Abstract
Purpose
To evaluate the association between aromatase inhibitor (AI) therapy and cognitive function (over a 6-month time period) in a cohort of patients age ≥ 60 compared with an age-matched healthy control group, and to evaluate changes in regional cerebral metabolism as measured by positron emission tomography (PET) scans of the brain done in a subset of the patient cohort.
Patients and Methods
Thirty-five patients (32 evaluable) and 35 healthy controls were recruited to this study. Patients with breast cancer completed a neuropsychological battery, self-reported memory questionnaire, and geriatric assessment prior to initiation of AI therapy and again 6 months later. Age-matched healthy control participants completed the same assessments at the same time points as the patient group.
Results
No significant decline in cognitive function was seen among individuals receiving an AI from pre-treatment to 6 months later compared with healthy controls. In the PET cohort over the same period, both standardized volume of interest (sVOI) and statistical parametric mapping (SPM) analyses detected specific changes in metabolic activity between baseline and follow-up uniquely in the AI patients, uniquely, most significantly in medial temporal lobes.
Conclusion
While patients undergoing AI treatment demonstrated few changes in neuropsychologic performance compared with healthy controls over a 6-month period during this interval, regionally specific changes in cerebral metabolic activity were identified in the patient group. Additional longitudinal follow-up is needed to understand the potential clinical implications of these findings.
Keywords: cognitive function, older adults, breast cancer, aromatase inhibitors, PET scan
Introduction
A growing body of literature has evaluated the potential effect of breast cancer therapy on cognitive function. There are limited data regarding the association between endocrine therapy and cognition, however, and despite the fact that breast cancer is a disease associated with aging, most studies have been performed with relatively young adults, so the impact of endocrine therapy for breast cancer on the cognition of older adults remains unknown.
Aromatase inhibitors – a mainstay of treatment for hormone receptor-positive, early-stage breast cancer in postmenopausal women – inhibit the enzyme aromatase, which leads to a reduction in estrogen levels throughout the body. Since estrogen receptors are spread throughout the brain, and studies have shown that estrogen promotes neuron growth and provides neuroprotective activity in vitro, there is a biologic reason to question whether aromatase inhibition might influence cognitive function.1–3
Conflicting data from randomized controlled studies exist concerning the impact of both estrogen replacement and estrogen deprivation on cognitive function in the clinical setting.4–8 Likewise, clinical studies examining the effects of endocrine therapy on cognitive function of patients with breast cancer have produced inconsistent results, with some,9–12 but not all,13 suggesting a decline in cognitive function resulting from treatment.
The biologic basis of cognitive change as a result of cancer therapies is poorly understood. Previously, Silverman et al. demonstrated that treatment-related regional changes in brain metabolism are associated with changes in neuropsychological performance.14 For example, diminished metabolism in the posterior inferior frontal gyrus in the vicinity of Broca’s area was specifically associated with diminished performance on a neuropsychological test of short-term memory in patients with breast cancer who had received adjuvant therapy.
In this study, we sought to use neuropsychological testing to examine the association between aromatase inhibitor (AI) therapy and cognitive function in a cohort of patients age ≥ 60 compared with an age-matched healthy control group and to evaluate changes in regional cerebral metabolism as measured by positron emission tomography (PET) scans of the brain performed for a subset of the patient cohort. We hypothesized that there would not be short-term changes in cognitive function among patients taking an AI compared to an age-matched healthy control group; however, regional changes in brain metabolism on PET imaging may be seen.
Materials and Methods
Study Population
Thirty-five patients (32 evaluable) and 35 healthy controls were recruited to the study. Patients age ≥ 60 with hormone receptor-positive stage I-III breast cancer who were about to receive adjuvant AI therapy as systemic therapy for breast cancer were eligible for the study and were recruited from the outpatient practice at City of Hope National Medical Center. These patients had received surgical treatment for their breast cancer and chemotherapy (if indicated). An age-matched healthy control group, solicited through the services of Marketing Systems Group, was recruited to participate in the study to enable comparison with the patients receiving AI therapy. Three patients who missed follow-up assessments were excluded from analysis. The study was approved by the institutional review board, and all study participants provided written, informed consent.
Patients were deemed ineligible if they had received estrogen replacement therapy within the past year or previous radiation treatment of the central nervous system. Other eligibility criteria included literacy in English, since many of the study measures were not validated in other languages.
Study Procedure
Study participants with breast cancer completed a neuropsychological battery, a self-reported memory questionnaire, and a geriatric assessment prior to initiation of AI therapy as well as 6 months later. Age-matched study participants in the healthy control group completed the same assessments at the same time points as the patient group.
The neuropsychological battery consisted of 13 standardized tests of neuropsychological function across seven domains: attention; verbal memory; visual memory; verbal, spatial, psychomotor, and executive functions (Table 1). The tests were chosen for succinctness, reliability, validity, and past use to enable comparison with normative data. This battery was previously tested in a study of older patients with breast cancer.15
Table 1.
