecutively. (c) DAPI was used for nucleus counter staining. (d) Merged image (scale bar = 10 μm).
Ex vivo imaging of excised organs and tumors showed similar biodistribution and tumor accumulation, consistent with the in vivo imaging results (Figure S11, Supporting Information). The dissected and fixed tumor tissues were incubated with the fusion protein and Cy-Him, generating colocalized FITC and NIR signals on the cellular membrane, whereas the tumor tissues treated with only Cy-Him showed weak fluorescence in confocal microscopy (Figures 6 and S12, Supporting Information). This ex vivo tissue staining confirmed that there was no loss of HER2 in the xenograft.
Discussion
A hapten is a small molecule (M.W. < 1000 Da) and is nonimmunogenic unless conjugated to a large carrier protein. As hapten molecules have many properties which make them attractive as reporter probes, they have been developed for a wide range of biological applications, specifically in molecular imaging by indirect targeting.29−31 For example, histamine-succinyl-glycine (HSG)-bivalent constructs have been developed for in vivo tumor pretargeting and have demonstrated good target antibody binding and low nontarget accumulations.32−34 In this study, a histamine-succinyl (Him-Suc)-conjugated peptide, Him-Suc-GSYK, was designed for biopanning with phage libraries to select for a Him-Suc hapten-specific scFv (Figure S2, Supporting Information).35 Moreover, the design incorporated a variety of functional groups in the amino acid side chains amenable to coupling with reporter molecules to allow molecular imaging. By incorporating a Lys residue within the hapten construct, the hapten could be conjugated via coupling reactions to a variety of dyes, chelators, or drugs that bear carboxylic acid functional groups. A second Lys residue could be added to the hapten construct to allow for dual modality imaging with an optical dye and a radiometal chelator by manipulation of orthogonal Lys protecting groups. Finally, the Tyr residue served not only as a chromophore for convenient quantification of the hapten but as a site for radiohalogenation, enabling multimodal imaging. In this work, cypate, a cyanine dye with absorption and emission in the near-infrared (NIR) region, was used as a label to follow Him-Suc-GSYK when conjugated to the ε-amino group of Lys for in vivo optical NIR imaging.
The hapten reporter binding partner, an anti-hapten scFv, was isolated from the human single fold scFv phage libraries (Tomlinson I + J). High affinity hapten binders were effectively obtained after background subtraction and four-round panning by indirect affinity selections. Increasing stringency in each successive round of panning with extensive washing resulted in selection for high affinity antibodies to the hapten with slow off rates (Table S1, Supporting Information, and Figure 1). The Tomlinson library has been used in the past for antigens and has contained antibodies with both strong and modest affinities for the targets.36 However, to our knowledge, this is the first investigation of the Tomlinson library regarding the discovery of antibodies for a hapten. The paratope of antibodies for haptens generally incorporate a cavity-like structure, and it is not clear whether this design feature is incorporated into the Tomlinson library.37
Screening the library against the hapten resulted in the discovery of four high affinity antibodies from 48 clones with KD values less than 4 nM (Figure 1) and with relatively slow off-rates (∼10–4 s–1), which is an excellent result for a library with a diversity of ∼1.4 × 108 (Figure 1).38 To achieve significant tumor retention in vivo, slow off-rates between the target and labeled ligand are desired. Therefore, in this study, an scFv from clone J42 that exhibited a slow dissociation rate (koff ∼10–4 s–1) and a high binding affinity was chosen for fusion with an affibody to generate a heterobivalent receptor molecule to test the efficacy of the scFv-hapten molecular imaging reporter system.
An affibody is designed to have high target specificity to its binding molecules that is comparable to the antibody, and its small size (∼7 kDa) is also beneficial for excellent tumor penetration and faster renal clearance than antibodies (∼150 kDa) while producing high tumor to normal tissue ratios.39 Currently, a few HER2-targeting fusion proteins from antibody fragments for direct imaging are in preclinical and clinical trials.2,40 The novel heterobivalent fusion protein scFv-L-Aff, consisting of anti-hapten scFv and an anti-HER2 affibody (ZHER2:477) with a linker, (G4S)3, was successfully produced and confirmed by SDS-gel and Western blotting (Figures 2 and S6, Supporting Information). Size exclusion chromatography indicates that the fusion protein is stable mostly in a monomeric state, although there was some dimerization during storage (Figure S7, Supporting Information). The SPR binding study showed a dual binding specificity to both hapten and HER2, with 2.52 nM and 185 pM affinities, respectively (Figures 1 and 2). For efficient tumor targeting, high affinity is desired, and the affinity of the fusion protein is 27-fold higher than that of the monoclonal antibody trastuzumab (KD ∼5 nM) against HER2. We conjugated the affibody at its N-terminus in anticipation of minimal disruption of binding affinity and found a 5.8-fold drop in affinity compared to that of the parent affibody.23 The reason for this may be destabilization of the three-helix bundle, which has been previously observed in a heterobivalent bis-affibody fusion and was shown to be dependent on which terminus bore the linker.21,41 Moreover, the 3.95 × 10–4 s–1 off rate of the fusion protein is favorable for in vivo HER2-tumor targeting, with a 29 min half-life that is advantageous for effective tumor accumulation. Conjugation of the C-terminus of the scFv had no effect on hapten binding affinity.
