Abstract
Monocular organization of the goldfish horizontal neural integrator was studied during spontaneous scanning saccadic and fixation behaviors. Analysis of neuronal firing rates revealed a population of ipsilateral (37%), conjugate (59%), and contralateral (4%) eye position neurons. When monocular optokinetic stimuli were employed to maximize disjunctive horizontal eye movements, the sampled population changed to 57, 39, and 4%. Monocular eye tracking could be elicited at different gain and phase with the integrator time constant independently modified for each eye by either centripetal (leak) or centrifugal (instability) drifting visual stimuli. Acute midline separation between the hindbrain oculomotor integrators did not affect either monocularity or time constant tuning, corroborating that left and right eye positions are independently encoded within each integrator. Together these findings suggest that the “ipsilateral” and “conjugate/contralateral” integrator neurons primarily target abducens motoneurons and internuclear neurons, respectively. The commissural pathway is proposed to select the conjugate/contralateral eye position neurons and act as a feedfoward inhibition affecting null eye position, oculomotor range, and saccade pattern.
INTRODUCTION
Spontaneous movement of both eyes in naïve goldfish appears to be highly correlated due to conjugate timing and direction of saccades (Easter 1971); however, after individual saccades, the two eyes are not in corresponding positions during the subsequent fixations (Easter 1971; Mensh et al. 2004). The central mechanisms underlying “disconjugate” eye positions can be envisioned as resulting from either separate encoding of each eye position in a common premotor pathway (Von Helmholtz 1910) or through a combination of signals arising from different premotor pathways (Hering et al. 1977). Oculomotor plasticity paradigms in a variety of vertebrates including fish have provided evidence for separate encoding of signals because visuomotor training has been shown to induce monocular changes in the vestibuloocular reflex (VOR), optokinetic reflex (OKR), and saccades (Averbuch-Heller et al. 1999; Lemij and Collewijn 1991b, 1992; Oohira and Zee 1992; Weiser et al. 1989). When eye position alignment in primates was changed by conjointly presenting vergence and versional cues, neurons in the vestibular and prepositus hypoglossi nuclei could be correlated with either ipsi- or contralateral eye movements (McConville et al. 1994; Sylvestre et al. 2003; Zhou and King 1996, 1998). Although clearly demonstrative of monocular encoding, the preceding studies could not be conclusive about the structural sites where conjugacy and/or monocularity occurs within oculomotor circuitry.
The goldfish horizontal eye position neural integrator (HPNI) provides an attractive site to rigorously test neuronal monocularity within the oculomotor system because neurons can be recorded during disconjugate eye movements (Aksay et al. 2000; Pastor et al. 1994). Afferent inputs to HPNI are largely, perhaps exclusively, from vestibular and reticular burst neurons as illustrated in Supplemental Fig. S1A.1 Efferent targets of HPNI consist of the ipsilateral abducens motoneurons, contralateral HPNI and abducens internuclear neurons including neurons in the saccadic pattern generator (Aksay et al. 2000). In contrast to the mammalian HPNI in which function is argued to be distributed between two nuclei (vestibular and prepositus hypoglossi) both sending and receiving cerebellar projections (McCrea and Horn 2005), the goldfish HPNI appears as a single nucleus without direct vestibular/cerebellar connections, thereby allowing an uncomplicated interpretation of monocular integrator performance and plasticity following experimental perturbations.
The experimental discrimination between separate or shared signal encoding of signals in the goldfish HPNI can be implemented entirely with the use of versional visual stimuli without recourse to vergence for producing monocular eye motion. Both converging and diverging eye movements can be produced with versional optokinetic stimuli because horizontal visual directional sensitive neurons identified in the fish pretectum respond to both nasal-temporal and temporal-nasal retinal slip (Klar and Hoffmann 2002; Masseck and Hoffmann 2009) (Supplementary Fig. S1A). Use of this behavioral paradigm allowed us to assess monocularity of individual HPNI neurons and eye position holding time constants by exploring the nasal and temporal halves of the oculomotor range for a given direction of eye motion (Supplemental Fig. S1B).
In spite of the preceding rationale, the firing rate properties of HPNI neurons make determination of monocularity challenging in the goldfish. HPNI neuronal mean firing rates at any given eye position have been shown to vary even when hysteresis is accounted for in eye position sensitivity (Aksay et al. 2003). Because HPNI neurons are irregular in firing rate, the variation within each fixation often can be greater than the small differences observed between the left and right eye because individual fixations are generally stable in most naïve animals (Major et al. 2004a,b; Mensh et al. 2004). Even when either disconjugate eye motions or small changes in eye position occurred, HPNI neurons appeared to be encoding a conjugate eye position, and thus it was not straightforward, even by statistical analysis, to distinguish whether HPNI neurons truly were encoding conjugate eye position or alternatively reflecting co- linearization of saccades tending to bring the eyes into a highly conjugate relationship (Aksay et al. 2000, 2007; Major et al. 2004b). In this study, we resolved the issue of eye-position integrator conjugacy at both the behavioral and neural level by using monocular visual stimulation to bring about disconjugate eye movements in naturally conjugate goldfish without involving viewing distance dependent vergence responses. This methodology is different from that employed in previous mammalian studies that utilized distance mediated vergence to induce monocular eye position and eye velocity changes (King and Zhou 2002; Sylvestre et al. 2003; Zhou and King 1996, 1998).
Monocularity of eye velocity and position signals has important implications concerning both extrinsic and intrinsic synaptic organization of HPNI that indirectly addresses mechanisms of neuronal persistence (Major and Tank 2004). If eye position was encoded conjugately, then each HPNI neuron potentially would control both the stability of nasal eye positions in the contralateral eye and temporal eye positions in the ipsilateral eye (Supplemental Fig. S1A). Accordingly, if eye position is separately encoded for the ipsilateral temporal and contralateral nasal hemifield within each HPNI, then two subpopulations of neurons are co-localized in each integrator. Bilaterally this would suggest at least four relatively independent sets of HPNI neurons. Inhibitory connections between HPNI might then be envisioned to coordinate the nasal and temporal eye position integrator subpopulations for each eye. This study also considered the role of HPNI commissural neurons to further assess the structural and functional basis of monocular time constant generation and plasticity.
METHODS
Animal care, maintenance, and surgical preparation
One hundred forty-four goldfish (Carassius auratus) 15–30 cm long were obtained from an institutionally approved supplier (Hunting Creek Fisheries, Thurmont, MD). Animal preparation and experimental analyses were adopted from those previously described (Aksay et al. 2000; Marsh and Baker 1997; Pastor et al. 1994). All goldfish were acclimated and maintained in a temperature controlled (18°C) tank, pH 7.0–7.6, exposed to a 12:12 h light/dark cycle. At least 2 days prior to neuronal recording, goldfish were prepared in advance for experiments that required open cranial exposure. Goldfish were anesthetized with buffered tricaine methanesulfonate solution (MS222; 1:5,000 wt/vol; Sigma, St. Louis, MO) and six self-tapping screws (Tx-000-1/8 in or Tx-00-1/8 in, Small Parts, Miami Lakes, FL) were placed in the skull and two stabilizing bolts (1–72) anchored with Durabase dental acrylic (Reliance Dental, Worth, IL). A small (3–6 mm) section of the skull covering the hindbrain was removed, and the bone flap was reset and sealed using Vetbond tissue adhesive (3M, St. Paul, MN). Goldfish were revived in anesthetic free water and then placed in an isolated temperature controlled recovery tank. The NYU School of Medicine Institutional Animal Care and Use Committee (IACUC) approved all protocols.
Eye movement measurements and behavioral experiments
On the day of the experiment, a 4% topical xylocaine gel (Astra Pharmaceutical Products, Westborough, MA) was applied by cotton to the eyes, mouth, and skull. Goldfish were placed inside a 38 cm diam temperature-controlled (18°C) aerated circular tank on top of a servocontrolled rate table and gently restrained in body-conforming acrylic holders. Eye movements were measured using the magnetic field search coil technique (Robinson 1963) by suturing (7–0 silk, Ethicon, Somerville, NJ) 80 turn 2.2 mm diam copper coils (Sokymat, SA, Switzerland) to the eyes after topical application of 4% xylocaine (Supplemental Fig. S1B). The experimental tank was placed within a dual axis driven magnetic field (C-N-C Engineering, Seattle, WA), and eye position signals were 150 Hz low-pass filtered.
VORs were elicited by using a servocontrolled rate table (Biomedical Engineering, Thornwood, NY). Optokinetic stimulation was elicited using either one or two servo-controlled planetariums (Biomedical Engineering) mounted above the center of the head and projecting 3–5° red visual spots on a white background (Supplemental Fig. S1). Stimulus waveforms were generated by a computer controlled function generator (LabView, National Instruments, Austin, TX) with individual control of the amplitude and phase of the vestibular and visual stimuli. A basic set of control data was recorded in every experiment that included spontaneous scanning in both light and darkness, sinusoidal VOR at 1/8 Hz 15.7°/s peak velocity, VOR/OKR (gain = 1.0), and OKR at 1/8 Hz 15.7°/s peak velocity. Goldfish were also placed in darkness before any experimental measurement to ensure that the visual threshold would be constant throughout data acquisition.
