Abstract
Obesity exacerbates the reproductive and metabolic manifestations of polycystic ovary syndrome (PCOS). The symptoms of PCOS often begin in adolescence, and the rising prevalence of peripubertal obesity has prompted concern that the prevalence and severity of adolescent PCOS is increasing in parallel. Recent data have disclosed a high prevalence of hyperandrogenemia among peripubertal adolescents with obesity, suggesting that such girls are indeed at risk for developing PCOS. Obesity may impact the risk of PCOS via insulin resistance and compensatory hyperinsulinemia, which augments ovarian/adrenal androgen production and suppresses sex hormone–binding globulin (SHBG), thereby increasing androgen bioavailability. Altered luteinizing hormone (LH) secretion plays an important role in the pathophysiology of PCOS, and although obesity is generally associated with relative reductions of LH, higher LH appears to be the best predictor of increased free testosterone among peripubertal girls with obesity. Other potential mechanisms of obesity-associated hyperandrogenemia include enhanced androgen production in an expanded fat mass and potential effects of abnormal adipokine/cytokine levels. Adolescents with PCOS are at risk for comorbidities such as metabolic syndrome and impaired glucose tolerance, and concomitant obesity compounds these risks. For all of these reasons, weight loss represents an important therapeutic target in obese adolescents with PCOS.
Keywords: polycystic ovary syndrome, hyperandrogenemia, obesity, overweight, adolescent
Polycystic ovary syndrome (PCOS) is a disorder of reproductive aged women characterized by hyperandrogenism (e.g., elevated free testosterone levels, hirsutism), ovulatory dysfunction (oligo/anovulation), and polycystic ovarian morphology. PCOS is exceedingly common: when defined by the “National Institutes of Health (NIH) criteria” (hyperandrogenism plus ovulatory dysfunction), the prevalence approximates 7% in adult women [1], but the apparent prevalence may be double that when using recent diagnostic criteria that incorporate ovarian morphology (e.g., “Rotterdam criteria”) [2]. PCOS is a major cause of subfertility, and it is associated with comorbidities such as obesity, the metabolic syndrome, and type 2 diabetes [3][4]. For these reasons, PCOS represents a major women’s health and public health issue.
Despite decades of extensive research, the precise origins of PCOS remain enigmatic, and underlying pathophysiological mechanisms are only partially understood. Nonetheless, obesity—defined as a body mass index (BMI) ≥ 30 kg/m2 in adults and a BMI-for-age percentile ≥ 95 in children—is widely believed to contribute to the pathophysiology of PCOS and to negatively influence its clinical expression. The manifestations of PCOS typically begin in early adolescence, and many investigators have hypothesized that peripubertal obesity promotes the development of adolescent PCOS [5][6]. Given that the prevalence of childhood obesity in the United States—now approximately 17% in girls aged 6 to 19 years—has markedly increased in recent decades [7], this relationship could have a major impact on public health.
The regulation of both adipose tissue mass/function and reproductive function is highly complex with innumerable genetic and environmental influences. Herein, we will review selected concepts relevant to the relationship between obesity and PCOS ([Fig. 1]).
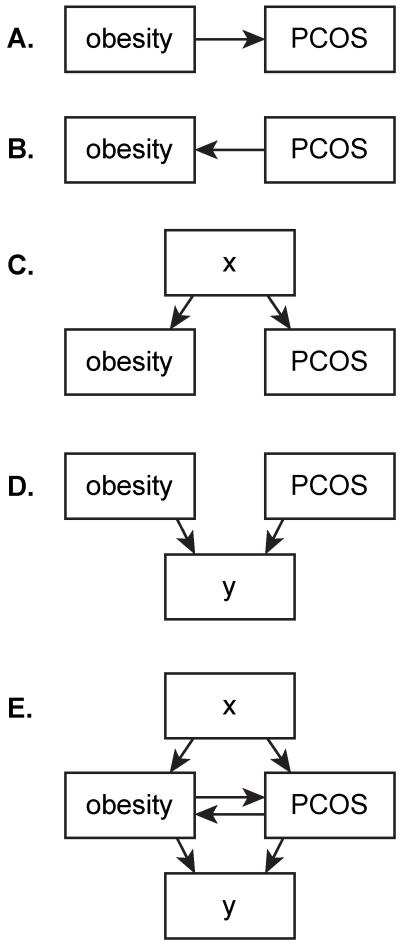
Figure 1.
(A–E) Basic diagrammatic representations of some of the ways that obesity could be related to PCOS. Importantly, relationships are not mutually exclusive. As described in the text, robust data support the relationship depicted in (A), although data also support the relationships shown in (B)–(D). Note that each arrow could represent a chain of relationships (e.g., for [A], obesity contributes to insulin resistance, which leads to hyperinsulinemia, which enhances ovarian androgen production). In addition, each arrow could encompass a vast number of different relationships (e.g., obesity could influence development of PCOS via its effects on sex steroid production, sex steroid bioavailability, follicular development, peripheral steroid metabolism, etc.). As an example of the relationship depicted in (D), both obesity and PCOS appear to have effects on insulin resistance that are at least partly independent of each other. PCOS, polycystic ovary syndrome.
The Influence of Obesity on the Prevalence of PCOS in Adults
Most data regarding the prevalence of PCOS were derived from adult studies, and while some studies suggest that the prevalence of PCOS is increased among women with obesity, others do not. For example, the estimated prevalence of PCOS was 28% among women with a BMI > 25 kg/m2 who were referred to an academic endocrinology clinic for only weight loss—much higher than the 5.5% estimated prevalence among lean women (BMI 18-25 kg/m2) in the same area (Madrid, Spain) [8]. In contrast, in a study of women undergoing pre-employment physicals at the University of Alabama at Birmingham—subjects who were not selected for either PCOS or obesity—the 12% prevalence of PCOS among women with BMI ≥ 35 kg/m2 was not demonstrably higher than the 9% prevalence among those with lower BMI [9].
Considering this issue from a different angle, the prevalence of obesity among U.S. women with PCOS has been estimated to be as high as 80% [10], well above the roughly one-third of the adult population with obesity [11]. Likewise, the prevalence of obesity among women diagnosed with PCOS in a University of Alabama at Birmingham clinic appeared to be three- to fourfold higher than in the surrounding population [9]. A recent meta-analysis of 35 studies suggested that women with PCOS had a nearly 2- and 2.8-fold higher prevalence of overweight (e.g., BMI 25–30 kg/m2) and obesity, respectively [4].
Similar rates of PCOS among populations with disparate obesity rates may imply that obesity does not play a major role in the development of PCOS [9][12]. Although it is clear that PCOS is not strictly tied to obesity, such studies cannot quantify the contribution of obesity to the development of PCOS since other important factors were likely to be different among these populations. A recent analysis suggested that the prevalence of obesity among unselected women with PCOS is (1) similar to that among unselected women without PCOS and (2) substantially lower than among women with PCOS referred to an academic reproductive endocrinology clinic [13]. These data imply that referral bias may contribute to inflated estimates of obesity prevalence among women with PCOS, but they remain consistent with the notion that obesity increases the severity of PCOS and, thus, the likelihood of requiring specialty assessment and/or treatment.
Importantly, data regarding obesity in PCOS are often based on assessments of BMI, yet BMI is an imperfect measure of metabolically important adiposity. PCOS has been associated with greater abdominal adiposity in many, but not all, studies [4][14]; a recent meta-analysis suggested that women with PCOS had a 1.73 relative risk of central obesity compared with women without PCOS [4]. However, the hypothesis that increased central obesity in PCOS reflects increased visceral adiposity has been challenged recently [15][16]. Nonetheless, when present, central adiposity may be associated with a more severe PCOS phenotype, especially with regard to metabolic phenotype [14]. Moreover, accumulating evidence suggests qualitative abnormalities of adipose tissue in PCOS. For example, adipocytes from women with PCOS are enlarged and exhibit reduced insulin sensitivity, lower lipoprotein lipase activity, and altered sensitivity to the lipolytic effects of catecholamines [10][16]. Thus, important quantitative and qualitative abnormalities of adipose tissue may have been missed in many clinical studies of PCOS.
The Influence of Obesity on the Prevalence of Diagnosed PCOS in Adolescents
The manifestations of PCOS often begin during puberty, suggesting that puberty is a critical developmental window during which the pathophysiology of PCOS unfolds. However, because the physiological changes of normal puberty overlap with the findings of PCOS, the diagnosis of PCOS in adolescents remains a controversial topic. Thus, it will be difficult to determine whether the prevalence of peripubertal obesity—and its recent increase—markedly influences the prevalence of adolescent PCOS.
A recent report suggests that intractable prepubertal obesity with severe insulin resistance can herald later development of PCOS [17]. Nonetheless, the degree to which peripubertal obesity—especially the more common forms of obesity—increases risk for PCOS remains unclear [18]. A recent population-based study of later adolescent girls (ages 15-19 years) suggested that the prevalence of PCOS (by NIH criteria) was approximately 3.0-, 6.7-, and 14.7-fold elevated in overweight, moderately obese, and extremely obese girls, respectively, and that the prevalence of obesity was significantly higher in adolescents with PCOS compared with those without (63.1 vs. 16.5%) [19]. However, this analysis was based on International Classification of Diseases-9 codes and medical records, which may be prone to both inaccuracy and bias. To our knowledge, though, no other studies have specifically addressed the impact of obesity on the prevalence of adolescent PCOS. Yet, studies have linked adolescent obesity with later PCOS/infertility: a longitudinal, population-based study suggested that obesity at age 14 years is associated with a 61% higher risk of having symptoms of PCOS at age 31 years [20], and in a large case-control study of U.S. nurses, higher BMI at age 18 years predicted later infertility related to ovulatory dysfunction [21]. Therefore, although obesity is commonly believed to be a risk factor for the development of adolescent PCOS, its quantitative impact remains unclear.
The Influence of Obesity on the Reproductive Manifestations of PCOS
Among women and adolescents with PCOS, increasing adiposity is associated with higher androgen concentrations and greater menstrual dysfunction [12][22][23][24]. Greater adiposity is also associated with higher androgen concentrations among those without PCOS [24][25][26][27]. This relationship is partly related to the negative influence of obesity on sex hormone–binding globulin (SHBG) concentration [28], since reductions of SHBG increase testosterone bioavailability. However, obesity is also associated with increased total testosterone concentrations [28]—a finding that implies increased androgen production.
Importantly, weight loss can improve hyperandrogenemia, ovulatory function, and fecundability in overweight and obese women with PCOS [29]. As a dramatic example, marked weight loss after bariatric surgery is usually associated with amelioration of PCOS—often to such a degree that the PCOS diagnosis (by NIH criteria) can no longer be substantiated [30][31][32].