To elucidate the epitope of J42 scFv, SPR experiments between scFv-L-Aff and the immobilized hapten were performed with the addition of competitive ligands (Figure 3). Histamine, the major mediator of acute inflammatory responses,42 was tested to verify whether free histamine affects the binding ability; racemic dl-histidine was also selected due to its structural similarity to histamine. Neither dl-histidine nor histamine suppressed the binding of Him-Suc-GSYK. HS containing probes showed high inhibitory capacity, indicating that the histamine-succinyl moiety is an essential recognition element in the J42 epitope.
The small peptide sequence GSYK appears in the LGSYKPS peptide, which is found in β-amyloid proteins, the pathological hallmark in Alzheimer’s disease (AD).43 In addition to AD, significant expression levels of amyloid proteins and its assemblies have been discovered in neurodegenerative brain diseases such as Parkinson’s disease or after traumatic brain injuries.44,45 Therefore, there would be a possibility of nonspecific binding of anti-hapten scFv to similar peptides or derivatives, such as β-amyloid proteins or its aggregates expressed in brain diseases. However, scFv-expressed phages binding to GSYK were initially removed by a background subtraction prior to the sequential panning with Him-Suc-GSYK. Additionally, the inhibition SPR study indicates the important role of Him-Suc in binding specificity (Figure 3). Therefore, cross-reactivity of J42 to amyloid proteins is not anticipated to be an interfering factor in this hapten reporter system.
To demonstrate the functionality of scFv-L-Aff, confocal microscopy and flow cytometric analysis were performed to verify the HER2-mediated binding of scFv-L-Aff and subsequent binding of the fluorescently labeled Him-Suc analogues Cy-Him and Cy5-Him on the HER2 positive human breast cancer cell line SK-BR3. There was considerable, but not complete, HER2-mediated endocytosis of scFv-L-Aff, indicated by immunofluorescence microcopy. Internalization of the fusion protein was further confirmed by flow cytometric analysis; both mean fluorescence intensity and fold increase were decreased in live cell labeling, demonstrating that ∼42% and ∼35.3% of the fusion proteins were available on the cell surface for labeling with FITC anti-His mAb and Cy5-Him, respectively (Figures 5). Although, the in vitro live cell imaging and flow cytometric analysis indicate HER2 receptor-mediated endocytosis, significant amounts of the scFv-L-Aff persisted on the cellular membrane after internalization. The efficiency and rate of internalization including pharmacokinetics involves complex mechanisms in vivo. Therefore, we attempted an in vivo study to validate HER2 pretargeting using a novel reporter system.
It was hypothesized that utilization of a pretargeted approach with bispecific anti-HER2 affibody fusion and cypate-labeled Him-Suc-GSYK would enable the detection of HER2-positive tumors and metastases and demonstrate the utility of the scFv and labeled hapten. However, the relatively rapid rate of HER-2-mediated internalization apparently resulted in no significant difference in tumor uptake between the pretargeted group and the control (Figure 6A). HER2-mediated endocytosis has been observed in other HER2 affibodies, which is beneficial in direct targeting for maximum cellular uptake by recycling and transmembrane relocation of HER2 receptors after internalization.27,28 However, internalization of the first targeting molecules decreases uptake of the second labeled probe in a pretargeted strategy. Therefore, controlling internalization and the pharmacokinetic profile by structural optimization of the fusion protein can enhance specific tumor uptake in stepwise targeting. Moreover, multivalent imaging probes fashioned from the hapten Him-Suc could be also designed to increase the binding affinity via the avidity effect in tumor targeting.46
In conclusion, a hapten-specific antibody fragment (scFv J42) exhibiting high affinity and slow dissociation rate was isolated from a phage display. J42 was recombinantly fused with anti-HER2 affibody (ZHER2:477) via a short peptide (G4S)3 linker for HER2 imaging to assess the efficacy of a universal imaging reporter system based on an scFv and hapten labeled with an NIR dye for molecular imaging. The binding affinity of anti-hapten scFv in the heterobivalent fusion protein scFv-L-Aff was largely preserved, confirming the stability of the scFv toward conjugation. The in vitro evaluation demonstrated that the novel fusion protein retained high affinity to both HER2 and hapten and was fully functional, enabling HER2 receptor labeling with the labeled hapten. The in vivo evaluation failed to demonstrate HER2-mediated uptake in the target tissues, most likely due to rapid internalization of the fusion protein. This is a first report of a unique hapten-specific scFv sequence which showed promising potential as a receptor–ligand pair that could play myriad roles in a universal hapten reporter system in molecular imaging and therapeutic applications.
Materials and Methods
All chemical reagents were purchased from commercial sources and used without further purification, unless otherwise stated. These include biotin-PEG NovaTag resin (Novabiochem), Rink Amide AM resin (Novabiochem), Pyclock (Novabiochem), and Dynabeads M-280 streptavidin (Invitrogen). Mass spectrometry was performed using ESI/MS (Waters 2998 photodiode array detector, Waters LCT premier XE mass spectrometry). Absorbance and fluorescence spectra were obtained using quartz fluorometer cuvettes (Starna cells, Inc., Atascadero, CA) at room temperature by a Cary 100 Bio UV/vis spectrophotometer and a Cary Eclipse fluorescence spectrophotometer, respectively.