Monocular optokinetic behavior and time constant plasticity
For monocular experiments, two independent servocontrolled planetariums were utilized that projected visual spots to ∼150° of the visual field. The average binocular overlap in goldfish (central ∼30°) was blocked from receiving any patterned visual stimuli (Trevarthen 1968). By necessity, each planetarium was slightly offset from table center with amplitude and phase separately adjusted. Eye positions were calibrated with pseudo-whole field conjugate stimulation in which the planetariums moved in equal phase and amplitude (Supplemental Fig. S1). A set of conjugate OKR controls were recorded for each experiment with the light blockers in place to minimize any gain changes due to an alteration of the optokinetic stimulus during the monocular paradigms. Disconjugate eye tracking was elicited when the planetariums differed in phase, frequency, or amplitude. Pathways monocular from the sensory (visual) periphery to the extraocular motor output were distinguished from those including central binocular suppression by selectively occluding one eye with a black painted hemi-sectioned ping-pong ball. Pattern blocking effectiveness was confirmed by the absence of eye movements in either eye during planetarium motion projecting to only the occluded eye as compared with the unblocked eye.
To modify eye position time constants, planetarium command signals induced visual slip by feeding eye position voltage into a custom built electronic device in which threshold could be adjusted for positive (left eye position) and negative (right eye position) voltage set points independently. For instability training, the planetarium was initially rotated at 20°/s until eye velocity eventually matched planetarium velocity, and then the training velocity was increased up to a maximum of 40°/s. For leak training, lower initial planetarium training velocities were utilized (2–5°/s) with a large central region not entrained (null region ±5°) to prevent unidirectional leak training as the saccadic pattern generator could not reset the eyes across the null position. This approach facilitated training by spontaneously switching between the nasal and temporal directions. As leak eye velocity increased over the course of the experiment to more closely follow the training paradigm, planetarium velocity was increased and the null region reduced until a maximal training stimulus of 40°/s was reached.
Neuronal recording
Extracellular recording from HPNI neurons was obtained using 3 mm OD glass electrodes pulled on a Narishige vertical puller with final tip diameters of 1–4 μM (resistances of 1–5 MΩ) filled with 2 M NaCl, 50 mM LiCl, and Fast Green. On the day of the experiment under local lidocaine anesthesia (2%), the surgical window was re-opened and a layer of Fluorinert (FC75, 3M) placed above the hindbrain. HPNI neurons exhibiting eye position sensitivity were recorded ∼400 μm from the midline, midway between the facial lobe and obex (Aksay et al. 2000, 2007; Major et al. 2004b). Electrical activity was amplified by 10,000 times and recorded with a band-pass of 100 Hz to 10 kHz. The raw voltage records of the neurons and behavior were digitized at 15 kHz using a DigitaData 1320A A-D board (Axon Instruments). The behavioral traces were subsampled off-line to 300 Hz using a custom written algorithm (AxonSqueeze; provided courtesy of D. W. Tank). Firing rates were determined off-line using custom written algorithms (Aksay et al. 2000) that distinguished neuronal populations by both spike amplitude and half-width time window including a histogram of the peak-peak amplitude (Supplemental Fig. S2). To check that a single neuron was isolated, the voltage records were superimposed (Supplemental Fig. S2D). Firing rates were computed from the interspike intervals (ISIs) and smoothed by a 125 ms triangle moving average window (program provided courtesy of. E. Aksay, Cornell University and D. W. Tank, Princeton University).
Acute/chronic midline lesion and histology
Control behavioral sets were recorded and the midline cut from slightly caudal to the facial lobe through the obex with either a small cover glass shard 0 (Electron Microscopy Sciences, Washington PA) or iridectomy scissors (Vannas type, Ted Pella, Redding, CA; Figs. 2–5). Immediately after the lesion, small Kimwipe sponges were utilized to control bleeding and minimize hemorrhagic effects. In the best surgical conditions, eye position stability could be assessed in both the presence and absence of visual feedback, on average, 20 min post lesion. If eye position time constants were markedly reduced (<3–5 s) and either the VOR or OKR also showed major deterioration (∼30%), the experimental protocols were discontinued. In nine experiments, oculomotor behaviors were recorded after the lesion and compared with the control conditions. In six cases, eye position drift was also trained toward instability, and in three goldfish, the bone flap was reattached and the animals were returned to the home tank for chronic observation.
Fig. 2.
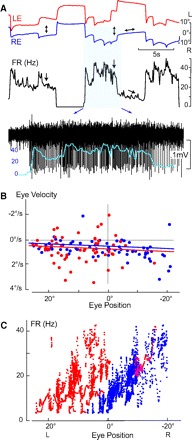
Ipsilateral right side HPNI neuron during spontaneous fixation behavior. A: LE and RE positions, FR (Hz) and neuronal activity of a right-side (ipsilateral) HPNI neuron with monocular eye positions indicated arrows. Neuronal activity is overlain by the expanded FR curve. B: P-V plot showing a τ for LE of 111.5 s and RE 124.5 s. C: plot of FR vs. eye position in which the coefficients for the LE were −0.52 (spike/s)/° and −0.41 (spike/s) per °/s, (r = 0.48) and the RE −1.59 (spike/s)/° and −0.30 (spike/s) per °/s, (r = 0.83).
Fig. 3.
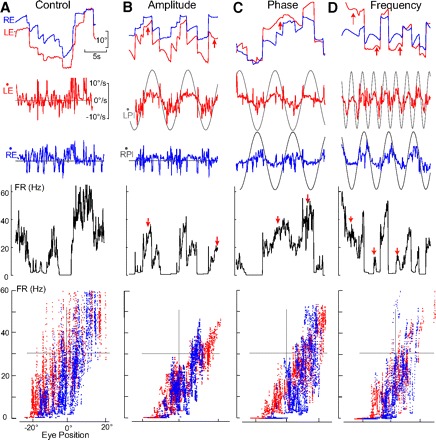
HPNI activity during monocular optokinetic reflex OKR behaviors. A–D: FR of an ipsilateral (left-side) neuron during spontaneous scanning (A) and differences in LE/RE OKR amplitude (B), phase (C), and frequency (D). Red arrows show regions of monocularity. A: FR sensitivity was found to be conjugate for the LE 1.06 (spike/s)/° and 0.21 (spike/s) per °/s, (r = 0.77) and RE 1.33 (spike/s)/° and −0.31 (spike/s) per °/s, (r = 0.74). B: PL (monocular planetarium) amplitude differences (LPl, 15.7°/s and RPl, 0°/s) produced monocular LE and RE velocity gains of 0.31 and 0.05 with similar phase leads of 7° and 14°. FR sensitivity was LE 1.13 (spike/s)/° and 1.02 (spike/s) per °/s, (r = 0.92) and for the RE 1.57 (spike/s)/° and 1.40 (spike/s) per °/s, (r = 0.72). C: LPl and RPl were of similar amplitude (15.7°/s) but 180° out-of-phase. Gains were similar (0.16) but with a phase lag of 166.2° resulting in oppositely directed velocity sensitivity. LE sensitivity was 1.28 (spike/s)/° and 0.40 (spike/s) per °/s, (r = 0.92) and RE 1.47 (spike/s)/° and −2.18 (spike/s) per °/s, (r = 0.75). D: LPl and RPl were of similar amplitude (15.7°/s), but at different frequency (0.4 and 0.18 Hz). LE and RE gains were similar (0.20 and 0.23) with a FR correlations of LE 1.32 (spike/s)/° and 1.42 (spike/s) per °/s, (r = 0.88) and RE 1.46 spike/s/° and 0.66 (spike/s) per °/s, (r = 0.66).
Fig. 4.
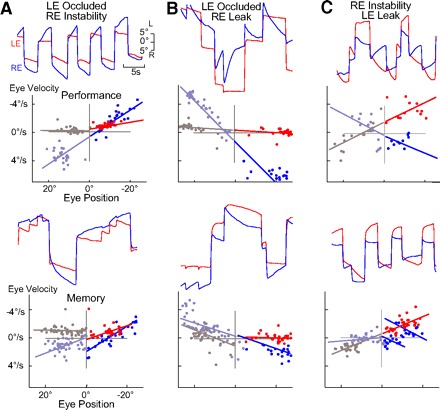
Monocular modification of integrator time constants. A–C: P-V plots and eye position records after training during performance (top) and memory (bottom) of monocular changes in the integrator time constant with + and − values indicating leak and instability, respectively. A: 6 h after the τ for RE (blue) instability performance was −5.2 s and for the occluded LE (red) −127.0 s. RE instability memory was −14.5 s and LE occlusion −194.4 s. B: τ for RE leak performance after 4 h was 3.5 s and LE stability 57.8 s. RE leak memory was 10.9 s and LE stability 107.2 s. C: τ for RE leak performance at 3 h was 7.3 s with LE instability at 8.1 s. RE memory leak was 43.2 s and LE instability −8.4 s.
Fig. 5.
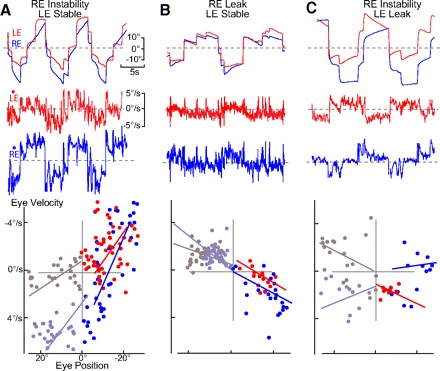
Monocular visual training changes in integrator time constants. A–C: P-V plots and behavioral records in darkness (memory) after 4–6 h of monocular training to instability (A), leak (B), and leak-instability (C). A: RE was trained toward instability and the LE to stable fixation. Based on largely nasal oculomotor ranges for LE and bidirectional for RE, τ was −15.3 and −4.4 s, respectively. B: RE was trained toward leak and the LE to stable fixation. Based on oculomotor ranges largely bidirectional for RE and temporal for LE (nasal drift), τ was 7.0 and 15.4 s, respectively. C: RE instability and LE leak nasal/temporal training significantly changed τ in RE to −26.7 s and LE 11.2.
