Abstract
To explore the influence of circadian rhythms on executive function during early adolescence, we administered a battery of executive function measures (including a Go-Nogo Task, the Iowa Gambling Task, a Self-ordered Pointing Task, and an Intra/Extradimensional Shift Task) to Morning-preference and Evening-preference participants (N = 80) between the ages of 11 and 14 years who were tested in the morning or afternoon. Significant Chronotype × Time of Day interactions (controlling for amount of sleep the previous night) revealed that adolescents tested at their optimal times of day performed better than those tested at their nonoptimal times. Implications for our understanding of physiological arousal, sleep, and executive function during adolescence are discussed.
Keywords: executive function, circadian rhythms, arousal, chronotype, synchrony
Physiological arousal, measured using a variety of biological and behavioral indices (e.g., core body temperature and sleep-wake cycles), rises and falls according to a regular circadian (daily) rhythm that is regulated by the suprachiasmatic nucleus via projections to the noradrenergic nucleus locus coeruleus (Aston-Jones, Chen, Zhu, & Oshinsky, 2001). Research has identified both individual differences and developmental changes in the character of this rhythm (e.g., Carskadon, Vieira, & Acebo, 1993; Ishihara, Honma, & Miyake, 1990; Kim, Dueker, Hasher, & Goldstein, 2002), which is influenced by both circadian timing and homeostatic sleep drive (e.g., Mongrain, Carrier, & Dumont, 2006).
Individual differences in circadian timing may be reliably and validly estimated in both adults and children using self-report measures in which participants are asked about when during the day they prefer to engage in various intellectual and physical activities (Carskadon et al., 1993; Horne & Östberg, 1976; Kim et al., 2002; Tankova, Adan, & Buela-Casal, 1994; Vitiello et al., 1986). A substantial literature has now accumulated establishing that young and elderly adults have different time of day preferences (i.e., chronotypes). Most young adults prefer afternoon or evening times, whereas most elderly adults prefer morning times (e.g., Hasher, Quig, & May, 1997; Intons-Peterson, Rocchi, West, McLellan, & Hackney, 1998; May & Hasher, 1998; Roenneberg et al., 2007).
Circadian fluctuations in arousal may be expected to influence human performance in a wide range of situations, but perhaps especially in those situations that require sustained, effortful cognition. Indeed, research by May, Hasher, and colleagues has shown that age-related time of day preferences are related to performance on measures of effortful cognition. In particular, elderly adults (usually, over 50 years of age) have been found to perform better on measures of recognition memory (May, Hasher, & Stoltzfus, 1993), verbal problem solving (May, 1999), word span (Yoon, May, Goldstein, & Hasher, in press; Yoon, May, & Hasher, 2000), and false memory rejection (Intons-Peterson et al., 1998) when tested in the morning (8 or 9 a.m.) compared to late afternoon (4 or 5 p.m.). These time of day differences have also been found among older adults on measures of executive function (EF), which refers to the effortful cognitive control of thought, action, and emotion (Hasher, Zacks, & May, 1999). May and Hasher (1998) suggested that these findings reflect the synchrony between test times and elderly participants’ preferred time of day (i.e., a synchrony effect): Elderly participants’ cognition is best when they are at or near their peak arousal, and it is poorer when they are far from peak arousal. This pattern of better cognitive performance at optimal times of the day has similarly been shown among younger adults (usually 18–25 years of age; Hasher et al., 1999; Hasher, Goldstein, & May, 2005).
Another developmental change in circadian rhythms occurs during the transition to adolescence, when many individuals move away from preferring mornings towards preferring evenings (Carskadon et al., 1993; Goldstein, Hahn, Hasher, Wiprzycka, & Zelazo, 2007; Ishihara et al., 1990; Kim et al., 2002; Roenneberg et al., 2004). The relative rapidity of this shift, together with normal variation in the timing of the shift, provides a unique opportunity to test both Morning-preference and Evening-preference adolescents at optimal and nonoptimal times of day, controlling for age. To examine the effects of chronotype, time of day, and the synchrony between chronotype and time of day, Goldstein and colleagues (2007) administered measures of fluid and crystallized intelligence to Morning- and Evening-preference adolescents who were tested either during a morning session or an afternoon session. A synchrony effect was found for the fluid-intelligence measures, with better performance at times that matched individuals’ preferences. As part of that study, adolescents were also administered a battery of EF measures and the corresponding data are presented here for the first time.
While EF develops rapidly during childhood, it continues to develop during adolescence in conjunction with the development of prefrontal cortex and related regions of the brain (Olson & Luciana, 2008; Zelazo, Carlson, & Kesek, 2008). Individual differences in EF in childhood predict important developmental outcomes, including academic achievement in school (e.g., Blair & Razza, 2007) and cognitive functioning in young adulthood (e.g., Eigsti et al., 2006). Indeed, impairments in EF are associated with a wide variety of disorders in childhood that interfere with learning and school success, including Attention Deficit Hyperactivity Disorder, Conduct Disorder, and autism, as well as specific problem behaviors, such as physical aggression and substance abuse. Furthermore, there is reason to believe that the development of EF may be especially vulnerable to disruption by a wide variety of perturbations (Zelazo et al., 2008).