Domains and Measures Assessed
| Measures | Description |
|---|---|
| Geriatric Assessment | |
|
| |
| Functional Status | |
|
|
| Comorbidity | |
| Physical Health Section (OARS Subscale) 23 | Evaluates the presence/absence of 13 comorbid illnesses and how much they interfere with daily activities. |
| Psychological | |
| Hospital Anxiety and Depression Scale 24–28 | Assessment of depression and anxiety levels based on mood, feelings, and emotions in the past week. |
| Cognition | |
| Squire Memory Self-Rating Questionnaire 16 | 18 item self-assessment of cognitive function |
|
| |
| Neuropsychological Battery | |
|
| |
| Verbal Function | |
|
|
| Verbal Learning and Memory | |
| Hopkins Verbal Learning Test – Revised 32 | A brief verbal learning and memory test which includes delayed recall and recognition trials. |
| Visual Memory | |
| Rey-Osterrieth Complex Figure Test 33 (copy, immediate, and delayed recall) | A measure of visuospatial construction and visual memory. |
| Spatial Function | |
|
|
| Psychomotor Function | |
|
|
| Attention | |
| Trail Making Test – Part A 35 | A measure of divided attention and cognitive flexibility. |
| Executive Function | |
|
|
Abbreviations: WRAT-4, Wide Range Achievement Test, 4th Edition; WAIS-III, Wechsler Adult Intelligence Scale, 3rd Edition.
The patients’ self-reported assessment of their cognitive function was collected through the Squire Memory Self-Rating Questionnaire.16 The questionnaire contains 18 items of self-reported cognitive function rated on a scale from −4 to +4. Three of the questions were found to have ambiguous loadings and were excluded from analysis, consistent with methodology used in a previously reported study 16. The participants also completed a geriatric assessment including validated measures of functional status, comorbid medical conditions, psychological state, social support, nutritional status, cognitive function, and medications.17,18
Ten patients and ten healthy controls completed a PET scan at both time points, to assess changes in regional cerebral metabolism. [F-18]-labeled fluorodeoxyglucose (FDG), was used as the tracer. At each time point, 5 millicuries of FDG were administered intravenously. After a 40-minute period of tracer uptake in a dimly-lit, quiet room, emission data were acquired for 30 minutes with an HR+ dedicated PET scanner (Siemens/CTI). Images were attenuation-corrected with emission data obtained from an external positron-emitting source, and summed over the acquisition period to yield a three-dimensional representation of the regional distribution of resting metabolism.19
Statistical Analysis
Using independent samples t-tests and chi-squared tests, 32 patients receiving adjuvant AI therapy were compared with 35 healthy controls by baseline demographic characteristics as well as the functional domains that constitute the geriatric assessment battery. The longitudinal analysis evaluated the change in neuropsychological performance between baseline and 6-month follow-up using paired t-tests. Standard scoring of neuropsychological tasks was based on population norms and adjusted for age, sex, and, in some cases, education. Comparisons between patients and their healthy counterparts were performed at baseline using independent sample t-tests. In order to control for the practice effects associated with repeated cognitive testing and to assess the clinical significance of changes in neuropsychological function, the degree of longitudinal change observed in patients was compared to that observed in controls using a t-test.
Prior to analysis, PET images were reoriented into standardized space, spatially smoothed (FWHM 8 mm), and normalized to mean whole-brain metabolic activity. As previously described,19,20 data were analyzed by (1) a standardized volume of interest (sVOI) approach using NeuroQ software (Syntermed Inc., Atlanta) and (2) a voxel-based statistical parametric mapping (spm) method using SPM8 software generously provided by the Wellcome Trust Centre for Neuroimaging (London). To statistically protect for multiple comparisons, regions identified by spm were noted only when containing voxels with significance P < 0.0005, and the sVOI approach was used for methodologically independent corroboration of location of changes in metabolism observed with spm. Furthermore, it was established a priori that only significant longitudinal changes in neuropsychological findings would be correlated with PET findings.
Results
Patient Characteristics
The healthy control group in this study did not significantly differ from our patient cohort with regard to age, race, education, employment and marital status, and previous hormone replacement therapy (Table 2). Fourteen study subjects had received prior hormone replacement therapy. Seven patients had received prior chemotherapy treatment and 12 patients had prior radiation therapy. Among the patient group that underwent PET imaging, only one had had prior chemotherapy and three had prior radiation therapy to the breast. Functional status, as evaluated by the geriatric assessment, statistically differed between the two groups on Instrumental Activities of Daily Living (IADLs) at both baseline and follow-up (P < 0.01), such that healthy controls reported a higher level of functioning than the patient group (Table 3).
Table 2.
Baseline Demographics
| Variable | Control (n = 35) | Case (n = 32)* | P-value | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Age, years | |||||
| Mean | 71.1 | NA | 72.4 | NA | |
| SD | 7.37 | NA | 7.01 | NA | 0.48 |
| Range | 60–85 | NA | 62–89 | NA | |
| Race | |||||
| White | 28 | 82.4 | 28 | 87.5 | |
| Asian | 3 | 8.8 | 1 | 3.1 | 0.63 |
| Other | 3 | 8.8 | 3 | 9.4 | |
| Marital Status | |||||
| Single | 2 | 5.7 | 2 | 6.3 | |
| Married | 18 | 51.4 | 19 | 59.4 | 0.81 |
| Widowed | 9 | 25.7 | 8 | 25.0 | |
| Separated/divorced | 6 | 17.1 | 3 | 9.4 | |
| Education | |||||
| High school or less | 7 | 20.0 | 7 | 21.9 | |
| Some college | 13 | 37.1 | 12 | 37.5 | |
| College degree | 3 | 8.6 | 6 | 18.8 | 0.52 |
| Advanced degree | 12 | 34.3 | 7 | 21.9 | |
| Employment | |||||
| Employed | 7 | 20.0 | 3 | 9.4 | |
| Homemaker | 4 | 11.4 | 4 | 12.5 | 0.32 |
| Retired | 24 | 68.6 | 23 | 71.9 | |
| Other | 0 | 0.0 | 2 | 6.3 | |
| Stage | |||||
| I | NA | NA | 15 | 46.9 | |
| II | NA | NA | 13 | 40.6 | NA |
| III | NA | NA | 4 | 12.5 | |
| Prior treatment | |||||
| Chemotherapy | NA | NA | 7 | 21.9 | |
| Radiation | NA | NA | 12 | 43.8 | NA |
| Hormone replacement | 14 | 40.0% | 14 | 43.8 | |
3 patients were excluded from the analysis due to missing follow-up assessments.
