Abstract
Hepatic resection remains the only curative option for the majority of patients with hepatocellular carcinoma (HCC) who do not meet criteria for transplantation or local ablative options. As the majority of patients with HCC also have underlying chronic liver disease and cirrhosis, post-hepatectomy complications can be significant, and in some prohibitive. The technique of portal vein embolization (PVE) has evolved to increase the candidacy of patients for major hepatectomy as well as improve postoperative outcomes and safety. This review will focus on PVE as well as discuss our institution’s experience with uses and limitations of this technique for HCC.
Introduction
The management of hepatocellular carcinoma (HCC) has evolved into a complex multidisciplinary practice with varying modalities of treatment dependent on patient characteristics, tumor features, and institutional preferences. Historically, the only possibility of long-term cure was realized via either orthotopic liver transplantation or formal hepatic resection, although there is some emerging evidence for the utility of local ablative therapies for small, unilocular lesions. Despite the controversy regarding the specific treatments for individual cases, most experts would agree that formal hepatic resection continues to offer significant long-term benefit in the majority of patients unsuitable for either liver transplantation (using standardized criteria) or local ablative therapies.
Major liver resection for both benign and malignant tumors is performed with increasing frequency throughout the world. The two most common sources of operative complications and subsequent mortality after major hepatectomy have traditionally been attributed to hemorrhage and postoperative liver failure – both correlating with the magnitude of resection as well as the complexity and duration of the procedure. With major advances in operative technique and instrumentation, as well as greater understanding of perioperative anesthetic management, significant bleeding complications have been considerably reduced.1 However the prevalence of postoperative liver dysfunction has not been correspondingly diminished, but rather observed with rising regularity, particularly in those with chronic liver disease or patients treated with preoperative chemotherapy. Although fatal liver failure after major hepatectomy is rare, as the limits of resection are tested and larger proportions of liver parenchyma are resected, complications (unrelated to technical failure) resulting from insufficient liver function contribute to the morbidity and prolonged recovery seen in these patients.2
Postoperative liver insufficiency is a particularly dreadful outcome as there is no formal treatment other than supportive measures, and if it should progress to failure and loss of total liver function, is incompatible with life. Our understanding of the etiologies of postoperative liver dysfunction after hepatectomy has led to the realization that it is not the absolute amount of parenchyma removed that is critical (contrary to popular conception), but rather the relative amount or volume of “functional” remnant liver tissue that remains. Patients with marginal remnant liver function (volume) after resection are also vulnerable to other significant surgical complications for which they may have greater difficulty recovering compared to those with adequate liver remnants.3,4 These observations are of maximal significance in patients undergoing hepatectomy for HCC as a large proportion of these patients have underlying chronic liver disease and thus are at highest risk. As a consequence, the technique of portal vein embolization (PVE) prior to hepatic resection for HCC has been developed to attempt to reduce these risks. The purpose of this review is to discuss the evidence for the uses and limitations of PVE for improving the perioperative outcomes for patients undergoing hepatectomy for HCC and to overview our institution’s experience with this technique. (Table 1)
Table 1.
Summary of our experience with PVE in HCC
| Portal Vein Embolization In Hepatocellular Carcinoma |
|---|
| Safe, Well Tolerated, and Established Technique |
| Increases Resectability Rates |
| Enables Improved Patient Selection and Safety |
| Powerful Negative Predictive Value |
| Indications: |
| ≤20% FLR(standardized) − Normal Liver |
| ≤40% FLR(standardized) − Cirrhotic (Childs A) |
| No Value in Normal Livers with an Adequate FLR |
| Augmented with Arterial Embolization |
The Future Liver Remnant (FLR)
The liver is essential for life in that there are no current alternatives of support in the absence of its function (outside of transplantation), in contrast to hemodialysis for renal failure, for example. As such, great care must be taken to assure adequate liver function postoperatively. In the earlier era of liver resection, many surgeons were hesitant to offer major or especially extended hepatectomy to patients they considered “borderline” or “high-risk” for postoperative liver dysfunction. To counter this apprehension many authors have attempted assessment of liver function by numerous biochemical methodologies and created algorithmic strategies in an attempt to quantify the risk of postoperative liver dysfunction preoperatively.5–7 All of these proposed methods vary according to geographic region as well as individual surgeon preference, and none have proven superior. Although traditionally most surgeons concentrated on the maximum volume of liver that could safely be resected, transplant surgeons have been well aware of the minimal sufficient volume of liver that must remain to assure patient survival.8 The interest in objectively quantifying adequate liver volume began with attempts to accurately assess appropriate graft size needed for transplantation.9 This was further developed as living donor transplantation evolved, creating the need to understand the minimum volume of liver needed to support post-transplant liver function in both the donor and the recipient.10 In an attempt to predict the minimum liver volume necessary for adequate function in the recipient, authors proposed a formula for calculating this volume from body surface area (BSA).11,12 This calculation was predicated on the known linear relationship between liver volume and patient size.13,14 As modern imaging and computational capabilities simultaneously evolved, the use of computed tomography (CT) volumetry for assessment of liver volume became possible and its accuracy was subsequently validated.15,16 This combination of creation of mathematical volume prediction formulas based on patient size and increased utilization of contemporary multi-detector helical CT imaging technology, led to a reassessment in those patients previously considered “borderline” or “high risk”, and a renewed interest in the volume-function relationship for major liver resection. With the increasing use of major hepatectomy and the need to minimize complications secondary to postoperative liver insufficiency, the principles and methods derived from this seminal work in the transplantation literature were applied to formal liver resection for either primary or metastatic cancer.