To determine the long-term effects of midline lesion on HPNI stability and monocularity, a midline lesion was performed more carefully during attachment of the head restraint under general anesthesia (n = 12). Including the acute lesioned goldfish (n = 3), eye position holding and leak/instability training was tested at various time intervals ≤3 mo. To verify that the lesion encompassed crossing midline HPNI projections, animals were perfused with 4% formaldehyde and 0.5% glutaraldehyde and sectioned coronally at 75–100 μm with a freezing microtome followed by staining with cresyl violet. Lesion dimensions were determined by graphic reconstruction. In several cases, biocytin crystals (Molecular Probes) were placed in the spinal cord 2 days prior to perfusion to better visualize hindbrain nuclei and commissural integrity. Biocytin labeled slices were incubated with an avidin-biotin complex (Vector) and reacted with diaminobenzidine as described in (Straka et al. 2006).
Data analysis and eye movement calibration
All data were recorded by an A-D board (DigitaData 1320A, Axon Instruments, Union City, CA) either at 300 Hz (behavioral recordings) or 15 kHz (neuronal recordings) and analyzed off-line using custom written Matlab algorithms (The Mathworks, Natick, MA; courtesy of G. Major, E. Aksay, and D.W. Tank). Eye and head velocity were determined by digitally differentiating the position records and smoothed by a moving average window of <50 ms. Eye position was always automatically de-saccaded for 200 ms before to 600 ms after each saccadic fast phase as determined by acceleration thresholds with movement artifacts manually removed from the quantitative analysis. During monocular tracking experiments, eye movements were more disconjugate, and a combined fixation index was used to exclude regions that could differ in time or the number of saccades.
Head velocity was produced by sinusoidally rotating the table at 0.125 Hz and ±20° (31.4°/s peak-to-peak velocity), and the planetarium was calibrated by adjusting the velocity and phase until the visual projection was stationary in the surrounding room during table rotation. The output voltages of the search coils were measured on a mechanical model before experimental use and then recalibrated during simultaneous visual-vestibular presentation of a sinusoidal stimulus (0.125 Hz 15.7°/s peak velocity) that presented a VOR gain of 1.0 (eye/head velocity). The de-saccaded eye position voltages were differentiated into eye velocity and fit by a linear regression to the sinusoidal waveform to compute a calibration constant. Zero eye position was determined within each orbit as the mean eye position during 3–5 min of scanning a stationary background. All conclusions in this paper were based on changes in oculomotor behavioral paradigms occurring within each experiment, thus a goldfish served as its own control, and any slight miscalibration (absolute value) would not affect either the qualitative or quantitative results.
VOR and OKR gain and phase were computed by a least square linear regression of the desaccaded eye velocity. Gain was a ratio of the amplitude of the eye velocity versus the command stimulus (table or planetarium). A least squares regression of the de-saccaded eye velocity was also computed for disconjugate OKR gain and phase; however, the gains were normalized to that during pseudo-whole field OKR (0.125 Hz 15.7°/s) to ascertain the percentage reduction in gain during monocular tracking.
Eye position time constants
To determine the time constants of eye fixations, position-velocity (P-V) plots were constructed using Matlab (Major et al. 2004a,b; Mensh et al. 2004). Mean eye position for each fixation lasting ≥150 ms was plotted against average eye drift velocity determined as the slope of the linear regression of eye position during the fixation to yield a scatter plot of eye velocity versus eye position (Supplemental Fig. S1B). The slope of linear regression of the P-V plot (k) is mathematically equal to the negative inverse of the time constant (τ = −1/k). This method was utilized previously to express stability of eye position holding and time constant plasticity (Major et al. 2004a,b; Mensh et al. 2004); however, one weakness is that all eye positions are assumed to have a single null point and time constant during a sampling period. In spite of this constraint, P-V plots were used for ease of implementation, interpretation, and comparison of time constants with those from previous studies.
Different slopes could be drawn for the nasal and temporal eye positions and significance was determined by ACOVA with a post hoc Tukey-Kramer analysis carried out in Matlab (P < 0.01). Similar results were obtained if a Scheffe test correction or a Bonferroni correction were utilized rather than a Tukey-Kramer test. Due to multiple observation points within the training time period, the Tukey Kramer test corrected for a type 1 error that could occur due to multiple sequential t-tests. This method was especially useful in monocular time constant plasticity studies that also compared interocular measurements. Time constants could not be used directly for statistical analysis because they are a noncontinuous function, so k values were employed in all comparisons. After analysis, k values were then reconverted into time constants with the consequence that SDs, and confidence intervals became asymmetrical due to the mathematical transformation. Average changes in time constants due to either experimental perturbation or training were analyzed using paired t-test in Microsoft Excel by comparing the initial to final observation. All average time constants are reported with either the SDs or range of values.
Determination of neuronal monocularity
Neurons were analyzed for monocular eye position and eye velocity sensitivity by plotting firing rates along with both the velocity and position records from each eye. Eye position and velocity for the right eye (RE) and left eye (LE) were regressed separately against the firing rate. The Pearson correlation coefficient of the right eye was compared for significance against the Pearson correlation coefficient for the left eye (Zar 1996). Neurons were determined to be correlated to eye position and eye velocity if the correlation coefficient (r) was >0.7. Correlation coefficients were used to determine monocularity rather than regression coefficients as used elsewhere (Sylvestre et al. 2003; Zhou and King 1996, 1998). The reasoning behind this strategy was that eye position versus firing rate plots of the right and left eye had similar slopes; however, the scatter of the data points and hence the correlation was much better for one eye. The high degree of collinearity between eye movements produced unreliable regression coefficients in the combined left/right eye multiple linear regression as seen by reversed polarities and negligibly small numbers. Collinearity was avoided by regressing eye motions of the right and left eye separately. To test that large qualitative difference did not exist between the methods utilized in this work and in previous studies of neuronal monocularity, a position ratio (Kr − Kl)/(Kr + Kl) was computed for the neurons as previously described (Zhou and King 1996). Analysis of the neurons yielded similar results regardless of the methodology employed.
RESULTS
A hallmark of HPNI activity in goldfish is proportionality of the firing rate to eye position and persistence in the absence of sensory feedback, i.e., in the dark (Fig. 1, A–C). Similar to previous work, scanning saccadic eye motions were conjugate in timing and direction (Fig. 1A); however, the amplitude frequently differed between the right and left eyes (Easter 1971; Mensh et al. 2004). Monocular saccades were occasionally observed at the extremes of the oculomotor range, and in contrast to the yoked onset of saccades, large differences occurred in post saccadic drift when both eyes were at nearly identical positions (Fig. 1A). The regression lines of the P-V plot (Fig. 1B) revealed an offset from zero indicating a nasal bias with the left eye drifting right and the right eye drifting left as previously observed (Easter 1971; Mensh et al. 2004). The nasal eye position drifts suggest two separate populations of neurons might be co-localized within HPNI, one projecting to the abducens motoneurons to control ipsilateral temporal eye position and the other projecting to the abducens internuclear interneurons to control nasal contralateral eye position (Supplemental Fig. S1A).
Fig. 1.
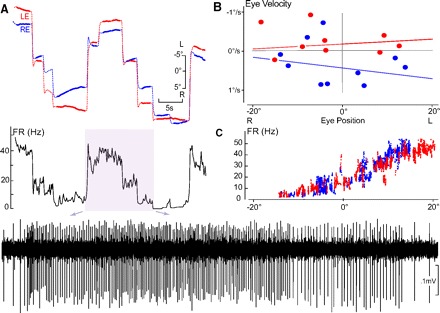
Conjugate left side horizontal eye position neural integrator (HPNI) neuron during spontaneous fixation behavior in darkness. A: FR (firing rate) in Hz correlated with left (LE, red) and right (RE, right) eye position following saccades of different amplitude. Inset: activity is expanded below as indicated by the arrows. B: P-V (eye position-velocity) plot constructed from only the fixations shown in A. Eye velocity (ordinate) was calculated from least squares regression of the eye position (filled part for each fixation). The time constant (τ) for LE was 159.7 s and RE 72.9 s. C: FR vs. eye position plot showing a correlation with both left and right eye position. Averaged LE and RE position and velocity coefficients were 1.59 (spike/s)/° and −0.29 (spike/s) per °/s, (r = 0.96) with + values leftward and – rightward.
Monocular HPNI neurons
To test if HPNI neurons preferentially encode for temporal positions of the ipsilateral eye and/or nasal positions of the contralateral eye, firing rates were recorded during naïve spontaneous scanning. Two distinct populations of neurons were distinguished, one correlated better with ipsilateral temporal eye position and thus is shown to be monocular (Fig. 2), and the other was shown to be conjugate because the correlation was not significantly different for either eye (Fig. 1). Conjugate neurons were recorded even when mild disconjugacy was observed in eye position records.