Peptide Synthesis
Peptides were synthesized using a standard fluorenylmethyl (Fmoc) procedure on solid support, as previously described.35 Briefly, biotinylated hapten peptide (histamine-succinyl-GSYK-Bt, Him-Suc-GSYK-Bt) was synthesized on Biotin-PEG NovaTag resin using a microwave synthesizer (CEM Discover Liberty 1), and histamine-succinyl capping was manually performed on the resin (Figure S1, Supporting Information). After precipitation in cold diethyl ether, the obtained white solid was purified by C18 reversed-phase HPLC (Proto 200, 150 × 20 mm; flow rate, 10 mL/min) using a gradient method: 80% A for 10 min and 65% A for 40 min (solvent A, 0.1% trifluoroacetic acid in water, and solvent B, 0.1% trifluoroacetic acid in acetonitrile). UV absorption was monitored at 230 and 270 nm, and the collected peptide obtained with >95% purity: GSYK-Bt, [M]+m/z, found m/z [M + H]+ 882.3, [M + 2H]2+ 442.2; Him-Suc-GSYK-Bt [M]+m/z 1074.6, found m/z [M + H]+ 1075.4, [M + 2H]2+ 538.2. The peptide without biotin was synthesized for further conjugation using Rink Amide AM resin using the same method and purified by C18 reversed-phase HPLC (Proto 300, 150 × 20 mm; flow rate, 7 mL/min) using an isocratic method (95% of solvent A); in 30 min >95% pure peptide was obtained: Him-Suc-GSYK m/z [M]+ 645.3, found m/z [M + H]+ 646.8, [M + 2H]2+ 323.4.
Optical Imaging Probe Synthesis
Cypate and the cypate conjugates were synthesized as previously described.20 To conjugate cypate to the hapten peptide, EDC-HCl (1.5 mg, 8.0 μmol) was added to the cypate (5 mg, 8.0 μmol) in DMF (100 μL). After 30 min, HOBt (1.1 mg, 8.0 μmol) and hapten peptide (4.3 mg, 6.7 μmol) in DMF (400 μL) were added. The reaction mixture was stirred overnight at room temperature and precipitated in cold diethyl ether to give a green powder. The mixture was purified by C18 reversed-phase HPLC (Proto 300, 150 × 4.6 mm; flow rate, 1 mL/min) using a gradient method from 40% B to 50% B for 30 min (solvent A, 0.1% trifluoroacetic acid in water, and solvent B, 0.1% trifluoroacetic acid in acetonitrile). UV absorption was monitored at 778 nm, and the cypate-conjugated hapten (Cy-Him) was obtained with >95% purity and 18% yield: Cy-Him m/z [M]+ 1252.62, found m/z [M + 2H]2+ 627.3, [M + H]+ 1252.7. Sulfo-Cy5-NHS (Luminoprobe) was conjugated to the ε-amine of Lys of the hapten. The mixture was purified by reversed-phase HPLC using a gradient method from 30% B to 60% B for 30 min. UV absorption was monitored at 645 nm, and the collected sulfo-Cy5-conjugated hapten (Cy5-Him) showed >95% purity: Cy5-Him (C61H78N11O15S2) m/z [M]+ 1269.47, found m/z [M + 2H]2+ 635.75, [M + H]+ 1270.49. The lyophilized hapten–dye conjugates were dissolved in DMSO and kept at −20 °C.
Phage Selections of Monoclonal Phage Libraries
The anti-hapten scFv was selected by a phage display technique and modified biopanning.47−50 Human single fold scFv libraries I + J (Tomlinson I + J, LifeSciences) phage stocks (1.2 × 1012 and 1.5 × 1012 phages in 150 μL, respectively) were diluted to 1 mL of MPBST [phosphate buffered saline (PBS) with 4% dried skimmed milk (Marvel) and 0.05% Tween-20] and incubated on a rotor for 1 h at room temperature prior to adding the biotinylated peptide (GSYK-Bt) for background subtraction. Streptavidin-coated magnetic beads (1 mg, (6–7) × 107 beads, Dynabeads M280, Invitrogen) were preblocked by incubation with MPBST (1 mL) for 1 h at room temperature and washed with MPBST, PBST (PBS with 0.05% Tween-20), and PBS three times for each solution. The washed Dynabeads were then added to the phage solution incubated with GSYK-Bt (253 μM) in MPBST and incubated on a rotor for 15 min at room temperature. Phages binding to GSYK-Bt was captured to the Dynabeads on a magnetic rack. The supernatant containing unbound phages from the background subtraction was transferred to a new tube and incubated with biotinylated hapten (Him-Suc-GSYK-Bt, 228 μM for the first round panning) for 1 h at room temperature. The hapten-bound phages were collected on the magnet, and the phage-bound magnetic beads were washed a total of 9 times with 1 mL each of MPBST, PBST, and PBS, 3 times for 5 min each for off-rate selection. The washed magnetic beads were transferred to a new tube between different washing solutions. The hapten-bound phages were eluted with 400 μL of triethylamine (100 mM) for 5 min of incubation followed by neutralization with 200 μL of Tris-HCl (1 M, pH 7.4). The selected phages were kept at 4 °C for amplification and the next round of biopanning. The E. coli bacteria strain TG1 was used as the host for phage amplification as described in previous studies.51,52 For the subsequent rounds of panning, the indirect selection method was repeated with the hapten at reduced concentrations (156 μM, 100 nM, and 50 nM of Him-Suc-GSYK-Bt for second, third, and fourth round pannings) to select high affinity binders.