Overall, these findings provide compelling evidence that, in many patients, obesity is an important factor in the development of PCOS. Thus, obesity likely contributes to the development of clinical PCOS in some at-risk adolescents who might have otherwise remained asymptomatic [6]. A study of sisters of PCOS patients is compatible with this notion: sisters who themselves developed PCOS had higher mean BMI compared with unaffected sisters (32.4 vs. 26.9 kg/m2, respectively) [33].
Obesity-Associated Hyperandrogenemia in Peripubertal Girls
It is difficult to identify peripubertal girls who will develop established and durable PCOS. However, peripubertal hyperandrogenemia can be a forerunner of full-blown adolescent PCOS. Accordingly, some investigators have focused on the relationship between peripubertal obesity and hyperandrogenemia [5][34][35].
Reinehr et al reported that total testosterone was 4-fold elevated in prepubertal girls with obesity (ages 7–9 years), and 1.75-fold elevated in pubertal obese girls (ages 10–12 years) [34]. Obesity was also associated with approximately 40% elevated dehydroepiandrosterone sulfate (DHEAS) levels. Importantly, weight loss was accompanied by a reduction of androgen concentrations—testosterone in particular [34]. Similar changes with weight loss have also been described in later pubertal obese girls [36].
In our cohort of obese peripubertal girls, BMI significantly and positively correlated with calculated free testosterone—a relationship that was partly independent of factors such as age and pubertal stage [5]. Compared with normal weight controls, mean free testosterone in Tanner stage-matched girls with obesity was elevated two- to ninefold, depending on pubertal stage [35] ([Fig. 2]), and some 60% of obese girls exhibited hyperandrogenemia [37] ([Fig. 3]). Importantly, in each of our studies [5][35][37], results were not materially different after excluding subjects with hirsutism and/or irregular menses (beyond 2 years of menarche), suggesting that recruitment bias was unlikely to have markedly influenced these results—particularly in the high proportion of asymptomatic early to mid-pubertal girls.
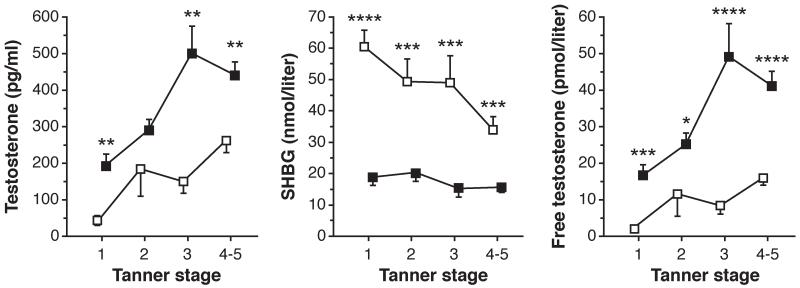
Figure 2.
Total testosterone, SHBG, and free testosterone concentrations in obese (BMI-for-age percentile ≥ 95; solid squares) and normal weight girls (BMI-for-age percentile < 85; open squares) grouped by Tanner stage. Data shown as mean ± SEM. *p < 0.05; **p ≤ 0.01; ***p ≤ 0.001; ****p ≤ 0.0001 before Bonferroni correction. SEM, standard error of the mean; SHBG, sex hormone–binding globulin. (Reprinted with permission from McCartney et al. [35])
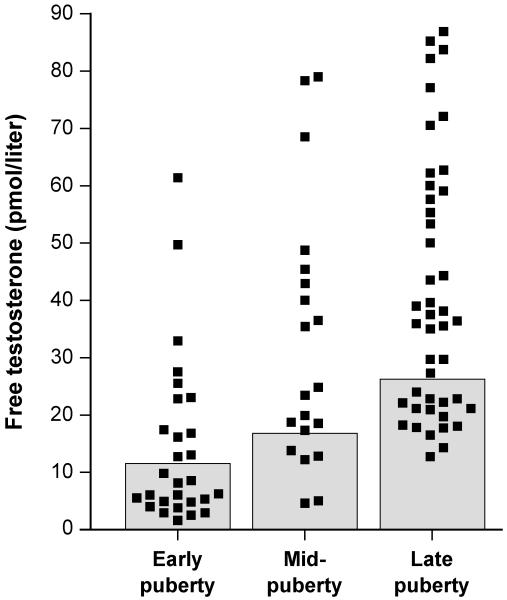
Figure 3.
Free testosterone concentration in obese peripubertal girls partitioned into pubertal stages as follows: early pubertal, Tanner breast stage 2 or Tanner stage 1 with estradiol ≥ 20 pg/mL; mid-pubertal, Tanner breast stage 3; and late pubertal, Tanner breast stages 4 or 5. Shaded boxes represent the normal range of free testosterone for each pubertal group. (Reprinted with permission from Knudsen et al. [37])
Importantly, not all peripubertal girls with obesity demonstrate hyperandrogenemia, and free testosterone concentrations are markedly variable among obese girls [37] ([Fig. 3]). It is, therefore, clear that obesity per se is not sufficient to cause hyperandrogenemia. Although the pathogenesis of hyperandrogenemia in some girls with obesity is unclear, several likely determinants are similar to those in PCOS in general, while others may be specifically related to an expanded fat mass. Some of the proposed obesity-related determinants of adolescent hyperandrogenemia are shown in [Fig. 4].
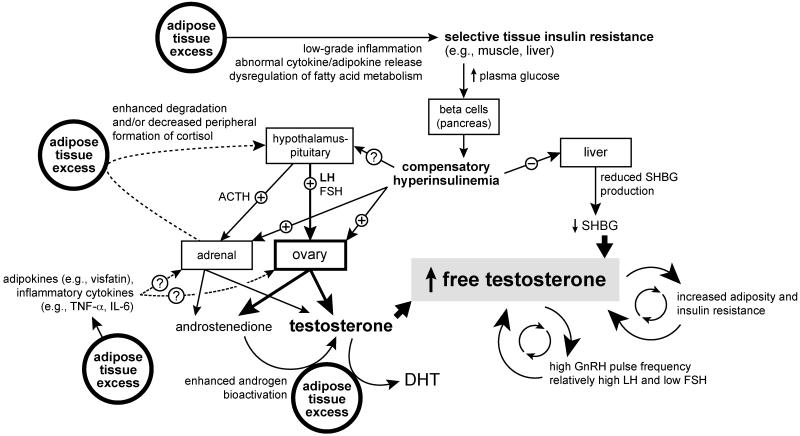
Figure 4.
Schematic of potential mechanisms by which obesity contributes to the development of adolescent PCOS. Please refer to the text for details. ACTH, adrenocorticotropic hormone; DHT, dihydrotestosterone; FSH, follicle-stimulating hormone; IL-6, interleukin 6; LH, luteinizing hormone; PCOS, polycystic ovary syndrome; SHBG, sex hormone binding globulin; TNF-α, tumor necrosis factor α.
Insulin Resistance and Hyperinsulinemia
Women with PCOS demonstrate insulin resistance as a group [10][38]. Although this insulin resistance appears to be partly independent of obesity, it is clear that concomitant obesity exacerbates insulin resistance in PCOS [10][38]. The resulting compensatory hyperinsulinemia can contribute to hyperandrogenemia in several ways. Insulin can augment androgen production from isolated ovarian theca cells [10], and experimental hyperinsulinemia can increase basal and gonadotropin releasing hormone (GnRH)-stimulated testosterone concentrations in women with PCOS [10]. Insulin also appears to potentiate basal and adrenocorticotropic hormone (ACTH)-stimulated adrenal androgen production (see below) [39]. Moreover, hyperinsulinemia inhibits hepatic synthesis of SHBG [10], which increases testosterone bioavailability, and hyperinsulinemia can also have untoward effects on granulosa cells and follicular maturation [10]. Notably, insulin reduction via a wide variety of approaches—weight loss, metformin, thiazolidinediones, diazoxide, somatostatin, and D-chiro-inositol—can improve hyperandrogenemia and/or menstrual function in PCOS [10][38][40]. Overall, these data constitute convincing evidence for a role of hyperinsulinemia in the pathogenesis of PCOS.
Although less abundant, published data in adolescents are consistent with observations in adults. Insulin resistance and hyperinsulinemia appear to be early findings in adolescent PCOS [41][42], and obese adolescents with PCOS demonstrate insulin resistance and hyperinsulinemia beyond that observed in obesity alone [42]. Also of note, some degree of insulin resistance and hyperinsulinemia is physiologic in normal pubertal development [43][44], and insulin resistance is exaggerated further in obese adolescents [45][46][47]. Among our cohort of peripubertal girls with obesity, fasting insulin was positively and significantly correlated with free testosterone, even after adjusting for differences in age, BMI z-score, and morning luteinizing hormone (LH) levels [37].
Peripubertal development of PCOS has been described in the setting of severe insulin resistance [17][48]. In this regard, it is also of interest that juvenile rats subjected to experimental hyperinsulinemia—producing a 66% increase of insulin levels across pubertal development—exhibited elevated serum testosterone and DHEAS, proliferation of the ovarian thecal and stromal compartments, and irregular estrous cycles [49]. In adolescents with PCOS, metformin concomitantly reduces hyperinsulinemia and hyperandrogenemia [50][51][52][53][54]. Taken together, these studies imply that obesity-related insulin resistance and hyperinsulinemia play an important role in the development of hyperandrogenemia and PCOS in some obese adolescents.
Abnormal Neuroendocrine Signaling
LH is the proximate stimulus for ovarian (theca cell) androgen production. As in adults, adolescents with hyperandrogenemia and/or PCOS exhibit increased LH (and by inference GnRH) pulse frequency, LH pulse amplitude, and LH-to-follicle-stimulating hormone (FSH) ratio, in addition to altered day-night patterns of LH secretion [55]. These abnormalities appear to begin during the pubertal transition [56][57]. The importance of LH in the hyperandrogenemia of adult PCOS is demonstrated by the use of long-acting GnRH agonists, which downregulate gonadotropin secretion and reduce androgens to levels observed in ovariectomized women [58][59]. In addition, transgenic mice studies suggest that excess ovarian stimulation by LH can produce hyperandrogenemia, oligo-ovulation, and ovarian enlargement with cystic changes [60]. The relationships among LH, androgens, and obesity are complex. Obesity is generally associated with reduced mean LH concentrations and LH pulse amplitude—with no clear effects on LH pulse frequency—as observed in women with and without PCOS [61][62] [63][64][65][66] and in peripubertal adolescents [57][67][68]. This phenomenon in adult PCOS partly relates to reduced pituitary responses to GnRH [64] and a shortened metabolic half-life of endogenous LH [65].