Abbreviations: SD: standard deviation; NA: not applicable.
Table 3.
Geriatric Assessment by Time Point
| Variable | n | Control (n = 35) | Case (n = 32)* | P-value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | n | Mean | SD | Range | |||
| Baseline | |||||||||
| IADL | 35 | 13.9 | 0.43 | 12–14 | 31 | 13.3 | 0.65 | 12–14 | 0.0003 |
| ADL | 35 | 11.9 | 0.36 | 11–12 | 32 | 11.9 | 0.39 | 10–12 | 0.5914 |
| Charlson | 35 | 3.3 | 1.25 | 2–7 | 31 | 3.5 | 1.21 | 2–6 | 0.4505 |
| Squire Memory Self-Rating Questionnaire | 33 | −1.5 | 17.02 | −38–49 | 30 | −4.8 | 18.78 | −45–50 | 0.4779 |
| HADS | 35 | 5.8 | 3.80 | 0–14 | 32 | 6.4 | 3.98 | 1–15 | 0.5281 |
| Follow-up | |||||||||
| IADL | 34 | 13.8 | 0.54 | 12–14 | 32 | 13.1 | 1.32 | 8–14 | 0.0057 |
| ADL | 35 | 11.9 | 0.24 | 11–12 | 31 | 11.8 | 0.45 | 10–12 | 0.2576 |
| Charlson | 35 | 3.5 | 1.46 | 2–9 | 32 | 3.3 | 1.04 | 2–5 | 0.7176 |
| Squire Memory Self-Rating Questionnaire | 33 | −8.7 | 12.29 | −49–11 | 29 | 6.2 | 30.44 | −28.5–96 | 0.0187 |
| HADS | 35 | 4.9 | 4.33 | 0–18 | 32 | 6.8 | 5.06 | 0–19 | 0.1088 |
3 patients were excluded from the analysis due to missing follow-up assessments.
Abbreviations: SD: standard deviation; IADL: Instrumental Activities of Daily Living; ADL: Activities of Daily Living; HADS: Hospital Anxiety and Depression Scale.
Neuropsychological Assessment (Tables 3–5)
Table 5.
Longitudinal Change in Neurocognitive Performance
| Variable | Baseline (T1) | 6-month follow-up (T2) | Difference (T2-T1) | Pa | Pb | ||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||
| WAIS Digit Symbol | |||||||
| Control | 12.4 | 2.93 | 12.7 | 3.09 | 0.3 | 0.3419 | 0.3261 |
| Patient | 10.8 | 2.52 | 11.4 | 2.63 | 0.6 | 0.0210 | |
| Block Design | |||||||
| Control | 11.4 | 3.03 | 11.3 | 2.93 | −0.1 | 0.8005 | 0.0227 |
| Patient | 10.9 | 2.79 | 11.8 | 3.43 | 0.9 | 0.0116 | |
| Trail Making Test A | |||||||
| Control | 10.1 | 2.66 | 10.2 | 2.85 | 0.1 | 0.8351 | 0.9885 |
| Patient | 9.6 | 2.88 | 9.7 | 2.23 | 0.1 | 0.8037 | |
| Trail Making Test B | |||||||
| Control | 10.5 | 2.86 | 10.5 | 2.97 | 0.0 | 0.9478 | 0.6814 |
| Patient | 9.3 | 3.44 | 9.5 | 3.06 | 0.2 | 0.4370 | |
| FAS | |||||||
| Control | −0.3 | 0.76 | −0.1 | 0.85 | 0.2 | 0.0735 | 0.2898 |
| Patient | −0.3 | 0.89 | −0.3 | 0.89 | 0.0 | 0.4720 | |
| WRAT-4 Reading Test | |||||||
| Control | 105.6 | 16.84 | 98.8 | 9.68 | −6.8 | 0.0004 | 0.0005 |
| Patient | 103.1 | 13.65 | 104.8 | 11.29 | 1.7 | 0.2626 | |
| Boston Naming Test | |||||||
| Control | 0.3 | 0.93 | 0.6 | 0.76 | 0.3 | 0.0567 | 0.1075 |
| Patient | −0.8 | 1.90 | −0.2 | 1.52 | 0.6 | 0.0030 | |
| STROOP-word page | |||||||
| Control | 43.1 | 14.07 | 42.6 | 13.12 | −0.5 | 0.6616 | 0.3999 |
| Patient | 45.4 | 9.79 | 43.5 | 9.98 | −1.9 | 0.1192 | |
| STROOP-color page | |||||||
| Control | 44.6 | 13.66 | 45.3 | 14.21 | 0.7 | 0.3830 | 0.2939 |
| Patient | 39.3 | 8.45 | 41.2 | 7.52 | 1.9 | 0.0368 | |
| SRTOOP-color word page | |||||||
| Control | 47.6 | 10.72 | 50.1 | 10.37 | 2.5 | 0.0133 | 0.4902 |
| Patient | 47.3 | 8.49 | 48.8 | 8.79 | 1.5 | 0.1669 | |
| STROOP-interference | |||||||
| Control | 45.7 | 8.70 | 47.9 | 7.62 | 2.2 | 0.0557 | 0.6020 |
| Patient | 46.2 | 7.57 | 47.6 | 8.60 | 1.4 | 0.2518 | |
| HVLT-total | |||||||
| Control | 52.6 | 8.69 | 51.3 | 10.50 | −1.3 | 0.3122 | 0.7509 |
| Patient | 50.5 | 10.10 | 48.5 | 11.35 | −2.0 | 0.2754 | |
| HVLT-delayed Recall | |||||||
| Control | 51.1 | 10.40 | 50.1 | 10.93 | −1.0 | 0.4863 | 0.9008 |