In the right-side monocular HPNI neuron shown in Fig. 2, ipsilateral right eye preference was observed when the left eye saccaded in the “off” direction, but the neuronal firing rate and right eye position remained constant (Fig. 2A, arrowsets). In other cases, the firing rate increased when the right eye was further in the “on direction,” whereas the left eye was further in the “off direction” thereby suggesting right eye position sensitivity. Combined eye position and eye velocity linear regression versus firing rate was obtained separately for the right and left eye with the graphical approximation of eye position versus firing rate plotted in Fig. 2C. A clear linear band was observed when right eye position (blue) was regressed against firing rate, as opposed, to the left eye regression (red) that was more diffuse and without a unique slope. When a combined left and right eye position and velocity analysis (Zhou and King 1996, 1998) was used to determine a ratio of the eye position/velocity regression coefficients, the HPNI neuron in Fig. 2 was also classified as ipsilateral-eye sensitive. By contrast the neuron illustrated to be ipsilateral in Fig. 3, B–D, showed during scanning behavior in A a large correlation between right and left eye positions (R2 > 0.92). No obvious ocular preference could be calculated by regressing the right and left eye positions separately due to the large overlap in eye position versus firing rate plots that would have supported a conjugate neuronal classification. When analyzed using the separate left and right eye position and velocity analysis, a position ratio of 0.21 was found also supporting a conjugate neuron classification.
Conjugate and ipsilateral HPNI neurons recorded during scanning saccades and fixations
Twenty-nine neurons, with correlation coefficients >0.7 were recorded and analyzed during spontaneous scanning. Thirty-seven percent of the neurons (11/29) were ipsilateral-eye sensitive and 59% of the neurons (17/29) were conjugate (Table 1). The average eye position sensitivity of the ipsilateral related neurons was 63% greater than the conjugate neurons (P = 0.004); however, the average velocity sensitivity during scanning was not significantly different. The thresholds of the conjugate neurons were slightly more nasal, although not significantly different based on population means (P = 0.22). Only a single HPNI neuron was found to be correlated with the contralateral eye. Conjugate neurons analyzed by position ratios were more likely to be categorized as either ipsilateral or contralateral eye sensitive. Twenty neurons (68%) exhibited a position ratio >0.3 and were classified as ipsilateral eye sensitive. Four neurons (14%) exhibited a position ratio between 0.3 and −0.3 and were classified as conjugate. Five neurons (17%) exhibited a position ratio below −0.3 and were classified as contralateral eye sensitive.
Table 1.
Eye preference during scanning
| Cell Type | Position, spike/s per °/s | Velocity, spike/s per °/s | Percentage Oculomotor Range | Position Ratio |
|---|---|---|---|---|
| Ipsilateral | 1.67 ± 0.51 | 0.95 ± 1.24 | 63.4 ± 22.6 | 0.70 ± 0.44 |
| Conjugate | 1.06 ± 0.44 (0.004) | 0.79 ± 0.72 (0.71) | 74.5 ± 22.4 (0.22) | 0.21 ± 0.63 (0.023) |
Values are means ± SD. P values are in parentheses. n = 11 and 17 for ipsilateral and conjugate, respectively.
Monocular optokinetic behavior and eye movements
Due to the high degree of conjugacy between the eyes during scanning behavior, separate optokinetic stimuli were presented to each eye to produce disconjugate behaviors for better distinguishing HPNI monocularity. Although optokinetic stimuli most likely reached abducens and medial rectus motoneurons directly before involvement of the eye velocity-to-position neural integrator (Cochran et al. 1984; Uchiyama et al. 1988), HPNI firing rates would be largely determined through vestibular neuron activity (Allum et al. 1976; Green et al. 1997). Monocular behavior was recorded in 23 experiments and analyzed in 39 experimental conditions in which the pseudowhole field optokinetic gain was >0.2 for 0.125 Hz sinusoids with a peak velocity of 15.7°/s. The average gain during whole field optokinetic tracking was 0.47 ± 0.16 for the LE and 0.43 ± 0.16 for the RE. The phase lag was 13.3 ± 5.9° for the LE and 13.3 ± 5.6° for the RE. The slightly larger phase lag and lower gain compared with previous OKR quantification in goldfish was likely due to the 30° occlusion of the binocular overlap region for visual stimuli (Supplemental Fig. S1B) (Marsh and Baker 1997). To account for interexperimental differences in optokinetic performance, the tracking gains were normalized to 1.0 for pseudo-whole field OKR at 0.125 Hz with a peak velocity of 15.7°/s.
Differences in tracking gain and phase were observed between the eyes, when the planetariums differed in either amplitude (Fig. 3B), phase (C), or frequency (D). Together these findings indicate that each eye could independently track the visual stimuli. Compared with pseudowhole field stimulation, both eyes exhibited a gain decrease when the stimulus differed between the planetariums, suggesting a binocular rivalry to exist in the central visual pathways. Differences in planetarium amplitude resulted in large differences in eye velocity between the stimulated (left) and stationary (right) eye (Fig. 3B). Significant differences were not observed in gain when either the left or right planetarium was stationary (P > 0.05), so the gain and phase data were pooled. The results of the monocular optokinetic stimulation experiments are summarized in Table 2. Disparity in eye velocity was due to independent optokinetic tracking and not gain suppression in one eye because contralateral eye occlusion exhibited similar eye velocity and phase differences (n = 4).
Table 2.
Monocular optokinetic reflex
| Stimulus | Gain (Normalized) | Phase, ° |
|---|---|---|
| Amplitude (n = 45) | 0.66 ± 0.12 | 9.5 ± 6.6 |
| 0.15 ± 0.07 (<0.0001) | 16.6 ± 18.7 (0.003) | |
| Occulsion (n = 4) | 0.67 ± 0.10 | 15.3 ± 4.9 |
| 0.21 ± 0.13 (0.01) | 28.0 ± 9.7 (0.11) | |
| 0.41 ± 0.12 | 109.5 ± 132.6 | |
| (n = 12) | 0.36 ± 0.12 (>0.2) | 1.3 ± 12.9 (0.018) |
| Phase (n = 20) | 0.47 ± 0.20 | 4.2 ± 6.5 |
| (n = 8) | 0.42 ± 0.10 (>0.2) | 1.4 ± 7.7 (0.19) |
P values in parentheses.
In the majority of experiments (12/20), goldfish followed phase lagged stimuli moving in equal and opposite directions with similar eye velocities (Fig. 3C) but with large phase differences during both convergence and divergence (arrows) in either half of the oculomotor range. The eyes also independently followed planetariums rotated at different frequencies (n = 3; Fig. 3D).
HPNI activity during monocular OKR
HPNI activity was recorded when the motion of each eye was driven by different optokinetic stimulation. Some HPNI neurons that were considered conjugate due to their high collinearity during scanning (Fig. 3A), now clearly encoded the ipsilateral eye during monocular OKR as collinearity decreased (Fig. 3, B–D, FR vs. Pos plots). During phase lag experiments, neuronal modulation correlated with slow phase movement of the left eye (Fig. 3C, red arrow). Velocity dependence of neuronal firing rate was clear, but saccadic sensitivity was not found (central region; Fig. 3C, FR). Monocularity was observed in the left eye position versus firing rate plot displaying a linear band and the right eye a cloud of points with greater vertical spread. Even in this monocular stimulus paradigm, a large covariation existed between eye positions due to saccadic conjugacy. The ipsilateral sensitivity of this HPNI neuron was most apparent during variations in either stimulus amplitude (Fig. 3B) or frequency (D).
Twenty-three position sensitive neurons were recorded during monocular OKR. Fifty-seven percent (13/23) of the neurons were ipsilateral sensitive and 39% of the neurons (9/23) were conjugate sensitive. Surprisingly, only one contralateral-sensitive neuron (4%) was found. When both scanning and stimulus evoked behaviors were recorded (n = 16), some neurons that were conjugate during scanning became ipsilateral during monocular OKR. By contrast, neurons that were determined to be ipsilateral during scanning were never found to be conjugate during monocular OKR.
Conjugate versus ipsilateral HPNI neurons during OKR
The average position sensitivity of ipsilateral HPNI neurons was larger than conjugate neurons [1.32 ± 0.58 vs. 1.02 ± 0.46 (spike/s)/°, P = 0.188]. However, the velocity sensitivity of conjugate neurons was larger than ipsilateral neurons [1.98 ± 1.42 1.11 ± 0.96 (spike /s) per °/s, P = 0.102]. Average sensitivity was not significantly different in either case due to the large range in values within each population of neurons.
When analyzed by position ratios, the ipsilateral neuron average (0.822 ± 0.0246) significantly differed from that of the conjugate neurons (0.147 ± 0.825, P = 0.01). The nine HPNI neurons determined to be conjugate by correlation analysis were also analyzed using position ratios. Three were found to be contralateral sensitive (ratio < −0.3), four were ipsilateral sensitive (ratio >0.3), and two were conjugate-sensitive. Eleven of the 13 neurons determined to be ipsilateral by correlation analysis had a position ratio >0.6. The average velocity ratio was not significantly different between conjugate (0.45 ± 0.49) and ipsilateral (0.70 ± 0.35 P = 0.189) neurons.
Neurons were calculated to be ipsilateral (69%), contralateral (17%), or conjugate (13%) using the multiple linear regression method of King and Zhou. Of the 16 neurons that were recorded during both scanning and monocular OKR, 9 changed their monocular preference. Three of the neurons correlated with one eye during scanning switched to the opposite eye during monocular optokinetic stimulation. Three of the neurons that were conjugate during spontaneous scanning appeared as monocular during monocular optokinetic evoked behaviors. In addition, three of the neurons that were monocular during spontaneous scanning were determined to be conjugate during optokinetically evoked behaviors. The change in eye preference and loss of monocularity observed when position and velocity ratios were used to determine monocularity questions this method's appropriateness to delineate eye sensitivity preference of HPNI neurons in goldfish.