When obese peripubertal girls are evaluated in isolation, early morning LH is positively associated with free testosterone, even after simultaneously adjusting for differences of fasting insulin, age, and BMI z-score; indeed, LH appears to be a better independent predictor of free testosterone than fasting insulin [37]. Although fasting insulin levels and early morning LH values are imprecise measures of total daily exposure to insulin and LH, respectively, our preliminary data in a small number of peripubertal obese girls (n = 11)—thus far presented in abstract form only [69]—suggest that estimates of 24-hour LH concentrations are significantly associated with free testosterone, while estimates of 24-hour insulin concentrations are not. This relationship between LH and free testosterone may reflect the ability of LH to stimulate androgen production. However, androgens can also antagonize the ability of progesterone to slow GnRH pulse frequency, as observed in animal models and in women with PCOS [55]. Since high GnRH pulse frequencies favor LH and slow frequencies favor FSH production, androgen-related increases of overall GnRH pulse frequency promote LH excess and relatively low FSH production [55]. We have hypothesized that some girls with obesity enter a vicious cycle in which peripubertal hyperandrogenemia enhances LH and limits FSH secretion, which in turn exacerbates hyperandrogenemia, interferes with follicular maturation, and supports a progression to PCOS [55][70]. Variable neuroendocrine sensitivity to this effect of androgens [71][72] may partly explain why some obese girls develop durable PCOS while others do not.
In mouse models, diet-induced obesity is associated with hyperinsulinemia, high serum LH concentrations, ovulatory dysfunction, and reduced fertility, but pituitary-specific insulin receptor knockout was found be protective in this regard, suggesting a role of pituitary insulin signaling in obesity-associated infertility [73]. However, human data regarding the potential influence of hyperinsulinemia on LH secretion are mixed [10]. Although obesity and hyperinsulinemia per se do not appear to influence LH pulse frequency in PCOS [63][64][74][75], data from our group suggest that hyperinsulinemia may interact with androgens to antagonize progesterone inhibition of pulsatile GnRH secretion in adolescents [72].
Taken together, the above data are consistent with the notion that hyperandrogenemia and (other facets of) obesity can have differential effects on LH secretion [76], and that LH is an important contributing and/or permissive factor relating to increased ovarian androgen production in some obese adolescents. Importantly, the LH dependence of ovarian hyperandrogenemia likely explains why PCOS often manifests around the time of puberty—the time when the reproductive hypothalamic-pituitary axis reawakens and LH secretion increases.
Abnormalities of Ovarian and Adrenal Steroidogenesis
Theca cells isolated from women with PCOS secrete excess androgens in response to LH and insulin [77][78], even after culture propagation over three or four passages in LH-free media [79]; these data suggest that abnormal steroidogenic activity may be an inherent (e.g., genetically and/or epigenetically determined) property of theca cells in PCOS. In vivo assessments of ovarian steroidogenesis involve acute GnRH agonism (to acutely increase LH) or human chorionic gonadotropin administration, and women with PCOS have exaggerated 17α-hydroxyprogesterone (17OHP) and androstenedione responses to these stimuli [80]. This “functional ovarian hyperandrogenism” (FOH) can be demonstrated in approximately two-thirds of women and late adolescents with PCOS. Obesity-related hyperinsulinemia may contribute to FOH. For example, short-term infusion of high-insulin doses (80 mU/m2 per minute for 17 hours) can augment androstenedione and testosterone responses to acute GnRH agonist challenge [81]. In a study of obese women with PCOS, diet-induced weight loss was associated with improved hyperinsulinemia and reduced 17OHP responses to acute GnRH agonism [82]. Similarly, metformin and troglitazone can simultaneously reduce hyperinsulinemia and improve FOH in women with PCOS [83][84][85][86], although these agents may also exert direct effects on steroidogenic cells [10].
Defects of adrenal steroidogenesis (functional adrenal hyperandrogenism [FAH]) contribute to hyperandrogenemia in some women with PCOS. Twenty to 30% of women with PCOS have elevated DHEAS levels, and some exhibit exaggerated 17OHP, androstenedione, and dehydroepiandrosterone (DHEA) responses to exogenous ACTH [39]. Adrenal androgen (DHEA, androstenedione) responsiveness to stimulation may be enhanced in obese women without PCOS.[87] In women with PCOS, short-term infusion of high-insulin doses (80 mU/m2 per minute for 3–8 hours) augment 17-hydroxypregnenolone and 17OHP responses to ACTH stimulation [88][89], while metformin and pioglitazone may reduce ACTH-stimulated 17OHP and androstenedione secretion [53][90].
To summarize, excessive activity of ovarian and/or adrenal steroidogenic enzyme pathways is likely an important factor contributing to the development of PCOS in some adolescents with obesity. These defects may be promoted or exacerbated by hyperinsulinemia and/or other factors related to obesity.
Alterations of Peripheral Steroid Metabolism
Beyond the ovaries and adrenal glands, several tissues participate in steroid metabolism. In normal women, approximately 50% of circulating testosterone is derived from such peripheral conversion [91]. Adipose tissue contains several steroidogenic enzymes including 17β-hydroxysteroid dehydrogenase type 5 (17HSD5), which can convert the weak androgen androstenedione to the more potent androgen testosterone. Accordingly, the expression of 17HSD5 in subcutaneous adipose tissue is proportional to overall adiposity (i.e., BMI) [92]. Thus, excessive adiposity may contribute to androgen excess via increased peripheral production of testosterone.
Of interest in this regard, women with PCOS but without apparent FOH tend to be more obese than those with FOH [93][94]. In addition, some obese young women with PCOS have no evidence for either FOH or FAH [94]. Rosenfield et al have hypothesized that in these particular subjects, (1) hyperandrogenism relates to excess 17HSD5 activity in adipose tissue and (2) ovulatory dysfunction reflects obesity-related decreases of LH [94]. Indeed, it is possible that these subjects represent a subtype of clinical PCOS—one whose prevalence may be increasing in parallel with that of obesity.
Increased 5α-reductase activity has been described in PCOS [95][96][97][98], and its activity appears to correlate with both adiposity (BMI) and insulin concentrations [98]. Given that 5α-reductase converts testosterone to the highly potent androgen dihydrotestosterone, increased 5α-reductase activity related to an expanded fat mass represents another way that obesity could promote hyperandrogenism. Another hypothesis holds that altered peripheral cortisol metabolism related to obesity leads to a compensatory increase of ACTH drive, contributing to increased adrenal androgen production. Specifically, reduced cortisol negative feedback on ACTH secretion could be related to (1) augmented cortisol inactivation by increased 5α-reductase activity and/or (2) decreased activity of 11β-hydroxysteroid dehydrogenase type 1 (11HSD1), which converts cortisone to cortisol. Evidence for impaired 11HSD1 activity has been described in the setting of central obesity [99], but data regarding possible abnormalities of 11HSD1 activity in PCOS are mixed [96][98][100].
Potential Roles of Adipokines and Inflammatory Cytokines
Obesity is associated with altered production of adipokines and inflammatory cytokines, which may promote obesity-associated insulin resistance and metabolic syndrome [101]. Much interest has focused on the possibility that these abnormalities may independently contribute to the genesis of PCOS and/or its metabolic complications. For example, circulating levels of adiponectin—an adipokine with insulin-sensitizing properties—are reduced in PCOS even after adjustment for BMI [102], and adiponectin gene polymorphisms may also be associated with PCOS [103]. Low adiponectin likely contributes to insulin resistance in women with PCOS, and since adiponectin may also constrain theca cell steroidogenesis [104], low adiponectin may, in effect, disinhibit ovarian androgen production. Similarly, the adipokine visfatin—implicated as a mediator of insulin resistance—has been found to be elevated in PCOS and may augment forskolin-stimulated 17-hydroxylase activity in human theca cells [105]. Some have hypothesized that leptin—an adipokine that circulates in concentrations that are proportional to fat mass and serves as an important hormonal marker of energy availability—may specifically contribute to the development of PCOS among obese women. Leptin is thought to act as a permissive factor regulating pubertal onset, and decreased levels may be important to the pathogenesis of anorexia-related hypogonadotropic hypogonadism. However, the potential role of leptin in PCOS remains unclear [106].
Regarding the possibility that obesity-related inflammatory cytokines may contribute to the pathogenesis of PCOS, elevated tumor necrosis factor α (TNF-α) concentrations observed in obesity may increase ovarian theca cell proliferation and steroidogenesis [107][108]. Similarly, high interleukin 6 (IL-6) levels may stimulate adrenal steroidogenesis [109][110]. In addition, polymorphisms of both the IL-6 receptor and the IL-6 gene promoter have been associated with hyperandrogenemia [111]. However, although some studies suggest TNF-α and IL-6 elevations in PCOS, the composite data do not clearly support this contention [112]. In summary, while cytokines and adipokines may be important factors in the pathophysiology of PCOS, their precise roles in its development remain unclear [106][113].
PCOS and Obesity May Share Some Common Determinants
Animal models have provided plausible evidence that intrauterine environment can contribute to the reproductive and metabolic changes of PCOS. As an intriguing example, when exogenous testosterone is administered to pregnant rhesus monkeys, the female offspring develop later LH hypersecretion, ovarian and adrenal hyperandrogenism, ovulatory dysfunction, and polycystic ovaries, as well as increased adiposity and altered insulin/glucose regulation [114][115]. Many of these same findings are also observed in prenatally androgenized sheep and rodents [116]. However, available data regarding the possibility of excessive in utero androgen exposure in humans are mixed [117][118][119]. Postnatal androgen excess may have important developmental effects as well. For example, early postnatal administration of testosterone to female rhesus monkeys has been reported to cause early accelerated weight gain and sexual precocity [120]. In addition, producing mild hyperandrogenemia via exogenous testosterone administration from 1 to 5 years of age resulted in increased LH pulse frequency at age 5 years (i.e., after puberty), although this study disclosed no differences in overall percent body fat or percent truncal fat [121].
Exposure to endocrine-disrupting chemicals may possibly contribute to the development of both obesity and PCOS [122]. For example, bisphenol A (BPA) has been implicated as a contributor to obesity [122][123]; BPA can increase testosterone synthesis in rat theca-interstitial cells [124]; and in women, BPA levels appear to correlate with free testosterone, androstenedione, and DHEAS [125].