| Patient | 51.7 | 9.22 | 51.0 | 10.65 | −0.7 | 0.6577 | |
| HVLT-retention | |||||||
| Control | 49.2 | 9.35 | 50.7 | 9.78 | 1.5 | .4804 | .7561 |
| Patient | 51.4 | 8.07 | 53.7 | 8.32 | 2.3 | .2001 | |
| HVLT-RDI | |||||||
| Control | 50.3 | 9.19 | 52.1 | 8.91 | 1.8 | 0.2440 | 0.4593 |
| Patient | 49.6 | 9.17 | 49.8 | 10.41 | 0.2 | 0.9407 | |
| ROCF-copy | |||||||
| Control | 28.7 | 4.00 | 29.0 | 3.89 | 0.3 | 0.5222 | 0.5852 |
| Patient | 28.0 | 6.05 | 28.7 | 5.18 | 0.7 | 0.2648 | |
| ROCF-immediate recall | |||||||
| Control | 49.7 | 11.95 | 54.2 | 11.86 | 4.5 | 0.0015 | 0.7285 |
| Patient | 46.8 | 13.76 | 50.5 | 12.89 | 3.7 | 0.0370 | |
| ROCF-delayed recall | |||||||
| Control | 48.5 | 12.30 | 54.3 | 12.24 | 5.8 | 0.0018 | 0.4208 |
| Patient | 46.5 | 16.26 | 50.2 | 14.33 | 3.7 | 0.0723 | |
Abbreviations: SD: standard deviation; WAIS: Wechsler Adult Intelligence Scale; WRAT: Wide Range Achievement Test, 4th edition; HVLT: Hopkins Verbal Learning Test; RDI: Recognition Discrimination Index; ROCF: Rey-Osterreith Complex Figure.
P-value for change between T1 and T2.
P-value for difference in slope between cases and controls.
At baseline, the control group had significantly higher scores than the patient group on the Wechsler Adult Intelligence Scale (WAIS) Digit Symbol test (P = 0.02) and the Boston Naming Test (P = 0.01); the latter difference persisted at 6 months, but not the former, as patient performance significantly improved from baseline to 6-month follow-up in WAIS Digit Symbol (P = 0.02). Patient performance also improved with respect to Block Design (P = 0.01), Boston Naming Test (P = 0.003), Stroop Color Page (P = 0.04), and Rey-Osterreith Complex Figure (ROCF) Immediate Recall (P = 0.04). Longitudinal change significantly differed between the two groups on Block Design (P = 0.02) and WRAT-4 reading test (P = 0.0005), the latter due to a significant decrease that was seen only in the control group. At follow-up, patients also reported significantly better memory function on the Squire Memory Self-Rating Questionnaire than the controls (P = 0.02; Table 3)
PET Imaging
sVOI analyses revealed change to four cortical regions between baseline and 6-month follow-up in patients with breast cancer who underwent AI therapy. Relative to baseline, anterior medial temporal activity tended to increase bilaterally (left, P = 0.02; right, P = 0.06), as did left posterior medial temporal activity (P = 0.03). In addition, a region in the vicinity of Broca’s area showed decreased activity (P = 0.02) following AI therapy. These changes were not observed in the control group.
SPM analyses revealed increased metabolism in bilateral medial temporal and cerebellar regions in patients who underwent AI therapy, with the largest and most significant cluster of increased metabolism occurring in the right medial temporal lobe (P < 0.0005). Direct statistical comparison of longitudinal changes between the patient group and control group further demonstrated that the change observed in this region differed significantly between groups (P < 0.0005) (Figure 1). Finally, in cancer patients who had received AI, a positive correlation between Block z-scores (an index of visuospatial ability) and bilateral occipital region activity was observed (P < 0.0005). This relationship also appeared to be therapy-specific, in that it was only present at follow-up in patients who had received AI, and was not observed at baseline, or at either time point in controls.
Fig 1.