Monocularity of time constant modification
In six of eight experiments, the time constant for fixation stability could be modified to monocular instability as measured in the absence of visual feedback. Monocular time constant plasticity was determined to have been successful when either: a statistical difference occurred in the ipsilateral eye time constant versus control while the contralateral eye was not significantly different from control or when a significant difference occurred in the time constants of both eyes versus their respective controls and between the right versus left eye after instability plasticity (Figs. 4A and 5A).
In five experiments, the time constant changes in the untrained eye (LE) were asymmetrical as there was a greater drift on the nasal side of the range (right) as compared with the temporal side (left; Fig. 4A). This difference was observed in the P-V plot in which different slopes could be drawn for left eye nasal (red) and temporal eye (gray) positions. The direction of conjugate changes in time constant corresponded to the position and velocity “on” direction of the HPNI neurons ipsilateral to the trained eye (Fig. 6A). The average changes in time constant were significant between the eyes and are summarized for all experiments in Table 3.
Fig. 6.
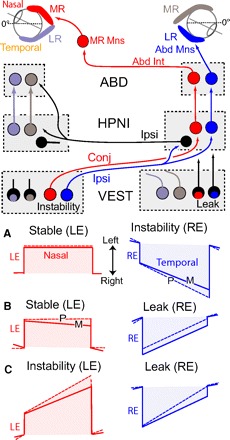
Schematic showing monocular fixation plasticity results and hypothesized neuronal connections. A–C: P (performance, dashed lines) and m (memory, solid lines) vignettes of monocular changes in time constant after 4 h visual training. Color-coding associates fixations in the LE and RE with the medial and lateral rectus eye muscles that can then be correlated with eye specific neurons in the vestibular nucleus (VEST), Area I (HPNI), and abducens (ABD) nuclei. A: RE instability and LE stability. B: RE leak and LE stability. C: RE leak and LE instability. In the diagram, monocular signaling is suggested for ipsilateral and conjugate eye movements with excitation (instability) originating from the left vestibular nucleus and inhibition (leak) from the right vestibular nucleus. Right inhibitory HPNI neurons (black) are shown to coordinate the proposed nasal and temporal eye position integrators. For simplification, connections are only shown to and from the right HPNI with direct vestibuloocular pathways to MR and Abd Mns omitted (see Supplemental Fig. S1A).
Table 3.
Time constant plasticity
| Behavior | Control, s | Training, s | Memory (P = LE vs RE) | Pτ | ||
|---|---|---|---|---|---|---|
| LE Instability | (n = 8) | 32.2 ± 14.8 | −3.7 ± 1.4 | −7.8 ± 5.5 | (0.005) | 0.002 |
| RE Stability | 31.2 ± 15.2 | 30.0 ± 11.1 | −19.0 ± 18.0 | 0.03 | ||
| LE Instability | (n = 3) | 96.8 ± 63.9 | −4.2 ± 1.9 | −8.1 ± 6.8 | (0.14) | 0.02 |
| RE Occlusion | 110 ± 82.5 | −20.8 ± 24.6 | −20.2 ± 11.2 | >0.2 | ||
| LE Leak | (n = 3) | −77.5 ± 26.2 | 1.96 ± 11.9 | 7.9 ± 4.0 | (0.03) | 0.04 |
| RE Stability | 24.1 ± 9.6 | 50.4 ± 10.4 | 15.8 ± 6.8 | >0.2 | ||
| LE Instability | (n = 3) | 283 ± 228 | −12.2 ± 12.9 | −19.2 ± 9.7 | (0.04) | 0.14 |
| RE Leak | −113 ± 85.2 | 4.7 ± 10.2 | 23.8 ± 13.0 | 0.18 |
P values in parentheses.
Time constants could be retrained to stability (P ≤ 0.01; n = 3) with no significant difference observed in left versus right eye. The average time constant after plasticity changed from –9.09 ± 5.3 s (trained) and −20.4 ± 12.6 s (untrained) to –35.9 ± 11.8 s (trained) and −178.5 ± 123.0 s (untrained). In three experiments, one eye was trained to nasal and temporal instability and the contralateral eye occluded. The training paradigm was verified by the absence of OKR in either eye when the stimulus was only shown to the occluded eye. In two cases, the unoccluded eye trained to instability (P < 0.01), implying that the changes in time constant were independent for each eye (both temporal and nasal). In addition, maintained presence of stability in the untrained eye could not be due to visual feedback.
Leak and instability leak
In two experiments, one eye was trained to both temporal and nasal leak, and the time constant significantly differed from initial stability (P < 0.01) without a statistical change in the contralateral eye time constant when spontaneous scanning was monitored in the dark (Figs. 4B and 5B). However, in a third experiment, both eyes exhibited a significant reduction in their time constants. After leak training, drift of the nontrained eye was more likely to occur on the temporal half of the oculomotor range rather than the nasal side as seen in instability training (Fig. 4B, left eye left). Nevertheless, the direction of visual slip for the asymmetrical changes in time constant was identical for instability and leak as illustrated schematically in Fig. 6.
In another series of plasticity experiments, both the left and right eye time constants were trained in opposite directions. One eye was trained to instability in both the temporal and nasal half of the oculomotor range and the other eye to leak over a similar range (Figs. 4C and 5C). The time constant was modified to a greater degree in the eye triggering the training stimulus in two of the three experiments with some memory observed in the contralateral (right eye) when the drift direction was nasal (leftward). During performance of the learning task, both eyes followed their respective stimuli (Fig. 4C), yet in all experiments, only one eye exhibited both temporal and nasal memory. Thus monocular time constant plasticity appeared to be dependent on the direction of visual slip rather than on the direction of time constant change. Nasal to temporal slip was conjugately encoded and temporal to nasal slip monocularly encoded as illustrated in Fig. 6. Because behavioral time constants were monocularly modified, parallel changes are assumed to have occurred in the firing rates of HPNI neurons. As such, monocular integrator plasticity then corroborates separate monocularly encoding populations of neurons within HPNI.
Midline interruption of HPNI
To determine whether the left and right side neural integrators function independently and encode the position of the eyes separately, oculomotor function was investigated in the absence of HPNI midline connections. Although this perturbation does not provide direct evidence for the role of inhibitory HPNI neurons, the ability to observe oculomotor behaviors within minutes of the lesion allows inferences about the possible roles of the crossed connection. Behavioral records (Fig. 7, D–H) and histology (A) are shown following an acute midline lesion completely severing bilateral HPNI. The naïve time constant of over 20 s (Fig. 7D) remained unaltered 20 min after the lesion (E); however, a slight instability was observed 10 min after the lesion (Fig. 7, D vs. E). Similarly, in this animal, low frequency VOR, 0.125 Hz 15.7°/s peak velocity, was minimally affected showing a small gain decrease and negligible phase shift of <3° 20 min after the lesion (Fig. 7, G and H).
Fig. 7.
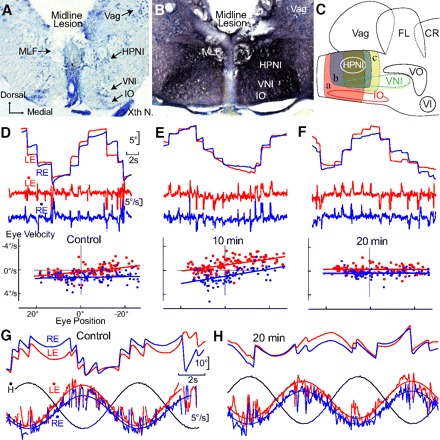
Integrator stability and time constant plasticity after acute midline lesion. A–C: anatomy of midline lesions between the bilateral HPNI nuclei. A: coronal photomicrograph showing an acute midline lesion encompassing ∼75% of the dorsal-ventral depth for behavior with P-V plots shown in D–H. B: 15 days post midline lesion photomicrograph showing extensive biocytin labeled axons in the medial longitudinal fasiculus (MLF) and reticular formation after spinal cord label. C: schematic illustrating 3 groups (a–c) of midline lesions (8 cases). D–F: P-V plots of eye position holding before (A) and 10 (B) and 30 min (C) after the midline lesion illustrated in A. Control LE τ was −127.8 s and RE −170.3 s in (A), −19.8 s and −16.6s in B, and 1,428.5 s and −384.3 s in C, respectively. G and H: vestibuloocular reflex (VOR) at 0.125 Hz and 15.7°/s before and 20 min after the lesion with least square regression fits of eye velocity. Head velocity shown in black. Gains changed minimally from (LE) 0.90 to 0.80 and (RE) 0.73 to 0.71 with negligible shifts in phase (LE) 3.2° to 2.3° and (RE) 0.2° to 3.0°. VNI, velocity neural integrator; FL, facial lobe; Vag, vagal lobe; VO, descending octaval nucleus; Xth N, vagus nerve; IO, inferior olive.
Chronic effects after the midline lesion were analyzed in 15 cases in which the lesion was determined adequate by histological criteria (Supplemental Fig. S3). Eye position holding remained an order of magnitude above the oculomotor plant time constant of 1 s. Occasionally the time constant became unstable chronically (Supplemental Fig. 3, D vs. F).
Time constant plasticity after midline lesion
Using binocular stimulation, integrator time constants could be successfully modified to either instability or leak during the first week as well as anytime examined thereafter (Fig. 8D). Average stability during the first week after lesion was found to be 22.9 ± 16.0 s (LE n = 8) and 40.2 ± 28.4 s (RE n = 9). Plasticity toward instability (n = 4) altered time constants to an average of −4.6 ± 34 s and −5.5 ± 30.1 (as in Fig. 8D). When time constants were modified toward leak (n = 5) by visual feedback, the average time constants were 13.9 ± 16.1 and 7.4 ± 3.4 s (Fig. 8D).