Preparation of Anti-Hapten scFv
After the affinity selections and panning, phage-infected clones were randomly selected and amplified in TG1 bacteria. A total of 56 phagemids were isolated using the QIAprep Spin Miniprep Kit (Qiagen) for sequencing (Genewiz Inc.), and the data were analyzed by the software VectorNTI (Invitrogen). The four scFv variants from clone J42, I4, I21, and I22 were expressed in HB2151 bacteria as described previously.49 Briefly, the phage–infected cells were grown overnight in 2YTGA containing 1% glucose and 100 μg/mL of ampicillin. The overnight culture was used for inoculation of fresh 2YTGA at 37 °C until OD600 ∼ 0.9. After the cells were harvested by centrifugation, the cell pellets were grown overnight in 2YT supplemented with 100 mM sucrose, 10% potassium phosphate buffer (v/v), 100 μM isopropyl-l-thio-β-d-galactopyranoside (IPTG), and 100 μg/mL of ampicillin at 30 °C.53 The supernatant containing the scFv was purified by protein-L affinity chromatography. The isolated scFv was quantified by BCA protein assay and characterized by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and Coomassie blue staining. The binding kinetics was determined by SPR.
Plasmid Construction and Fusion Protein Expression (HER2 Affibody × Anti-Hapten scFv)
To construct an affibody and scFv fusion protein with specificities to both the extracellular domain of HER2 and the hapten, a DNA plasmid of the monomeric HER2 affibody (ZHER2:477) with the His6-tag at the C-terminus and linker (G4S)3 was first custom synthesized (GenScript). Then, the scFv (clone J42) was recombinantly inserted into the pIT2 vector by double digestion of plasmids with SalI and NotI enzymes according to the directional plasmid cloning method.54 After transformation to the E. coli strain BL21 competent cells, the synthesized plasmids were confirmed by PCR and DNA sequencing (Genewiz Inc.). The transformed cells were grown overnight in 2YTGA at 37 °C. The next day, the overnight bacteria culture was inoculated in fresh 2YTGA until OD600 ∼0.6. Bacterial pellets were harvested and after centrifugation were resuspended with 2YTA containing 1 mM IPTG and cultured overnight at 30 °C. His6-tagged fusion protein in the medium was purified by a QIAexpress Ni-NTA Fast Start kit (Qiagen) under denaturing conditions, and protein concentration was measured by a BCA protein assay (Pierce).
SDS–PAGE and Western Blotting Analysis
Protein samples were loaded onto 4–15% Mini-protein TGX precast gel (Biorad) in Tris/glycine buffer. After migration, protein bands were visualized by Coomassie blue staining (Biorad). For Western blotting, the proteins in the SDS–PAGE gel were transferred onto nitrocellulose membranes (Biorad) using a Trans-Blot Turbo Transfer system (Biorad). Membranes were blocked with 3% bovine serum albumin (BSA) in TBS for 1 h at room temperature and incubated with anti His-tag antibody (1:2000, Qiagen) for 1 h. The membranes were washed and incubated with an HRP-conjugated anti-mouse secondary antibody (1:2000, Invitrogen) in 10% nonfat dried milk. After washing, the protein bands were imaged with the ChemiDoc MP system (Biorad) after incubation with Immobilion Western HRP Chemiluminescent Substrates (Millipore).
Measurement of Affinity and Kinetics Using SPR
A Biacore X100 (GE Healthcare) was used for studying the binding of the purified anti-hapten scFv and the heterobivalent fusion protein. Biotinylated hapten peptide (Him-Suc-GSYK-Bt) was captured on the streptavidin-immobilized SA sensor chip (GE Healthcare). The purified scFvs and fusion protein, scFv-L-Aff, were diluted in HBS-EP buffer (10 mM HEPES, 150 mM NaCl, 3 mM EDTA, and 0.005% surfactant P20, pH 7.4) and injected over a hapten-immobilized surface (∼230 RU) for 180 s at a rate of 10 μL/min, followed by dissociation for 800 s. After each sample injection, the surface was regenerated with an injection of 2.5 μL of 1 M NaCl solution.
To evaluate the binding affinity of the heterobivalent fusion protein scFv-L-Aff to HER2, recombinant Human ErbB2/HER2 Fc Chimera (96.8 kDa, R&D Systems) was immobilized via amine coupling (∼3000 RU) on a CM5 sensor chip (GE Healthcare). Serial dilutions of fusion proteins were flowed for 180 s at a rate of 10 μL/min followed by dissociation for 600 s. For surface regeneration, 0.33% SDS solution (30 μL) was used.
A competitive SPR binding study was performed to verify the hapten specificity of the fusion protein. First, scFv-L-Aff (final concentration: 100 nM) was mixed with serial dilutions of succinyl histamine (HS), hapten (Him-Suc-GSYK, Him), histamine dichloride, and dl-histidine; the mixture was then injected over the biotinylated hapten-captured SA chip under the same SPR condition that was used for the hapten binding study. After each sample injection, the surface was regenerated with 2.5 μL of 1 M NaCl solution. All sensorgrams were double referenced by subtracting the surface effect from the control flow cell and the buffer effect from the blank buffer. The kinetic values kon, koff, and KD were obtained using Biacore X100 Evaluation Software (GE Healthcare) assuming the Langmuir 1:1 binding model.