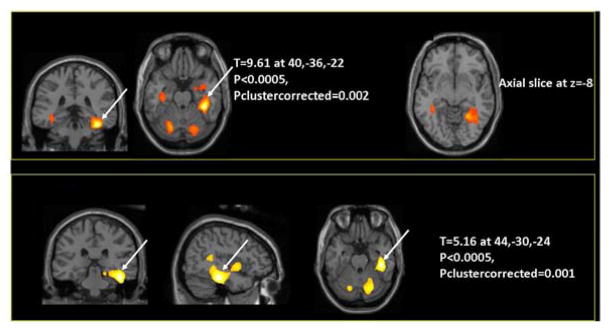
The color scale in all images represents a statistical mapping of voxels in subjects' brain tissue, which are overlaid upon the structural images of the gray scale for anatomical reference. Top panel: regional increases in cerebral metabolic activity within the patient group from before initiation of AI therapy to six months after AI therapy was initiated, demonstrating increased metabolism in bilateral medial temporal (P < 0.0005) and bilateral cerebellar (P < 0.0005) areas, with the largest and most significant increase occurring in the right medial temporal region. Bottom panel: direct statistical comparison of longitudinal changes in the patient group relative to changes over the same time interval in the control group confirming that patients who received AI therapy experienced increases in metabolism that were greater than any increases occurring among control subjects, with the most significant inter-group difference also occurring in the right medial temporal area (P < 0.0005).
Discussion
The literature regarding the association of endocrine treatment with cognitive function is conflicting. Several studies support the idea that treatment is associated with cognitive decline in patients with breast cancer. A study by Jenkins et al. indicated that patients taking anastrozole, tamoxifen, or the combination, experienced cognitive impairments compared with a healthy control group, specifically in processing speed and immediate verbal memory.9 A study by Collins et al. found similar results, with patients taking anastrozole or tamoxifen experiencing a decline in cognitive function (from start of endocrine therapy to 6 months later) when compared with healthy controls.11 Relative to healthy controls, patients receiving anastrozole demonstrated a nine-fold increase in risk for cognitive decline. Also, in the BIG 1-98 trial, Phillips et al. found that cognition significantly improved from end of endocrine therapy to one year after treatment stopped.21
Other studies, however, have demonstrated no association between AI treatment and cognitive function. A study by Jenkins et al. concluded that use of anastrozole had little or no association with impairment of cognitive performance compared with a placebo in women at increased risk for developing breast cancer.13 Also, a study by Schilder et al. examined tamoxifen and exemestane as adjuvant therapies in postmenopausal patients with breast cancer.12 That study found that one year of exemestane treatment was not associated with significant negative effects on cognitive function, although patients receiving tamoxifen did show lower functioning in verbal memory and executive functioning compared with healthy controls. This study did suggest age-dependent effects of tamoxifen on cognition.
The PET scan findings showed an increase in bilateral anterior medial temporal activity, left posterior medial temporal activity, and cerebellar region activity, and a decrease in the Broca’s area activity following AI therapy. These changes were not observed in the controls. In order to put these results into a clinical context, the medial temporal area is responsible for long-term memory. The cerebellar area is primarily responsible for motor control and coordination, with lesser roles in attention and language. Broca’s area is responsible for speech production. Although the estrogen pathway plays a role in verbal memory and language, the significance of these PET findings is unclear in the relative absence of neuropsychological test findings. The PET scan did show a correlation between scores on the Block Design and occipital region activity. The Block Design test is a test of visuospatial ability and the occipital region of the brain, as well as the right temporal lobe, plays a key role in this function.
Most prior studies examining the effects of endocrine therapy on cognition have involved subjects treated with tamoxifen, or a mixture of subjects treated with either tamoxifen or aromatase inhibitors. This is the first study, to our knowledge, to utilize PET scans to understand cognitive function in patients with breast cancer who are receiving AI therapy. To date, only one other study has incorporated a PET scan component to investigate cerebral dysfunction through examination of brain metabolism in patients with breast cancer. That study, by Silverman and colleagues, showed that breast cancer survivors (5–10 years after completion of chemotherapy) had alterations in basal ganglia, frontocortical, and cerebellar activity. However, patients who received endocrine therapy received tamoxifen (not an AI).14 In sum, the PET scan findings of this study are intriguing, and represent the first reported data regarding changes in regional cerebral metabolism among patients receiving AI therapy. Further follow-up is warranted.
Limitations to this study include its modest sample size, and the PET scans of the brain were only performed in a subset of our cohort. The sample was also heterogeneous, with administration of chemotherapy in 21% of the patients. Furthermore, although the study design did include a healthy control group, we did not accrue a cancer group who did not undergo endocrine therapy. A correction for multiple comparisons was performed for the PET scan findings; however, for the neuropsychological tests results we reported any results with P < 0.05, without doing a correction for multiple comparisons, in order to identify whether there were any signals from the neuropsychological testing to guide the selection of the neuropsychological tests to compare with brain metabolism.
Despite these limitations, this study has some notable strengths. We specifically sought to study older patients whose age would be representative of the majority of patients with breast cancer, and compared these results with that of an age-matched healthy control group. We sought to further our understanding of the biology of aromatase inhibition and estrogen deprivation via PET scans of the brain, which confirmed changes in central nervous system (CNS) glucose metabolism. The clinical significance of these findings is unknown, and further long-term follow-up is warranted.
Conclusion
This study evaluated the impact of aromatase inhibition on cerebral function, as assessed by changes in neuropsychologic performance, and regional cerebral metabolism over six months. Overall, no dramatic effects of AI therapy on neuropsychologic performance were seen, and the few changes that were observed tended to be more favorable for the patient group than the control group. At the same time, both sVOI and SPM analyses detected specific changes in metabolic activity between baseline and follow-up in the patient group receiving AI, and not in the control group, with the largest and most significant change being an increase in medial temporal metabolism. In light of the similarity between the groups on neuropsychological testing, the PET findings may represent early detection of neuropsychological changes that had not yet manifested, or reflect a higher sensitivity for the detection of cerebral metabolic changes than changes in neuropsychologic performance. Alternatively, these findings could provide insight into compensatory mechanisms employed by patients to maintain neuropsychological performance.
