Abstract
Background
A substantial number of surveillance studies have documented rotavirus prevalence among children admitted for dehydrating diarrhea. We sought to establish global seasonal patterns of rotavirus disease before widespread vaccine introduction.
Methods
We reviewed studies of rotavirus detection in children with diarrhea published since 1995. We assessed potential relationships between seasonal prevalence and locality by plotting the average monthly proportion of diarrhea cases positive for rotavirus according to geography, country development, and latitude. We used linear regression to identify variables that were potentially associated with the seasonal intensity of rotavirus.
Results
Among a total of 99 studies representing all six geographical regions of the world, patterns of year-round disease were more evident in low- and low-middle income countries compared with upper-middle and high income countries where disease was more likely to be seasonal. The level of country development was a stronger predictor of strength of seasonality (P=0.001) than geographical location or climate. However, the observation of distinctly different seasonal patterns of rotavirus disease in some countries with similar geographical location, climate and level of development indicate that a single unifying explanation for variation in seasonality of rotavirus disease is unlikely.
Conclusion
While no unifying explanation emerged for varying rotavirus seasonality globally, the country income level was somewhat more predictive of the likelihood of having seasonal disease than other factors. Future evaluation of the effect of rotavirus vaccination on seasonal patterns of disease in different settings may help understand factors that drive the global seasonality of rotavirus disease.
Keywords: rotavirus, seasonality, season, diarrhea, global, surveillance
Background
Rotavirus infection is the most common cause of severe diarrhea among children under 5 years of age in both developing and developed regions of the world.(1, 2) Seasonality of rotavirus infection has been shown to differ widely across the world. Several previous studies have undertaken efforts to improve understanding of factors favoring the variation in occurrence of rotavirus disease by season and locality. In a review of 34 studies conducted prior to 1990, Cook et al. found that rotavirus infection typically occurred during the winter months of the year in temperate regions, while year-round patterns were common in the warmer tropical regions.(3) Attempts to find a link between seasonal incidence of rotavirus disease and many aspects of climate, such as temperature, relative humidity, rainfall, and barometric pressure have yielded conflicting findings.(4–8)
The difficulty in finding an association between rotavirus disease and climatic, geographic, economic, or behavioral factors is related to the fact that most human activity and condition is intricately linked with or affected by climate. Another challenging aspect of findings explanations for rotavirus seasonality has been that, until recently, studies examining rotavirus prevalence have been limited in number from many regions of the world, and widely variable in terms of study design, use of laboratory assays, and reporting of data.(9) To address these concerns and better assess the burden of rotavirus disease in anticipation of vaccine introduction, in 2001, the World Health Organization (WHO) developed a generic protocol for conducting sentinel hospital-based rotavirus surveillance using standardized site selection procedures, surveillance design, enzyme immunoassays for rotavirus detection, and reporting parameters.(10) Simultaneously, funding from international donors has enabled the development of global epidemiology and laboratory networks of rotavirus surveillance. As a result of these two concerted efforts, an abundance of studies have been published in recent years, which have applied uniform procedures to examine the burden and seasonality of rotavirus disease.(1, 11)
We used the wealth of information that has recently become available to review the global prevalence of rotavirus disease before the widespread use of recently available rotavirus vaccines.(12) In addition, we reexamined the influence of localities on the seasonality of rotavirus disease on a global scale. We found that the recently published studies were broadly in agreement with previous observations that rotavirus disease tends to have a more strongly seasonal occurrence in temperate climates compared to tropical climates, where disease tends to occur year-round. However, we found that income level, rather than latitude or geographic region, was the strongest predictor of seasonality. Disentangling the effect of climate from other ecological factors such as income level, access to clean water, level of sanitation, birth rates, and interactions with animals (which could serve as proxies for the overall transmission rate) has been proven to be difficult, but could perhaps be better understood as these surveillance data accumulate.
METHODS
Search strategy and selection criteria
We reviewed all epidemiological studies published between January 1995 and December 2010 that assessed the prevalence of rotavirus among children with diarrhea. We searched PubMed MEDLINE using the following keywords: “rotavirus and season”, and “rotavirus and seasonality.” We did not limit our search by language. We limited our analysis to studies that met the following criteria: conducted in full-year increments; tested at least 50 children with acute gastroenteritis for rotavirus, using an enzyme immunoassay, polyacrylamide gel electrophoresis, or reverse-transcriptase polymerase chain reaction; provided monthly data on the proportion of all patients with diarrhea caused by rotavirus. We included studies from both inpatient and outpatient settings because seasonality is not expected to vary by treatment setting. If studies reported data from more than one city, we considered results from each city as a separate data point. We restricted our analysis to studies published since 1995 because studies prior to this date have previously been reviewed(3) and because, since the mid-1990s, most studies have employed sensitive and specific enzyme immunoassays when conducting surveillance for rotavirus, typically using the standardized surveillance methods recommended by the WHO. Eligible studies were selected and abstracted by a single author (DV) and reviewed by another author (MP). For each study that satisfied our criteria, we recorded the study city, country, duration, age of enrollees, proportion of rotavirus positive results by month, and the number of specimens tested and number of positive results if available.
Most studies provided proportion of diarrhea events positive for rotavirus, rather than the absolute numbers of rotavirus-positive tests. Among the few studies that presented both, the seasonal pattern of rotavirus infection was similar (data not shown). For studies longer than 12 months in duration, we averaged the proportion of diarrhea events positive for rotavirus during each month.
Data analysis
Previous studies have determined that seasonality of rotavirus disease may vary by region. To assess potential relationships between seasonal prevalence and locality, we plotted the average monthly proportion of diarrhea cases positive for rotavirus according to three inter-related groups: geographical area, level of country development, and latitude. First, we grouped studies according to six geographic regions of the world: North & Central America, South America, Asia, Africa, Europe, and Oceania. Second, we grouped studies according to the World Bank income stratification for the country represented by each study: low and low-middle income; high-middle income; and high-income countries. By summing monthly prevalence data across regions with rotavirus detection during opposing seasons could be misinterpreted as “year-round” disease. Thus, the grouping of studies by income level was restricted to the Northern Hemisphere and the tropical belt (i.e., latitudes 10° North and 10° South of the equator), where peak prevalence of disease was between December and May, and ignored countries in the Southern Hemisphere where peak prevalence of rotavirus disease occurred during May–October. Third, we grouped studies according to latitudes with broadly similar seasons: 36–70° North, 11–35° North, 10° North–10° South, 11°–35° South, and 36°–55° South. To account for the undue influence of studies with a smaller sample, the pooled averages for these groups were weighted according to study size.
We were also interested in determining predictors at the ecological level for apparent differences in the strength of seasonality of rotavirus disease on a global scale. To characterize seasonal variability in rotavirus disease, we used an indicator of “seasonal intensity” which is the ratio of amplitude of peak rotavirus activity (i.e., peak monthly prevalence of rotavirus) to the average annual prevalence of rotavirus. This ratio reduces noise from months with little or no rotavirus testing in some settings. Higher seasonal intensity reflected greater deviation from the annual mean, and thus greater seasonal variation. We also explored the seasonality patterns of each site by measuring the amplitude (peak – trough/peak) and primary peak timing of the ‘average’ year calculated using Fourier analysis. Briefly, by this method we extracted the frequencies of the first (annual), second (semi-annual) and third (quarterly) harmonics in the data, which were then summed to produce the seasonal signature of the series.(13) Amplitudes and primary peak timings of each site were inspected against their corresponding latitudes.(14)
We used multivariable linear regression to identify independent variables that were potentially associated with the seasonal intensity of rotavirus. Possible predictors were identified on the basis of previous studies and hypotheses for explaining rotavirus seasonality. These included temperature, rainfall, altitude, geographic location (latitude), population density, and level of country development as measured by 2001’s Gross National Income [GNI] per capita adjusted for purchasing power parity, retaining all variables in the final model. Birth rate was considered as a potential explanatory variable but was not included due to collinearity with GNI. Including study size in the model did not alter the findings and thus was not retained in the final model. Data on weather, latitude, and altitude were abstracted from Weatherbase(15) and the World Weather Information Service.(16) For cities that were not listed in the weather databases, meteorological information was based on a nearby city within the same climatic region. For studies that reported aggregate results of multiple cities, the average weather pattern was calculated. GNI per capita based on purchasing power parity was obtained from the World Bank.(17) The year of 2001 was chosen as it is the average year of the focused studies. Analyses were conducted using Matlab R2007b, and SAS 9.1.
RESULTS
We reviewed 522 abstracts to identify a total of 186 potential studies, from which 99 studies (representing 153 separate study sites) met the inclusion criteria of our analysis (Table 1). These studies represented all six regions of the world, with sites being most common in Asia (59%) and South America (20%), and less common in Europe (8%), Africa (7%), North/Central America (4%), and Oceania (3%). Most studies had similar methodology in that they followed the WHO recommendations for conducting rotavirus surveillance, used enzyme linked immunoassays to detect rotavirus, and limited the sample population to children less than 5 years of age. Studies reported data from the hospital setting (83%), outpatient setting (3%), or both (14%). In total, these studies reported data on ~428,000 samples of stool from children with diarrhea, of which ~111,000 (26%) tested positive for rotavirus.
Table 1.