In Vitro Cell Labeling and Confocal Microscopy
The SK-BR3 human breast carcinoma cell line was purchased from ATCC (American Type Culture Collection) and cultured in McCoy’s complete medium (ATCC) supplemented with 10% fetal bovine serum (FBS, Invitrogen), penicillin (100 IU/mL), and streptomycin (100 μg/mL, Lonza) at 37 °C with 5% CO2. HER2-negative breast cancer cells (MDA-MB-23) were cultured in RPMI medium containing the same supplements as those in the McCoy’s complete medium. Cells were plated onto poly-L-Lysine (PLL)-coated coverslips (BD Biocoat cellware) and incubated overnight. The medium was gently removed, and 3% paraformaldehyde (PFA) in PBS was added for fixation. After washing with PBS, cells were incubated with scFv-L-Aff fusion protein (7 μg/mL) for 1 h at room temperature. After washing with PBS, a saturated labeling condition was used for labeling: FITC-anti-His6 tag mAb (33 μg/mL, abcam) and cypate-conjugated hapten (Cy-Him, 1 μM) were added for His6-tag and anti-hapten scFv labeling, respectively, and incubated for 30 min at room temperature. An FITC-labeled anti-HER2 affibody (2 μg/mL, abcam) was used to visualize the membrane HER2 as a control. Nuclei were counterstained with DAPI followed by washing with excess PBS. Coverslips were mounted on slides using ProLong Gold antifade reagent (Invitrogen) and kept at 4 °C. The same procedure was used for live cell labeling; after sequential incubation and labeling, cells were fixed with 3% PFA, and nuclei were counterstained with DAPI. Fluorescence microscopic images were taken with a Zeiss Observer.Z1/Apotome 2 microscope (Carl Zeiss) equipped with a QuantEM 512SC camera, and the images were analyzed using ZEN 2011 software.
Flow Cytometry
SK-BR3 cells were fixed with 4% PFA, and 5 × 104 cells were used for HER2 labeling (n ≥ 3). Cells were preincubated with scFv-L-Aff (10 μg) in PBS (120 μL) containing 3% BSA for 30 min at room temperature. After washing and centrifugation, the cell pellets were resuspended and incubated with Cy5-Him (1–2 μM) and an FITC-anti-His6 tag mAb (abcam, 2 μg) in a total volume of 120 μL for 30 min at room temperature. FITC-conjugated anti-HER2 affibody (abcam, 1.5 μg) was used as a positive control. The same labeling conditions were performed for live cell incubation without fixation. Flow cytometry analysis was performed on a FACS LSR Fortessa flow cytometer (BD) using FACSDiva software, and the data were analyzed using VenturiOne software (Applied Cytometry).
Small Animal Imaging Using Fluorescent Molecular Tomography (FMT)
The experimental protocols were approved by the Animal Care and Use Committee at the University of Pittsburgh. SK-BR3 xenografts were established on female Balb/c nude mice (4–5 weeks of age) from Taconic Lab Animals and Services. HER2 overexpressing breast carcinoma cells, SK-BR3 (7–10 × 106), in 0.2 mL of matrigel (BD Biosciences) were injected subcutaneously onto the right shoulders of the mice. Tumors were allowed to grow to 5–7 mm in diameter for the in vivo imaging study. For HER2 pretargeting, scFv-L-Aff (2.5 nmol) was given to the mice via intraperitoneal injections, and Cy-Him (3 nmol) was administered intravenously to the mice 5 h post-fusion protein injection. NIR tomographic imaging was conducted by a VISEN FMT 2500 in vivo imaging system (PerkinElmer Inc., Boston, MA) while the mice were anesthetized with isoflurane. Three-dimensional region of interests (ROIs) were drawn around the tumors, and the images were analyzed by TrueQuant 3.0 software.
Preparation of Frozen Tissue Sections and Immunofluorescence Staining
The tumors from the SK-BR3 xenograft mice were dissected after sacrificing occurred by carbon dioxide asphyxiation. The tumors were quickly frozen in cold hexane at −60 °C and embedded in Tissue-Tek optimal cutting temperature (OCT) compound (Andwin Scientific). The frozen tissue was cut into 10 μM thickness sections on a cryostat (Microm HM 500OM Cryostat). Tissue sections on the glass slides were kept at −80 °C and hydrolyzed in PBS before staining followed by fixation in acetone at −20 °C. The sections were incubated with 3% horse serum blocking solution in PBS for 1 h at room temperature and washed with PBST (0.1% Tween in PBS). For tissue staining, 100 μg/mL of scFv-L-Aff in the incubation solution (3% BSA, 0.01% sodium azide, and 0.3% Tween in PBS) was applied onto the tissue slides and incubated overnight at 4 °C. After washing, the FITC-labeled anti-HER2 affibody (abcam, 50 μg/mL), anti-His6 tag antibody FITC (abcam, 50 μg/mL), and cypate-conjugated hapten (Cy-Him, 300 μM) were incubated for 3 h at 4 °C in the dark. Nuclei were counterstained with DAPI, and excess PBS was used for washing. Stained sections were mounted with coverslips using ProLong Gold antifade reagent (Invitrogen). Fluorescence microscopic images were taken with a Zeiss Observer.Z1/Apotome 2 microscope (Carl Zeiss) equipped with an EMCCD camera (Evolve 512 Delta, Photometrics, Tuscon, AZ). Fluorescence signals from DAPI, FITC, and Cy-Him were acquired at an excitation of 350, 490, and 745 nm respectively, and the images were analyzed with ZEN 2011 software.
Data Analysis
All numeric data are presented as the mean ± SEM. Statistical analysis was carried out using paired Student’s t-test.
Acknowledgments
This work was funded by an award from the NCI (1R21CA15902, to W.B.E.). This project used the UPCI FACS LSR Fortessa flow cytometer (BD) that is supported in part by award P30CA047904.