We hypothesized that young adolescent participants (ages 11–14 years) tested at their optimal (i.e., preferred) time of day would show better EF than participants tested at their nonoptimal time of day. To provide a reasonably comprehensive assessment of EF, we included measures of three aspects of EF that have been shown to be partially independent in factor-analytic work with adults, and that can be assessed with some specificity: inhibitory control, updating/working memory, and set shifting (Miyake et al., 2000). Inhibitory control was assessed via a Go-Nogo Task (e.g., Davis, Bruce, Snyder, & Nelson, 2003), updating/working memory was assessed via the Self-ordered Pointing task (Petrides & Milner, 1982), and set shifting was assessed via the Intra- and Extra-Dimensional (ID/ED) Shift Task (e.g., Dias, Robbins, & Roberts, 1996). These three measures might be considered measures of relatively “cool” cognitive aspects of EF associated more with activity in lateral prefrontal cortex (Zelazo et al., 2008). Additionally, we administered a measure of more orbitofrontal “hot” EF, the Iowa Gambling Task (Bechara, Damasio, Damasio, & Anderson, 1994), to capture variations in cognitive control in the context of motivationally significant rewards and losses (e.g., Happaney, Zelazo, & Stuss, 2004).
The presence of synchrony effects on these measures would be consistent with previously reported effects on fluid intelligence in this sample, as well as with earlier findings on EF in younger and older adults (e.g., Hasher et al., 1999). Importantly, such findings may shed light on the dynamic interaction between arousal and EF during a key developmental transition.
Method
Participants and Recruitment
Using a telephone interview protocol, we administered the Children’s Morningness-Eveningness Preferences scale (CMEP; Carskadon et al., 1993) to 259 young adolescents (132 male, 127 female) ranging in age from 11 to 14 years (M = 12.48, SD = 1.07). From this pool of participants, the scores of 41 boys and 39 girls at ages 11 (n = 20), 12 (n = 21), 13, (n = 19), and 14 (n = 20) years fell into the two outer quartiles on the CMEP (see Results), and these participants were invited to the lab for further testing. The numbers of participants at each age classified as having morning or evening preferences are presented in Table 1. As reported in Goldstein et al. (2007), we administered the Vocabulary, Block Design, and Digit Span subtests from the WISC-III (Wechsler, 1991) to all participants who came to the lab. These subtests were administered according to the Chronotype × Testing Time design described below, and Morning- and Evening-preference participants did not differ on any of these subtests.
Table 1.
Sleep Durations by Age and Chronotype
| Age (years) | Chronotype (n) | Sleep Previous Evening (M [SD]) |
|---|---|---|
| 11 | Morning (13) | 9.49 (.68) |
| Evening (7) | 10.64 (1.24) | |
| 12 | Morning (12) | 9.93 (1.30) |
| Evening (9) | 8.84 (1.19) | |
| 13 | Morning (8) | 8.77 (1.06) |
| Evening (11) | 7.53 (1.86) | |
| 14 | Morning (7) | 9.05 (1.20) |
Design
Twenty participants were assigned to each of four conditions created by crossing chronotype (Morning- or Evening-preference) and testing time (morning or afternoon). Participants of each chronotype were assigned randomly without replacement to a testing time, except that an effort was made to balance the conditions by age and gender. There were no significant differences between those assigned to optimal and nonoptimal testing times in age, gender, duration of sleep the previous night, or parental education levels. All participants were tested during the summer.
Materials
Children’s Morningness-Eveningness Preferences (CMEP) scale
This 10-item, multiple-choice scale was adapted by Carskadon and colleagues (1993) from the widely used Horne-Östberg Morningness-Eveningness Questionnaire (MEQ; Horne & Östberg, 1976). Scores range from 10 (Extreme Evening preference) to 42 (Extreme Morning preference). Cut-off scores for Morningness and Eveningness, based on the outer quartiles of CMEP scores of the telephone sample, were 32 and above for Morning-preference, and 24 and below for Evening- preference.
Go-Nogo Task
In this Go-Nogo Task (e.g., Davis et al., 2003), a series of letters were flashed on the center of a computer screen. Participants were instructed to respond by pressing the space bar as quickly as possible when they saw any letter except for X, and to withhold responses when presented with an X. The dependent measures were the percentage of errors of commission (incorrect Nogo trials) and omission (incorrect Go trials).
Self-ordered Pointing (SOP) Task
In this computerized version of Petrides and Milner’s (1982) SOP Task, 12 different pictures were presented on 12 pages subsequently appearing on the computer screen. Each one of the 12 pictures appeared on every screen, but the location changed from page to page. Participants’ task was to click to a different picture on each screen. By pointing to a different picture on each screen, after 12 pages, participants should have pointed to each picture once. The computer program prevented participants from pointing to the same screen position on more than two pages in a row. Two versions of the SOP task were administered: a concrete version displaying specific objects (e.g., pictures of cars), and an abstract version (e.g., pictures of black circles). The dependent measures for each version were the total number of errors participants made over the 12 trials.
Intra- and Extra-Dimensional (ID/ED) Shift Task
In the computerized ID/ED Shift Task (e.g., Dias et al., 1996), participants first learned to make visual discriminations between two compound stimuli, based on positive or negative feedback from the computer. In this initial phase, participants had to select a particular color or shape consistently. After reaching a criterion of six consecutive correct responses, novel exemplars were introduced (new colors and new shapes) and participants then had to learn to respond to the previously irrelevant dimension (reversal shift). The criterion for passing a phase was to reach criterion at or before 50 trials. The ID/ED Shift Task was designed to get increasingly more difficult across eight phases, beginning with simple visual discrimination between stimuli, moving to more complex rule-based discriminations, and finally, constantly alternating discriminations (color rule/ shape rule/ color rule). Participants’ scores were based on the most difficult phase (i.e., highest level) reached.