Summary of studies published between 1999–2010 that assessed rotavirus seasonality globally
| Ref | Country | City | Study Dates | Age | Site | No. AGE | %RV | Peak RV month | GNI |
|---|---|---|---|---|---|---|---|---|---|
| AFRICA | |||||||||
|
| |||||||||
| (41) | Egypt | Abu Homos | 05/2000–05/2002 | <5 | H | 714 | 10% | 11 | 2016 |
| (41) | Egypt | Benha | 05/2000–05/2002 | <5 | H | 561 | 23% | 11 | 2016 |
| (42) | Morocco | Tanger, Rabat, Oujda, Benimellal | 06/2006–07/2007 | <5 | H | 314 | 44% | 1 | 2696 |
| (43) | Nigeria | NW region | 07/2002–07/2004 | <5 | H | 869 | 18% | 11 | 1393 |
| (44) | Tunisia | Eastern-Center | 10/2003–09/2005 | <5 | B | 638 | 21% | 2 | 3680 |
| (28) | Kenya | Nairobi | 08/1991–07/1994 | <6 | H | 656 | 28% | 7 | 783 |
| (28) | Kenya | Kitui | 08/1991–07/1994 | <6 | H | 490 | 14% | 1 | 783 |
| (28) | Kenya | Nanyuki | 08/1991–07/1994 | <6 | H | 285 | 22% | 3 | 783 |
| (45) | Zambia | Lusaka | 01/1992–12/1992 | <5 | H | 1067 | 24% | 5 | 1053 |
| (46) | Botwsana | Gaborone | 03/2001–02/2002 | <5 | H | 346 | 9% | 8 | 5630 |
| (47) | Tunisia | Tunis | 01/2007–12/2007 | <5 | H | 117 | 26% | 7 | 3680 |
|
| |||||||||
| ASIA | |||||||||
|
| |||||||||
| (48) | Malaysia | Kuala Lumpur | 01/2001–04/2003 | <5 | H | 2668 | 47% | 2 | 7921 |
| (49) | Thailand | Country-wide | 07/2001–06/2003 | <5 | B | 1950 | 43% | 1 | 4046 |
| (50) | Saudi Arabia | Medina | 04/2004–04/2005 | <5 | B | 984 | 19% | 1 | 18718 |
| (51) | Indonesia | Palembang, Jakarta, Bandung, Yogyakarta, Denpasar, Mataram | 01/2006–12/2006 | <5 | B | 2416 | 59% | 7 | 2150 |
| (52) | Indonesia | Jakarta | 02/2004–02/2005 | <5 | O | 1660 | 45% | 3 | 2150 |
| (53) | Oman | Muscat, Salalah, Sohar, Rustaq, Nizwa, Ibra, Sur, Ibri | 07/2006–06/2008 | <5 | H | 3470 | 49% | 3 | 17884 |
| (54) | Phillipines | Muntinlupa City | 01/2005–12/2006 | <5 | B | 2946 | 21% | 1 | 2063 |
| (55) | Japan | Maizuru, Tokyo, Sapporo, Saga and Osaka | 07/2006–06/2007 | <10 | H | 628 | 20% | 7 | 39853 |
| (56) | China | Various cities throughout | 08/2003–07/2007 | <5 | H | 7846 | 48% | 1 | 3213 |
| (57) | Iran | Tabriz, Mashhad, Tehran, Shiraz, Bandar Abbas | 05/2006–04/2007 | <5 | H | 2198 | 59% | 1 | 4651 |
| (58) | Uzbekistan | Tashkent, Bukhara city | 01/2005–12/2006 | <5 | H | 3537 | 30% | 10 | 948 |
| (59) | South Korea | Gyeonggi | 01/2001–12/2005 | All ages | B | 10028 | 67% | 3 | 19422 |
| (23) | Georgia | Tbilisi | 01/2007–12/2007 | <5 | H | 703 | 40% | 1 | 2948 |
| (23) | Tajikstan | Dushanbe City | 01/2007–12/2007 | <5 | H | 702 | 38% | 11 | 468 |
| (60) | Mongolia | Ulaanbaatar city | 03/2005–02/2007 | <5 | H | 1152 | 40% | 9 | 2052 |
| (60) | Sri Lanka | Colombo | 03/2005–02/2007 | <5 | H | 1806 | 24% | 2 | 2007 |
| (61) | Nepal | Kathmandu | 11/2005–10/2007 | <5 | H | 1139 | 33% | 1 | 468 |
| (61) | Nepal | Kathmandu | 11/2005–10/2007 | <5 | H | 1139 | 33% | 1 | 468 |
| (62) | Turkey | Ankara, Istanbul, Izmir, and Adana | 06/2005–06/2006 | <5 | H | 338 | 53% | 2 | 10007 |
| (63) | India | Delhi | 12/2005–11/2007 | <5 | H | 633 | 37% | 12 | 1054 |
| (63) | India | Trichy | 12/2005–11/2007 | <5 | H | 406 | 53% | 9 | 1054 |
| (64) | Japan | Tsu City | 01/2003–12/2007 | <5 | H | 551 | 46% | 3 | 39853 |
| (64) | Japan | Ise City | 01/2003–12/2007 | <5 | H | 327 | 59% | 3 | 39853 |
| (65) | Bangladesh | Dhaka | 01/2001–05/2006 | <5 | H | 19039 | 24% | 2 | 533 |
| (66) | Pakistan | Karachi | 06/2005–05/2007 | <5 | O | 575 | 17% | 12 | 1031 |
| (24) | Kyrgyzstan | Bishkek | 01/2005–12/2007 | <5 | H | 1959 | 29% | 9 | 909 |
| (24) | Kyrgyzstan | Osh | 01/2005–12/2007 | <5 | H | 1797 | 22% | 9 | 909 |
| (67) | China | Changchun, Lulong, Lanzhou | 01/2006–12/2007 | <5 | H | 2328 | 52% | 12 | 3213 |
| (68) | Taiwan | Various | 01/2005–12/2007 | <5 | H | 3435 | 25% | 2 | 18000 |
| (69) | Vietnam | Haiphong | 09/2006–08/2007 | <5 | H | 978 | 52% | 1 | 1019 |
| (70) | Cambodia | Phnom Penh | 03/2005–02/2007 | <5 | H | 2817 | 45% | 12 | 660 |
| (71) | Laos | Vientiane | 03/2005–02/2007 | <5 | H | 1158 | 55% | 2 | 790 |
| (72) | Myanmar | Yangon | 01/2004–12/2005 | <5 | H | 2179 | 56% | 12 | 578 |
| (73) | Indonesia | Java | 08/2001–04/2004 | <3 | H | 1321 | 53% | 8 | 2150 |
| (74) | South Korea | Gyeonggi | 07/2001–06/2002 | <18 | H | 1031 | 27% | 4 | 19422 |
| (75) | Taiwan | Taipei | 01/2004–03/2005 | <14 | H | 201 | 26% | 4 | 18000 |
| (76) | Bangladesh | Dhaka | 01/1993–12/2004 | <5 | B | 18300 | 33% | 1 | 533 |
| (77) | China | Wuhan, Changling | 12/2000–4/2006* | All ages | B | 3174 | 16% | 11 | 3213 |
| (78) | Hong Kong | Hong Kong | 04/2001–3/2003 | <5 | H | 5881 | 30% | 1 | 32900 |
| (79) | Japan | Akita | 01/2001–12/2002 | <5 | H | 422 | 58% | 4 | 39853 |
| (80) | India | New Delhi | 08/2000–07/2001 | <5 | H | 584 | 23% | 12 | 1054 |
| (81) | Bangladesh | Dhaka | 02/1993–06/1994 | <5 | H | 814 | 20% | 1 | 533 |
| (82) | Turkey | Ankara | 03/1999–12/2002 | <16 | H | 1099 | 37% | 12 | 10007 |
| (83) | Saudi Arabia | Jeddah | 1/1992–12/1992 | <5 | H | 1031 | 43% | 11 | 18718 |
| (83) | Saudi Arabia | Al-taif | 07/1992–06/1993 | <5 | H | 354 | 42% | 8 | 18718 |
| (84) | Thailand | Chiang Mai | 01/1995–12/1996 | Pedi atric | H | 164 | 34% | 1 | 4046 |
| (85) | Pakistan | Karachi | 01/1990–12/1997 | <5 | H | 818 | 14% | 11 | 1031 |
| (86) | Vietnam | Hanoi | 07/1998–06/2000 | <5 | H | 1233 | 53% | 5 | 1019 |
| (86) | Vietnam | Hanoi | 07/1998–06/2000 | <5 | H | 390 | 47% | 2 | 1019 |
| (86) | Vietnam | Haiphong | 07/1998–06/2000 | <5 | H | 886 | 60% | 5 | 1019 |
| (86) | Vietnam | Nha Trang | 07/1998–06/2000 | <5 | H | 589 | 59% | 3 | 1019 |
| (86) | Vietnam | Ho Chi Minh City | 07/1998–06/2000 | <5 | H | 1724 | 57% | 1 | 1019 |
| (86) | Vietnam | Ho Chi Minh City | 07/1998–06/2000 | <5 | H | 946 | 58% | 2 | 1019 |
| (87) | South Korea | Jeongeub | 07/2002–06/2004 | <5 | H | 2232 | 21% | 2 | 19422 |
| (88) | China | Taiwan | 04/2001–03/2003 | <5 | H | 2600 | 43% | 2 | 3213 |
| (89) | China | MaAnShan | 08/2001–07/2003 | <5 | H | 158 | 28% | 4 | 3213 |
| (89) | China | Beijing | 08/2001–07/2003 | <5 | H | 70 | 36% | 9 | 3213 |
| (89) | China | Lulong | 08/2001–07/2003 | <5 | H | 667 | 45% | 5 | 3213 |
| (89) | China | Suzhou | 08/2001–07/2003 | <5 | H | 703 | 49% | 5 | 3213 |
| (89) | China | Changchun | 08/2001–07/2003 | <5 | H | 904 | 65% | 5 | 3213 |
| (89) | China | Kunming | 08/2001–07/2003 | <5 | H | 647 | 46% | 3 | 3213 |
| (90) | Myanmar | Yangon | 01/2002–12/2003 | <5 | H | 1736 | 53% | 10 | 578 |
| (91) | Vietnam | Hanoi | 07/2000–06/2003 | <5 | H | 1690 | 56% | 2 | 1019 |
| (91) | Vietnam | Haiphong | 07/2000–06/2003 | <5 | H | 1095 | 44% | 1 | 1019 |
| (91) | Vietnam | Khanh Hoa | 07/2000–06/2003 | <5 | H | 625 | 50% | 8 | 1019 |
| (91) | Vietnam | Ho Chi Minh City | 07/2000–06/2003 | <5 | H | 1508 | 59% | 2 | 1019 |
| (91) | Vietnam | Ho Chi Minh City | 07/2000–06/2003 | <5 | H | 891 | 62% | 2 | 1019 |
| (92) | Hong Kong | Hong Kong | 01/1987–12/1996 | <5 | H | 7822 | 28% | 12 | 32900 |
| (93) | Bangladesh | Dhaka | 01/1990–12/1993 | <5 | B | 7709 | 20% | 12 | 533 |
| (9) | China | Beijing, Changchun, Lulong, Kunming, Ma-An-Shan, Suzhou | 08/2001–07/2002 | <5 | H | 2079 | 44% | 1 | 3213 |
| (9) | Vietnam | Hanoi, Haiphong, Khan Hoa, Ho Chi | 08/2001–07/2002 | <5 | H | 1570 | 59% | 8 | 1019 |
| (9) | Taiwan | Minh City, Taipei | 08/2001–07/2002 | <5 | H | 1532 | 49% | 2 | 18000 |
| (9) | Thailand | Nongkhai, Maesod, Prapokklao, Ramathibodi, Hadyai, Srakaew | 08/2001–07/2002 | <5 | H | 992 | 44% | 1 | 4046 |
| (9) | Hong Kong | Hong Kong | 08/2001–07/2002 | <5 | H | 2986 | 28% | 5 | 32900 |
| (9) | Malaysia | Kuala Lumpur, Sarawak | 08/2001–07/2002 | <5 | H | 1374 | 57% | 1 | 7921 |