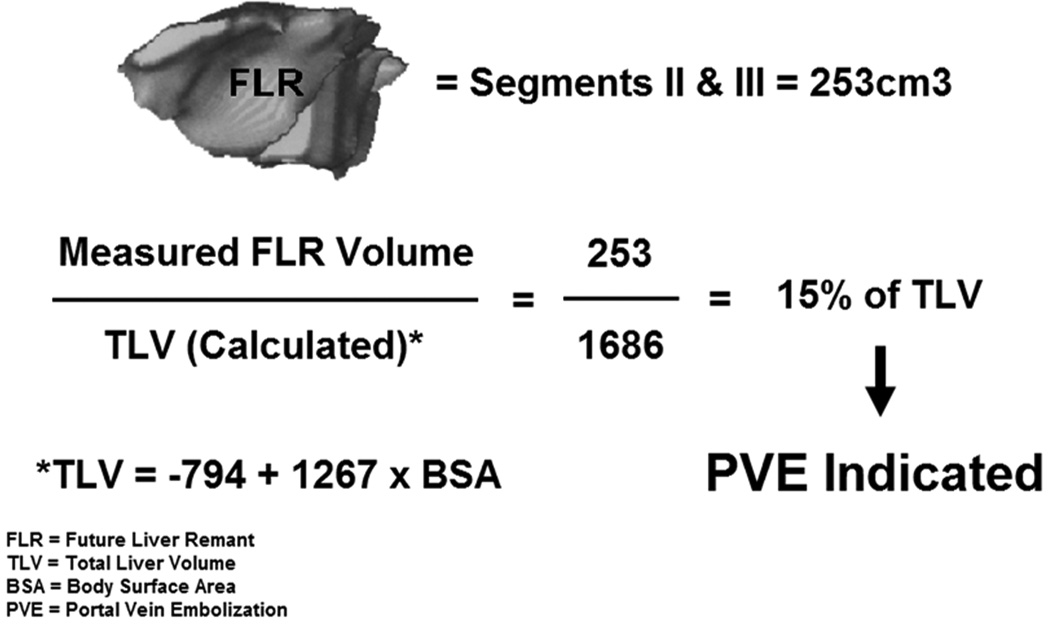
The most important concept in this initial work was the shift in importance from volume of liver resected to the FLR (future liver remnant or functional liver remnant) as the determinant of postoperative liver function.17 The exact FLR volume (which is not compromised by tumor) can easily be directly measured by CT three-dimensional volumetry reconstruction. However, in those who are candidates for major hepatectomy, the absolute FLR volume (needed for adequate postoperative function) varies significantly from patient to patient and the safe minimal absolute volume for FLR also varies from patient to patient. This safe minimum had not been well defined or universally accepted. The initial classic estimation of liver volumes was as follows: Measured FLR volume/ (TLV − tumor volume). This calculation has several inherent errors: cumulative error associated with measurement of non-functioning parenchyma (tumor nodules), error in measurement in the presence of dilated bile ducts, or of atrophic lobes secondary to portal or bile duct invasion/compression, as well as cirrhotic or steatotic liver volumes do not estimate accurate liver function. As a result a standardized simple method was developed to express FLR as a percentage of total liver volume (TLV).18,19 (Figure 1) This methodology allows for a stable denominator to provide a fixed reference for total liver volume before and after intervention. The total liver volume (TLV) is based on patient size, as previously discussed, specifically using BSA (body surface area). We were the first to show that hepatic three-dimensional volume reconstruction based on helical CT reliably predicts TLV based on BSA or body weight, and the new formulas derived from this correlation contribute to the estimation of TLV before major hepatic resection.19 Our favored approach of TLV based on BSA has been shown in a meta-analysis to be the least biased and most precise formula to calculate TLV.13 And most importantly we have previously shown a strong correlation between the FLR/TLV measurements calculated from BSA and their ability to predict postoperative hepatic dysfunction.20 Subsequent early retrospective studies have shown that preoperative volumetric assessment of the future liver remnant volume is in fact able to predict hepatic dysfunction in patients undergoing major liver resection.18,21–23 Specifically these studies demonstrated that postoperative liver function abnormalities and subsequent patient recovery were related to liver volume, and most importantly that major complications and death correlated not to the volume resected, but rather to the standardized FLR volume. This correlation of FLR and subsequent outcomes was found evident both in patients with normal livers as well as in those with underlying liver disease or cirrhosis.4,24 These and subsequent results have firmly established the direct relationship between remnant volume and remnant function. The exact determination of the specific minimal limit of liver volume which permits safe resection warrants further discussion.
Figure 1.
Standardized Future Liver Remnant Formula
The “Minimum” Adequate FLR
In the transplantation literature a variety of measures have been reported to estimate the minimal safe graft size. One of the simplest is the graft-to-recipient body weight ratio (GRBWR). A GRBWR of at least 0.8% (approximately 40% of the standardized TLV) has been described as optimal, as a ratio below 0.8% has been found to result in small-for-size graft dysfunction.25–27 More recent data has challenged this arbitrary dogma based on weak supporting data from earlier studies.28 Not surprisingly, similar intense debate exists regarding the limits of safe resection and the minimal FLR required for adequate liver function postoperatively. Previous reports have suggested a wide range of minimal volumes conditional on the presence and degree of underlying parenchymal liver disease. The adequacy of FLR in the past had been based largely on surgeon’s experience and inexact estimations. In the early era of liver resection, it was observed that patients with normal livers are able to tolerate more extensive resections with a lower frequency of complications compared those patients with chronic liver disease or cirrhosis, even those undergoing less significant resections. This observation was both logical and intuitive as the more severe the underlying cirrhosis, the larger the volume of functional liver needed to maintain adequate function postoperatively. A healthy liver is capable of not only excellent regeneration but also adjusts to the metabolic requirements needed after major surgery, in stark contrast to someone with chronic liver disease whose postoperative recovery is not as robust. There are 3 potential outcomes after major liver resection: an adequate FLR – the ideal situation and goal with minimal liver function related complications; an inadequate FLR – necessitating either emergent liver transplantation or resulting in eventual death; and a marginal FLR – with patients developing cholestasis, impaired synthetic function, and frequently a cascade of complications (jaundice, fluid retention, pulmonary complications, and multiorgan dysfunction – which in patients with underlying liver disease or other significant comorbidities, may progress to an inadequate FLR and death). Death secondary to liver failure after hepatectomy is a simple outcome to measure, and fortunately is rather rare. However to more accurately determine appropriate minimal FLR’s for resection, one must determine an objective definition of non-fatal postoperative “liver insufficiency” based on validated defined measures of hepatic dysfunction, unfortunately not all authors are in agreement on this definition and the literature varies from study to study.3,29–31 Although no standard definition of postoperative liver dysfunction has been applied uniformly to the studies available for review, the persistent postoperative biochemical abnormalities and clinical observations without other known technical causes, suffice for the majority of cases. The current data that supports the definition of an “adequate” FLR should be discussed categorically, that is in those with normal and those with diseased livers (acknowledging the existence of variability of disease severity).