The Iowa Gambling Task
In this computerized version of the Iowa Gambling Task (Bechara et al., 1994), participants chose cards from any of four decks, two of which offered large rewards but occasional larger losses (disadvantageous overall) and two of which offered low rewards but also lower losses (advantageous overall). In particular, selection of a card from decks A and B resulted in a large reward (i.e., $100 on each trial) but occasional large losses (e.g. $1,250 on deck B), whereas selection of a card from decks C and D resulted in a smaller reward (i.e., $50 on each trial) but smaller losses (e.g., $25). Thus, repeated selection of cards from decks A and B resulted in an overall loss of money, whereas repeated selection of cards from decks C and D resulted in an overall profit. Participants received a $2,000 credit to start the game. There were 100 trials in total. A net score was calculated for each block of 20 trials by taking the difference between the number of trials on which participants chose an advantageous deck and the number of trials on which they chose a disadvantageous deck.
Procedure
Morning- and Evening-preference participants were randomly assigned to either a morning session (8 to 10 a.m.) or an afternoon session (1 to 3 p.m.). These times were chosen to reflect the limits of the average school day schedule. Participants were administered the CMEP, a sleep questionnaire, and a battery of four EF tasks presented in a counterbalanced order. Testing was individually administered. Participants were compensated $5 and reimbursed for transportation.
Results
Time of Day Preferences
Telephone interview assessment
Mean CMEP scores declined (i.e., from Morningness to Eveningness) monotonically with age: 11 years (M = 29.69; SD = 4.96; n = 59); 12 years (M = 27.84; SD = 4.57; n = 73); 13 years (M = 27.30; SD = 5.49; n = 70); 14 years (M = 26.23; SD = 4.08; n = 57). The effect of age was reliable, F(3, 255) = 5.30, p = .001, η2 = .06, replicating previous findings (e.g., Kim et al., 2002; Roenneberg et al., 2004). Moreover, the relation between eveningness and age was also significant, (r = .93, p < .001).
The CMEP was re-administered to the 80 adolescents who came into the laboratory, and laboratory CMEP scores were highly correlated with initial telephone CMEP scores, r = .93, p < .001. The initial CMEP scores were used in all subsequent analyses, following an intent-to-treat approach, because it was on the basis of these scores that participants were invited to the lab. The initial CMEP scores for the 80 participants included in the final sample were as follows: 11 years (M = 29.80; SD = 6.88; n = 20); 12 years (M = 28.48; SD = 6.23; n = 21); 13 years (M = 26.79; SD = 7.56; n = 19); 14 years (M = 25.50; SD = 6.42; n = 20).
To examine whether amount of sleep the night before the study varied as a function of our independent variables, we conducted a 2 (Chronotype: Morning-preference vs. Evening- preference) × 2 (Time of Day: morning vs. afternoon) analysis of variance (ANOVA) with amount of sleep as the dependent variable. This sleep score was computed as the difference between self-reported sleep and rising times on the night before and morning of the laboratory session, respectively. Morning-preference adolescents (M = 9.40 h, SD = 1.11) reported longer sleep times than Evening-preference adolescents (M = 8.72 h, SD = 1.91), F(1, 76) = 3.97, p < .05, η2 = .05. Also, adolescents tested in the morning (M = 8.71 h, SD = 1.41) reported shorter sleep times than adolescents tested in the afternoon (M = 9.41 h, SD = 1.70), F(1, 76) = 4.11, p < .05, η2 = .05. Importantly, however, the interaction between chronotype and time of day was not significant (F < 1), confirming that adolescents tested at their optimal time of day (M = 9.00 h) did not differ in the amount of sleep from those tested at their nonoptimal time of day (M = 9.12 h). In addition, amount of sleep was not significantly related to any of our dependent variables (see below). A full report of sleep duration as a function of age and chronotype can be found in Table 1. Nonetheless, as an extra precaution and in an attempt to begin to account for the role of sleep in cognition, we entered reported sleep duration as a covariate into subsequent analyses. Controlling for sleep duration did not change the results.
Executive Function Measures
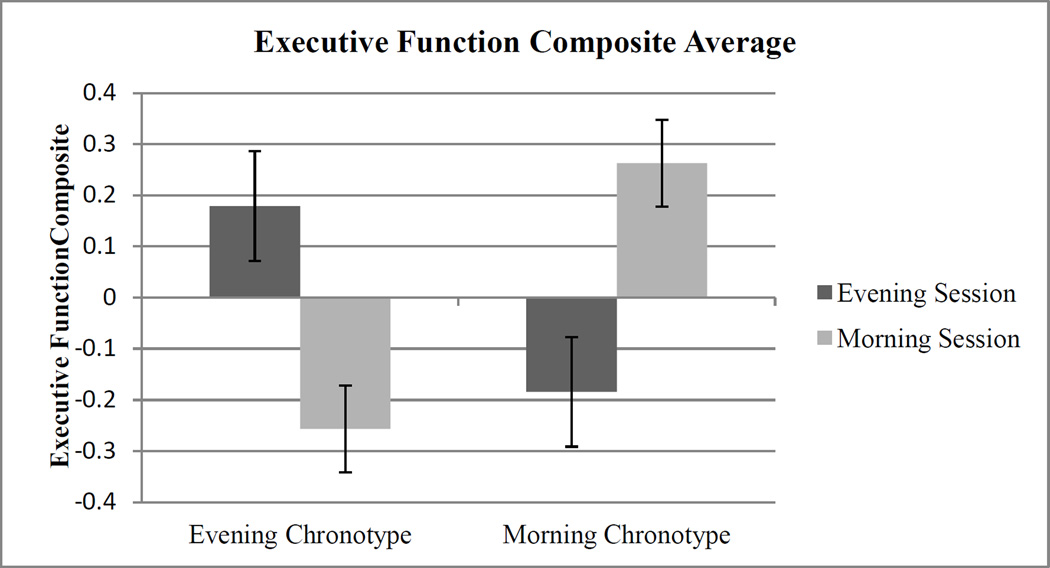
We calculated an overall EF composite score by standardizing the scores for Go-Nogo percentage of commission errors, Iowa Gambling Task net score, SOP total number of errors on the concrete version, and ID/ED Shift Task highest level reached, and creating a standardized composite score from the mean of the z-scores. A 2 (Chronotype) × 2 (Time of Day) ANCOVA with sleep the previous night as a covariate revealed a highly significant Chronotype × Time of Day interaction, F(1,79) = 12.14, p < .001, η2 = .138. Post-hoc Least Squared Differences (LSD) analyses revealed effects of chronotype in participants tested in both the evening (LSD = .3633, p < .05) and morning (LSD = .5193, p < .01) sessions; Evening-preference individuals outperformed Morning-preference individuals on overall EF during afternoon sessions, whereas Morning-preference individuals outperformed Evening-preference individuals on overall EF during morning sessions. Also, effects of time of day emerged in Evening-preference (LSD = .4357, p < .05) and Morning-preference (LSD = .4468, p < .05) participants; Evening-preference individuals tested during afternoon sessions outperformed Evening-preference individuals tested during morning sessions on overall EF, whereas Morning-preference participants tested during morning sessions outperformed Morning-preference individuals tested during afternoon sessions on overall EF. See Figure 1.