Fig. 8.
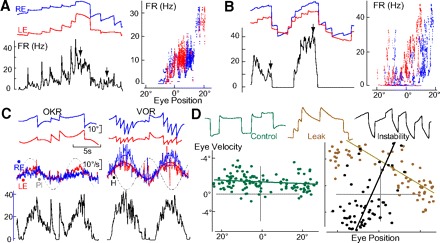
HPNI persistence and plasticity without commissure. A and B: FR (Hz) eye position records, and FR versus eye position plots during spontaneous fixation in darkness for 2 ipsilateral-eye related HPNI neurons. A: LE FR coefficients were 0.84 (spike/s)/° and 0.23 (spike/s) per °/s, (r = 0.81). RE FR constants were 0.72 (spike/s)/° and 0.33 spike/s [per] °/s, (r = 0.58). B: LE FR 1.45 (spike/s)/° and 0.63 (spike/s) per °/s, (r = 0.76) with RE FR 1.18 (spike/s)/° and 0.17 (spike/s) per °/s, (r = 0.49). C: HPNI modulation in A by head and eye velocity at 0.125 Hz during OKR and VOR. D: P-V plots and behavior 5 days after a midline lesion (c in Fig. 7C) of RE in darkness (control: 79.4 s), 2 h after leak training (5.3 s), and 4 h after instability training (−1.5 s).
In five fish, the average time constants 2–3 wk after lesion were LE, 95.2 ± 81.5 s, and RE, 434.8 ± 411.5 s. Four animals were trained toward instability, and a robust performance was observed in all cases. In three experiments, the time constant during memory was significant (P ≤ 0.01) when compared with initial stability. The average time constant during memory was −5.4 ± 43.4 s for the left eye and −4.1 ± 22.6 s for the right eye. When subsequently re-trained toward leak (n = 3), the average time constants were modified from −4.2 ± 8.1 to 14.2 ± 42.7 s (LE, P = 0.018) and −3.3 ± 7.4 to 13.4 ± 21.5 s (RE, P = 0.6). The ability to change the fixation time constant in the nasal and temporal oculomotor ranges for both eyes implies that midline inhibitory pathways are not necessary for time constant plasticity.
HPNI activity after midline lesion
The presence of continued stability at all times after midline lesion implied but did not prove that HPNI was functional. HPNI neurons were recorded after midline lesion (n = 9) to determine if firing rates remained correlated with the behavioral effects. Five neurons exhibited correlation coefficients >0.7 and were concluded to be eye position sensitive. HPNI neuronal activity could be observed after midline lesion in the absence of visual feedback as shown in Fig. 8, A and B. The left-sided HPNI neurons demonstrated multi-stable persistent activity implying an intact horizontal eye velocity-to-position integration. Activity of the neuron shown in Fig. 8A was modulated during VOR and OKR (C), but the firing rate appeared to be dominated more by eye/head velocity. The velocity dominance of the firing rates was largely due to a limited oculomotor range available to produce the compensatory response. Together the results after midline separation suggest that eye position is independently encoded in HPNI for each eye.
DISCUSSION
Monocular versus congugate HPNI
The general issue addressed in this study was conjugate versus monocular signal processing in the goldfish eye position neural integrator during versional eye movements. Seminal papers showed that monocular and convergence-like eye movements occurred spontaneously (Easter 1971, 1972; Hermann and Constantine 1971), but none simultaneously described neuronal activity, monocular learning, and memory During scanning saccades and fixations, the eyes exhibit opposite drift directions (Fig. 1), supporting a hypothesis that each HPNI may be composed of two independent populations of neurons, controlling the ipsilateral eye in the temporal half of the oculomotor range (Fig. 6, LR) and the contralateral eye in the nasal half of the oculomotor range (Fig. 6, MR). However, because all previously recorded HPNI neurons in “naïve” goldfish demonstrated a “leaky” persistent activity without the appearance of unstable firing rates (Aksay et al. 2000; Pastor et al. 1994), nasal instability cannot be explained solely by HPNI activity. A parsimonious explanation would be midbrain vergence signals superimposed on either the abducens internuclear neurons or directly onto the medial rectus motoneurons independent of HPNI.
Monocular eye velocity-to-position integrator persistence
Saccades and ensuing fixations are generated by burst neurons projecting both directly to motoneurons and HPNI neurons with minimal, if any, connections through the vestibular nuclei (Green et al. 1997; Pastor et al. 1994). Both anatomical and electrophysiological evidence suggests that pretectal signals are largely relayed through hindbrain vestibular pathways (Allum et al. 1976; Dichgans et al. 1973). Because preferred axes were not segregated in the APT, both directions of horizontal eye motion, temporal and nasal, can be elicited with monocular visual stimuli as illustrated in Supplemental Fig. S1A.
HPNI firing rates during monocular saccades were first reported to mimic ipsilateral eye position, suggesting some HPNI neurons were distinctly monocular (Pastor et al. 1994). Herein systematically testing for monocularity using disconjugate visual stimulation along with correlation analysis (Figs. 1–3) proved that assertion to be true, but an equally distinct, comparably sized population of contralateral neurons was not found. A high degree of monocularity exists in at least one premotor pathway as >50% of the HPNI neurons were significantly related to the ipsilateral eye. The few contralateral HPNI neurons (∼4%) and many more conjugate (∼36%) likely are accounted for largely by saccades that were conjugate in direction and timing along with firing rates that were independently correlated with the left and right eye position and velocity.
The lower percentage of ipsilateral HPNI neurons seen during scanning saccadic and fixation behavior as compared with monocular OKR (Fig. 3, B–D) could be due to a different extent of monocularity in each of the premotor HPNI pathways. Alternatively, the HPNI neurons may encode a conjugate eye position, but monocular eye velocity sensitivity. Due to the lack of significant post saccadic eye velocity during fixations, the velocity sensitivity of the HPNI neurons would only have been revealed during monocular OKR. HPNI neurons with a conjugate eye position and ipsilateral eye velocity sensitivity would have been classified as conjugate during scanning but as ipsilateral during monocular OKR. Although the neurons were grouped into three discrete classes (ipsilateral, conjugate, and contralateral), the actual ranges of sensitivity formed a continuum from monocular through conjugate encoding. Given the conservative analytical methods used in this study, the percentage of conjugate HPNI neurons was, if anything, overestimated in respect to separate populations of ipsilateral and contralateral neurons. In spite of this caveat, the clear conclusion is that two distinct populations of HPNI neurons are present within HPNI. One population is quite closely related to the ipsilateral eye, and a second is more closely related to the contralateral eye albeit by conjugate, not selective contralateral, eye position signaling.
Comparison of analysis methods in goldfish with mammals
The analytical methods used to determine monocularity of HPNI neurons differed from studies that utilized regression coefficients (Sylvestre et al. 2003; Zhou and King 1996, 1998). In better agreement with the literature, analysis of HPNI neurons using multiple linear regression resulted in a greater percentage of ipsilateral (69%) and contralateral (16%) neurons than by correlation coefficient analysis. Even so, the majority of monocular neurons were ipsilateral eye sensitive during monocular saccades and fixations as opposed to being equally distributed between ipsi- and contralateral populations (Sylvestre et al. 2003; Zhou and King 1996, 1998). In goldfish, the high degree of collinearity in eye position regression analysis during both spontaneous scanning and monocular visual tracking made computation of the ipsi- and contralateral populations by regression coefficients less useful. The methodological constraints were minimized in mammals because the spontaneous firing rates of the neurons were higher and over a far greater oculomotor range. In goldfish, thresholds for many HPNI neurons were closely aligned at the center of the oculomotor range that reduced the extent over which neuron monocularity could be measured (Aksay et al. 2000).
Monocularity of time constant modification
The results of the monocular plasticity experiments changing differentially the time constant of drift between the two eyes are highly consistent with the presence of two distinct populations of Area I neurons (Fig. 4). When trained to oppositely directed eye position drifts (leak-instability), the monocular time constant measurements suggested separate encoding of eye position sensitivity within HPNI (Figs. 4, C and F, and 5C). These findings are comparable to, and consistent with, the results from either monocular surgical muscle weakening or retinal disparity training in primates (Lemij and Collewijn 1991a,b; Oohira and Zee 1992; Viirre et al. 1988).
Because both the learning and memory phases of monocular instability were similar when one eye was either occluded (Fig. 4) or viewed a stationary pattern (Fig. 5), independent changes in right eye and left eye time constants were not due to central binocular suppression of plasticity but rather the existence of eye-specific monocular visual and oculomotor hindbrain pathways. Although clear differences could be observed in the time constants between the two eyes after monocular plasticity paradigms, there were limitations to the extent which monocularity could be obtained. Consistent with the presence of conjugate HPNI neurons, the time constant was detuned in the “untrained” eye to a greater extent, as tested during memory, than during either the instability or leak training (Fig. 4, A and B). During training to an oppositely directed time constant for each eye, bidirectional behavior was evident in both eyes; however, memory was found to be bidirectional in only one eye (Fig. 4C). Because no error signal would be present during memory, the absence of a monocular time constant modification could be due to circuitry constraints within either the pretectum or hindbrain. In the latter case, this would likely occur through vestibulocerebellar pathways during acquisition of the modified behaviors, which would, then become apparent as a conjugate eye position plasticity.