| (9) | Indonesia | Yogyakarta, Purworejo | 08/2001–07/2002 | <5 | H | 577 | 52% | 8 | 2150 |
| (94) | China | Lanzhou | 01/2001–12/2006 | <5 | H | 1019 | 52% | 10 | 3213 |
| (95) | Turkey | Izmir | 01/2000–01/2001 | <5 | H | 920 | 40% | 2 | 10007 |
| (96) | Taiwan | Taichung | 12/2004–06/2006 | <5 | H | 763 | 46% | 1 | 18000 |
| (97) | India | Lucknow | 09/2004–04/2008 | <3 | H | 412 | 19% | 12 | 1054 |
| (98) | Japan | Sapporo | 07/1984–06/1999 | <14 | H | 445 | 49% | 3 | 39853 |
| (98) | Japan | Tokyo | 07/1984–06/1999 | <14 | H | 1172 | 67% | 3 | 39853 |
| (98) | Japan | Maizuru | 07/1984–06/1999 | <14 | H | 2311 | 50% | 3 | 39853 |
| (98) | Japan | Osaka | 07/1984–06/1999 | <14 | H | 519 | 54% | 2 | 39853 |
| (98) | Japan | Kurume | 07/1984–06/1999 | <14 | H | 1188 | 69% | 3 | 39853 |
| (98) | Japan | Saga | 07/1984–06/1999 | <14 | H | 296 | 76% | 2 | 39853 |
| (99) | Thailand | Bangkok | 04/2001–03/2002 | <5 | H | 85 | 48% | 11 | 4046 |
| (100) | South Korea | Country-wide | 09/2000–08/2007 | All ages | H | 164081 | 12% | 2 | 19422 |
|
| |||||||||
| EUROPE | |||||||||
|
| |||||||||
| (101) | Israel | Netanya, Hadera, Haifa | 11/2007–10/2008 | <5 | H | 412 | 34% | 3 | 28190 |
| (102) | Italy | Padova | 10/2004–09/2005 | <5 | B | 725 | 46% | 3 | 38047 |
| (23) | Ukraine | Kyiv | 01/2007–12/2007 | <5 | H | 947 | 49% | 3 | 3879 |
| (23) | Ukraine | Odessa | 01/2007–12/2007 | <5 | H | 1022 | 41% | 12 | 3879 |
| (103) | Spain | Valladolid | 01/2000–12/2004 | <5 | H | 2233 | 24% | 1 | 35098 |
| (104) | Saudi Arabia | Jeddah, Makkah, Riyadh | 09/2002–08/2003 | <6 | B | 1000 | 6% | 4 | 18718 |
| (105) | Spain | Country-wide | 01/1999–12/2000 | All ages | H | 32541 | 14% | 2 | 35098 |
| (106) | Hungary | Budapest | 01/1993–12/1996 | <14 | H | 6022 | 26% | 12 | 14387 |
| (106) | Hungary | Pecs | 01/1993–12/1996 | <14 | H | 845 | 11% | 2 | 14387 |
| (106) | Hungary | Szeged | 01/1993–10/1996 | <14 | H | 2315 | 12% | 12 | 14387 |
| (27) | Spain | Madrid | 10/1998–10/2002 | <5 | H | 3760 | 31% | 12 | 35098 |
| (26) | France | Dijon | 10/2004–09/2005 | <5 | H | 218 | 33% | 3 | 44972 |
|
| |||||||||
| NORTH & CENTRAL AMERICA | |||||||||
|
| |||||||||
| (107) | USA | Cincinnati | 03/1999–05/2000 | <5 | H | 199 | 37% | 3 | 45836 |
| (107) | USA | Oakland | 03/1999–05/2000 | <6 | H | 83 | 46% | 2 | 45836 |
| (108) | United States | Jacksonville | 12/1991–11/1995 | <1 | B | 194 | 49% | 1 | 45836 |
| (29) | El Salvador | San Salvador | 05/2001–04/2002 | <5 | H | 322 | 21% | 12 | 3499 |
| (25) | United States | Cincinnati and Durham | 02/2005–06/2006 | <5 | B | 1601 | 44% | 3 | 45836 |
| (109) | Latin America--north hemisphere* | El Salvador, Guatemala, Honduras, Venezuela, Guyana | 01/2006–12/2007 | <5 | H | 15071 | 41% | 3 | 4114a |
|
| |||||||||
| OCEANIA | |||||||||
|
| |||||||||
| (110) | New Zealand | Auckland, Christchruch, Wellington | 05/1998–04/2000 | <3 | H | 1138 | 43% | 7 | 30447 |
| (111) | Fiji | Suva | 01/2006–12/2007 | <5 | H | 454 | 39% | 7 | 4018 |
| (112) | Australia | Melbourne | 04/1980–03/1993 | <14 | H | 3503 | 42% | 9 | 46574 |
| (113) | Australia | Sydney | 07/1997–06/1998 | <5 | B | 412 | 14% | 9 | 46574 |
| (114) | New Zealand | Christchurch, Wellington | 05/1998–04/2000 | <3 | H | 1138 | 43% | 8 | 30447 |
|
| |||||||||
| SOUTH AMERICA | |||||||||
|
| |||||||||
| (115) | Chile | Iquique, Santiago | 08/2004–10/2005 | <5 | O | 1313 | 14% | 5 | 9147 |
| (116) | Brazil | Goiania | 08/2005–08/2006 | <5 | B | 98 | 42% | 11 | 8136 |
| (116) | Brazil | Porto Alegre | 08/2005–08/2006 | <5 | B | 56 | 21% | 10 | 8136 |
| (116) | Brazil | Salvador | 08/2005–08/2006 | <5 | B | 147 | 38% | 2 | 8136 |
| (116) | Brazil | Sao Paulo | 08/2005–08/2006 | <5 | B | 209 | 54% | 9 | 8136 |
| (117) | Venezuela | Merida | 06/1993–05/1995 | <5 | H | 377 | 15% | 12 | 11444 |
| (117) | Venezuela | Valencia | 06/1998–05/2000 | <5 | H | 4414 | 25% | 1 | 11444 |
| (117) | Venezuela | Cumana | 06/1993–05/1995 | <5 | H | 376 | 46% | 12 | 11444 |
| (117) | Venezuela | Puerto Ordaz | 06/1993–05/1995 | <5 | H | 336 | 29% | 11 | 11444 |
| (117) | Venezuela | Caracas | 06/1993–05/1995 | <5 | H | 1239 | 31% | 1 | 11444 |
| (118) | Venezuela | Caracas | 01/1988–12/1993 | <3yr | B | 2101 | 26% | 2 | 11444 |
| (119) | Argentina | Tucuman | 10/1996–9/1998 | <3 | H | 435 | 53% | 6 | 8027 |
| (119) | Argentina | Mendoza | 10/1996–9/1998 | <3 | H | 151 | 23% | 6 | 8027 |
| (119) | Argentina | La Plata | 10/1996–9/1998 | <3 | H | 188 | 48% | 4 | 8027 |
| (119) | Argentina | Rosario | 10/1996–9/1998 | <3 | H | 174 | 34% | 6 | 8027 |
| (119) | Argentina | Cordoba | 10/1996–9/1998 | <3 | H | 231 | 32% | 6 | 8027 |
| (120) | Argentina | Buenos Aires | 08/1997–07/1999 | <3 | H | 1133 | 39% | 5 | 8027 |
| (120) | Chile | Santiago | 08/1997–07/1999 | <3 | H | 1739 | 34% | 8 | 9147 |
| (120) | Venezuela | Valencia | 08/1997–07/1999 | <3 | H | 2929 | 29% | 1 | 11444 |
| (121) | Argentina | Buenos Aires | 09/1997–08/1998 | <3 | O | 66 | 26% | 7 | 8027 |
| (122) | Paraguay | Asuncion | 01/1999–03/2000 | <3 | B | 220 | 32% | 8 | 2688 |
| (123) | Brazil | Botucatu | 06/1997–05/1998 | <5 | H | 54 | 41% | 9 | 8136 |
| (124) | Venezuela | Valencia | 01/1998–12/2002 | <5 | O | 9109 | 20% | 1 | 11444 |
| (124) | Venezuela | Valencia | 01/1998–12/2002 | <5 | H | 2879 | 31% | 1 | 11444 |
| (125) | Brazil | Sao Luis | 06/1997–06/1999 | <3 | H | 128 | 32% | 10 | 8136 |
| (126) | Paraguay | Asuncion | 08/1998–12/1999 | <4 | H | 393 | 19% | 7 | 2688 |
| (127) | Brazil | Salvador | 11/2003–11/2004 | <19 | H | 558 | 16% | 1 | 8136 |
| (128) | Argentina | Buenos Aires | 01/1997–06/2003 | <17 | H | 1579 | 27% | 5 | 8027 |
| (129) | Brazil | Juiz de Fora | 01/1998–12/1998 | <5 | B | 656 | 12% | 7 | 8136 |
| (109) | Latin America--south hemisphere* | Bolivia, Paraguay, Chile | 01/2006–12/2007 | <5 | H | 4532 | 36% | 5 | 4450a |
| (130) | Argentina | Buenos Aires | 09/1997–08/1998 | <3 | H | 648 | 36% | 6 | 8027 |
H denotes hospitalization; O denotes outpatient visit; B denotes both; GNI denotes gross national income
: individual data not presented for these countries, thus average GNI of the countries was taken.
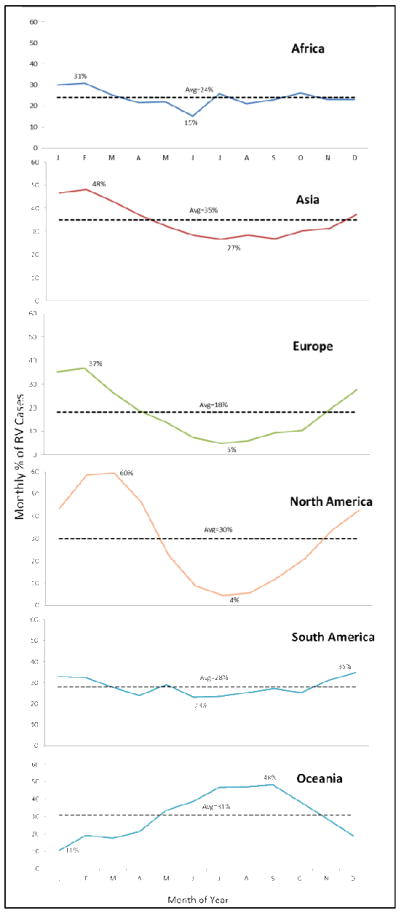
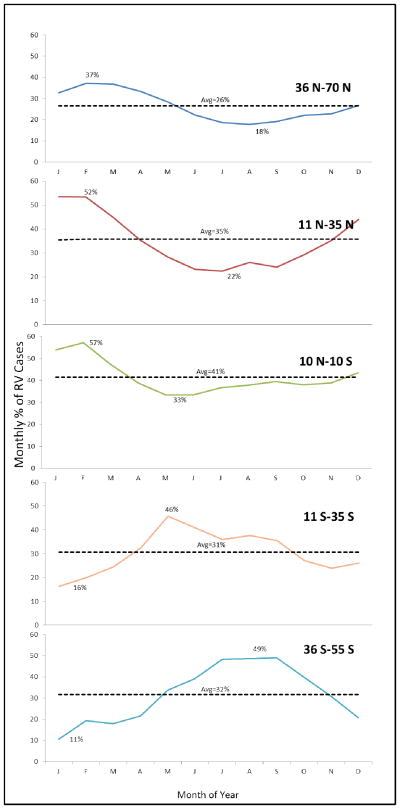
When studies were examined by regions, in general, detection of rotavirus occurred year-round in Africa, Asia, and South America, with some month-to-month fluctuations (Figure 1). In these continents, the mean prevalence of rotavirus ranged between 31%–48% during peak month and 15%–23% during the nadir (i.e., lowest prevalence) month. In contrast, seasonality was stronger in Europe, North America, and Oceania, with prevalence of disease ranging between 37%–60% during peak winter months and 4%–11% during the nadir summer months. Detection of rotavirus overlapped with the winter months (November–April) in higher Northern latitudes, and the peak times in the tropical belt were also in phase with the peak times of higher latitudes (Figures 2&3). In contrast, peak prevalence in the Southern hemisphere (below 10° South) typically occurred between May and October, the winter months in this hemisphere. The intensity of seasonality was quite strong through all latitudes, although overall a bit lower in the tropical belt (Figures 4&5), where the monthly prevalence never dropped below 33%, compared with countries further north or south of these latitudes where minimum monthly prevalence ranged between 11%–22% (Figure 2).
Figure 1.
Seasonal variation in rotavirus prevalence globally by region, 1996–2009.
Figure 2.
Seasonal variation in rotavirus prevalence globally by latitude, 1996–2009.
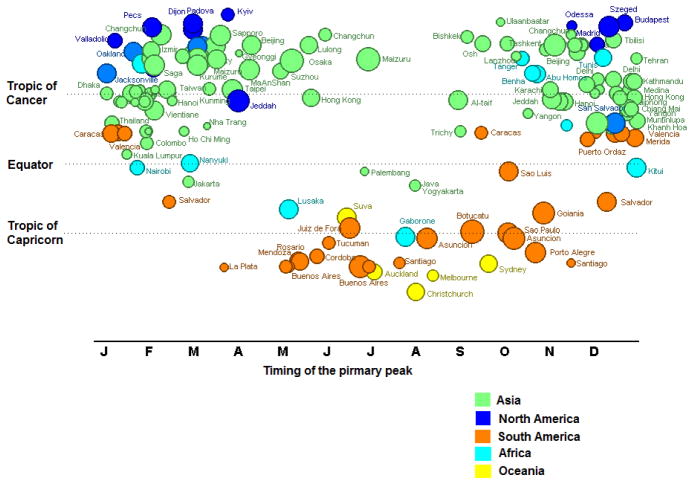
Figure 3. Timing of the primary peak of rotavirus disease globally, by latitude.