It is also possible that genetic determinants of obesity may independently influence PCOS risk. For example, there may be an association between FTO (i.e., fat mass and obesity-associated) gene polymorphisms and PCOS risk [126], and a recent analysis suggested that FTO variants can independently impact hyperandrogenemia in PCOS [127]. However, other studies suggest that the influence of FTO on hyperandrogenemia and PCOS risk is mediated primarily through an influence on obesity [126][128][129]. Of interest, the apparent influence of FTO polymorphisms on obesity may be greater in women with PCOS compared with the general population [130][131], suggesting an interaction between FTO and PCOS with regard to obesity risk.
Influence of Obesity on the Common Comorbidities of PCOS
Obesity significantly impacts metabolic (and other) complications of PCOS. For example, women with PCOS as a group have an estimated 2.2-, 2.5-, and 4-fold increased prevalence of metabolic syndrome, impaired glucose tolerance, and type 2 diabetes, respectively [3]. Importantly, accompanying obesity negatively impacts these risks [3]— likely a reflecting an independent contribution of obesity to insulin resistance. For example, in a study of adolescents with PCOS, the BMI-adjusted risk of metabolic syndrome was 4.5 times elevated in PCOS compared with the reference population; however, the prevalence in girls with PCOS—0, 11, and 63% in girls who were normal weight, overweight, and obese, respectively—was at least partly related to BMI [132]. The impact of obesity on the risk of impaired glucose tolerance in adolescents with PCOS is currently unclear [133][134].
PCOS is associated with dyslipidemia even after adjustment for BMI [135], but dyslipidemia is worsened with concomitant obesity [28]. An increased risk of obstructive sleep apnea (OSA) has been described in women with PCOS; and although this risk appears to be largely independent of BMI, both hyperandrogenemia and obesity— especially visceral adiposity—may contribute to this risk [136]. However, it is unclear to what degree OSA risk is increased in adolescents with PCOS [137][138]. Depression and anxiety are also more common in women with PCOS [139], and adolescent PCOS is associated with reduced health-related quality of life [140]. Obesity is likely an important contributing factor in this regard [28][141][142][143].
Impact of Hyperandrogenemia on Adiposity and the Metabolic Complications of PCOS
It is important to note that hyperandrogenemia—a cardinal feature of PCOS—may itself influence adiposity and induce metabolic disturbances [10]. Androgen receptors are found in adipocytes, especially from visceral compartments [144]. In a cross-sectional study of middle-aged women (ages 42–60 years), bioavailable testosterone correlated with visceral adiposity, consistent with the notion that androgens may influence regional fat distribution [145]. Likewise, exogenous androgen administration can increase visceral adiposity in women [146][147], while the androgen receptor antagonist flutamide may decrease visceral fat in women with PCOS [148][149].
Hyperandrogenemia may also negatively affect insulin sensitivity [10][150] and the risk of metabolic syndrome [132][151][152][153]. For example, testosterone can rapidly impair insulin sensitivity in ovariectomized rats [154][155]; testosterone reduces maximal insulin-stimulated glucose uptake in subcutaneous preadipocytes from normal women [156]; and exogenous administration of testosterone [157] or methyltestosterone [158] to women can reduce insulin sensitivity. However, data regarding the effects of androgen reduction (via long-acting GnRH agonists) or androgen receptor antagonism on insulin sensitivity are mixed [10][150]. Overall, though, the above data suggest the possibility of a vicious circle in which hyperandrogenemia contributes to increased adiposity and insulin resistance, which in turn support or exacerbate hyperandrogenemia.
Conclusion
PCOS is a heterogeneous disorder—a collection of clinical manifestations that are determined by numerous influences. As such, the presence or absence of the syndrome, in addition to the degree of pathophysiologic manifestations, represents the cumulative effect of multiple interacting components. Although obesity is neither sufficient nor necessary for the development of PCOS, it is an important factor contributing to the presence and severity of PCOS in adolescents.
We suggest that puberty represents a critical developmental window during which obesity-related hyperinsulinemia and hyperandrogenemia may promote the development of PCOS. In particular, we hypothesize that in some girls with obesity, hyperinsulinemia-related augmentation of adrenal and ovarian androgen production—along with other sources of obesity-related hyperandrogenemia—promotes a rapid day-night GnRH pulse frequency [55][70], which in turn leads to relative LH excess and FSH deficiency. These abnormalities of gonadotropin secretion further consolidate hyperandrogenemia and interfere with follicular maturation, thus promoting the development of full-blown PCOS. Some important objectives for future research include (1) understanding the relative importance of various possible sources and determinants of hyperandrogenemia in some peripubertal girls with obesity; (2) delineating the natural history of hyperandrogenemia in peripubertal girls with obesity; (3) elucidating mechanisms by which androgens may alter pubertal GnRH secretion; and (4) discovering early predictors for the development of established PCOS in girls with obesity.
We suggest that all adolescent girls with obesity should be asked about possible symptoms of PCOS, and that all adolescents with diagnosed PCOS should be screened for obesity (e.g., BMI, waist circumference). We also believe that it is prudent to screen adolescents with PCOS—especially those with concomitant obesity—for other comorbidities such as impaired glucose tolerance, type 2 diabetes, dyslipidemia, hypertension, OSA, and psychological morbidity (e.g., depression). In the setting of obesity, weight loss can ameliorate many of the reproductive and metabolic manifestations of PCOS. Accordingly, this represents an important therapeutic target for all overweight and obese adolescents with PCOS.
Acknowledgments
This work was supported in part by NIH R01 HD058671 (C.R.M.); the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health through cooperative agreement U54 HD28934 (C.R.M. and C.M.B.S.) as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research; NIH K32 HD070854 (C.M.B.S.); NIH T32 DK007646 (A.D.A.); and an Endocrine Research Grant from the Endocrine Fellows Foundation (A.D.A.).
References
- 1.[Anonymous], ACOG Committee on Practice Bulletins--Gynecology ACOG Practice Bulletin No. 108: polycystic ovary syndrome. Obstet Gynecol. 2009;114(4):936–949. doi: 10.1097/AOG.0b013e3181bd12cb. [DOI] [PubMed] [Google Scholar]
- 2.March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25(2):544–551. doi: 10.1093/humrep/dep399. [DOI] [PubMed] [Google Scholar]
- 3.Moran LJ, Misso ML, Wild RA, Norman RJ. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2010;16(4):347–363. doi: 10.1093/humupd/dmq001. [DOI] [PubMed] [Google Scholar]
- 4.Lim SS, Davies MJ, Norman RJ, Moran LJ. Overweight, obesity and central obesity in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2012;18(6):618–637. doi: 10.1093/humupd/dms030. [DOI] [PubMed] [Google Scholar]
- 5.McCartney CR, Prendergast KA, Chhabra S, et al. The association of obesity and hyperandrogenemia during the pubertal transition in girls: obesity as a potential factor in the genesis of postpubertal hyperandrogenism. J Clin Endocrinol Metab. 2006;91(5):1714–1722. doi: 10.1210/jc.2005-1852. [DOI] [PubMed] [Google Scholar]
- 6.Franks S. Polycystic ovary syndrome in adolescents. Int J Obes (Lond) 2008;32(7):1035–1041. doi: 10.1038/ijo.2008.61. [DOI] [PubMed] [Google Scholar]
- 7.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. JAMA. 2012;307(5):483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alvarez-Blasco F, Botella-Carretero JI, San Millán JL, Escobar-Morreale HF. Prevalence and characteristics of the polycystic ovary syndrome in overweight and obese women. Arch Intern Med. 2006;166(19):2081–2086. doi: 10.1001/archinte.166.19.2081. [DOI] [PubMed] [Google Scholar]
- 9.Yildiz BO, Knochenhauer ES, Azziz R. Impact of obesity on the risk for polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93(1):162–168. doi: 10.1210/jc.2007-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. 2012;33(6):981–1030. doi: 10.1210/er.2011-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009-2010. NCHS Data Brief. 2012;82:1–8. [PubMed] [Google Scholar]
- 12.Legro RS. Obesity and PCOS: implications for diagnosis and treatment. Semin Reprod Med. 2012;30(6):496–506. doi: 10.1055/s-0032-1328878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ezeh U, Yildiz BO, Azziz R. Referral bias in defining the phenotype and prevalence of obesity in polycystic ovary syndrome. J Clin Endocrinol Metab. 2013;98(6):E1088–E1096. doi: 10.1210/jc.2013-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Escobar-Morreale HF, San Millán JL. Abdominal adiposity and the polycystic ovary syndrome. Trends Endocrinol Metab. 2007;18(7):266–272. doi: 10.1016/j.tem.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Barber TM, Golding SJ, Alvey C, et al. Global adiposity rather than abnormal regional fat distribution characterizes women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93(3):999–1004. doi: 10.1210/jc.2007-2117. [DOI] [PubMed] [Google Scholar]