Table 4.
Neuropsychological Assessment by Time Point
| Variable | Control (n=35) | Case (n=32)* | P-value | ||||
|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | ||
| Baseline | |||||||
| WAIS Digit Symbol | 35 | 12.4 | 2.93 | 32 | 10.8 | 2.52 | 0.0189 |
| Block Design | 35 | 11.4 | 3.03 | 32 | 10.9 | 2.79 | 0.5455 |
| Trail Making Test A | 35 | 10.1 | 2.66 | 32 | 9.6 | 2.88 | 0.4987 |
| Trail Making Test B | 35 | 10.5 | 2.86 | 32 | 9.3 | 3.44 | 0.1135 |
| FAS | 35 | −0.3 | 0.76 | 32 | −0.3 | 0.89 | 0.9288 |
| WRAT-4 Naming list | 35 | 105.6 | 16.84 | 32 | 103.1 | 13.65 | 0.5130 |
| Boston Naming Test | 35 | 0.2 | 1.18 | 32 | −0.8 | 1.90 | 0.0141 |
| STROOP | |||||||
| -Word Page | 35 | 43.1 | 14.07 | 32 | 45.4 | 9.79 | 0.4388 |
| -Color Page | 35 | 44.6 | 13.66 | 32 | 39.3 | 8.45 | 0.0582 |
| -Color Word Page | 35 | 47.6 | 10.72 | 32 | 47.3 | 8.49 | 0.8947 |
| -Interference | 35 | 45.7 | 8.70 | 32 | 46.2 | 7.57 | 0.7798 |
| HVLT | |||||||
| -Total | 35 | 52.6 | 8.69 | 32 | 50.5 | 10.10 | 0.3572 |
| -Delayed Recall | 35 | 51.1 | 10.40 | 31 | 51.7 | 9.22 | 0.7894 |
| -Retention | 35 | 49.2 | 9.35 | 31 | 51.4 | 8.07 | 0.3296 |
| -RDI | 35 | 50.3 | 9.19 | 31 | 49.6 | 9.17 | 0.7878 |
| ROCF | |||||||
| -Copy | 35 | 28.7 | 4.00 | 32 | 28.0 | 6.05 | 0.5837 |
| -Immediate Recall | 35 | 49.7 | 11.95 | 32 | 46.8 | 13.76 | 0.3444 |
| -Delayed Recall | 35 | 48.5 | 12.30 | 32 | 46.5 | 16.26 | 0.5558 |
| Follow-up | |||||||
| WAIS Digit Symbol | 35 | 12.7 | 3.09 | 32 | 11.4 | 2.63 | 0.0812 |
| Block Design | 35 | 11.3 | 2.93 | 32 | 11.8 | 3.43 | 0.5768 |
| Trail Making Test A | 35 | 10.2 | 2.85 | 32 | 9.7 | 2.23 | 0.4751 |
| Trail Making Test B | 35 | 10.5 | 2.97 | 32 | 9.5 | 3.06 | 0.1737 |
| FAS | 35 | −0.1 | 0.85 | 32 | −0.3 | 0.89 | 0.4259 |
| WRAT-4 Naming list | 35 | 98.8 | 9.68 | 32 | 104.8 | 11.29 | 0.0228 |
| Boston Naming Test | 33 | 0.6 | 0.76 | 32 | −0.2 | 1.52 | 0.0135 |
| STROOP | |||||||
| -Word Page | 35 | 42.6 | 13.12 | 32 | 43.5 | 9.98 | 0.7630 |
| -Color Page | 35 | 45.3 | 14.21 | 32 | 41.2 | 7.52 | 0.1386 |
| -Color word Page | 35 | 50.1 | 10.37 | 32 | 48.8 | 8.79 | 0.5831 |
| -Interference | 35 | 47.9 | 7.62 | 32 | 47.6 | 8.60 | 0.8833 |
| HVLT | |||||||
| -Total | 35 | 51.3 | 10.50 | 32 | 48.5 | 11.35 | 0.2962 |
| -Delayed recall | 35 | 50.1 | 10.93 | 32 | 50.6 | 10.77 | 0.8580 |
| -Retention | 35 | 50.7 | 9.78 | 32 | 52.7 | 10.12 | 0.4275 |
| -RDI | 35 | 52.1 | 8.91 | 32 | 49.8 | 10.25 | 0.3359 |
| ROCF | |||||||
| -Copy | 35 | 29.0 | 3.89 | 32 | 28.7 | 5.18 | 0.7681 |
| -Immediate recall | 35 | 54.2 | 11.86 | 32 | 50.5 | 12.89 | 0.2217 |
| -Delayed recall | 35 | 54.3 | 12.24 | 32 | 50.2 | 14.33 | 0.2019 |
3 patients were excluded from the analysis due to missing follow-up assessments.
Abbreviations: SD: standard deviation; WAIS: Wechsler Adult Intelligence Scale; WRAT: Wide Range Achievement Test, 4th edition; HVLT: Hopkins Verbal Learning Test; RDI: Recognition Discrimination Index; ROCF: Rey-Osterreith Complex Figure.
Clinical Practice Points.