FLR in the Absence of Liver Disease
A contemporary analysis of outcome after liver resection based on FLR volume found a critical minimal FLR of 26.6% (using non-standardized CT volumetry) based on receiver operator characteristic (ROC) curve analysis to predict severe postoperative hepatic dysfunction.32 Our institutions preliminary data indicated an increase in major complications for liver volumes ≤25% (using standardized volumetry as previously described).18 These results prompted additional studies that supported a subsequent decrease in the minimal cutoff of 20% FLR prior to hepatic resection in patients with normal livers.22,23 Importantly, these results (using standardized FLR’s) revealed that postoperative liver insufficiency or death from liver failure is no more common in patients with intermediate FLR’s (20.1 – 30%) than in patients with FLR’s >30%. Accordingly this safe limit of resection in patients with normal livers has been validated and is now firmly established.
FLR in Chronic Liver Disease
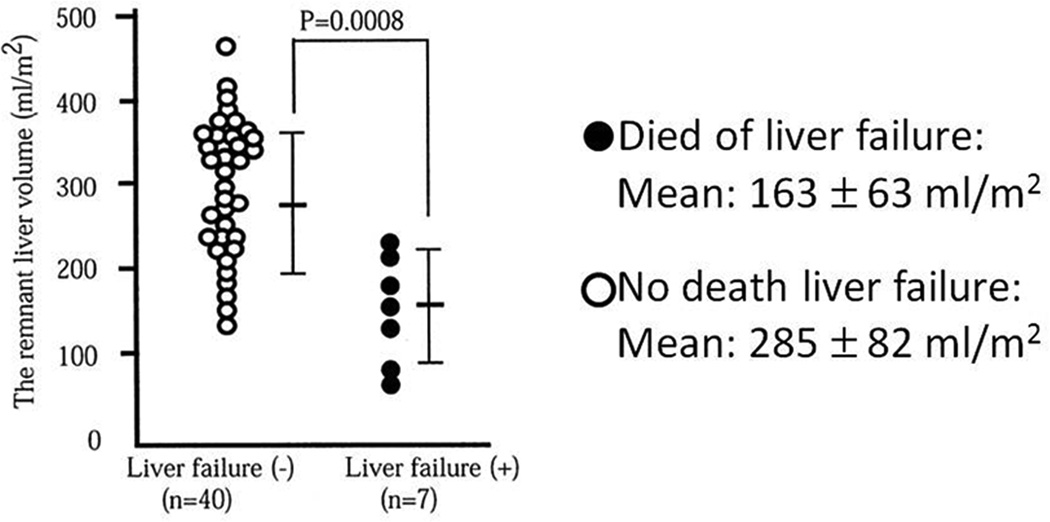
Resection of the hepatocellular carcinoma and its portal territory is necessary to achieve adequate disease-free survival.33 Major hepatectomy is required in most patients with larger tumors or those with smaller tumors that are situated deep in the parenchyma. Early studies have underscored the very significant morbidity and mortality in patients undergoing major hepatic resection for HCC with significant liver disease.34,35 As the majority of patients with HCC have underlying chronic liver disease or frank cirrhosis secondary to infectious, alcohol, or other causes, the question of minimal FLR volume (function) is critical in this cohort to prevent postoperative liver failure. Unfortunately the evidence for this FLR is markedly limited given the significant variability in liver disease among patients and heterogeneity of studies available regarding tumor type. One point that all authors agree on is that major resection should only be considered in well-compensated Child-Pugh grade A patients. One of the initial studies to outline a minimal FLR looked at outcomes following hepatic resection in cirrhotic patients and found no patients with liver insufficiency or mortality with FLR’s ≥40% implying this as the minimal safe standard for cirrhotics.24 Another early report of safe minimal FLR for resection for HCC in patients with liver disease suggested remnant liver volume of greater than 250 mL/m2 (non-standardized volume), due to the incidence of liver failure as high as 35% in smaller remnants.4 (Figure 2) Subsequent analyses of small series from other centers and authoritative opinions have concluded the high risk of liver failure in patients with cirrhosis with FLR of < 40%, thus this minimum safe threshold is now considered appropriate for those with chronic liver disease.36,37 Although certainly some well compensated patients may tolerate resection with smaller remnant volumes, due to the significant risk involved any further studies to challenge this minimum would be unethical and inappropriate. As a consequence of this work the current accepted minimal %FLR required for safe major hepatic resection in normal and diseased livers is 20% and 40% respectively in those institutions utilizing standardized volumetry, understanding that other groups prefer to utilize volumetric data selectively in favor of ICG clearance studies. How to achieve these critical volumes in those patients whose predicted postoperative FLR volume is less then these established guidelines is the next topic of discussion.
Figure 2.
No liver failures or mortality in patients with FLR >250ml/m2. (Reprinted with permission from: Shirabe K, Shimada M, Gion T, et al. J Am Coll Surg 1999;188:304-9)
Portal Vein Embolization
Intrahepatic dissemination of the tumor via the portal circulation is thought to be the mechanism for the large majority of in-liver recurrences of hepatocellular carcinoma. As a consequence of this hypothesis, early authors attempted to embolize the portal vein of the involved lobe in efforts to minimize tumor seeding.38 The thought was to suppress intrahepatic spread during hepatic resection procedures and improve prognosis after surgery. What the authors found was contralateral lobe hypertrophy. This observation was then deliberately exploited in preparation prior to extended hepatectomy to allow growth of the FLR in order to improve outcomes and hence the era of portal vein embolization (PVE) was introduced.39 Although met with much skepticism as to its utility early on, there has been a plethora of literature that has definitively shown PVE correlates with increased function of the resulting hypertrophied FLR via manifold biochemical, functional, and radiographic analyses. These have included increase in percentage of ICG excretion of the FLR, increase in bile flow and clearance, and increase in 99mTc-GSA scintigraphy supporting the shift and increase of function to the non-embolized lobe (FLR).40–42 The vast majority of literature regarding PVE and liver resection involves either formal right hepatectomy or extended right hepatectomy, as the right lobe of the liver comprises approximately 60% to 70% of the total liver volume thus leaving a potentially smaller FLR volume.