Colors denote continents to which each point belongs and size of circle is proportion to the number of months rotavirus was in circulation.
Figure 4. Amplitude of the primary peak of the seasonal signature of the series as a function of latitude.
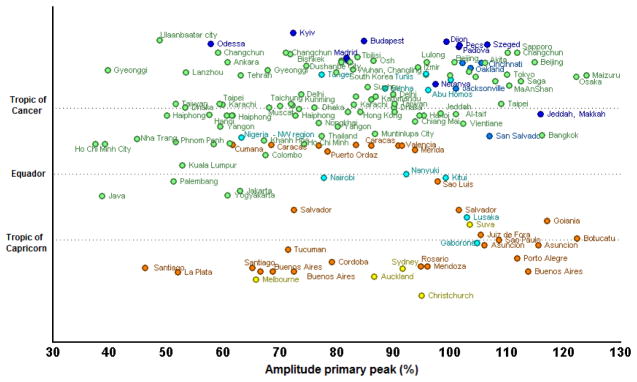
Amplitude is peak-trough/peak ratio. Colors denote continents to which each point belongs.
Figure 5.
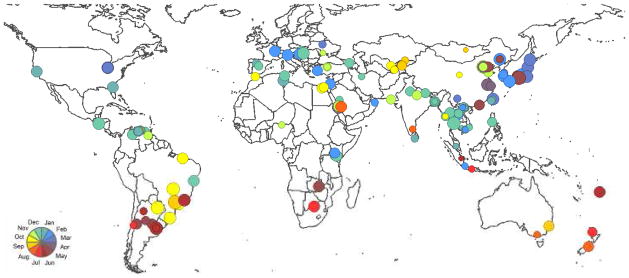
Map with the same information, but here the relative amplitudes are expressed in circle sizes.
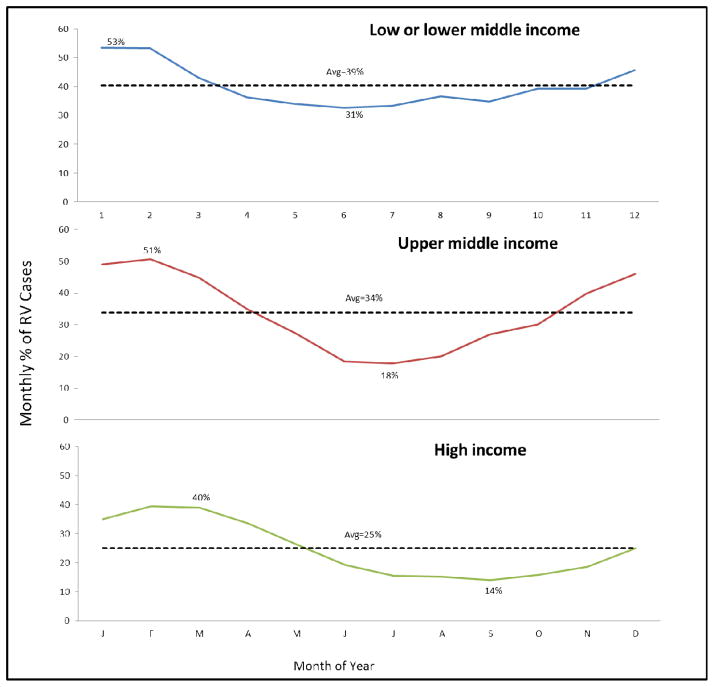
When countries were grouped according to their level of economic development (Figure 6), the low- and low-middle income countries had a higher mean minimum prevalence of rotavirus at 31% during the nadir month compared with 14% in high-income countries and 18% in upper-middle income countries. In the multivariate regression model, no single factor explained most of the seasonality at this ecological level (R2=0.13, P<0.001). Among considered factors, the country GNI emerged as the strongest predictor of the seasonality intensity (P=0.001), after adjusting for latitude, yearly rainfall, average, temperature, altitude, and population density. Although latitude and climate were not significant predictors of the seasonality intensity in the multivariate model overall, a distinct pattern existed with regard to the timing of the peak prevalence of rotavirus disease (Figures 3&6) which was not assessed in the model.
Figure 6. Seasonal variation in rotavirus in tropical and northern temperate zones, by World Bank income groups.
We restricted the analysis to studies conducted in the Northern Hemisphere and the tropical belt (i.e., latitudes 10° North and 10° South of the equator) to avoid falsely representing seasonality by summing monthly prevalence across regions with peak rotavirus detections during opposing seasons (which would result in a bias towards weaker seasonality).
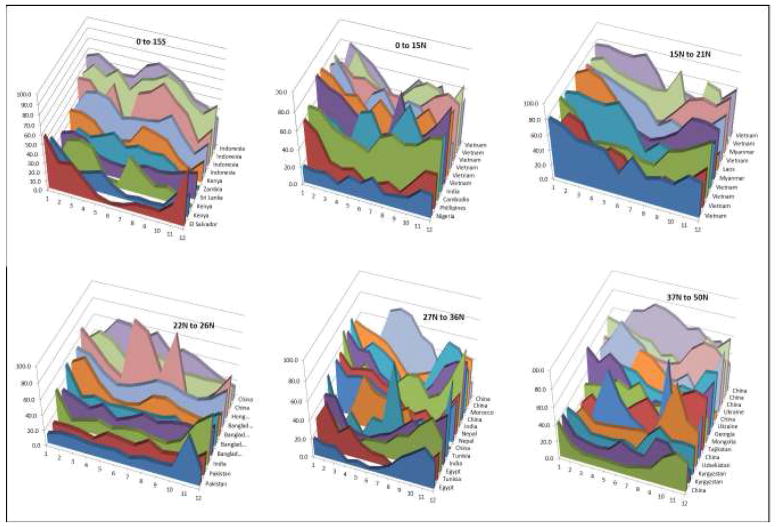
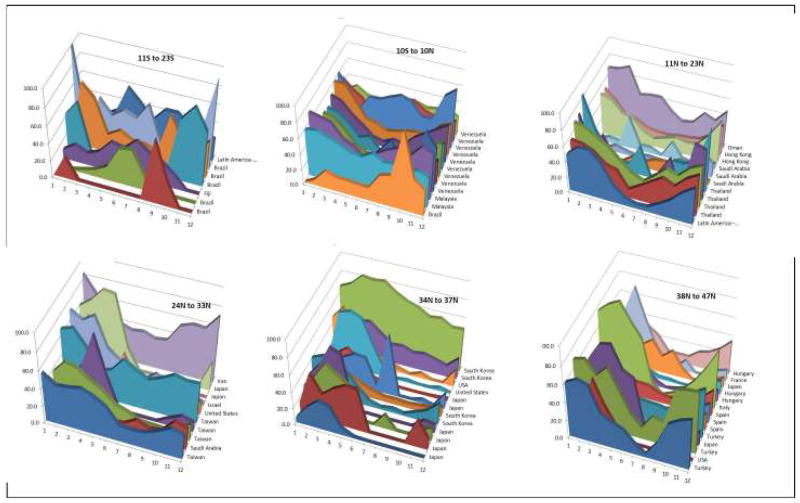
The country-specific data indicated that rotavirus infection had a predictable annual pattern in some countries while in others peak timing of infection was more sporadic (Figures 6&7). For instance, some studies reported year-round prevalence of rotavirus disease with possible peaks during disparate months (e.g., Vietnam, China), while others had most rotavirus events confined to a few months of the year (e.g., El Salvador, United States, Japan). Neither latitude nor the country’s level of economic development perfectly explained seasonality. For example, year-round disease was common in several poor countries closer to the equator (e.g., Indonesia, Vietnam, Cambodia), whereas other poor countries had prominent seasonal variability (e.g., El Salvador, Kenya, and Philippines) (Figure 8). Likewise, among poor countries >37° North of the equator, year-round disease was present in some regions (e.g., Ukraine, Mongolia, Tajikistan, Kyrgyzstan), but others also had marked seasonal variability (e.g., Georgia, Uzbekistan). Interestingly, year-round circulation of rotavirus was not confined to low-income countries in the tropics, but was also observed in several upper-middle and high-income countries (Figures 9&10) from various regions and latitudes (e.g., Taiwan, South Korea, Brazil, Iran, Oman, and Malaysia). The year-round patterns of rotavirus disease were similar among studies conducting surveillance for 12 months compared to those conducting surveillance for more than 12 months, indicating that this year-round pattern was not due to averaging of prevalence over multiple years with potentially different timing of peak incidence.
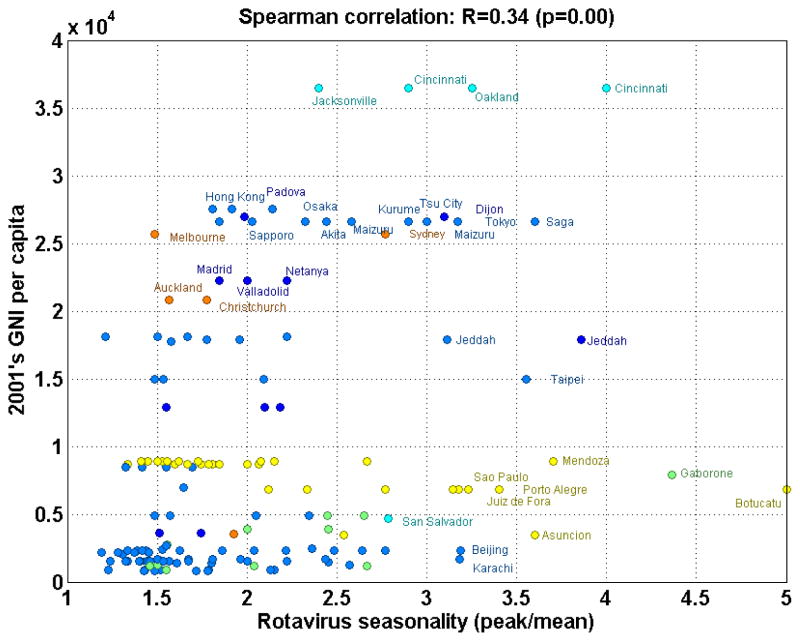
Figure 7. Scatter plot of gross national income per capita plotted against the strength of rotavirus seasonality.
Gross national income per capita is adjusted for purchasing power parity. Strength of seasonality was defined by the “seasonality ratio” (peak/mean prevalence). Colors denote continents to which each point belongs. Site legends are displayed for “outliers” (where values are more than one standard deviation above the average).
Figure 8.
Seasonality in low and low-middle income countries, by latitude.
Figure 9.
Seasonality in high-middle and high-income countries, by latitude.