- 16.Mannerås-Holm L, Leonhardt H, Kullberg J, et al. Adipose tissue has aberrant morphology and function in PCOS: enlarged adipocytes and low serum adiponectin, but not circulating sex steroids, are strongly associated with insulin resistance. J Clin Endocrinol Metab. 2011;96(2):E304–E311. doi: 10.1210/jc.2010-1290. [DOI] [PubMed] [Google Scholar]
- 17.Littlejohn EE, Weiss RE, Deplewski D, Edidin DV, Rosenfield R. Intractable early childhood obesity as the initial sign of insulin resistant hyperinsulinism and precursor of polycystic ovary syndrome. J Pediatr Endocrinol Metab. 2007;20(1):41–51. doi: 10.1515/jpem.2007.20.1.41. [DOI] [PubMed] [Google Scholar]
- 18.Rosenfield RL. Clinical review: identifying children at risk for polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92(3):787–796. doi: 10.1210/jc.2006-2012. [DOI] [PubMed] [Google Scholar]
- 19.Christensen SB, Black MH, Smith N, et al. Prevalence of polycystic ovary syndrome in adolescents. Fertil Steril. 2013;100(2):470–477. doi: 10.1016/j.fertnstert.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laitinen J, Taponen S, Martikainen H, et al. Body size from birth to adulthood as a predictor of self-reported polycystic ovary syndrome symptoms. Int J Obes Relat Metab Disord. 2003;27(6):710–715. doi: 10.1038/sj.ijo.0802301. [DOI] [PubMed] [Google Scholar]
- 21.Rich-Edwards JW, Goldman MB, Willett WC, et al. Adolescent body mass index and infertility caused by ovulatory disorder. Am J Obstet Gynecol. 1994;171(1):171–177. doi: 10.1016/0002-9378(94)90465-0. [DOI] [PubMed] [Google Scholar]
- 22.Barber TM, McCarthy MI, Wass JA, Franks S. Obesity and polycystic ovary syndrome. Clin Endocrinol (Oxf) 2006;65(2):137–145. doi: 10.1111/j.1365-2265.2006.02587.x. [DOI] [PubMed] [Google Scholar]
- 23.Pasquali R, Gambineri A, Pagotto U. The impact of obesity on reproduction in women with polycystic ovary syndrome. BJOG. 2006;113(10):1148–1159. doi: 10.1111/j.1471-0528.2006.00990.x. [DOI] [PubMed] [Google Scholar]
- 24.Pinola P, Lashen H, Bloigu A, et al. Menstrual disorders in adolescence: a marker for hyperandrogenaemia and increased metabolic risks in later life? Finnish general population-based birth cohort study. Hum Reprod. 2012;27(11):3279–3286. doi: 10.1093/humrep/des309. [DOI] [PubMed] [Google Scholar]
- 25.Strain GW, Zumoff B, Miller LK, Rosner W. Sex difference in the effect of obesity on 24-hour mean serum gonadotropin levels. Horm Metab Res. 2003;35(6):362–366. doi: 10.1055/s-2003-41358. [DOI] [PubMed] [Google Scholar]
- 26.Taponen S, Martikainen H, Järvelin MR, et al. Hormonal profile of women with self-reported symptoms of oligomenorrhea and/or hirsutism: Northern Finland birth cohort 1966 study. J Clin Endocrinol Metab. 2003;88(1):141–147. doi: 10.1210/jc.2002-020982. [DOI] [PubMed] [Google Scholar]
- 27.Randolph JF, Jr, Sowers M, Gold EB, et al. Reproductive hormones in the early menopausal transition: relationship to ethnicity, body size, and menopausal status. J Clin Endocrinol Metab. 2003;88(4):1516–1522. doi: 10.1210/jc.2002-020777. [DOI] [PubMed] [Google Scholar]
- 28.Lim SS, Norman RJ, Davies MJ, Moran LJ. The effect of obesity on polycystic ovary syndrome: a systematic review and meta-analysis. Obes Rev. 2013;14(2):95–109. doi: 10.1111/j.1467-789X.2012.01053.x. [DOI] [PubMed] [Google Scholar]
- 29.Moran LJ, Pasquali R, Teede HJ, Hoeger KM, Norman RJ. Treatment of obesity in polycystic ovary syndrome: a position statement of the Androgen Excess and Polycystic Ovary Syndrome Society. Fertil Steril. 2009;92(6):1966–1982. doi: 10.1016/j.fertnstert.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 30.Escobar-Morreale HF, Botella-Carretero JI, Alvarez-Blasco F, Sancho J, San Millán JL. The polycystic ovary syndrome associated with morbid obesity may resolve after weight loss induced by bariatric surgery. J Clin Endocrinol Metab. 2005;90(12):6364–6369. doi: 10.1210/jc.2005-1490. [DOI] [PubMed] [Google Scholar]
- 31.Eid GM, Cottam DR, Velcu LM, et al. Effective treatment of polycystic ovarian syndrome with Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2005;1(2):77–80. doi: 10.1016/j.soard.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 32.Jamal M, Gunay Y, Capper A, Eid A, Heitshusen D, Samuel I. Roux-en-Y gastric bypass ameliorates polycystic ovary syndrome and dramatically improves conception rates: a 9-year analysis. Surg Obes Relat Dis. 2012;8(4):440–444. doi: 10.1016/j.soard.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 33.Legro RS, Bentley-Lewis R, Driscoll D, Wang SC, Dunaif A. Insulin resistance in the sisters of women with polycystic ovary syndrome: association with hyperandrogenemia rather than menstrual irregularity. J Clin Endocrinol Metab. 2002;87(5):2128–2133. doi: 10.1210/jcem.87.5.8513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reinehr T, de Sousa G, Roth CL, Andler W. Androgens before and after weight loss in obese children. J Clin Endocrinol Metab. 2005;90(10):5588–5595. doi: 10.1210/jc.2005-0438. [DOI] [PubMed] [Google Scholar]
- 35.McCartney CR, Blank SK, Prendergast KA, et al. Obesity and sex steroid changes across puberty: evidence for marked hyperandrogenemia in pre- and early pubertal obese girls. J Clin Endocrinol Metab. 2007;92(2):430–436. doi: 10.1210/jc.2006-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wabitsch M, Hauner H, Heinze E, et al. Body fat distribution and steroid hormone concentrations in obese adolescent girls before and after weight reduction. J Clin Endocrinol Metab. 1995;80(12):3469–3475. doi: 10.1210/jcem.80.12.8530585. [DOI] [PubMed] [Google Scholar]
- 37.Knudsen KL, Blank SK, Burt Solorzano C, et al. Hyperandrogenemia in obese peripubertal girls: correlates and potential etiological determinants. Obesity (Silver Spring) 2010;18(11):2118–2124. doi: 10.1038/oby.2010.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev. 1997;18(6):774–800. doi: 10.1210/edrv.18.6.0318. [DOI] [PubMed] [Google Scholar]
- 39.Yildiz BO, Azziz R. The adrenal and polycystic ovary syndrome. Rev Endocr Metab Disord. 2007;8(4):331–342. doi: 10.1007/s11154-007-9054-0. [DOI] [PubMed] [Google Scholar]
- 40.Poretsky L, Cataldo NA, Rosenwaks Z, Giudice LC. The insulin-related ovarian regulatory system in health and disease. Endocr Rev. 1999;20(4):535–582. doi: 10.1210/edrv.20.4.0374. [DOI] [PubMed] [Google Scholar]
- 41.Arslanian SA, Lewy VD, Danadian K. Glucose intolerance in obese adolescents with polycystic ovary syndrome: roles of insulin resistance and beta-cell dysfunction and risk of cardiovascular disease. J Clin Endocrinol Metab. 2001;86(1):66–71. doi: 10.1210/jcem.86.1.7123. [DOI] [PubMed] [Google Scholar]
- 42.Lewy VD, Danadian K, Witchel SF, Arslanian S. Early metabolic abnormalities in adolescent girls with polycystic ovarian syndrome. J Pediatr. 2001;138(1):38–44. doi: 10.1067/mpd.2001.109603. [DOI] [PubMed] [Google Scholar]
- 43.Bloch CA, Clemons P, Sperling MA. Puberty decreases insulin sensitivity. J Pediatr. 1987;110(3):481–487. doi: 10.1016/s0022-3476(87)80522-x. [DOI] [PubMed] [Google Scholar]
- 44.Moran A, Jacobs DR, Jr, Steinberger J, et al. Insulin resistance during puberty: results from clamp studies in 357 children. Diabetes. 1999;48(10):2039–2044. doi: 10.2337/diabetes.48.10.2039. [DOI] [PubMed] [Google Scholar]
- 45.Roemmich JN, Clark PA, Lusk M, et al. Pubertal alterations in growth and body composition. VI. Pubertal insulin resistance: relation to adiposity, body fat distribution and hormone release. Int J Obes Relat Metab Disord. 2002;26(5):701–709. doi: 10.1038/sj.ijo.0801975. [DOI] [PubMed] [Google Scholar]
- 46.Brufani C, Tozzi A, Fintini D, et al. Sexual dimorphism of body composition and insulin sensitivity across pubertal development in obese Caucasian subjects. Eur J Endocrinol. 2009;160(5):769–775. doi: 10.1530/EJE-08-0878. [DOI] [PubMed] [Google Scholar]
- 47.Pilia S, Casini MR, Foschini ML, et al. The effect of puberty on insulin resistance in obese children. J Endocrinol Invest. 2009;32(5):401–405. doi: 10.1007/BF03346475. [DOI] [PubMed] [Google Scholar]
- 48.Vambergue A, Lautier C, Valat AS, Cortet-Rudelli C, Grigorescu F, Dewailly D. Follow-up study of two sisters with type A syndrome of severe insulin resistance gives a new insight into PCOS pathogenesis in relation to puberty and pregnancy outcome: a case report. Hum Reprod. 2006;21(5):1274–1278. doi: 10.1093/humrep/dei455. [DOI] [PubMed] [Google Scholar]
- 49.Chakrabarty S, Miller BT, Collins TJ, Nagamani M. Ovarian dysfunction in peripubertal hyperinsulinemia. J Soc Gynecol Investig. 2006;13(2):122–129. doi: 10.1016/j.jsgi.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 50.Ibáñez L, Valls C, Potau N, Marcos MV, de Zegher F. Sensitization to insulin in adolescent girls to normalize hirsutism, hyperandrogenism, oligomenorrhea, dyslipidemia, and hyperinsulinism after precocious pubarche. J Clin Endocrinol Metab. 2000;85(10):3526–3530. doi: 10.1210/jcem.85.10.6908. [DOI] [PubMed] [Google Scholar]
- 51.Ibáñez L, Valls C, Ferrer A, Marcos MV, Rodriguez-Hierro F, de Zegher F. Sensitization to insulin induces ovulation in nonobese adolescents with anovulatory hyperandrogenism. J Clin Endocrinol Metab. 2001;86(8):3595–3598. doi: 10.1210/jcem.86.8.7756. [DOI] [PubMed] [Google Scholar]
- 52.Glueck CJ, Wang P, Fontaine R, Tracy T, Sieve-Smith L. Metformin to restore normal menses in oligo-amenorrheic teenage girls with polycystic ovary syndrome (PCOS) J Adolesc Health. 2001;29(3):160–169. doi: 10.1016/s1054-139x(01)00202-6. [DOI] [PubMed] [Google Scholar]