A recent meta-analysis reviewing 75 publications and including >1000 patients undergoing PVE with a variety of indications, concluded that PVE is a safe and effective procedure in inducing liver hypertrophy to prevent post-resection liver failure due to insufficient liver remnant.43. The overall morbidity was 2.2% without any deaths related to the technique. Over 85% of those patients treated ultimately were able to undergo resection and the incidence of transient liver failure following resection was only 2.5% with a mortality rate from liver failure of 0.8%. As a result of this robust data, the integration of preoperative PVE into the multidisciplinary individualized treatment plan for patients with HCC is now considered standard of care in the majority of major liver centers.
Portal Vein Embolization in HCC
The initial observations with PVE for HCC, that continue to hold true, were that resectability rates can be increased and that major hepatectomy would not be possible without PVE in the majority of patients with advanced HCC or patients with previously unresectable tumors.44 Some groups have even suggested that PVE improves prognosis after right hepatectomy in HCC patients.45 Others have refuted this claim reporting similar outcomes in regards to recurrent foci in the non-embolized lobe following extended hepatectomy between the patient groups with and without preoperative portal embolization.46,47 Our own recently published experience with PVE in hepatocellular carcinoma found that PVE is associated with improved perioperative outcome with significant differences in major complications in the non-PVE 35% vs. PVE 10% (p = .028) as well as mortality non-PVE 18% vs. PVE 0% (p=0.38).48 Furthermore the overall and disease-free survivals were comparable. As the vast majority of those in the PVE group would not have gone to hepatectomy otherwise, our conclusion that PVE increases the safety of major hepatectomy in patients with HCC without compromising long-term oncologic outcomes is justified. (Figure 3)
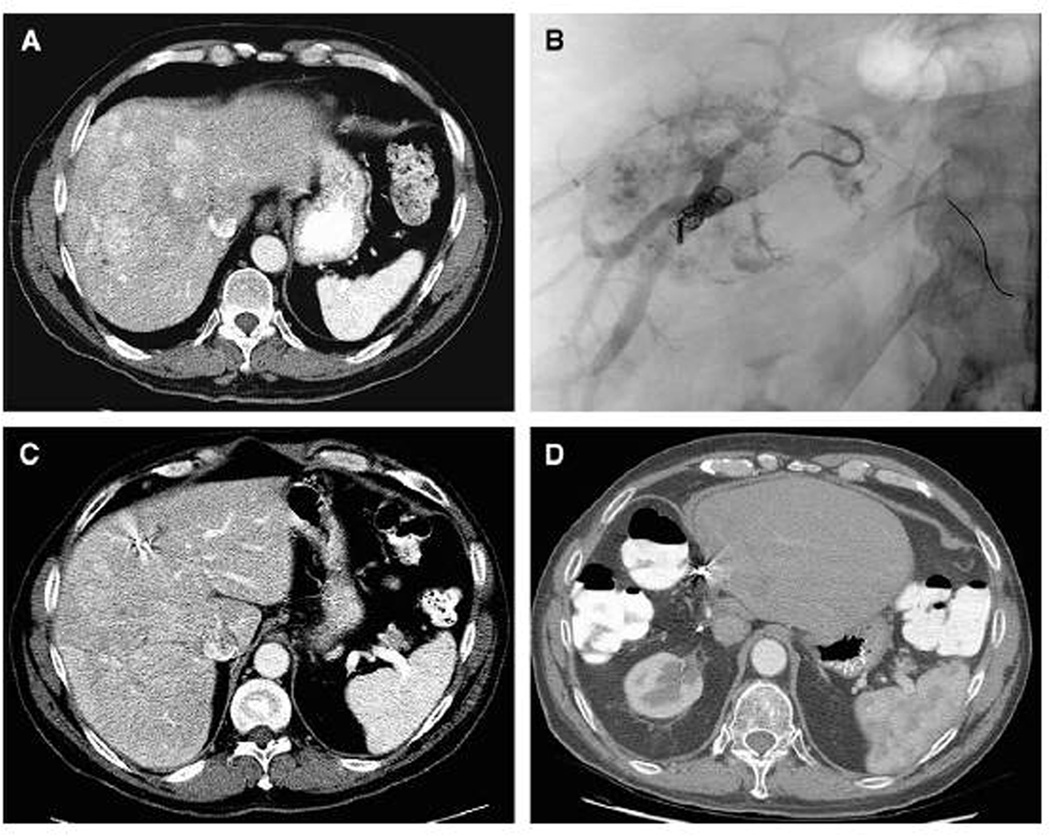
Figure 3.
A 59-year-old male patient had a 16-cm hepatocellular carcinoma in the right liver without evidence of extrahepatic disease. (A) Computed tomography revealed that the standardized future liver remnant (sFLR) volume was 12%.