Figure 1.
Executive function composite scores (based on Go-Nogo Task, the Iowa Gambling Task, Self-ordered Pointing Task [concrete version], and the Intra/Extradimensional Shift Task; see text for details), as a function of chronotype and time of day. Error bars represent standard errors.
We also analyzed data from each EF measure separately to explore which measures might be contributing to the synchrony effect on overall EF. In general, only significant results are reported.
Go-Nogo Task
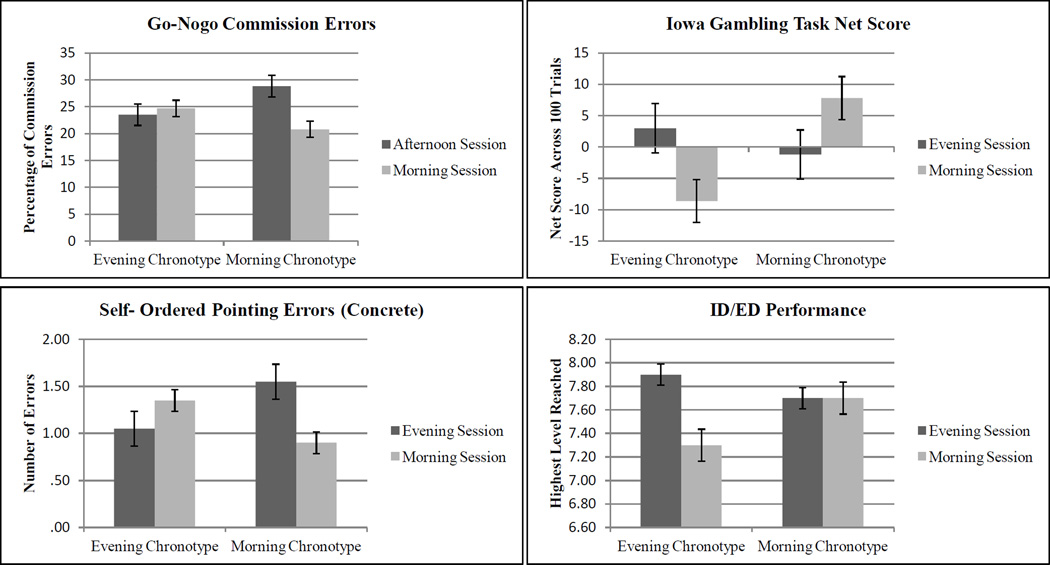
A trend towards significance emerged for the Chronotype × Time of Day interaction in percentage of commission errors, F(1, 79) = 3.43, p < .07, η2 =.044, and percentage of total errors (commission and omission), F(1, 79) = 3.779, p < .06, η2 = .048. See Figure 2A. Post-hoc LSD tests revealed an effect of time of day for Morning-preference individuals on both percentage of commission errors (LSD = −8.047, p < .05) and percentage of total errors (LSD = −3.389, p < .05); Morning-preference individuals tested during morning sessions committed fewer errors than Morning-preference individuals tested during afternoon sessions.
Figure 2.
(A) Percentage of commission errors on the Go-Nogo Task, as a function of chronotype and time of day. Error bars represent standard errors. (B) Performance on the Iowa Gambling Task (net score across 100 trials), as a function of chronotype and time of day. Error bars represent standard errors. (C) Performance (total errors) on the Self-ordered Pointing Task (concrete version), as a function of chronotype and time of day. Error bars represent standard errors. (D) Performance on the Intra/Extradimensional Shift Task (highest level reached, as a function of chronotype and time of day. Error bars represent standard errors.
Iowa Gambling Task
There was a significant Chronotype × Time of Day interaction on overall net score (across 100 trials), F (1, 79) = 4.178, p <.05, η2 =.052. See Figure 2B. Post-hoc LSD tests indicated that there was an effect of chronotype for morning testing sessions (LSD = −8.20, p < .05); Morning-preference individuals obtained higher overall net scores than Evening-preference individuals when tested during morning sessions.
SOP Task
A significant Chronotype × Time of Day interaction was revealed for the number of errors on the concrete version, F (1, 79) = 4.812, p < .05, η2 =.06 (see Figure 2C); no significant differences were shown for the abstract version, F (1, 79) = .000, ns. Post-hoc LSD tests revealed an effect of time of day for Morning-preference individuals (LSD = −.650, p < .05), but not for Evening-preference individuals (LSD = .30, ns); Morning-preference individuals tested during morning sessions made fewer errors than Morning-preference individuals tested in the afternoon.