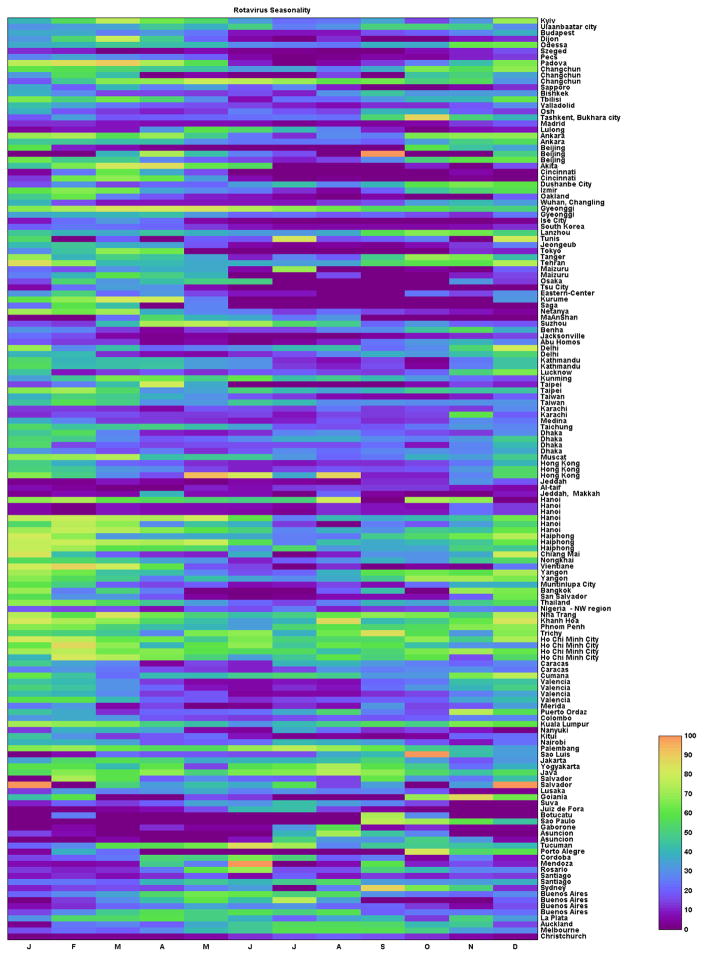
Figure 10. Visualization of relative detection of rotavirus per month in each site globally, sorted by latitude.
The colors represent the monthly prevalence of rotavirus among diarrhea cases, as indicated by the color bar.
DISCUSSION
In 1990, Cook et al. demonstrated that rotavirus had a distinct seasonal peak in countries with temperate climates but was year-round in the tropics.(3) Our review of a large body of data published since the paper by Cook et al. identified that such a clear distinction between seasonal patterns in tropical and temperate settings may not exist, as many countries in the tropics exhibited very seasonal disease patterns while several temperate countries showed year-round disease. The level of country development was a stronger predictor of seasonal intensity of rotavirus disease than latitude or geographical location per se —poorer countries, particularly those in Africa, Asia and South America had lesser seasonal variation in disease than more developed countries from Europe, North America, and Oceania, even after taking into account local climate and geographical location. These data are not in complete disagreement with findings by Cook et al. in that tropical countries are in general less developed than those in temperate regions. Our findings are also consistent with a recent modeling study by Pitzer et al.(18) which demonstrated that high transmission rates and high birth rates, factors common to poor countries, explained, in part, a relative lack of seasonality in these countries. However, on closer examination of data from individual countries, we found several exceptions to these general patterns, and thus it is possible that factors other than income level and transmission patterns could also influence seasonal patterns of rotavirus disease to some extent.
Some data have suggested that local climatologic factors may be associated with seasonality of rotavirus disease, with increased incidence during cool, dry seasons, particularly in settings where the annual rotavirus epidemic coincides with the winter season.(5, 19, 20) Because survival of infective rotavirus is favored in cooler conditions with low relative humidity, it has been hypothesized that a relative drop in humidity and rainfall combined with drying of soils might increase the aerial transport of dried, contaminated fecal material.(21, 22) In our analysis, detection of rotavirus overlapped with the cooler months in higher latitudes but, surprisingly (given that the winter concept is less common closer to the equator) peak times of rotavirus detection in the tropical belt were generally in phase with the peak times of higher latitudes (Figure 3). While subtle changes in local climate may play a role in explaining seasonal cycling of rotavirus disease in some settings, the gross differences in seasonal prevalence of rotavirus disease globally cannot be attributed to climate alone. For example, several countries with temperate climates such as Ukraine(23) and Kyrgyzstan(24) had year-round presence of rotavirus disease whereas disease tends to be highly concentrated during the winter months in other temperate regions of North America(25) and Western Europe(26, 27). Likewise, several poor tropical countries such as Kenya(28) and El Salvador(29) also have notable seasonal disease similar to some wealthier countries in temperate climates such as the US. The fact that rotavirus peaks occur on a yearly basis would suggest that some factor that cycles annually (potentially related to weather), must play a role underpinning the timing and regularity of patterns. However, any single explanation for seasonal differences in rotavirus disease globally is difficult to reconcile with these observations.
At a regional level, many factors may interact and explain seasonality including climate, transmission patterns, host behavior, and susceptibility. The influence of these factors may also be context specific. It is possible that multiple factors related to transmission interact in some settings and have opposing climatic influences. For instance, contaminated water may be an important source of transmission in settings with poor sanitation.(30, 31) Rotavirus can be isolated from water sources, and waterborne outbreaks of rotavirus have been described.(31–34) Furthermore, some studies have noted secondary seasonal peaks in rotavirus incidence associated with periods of high rainfall and flooding.(6, 35) The waterborne transmission route may dominate during times of increased rainfall in such settings, whereas more direct forms of transmission may dominate during the cooler, drier seasons (when virus survival is increased) across all settings. Rotavirus strains also tend to be more diverse in low-income settings.(36) Although no data exist assessing the variation in seasonality between the different rotavirus serotypes, factors driving the global seasonality of influenza have been determined to differ between the specific types and subtypes of the influenza virus.(37) Similar differences between different strains of rotavirus, if they were to exist, might partly explain the weaker seasonality in low-income settings.
Some limitations must be considered when interpreting our results. While we provided a descriptive account of differences in seasonality of rotavirus by region, we did not explicitly account for factors such as local transmission dynamics; a closer examination of regional data may provide an explanation for the timing of onset and variation in disease in any given country or geographic region. Furthermore, we only examined associations between a crude indicator of seasonality and possible predictors. A more detailed examination of the associations between annual variation in rotavirus prevalence in each setting and possible predictors, taking into account population immunity, possible lags, and the non-linear effects of transmission(8), was beyond the scope of the current study and would require more detailed data. Heterogeneity in data quality is likely to exist given the large number of studies. However, of specific importance to our objective is that most studies in the past decade have employed validated diagnostic assays and a similar case-definition for diarrhea, and were conducted for a minimum of 12 calendar months among children <5 years of age. Another potential limitation is that most studies presented monthly prevalence of rotavirus detection (proportion of tests positive) among children with diarrhea rather than the actual number of events. Monthly prevalence of rotavirus might be affected by the incidence of non-rotavirus pathogens, which will influence the number of acute gastroenteritis events that are tested. However, our conclusions were unchanged when we assessed this potential effect in a subset of studies that presented both case-counts and percent of tests positive for rotavirus. Lastly, the number of children enrolled in individual studies varied markedly. To account for the undue influence of studies with a smaller sample, we used weighted averages when pooling data across regions; however, patterns of seasonality remained unchanged when we used unweighted averages.
Many countries, mostly developed countries with seasonal disease, have already introduced rotavirus vaccines, and introductions are expected in poorer regions of Asia and Africa in coming years.(38) Changes in seasonality of rotavirus disease after large-scale rollout of rotavirus vaccines particularly in countries with year-round disease versus highly seasonal disease should allow us to test the various proposed hypotheses for the seasonal cycling of rotavirus disease. For example, Pitzer et al have demonstrated through modeling that variation in birth rates within the US strongly influences the timing and spread of the annual rotavirus epidemic.(39) Since the introduction of vaccine in the US, studies have observed a remarkable alteration in the timing of the annual winter epidemic and spatiotemporal spread of rotavirus disease(40), which confirmed the influence of birth rates on seasonality in the US in so far as vaccination is effectively similar to reducing the birth rate by reducing the number of fully susceptible children entering the population. Under the hypothesis that the apparent lack of seasonality in some countries is related to higher birth rates, vaccination could alter the seasonal cycling of rotavirus in some developing countries and lead to the appearance of more strongly seasonal disease during the first few years after vaccine introduction(18). Use of vaccine particularly in countries with year-round disease may help elucidate the competing explanations of seasonality, thereby providing insights into the transmission dynamics of rotavirus.
In summary, marked differences in seasonal prevalence of rotavirus disease occur globally. Observations from several countries challenged the traditional dogma that rotavirus disease is seasonal in temperate settings but not in tropical countries. We found that country income level was somewhat more predictive of the likelihood of having seasonal disease than other factors. However, the observations of distinctly different seasonal patterns of rotavirus disease in some countries with similar geographical location, climate and level of development are difficult to reconcile, indicating that a single unifying explanation for variation in seasonality of rotavirus disease is unlikely. Higher birth rates and higher transmission rates have emerged as a potential explanation for sustained year-round circulation of rotavirus, but an interaction of other local factors between the host and the environment likely also contribute to the seasonality.
Table 2.
Results of the multiple linear regression modela assessing predictors of seasonality
| Variable | Coefficient (β) | Standard Error | P |
|---|---|---|---|
| Intercept | 0.7016 | 0.9598 | 0.466 |
| Latitude | 0.0200 | 0.0147 | 0.1749 |
| Yearly rainfall | 0.0001 | 0.0002 | 0.7116 |
| Average temperature | 0.0357 | 0.0249 | 0.155 |
| Altitude | 0.0002 | 0.0002 | 0.3892 |
| GNIb per capita × 1000 | 0.0272 | 0.0826 | 0.0012 |
| Population density | −0.0007 | 0.0005 | 0.1178 |
: Response variable was seasonality ratio of rotavirus (peak monthly prevalence/mean prevalence). Model R2 = 0.13. Study size but was also evaluated in a separate model but was not significant (P=0.47) and did not alter the current model findings.
: GNI denotes gross national income adjusted for purchasing power parity
Acknowledgments
Role of the funding source: This work was partially supported by the Bill and Melinda Gates Foundation and the RAPIDD program of the Science & Technology Directorate, Department of Homeland Security, and the Fogarty International Center, National Institutes of Health (V.E.P.).
This work was supported by the Bill and Melinda Gates Foundation and the RAPIDD program of the Science & Technology Directorate, Department of Homeland Security, and the Fogarty International Center, National Institutes of Health (V.E.P.). The findings and conclusions of this report are those of the authors and do not necessarily represent the views of the US Centers for Disease Control and Prevention. We thank Jazmin Vojdani and Sharla McDonald for their contributions to part of the data collection.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention (CDC).
conflicts of interest: The funders had no role in study design, data collection, analysis and interpretation, or writing of the report. Manish Patel had full access to all the data in the study and had final responsibility for the decision to submit for publication.
No conflicts of interest are declared by any of the authors.
Contributors
MP, UP, VP, and CV created and designed the study. MP and DV collected the data. MP, JT, WA analyzed the data. MP, VP, WA, DV, JT, BL, CV, and UP interpreted the data. MP drafted the report. MP, VP, WA, DV, JT, BL, CV, and UP critically revised the report.
Conflicts of interest
None of the authors had any conflicts of interest relevant to this manuscript.