(B) Right portal vein embolization (PVE) was performed. (C) Four weeks after PVE, the sFLR was 21%. (D) The patient had no evidence of disease 6 years post-resection. (Reprinted with permission from: Palavecino M, Chun YS, Madoff DC, et al. Surgery 2009;145:399–405)
Normal livers have excellent regenerative capacity, however studies have shown that PVE induces clinically important hypertrophy of the FLR in patients with chronic liver disease as well. Results of the only randomized trial of PVE versus immediate surgery found a mean increase in FLR of 9% in cirrhotic livers versus 16% in patients with normal livers with the majority of those with cirrhosis experiencing some degree of hypertrophy.49 The demonstrable benefit of PVE in cirrhotics is the development hypertrophy itself after treatment, as it provides extremely valuable useful pre-resection information about liver function in a relatively low-risk setting. In our initial experience using volumetric data to analyze the kinetics of FLR growth after PVE, the FLR volume significantly increases in the first 3 weeks after the procedure after which the degree of hypertrophy reaches a plateau phase of minimal regeneration.50 There is a considerable amount of data emerging regarding the significance of the specific extent of hypertrophy after PVE to predict postoperative outcome, however the specific cutoff values have not been universally accepted or defined. In our experience >5% hypertrophy (or DH - degree of hypertrophy) after PVE is predictive of success.50 The absolute total portal blood flow is unchanged after PVE, thus all portal flow is completely redistributed to the FLR.51 This fact is exemplified by a study that found hypertrophy of the FLR following PVE was correlated with the total embolized liver volume suggesting that the increased flow was critical to hypertrophy.52
Approximately 10–20% of patients undergoing PVE do not experience adequate hypertrophy of the FLR as a result of either inadequate regeneration capability in those with cirrhosis or the presence of collaterals, which prevents increase in portal flow to FLR. Such patients are thus at high risk of postoperative complications and mortality and considered a contraindication to major liver resection. For those patients without chronic liver disease in whom we anticipate extended right hepatectomy, we recommend embolization of segment 4 branches as well, as we have had improved post-PVE hypertrophy of segments 2/3 with this approach.20
There is a compensatory increase in hepatic arterial blood flow that occurs in the embolized lobe after PVE.53,54 Understanding that HCC is a hypervascular tumor whose growth relies on angiogenesis, PVE may induce increased tumor growth in the embolized lobe prior to resection as well induce microscopic tumor growth in the FLR of occult lesions as a result of bystander effect of induced regenerative growth factors. And in fact, HCC growth rate in the embolized lobe after PVE has been found to be accelerated by as much as two-fold.55 Similar observations have also been observed which identified increased tumor growth after PVE in hepatic colorectal metastases, which are also dependent on hepatic arterial supply. The hypothesis suggests that growth factors elicited from the regenerating FLR now stimulate tumor growth. Some have suggested that to prevent this possibility it is important to embolize all tumor-bearing segments of the liver, sometimes segment IV branches in anticipation of extended right hepatectomy. Others as a result recommend that patients with hepatocellular carcinoma (HCC) undergo transcatheter arterial chemoembolization (TACE) to suppress tumor growth before PVE is performed. TACE before PVE is a reasonable and feasible technique to provide more adequate hypertrophy of the non-embolized lobe in cases of HCC, as the degree of hypertrophy associated with PVE is further enhanced by arterial embolization. (Figure 4) This assertion is supported by previously reported data in which patients who undergo TACE before PVE have significantly increased FLR hypertrophy (>20%). This technique also offers a significantly higher rate of complete tumor necrosis and in some series a higher 5-year disease-free survival rate.56,57 The tissue damage to the embolized lobe induced by TACE is thought to promote liver regeneration in the FLR. It has been shown that hypertrophy of the FLR to be significantly greater in HCC patients who received TACE before PVE than in other hepatic tumors.52 Furthermore TACE before PVE is also reasonable in HCC if the FLR is at risk of tumor invasion from adjacent lesions that may grow after PVE, or if there is risk of tumor rupture because of tumor growth acceleration.
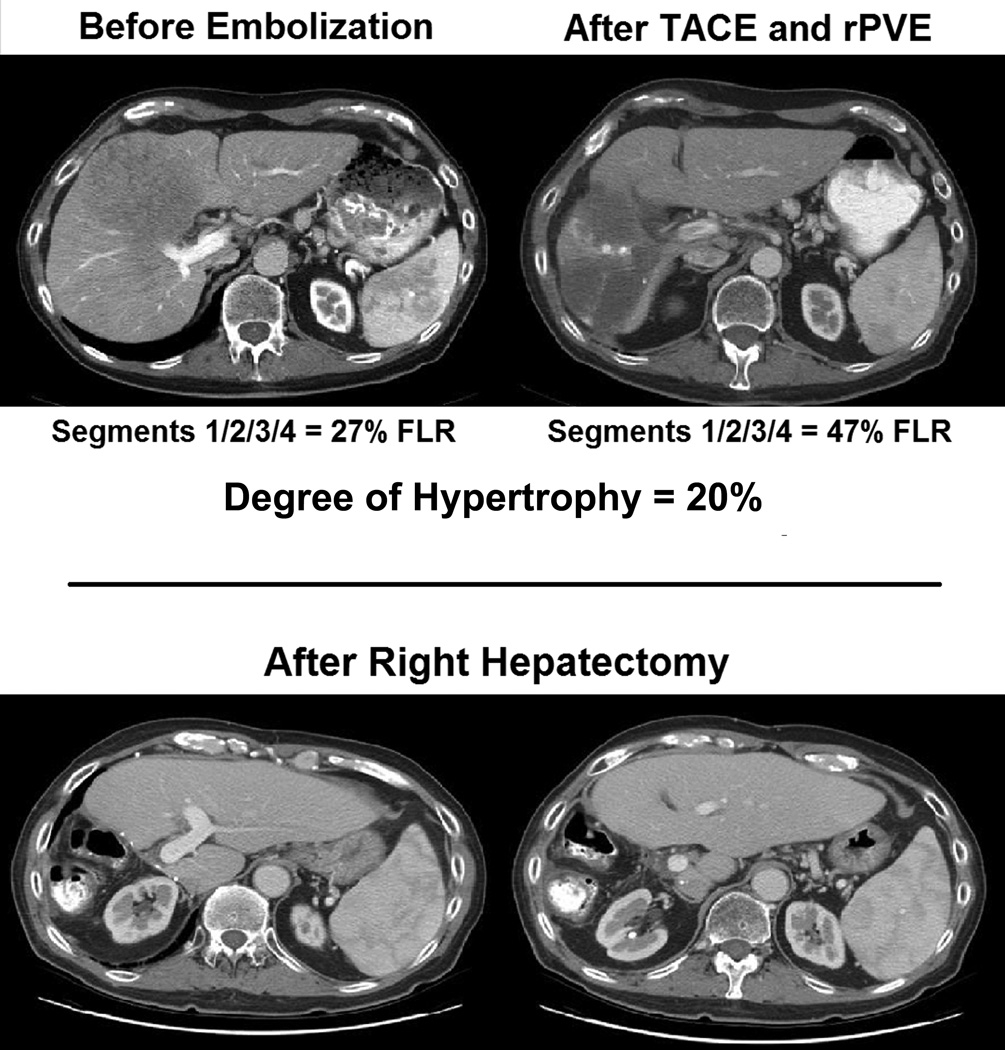
Figure 4.
Sequential arterial and portal vein embolization prior to resection for 12.5 cm solitary HCC with HCV cirrhosis. No evidence of recurrence after 3 years.