Aromatase inhibitors (AIs) are a mainstay of treatment for hormone receptor-positive, early-stage breast cancer in post-menopausal women. Conflicting data are available regarding the effects of endocrine therapy on cognitive function; some studies suggest a decline in cognitive function associated with treatment and others indicate no significant change. This study evaluated the short-term impact of aromatase inhibition on cognitive function of older patients via neuropsychological testing and sought to elucidate effects of aromatase inhibition on cerebral metabolic activity, using PET scans of the brain performed pre-initiation of aromatase inhibition and 6 months later. No worsening of cognitive function was seen among patients receiving an AI (from before treatment to 6 months later) and the few differences that were observed compared with healthy controls were actually in the direction of being more favorable for the patients. At the same time, there were localized increases in cerebral metabolic activity uniquely seen among patients receiving an AI. Additional long-term follow-up of this cohort is of interest.
Acknowledgments
Funding Source
The authors would like to acknowledge the generous support of the Hagle family, who helped to make this research possible. Dr. Hurria’s efforts are supported by R01 AG037037, the Breast Cancer Research Foundation, the Hearst Foundation, and the William Randolph Hearst Foundation.
Footnotes
www.ClinicalTrials.gov registration ID: NCT00681928
Disclosure of Potential Conflicts of Interest
AH has received research support from Celgene Corporation and GlaxoSmithKline, and consulting fees from Seattle Genetics and GTx, Inc., for work performed outside the scope of this manuscript. All remaining authors declare that they have no conflicts of interest.
Author Contributions
AH, SP, and DS were responsible for the conception, design, development of methodology, and supervision of the study. VK, RR, KH, and CG provided administrative, technical, or material support. All authors except TF and CC were involved in the acquisition of data, and all authors were involved in data analysis and interpretation. All authors except KH and RR participated in the writing, review, and revision of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chowen JA, Torres-Aleman I, Garcia-Segura LM. Trophic effects of estradiol on fetal rat hypothalamic neurons. Neuroendocrinology. 1992;56:895–901. doi: 10.1159/000126321. [DOI] [PubMed] [Google Scholar]
- 2.Zaulyanov LL, Green PS, Simpkins JW. Glutamate receptor requirement for neuronal death from anoxia-reoxygenation: an in Vitro model for assessment of the neuroprotective effects of estrogens. Cellular and molecular neurobiology. 1999;19:705–18. doi: 10.1023/A:1006948921855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Behl C, Skutella T, Lezoualc’h F, et al. Neuroprotection against oxidative stress by estrogens: structure-activity relationship. Molecular pharmacology. 1997;51:535–41. [PubMed] [Google Scholar]
- 4.Yaffe K, Krueger K, Sarkar S, et al. Cognitive function in postmenopausal women treated with raloxifene. The New England journal of medicine. 2001;344:1207–13. doi: 10.1056/NEJM200104193441604. [DOI] [PubMed] [Google Scholar]
- 5.Grady D, Yaffe K, Kristof M, Lin F, Richards C, Barrett-Connor E. Effect of post menopausal hormone therapy on cognitive function: the Heart and Estrogen/progestin Replacement Study. Am J Med. 2002;113:543–8. doi: 10.1016/s0002-9343(02)01270-6. [DOI] [PubMed] [Google Scholar]
- 6.Rice MM, Graves AB, McCurry SM, et al. Postmenopausal estrogen and estrogen-progestin use and 2-year rate of cognitive change in a cohort of older Japanese American women: The Kame Project. Arch Intern Med. 2000;160:1641–9. doi: 10.1001/archinte.160.11.1641. [DOI] [PubMed] [Google Scholar]
- 7.Yaffe K, Haan M, Byers A, Tangen C, Kuller L. Estrogen use, APOE, and cognitive decline: evidence of gene-environment interaction. Neurology. 2000;54:1949–54. doi: 10.1212/wnl.54.10.1949. [DOI] [PubMed] [Google Scholar]
- 8.Matthews K, Cauley J, Yaffe K, Zmuda JM. Estrogen replacement therapy and cognitive decline in older community women. J Am Geriatr Soc. 1999;47:518–23. doi: 10.1111/j.1532-5415.1999.tb02563.x. [DOI] [PubMed] [Google Scholar]
- 9.Jenkins V, Shilling V, Fallowfield L, Howell A, Hutton S. Does hormone therapy for the treatment of breast cancer have a detrimental effect on memory and cognition? A pilot study. Psycho-oncology. 2004;13:61–6. doi: 10.1002/pon.709. [DOI] [PubMed] [Google Scholar]
- 10.Bender CM, Sereika SM, Berga SL, et al. Cognitive impairment associated with adjuvant therapy in breast cancer. Psycho-oncology. 2006;15:422–30. doi: 10.1002/pon.964. [DOI] [PubMed] [Google Scholar]
- 11.Collins B, Mackenzie J, Stewart A, Bielajew C, Verma S. Cognitive effects of hormonal therapy in early stage breast cancer patients: a prospective study. Psycho-oncology. 2009;18:811–21. doi: 10.1002/pon.1453. [DOI] [PubMed] [Google Scholar]