ID/ED Shift Task
A trend towards significance emerged for Chronotype × Time of Day interaction for the highest level reached, F (1, 79) = 3.556, p <.06, η2 =.045. See Figure 2D. Post-hoc LSD tests revealed an effect of time of day for Evening-preference individuals (LSD = −.600, p < .01); Evening-preference individuals tested during afternoon sessions reached higher levels than Evening-preference individuals tested during morning sessions.
Discussion
The aim of the current study was to explore the effect of Chronotype × Time of Day synchrony on EF during a developmental period marked by a shift towards Eveningness (e.g., Ishihara et al., 1990; Kim et al., 2002; Roenneberg et al., 2004). Our findings extend previous literature showing a difference in cognitive performance when participants are tested at optimal versus nonoptimal times of day (Goldstein et al., 2007; Hasher et al., 1999). Participants tested at their optimal time performed better on a composite measure of EF, as well as on individual measures of affective decision making (the Iowa Gambling Task) and working memory (the SOP task, concrete version). Trends were observed for measures of inhibitory control (Go-Nogo) and shifting (ID/ED Shift).
Whereas most previous research has compared young Evening-preference adults to elderly Morning-preference adults (e.g., Hasher et al., 1999; Intons-Peterson et al., 1998; May & Hasher, 1998), the current study capitalized on the chronotypic variability during early adolescence and included both Morning-preference and Evening-preference participants within each age group. As with our previously reported findings for fluid intelligence (Goldstein et al., 2007), the current findings reveal synchrony effects that are not confounded with age.
The importance of sleep for cognitive function is well documented in both adults (e.g., Durmer & Dinges, 2005; for a review, see Dahl, 2004) and adolescents (Wolfson & Carskadon, 1998). In addition to controlling for age, we also controlled for self-reported sleep on the night prior to participants’ visit to the lab. Participants with evening preferences did report less sleep than those with Morning preferences, consistent with a large body of previous findings (e.g., Carskadon et al., 1993; Laberge et al., 2001; Sadeh, Dahl, Shahar, & Rosenblat-Stein, 2009; Wolfson & Carskadon, 1998), and participants tested in the morning reported less sleep than participants tested in the afternoon (suggesting that they may have awakened early in order to participate in the study). Sleep duration on the night prior to testing did not differ between optimal and nonoptimal testing times, however, and sleep duration had no effect on children’s performance. Moreover, synchrony effects were found for both Evening-preference participants tested in the morning and for Morning-preference participants tested in the afternoon. Nonetheless, in order better to differentiate between homeostatic and circadian arousal influences on EF, future studies investigating the role of synchrony between circadian arousal and testing time would benefit from the imposition of a week-long sleep schedule prior to the testing session, as well as the inclusion of measures of homeostatic processes pertaining to sleep (e.g., dissipation of homeostatic sleep pressure; Mongrain et al., 2006; for a review, see Dijk & Archer, 2009). Imposing a sleep schedule that is in accord with participants’ preferred schedule would help reduce sleep debt and allow for a cleaner assessment of synchrony effects than was possible in the current study (which only assessed sleep duration on the night prior to testing).
It should be noted that all participants in the current study were tested during the summer, when adolescent sleep patterns (and potentially circadian rhythms) are more self-regulated and less susceptible to constraints from school day start times, potentially leading to less sleep deprivation when compared to during the school year (e.g., Roenneberg et al., 2004, 2007). That said, however, the main effects of chronotype and time of day on amount of sleep (greater for Morning-preference participants and for participants tested in the afternoon) suggest that there was a degree of circadian misalignment even during the summer.
The influence of arousal on cognitive function has long been recognized (for review, see Carrier & Monk, 2000; Yerkes & Dodson, 1908), and this influence may be particularly pronounced in school children (Guerin et al., 1991; Montagner, Restoin, De Roquefeuil, & Djakovic, 1992; Montagner & Testu, 1996; Reinberg, Ugolini, Motohashi, & Drawigny, 1988). The synchrony effects reported here support the assumption that EF is an effortful process, dependent on arousal. The nature of this dependence has recently been explored in the context of several lines of research, including work on ego depletion (for a review, see Gaillot et al., 2007), work on “hot” EF (e.g., Prencipe et al., 2011), and work on the integration of emotional processing and EF (e.g., Blair & Dennis, 2010; Calkins & Marcovitch, 2010). Physiological arousal has been shown, for example, to affect self-regulation in several domains, including appetite regulation (Baumeister, Bratslavsky. Muraven, & Tice, 1998) and the suppression of stereotypes (Richeson, Trawalter, & Shelton, 2005). These findings, like those reported here, highlight the need to consider the way in which EF interacts with arousal and related processes, such as motivation (e.g., Hull, Wright, & Czeisler, 2003).
The current data suggest that variations in arousal affect multiple aspects of EF, as assessed by both hot and cool EF measures. The Iowa Gambling Task is a classic measure of hot EF associated with risk-taking behavior, and it has been linked to neural activation of medial orbitofrontal cortex (Bechara et al., 1994; Cohen, Heller, & Ranganath, 2005; Ursu & Carter, 2005). A synchrony effect was observed for the Iowa Gambling Task, such that young adolescents tested at their optimal time outperformed their peers tested at their nonoptimal time. Participants tested at nonoptimal times chose more frequently from disadvantageous decks where they earned more rewards on every trial but suffered larger losses in the long run.