Contraindications to PVE for HCC in our practice include: portal vascular invasion or thrombosis (portal flow already diverted), tumor extension to the FLR, uncorrectable coagulopathy, renal failure, and portal hypertension (in those with chronic liver disease). In those patients with biliary obstruction, drainage should be performed prior to proceeded with embolization. The increased risk of variceal bleeding resulting from acute portal hypertension following PVE, although in theory possible, has not been a concern that has materialized to any significance in our experience. PVE is not necessarily indicated for FLR ≥ 30% in patients with non-diseased livers as this has not been shown to be of any benefit in a clinical trial and does subject patients to risk, albeit low, from the embolization procedure.49
Conclusion
In summary, PVE for HCC is a recognized modality which is well accepted and recognized to increase the functional liver remnant preoperatively in an effort to improve postoperative outcomes, as well as predict which patients are at excessive risk prior to surgical intervention. (Table 1) There are persistent critics who continue to deny the tangible benefits of PVE and demand additional justification in the form of a randomized clinical trial. The unethical denial of the benefit of this technique to allow safe resection to patients who are otherwise poor candidates for resection based on inadequate liver size or function makes such a study impossible.2 This advancement of the field with modern technology is a coordinated and compassionate attempt to quantifiably increase the potential pool of patients that can be offered a chance for possible curative resection, as the overall prognosis of patients with HCC is poor. The future of PVE in HCC rests with data and techniques emerging from basic and translational laboratories and animal models with the use of agents to further stimulate FLR growth while simultaneously preventing tumor propagation. All of these concerted efforts will serve to further increase the numbers of those able to undergo curative resection as well as improve the postoperative outcomes as well as subsequent survival.
References
- 1.Cunningham JD, Fong Y, Shriver C, Melendez J, Marx WL, Blumgart LH. One hundred consecutive hepatic resections. Blood loss, transfusion, and operative technique. Arch Surg. 1994;129:1050–1056. doi: 10.1001/archsurg.1994.01420340064011. [DOI] [PubMed] [Google Scholar]
- 2.Abdalla EK, Hicks ME, Vauthey JN. Portal vein embolization: rationale, technique and future prospects. Br J Surg. 2001;88:165–175. doi: 10.1046/j.1365-2168.2001.01658.x. [DOI] [PubMed] [Google Scholar]
- 3.Mullen JT, Ribero D, Reddy SK, Donadon M, Zorzi D, Gautam S, et al. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg. 2007;204:854–862. doi: 10.1016/j.jamcollsurg.2006.12.032. discussion 62-4. [DOI] [PubMed] [Google Scholar]
- 4.Shirabe K, Shimada M, Gion T, Hasegawa H, Takenaka K, Utsunomiya T, et al. Postoperative liver failure after major hepatic resection for hepatocellular carcinoma in the modern era with special reference to remnant liver volume. J Am Coll Surg. 1999;188:304–309. doi: 10.1016/s1072-7515(98)00301-9. [DOI] [PubMed] [Google Scholar]
- 5.Seyama Y, Kokudo N. Assessment of liver function for safe hepatic resection. Hepatol Res. 2009;39:107–116. doi: 10.1111/j.1872-034X.2008.00441.x. [DOI] [PubMed] [Google Scholar]
- 6.Teh SH, Christein J, Donohue J, Que F, Kendrick M, Farnell M, et al. Hepatic resection of hepatocellular carcinoma in patients with cirrhosis: Model of End-Stage Liver Disease (MELD) score predicts perioperative mortality. J Gastrointest Surg. 2005;9:1207–1215. doi: 10.1016/j.gassur.2005.09.008. discussion 15. [DOI] [PubMed] [Google Scholar]
- 7.Makuuchi M, Kosuge T, Takayama T, Yamazaki S, Kakazu T, Miyagawa S, et al. Surgery for small liver cancers. Semin Surg Oncol. 1993;9:298–304. doi: 10.1002/ssu.2980090404. [DOI] [PubMed] [Google Scholar]
- 8.Kiuchi T, Kasahara M, Uryuhara K, Inomata Y, Uemoto S, Asonuma K, et al. Impact of graft size mismatching on graft prognosis in liver transplantation from living donors. Transplantation. 1999;67:321–327. doi: 10.1097/00007890-199901270-00024. [DOI] [PubMed] [Google Scholar]
- 9.Kiuchi T, Tanaka K, Ito T, Oike F, Ogura Y, Fujimoto Y, et al. Small-for-size graft in living donor liver transplantation: how far should we go? Liver Transpl. 2003;9:S29–S35. doi: 10.1053/jlts.2003.50198. [DOI] [PubMed] [Google Scholar]
- 10.Soejima Y, Shimada M, Suehiro T, Hiroshige S, Ninomiya M, Shiotani S, et al. Outcome analysis in adult-to-adult living donor liver transplantation using the left lobe. Liver Transpl. 2003;9:581–586. doi: 10.1053/jlts.2003.50114. [DOI] [PubMed] [Google Scholar]
- 11.Urata K, Kawasaki S, Matsunami H, Hashikura Y, Ikegami T, Ishizone S, et al. Calculation of child and adult standard liver volume for liver transplantation. Hepatology. 1995;21:1317–1321. [PubMed] [Google Scholar]
- 12.Urata K, Hashikura Y, Ikegami T, Terada M, Kawasaki S. Standard liver volume in adults. Transplant Proc. 2000;32:2093–2094. doi: 10.1016/s0041-1345(00)01583-9. [DOI] [PubMed] [Google Scholar]
- 13.Johnson TN, Tucker GT, Tanner MS, Rostami-Hodjegan A. Changes in liver volume from birth to adulthood: a meta-analysis. Liver Transpl. 2005;11:1481–1493. doi: 10.1002/lt.20519. [DOI] [PubMed] [Google Scholar]
- 14.Deland FH, North WA. Relationship between Liver Size and Body Size. Radiology. 1968;91:1195–1968. doi: 10.1148/91.6.1195. [DOI] [PubMed] [Google Scholar]
- 15.Kawasaki S, Makuuchi M, Matsunami H, Hashikura Y, Ikegami T, Chisuwa H, et al. Preoperative measurement of segmental liver volume of donors for living related liver transplantation. Hepatology. 1993;18:1115–1120. [PubMed] [Google Scholar]