- 12.Schilder CM, Eggens PC, Seynaeve C, et al. Neuropsychological functioning in postmenopausal breast cancer patients treated with tamoxifen or exemestane after AC-chemotherapy: Cross-sectional findings from the neuropsychological TEAM-side study. Acta oncologica (Stockholm, Sweden) 2008:1–10. doi: 10.1080/02841860802314738. [DOI] [PubMed] [Google Scholar]
- 13.Jenkins VA, Ambroisine LM, Atkins L, Cuzick J, Howell A, Fallowfield LJ. Effects of anastrozole on cognitive performance in post menopausal women: a randomised, double-blind chemoprevention trial (IBIS II) The lancet oncology. 2008 doi: 10.1016/S1470-2045(08)70207-9. [DOI] [PubMed] [Google Scholar]
- 14.Silverman DH, Dy CJ, Castellon SA, et al. Altered frontocortical, cerebellar, and basal ganglia activity in adjuvant-treated breast cancer survivors 5–10 years after chemotherapy. Breast Cancer Res Treat. 2007;103:303–11. doi: 10.1007/s10549-006-9380-z. [DOI] [PubMed] [Google Scholar]
- 15.Hurria A, Rosen C, Hudis C, et al. Cognitive function of older patients receiving adjuvant chemotherapy for breast cancer: a pilot prospective longitudinal study. J Am Geriatr Soc. 2006;54:925–31. doi: 10.1111/j.1532-5415.2006.00732.x. [DOI] [PubMed] [Google Scholar]
- 16.Squire L, Wetzel C, PCS Memory complaints after electroconvulsive therapy: assessment with a new self-rating instrument. Biol Psychiatry. 1979;14:791–801. [PubMed] [Google Scholar]
- 17.Hurria A, Gupta S, Zauderer M, et al. Developing a cancer-specific geriatric assessment: a feasibility study. Cancer. 2005;104:1998–2005. doi: 10.1002/cncr.21422. [DOI] [PubMed] [Google Scholar]
- 18.Hurria A, Cirrincione CT, Muss HB, et al. Implementing a geriatric assessment in cooperative group clinical cancer trials: CALGB 360401. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:1290–6. doi: 10.1200/JCO.2010.30.6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boellaard R. Standards for PET Image Acquisition and Quantitative Data Analysis. Journal of Nuclear Medicine. 2009;50:11S–20S. doi: 10.2967/jnumed.108.057182. [DOI] [PubMed] [Google Scholar]
- 20.Silverman DHS, Geist CL, Kenna HA, et al. Differences in regional brain metabolism associated with specific formulations of hormone therapy in postmenopausal women at risk for AD. Psychoneuroendocrinology. 2011;36:502–13. doi: 10.1016/j.psyneuen.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phillips KA, Aldridge J, Ribi K, et al. Cognitive function in postmenopausal breast cancer patients one year after completing adjuvant endocrine therapy with letrozole and/or tamoxifen in the BIG 1–98 trial. Breast Cancer Res Treat. 2011;126:221–6. doi: 10.1007/s10549-010-1235-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stewart ALKCJ. Measuring functioning and well-being; the Medical Outcomes Study approach. 1992. Durham: North Carolina Duke University Press; 1992. [Google Scholar]
- 23.Fillenbaum GG, Smyer MA. The development, validity, and reliability of the OARS multidimensional functional assessment questionnaire. J Gerontol. 1981;36:428–34. doi: 10.1093/geronj/36.4.428. [DOI] [PubMed] [Google Scholar]
- 24.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 25.Carroll BT, Kathol RG, Noyes R, Jr, Wald TG, Clamon GH. Screening for depression and anxiety in cancer patients using the Hospital Anxiety and Depression Scale. Gen Hosp Psychiatry. 1993;15:69–74. doi: 10.1016/0163-8343(93)90099-a. [DOI] [PubMed] [Google Scholar]
- 26.Hopwood P, Howell A, Maguire P. Screening for psychiatric morbidity in patients with advanced breast cancer: validation of two self-report questionnaires. Br J Cancer. 1991;64:353–6. doi: 10.1038/bjc.1991.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Razavi D, Delvaux N, Farvacques C, Robaye E. Screening for adjustment disorders and major depressive disorders in cancer in-patients. Br J Psychiatry. 1990;156:79–83. doi: 10.1192/bjp.156.1.79. [DOI] [PubMed] [Google Scholar]
- 28.Ibbotson T, Maguire P, Selby P, Priestman T, Wallace L. Screening for anxiety and depression in cancer patients: the effects of disease and treatment. Eur J Cancer. 1994;30A:37–40. doi: 10.1016/s0959-8049(05)80015-2. [DOI] [PubMed] [Google Scholar]
- 29.Wilkinson GS. Wide Range Achievement Test-Revision 3. Wilmington, DE: Jastak Association; 1993. [Google Scholar]
- 30.Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- 31.Benton AL, Hamsher K. Multilingual aphasia examination. Iowa City: University of Iowa; 1976. [Google Scholar]
- 32.Benedict RHB, Schretlen D, Groninger L, Brandt J. The Hopkins Verbal Learning Test-Revised: Normative data and analysis of interform and test-retest reliability. Clinical Neuropsychologist. 1998;12:43–55. [Google Scholar]
- 33.Osterrieth PA. Filetest de copie d'une figure complex: contribution a l'étude de la perception et de la memoire. [The test of copying a complex figure: a contribution to the study of perception and memory]. Archives de Psychologie. 1944;30:286–356. [Google Scholar]
- 34.Wechsler D. Wechsler Adult Intelligence Scale Co. 1997. [Google Scholar]
- 35.RR The validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–6. [Google Scholar]
- 36.Golden CJ. The Stroop color and word test: A manual for clinical and experimental uses. Chicago, IL: Stoelting Co; 1978. [Google Scholar]