References
- 1.Rotavirus surveillance worldwide - 2009. Wkly Epidemiol Rec. 2011;86:174–176. [PubMed] [Google Scholar]
- 2.Forster J, Guarino A, Parez N, et al. Hospital-based surveillance to estimate the burden of rotavirus gastroenteritis among European children younger than 5 years of age. Pediatrics. 2009;123:e393–400. doi: 10.1542/peds.2008-2088. [DOI] [PubMed] [Google Scholar]
- 3.Cook SM, Glass RI, LeBaron CW, Ho MS. Global seasonality of rotavirus infections. Bull World Health Organ. 1990;68:171–177. [PMC free article] [PubMed] [Google Scholar]
- 4.Purohit SG, Kelkar SD, Simha V. Time series analysis of patients with rotavirus diarrhoea in Pune, India. J Diarrhoeal Dis Res. 1998;16:74–83. [PubMed] [Google Scholar]
- 5.Levy K, Hubbard AE, Eisenberg JN. Seasonality of rotavirus disease in the tropics: a systematic review and meta-analysis. Int J Epidemiol. 2009;38:1487–1496. doi: 10.1093/ije/dyn260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hashizume M, Armstrong B, Wagatsuma Y, et al. Rotavirus infections and climate variability in Dhaka, Bangladesh: a time-series analysis. Epidemiol Infect. 2007:1–9. doi: 10.1017/S0950268807009776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Souza RM, Hall G, Becker NG. Climatic factors associated with hospitalizations for rotavirus diarrhoea in children under 5 years of age. Epidemiol Infect. 2008;136:56–64. doi: 10.1017/S0950268807008229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atchison CJ, Tam CC, Hajat S, et al. Temperature-dependent transmission of rotavirus in Great Britain and The Netherlands. Proc Biol Sci. 2010;277:933–942. doi: 10.1098/rspb.2009.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bresee J, Fang ZY, Wang B, et al. First report from the Asian Rotavirus Surveillance Network. Emerg Infect Dis. 2004;10:988–995. doi: 10.3201/eid1006.030519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO. Generic protocols for (i) hospital-based surveillance to estimate the burden of rotavirus gastroenteritis in children and (ii) a community-based survey on utilization of health care services for gastroenteritis in children. Document WHO/V & B/0215 Geneva. 2002:1–67. [Google Scholar]
- 11.Widdowson MA, Steele D, Vojdani J, Wecker J, Parashar U. Global rotavirus surveillance: determining the need and measuring the impact of rotavirus vaccines. J Infect Dis. 2009;200 (Suppl 1):S1–8. doi: 10.1086/605061. [DOI] [PubMed] [Google Scholar]
- 12.Rotavirus surveillance worldwide. Wkly Epidemiol Rec. 2009;86:174–176. [PubMed] [Google Scholar]
- 13.Rogers DJ, Hay SI, Packer MJ. Predicting the distribution of tsetse flies in West Africa using temporal Fourier processed meteorological satellite data. Ann Trop Med Parasitol. 1996;90:225–241. doi: 10.1080/00034983.1996.11813049. [DOI] [PubMed] [Google Scholar]
- 14.Alonso WJ, Viboud C, Simonsen L, et al. Seasonality of influenza in Brazil: a traveling wave from the Amazon to the subtropics. Am J Epidemiol. 2007;165:1434–1442. doi: 10.1093/aje/kwm012. [DOI] [PubMed] [Google Scholar]
- 15. [Last accessed January 3, 2012]; Available at http://www.weatherbase.com/
- 16. [Last accessed January 3, 2012]; Available at http://worldweather.wmo.int/
- 17. [Last accessed January 3, 2012]; Available at http://data.worldbank.org/indicator/NY.GNP.PCAP.PP.CD/countries?page=1.
- 18.Pitzer VE, Viboud C, Lopman BA, et al. Influence of birth rates and transmission rates on the global seasonality of rotavirus incidence. J R Soc Interface. 2011 doi: 10.1098/rsif.2011.0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brandt CD, Kim HW, Rodriguez WJ, et al. Rotavirus gastroenteritis and weather. J Clin Microbiol. 1982;16:478–482. doi: 10.1128/jcm.16.3.478-482.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haffejee IE. The epidemiology of rotavirus infections: a global perspective. J Pediatr Gastroenterol Nutr. 1995;20:275–286. doi: 10.1097/00005176-199504000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Moe K, Shirley JA. The effects of relative humidity and temperature on the survival of human rotavirus in faeces. Arch Virol. 1982;72:179–186. doi: 10.1007/BF01348963. [DOI] [PubMed] [Google Scholar]
- 22.Ansari SA, Springthorpe VS, Sattar SA. Survival and vehicular spread of human rotaviruses: possible relation to seasonality of outbreaks. Rev Infect Dis. 1991;13:448–461. doi: 10.1093/clinids/13.3.448. [DOI] [PubMed] [Google Scholar]
- 23.Mirzayeva R, Cortese MM, Mosina L, et al. Rotavirus burden among children in the newly independent states of the former union of soviet socialist republics: literature review and first-year results from the rotavirus surveillance network. J Infect Dis. 2009;200 (Suppl 1):S203–214. doi: 10.1086/605041. [DOI] [PubMed] [Google Scholar]
- 24.Flem ET, Kasymbekova KT, Vainio K, et al. Rotavirus infection in hospitalized children and estimates of disease burden in Kyrgyzstan, 2005–2007. Vaccine. 2009;27 (Suppl 5):F35–39. doi: 10.1016/j.vaccine.2009.08.087. [DOI] [PubMed] [Google Scholar]
- 25.Mast TC, Walter EB, Bulotsky M, et al. Burden of childhood rotavirus disease on health systems in the United States. Pediatr Infect Dis J. 2010;29:e19–25. doi: 10.1097/inf.0b013e3181ca7e2e. [DOI] [PubMed] [Google Scholar]
- 26.Huet F, Chouchane M, Cremillieux C, et al. Prospective epidemiological study of rotavirus gastroenteritis in Europe (REVEAL study). Results in the French area of the study. Arch Pediatr. 2008;15:362–374. doi: 10.1016/j.arcped.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 27.Sanchez-Fauquier A, Wilhelmi I, Colomina J, Cubero E, Roman E. Diversity of group A human rotavirus types circulating over a 4-year period in Madrid, Spain. J Clin Microbiol. 2004;42:1609–1613. doi: 10.1128/JCM.42.4.1609-1613.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakata S, Gatheru Z, Ukae S, et al. Epidemiological study of the G serotype distribution of group A rotaviruses in Kenya from 1991 to 1994. J Med Virol. 1999;58:296–303. doi: 10.1002/(sici)1096-9071(199907)58:3<296::aid-jmv17>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 29.Guardado JA, Clara WA, Turcios RM, et al. Rotavirus in El Salvador: an outbreak, surveillance and estimates of disease burden, 2000–2002. Pediatr Infect Dis J. 2004;23:S156–160. doi: 10.1097/01.inf.0000142464.83628.8e. [DOI] [PubMed] [Google Scholar]
- 30.Black RE, Lopez de Romana G, Brown KH, et al. Incidence and etiology of infantile diarrhea and major routes of transmission in Huascar, Peru. Am J Epidemiol. 1989;129:785–799. doi: 10.1093/oxfordjournals.aje.a115193. [DOI] [PubMed] [Google Scholar]
- 31.Divizia M, Gabrieli R, Donia D, et al. Waterborne gastroenteritis outbreak in Albania. Water Sci Technol. 2004;50:57–61. [PubMed] [Google Scholar]
- 32.Hopkins RS, Gaspard GB, Williams FP, Jr, et al. A community waterborne gastroenteritis outbreak: evidence for rotavirus as the agent. Am J Public Health. 1984;74:263–265. doi: 10.2105/ajph.74.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koroglu M, Yakupogullari Y, Otlu B, et al. A waterborne outbreak of epidemic diarrhea due to group A rotavirus in Malatya, Turkey. New Microbiol. 2011;34:17–24. [PubMed] [Google Scholar]
- 34.Verheyen J, Timmen-Wego M, Laudien R, et al. Detection of adenoviruses and rotaviruses in drinking water sources used in rural areas of Benin, West Africa. Appl Environ Microbiol. 2009;75:2798–2801. doi: 10.1128/AEM.01807-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pinfold JV, Horan NJ, Mara DD. Seasonal effects on the reported incidence of acute diarrhoeal disease in northeast Thailand. Int J Epidemiol. 1991;20:777–786. doi: 10.1093/ije/20.3.777. [DOI] [PubMed] [Google Scholar]
- 36.Gentsch JR, Laird AR, Bielfelt B, et al. Serotype diversity and reassortment between human and animal rotavirus strains: implications for rotavirus vaccine programs. J Infect Dis. 2005;192 (Suppl 1):S146–159. doi: 10.1086/431499. [DOI] [PubMed] [Google Scholar]
- 37.Finkelman BS, Viboud C, Koelle K, et al. Global patterns in seasonal activity of influenza A/H3N2, A/H1N1, and B from 1997 to 2005: viral coexistence and latitudinal gradients. PLoS One. 2007;2:e1296. doi: 10.1371/journal.pone.0001296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel MM, Steele D, Gentsch JR, et al. Real-world impact of rotavirus vaccination. Pediatr Infect Dis J. 2011;30:S1–5. doi: 10.1097/INF.0b013e3181fefa1f. [DOI] [PubMed] [Google Scholar]
- 39.Pitzer VE, Viboud C, Simonsen L, et al. Demographic variability, vaccination, and the spatiotemporal dynamics of rotavirus epidemics. Science. 2009;325:290–294. doi: 10.1126/science.1172330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Curns AT, Panozzo CA, Tate JE, et al. Remarkable postvaccination spatiotemporal changes in United States rotavirus activity. Pediatr Infect Dis J. 2011;30:S54–55. doi: 10.1097/INF.0b013e3181fefda9. [DOI] [PubMed] [Google Scholar]
- 41.Wierzba TF, Abdel-Messih IA, Abu-Elyazeed R, et al. Clinic-based surveillance for bacterial- and rotavirus-associated diarrhea in Egyptian children. Am J Trop Med Hyg. 2006;74:148–153. [PubMed] [Google Scholar]
- 42.Benhafid M, Youbi M, Klena JD, et al. Epidemiology of rotavirus gastroenteritis among children <5 years of age in Morocco during 1 year of sentinel hospital surveillance, June 2006-May 2007. J Infect Dis. 2009;200 (Suppl 1):S70–75. doi: 10.1086/605048. [DOI] [PubMed] [Google Scholar]
- 43.Aminu M, Ahmad AA, Umoh JU, et al. Epidemiology of rotavirus infection in north-western Nigeria. J Trop Pediatr. 2008;54:340–342. doi: 10.1093/tropej/fmn021. [DOI] [PubMed] [Google Scholar]
- 44.Fodha I, Chouikha A, Peenze I, et al. Identification of viral agents causing diarrhea among children in the Eastern Center of Tunisia. J Med Virol. 2006;78:1198–1203. doi: 10.1002/jmv.20681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mpabalwani M, Oshitani H, Kasolo F, et al. Rotavirus gastro-enteritis in hospitalized children with acute diarrhoea in Zambia. Ann Trop Paediatr. 1995;15:39–43. doi: 10.1080/02724936.1995.11747747. [DOI] [PubMed] [Google Scholar]