- 16.Heymsfield SB, Fulenwider T, Nordlinger B, Barlow R, Sones P, Kutner M. Accurate measurement of liver, kidney, and spleen volume and mass by computerized axial tomography. Ann Intern Med. 1979;90:185–187. doi: 10.7326/0003-4819-90-2-185. [DOI] [PubMed] [Google Scholar]
- 17.Starzl TE, Putnam CW, Groth CG, Corman JL, Taubman J. Alopecia, ascites, and incomplete regeneration after 85 to 90 per cent liver resection. Am J Surg. 1975;129:587–590. doi: 10.1016/0002-9610(75)90323-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vauthey JN, Chaoui A, Do KA, Bilimoria MM, Fenstermacher MJ, Charnsangavej C, et al. Standardized measurement of the future liver remnant prior to extended liver resection: methodology and clinical associations. Surgery. 2000;127:512–519. doi: 10.1067/msy.2000.105294. [DOI] [PubMed] [Google Scholar]
- 19.Vauthey JN, Abdalla EK, Doherty DA, Gertsch P, Fenstermacher MJ, Loyer EM, et al. Body surface area and body weight predict total liver volume in Western adults. Liver Transpl. 2002;8:233–240. doi: 10.1053/jlts.2002.31654. [DOI] [PubMed] [Google Scholar]
- 20.Kishi Y, Madoff DC, Abdalla EK, Palavecino M, Ribero D, Chun YS, et al. Is embolization of segment 4 portal veins before extended right hepatectomy justified? Surgery. 2008;144:744–751. doi: 10.1016/j.surg.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shoup M, Gonen M, D'Angelica M, Jarnagin WR, DeMatteo RP, Schwartz LH, et al. Volumetric analysis predicts hepatic dysfunction in patients undergoing major liver resection. J Gastrointest Surg. 2003;7:325–330. doi: 10.1016/s1091-255x(02)00370-0. [DOI] [PubMed] [Google Scholar]
- 22.Abdalla EK, Barnett CC, Doherty D, Curley SA, Vauthey JN. Extended hepatectomy in patients with hepatobiliary malignancies with and without preoperative portal vein embolization. Arch Surg. 2002;137:675–680. doi: 10.1001/archsurg.137.6.675. discussion 80-1. [DOI] [PubMed] [Google Scholar]
- 23.Kishi Y, Abdalla EK, Chun YS, Zorzi D, Madoff DC, Wallace MJ, et al. Three Hundred and One Consecutive Extended Right Hepatectomies: Evaluation of Outcome Based on Systematic Liver Volumetry. Ann Surg. 2009 doi: 10.1097/SLA.0b013e3181b674df. [DOI] [PubMed] [Google Scholar]
- 24.Kubota K, Makuuchi M, Kusaka K, Kobayashi T, Miki K, Hasegawa K, et al. Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology. 1997;26:1176–1181. doi: 10.1053/jhep.1997.v26.pm0009362359. [DOI] [PubMed] [Google Scholar]
- 25.Marcos A, Fisher RA, Ham JM, Olzinski AT, Shiffman ML, Sanyal AJ, et al. Selection and outcome of living donors for adult to adult right lobe transplantation. Transplantation. 2000;69:2410–2415. doi: 10.1097/00007890-200006150-00034. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez-Chamorro A, Loinaz Segurola C, Moreno Gonzalez E, Jimenez Romero C, Gonzalez-Pinto Arrillaga I, Gomez Sanz R, et al. Graft mass and volume calculation in living related donors for liver transplantation. Hepatogastroenterology. 1998;45:510–513. [PubMed] [Google Scholar]
- 27.Gruttadauria S, di Francesco F, Vizzini GB, Luca A, Spada M, Cintorino D, et al. Early graft dysfunction following adult-to-adult living-related liver transplantation: predictive factors and outcomes. World J Gastroenterol. 2009;15:4556–4560. doi: 10.3748/wjg.15.4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Selzner M, Kashfi A, Cattral MS, Selzner N, Greig PD, Lilly L, et al. A graft to body weight ratio less than 0.8 does not exclude adult-to-adult right-lobe living donor liver transplantation. Liver Transpl. 2009;15:1776–1782. doi: 10.1002/lt.21955. [DOI] [PubMed] [Google Scholar]
- 29.Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy--definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716–7121. doi: 10.1053/jhep.2002.31250. [DOI] [PubMed] [Google Scholar]
- 30.Paugam-Burtz C, Janny S, Delefosse D, Dahmani S, Dondero F, Mantz J, et al. Prospective validation of the "fifty-fifty" criteria as an early and accurate predictor of death after liver resection in intensive care unit patients. Ann Surg. 2009;249:124–128. doi: 10.1097/SLA.0b013e31819279cd. [DOI] [PubMed] [Google Scholar]
- 31.Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, et al. The "50-50 criteria" on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242:824–828. doi: 10.1097/01.sla.0000189131.90876.9e. discussion 8–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schindl MJ, Redhead DN, Fearon KC, Garden OJ, Wigmore SJ. The value of residual liver volume as a predictor of hepatic dysfunction and infection after major liver resection. Gut. 2005;54:289–296. doi: 10.1136/gut.2004.046524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Regimbeau JM, Kianmanesh R, Farges O, Dondero F, Sauvanet A, Belghiti J. Extent of liver resection influences the outcome in patients with cirrhosis and small hepatocellular carcinoma. Surgery. 2002;131:311–317. doi: 10.1067/msy.2002.121892. [DOI] [PubMed] [Google Scholar]
- 34.Tjandra JJ, Fan ST, Wong J. Peri-operative mortality in hepatic resection. Aust N Z J Surg. 1991;61:201–206. doi: 10.1111/j.1445-2197.1991.tb07592.x. [DOI] [PubMed] [Google Scholar]
- 35.Tanabe G, Sakamoto M, Akazawa K, Kurita K, Hamanoue M, Ueno S, et al. Intraoperative risk factors associated with hepatic resection. Br J Surg. 1995;82:1262–1265. doi: 10.1002/bjs.1800820935. [DOI] [PubMed] [Google Scholar]
- 36.Kokudo N, Makuuchi M. Current role of portal vein embolization/hepatic artery chemoembolization. Surg Clin North. Am. 2004;84:643–657. doi: 10.1016/j.suc.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 37.Belghiti J, Ogata S. Assessment of hepatic reserve for the indication of hepatic resection. J Hepatobiliary Pancreat Surg. 2005;12:1–3. doi: 10.1007/s00534-004-0951-2. [DOI] [PubMed] [Google Scholar]