The presence of both monocular and conjugate time constant modification during integrator plasticity is consistent with the presence of monocular (Figs. 2 and 3, B–D) and conjugate HPNI neurons (Figs. 1 and 3A). Conjugate time constant modification tended to be stronger in that part of the oculomotor range in which nasal-to-temporal drift was imposed in the training eye. Conjugate training was direction dependent rather than orbital eye position dependent (Fig. 5), indicating that conjugate modifications occurred “upstream” of HPNI. This finding is in agreement with the suggestion that time constant plasticity includes pathways through the vestibular nucleus as well as the cerebellum (Beck et al. 2006; Straka et al. 2006). An alternative, but far less likely explanation, is that conjugate behaviors result from monocular training paradigms accentuating a natural nasal drift observed during goldfish fixation.
Based on the anatomy and physiology of the known vestibular connections to HPNI, it can be suggested that instability is predominantly entrained through excitatory vestibular neurons projecting to the contralateral HPNI, whereas leak is largely produced through the ipsilateral inhibitory projections (Fig. 6). Conjugate behaviors would appear to be mediated by the vestibular commissural neurons.
Role of HPNI interconnections and persistence
Most early models of eye velocity-to-position integrator function postulated feedback inhibition between bilateral HPNI neurons as vital for production of neuronal persistence and thus eye position stability (Arnold and Robinson 1997; Cannon et al. 1983; Cova and Galiana 1995). Accordingly, an interruption of this connection would cause a large decrease in the time constant of eye position holding. Midline lesions between the eye velocity-to-position integrator in mammals yielded conflicting results, ranging from negligible effects (Cheron et al. 1986a,b) to a drastic reduction in eye time constant stability (Anastasio and Robinson 1991; Arnold and Robinson 1997). Anecdotally, eye position holding was noted to be reduced, but still present, in goldfish when either the midline was severed or HPNI unilaterally inactivated (Pastor et al. 1994).
A commissural mediated integrator stability was supported by pharmacological studies because unilateral inactivation caused bilateral leak in the eye position time constants (Arnold et al. 1999; Mettens et al. 1994a–c). These studies were thus more consistent with eye movements encoded for both eyes by a bilateral interaction between HPNI; however, partial ipsilateral inactivation of the goldfish eye velocity-to-position integrator (Area I) affected high firing rates while contralateral silencing influenced low firing rates suggesting that the two integrators function semi-autonomously (Aksay et al. 2007). This finding also provided indirect evidence suggesting left and right eye positions were encoded separately within the hindbrain. As demonstrated in this study following midline lesions, persistence and time constant plasticity are not essential for HPNI monocularity (Fig. 8).
Binocular time constant stability and plasticity were observed within 20 min after a midline lesion and when tested ≤3 mo after midline lesion (Supplemental Fig. S3). In a majority of cases (7/9), stability decreased slightly toward leak, with the time constant remaining >10 s, when measured 20–30 min after midline lesion. Pharmacological inactivation of HPNI demonstrated that the time constant of the oculomotor plant was between 1 and 2 s (Aksay et al. 2001; Pastor et al. 1994). After midline lesion, the time constant of eye position holding remained an order of magnitude above the upper limit of the plant time constant (>10 s). Because long time constants are assumed, a priori, to be a behavioral indicator of persistence in the eye position integrator (Major and Tank 2004), midline connections can be concluded to contribute minimally, if at all, to fixation stability and neuronal persistence, supporting the finding that neuronal plasticity is encoded separately for each eye within each Area I (HPNI) nucleus. The combined results of the monocular fixation plasticity experiments are consistent with the presence of monocular and conjugate pathways but not with the observations from either chameleons or sandlances said to exhibit complete independence of eye movements (Ott 2001; Pettigrew et al. 1999).
Role of abducens internuclear neurons in conjugate eye movements
Based on exclusive connections to medial rectus motoneurons, the role of abducens internuclear neurons was envisioned as an essential neuronal link to ensure conjugacy between temporal movements of the ipsilateral eye and nasal movements of the contralateral eye. Afferent inputs from the vestibular and saccadic systems were envisioned to be shared equally between the abducens motoneurons and abducens internuclear neurons (Baker and Highstein 1975; Delgado-Garcia et al. 1986; Fuchs et al. 1988). This conjugate view was strengthened by the finding that firing rates of abducens internuclear neurons were similar to those of the abducens motoneurons in primates during vergence-induced disconjugate eye movements (Gamlin et al. 1989). However, cat abducens internuclear neurons were found to be contralateral eye motion sensitive as they responded during monocular saccades/fixations of the contralateral eye (Delgado-Garcia et al. 1977, 1986). Goldfish abducens internuclear neurons appear to be targeted by both excitatory and inhibitory HPNI neurons (Fig. 6). Thus any time constant change in nasal eye motion should be reflected and encoded through the abducens internuclear neurons. If ipsilateral HPNI neurons projected equally to abducens motoneurons and internuclear neurons, the latter would receive an inappropriate signal during monocular behavior. Because time constant monocularity is stronger during nasal than temporal instability, abducens internuclear neurons cannot be viewed as part of an “obligatory conjugacy” mechanism as they must encode monocular time constant changes.
In many mammalian species, abducens motoneurons and internuclear neurons are intimately intermingled suggestive of a structurally determined conjugacy. (McCrea et al. 1986). By contrast, in goldfish the two nuclei are anatomically separated (Cabrera et al. 1992) making shared afferent inputs between the motoneurons and internuclear neurons more unlikely. In addition this arrangement provides a good model system to easily test the hypothesis that abducens internuclear neurons preferentially encode contralateral eye motion through use of monocular versional stimuli.
Function of HPNI in goldfish oculomotor behaviors
The monocular behaviors observed in goldfish indicate that abducens motoneurons and internuclear neurons receive different amounts, hence sources, of vestibular and HPNI input. The monocularity results in goldfish imply ipsilateral temporal and contralateral nasal eye movements are synchronous in timing and direction during version due to a common input of excitatory/inhibitory saccadic burst neurons, but downstream eye position holding as initiated by different saccadic amplitudes is separately encoded for the left and right eyes. We conclude that horizontal eye movement pathways may have been inherently monocular in developmental design from their evolutionary appearance in vertebrates more primitive than goldfish. By and large, this structural plan has been retained to some extent in nearly all species including mammals (McConville et al. 1994; Sylvestre et al. 2003; Zhou and King 1996, 1998).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Aksay E, Baker R, Seung HS, Tank DW. Anatomy and discharge properties of pre-motor neurons in the goldfish medulla that have eye-position signals during fixations. J Neurophysiol 84: 1035–1049, 2000. [DOI] [PubMed] [Google Scholar]
- Aksay E, Gamkrelidze G, Seung HS, Baker R, Tank DW. In vivo intracellular recording and perturbation of persistent activity in a neural integrator. Nat Neurosci 4: 184–193, 2001. [DOI] [PubMed] [Google Scholar]
- Aksay E, Major G, Goldman MS, Baker R, Seung HS, Tank DW. History dependence of rate covariation between neurons during persistent activity in an oculomotor integrator. Cereb Cortex 13: 1173–1184, 2003. [DOI] [PubMed] [Google Scholar]
- Aksay E, Olasagasti I, Mensh BD, Baker R, Goldman MS, Tank DW. Functional dissection of circuitry in a neural integrator. Nat Neurosci 10: 494–504, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allum JH, Graf W, Dichgans J, Schmidt CL. Visual-vestibular interactions in the vestibular nuclei of the goldfish. Exp Brain Res 26: 463–485, 1976. [DOI] [PubMed] [Google Scholar]
- Anastasio TJ, Robinson DA. Failure of the oculomotor neural integrator from a discrete midline lesion between the abducens nuclei in the monkey. Neurosci Lett 127: 82–86, 1991. [DOI] [PubMed] [Google Scholar]
- Arnold DB, Robinson DA. The oculomotor integrator: testing of a neural network model. Exp Brain Res 113: 57–74, 1997. [DOI] [PubMed] [Google Scholar]
- Arnold DB, Robinson DA, Leigh RJ. Nystagmus induced by pharmacological inactivation of the brainstem ocular motor integrator in monkey. Vision Res 39: 4286–4295, 1999. [DOI] [PubMed] [Google Scholar]
- Averbuch-Heller L, Lewis RF, Zee DS. Disconjugate adaptation of saccades: contribution of binocular and monocular mechanisms. Vision Res 39: 341–352, 1999. [DOI] [PubMed] [Google Scholar]