- 46.Basu G, Rossouw J, Sebunya TK, et al. Prevalence of rotavirus, adenovirus and astrovirus infection in young children with gastroenteritis in Gaborone, Botswana. East Afr Med J. 2003;80:652–655. doi: 10.4314/eamj.v80i12.8783. [DOI] [PubMed] [Google Scholar]
- 47.Tinsa F, Brini I, Yahyaoui S, et al. Infectious diarrhoea in children under five years. Tunis Med. 2009;87:599–602. [PubMed] [Google Scholar]
- 48.Hung LC, Wong SL, Chan LG, et al. Epidemiology and strain characterization of rotavirus diarrhea in Malaysia. Int J Infect Dis. 2006;10:470–474. doi: 10.1016/j.ijid.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 49.Jiraphongsa C, Bresee JS, Pongsuwanna Y, et al. Epidemiology and burden of rotavirus diarrhea in Thailand: results of sentinel surveillance. J Infect Dis. 2005;192 (Suppl 1):S87–93. doi: 10.1086/431508. [DOI] [PubMed] [Google Scholar]
- 50.Kheyami AM, Nakagomi T, Nakagomi O, et al. Molecular epidemiology of rotavirus diarrhea among children in Saudi Arabia: first detection of G9 and G12 strains. J Clin Microbiol. 2008;46:1185–1191. doi: 10.1128/JCM.02244-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Soenarto Y, Aman AT, Bakri A, et al. Burden of severe rotavirus diarrhea in indonesia. J Infect Dis. 2009;200 (Suppl 1):S188–194. doi: 10.1086/605338. [DOI] [PubMed] [Google Scholar]
- 52.Putnam SD, Sedyaningsih ER, Listiyaningsih E, et al. Group A rotavirus-associated diarrhea in children seeking treatment in Indonesia. J Clin Virol. 2007;40:289–294. doi: 10.1016/j.jcv.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 53.Al Awaidy SA, Bawikar S, Al Busaidy S, et al. Considerations for introduction of a rotavirus vaccine in Oman: rotavirus disease and economic burden. J Infect Dis. 2009;200 (Suppl 1):S248–253. doi: 10.1086/605339. [DOI] [PubMed] [Google Scholar]
- 54.Carlos CC, Inobaya MT, Bresee JS, et al. The burden of hospitalizations and clinic visits for rotavirus disease in children aged <5 years in the Philippines. J Infect Dis. 2009;200 (Suppl 1):S174–181. doi: 10.1086/605044. [DOI] [PubMed] [Google Scholar]
- 55.Dey SK, Thongprachum A, Ota Y, et al. Molecular and epidemiological trend of rotavirus infection among infants and children in Japan. Infect Genet Evol. 2009;9:955–961. doi: 10.1016/j.meegid.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 56.Duan ZJ, Liu N, Yang SH, et al. Hospital-Based Surveillance of Rotavirus Diarrhea in the People’s Republic of China, August 2003-July 2007. J Infect Dis. 2009;200 (Suppl 1):S167–173. doi: 10.1086/605039. [DOI] [PubMed] [Google Scholar]
- 57.Eesteghamati A, Gouya M, Keshtkar A, et al. Sentinel hospital-based surveillance of rotavirus diarrhea in iran. J Infect Dis. 2009;200 (Suppl 1):S244–247. doi: 10.1086/605050. [DOI] [PubMed] [Google Scholar]
- 58.Flem ET, Musabaev E, Juraev R, et al. Rotavirus gastroenteritis in uzbekistan: implications for vaccine policy in central Asia. J Infect Dis. 2009;200 (Suppl 1):S154–159. doi: 10.1086/605032. [DOI] [PubMed] [Google Scholar]
- 59.Huh JW, Kim WH, Moon SG, Lee JB, Lim YH. Viral etiology and incidence associated with acute gastroenteritis in a 5-year survey in Gyeonggi province, South Korea. J Clin Virol. 2009;44:152–156. doi: 10.1016/j.jcv.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 60.Nyambat B, Gantuya S, Batuwanthudawe R, et al. Epidemiology of rotavirus diarrhea in mongolia and sri lanka, march 2005-february 2007. J Infect Dis. 2009;200 (Suppl 1):S160–166. doi: 10.1086/605030. [DOI] [PubMed] [Google Scholar]
- 61.Sherchand JB, Nakagomi O, Dove W, et al. Molecular epidemiology of rotavirus diarrhea among children aged <5 years in nepal: predominance of emergent G12 strains during 2 years. J Infect Dis. 2009;200 (Suppl 1):S182–187. doi: 10.1086/605046. [DOI] [PubMed] [Google Scholar]
- 62.Ceyhan M, Alhan E, Salman N, et al. Multicenter prospective study on the burden of rotavirus gastroenteritis in Turkey, 2005–2006: a hospital-based study. J Infect Dis. 2009;200 (Suppl 1):S234–238. doi: 10.1086/605056. [DOI] [PubMed] [Google Scholar]
- 63.Kang G, Arora R, Chitambar SD, et al. Multicenter, hospital-based surveillance of rotavirus disease and strains among indian children aged <5 years. J Infect Dis. 2009;200 (Suppl 1):S147–153. doi: 10.1086/605031. [DOI] [PubMed] [Google Scholar]
- 64.Kamiya H, Nakano T, Inoue M, et al. A retrospective evaluation of hospitalizations for acute gastroenteritis at 2 sentinel hospitals in central Japan to estimate the health burden of rotavirus. J Infect Dis. 2009;200 (Suppl 1):S140–146. doi: 10.1086/605028. [DOI] [PubMed] [Google Scholar]
- 65.Rahman M, Sultana R, Ahmed G, et al. Prevalence of G2P[4] and G12P[6] rotavirus, Bangladesh. Emerg Infect Dis. 2007;13:18–24. doi: 10.3201/eid1301.060910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Qazi R, Sultana S, Sundar S, et al. Population-based surveillance for severe rotavirus gastroenteritis in children in Karachi, Pakistan. Vaccine. 2009;27 (Suppl 5):F25–30. doi: 10.1016/j.vaccine.2009.08.064. [DOI] [PubMed] [Google Scholar]
- 67.Li DD, Liu N, Yu JM, et al. Molecular epidemiology of G9 rotavirus strains in children with diarrhoea hospitalized in Mainland China from January 2006 to December 2007. Vaccine. 2009;27 (Suppl 5):F40–45. doi: 10.1016/j.vaccine.2009.08.073. [DOI] [PubMed] [Google Scholar]
- 68.Wu FT, Liang SY, Tsao KC, et al. Hospital-based surveillance and molecular epidemiology of rotavirus infection in Taiwan, 2005–2007. Vaccine. 2009;27 (Suppl 5):F50–54. doi: 10.1016/j.vaccine.2009.08.090. [DOI] [PubMed] [Google Scholar]
- 69.Ngo TC, Nguyen BM, Dang DA, et al. Molecular epidemiology of rotavirus diarrhoea among children in Haiphong, Vietnam: the emergence of G3 rotavirus. Vaccine. 2009;27 (Suppl 5):F75–80. doi: 10.1016/j.vaccine.2009.08.074. [DOI] [PubMed] [Google Scholar]
- 70.Nyambat B, Meng CY, Vansith K, et al. Hospital-based surveillance for rotavirus diarrhoea in Phnom Penh, Cambodia, March 2005 through February 2007. Vaccine. 2009;27 (Suppl 5):F81–84. doi: 10.1016/j.vaccine.2009.08.085. [DOI] [PubMed] [Google Scholar]
- 71.Aloun DS, Nyambat B, Phetsouvanh R, et al. Rotavirus diarrhoea among children aged less than 5 years at Mahosot Hospital, Vientiane, Lao PDR. Vaccine. 2009;27 (Suppl 5):F85–88. doi: 10.1016/j.vaccine.2009.08.100. [DOI] [PubMed] [Google Scholar]
- 72.Moe K, Thu HM, Oo WM, et al. Genotyping of rotavirus isolates collected from children less than 5 years of age admitted for diarrhoea at the Yangon Children’s Hospital, Myanmar. Vaccine. 2009;27 (Suppl 5):F89–92. doi: 10.1016/j.vaccine.2009.08.068. [DOI] [PubMed] [Google Scholar]
- 73.Wilopo SA, Soenarto Y, Bresee JS, et al. Rotavirus surveillance to determine disease burden and epidemiology in Java, Indonesia, August 2001 through April 2004. Vaccine. 2009;27 (Suppl 5):F61–66. doi: 10.1016/j.vaccine.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 74.Kang JO, Kim CR, Kilgore PE, Choi TY. G and P genotyping of human rotavirus isolated in a university hospital in Korea: implications for nosocomial infections. J Korean Med Sci. 2006;21:983–988. doi: 10.3346/jkms.2006.21.6.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu TC, Liu HH, Chen YJ, et al. Comparison of clinical features of childhood norovirus and rotavirus gastroenteritis in Taiwan. J Chin Med Assoc. 2008;71:566–570. doi: 10.1016/S1726-4901(08)70170-9. [DOI] [PubMed] [Google Scholar]
- 76.Tanaka G, Faruque AS, Luby SP, et al. Deaths from rotavirus disease in Bangladeshi children: estimates from hospital-based surveillance. Pediatr Infect Dis J. 2007;26:1014–1018. doi: 10.1097/INF.0b013e318125721c. [DOI] [PubMed] [Google Scholar]
- 77.Wang YH, Kobayashi N, Zhou DJ, et al. Molecular epidemiologic analysis of group A rotaviruses in adults and children with diarrhea in Wuhan city, China, 2000–2006. Arch Virol. 2007;152:669–685. doi: 10.1007/s00705-006-0904-y. [DOI] [PubMed] [Google Scholar]
- 78.Nelson EA, Tam JS, Bresee JS, et al. Estimates of rotavirus disease burden in Hong Kong: hospital-based surveillance. J Infect Dis. 2005;192 (Suppl 1):S71–79. doi: 10.1086/431492. [DOI] [PubMed] [Google Scholar]
- 79.Nakagomi T, Nakagomi O, Takahashi Y, et al. Incidence and burden of rotavirus gastroenteritis in Japan, as estimated from a prospective sentinel hospital study. J Infect Dis. 2005;192 (Suppl 1):S106–110. doi: 10.1086/431503. [DOI] [PubMed] [Google Scholar]
- 80.Bahl R, Ray P, Subodh S, et al. Incidence of severe rotavirus diarrhea in New Delhi, India, and G and P types of the infecting rotavirus strains. J Infect Dis. 2005;192 (Suppl 1):S114–119. doi: 10.1086/431497. [DOI] [PubMed] [Google Scholar]
- 81.Albert MJ, Faruque AS, Faruque SM, Sack RB, Mahalanabis D. Case-control study of enteropathogens associated with childhood diarrhea in Dhaka, Bangladesh. J Clin Microbiol. 1999;37:3458–3464. doi: 10.1128/jcm.37.11.3458-3464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Karadag A, Acikgoz ZC, Avci Z, et al. Childhood diarrhoea in Ankara, Turkey: epidemiological and clinical features of rotavirus-positive versus rotavirus-negative cases. Scand J Infect Dis. 2005;37:269–275. doi: 10.1080/00365540410020983. [DOI] [PubMed] [Google Scholar]
- 83.Milaat WA, Elassouli SM. Epidemiology of diarrhoea in two major cities in Saudi Arabia. J Commun Dis. 1995;27:84–91. [PubMed] [Google Scholar]
- 84.Maneekarn N, Ushijima H. Epidemiology of rotavirus infection in Thailand. Pediatr Int. 2000;42:415–421. doi: 10.1046/j.1442-200x.2000.01251.x. [DOI] [PubMed] [Google Scholar]
- 85.Nishio O, Matsui K, Oka T, et al. Rotavirus infection among infants with diarrhea in Pakistan. Pediatr Int. 2000;42:425–427. doi: 10.1046/j.1442-200x.2000.01256.x. [DOI] [PubMed] [Google Scholar]