- 38.Kinoshita H, Sakai K, Hirohashi K, Igawa S, Yamasaki O, Kubo S. Preoperative portal vein embolization for hepatocellular carcinoma. World J Surg. 1986;10:803–808. doi: 10.1007/BF01655244. [DOI] [PubMed] [Google Scholar]
- 39.Makuuchi M, Thai BL, Takayasu K, Takayama T, Kosuge T, Gunven P, et al. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery. 1990;107:521–527. [PubMed] [Google Scholar]
- 40.Uesaka K, Nimura Y, Nagino M. Changes in hepatic lobar function after right portal vein embolization. An appraisal by biliary indocyanine green excretion. Ann Surg. 1996;223:77–83. doi: 10.1097/00000658-199601000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hirai I, Kimura W, Fuse A, Suto K, Urayama M. Evaluation of preoperative portal embolization for safe hepatectomy, with special reference to assessment of nonembolized lobe function with 99mTc-GSA SPECT scintigraphy. Surgery. 2003;133:495–506. doi: 10.1067/msy.2003.138. [DOI] [PubMed] [Google Scholar]
- 42.Ijichi M, Makuuchi M, Imamura H, Takayama T. Portal embolization relieves persistent jaundice after complete biliary drainage. Surgery. 2001;130:116–118. doi: 10.1067/msy.2001.115358. [DOI] [PubMed] [Google Scholar]
- 43.Abulkhir A, Limongelli P, Healey AJ, Damrah O, Tait P, Jackson J, et al. Preoperative portal vein embolization for major liver resection: a meta-analysis. Ann Surg. 2008;247:49–57. doi: 10.1097/SLA.0b013e31815f6e5b. [DOI] [PubMed] [Google Scholar]
- 44.Wakabayashi H, Okada S, Maeba T, Maeta H. Effect of preoperative portal vein embolization on major hepatectomy for advanced-stage hepatocellular carcinomas in injured livers: a preliminary report. Surg Today. 1997;27:403–410. doi: 10.1007/BF02385702. [DOI] [PubMed] [Google Scholar]
- 45.Tanaka H, Hirohashi K, Kubo S, Shuto T, Higaki I, Kinoshita H. Preoperative portal vein embolization improves prognosis after right hepatectomy for hepatocellular carcinoma in patients with impaired hepatic function. Br J Surg. 2000;87:879–882. doi: 10.1046/j.1365-2168.2000.01438.x. [DOI] [PubMed] [Google Scholar]
- 46.Wakabayashi H, Ishimura K, Okano K, Izuishi K, Karasawa Y, Goda F, et al. Is preoperative portal vein embolization effective in improving prognosis after major hepatic resection in patients with advanced-stage hepatocellular carcinoma? Cancer. 2001;92:2384–2390. doi: 10.1002/1097-0142(20011101)92:9<2384::aid-cncr1586>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 47.Azoulay D, Castaing D, Krissat J, Smail A, Hargreaves GM, Lemoine A, et al. Percutaneous portal vein embolization increases the feasibility and safety of major liver resection for hepatocellular carcinoma in injured liver. Ann Surg. 2000;232:665–672. doi: 10.1097/00000658-200011000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palavecino M, Chun YS, Madoff DC, Zorzi D, Kishi Y, Kaseb AO, et al. Major hepatic resection for hepatocellular carcinoma with or without portal vein embolization: Perioperative outcome and survival. Surgery. 2009;145:399–405. doi: 10.1016/j.surg.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 49.Farges O, Belghiti J, Kianmanesh R, Regimbeau JM, Santoro R, Vilgrain V, et al. Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Surg. 2003;237:208–217. doi: 10.1097/01.SLA.0000048447.16651.7B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ribero D, Abdalla EK, Madoff DC, Donadon M, Loyer EM, Vauthey JN. Portal vein embolization before major hepatectomy and its effects on regeneration, resectability and outcome. Br J Surg. 2007;94:1386–1394. doi: 10.1002/bjs.5836. [DOI] [PubMed] [Google Scholar]
- 51.Goto Y, Nagino M, Nimura Y. Doppler estimation of portal blood flow after percutaneous transhepatic portal vein embolization. Ann Surg. 1998;228:209–213. doi: 10.1097/00000658-199808000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamakado K, Takeda K, Matsumura K, Nakatsuka A, Hirano T, Kato N, et al. Regeneration of the un-embolized liver parenchyma following portal vein embolization. J Hepatol. 1997;27:871–880. doi: 10.1016/s0168-8278(97)80325-x. [DOI] [PubMed] [Google Scholar]
- 53.Denys AL, Abehsera M, Leloutre B, Sauvanet A, Vilgrain V, O'Toole D, et al. Intrahepatic hemodynamic changes following portal vein embolization: a prospective Doppler study. Eur Radiol. 2000;10:1703–1707. doi: 10.1007/s003300000577. [DOI] [PubMed] [Google Scholar]
- 54.Nagino M, Nimura Y, Kamiya J, Kanai M, Hayakawa N, Yamamoto H. Immediate increase in arterial blood flow in embolized hepatic segments after portal vein embolization: CT demonstration. AJR Am J Roentgenol. 1998;171:1037–1039. doi: 10.2214/ajr.171.4.9762992. [DOI] [PubMed] [Google Scholar]
- 55.Hayashi S, Baba Y, Ueno K, Nakajo M, Kubo F, Ueno S, et al. Acceleration of primary liver tumor growth rate in embolized hepatic lobe after portal vein embolization. Acta Radiol. 2007;48:721–727. doi: 10.1080/02841850701424514. [DOI] [PubMed] [Google Scholar]
- 56.Ogata S, Belghiti J, Farges O, Varma D, Sibert A, Vilgrain V. Sequential arterial and portal vein embolizations before right hepatectomy in patients with cirrhosis and hepatocellular carcinoma. Br J Surg. 2006;93:1091–1098. doi: 10.1002/bjs.5341. [DOI] [PubMed] [Google Scholar]
- 57.Aoki T, Imamura H, Hasegawa K, Matsukura A, Sano K, Sugawara Y, et al. Sequential preoperative arterial and portal venous embolizations in patients with hepatocellular carcinoma. Arch Surg. 2004;139:766–774. doi: 10.1001/archsurg.139.7.766. [DOI] [PubMed] [Google Scholar]