- 53.Arslanian SA, Lewy V, Danadian K, Saad R. Metformin therapy in obese adolescents with polycystic ovary syndrome and impaired glucose tolerance: amelioration of exaggerated adrenal response to adrenocorticotropin with reduction of insulinemia/insulin resistance. J Clin Endocrinol Metab. 2002;87(4):1555–1559. doi: 10.1210/jcem.87.4.8398. [DOI] [PubMed] [Google Scholar]
- 54.De Leo V, Musacchio MC, Morgante G, Piomboni P, Petraglia F. Metformin treatment is effective in obese teenage girls with PCOS. Hum Reprod. 2006;21(9):2252–2256. doi: 10.1093/humrep/del185. [DOI] [PubMed] [Google Scholar]
- 55.McCartney CR. Maturation of sleep-wake gonadotrophin-releasing hormone secretion across puberty in girls: potential mechanisms and relevance to the pathogenesis of polycystic ovary syndrome. J Neuroendocrinol. 2010;22(7):701–709. doi: 10.1111/j.1365-2826.2010.02029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Apter D, Bützow T, Laughlin GA, Yen SS. Accelerated 24-hour luteinizing hormone pulsatile activity in adolescent girls with ovarian hyperandrogenism: relevance to the developmental phase of polycystic ovarian syndrome. J Clin Endocrinol Metab. 1994;79(1):119–125. doi: 10.1210/jcem.79.1.8027216. [DOI] [PubMed] [Google Scholar]
- 57.McCartney CR, Prendergast KA, Blank SK, Helm KD, Chhabra S, Marshall JC. Maturation of luteinizing hormone (gonadotropin-releasing hormone) secretion across puberty: evidence for altered regulation in obese peripubertal girls. J Clin Endocrinol Metab. 2009;94(1):56–66. doi: 10.1210/jc.2008-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chang RJ, Laufer LR, Meldrum DR, et al. Steroid secretion in polycystic ovarian disease after ovarian suppression by a long-acting gonadotropin-releasing hormone agonist. J Clin Endocrinol Metab. 1983;56(5):897–903. doi: 10.1210/jcem-56-5-897. [DOI] [PubMed] [Google Scholar]
- 59.Steingold K, De Ziegler D, Cedars M, et al. Clinical and hormonal effects of chronic gonadotropin-releasing hormone agonist treatment in polycystic ovarian disease. J Clin Endocrinol Metab. 1987;65(4):773–778. doi: 10.1210/jcem-65-4-773. [DOI] [PubMed] [Google Scholar]
- 60.Risma KA, Clay CM, Nett TM, Wagner T, Yun J, Nilson JH. Targeted overexpression of luteinizing hormone in transgenic mice leads to infertility, polycystic ovaries, and ovarian tumors. Proc Natl Acad Sci U S A. 1995;92(5):1322–1326. doi: 10.1073/pnas.92.5.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Holte J, Bergh T, Gennarelli G, Wide L. The independent effects of polycystic ovary syndrome and obesity on serum concentrations of gonadotrophins and sex steroids in premenopausal women. Clin Endocrinol (Oxf) 1994;41(4):473–481. doi: 10.1111/j.1365-2265.1994.tb02578.x. [DOI] [PubMed] [Google Scholar]
- 62.Taylor AE, McCourt B, Martin KA, et al. Determinants of abnormal gonadotropin secretion in clinically defined women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1997;82(7):2248–2256. doi: 10.1210/jcem.82.7.4105. [DOI] [PubMed] [Google Scholar]
- 63.Arroyo A, Laughlin GA, Morales AJ, Yen SS. Inappropriate gonadotropin secretion in polycystic ovary syndrome: influence of adiposity. J Clin Endocrinol Metab. 1997;82(11):3728–3733. doi: 10.1210/jcem.82.11.4377. [DOI] [PubMed] [Google Scholar]
- 64.Pagán YL, Srouji SS, Jimenez Y, Emerson A, Gill S, Hall JE. Inverse relationship between luteinizing hormone and body mass index in polycystic ovarian syndrome: investigation of hypothalamic and pituitary contributions. J Clin Endocrinol Metab. 2006;91(4):1309–1316. doi: 10.1210/jc.2005-2099. [DOI] [PubMed] [Google Scholar]
- 65.Srouji SS, Pagán YL, D’Amato F, et al. Pharmacokinetic factors contribute to the inverse relationship between luteinizing hormone and body mass index in polycystic ovarian syndrome. J Clin Endocrinol Metab. 2007;92(4):1347–1352. doi: 10.1210/jc.2006-2716. [DOI] [PubMed] [Google Scholar]
- 66.Jain A, Polotsky AJ, Rochester D, et al. Pulsatile luteinizing hormone amplitude and progesterone metabolite excretion are reduced in obese women. J Clin Endocrinol Metab. 2007;92(7):2468–2473. doi: 10.1210/jc.2006-2274. [DOI] [PubMed] [Google Scholar]
- 67.Silfen ME, Denburg MR, Manibo AM, et al. Early endocrine, metabolic, and sonographic characteristics of polycystic ovary syndrome (PCOS): comparison between nonobese and obese adolescents. J Clin Endocrinol Metab. 2003;88(10):4682–4688. doi: 10.1210/jc.2003-030617. [DOI] [PubMed] [Google Scholar]
- 68.Bordini B, Littlejohn E, Rosenfield RL. Blunted sleep-related luteinizing hormone rise in healthy premenarcheal pubertal girls with elevated body mass index. J Clin Endocrinol Metab. 2009;94(4):1168–1175. doi: 10.1210/jc.2008-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Anderson AD, Burt Solorzano C, Bhabhra R, et al. The role of hyperinsulinemia vs. insulin resistance in obese girls with hyperandrogenemia; 95th Meeting of the Endocrine Society; San Francisco, CA. 2013; Jun 15-18, Abstract SUN-505. [Google Scholar]
- 70.Burt Solorzano CM, Beller JP, Abshire MY, Collins JS, McCartney CR, Marshall JC. Neuroendocrine dysfunction in polycystic ovary syndrome. Steroids. 2012;77(4):332–337. doi: 10.1016/j.steroids.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chhabra S, McCartney CR, Yoo RY, Eagleson CA, Chang RJ, Marshall JC. Progesterone inhibition of the hypothalamic gonadotropin-releasing hormone pulse generator: evidence for varied effects in hyperandrogenemic adolescent girls. J Clin Endocrinol Metab. 2005;90(5):2810–2815. doi: 10.1210/jc.2004-2359. [DOI] [PubMed] [Google Scholar]
- 72.Blank SK, McCartney CR, Chhabra S, et al. Modulation of gonadotropin-releasing hormone pulse generator sensitivity to progesterone inhibition in hyperandrogenic adolescent girls—implications for regulation of pubertal maturation. J Clin Endocrinol Metab. 2009;94(7):2360–2366. doi: 10.1210/jc.2008-2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brothers KJ, Wu S, DiVall SA, et al. Rescue of obesity-induced infertility in female mice due to a pituitary-specific knockout of the insulin receptor. Cell Metab. 2010;12(3):295–305. doi: 10.1016/j.cmet.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dunaif A, Mandeli J, Fluhr H, Dobrjansky A. The impact of obesity and chronic hyperinsulinemia on gonadotropin release and gonadal steroid secretion in the polycystic ovary syndrome. J Clin Endocrinol Metab. 1988;66(1):131–139. doi: 10.1210/jcem-66-1-131. [DOI] [PubMed] [Google Scholar]
- 75.Morales AJ, Laughlin GA, Bützow T, Maheshwari H, Baumann G, Yen SS. Insulin, somatotropic, and luteinizing hormone axes in lean and obese women with polycystic ovary syndrome: common and distinct features. J Clin Endocrinol Metab. 1996;81(8):2854–2864. doi: 10.1210/jcem.81.8.8768842. [DOI] [PubMed] [Google Scholar]
- 76.Rosenfield RL, Bordini B. Evidence that obesity and androgens have independent and opposing effects on gonadotropin production from puberty to maturity. Brain Res. 2010;1364:186–197. doi: 10.1016/j.brainres.2010.08.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barbieri RL, Makris A, Randall RW, Daniels G, Kistner RW, Ryan KJ. Insulin stimulates androgen accumulation in incubations of ovarian stroma obtained from women with hyperandrogenism. J Clin Endocrinol Metab. 1986;62(5):904–910. doi: 10.1210/jcem-62-5-904. [DOI] [PubMed] [Google Scholar]
- 78.Gilling-Smith C, Willis DS, Beard RW, Franks S. Hypersecretion of androstenedione by isolated thecal cells from polycystic ovaries. J Clin Endocrinol Metab. 1994;79(4):1158–1165. doi: 10.1210/jcem.79.4.7962289. [DOI] [PubMed] [Google Scholar]
- 79.Nelson VL, Legro RS, Strauss JF, III, McAllister JM. Augmented androgen production is a stable steroidogenic phenotype of propagated theca cells from polycystic ovaries. Mol Endocrinol. 1999;13(6):946–957. doi: 10.1210/mend.13.6.0311. [DOI] [PubMed] [Google Scholar]
- 80.Rosenfield RL. Ovarian and adrenal function in polycystic ovary syndrome. Endocrinol Metab Clin North Am. 1999;28(2):265–293. doi: 10.1016/s0889-8529(05)70070-0. [DOI] [PubMed] [Google Scholar]
- 81.Tosi F, Negri C, Perrone F, et al. Hyperinsulinemia amplifies GnRH agonist stimulated ovarian steroid secretion in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2012;97(5):1712–1719. doi: 10.1210/jc.2011-2939. [DOI] [PubMed] [Google Scholar]
- 82.Jakubowicz DJ, Nestler JE. 17 alpha-Hydroxyprogesterone responses to leuprolide and serum androgens in obese women with and without polycystic ovary syndrome offer dietary weight loss. J Clin Endocrinol Metab. 1997;82(2):556–560. doi: 10.1210/jcem.82.2.3753. [DOI] [PubMed] [Google Scholar]
- 83.Nestler JE, Jakubowicz DJ. Decreases in ovarian cytochrome P450c17 alpha activity and serum free testosterone after reduction of insulin secretion in polycystic ovary syndrome. N Engl J Med. 1996;335(9):617–623. doi: 10.1056/NEJM199608293350902. [DOI] [PubMed] [Google Scholar]
- 84.Nestler JE, Jakubowicz DJ. Lean women with polycystic ovary syndrome respond to insulin reduction with decreases in ovarian P450c17 alpha activity and serum androgens. J Clin Endocrinol Metab. 1997;82(12):4075–4079. doi: 10.1210/jcem.82.12.4431. [DOI] [PubMed] [Google Scholar]
- 85.Ehrmann DA, Schneider DJ, Sobel BE, et al. Troglitazone improves defects in insulin action, insulin secretion, ovarian steroidogenesis, and fibrinolysis in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1997;82(7):2108–2116. doi: 10.1210/jcem.82.7.4069. [DOI] [PubMed] [Google Scholar]