- Baker R, Highstein SM. Physiological identification of interneurons and motoneurons in the abducens nucleus. Brain Res 91: 292–298, 1975. [DOI] [PubMed] [Google Scholar]
- Beck JC, Rothnie P, Straka H, Wearne SL, Baker R. Precerebellar hindbrain neurons encoding eye velocity during vestibular and optokinetic behavior in the goldfish. J Neurophysiol 96: 1370–1382, 2006. [DOI] [PubMed] [Google Scholar]
- Cabrera B, Torres B, Pasaro R, Pastor AM, Delgado-Garcia JM. A morphological study of abducens nucleus motoneurons and internuclear neurons in the goldfish (Carassius auratus). Brain Res Bull 28: 137–144, 1992. [DOI] [PubMed] [Google Scholar]
- Cannon SC, Robinson DA, Shamma S. A proposed neural network for the integrator of the oculomotor system. Biol Cybern 49: 127–136, 1983. [DOI] [PubMed] [Google Scholar]
- Cheron G, Gillis P, Godaux E. Lesions in the cat prepositus complex: effects on the optokinetic system. J Physiol 372: 95–111, 1986a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheron G, Godaux E, Laune JM, Vanderkelen B. Lesions in the cat prepositus complex: effects on the vestibulo-ocular reflex and saccades. J Physiol 372: 75–94, 1986b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran SL, Dieringer N, Precht W. Basic optokinetic-ocular reflex pathways in the frog. J Neurosci 4: 43–57, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cova A, Galiana HL. Providing distinct vergence and version dynamics in a bilateral oculomotor network. Vision Res 35: 3359–3371, 1995. [DOI] [PubMed] [Google Scholar]
- Delgado-Garcia J, Baker R, Highstein SM. The activity of internuclear neurons identified within the abducens nucleus of the alert cat. In: Control of Gaze by Brainstem Neurons, edited by Baker R, Berthoz A. Amsterdam: Elsevier/North-Holland Biomedical Press, 1977, p. 291–300. [Google Scholar]
- Delgado-Garcia JM, del Pozo F, Baker R. Behavior of neurons in the abducens nucleus of the alert cat. II. Internuclear neurons. Neuroscience 17: 953–973, 1986. [DOI] [PubMed] [Google Scholar]
- Dichgans J, Schmidt CL, Graf W. Visual input improves the speedometer function of the vestibular nuclei in the goldfish. Exp Brain Res 18: 319–322, 1973. [DOI] [PubMed] [Google Scholar]
- Easter SS., Jr Spontaneous eye movements in restrained goldfish. Vision Res 11: 333–342, 1971. [DOI] [PubMed] [Google Scholar]
- Easter SS., Jr Pursuit eye movements in goldfish (Carassius auratus). Vision Res 12: 673–688, 1972. [DOI] [PubMed] [Google Scholar]
- Fuchs AF, Scudder CA, Kaneko CR. Discharge patterns and recruitment order of identified motoneurons and internuclear neurons in the monkey abducens nucleus. J Neurophysiol 60: 1874–1895, 1988. [DOI] [PubMed] [Google Scholar]
- Gamlin PD, Gnadt JW, Mays LE. Abducens internuclear neurons carry an inappropriate signal for ocular convergence. J Neurophysiol 62: 70–81, 1989. [DOI] [PubMed] [Google Scholar]
- Green A, Suwa H, Baker R, Galiana H. Characterization of second order vestibular neurons related to horizontal eye movement in the goldfish. Soc Neurosci Abstr 23: 751, 1997. [Google Scholar]
- Hering E, Bridgeman B, Stark L. The Theory of Binocular Vision. New York: Plenum, 1977. [Google Scholar]
- Hermann HT, Constantine MM. Eye movements in the goldfish. Vision Res 11: 313–331, 1971. [DOI] [PubMed] [Google Scholar]
- King WM, Zhou W. Neural basis of disjunctive eye movements. Ann NY Acad Sci 956: 273–283, 2002. [DOI] [PubMed] [Google Scholar]
- Klar M, Hoffmann KP. Visual direction-selective neurons in the pretectum of the rainbow trout. Brain Res Bull 57: 431–433, 2002. [DOI] [PubMed] [Google Scholar]
- Lemij HG, Collewijn H. Long-term nonconjugate adaptation of human saccades to anisometropic spectacles. Vision Res 31: 1939–1954, 1991a. [DOI] [PubMed] [Google Scholar]
- Lemij HG, Collewijn H. Short-term nonconjugate adaptation of human saccades to anisometropic spectacles. Vision Res 31: 1955–1966, 1991b. [DOI] [PubMed] [Google Scholar]
- Lemij HG, Collewijn H. Nonconjugate adaptation of human saccades to anisometropic spectacles: meridian-specificity. Vision Res 32: 453–464, 1992. [DOI] [PubMed] [Google Scholar]
- Major G, Baker R, Aksay E, Mensh B, Seung HS, Tank DW. Plasticity and tuning by visual feedback of the stability of a neural integrator. Proc Natl Acad Sci USA 101: 7739–7744, 2004a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major G, Baker R, Aksay E, Seung HS, Tank DW. Plasticity and tuning of the time course of analog persistent firing in a neural integrator. Proc Natl Acad Sci USA 101: 7745–7750, 2004b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major G, Tank D. Persistent neural activity: prevalence and mechanisms. Curr Opin Neurobiol 14: 675–684, 2004. [DOI] [PubMed] [Google Scholar]
- Marsh E, Baker R. Normal and adapted visuooculomotor reflexes in goldfish. J Neurophysiol 77: 1099–1118, 1997. [DOI] [PubMed] [Google Scholar]
- Masseck OA, Hoffmann KP. Question of reference frames: visual direction-selective neurons in the accessory optic system of goldfish. J Neurophysiol 102: 2781–2789, 2009. [DOI] [PubMed] [Google Scholar]
- McConville K, Tomlinson RD, King WM, Paige G, Na EQ. Eye position signals in the vestibular nuclei: consequences for models of integrator function. J Vestib Res 4: 391–400, 1994. [PubMed] [Google Scholar]
- McCrea RA, Horn AK. Nucleus prepositus. Prog Brain Res 151: 205–230, 2005. [DOI] [PubMed] [Google Scholar]
- McCrea RA, Strassman A, Highstein SM. Morphology and physiology of abducens motoneurons and internuclear neurons intracellularly injected with horseradish peroxidase in alert squirrel monkeys. J Comp Neurol 243: 291–308, 1986. [DOI] [PubMed] [Google Scholar]
- Mensh BD, Aksay E, Lee DD, Seung HS, Tank DW. Spontaneous eye movements in goldfish: oculomotor integrator performance, plasticity, and dependence on visual feedback. Vision Res 44: 711–726, 2004. [DOI] [PubMed] [Google Scholar]
- Mettens P, Cheron G, Godaux E. NMDA receptors are involved in temporal integration in the oculomotor system of the cat. Neuroreport 5: 1333–1336, 1994a. [PubMed] [Google Scholar]
- Mettens P, Cheron G, Godaux E. Role of the vestibular commissure in gaze-holding in the cat: a pharmacological evaluation. Neuroreport 5: 1421–1424, 1994b. [DOI] [PubMed] [Google Scholar]
- Mettens P, Godaux E, Cheron G, Galiana HL. Effect of muscimol microinjections into the prepositus hypoglossi and the medial vestibular nuclei on cat eye movements. J Neurophysiol 72: 785–802, 1994c. [DOI] [PubMed] [Google Scholar]
- Oohira A, Zee DS. Disconjugate ocular motor adaptation in rhesus monkey. Vision Res 32: 489–497, 1992. [DOI] [PubMed] [Google Scholar]
- Ott M. Chameleons have independent eye movements but synchronise both eyes during saccadic prey tracking. Exp Brain Res 139: 173–179, 2001. [DOI] [PubMed] [Google Scholar]
- Pastor AM, De la Cruz RR, Baker R. Eye position and eye velocity integrators reside in separate brain stem nuclei. Proc Natl Acad Sci USA 91: 807–811, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettigrew JD, Collin SP, Ott M. Convergence of specialised behaviour, eye movements and visual optics in the sandlance (Teleostei) and the chameleon (Reptilia). Curr Biol 9: 421–424, 1999. [DOI] [PubMed] [Google Scholar]
- Robinson DA. A method of measuring eye movement using a scleral search coil in a magnetic field. IEEE Trans Biomed Eng 10: 137–145, 1963. [DOI] [PubMed] [Google Scholar]
- Straka H, Beck JC, Pastor AM, Baker R. Morphology and physiology of the cerebellar vestibulolateral lobe pathways linked to oculomotor function in the goldfish. J Neurophysiol 96: 1963–1980, 2006. [DOI] [PubMed] [Google Scholar]
- Suwa H, Gilland E, Baker R. Segmental organization of vestibular and reticular projections to spinal and oculomotor nuclei in the zebrafish and goldfish. Biol Bull 191: 257–259, 1996. [DOI] [PubMed] [Google Scholar]
- Sylvestre PA, Choi JT, Cullen KE. Discharge dynamics of oculomotor neural integrator neurons during conjugate and disjunctive saccades and fixation. J Neurophysiol 90: 739–754, 2003. [DOI] [PubMed] [Google Scholar]
- Trevarthen C. Vision in fish: the origins of the visual frame for action in vertebrates. In: The Central Nervous System and Fish Behavior, edited by Ingle D. Chicago: University of Chicago Press, 1968, p. 61–94. [Google Scholar]
- Uchiyama H, Matsutani S, Ito H. Pretectum and accessory optic system in the filefish Navodon modestus (Balistidae, Teleostei) with special reference to visual projections to the cerebellum and oculomotor nuclei. Brain Behav Evol 31: 170–180, 1988. [DOI] [PubMed] [Google Scholar]
- Viirre E, Cadera W, Vilis T. Monocular adaptation of the saccadic system and vestibulo-ocular reflex. Invest Ophthalmol Vis Sci 29: 1339–1347, 1988. [PubMed] [Google Scholar]
- Von Helmholtz H. Treatise on Physiological Optics. New York: Dover, 1910. [Google Scholar]
- Weiser M, Pastor AM, Baker R. Monocular adaptive gain control of the vestibulo-ocular reflex in goldfish Carassius auratus. Soc Neurosci Abstr 15: 807, 1989. [Google Scholar]
- Zar JH. Biostatistical Analysis. Upper Saddle River, NJ: Prentice Hall, 1996. [Google Scholar]
- Zhou W, King WM. Ocular selectivity of units in oculomotor pathways. Ann NY Acad Sci 781: 724–728, 1996. [DOI] [PubMed] [Google Scholar]
- Zhou W, King WM. Premotor commands encode monocular eye movements. Nature 393: 692–695, 1998. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