The SOP task, in contrast, is a relatively cool measure of working memory, which has been related to neural activity in dorsolateral prefrontal cortex (Petrides & Milner, 1982). Although no synchrony effect was found for the abstract version of the SOP, a clear synchrony effect was revealed for the concrete version. A possible explanation of this dissociation is that the abstract stimuli, which are difficult to label, are less likely to elicit an analytic strategy, as opposed to a more holistic and intuitive approach (e.g., Chaiken & Trope, 1999; Stanovich, 2009). Evidence from the time of day literature (e.g., Bodenhausen, 1990; Hasher et al., 1999; Yoon, 1997) suggests that both younger and older adults tend to use a more detailed and analytic processing strategy at optimal times, and tend to use more schema-based, heuristic processing at nonoptimal times.
In contrast to both the Iowa Gambling Task and the concrete version of the SOP task, there were only trends towards significant effects for the Go-Nogo and ID/ED tasks. Nonetheless, taken together, the current data are consistent with an influence of circadian fluctuations in arousal on measures of effortful cognition, and encourage further research on the practical implications of these influences on children’s behavior (e.g., Goldstein et al., 2007). For example, adolescence is a period of increasing autonomy and self-regulatory demands that is often accompanied by risky behavior and poor decisions that can have lifelong negative consequences (e.g., Dahl, 2004; Ernst & Hardin, 2010; Steinberg, 2005; Steinberg et al., 2009; Van Leijenhorst & Crone, 2009). One factor contributing to the high incidence of such risky behaviors may be the continued immaturity of EF. A possible implication of the current findings, however, is that executive dysfunction in school settings may result, at least in part, from a mismatch between changing school demands (e.g., earlier school start times) and children’s rapidly changing chronotypes (i.e., a change toward eveningness) during the transition to adolescence. That is, adolescents’ EF in school may be especially likely to be compromised in the morning. In any event, these results, as well as previous findings on self-regulation under external and internal constraints (e.g., Vohs & Heatherton, 2000), underscore the fragility of EF—the fact that it seems especially sensitive to disruption from a variety of perturbations.
An important aspect of EF is the ability to respond flexibly and adaptively in situations that prime maladaptive and/or prepotent responses, leading to impulsive acts and errors in judgment. In these cases, highly salient information often introduces an element of emotion that can cloud otherwise good judgment. Adolescence may mark a period of particular vulnerability to such errors, in part because the transition to adolescence is often accompanied by a new set of challenging emotional experiences, related to puberty, which may further undermine emerging cognitive control. In future research, it would be useful to assess puberty directly, rather than relying on age. Understanding the possible role of circadian synchrony on EF during puberty may shed light on the dynamic interaction between arousal and EF during a key developmental transition, providing insight into individual differences in educational achievement and the possible onset of behavioral problems.
Acknowledgments
This research was supported by National Institute of Aging Grant NIA R37 AGO 4306 awarded to LH and by grants from the Natural Sciences and Engineering Research Council (NSERC) and the Canada Research Chairs Program to PDZ. For assistance with data collection we thank Silvia Celucci, Sarah Douglas, Anoop Ganda, Nicole Recel, and David Vitale. CH was financially supported by the DAAD (German Academic Exchange Service) and Studienstiftung des deutschen Volkes (German National Academic Foundation). JMC was supported by an NIH/NICHD NRSA predoctoral fellowship through the UMN Center for Cognitive Science (“Interdisciplinary Training Program in Cognitive Science”) NIH Grant No: T32 HD007151.
References
- Aston-Jones G, Chen S, Zhu Y, Oshinsky ML. A neural circuit for circadian regulation of arousal. Nature Neuroscience. 2001;4:732–738. doi: 10.1038/89522. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Bratslavsky E, Muraven M, Tice DM. Self-control depletion: Is the active self a limited resource? Journal of Personality and Social Psychology. 1998;74I:1252–1265. doi: 10.1037//0022-3514.74.5.1252. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50(1–3):7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Blair C, Dennis T. An optimal balance: Emotion-cognition integration in context. In: Calkins SD, Bell MA, editors. Child development at the intersection of cognition and emotion. Washington DC: American Psychological Association; 2010. pp. 17–36. [Google Scholar]
- Blair C, Razza RP. Relating effortful control, executive function, and false belief understanding to emerging math and literacy ability in kindergarten. Child Development. 2007;78(2):647–663. doi: 10.1111/j.1467-8624.2007.01019.x. [DOI] [PubMed] [Google Scholar]
- Bodenhausen GV. Stereotypes as judgmental heuristics: Evidence of circadian variations in discrimination. Psychological Science. 1990;1(5):319–322. [Google Scholar]
- Calkins SD, Marcovitch S. Emotion regulation and executive functioning in early development: Integrated mechanisms of control supporting adaptive functioning. In: Calkins SD, Bell MA, editors. Child development at the intersection of emotion and cognition. Washington, DC: American Psychological Association; 2010. pp. 37–58. [Google Scholar]