- 86.Moghetti P, Castello R, Negri C, et al. Metformin effects on clinical features, endocrine and metabolic profiles, and insulin sensitivity in polycystic ovary syndrome: a randomized, double-blind, placebo-controlled 6-month trial, followed by open, long-term clinical evaluation. J Clin Endocrinol Metab. 2000;85(1):139–146. doi: 10.1210/jcem.85.1.6293. [DOI] [PubMed] [Google Scholar]
- 87.Komindr S, Kurtz BR, Stevens MD, Karas JG, Bittle JB, Givens JR. Relative sensitivity and responsivity of serum cortisol and two adrenal androgens to alpha-adrenocorticotropin-(1-24) in normal and obese, nonhirsute, eumenorrheic women. J Clin Endocrinol Metab. 1986;63(4):860–864. doi: 10.1210/jcem-63-4-860. [DOI] [PubMed] [Google Scholar]
- 88.Moghetti P, Castello R, Negri C, et al. Insulin infusion amplifies 17 alpha-hydroxycorticosteroid intermediates response to adrenocorticotropin in hyperandrogenic women: apparent relative impairment of 17,20-lyase activity. J Clin Endocrinol Metab. 1996;81(3):881–886. doi: 10.1210/jcem.81.3.8772544. [DOI] [PubMed] [Google Scholar]
- 89.Tosi F, Negri C, Brun E, et al. Insulin enhances ACTH-stimulated androgen and glucocorticoid metabolism in hyperandrogenic women. Eur J Endocrinol. 2011;164(2):197–203. doi: 10.1530/EJE-10-0782. [DOI] [PubMed] [Google Scholar]
- 90.Romualdi D, Giuliani M, Draisci G, et al. Pioglitazone reduces the adrenal androgen response to corticotropin-releasing factor without changes in ACTH release in hyperinsulinemic women with polycystic ovary syndrome. Fertil Steril. 2007;88(1):131–138. doi: 10.1016/j.fertnstert.2006.11.076. [DOI] [PubMed] [Google Scholar]
- 91.Burger HG. Androgen production in women. Fertil Steril. 2002;77(Suppl. 04):S3–S5. doi: 10.1016/s0015-0282(02)02985-0. [DOI] [PubMed] [Google Scholar]
- 92.Quinkler M, Sinha B, Tomlinson JW, Bujalska IJ, Stewart PM, Arlt W. Androgen generation in adipose tissue in women with simple obesity—a site-specific role for 17beta-hydroxysteroid dehydrogenase type 5. J Endocrinol. 2004;183(2):331–342. doi: 10.1677/joe.1.05762. [DOI] [PubMed] [Google Scholar]
- 93.Hirshfeld-Cytron J, Barnes RB, Ehrmann DA, Caruso A, Mortensen MM, Rosenfield RL. Characterization of functionally typical and atypical types of polycystic ovary syndrome. J Clin Endocrinol Metab. 2009;94(5):1587–1594. doi: 10.1210/jc.2008-2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rosenfield RL, Mortensen M, Wroblewski K, Littlejohn E, Ehrmann DA. Determination of the source of androgen excess in functionally atypical polycystic ovary syndrome by a short dexamethasone androgen-suppression test and a low-dose ACTH test. Hum Reprod. 2011;26(11):3138–3146. doi: 10.1093/humrep/der291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stewart PM, Shackleton CH, Beastall GH, Edwards CR. 5 alpha-reductase activity in polycystic ovary syndrome. Lancet. 1990;335(8687):431–433. doi: 10.1016/0140-6736(90)90664-q. [DOI] [PubMed] [Google Scholar]
- 96.Tsilchorozidou T, Honour JW, Conway GS. Altered cortisol metabolism in polycystic ovary syndrome: insulin enhances 5alpha-reduction but not the elevated adrenal steroid production rates. J Clin Endocrinol Metab. 2003;88(12):5907–5913. doi: 10.1210/jc.2003-030240. [DOI] [PubMed] [Google Scholar]
- 97.Fassnacht M, Schlenz N, Schneider SB, Wudy SA, Allolio B, Arlt W. Beyond adrenal and ovarian androgen generation: increased peripheral 5 alpha-reductase activity in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88(6):2760–2766. doi: 10.1210/jc.2002-021875. [DOI] [PubMed] [Google Scholar]
- 98.Vassiliadi DA, Barber TM, Hughes BA, et al. Increased 5 alpha-reductase activity and adrenocortical drive in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2009;94(9):3558–3566. doi: 10.1210/jc.2009-0837. [DOI] [PubMed] [Google Scholar]
- 99.Stewart PM, Boulton A, Kumar S, Clark PM, Shackleton CH. Cortisol metabolism in human obesity: impaired cortisone—>cortisol conversion in subjects with central adiposity. J Clin Endocrinol Metab. 1999;84(3):1022–1027. doi: 10.1210/jcem.84.3.5538. [DOI] [PubMed] [Google Scholar]
- 100.Rodin A, Thakkar H, Taylor N, Clayton R. Hyperandrogenism in polycystic ovary syndrome. Evidence of dysregulation of 11 beta-hydroxysteroid dehydrogenase. N Engl J Med. 1994;330(7):460–465. doi: 10.1056/NEJM199402173300703. [DOI] [PubMed] [Google Scholar]
- 101.DeBoer MD. Obesity, systemic inflammation, and increased risk for cardiovascular disease and diabetes among adolescents: a need for screening tools to target interventions. Nutrition. 2013;29(2):379–386. doi: 10.1016/j.nut.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Toulis KA, Goulis DG, Farmakiotis D, et al. Adiponectin levels in women with polycystic ovary syndrome: a systematic review and a meta-analysis. Hum Reprod Update. 2009;15(3):297–307. doi: 10.1093/humupd/dmp006. [DOI] [PubMed] [Google Scholar]
- 103.Gao L, Zhang Y, Cui Y, Jiang Y, Wang X, Liu J. Association of the T45G and G276T polymorphisms in the adiponectin gene with PCOS: a meta-analysis. Gynecol Endocrinol. 2012;28(2):106–110. doi: 10.3109/09513590.2010.508543. [DOI] [PubMed] [Google Scholar]
- 104.Lagaly DV, Aad PY, Grado-Ahuir JA, Hulsey LB, Spicer LJ. Role of adiponectin in regulating ovarian theca and granulosa cell function. Mol Cell Endocrinol. 2008;284(1-2):38–45. doi: 10.1016/j.mce.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 105.Munir I, Yen HW, Baruth T, et al. Resistin stimulation of 17alpha-hydroxylase activity in ovarian theca cells in vitro: relevance to polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90(8):4852–4857. doi: 10.1210/jc.2004-2152. [DOI] [PubMed] [Google Scholar]
- 106.Barber TM, Franks S. Adipocyte biology in polycystic ovary syndrome. Mol Cell Endocrinol. 2013;373(1-2):68–76. doi: 10.1016/j.mce.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 107.Roby KF, Terranova PF. Effects of tumor necrosis factor-alpha in vitro on steroidogenesis of healthy and atretic follicles of the rat: theca as a target. Endocrinology. 1990;126(5):2711–2718. doi: 10.1210/endo-126-5-2711. [DOI] [PubMed] [Google Scholar]
- 108.Spaczynski RZ, Arici A, Duleba AJ. Tumor necrosis factor-alpha stimulates proliferation of rat ovarian theca-interstitial cells. Biol Reprod. 1999;61(4):993–998. doi: 10.1095/biolreprod61.4.993. [DOI] [PubMed] [Google Scholar]
- 109.Mastorakos G, Chrousos GP, Weber JS. Recombinant interleukin-6 activates the hypothalamic-pituitary-adrenal axis in humans. J Clin Endocrinol Metab. 1993;77(6):1690–1694. doi: 10.1210/jcem.77.6.8263159. [DOI] [PubMed] [Google Scholar]
- 110.Päth G, Bornstein SR, Ehrhart-Bornstein M, Scherbaum WA. Interleukin-6 and the interleukin-6 receptor in the human adrenal gland: expression and effects on steroidogenesis. J Clin Endocrinol Metab. 1997;82(7):2343–2349. doi: 10.1210/jcem.82.7.4072. [DOI] [PubMed] [Google Scholar]
- 111.Escobar-Morreale HF, Luque-Ramírez M, San Millán JL. The molecular-genetic basis of functional hyperandrogenism and the polycystic ovary syndrome. Endocr Rev. 2005;26(2):251–282. doi: 10.1210/er.2004-0004. [DOI] [PubMed] [Google Scholar]
- 112.Escobar-Morreale HF, Luque-Ramírez M, González F. Circulating inflammatory markers in polycystic ovary syndrome: a systematic review and metaanalysis. Fertil Steril. 2011;95(3):1048–1058. e1–e2. doi: 10.1016/j.fertnstert.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Repaci A, Gambineri A, Pasquali R. The role of low-grade inflammation in the polycystic ovary syndrome. Mol Cell Endocrinol. 2011;335(1):30–41. doi: 10.1016/j.mce.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 114.Dumesic DA, Abbott DH, Padmanabhan V. Polycystic ovary syndrome and its developmental origins. Rev Endocr Metab Disord. 2007;8(2):127–141. doi: 10.1007/s11154-007-9046-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Abbott DH, Tarantal AF, Dumesic DA. Fetal, infant, adolescent and adult phenotypes of polycystic ovary syndrome in prenatally androgenized female rhesus monkeys. Am J Primatol. 2009;71(9):776–784. doi: 10.1002/ajp.20679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Padmanabhan V, Veiga-Lopez A. Animal models of the polycystic ovary syndrome phenotype. Steroids. 2013;78(8):734–740. doi: 10.1016/j.steroids.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hickey M, Sloboda DM, Atkinson HC, et al. The relationship between maternal and umbilical cord androgen levels and polycystic ovary syndrome in adolescence: a prospective cohort study. J Clin Endocrinol Metab. 2009;94(10):3714–3720. doi: 10.1210/jc.2009-0544. [DOI] [PubMed] [Google Scholar]
- 118.Anderson H, Fogel N, Grebe SK, Singh RJ, Taylor RL, Dunaif A. Infants of women with polycystic ovary syndrome have lower cord blood androstenedione and estradiol levels. J Clin Endocrinol Metab. 2010;95(5):2180–2186. doi: 10.1210/jc.2009-2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Barry JA, Kay AR, Navaratnarajah R, et al. Umbilical vein testosterone in female infants born to mothers with polycystic ovary syndrome is elevated to male levels. J Obstet Gynaecol. 2010;30(5):444–446. doi: 10.3109/01443615.2010.485254. [DOI] [PubMed] [Google Scholar]
- 120.Van Wagenen G. Accelerated growth with sexual precocity in female monkeys receiving testosterone propionate. Endocrinology. 1949;45(5):544–546. doi: 10.1210/endo-45-5-544. [DOI] [PubMed] [Google Scholar]