Glossary
Abbreviations
- HER2
human epidermal growth factor 2
- EGFR
endothelial growth factor receptor
- scFv
single chain variable fragment
- NIR
near-infrared
Supporting Information Available
Synthetic scheme and characterization of haptens, SEC chromatography, fluorescence labeling images, phage titration, and ex vivo data. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Barbet J., Bardiès M., Bourgeois M., Chatal J.-F., Chérel M., Davodeau F., Faivre-Chauvet A., Gestin J.-F., and Kraeber-Bodéré F. (2012) Radiolabeled Antibodies for Cancer Imaging and Therapy, in Antibody Engineering (Chames P., Ed.) pp 681–697, Humana Press, Totowa, NJ. [DOI] [PubMed] [Google Scholar]
- Kaur S.; Venktaraman G.; Jain M.; Senapati S.; Garg P. K.; Batra S. K. (2012) Recent trends in antibody-based oncologic imaging. Cancer Lett. 315, 97–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuhmacher J.; Klivenyi G.; Matys R.; Stadler M.; Regiert T.; Hauser H.; Doll J.; Maier-Borst W.; Zoller M. (1995) Multistep tumor targeting in nude mice using bispecific antibodies and a gallium chelate suitable for immunoscintigraphy with positron emission tomography. Cancer Res. 55, 115–123. [PubMed] [Google Scholar]
- Moosmayer D.; Berndorff D.; Chang C. H.; Sharkey R. M.; Rother A.; Borkowski S.; Rossi E. A.; McBride W. J.; Cardillo T. M.; Goldenberg D. M.; Dinkelborg L. M. (2006) Bispecific antibody pretargeting of tumor neovasculature for improved systemic radiotherapy of solid tumors. Clin. Cancer Res. 12, 5587–5595. [DOI] [PubMed] [Google Scholar]
- Kontermann R. E. (2012) Dual targeting strategies with bispecific antibodies. mAbs 4, 182–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G. Z.; Hnatowich D. J. (2008) A Semiempirical model of tumor pretargeting. Bioconjugate Chem. 19, 2095–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paganelli G.; Riva P.; Deleide G.; Clivio A.; Chiolerio F.; Scassellati G. A.; Malcovati M.; Siccardi A. G. (1988) In vivo labelling of biotinylated monoclonal antibodies by radioactive avidin: a strategy to increase tumor radiolocalization. Int. J. Cancer (Suppl 2), 121–125. [DOI] [PubMed] [Google Scholar]
- Boerman O. C.; van Schaijk F. G.; Oyen W. J. G.; Corstens F. H. M. (2003) Pretargeted radioimmunotherapy of cancer: progress step by step. J. Nucl. Med. 44, 400–411. [PubMed] [Google Scholar]
- Breitz H. B.; Fisher D. R.; Goris M. L.; Knox S.; Ratliff B.; Murtha A. D.; Weiden P. L. (1999) Radiation absorbed dose estimation for Y-90-DOTA-biotin with pretargeted NR-LU-10/streptavidin. Cancer Biother. Radiopharm. 14, 381–395. [DOI] [PubMed] [Google Scholar]
- Goshorn S.; Sanderson J.; Axworthy D.; Lin Y. K.; Hylarides M.; Schultz J. (2001) Preclinical evaluation of a humanized NR-LU-10 antibody-streptavidin fusion protein for pretargeted cancer therapy. Cancer Biother. Radiopharm. 16, 109–123. [DOI] [PubMed] [Google Scholar]
- Weiden P. L.; Breitz H. B. (2001) Pretargeted radioimmunotherapy (PRIT (TM)) for treatment of non-Hodgkin’s lymphoma (NHL). Crit. Rev. Oncol. Hematol. 40, 37–51. [DOI] [PubMed] [Google Scholar]
- Rusckowski M.; Fogarasi M.; Fritz B.; Hnatowich D. J. (1997) Effect of endogenous biotin on the applications of streptavidin and biotin in mice. Nucl. Med. Biol. 24, 263–268. [DOI] [PubMed] [Google Scholar]
- Nolan O.; Okennedy R. (1990) Bifunctional antibodies - concept, production and applications. Biochim. Biophys. Acta 1040, 1–11. [DOI] [PubMed] [Google Scholar]
- Bird R. E.; Walker B. W. (1991) Single chain antibody variable regions. Trends Biotechnol. 9, 132–137. [DOI] [PubMed] [Google Scholar]
- Hudson P. J.; Souriau C. (2003) Engineered antibodies. Nature Med. 9, 129–134. [DOI] [PubMed] [Google Scholar]
- Yarden Y. (2001) The EGFR family and its ligands in human cancer. signalling mechanisms and therapeutic opportunities. Eur. J. Cancer 37(Suppl 4), S3–S8. [DOI] [PubMed] [Google Scholar]
- Slamon D.; Clark G.; Wong S.; Levin W.; Ullrich A.; McGuire W. (1987) Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235, 177–182. [DOI] [PubMed] [Google Scholar]
- Iván Peñuelas I. D.-P.; García-Velloso M. J.; Martí-Climent J. M.; Rodríguez-Fraile M.; Caicedo C.; Sánchez-Martínez M.; Richter J. A. (2012) PET tracers for clinical imaging of breast cancer. J. Oncol. 2012, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath L.; Kwon S.; Ke S.; Wang W.; Schiff R.; Mawad M. E.; Sevick-Muraca E. M. (2007) Dual-labeled trastuzumab-based imaging agent for the detection of human epidermal growth factor receptor 2 overexpression in breast cancer. J. Nucl. Med. 48, 1501–1510. [DOI] [PubMed] [Google Scholar]