- Carrier J, Monk TH. Circadian rhythms of performance: New trends. Chronobiology International. 2000;17(6):719–732. doi: 10.1081/cbi-100102108. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Vieira C, Acebo C. Association between puberty and delayed phase preference. Sleep. 1993;16(3):258–262. doi: 10.1093/sleep/16.3.258. [DOI] [PubMed] [Google Scholar]
- Chaiken S, Trope Y. Dual-process theories in social psychology. New York: Guilford Press; 1999. [Google Scholar]
- Cohen MX, Heller AS, Ranganath C. Functional connectivity with anterior cingulate and orbitofrontal cortices during decision-making. Cognitive Brain Research. Special Issue: Multiple Perspectives on Decision Making. 2005;23(1):61–70. doi: 10.1016/j.cogbrainres.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Dahl RE. Adolescent brain development: A period of vulnerabilities and opportunities. Annals of the New York Academy of Sciences. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- Davis EP, Bruce J, Snyder K, Nelson CA. The X-trials: Neural correlates of an inhibitory control task in children and adults. Journal of Cognitive Neuroscience. 2003;15(3):432–443. doi: 10.1162/089892903321593144. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380(6569):69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Archer SN. Light, sleep, and circadian hits: Together again. Public Library of Science: Biology. 2009;7(6):e1000145. doi: 10.1371/journal.pbio.1000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Seminars in Neurology. 2005;25:117–129. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- Eigsti IM, Zayas V, Mischel W, Shoda Y, Ayduk O, Dadlani MB, et al. Predicting cognitive control from preschool to late adolescence and young adulthood. Psychological Science. 2006;17(6):478–484. doi: 10.1111/j.1467-9280.2006.01732.x. [DOI] [PubMed] [Google Scholar]
- Ernst M, Hardin M. Neurodeveloment underlying adolescent behavior: A neurobiological model. In: Zelazo PD, Chandler M, Crone E, editors. Developmental social cognitive neuroscience. New York: Psychology Press; 2010. pp. 165–190. [Google Scholar]
- Gailliot MT, Baumeister RF, DeWall N, Maner JK, Ashby Plant E, Tice DM, et al. Self-control relies on a limited energy source: Willpower is more than a metaphor. Journal of Personality and Social Psychology. 2007;92:325–336. doi: 10.1037/0022-3514.92.2.325. [DOI] [PubMed] [Google Scholar]
- Goldstein D, Hahn CS, Hasher L, Wiprzycka UJ, Zelazo PD. Time of day, intellectual performance, and behavioral problems in morning versus evening type adolescents: Is there a synchrony effect? Personality and Individual Differences. 2007;42(3):431–440. doi: 10.1016/j.paid.2006.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerin N, Boulenguiez S, Reinberg A, Di Constanzo G, Guran P, Touitou Y. Diurnal changes in psychophysiological variables of school girls: Comparison with regard to age and teachers appreciation of learning. Chronobiology International. 1991;8:131–148. doi: 10.3109/07420529109059164. [DOI] [PubMed] [Google Scholar]
- Happaney K, Zelazo PD, Stuss DT. Development of orbitofrontal function: Current themes and future directions. Brain and Cognition. 2004;55:1–10. doi: 10.1016/j.bandc.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Hasher L, Goldstein D, May C. It’s about time: Circadian rhythms, memory and aging. In: Izawa C, Ohta N, editors. Human learning and memory: Advances in theory and application. Mahwah, NJ: Lawrence Erlbaum Associates; 2005. pp. 199–218. [Google Scholar]
- Hasher L, Quig MB, May CP. Inhibitory control over no-longer relevant information: Adult age differences. Memory & Cognition. 1997;25(3):286–295. doi: 10.3758/bf03211284. [DOI] [PubMed] [Google Scholar]
- Hasher L, Zacks RT, May C. Inhibitory control, circadian arousal, and age. In: Gopher D, Koriat A, editors. Attention & performance xvii: cognitive regulation of performance: interaction of theory and application. Cambridge, MA: MIT Press; 1999. pp. 653–675. [Google Scholar]
- Horne JA, Östberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. International Journal of Chronobiology. 1976;4(2):97–110. [PubMed] [Google Scholar]
- Hull JT, Wright KP, Czeisler CA. The influence of subjective alertness and motivation on human performance independent of circadian and homeostatic regulation. Journal of Biological Rhythms. 2003;18(4):329–338. doi: 10.1177/0748730403253584. [DOI] [PubMed] [Google Scholar]
- Intons-Peterson MJ, Rocchi P, West T, McLellan K, Hackney A. Aging, optimal testing times, and negative priming. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1998;24:362–376. doi: 10.1037//0278-7393.25.1.23. [DOI] [PubMed] [Google Scholar]
- Ishihara K, Honma Y, Miyake S. Investigation of the children's version of the morningness-eveningness questionnaire with primary and junior high school pupils in Japan. Perceptual and Motor Skills. 1990;71:1353–1354. [Google Scholar]
- Kim S, Dueker GL, Hasher L, Goldstein D. Children’s time of day preference: Age, gender and ethnic differences. Personality and Individual Differences. 2002;33:1083–1090. [Google Scholar]
- Laberge L, Petit D, Simard C, Vitaro F, Tremblay RE, Montplaisir J. Development of sleep patterns in early adolescence. Journal of Sleep Research. 2001;10:59–67. doi: 10.1046/j.1365-2869.2001.00242.x. [DOI] [PubMed] [Google Scholar]
- May CP. Synchrony effect in cognition: The costs and a benefit. Psychonomic Bulletin and Review. 1999;6:142–147. doi: 10.3758/bf03210822. [DOI] [PubMed] [Google Scholar]