- 121.McGee WK, Bishop CV, Bahar A, et al. Elevated androgens during puberty in female rhesus monkeys lead to increased neuronal drive to the reproductive axis: a possible component of polycystic ovary syndrome. Hum Reprod. 2012;27(2):531–540. doi: 10.1093/humrep/der393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30(4):293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Eng DS, Lee JM, Gebremariam A, Meeker JD, Peterson K, Padmanabhan V. Bisphenol A and chronic disease risk factors in US children. Pediatrics. 2013;132(3):e637–e645. doi: 10.1542/peds.2013-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhou W, Liu J, Liao L, Han S, Liu J. Effect of bisphenol A on steroid hormone production in rat ovarian theca-interstitial and granulosa cells. Mol Cell Endocrinol. 2008;283(1-2):12–18. doi: 10.1016/j.mce.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 125.Takeuchi T, Tsutsumi O, Ikezuki Y, Takai Y, Taketani Y. Positive relationship between androgen and the endocrine disruptor, bisphenol A, in normal women and women with ovarian dysfunction. Endocr J. 2004;51(2):165–169. doi: 10.1507/endocrj.51.165. [DOI] [PubMed] [Google Scholar]
- 126.Barber TM, Bennett AJ, Groves CJ, et al. Association of variants in the fat mass and obesity associated (FTO) gene with polycystic ovary syndrome. Diabetologia. 2008;51(7):1153–1158. doi: 10.1007/s00125-008-1028-6. [DOI] [PubMed] [Google Scholar]
- 127.Wehr E, Schweighofer N, Möller R, Giuliani A, Pieber TR, Pietsch B. Association of FTO gene with hyperandrogenemia and metabolic parameters in women with polycystic ovary syndrome. Metabolism. 2010;59(4):575–580. doi: 10.1016/j.metabol.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 128.Kowalska I, Malecki MT, Straczkowski M, et al. The FTO gene modifies weight, fat mass and insulin sensitivity in women with polycystic ovary syndrome, where its role may be larger than in other phenotypes. Diabetes Metab. 2009;35(4):328–331. doi: 10.1016/j.diabet.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 129.Ewens KG, Jones MR, Ankener W, et al. FTO and MC4R gene variants are associated with obesity in polycystic ovary syndrome. PLoS ONE. 2011;6(1):e16390. doi: 10.1371/journal.pone.0016390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tan S, Scherag A, Janssen OE, et al. Large effects on body mass index and insulin resistance of fat mass and obesity associated gene (FTO) variants in patients with polycystic ovary syndrome (PCOS) BMC Med Genet. 2010;11:12. doi: 10.1186/1471-2350-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wojciechowski P, Lipowska A, Rys P, et al. GIANT Consortium Impact of FTO genotypes on BMI and weight in polycystic ovary syndrome: a systematic review and meta-analysis. Diabetologia. 2012;55(10):2636–2645. doi: 10.1007/s00125-012-2638-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Coviello AD, Legro RS, Dunaif A. Adolescent girls with polycystic ovary syndrome have an increased risk of the metabolic syndrome associated with increasing androgen levels independent of obesity and insulin resistance. J Clin Endocrinol Metab. 2006;91(2):492–497. doi: 10.1210/jc.2005-1666. [DOI] [PubMed] [Google Scholar]
- 133.Palmert MR, Gordon CM, Kartashov AI, Legro RS, Emans SJ, Dunaif A. Screening for abnormal glucose tolerance in adolescents with polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;87(3):1017–1023. doi: 10.1210/jcem.87.3.8305. [DOI] [PubMed] [Google Scholar]
- 134.Flannery CA, Rackow B, Cong X, Duran E, Selen DJ, Burgert TS. Polycystic ovary syndrome in adolescence: impaired glucose tolerance occurs across the spectrum of BMI. Pediatr Diabetes. 2013;14(1):42–49. doi: 10.1111/j.1399-5448.2012.00902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wild RA, Rizzo M, Clifton S, Carmina E. Lipid levels in polycystic ovary syndrome: systematic review and meta-analysis. Fertil Steril. 2011;95(3):1073–1079. e1–e11. doi: 10.1016/j.fertnstert.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 136.Tasali E, Van Cauter E, Ehrmann DA. Polycystic ovary syndrome and obstructive sleep apnea. Sleep Med Clin. 2008;3(1):37–46. doi: 10.1016/j.jsmc.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.de Sousa G, Schlüter B, Buschatz D, et al. A comparison of polysomnographic variables between obese adolescents with polycystic ovarian syndrome and healthy, normal-weight and obese adolescents. Sleep Breath. 2010;14(1):33–38. doi: 10.1007/s11325-009-0276-0. [DOI] [PubMed] [Google Scholar]
- 138.Nandalike K, Agarwal C, Strauss T, et al. Sleep and cardiometabolic function in obese adolescent girls with polycystic ovary syndrome. Sleep Med. 2012;13(10):1307–1312. doi: 10.1016/j.sleep.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dokras A. Mood and anxiety disorders in women with PCOS. Steroids. 2012;77(4):338–341. doi: 10.1016/j.steroids.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 140.Trent ME, Rich M, Austin SB, Gordon CM. Quality of life in adolescent girls with polycystic ovary syndrome. Arch Pediatr Adolesc Med. 2002;156(6):556–560. doi: 10.1001/archpedi.156.6.556. [DOI] [PubMed] [Google Scholar]
- 141.McElroy SL, Kotwal R, Malhotra S, Nelson EB, Keck PE, Nemeroff CB. Are mood disorders and obesity related? A review for the mental health professional. J Clin Psychiatry. 2004;65(5):634–651. doi: 10.4088/jcp.v65n0507. quiz 730. [DOI] [PubMed] [Google Scholar]
- 142.Trent M, Austin SB, Rich M, Gordon CM. Overweight status of adolescent girls with polycystic ovary syndrome: body mass index as mediator of quality of life. Ambul Pediatr. 2005;5(2):107–111. doi: 10.1367/A04-130R.1. [DOI] [PubMed] [Google Scholar]
- 143.Hollinrake E, Abreu A, Maifeld M, Van Voorhis BJ, Dokras A. Increased risk of depressive disorders in women with polycystic ovary syndrome. Fertil Steril. 2007;87(6):1369–1376. doi: 10.1016/j.fertnstert.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 144.Dieudonne MN, Pecquery R, Boumediene A, Leneveu MC, Giudicelli Y. Androgen receptors in human preadipocytes and adipocytes: regional specificities and regulation by sex steroids. Am J Physiol. 1998;274(6 Pt 1):C1645–C1652. doi: 10.1152/ajpcell.1998.274.6.C1645. [DOI] [PubMed] [Google Scholar]
- 145.Janssen I, Powell LH, Kazlauskaite R, Dugan SA. Testosterone and visceral fat in midlife women: the Study of Women’s Health Across the Nation (SWAN) fat patterning study. Obesity (Silver Spring) 2010;18(3):604–610. doi: 10.1038/oby.2009.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lovejoy JC, Bray GA, Bourgeois MO, et al. Exogenous androgens influence body composition and regional body fat distribution in obese postmenopausal women—a clinical research center study. J Clin Endocrinol Metab. 1996;81(6):2198–2203. doi: 10.1210/jcem.81.6.8964851. [DOI] [PubMed] [Google Scholar]
- 147.Elbers JM, Asscheman H, Seidell JC, Megens JA, Gooren LJ. Long-term testosterone administration increases visceral fat in female to male transsexuals. J Clin Endocrinol Metab. 1997;82(7):2044–2047. doi: 10.1210/jcem.82.7.4078. [DOI] [PubMed] [Google Scholar]
- 148.Gambineri A, Pelusi C, Genghini S, et al. Effect of flutamide and metformin administered alone or in combination in dieting obese women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 2004;60(2):241–249. doi: 10.1111/j.1365-2265.2004.01973.x. [DOI] [PubMed] [Google Scholar]
- 149.Gambineri A, Patton L, Vaccina A, et al. Treatment with flutamide, metformin, and their combination added to a hypocaloric diet in overweight-obese women with polycystic ovary syndrome: a randomized, 12-month, placebo-controlled study. J Clin Endocrinol Metab. 2006;91(10):3970–3980. doi: 10.1210/jc.2005-2250. [DOI] [PubMed] [Google Scholar]
- 150.Corbould A. Effects of androgens on insulin action in women: is androgen excess a component of female metabolic syndrome? Diabetes Metab Res Rev. 2008;24(7):520–532. doi: 10.1002/dmrr.872. [DOI] [PubMed] [Google Scholar]
- 151.Korhonen S, Hippeläinen M, Vanhala M, Heinonen S, Niskanen L. The androgenic sex hormone profile is an essential feature of metabolic syndrome in premenopausal women: a controlled community-based study. Fertil Steril. 2003;79(6):1327–1334. doi: 10.1016/s0015-0282(03)00347-9. [DOI] [PubMed] [Google Scholar]
- 152.Apridonidze T, Essah PA, Iuorno MJ, Nestler JE. Prevalence and characteristics of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90(4):1929–1935. doi: 10.1210/jc.2004-1045. [DOI] [PubMed] [Google Scholar]
- 153.Fruzzetti F, Perini D, Lazzarini V, Parrini D, Genazzani AR. Hyperandrogenemia influences the prevalence of the metabolic syndrome abnormalities in adolescents with the polycystic ovary syndrome. Gynecol Endocrinol. 2009;25(5):335–343. doi: 10.1080/09513590802630146. [DOI] [PubMed] [Google Scholar]
- 154.Holmäng A, Svedberg J, Jennische E, Björntorp P. Effects of testosterone on muscle insulin sensitivity and morphology in female rats. Am J Physiol. 1990;259(4 Pt 1):E555–E560. doi: 10.1152/ajpendo.1990.259.4.E555. [DOI] [PubMed] [Google Scholar]
- 155.Holmäng A, Larsson BM, Brzezinska Z, Björntorp P. Effects of short-term testosterone exposure on insulin sensitivity of muscles in female rats. Am J Physiol. 1992;262(6 Pt 1):E851–E855. doi: 10.1152/ajpendo.1992.262.6.E851. [DOI] [PubMed] [Google Scholar]
- 156.Corbould A. Chronic testosterone treatment induces selective insulin resistance in subcutaneous adipocytes of women. J Endocrinol. 2007;192(3):585–594. doi: 10.1677/joe.1.07070. [DOI] [PubMed] [Google Scholar]
- 157.Polderman KH, Gooren LJ, Asscheman H, Bakker A, Heine RJ. Induction of insulin resistance by androgens and estrogens. J Clin Endocrinol Metab. 1994;79(1):265–271. doi: 10.1210/jcem.79.1.8027240. [DOI] [PubMed] [Google Scholar]
- 158.Diamond MP, Grainger D, Diamond MC, Sherwin RS, Defronzo RA. Effects of methyltestosterone on insulin secretion and sensitivity in women. J Clin Endocrinol Metab. 1998;83(12):4420–4425. doi: 10.1210/jcem.83.12.5333. [DOI] [PubMed] [Google Scholar]