- Ye Y.; Bloch S.; Achilefu S. (2004) Polyvalent carbocyanine molecular beacons for molecular recognitions. J. Am. Chem. Soc. 126, 7740–7741. [DOI] [PubMed] [Google Scholar]
- Ekerljung L.; Wållberg H.; Sohrabian A.; Andersson K.; Friedman M.; Frejd F. Y.; Ståhl S.; Gedda L. (2012) Generation and evaluation of bispecific affibody molecules for simultaneous targeting of EGFR and HER2. Bioconjugate Chem. 23, 1802–1811. [DOI] [PubMed] [Google Scholar]
- Steiner M.; Gutbrodt K.; Krall N.; Neri D. (2013) Tumor-targeting antibody-anticalin fusion proteins for in vivo pretargeting applications. Bioconjugate Chem. 24, 234–241. [DOI] [PubMed] [Google Scholar]
- Orlova A.; Magnusson M.; Eriksson T. L.; Nilsson M.; Larsson B.; Hoiden-Guthenberg I.; Widstrom C.; Carlsson J.; Tolmachev V.; Stahl S.; Nilsson F. Y. (2006) Tumor imaging using a picomolar affinity HER2 binding affibody molecule. Cancer Res. 66, 4339–4348. [DOI] [PubMed] [Google Scholar]
- Morandeau L.; Benoist E.; Loussouarn A.; Ouadi A.; Lesaec P.; Mougin M.; Faivre-Chauvet A.; Le Boterff J.; Chatal J. F.; Barbet J.; Gestin J. F. (2004) Synthesis of new bivalent peptides for applications in the affinity enhancement system. Bioconjugate Chem. 16, 184–193. [DOI] [PubMed] [Google Scholar]
- Rusnak D. W.; Alligood K. J.; Mullin R. J.; Spehar G. M.; Arenas-Elliott C.; Martin A. M.; Degenhardt Y.; Rudolph S. K.; Haws T. F. Jr.; Hudson-Curtis B. L.; Gilmer T. M. (2007) Assessment of epidermal growth factor receptor (EGFR, ErbB1) and HER2 (ErbB2) protein expression levels and response to lapatinib (Tykerb, GW572016) in an expanded panel of human normal and tumour cell lines. Cell Proliferation 40, 580–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekerljung L.; Wallberg H.; Sohrabian A.; Andersson K.; Friedman M.; Frejd F. Y.; Stahl S.; Gedda L. (2012) Generation and evaluation of bispecific affibody molecules for simultaneous targeting of EGFR and HER2. Bioconjugate Chem. 23, 1802–1811. [DOI] [PubMed] [Google Scholar]
- Nordberg E.; Friedman M.; Göstring L.; Adams G. P.; Brismar H.; Nilsson F. Y.; Ståhl S.; Glimelius B.; Carlsson J. (2007) Cellular studies of binding, internalization and retention of a radiolabeled EGFR-binding affibody molecule. Nucl. Med. Biol. 34, 609–618. [DOI] [PubMed] [Google Scholar]
- Zhu W.; Okollie B.; Artemov D. (2007) Controlled internalization of Her-2/ neu receptors by cross-linking for targeted delivery. Cancer Biol. Ther. 6, 1960–1966. [DOI] [PubMed] [Google Scholar]
- Charlton K. A.; Porter A. J. (2002) Isolation of anti-hapten specific antibody fragments from combinatorial libraries. Methods Mol. Biol. 178, 159–171. [DOI] [PubMed] [Google Scholar]
- Wei L. H.; Olafsen T.; Radu C.; Hildebrandt I. J.; McCoy M. R.; Phelps M. E.; Meares C.; Wu A. M.; Czernin J.; Weber W. A. (2008) Engineered antibody fragments with infinite affinity as reporter genes for PET imaging. J. Nucl. Med. 49, 1828–35. [DOI] [PubMed] [Google Scholar]
- Morel A.; Darmon M.; Delaage M. (1990) Recognition of imidazole and histamine derivatives by monoclonal antibodies. Mol. Immunol. 27, 995–1000. [DOI] [PubMed] [Google Scholar]
- Griffiths G. L.; Chang C. H.; McBride W. J.; Rossi E. A.; Sheerin A.; Tejada G. R.; Karacay H.; Sharkey R. M.; Horak I. D.; Hansen H. J.; Goldenberg D. M. (2004) Reagents and methods for PET using bispecific antibody pretargeting and 68Ga-radiolabeled bivalent hapten-peptide-chelate conjugates. J. Nucl. Med. 45, 30–39. [PubMed] [Google Scholar]
- Gold D. V.; Goldenberg D. M.; Karacay H.; Rossi E. A.; Chang C. H.; Cardillo T. M.; McBride W. J.; Sharkey R. M. (2008) A novel bispecific, trivalent antibody construct for targeting pancreatic carcinoma. Cancer Res. 68, 4819–4826. [DOI] [PubMed] [Google Scholar]
- Sharkey R. M.; Karacay H.; Chang C. H.; McBride W. J.; Horak I. D.; Goldenberg D. M. (2005) Improved therapy of non-Hodgkin’s lymphoma xenografts using radionuclides pretargeted with a new anti-CD20 bispecific antibody. Leukemia 19, 1064–1069. [DOI] [PubMed] [Google Scholar]