- May CP, Hasher L. Synchrony effects in inhibitory control over thought and action. Journal of Experimental Psychology: Human Perception and Performance. 1998;24(2):363–379. doi: 10.1037//0096-1523.24.2.363. [DOI] [PubMed] [Google Scholar]
- May CP, Hasher L, Stoltzfus ER. Optimal time of day and the magnitude of age differences in memory. Psychological Science. 1993;4:326–330. [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex "frontal lobe" tasks: A latent variable analysis. Cognitive Psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Mongrain V, Carrier J, Dumont M. Circadian and homeostatic sleep regulation in morningness-eveningness. Journal of Sleep Research. 2006;15:162–166. doi: 10.1111/j.1365-2869.2006.00532.x. [DOI] [PubMed] [Google Scholar]
- Montagner H, Restoin A, de Roquefeuil G, Djakovic M. Fluctuations of biological rhythms, behavior and intellectual activity in children in different environments. Pediatrie. 1992;47:85–104. [PubMed] [Google Scholar]
- Montagner H, Testu F. Biological, behavioral and intellectual rhythms in pupils during the school day. Pathologie-biologie. 1996;44(6):519–533. [PubMed] [Google Scholar]
- Olson EA, Luciana M. The development of prefrontal cortex functions in adolescence: Theoretical models and a possible dissociation of dorsal versus ventral subregions. In: Nelson CA, Luciana M, editors. Handbook of developmental cognitive neuroscience. 2nd ed. Cambridge, MA: MIT Press; 2008. pp. 575–590. [Google Scholar]
- Petrides M, Milner B. Deficits on subject-ordered tasks after frontal- and temporal-lobe lesions in man. Neuropsychologia. 1982;20(3):249–262. doi: 10.1016/0028-3932(82)90100-2. [DOI] [PubMed] [Google Scholar]
- Prencipe A, Kesek A, Cohen J, Lamm C, Lewis M, Zelazo PD. Development of executive function in adolescence. Journal of Experimental Child Psychology. 2011;108:621–637. doi: 10.1016/j.jecp.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Reinberg A, Ugolini C, Motohashi Y, Drawigny C. Diurnal rhythms in performance tests of school children with and without language disorders. Chronobiology International. 1988;5(3):291–299. doi: 10.3109/07420528809079567. [DOI] [PubMed] [Google Scholar]
- Richeson JA, Trawalter S, Shelton JN. African Americans’ implicit racial attitudes and the depletion of executive function after interracial interactions. Social Cognition. 2005;23:336–352. [Google Scholar]
- Roenneberg T, Kuehnle T, Juda M, Kantermann T, Allebrandt K, Gordijn M, et al. Epidemiology of the human circadian clock. Sleep Medicine Reviews. 2007;11:429–438. doi: 10.1016/j.smrv.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Kuehnle T, Pramstaller PP, Ricken J, Havel M, Guth A, et al. A marker for the end of adolescence. Current Biology. 2004;14:r1038–r1039. doi: 10.1016/j.cub.2004.11.039. [DOI] [PubMed] [Google Scholar]
- Sadeh A, Dahl RE, Shahar G, Rosenblat-Stein S. Sleep and the transition to adolescence: A longitudinal study. Sleep. 2009;32(12):1602–1609. doi: 10.1093/sleep/32.12.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanovich KE. Distinguishing the reflective, algorithmic, and autonomous minds: Is it time for a tri-process theory? In: St J, Evans BT, Frankish K, editors. In two minds: Dual processes and beyond. New York: Oxford University Press; 2009. [Google Scholar]
- Steinberg L. Cognitive and affective development in adolescence. Trends in Cognitive Sciences. 2005;9:69–74. doi: 10.1016/j.tics.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Graham S, O'Brien L, Woolard J, Cauffman E, Banich M. Age differences in future orientation and delay discounting. Child Development. 2009;80:28–44. doi: 10.1111/j.1467-8624.2008.01244.x. [DOI] [PubMed] [Google Scholar]
- Tankova I, Adan A, Buela-Casal G. Circadian typology and individual differences: A review. Personality and Individual Differences. 1994;16(5):671–684. [Google Scholar]
- Ursu S, Carter CS. Outcome representations, counterfactual comparisons and the human orbitofrontal cortex: Implications for neuroimaging studies of decision-making. Cognitive Brain Research. 2005;23:51–60. doi: 10.1016/j.cogbrainres.2005.01.004. [DOI] [PubMed] [Google Scholar]
- van Leijenhorst L, Crone EAM. Paradoxes in adolescent risk taking. In: Zelazo PD, Chandler M, Crone E, editors. Developmental social cognitive neuroscience. New York: Psychology Press; 2009. pp. 209–226. [Google Scholar]
- Vitiello MV, Smallwood RG, Avery DH, Pascualy RA, Martin DC, Prinz PN. Circadian temperature rhythms in young adult and aged men. Neurobiology of Aging. 1986;7(2):97–100. doi: 10.1016/0197-4580(86)90146-6. [DOI] [PubMed] [Google Scholar]
- Vohs KD, Heatherton TF. Self-regulatory failure: A resource-depletion approach. Psychological Science. 2000;11(3):249–254. doi: 10.1111/1467-9280.00250. [DOI] [PubMed] [Google Scholar]
- Wolfson AR, Carskadon MA. Sleep schedules and daytime functioning in adolescents. Child Development. 1998;69(4):875–887. [PubMed] [Google Scholar]
- Yerkes RM, Dodson JD. The relation of strength of stimulus to rapidity of habit-formation. Journal of Comparative Neurology. 1908;18:459–482. [Google Scholar]
- Yoon C. Age differences in consumers’ processing strategies: An investigation of moderating influences. Journal of Consumer Research. 1997;24:329–342. [Google Scholar]
- Yoon C, May CP, Goldstein D, Hasher L. Aging, circadian arousal patterns and cognition. In: Park D, Schwarz N, editors. Cognitive aging: A primer. 2nd edition. New York: Psychology Press; (in press). [Google Scholar]
- Zelazo PD, Carlson SM, Kesek A. Development of executive function in childhood. In: Nelson CA, Luciana M, editors. Handbook of developmental cognitive neuroscience. 2nd ed. Cambridge, MA: MIT Press; 2008. pp. 553–574. [Google Scholar]