- Janevik-Ivanovska E.; Gautherot E.; Hillairet de Boisferon M.; Cohen M.; Milhaud G.; Tartar A.; Rostene W.; Barbet J.; Gruaz-Guyon A. (1997) Bivalent hapten-bearing peptides designed for iodine-131 pretargeted radioimmunotherapy. Bioconjugate Chem. 8, 526–533. [DOI] [PubMed] [Google Scholar]
- Barderas R.; Shochat S.; Martínez-Torrecuadrada J.; Altschuh D.; Meloen R.; Ignacio Casal J. (2006) A fast mutagenesis procedure to recover soluble and functional scFvs containing amber stop codons from synthetic and semisynthetic antibody libraries. J. Immunol. Methods 312, 182–189. [DOI] [PubMed] [Google Scholar]
- Persson H.; Flicker S.; Sadegh M. K.; Greiff L.; Valenta R.; Ohlin M. (2008) A common idiotype in IgE and its relation to recognition of the grass pollen allergen Phl p 2. Mol. Immunol. 45, 2715–2720. [DOI] [PubMed] [Google Scholar]
- Ling M. M. (2003) Large antibody display libraries for isolation of high-affinity antibodies. Comb. Chem. High Throughput Screening 6, 421–432. [DOI] [PubMed] [Google Scholar]
- Tolmachev V.; Orlova A.; Nilsson F. Y.; Feldwisch J.; Wennborg A.; Abrahmsen L. (2007) Affibody molecules: potential for in vivo imaging of molecular targets for cancer therapy. Expert Opin. Biol. Ther. 7, 555–568. [DOI] [PubMed] [Google Scholar]
- Holliger P.; Hudson P. J. (2005) Engineered antibody fragments and the rise of single domains. Nat. Biotechnol. 23, 1126–1136. [DOI] [PubMed] [Google Scholar]
- Josan J. S.; Handl H. L.; Sankaranarayanan R.; Xu L.; Lynch R. M.; Vagner J.; Mash E. A.; Hruby V. J.; Gillies R. J. (2011) Cell-specific targeting by heterobivalent ligands. Bioconjugate Chem. 22, 1270–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutel M.; Akdis M.; Akdis C. A. (2009) Histamine, histamine receptors and their role in immune pathology. Clin. Exp. Allergy 39, 1786–1800. [DOI] [PubMed] [Google Scholar]
- Nelson T. J.; Alkon D. L. (2007) Protection against β-amyloid-induced apoptosis by peptides interacting with β-amyloid. J. Biol. Chem. 282, 31238–31249. [DOI] [PubMed] [Google Scholar]
- Koo E. H.; Lansbury P. T. Jr.; Kelly J. W. (1999) Amyloid diseases: abnormal protein aggregation in neurodegeneration. Proc. Natl. Acad. Sci. U.S.A. 96, 9989–9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T.; Satou T.; Nishida S.; Tsubaki M.; Hashimoto S.; Ito H. (2009) Expression of amyloid precursor protein after rat traumatic brain injury. Neurol. Res. 31, 103–109. [DOI] [PubMed] [Google Scholar]
- Chang C.-H.; Sharkey R. M.; Rossi E. A.; Karacay H.; McBride W.; Hansen H. J.; Chatal J.-F.; Barbet J.; Goldenberg D. M. (2002) Molecular advances in pretargeting radioimunotherapy with bispecific antibodies. Mol. Cancer Ther. 1, 553–563. [PubMed] [Google Scholar]
- McCall A. M.; Adams G. P.; Amoroso A. R.; Nielsen U. B.; Zhang L.; Horak E.; Simmons H.; Schier R.; Marks J. D.; Weiner L. M. (1999) Isolation and characterization of an anti-CD16 single-chain Fv fragment and construction of an anti-HER2/neu/anti-CD16 bispecific scFv that triggers CD16-dependent tumor cytolysis. Mol. Immunol. 36, 433–445. [DOI] [PubMed] [Google Scholar]
- Hawkins R. E.; Russell S. J.; Winter G. (1992) Selection of phage antibodies by binding-affinity - mimicking affinity maturation. J. Mol. Biol. 226, 889–896. [DOI] [PubMed] [Google Scholar]
- Rouet R.; Lowe D.; Dudgeon K.; Roome B.; Schofield P.; Langley D.; Andrews J.; Whitfeld P.; Jermutus L.; Christ D. (2012) Expression of high-affinity human antibody fragments in bacteria. Nat. Protoc. 7, 364–373. [DOI] [PubMed] [Google Scholar]
- Chames P., and Baty D.. Antibody Engineering; Springer Berlin Heidelberg: Berlin and Heidelberg, Vol. 1, pp 151–164. [Google Scholar]
- Lee C. M.; Iorno N.; Sierro F.; Christ D. (2007) Selection of human antibody fragments by phage display. Nat. Protoc. 2, 3001–3008. [DOI] [PubMed] [Google Scholar]
- Goletz S.; Christensen P. A.; Kristensen P.; Blohm D.; Tomlinson I.; Winter G.; Karsten U. (2002) Selection of large diversities of antiidiotypic antibody fragments by phage display. J. Mol. Biol. 315, 1087–1097. [DOI] [PubMed] [Google Scholar]
- Hust M.; Steinwand M.; Al-Halabi L.; Helmsing S.; Schirrmann T.; Dubel S. (2009) Improved microtitre plate production of single chain Fv fragments in Escherichia coli. New Biotechnol. 25, 424–428. [DOI] [PubMed] [Google Scholar]
- Sambrook J., and Russell D. W. (2001) Molecular Cloning: A Laboratory Manual, 3rd ed., Cold Spring Harbor, New York. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.