- 86.Nguyen VM, Nguyen VT, Huynh PL, et al. The epidemiology and disease burden of rotavirus in Vietnam: sentinel surveillance at 6 hospitals. J Infect Dis. 2001;183:1707–1712. doi: 10.1086/320733. [DOI] [PubMed] [Google Scholar]
- 87.Kim JS, Kang JO, Cho SC, et al. Epidemiological profile of rotavirus infection in the Republic of Korea: results from prospective surveillance in the Jeongeub District, 1 July 2002 through 30 June 2004. J Infect Dis. 2005;192 (Suppl 1):S49–56. doi: 10.1086/431506. [DOI] [PubMed] [Google Scholar]
- 88.Chen KT, Chen PY, Tang RB, et al. Sentinel hospital surveillance for rotavirus diarrhea in Taiwan, 2001–2003. J Infect Dis. 2005;192 (Suppl 1):S44–48. doi: 10.1086/431495. [DOI] [PubMed] [Google Scholar]
- 89.Fang ZY, Wang B, Kilgore PE, et al. Sentinel hospital surveillance for rotavirus diarrhea in the People’s Republic of China, August 2001-July 2003. J Infect Dis. 2005;192 (Suppl 1):S94–99. doi: 10.1086/431505. [DOI] [PubMed] [Google Scholar]
- 90.Moe K, Hummelman EG, Oo WM, Lwin T, Htwe TT. Hospital-based surveillance for rotavirus diarrhea in children in Yangon, Myanmar. J Infect Dis. 2005;192 (Suppl 1):S111–113. doi: 10.1086/431509. [DOI] [PubMed] [Google Scholar]
- 91.Van Man N, Luan le T, Trach DD, et al. Epidemiological profile and burden of rotavirus diarrhea in Vietnam: 5 years of sentinel hospital surveillance, 1998–2003. J Infect Dis. 2005;192 (Suppl 1):S127–132. doi: 10.1086/431501. [DOI] [PubMed] [Google Scholar]
- 92.Chan PK, Tam JS, Nelson EA, et al. Rotavirus infection in Hong Kong: epidemiology and estimates of disease burden. Epidemiol Infect. 1998;120:321–325. doi: 10.1017/s0950268898008747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Unicomb LE, Kilgore PE, Faruque SG, et al. Anticipating rotavirus vaccines: hospital-based surveillance for rotavirus diarrhea and estimates of disease burden in Bangladesh. Pediatr Infect Dis J. 1997;16:947–951. doi: 10.1097/00006454-199710000-00008. [DOI] [PubMed] [Google Scholar]
- 94.Jin Y, Ye XH, Fang ZY, et al. Molecular epidemic features and variation of rotavirus among children with diarrhea in Lanzhou, China, 2001–2006. World J Pediatr. 2008;4:197–201. doi: 10.1007/s12519-008-0036-4. [DOI] [PubMed] [Google Scholar]
- 95.Kurugol Z, Geylani S, Karaca Y, et al. Rotavirus gastroenteritis among children under five years of age in Izmir, Turkey. Turk J Pediatr. 2003;45:290–294. [PubMed] [Google Scholar]
- 96.Mast TC, Chen PY, Lu KC, et al. Epidemiology and economic burden of rotavirus gastroenteritis in hospitals and paediatric clinics in Taiwan, 2005–2006. Vaccine. 2010;28:3008–3013. doi: 10.1016/j.vaccine.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 97.Mishra V, Awasthi S, Nag VL, Tandon R. Genomic diversity of group A rotavirus strains in patients aged 1–36 months admitted for acute watery diarrhoea in northern India: a hospital-based study. Clin Microbiol Infect. 2010;16:45–50. doi: 10.1111/j.1469-0691.2009.02772.x. [DOI] [PubMed] [Google Scholar]
- 98.Zhou Y, Li L, Kim B, et al. Rotavirus infection in children in Japan. Pediatr Int. 2000;42:428–439. doi: 10.1046/j.1442-200x.2000.01247.x. [DOI] [PubMed] [Google Scholar]
- 99.Intusoma U, Sornsrivichai V, Jiraphongsa C, Varavithaya W. Epidemiology, clinical presentations and burden of rotavirus diarrhea in children under five seen at Ramathibodi Hospital, Thailand. J Med Assoc Thai. 2008;91:1350–1355. [PubMed] [Google Scholar]
- 100.Jeong HS, Lee KB, Jeong AY, et al. Genotypes of the circulating rotavirus strains in the seven prevaccine seasons from September 2000 to August 2007 in South Korea. Clin Microbiol Infect. 2011;17:232–235. doi: 10.1111/j.1469-0691.2010.03232.x. [DOI] [PubMed] [Google Scholar]
- 101.Muhsen K, Shulman L, Rubinstein U, et al. Incidence, characteristics, and economic burden of rotavirus gastroenteritis associated with hospitalization of israeli children <5 years of age, 2007–2008. J Infect Dis. 2009;200 (Suppl 1):S254–263. doi: 10.1086/605425. [DOI] [PubMed] [Google Scholar]
- 102.Giaquinto C, Callegaro S, Andreola B, et al. Prospective study of the burden of acute gastroenteritis and rotavirus gastroenteritis in children less than 5 years of age, in Padova, Italy. Infection. 2008;36:351–357. doi: 10.1007/s15010-008-7200-6. [DOI] [PubMed] [Google Scholar]
- 103.Luquero Alcalde FJ, Eiros Bouza JM, Rubio AP, et al. Gastroenteritis by rotavirus in Spanish children. Analysis of the disease burden. Eur J Pediatr. 2007 doi: 10.1007/s00431-007-0550-8. [DOI] [PubMed] [Google Scholar]
- 104.Tayeb HT, Dela Cruz DM, Al-Qahtani A, Al-Ahdal MN, Carter MJ. Enteric viruses in pediatric diarrhea in Saudi Arabia. J Med Virol. 2008;80:1919–1929. doi: 10.1002/jmv.21291. [DOI] [PubMed] [Google Scholar]
- 105.Gil A, Carrasco P, Jimenez R, et al. Burden of hospitalizations attributable to rotavirus infection in children in Spain, period 1999–2000. Vaccine. 2004;22:2221–2225. doi: 10.1016/j.vaccine.2003.11.037. [DOI] [PubMed] [Google Scholar]
- 106.Szucs G, Uj M, Mihaly I, Deak J. Burden of human rotavirus-associated hospitalizations in three geographic regions of Hungary. Acta Paediatr Suppl. 1999;88:61–65. doi: 10.1111/j.1651-2227.1999.tb14328.x. [DOI] [PubMed] [Google Scholar]
- 107.Yee EL, Staat MA, Azimi P, et al. Burden of rotavirus disease among children visiting pediatric emergency departments in Cincinnati, Ohio, and Oakland, California, in 1999–2000. Pediatrics. 2008;122:971–977. doi: 10.1542/peds.2007-1609. [DOI] [PubMed] [Google Scholar]
- 108.Sharma R, Hudak ML, Premachandra BR, et al. Clinical manifestations of rotavirus infection in the neonatal intensive care unit. Pediatr Infect Dis J. 2002;21:1099–1105. doi: 10.1097/00006454-200212000-00003. [DOI] [PubMed] [Google Scholar]
- 109.de Oliveira LH, Danovaro-Holliday MC, Andrus JK, et al. Sentinel hospital surveillance for rotavirus in latin american and Caribbean countries. J Infect Dis. 2009;200 (Suppl 1):S131–139. doi: 10.1086/605060. [DOI] [PubMed] [Google Scholar]
- 110.Grimwood K, Huang QS, Cohet C, et al. Rotavirus hospitalisation in New Zealand children under 3 years of age. J Paediatr Child Health. 2006;42:196–203. doi: 10.1111/j.1440-1754.2006.00829.x. [DOI] [PubMed] [Google Scholar]
- 111.Jenney A, Tikoduadua L, Buadromo E, et al. The burden of hospitalised rotavirus infections in Fiji. Vaccine. 2009;27 (Suppl 5):F108–111. doi: 10.1016/j.vaccine.2009.08.071. [DOI] [PubMed] [Google Scholar]
- 112.Barnes GL, Uren E, Stevens KB, Bishop RF. Etiology of acute gastroenteritis in hospitalized children in Melbourne, Australia, from April 1980 to March 1993. J Clin Microbiol. 1998;36:133–138. doi: 10.1128/jcm.36.1.133-138.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.McIver CJ, Hansman G, White P, et al. Diagnosis of enteric pathogens in children with gastroenteritis. Pathology. 2001;33:353–358. [PubMed] [Google Scholar]
- 114.Chen YE, Beasley S, Grimwood K. Intussusception and rotavirus associated hospitalisation in New Zealand. Arch Dis Child. 2005;90:1077–1081. doi: 10.1136/adc.2005.074104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.O’Ryan M, Diaz J, Mamani N, Navarrete M, Vallebuono C. Impact of rotavirus infections on outpatient clinic visits in chile. Pediatr Infect Dis J. 2007;26:41–45. doi: 10.1097/01.inf.0000247104.01291.71. [DOI] [PubMed] [Google Scholar]
- 116.Munford V, Gilio AE, de Souza EC, et al. Rotavirus gastroenteritis in children in 4 regions in Brazil: a hospital-based surveillance study. J Infect Dis. 2009;200 (Suppl 1):S106–113. doi: 10.1086/605037. [DOI] [PubMed] [Google Scholar]
- 117.Schael IP, Gonzalez R, Salinas B. Severity and age of rotavirus diarrhea, but not socioeconomic conditions, are associated with rotavirus seasonality in Venezuela. J Med Virol. 2009;81:562–567. doi: 10.1002/jmv.21420. [DOI] [PubMed] [Google Scholar]
- 118.Perez-Schael I, Gonzalez R, Fernandez R, et al. Epidemiological features of rotavirus infection in Caracas, Venezuela: implications for rotavirus immunization programs. J Med Virol. 1999;59:520–526. doi: 10.1002/(sici)1096-9071(199912)59:4<520::aid-jmv16>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 119.Bok K, Castagnaro N, Borsa A, et al. Surveillance for rotavirus in Argentina. J Med Virol. 2001;65:190–198. [PubMed] [Google Scholar]
- 120.O’Ryan M, Perez-Schael I, Mamani N, et al. Rotavirus-associated medical visits and hospitalizations in South America: a prospective study at three large sentinel hospitals. Pediatr Infect Dis J. 2001;20:685–693. doi: 10.1097/00006454-200107000-00009. [DOI] [PubMed] [Google Scholar]
- 121.Bereciartu A, Bok K, Gomez J. Identification of viral agents causing gastroenteritis among children in Buenos Aires, Argentina. J Clin Virol. 2002;25:197–203. doi: 10.1016/s1386-6532(02)00010-0. [DOI] [PubMed] [Google Scholar]
- 122.Coluchi N, Munford V, Manzur J, et al. Detection, subgroup specificity, and genotype diversity of rotavirus strains in children with acute diarrhea in Paraguay. J Clin Microbiol. 2002;40:1709–1714. doi: 10.1128/JCM.40.5.1709-1714.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rodrigues J, Acosta VC, Candeias JM, Souza LO, Filho FJ. Prevalence of diarrheogenic Escherichia coli and rotavirus among children from Botucatu, Sao Paulo State, Brazil. Braz J Med Biol Res. 2002;35:1311–1318. doi: 10.1590/s0100-879x2002001100008. [DOI] [PubMed] [Google Scholar]
- 124.Salinas B, Gonzalez G, Gonzalez R, et al. Epidemiologic and clinical characteristics of rotavirus disease during five years of surveillance in Venezuela. Pediatr Infect Dis J. 2004;23:S161–167. doi: 10.1097/01.inf.0000142465.25992.c3. [DOI] [PubMed] [Google Scholar]
- 125.Luz CR, Mascarenhas JD, Gabbay YB, et al. Rotavirus serotypes and electropherotypes identified among hospitalised children in Sao Luis, Maranhao, Brazil. Rev Inst Med Trop Sao Paulo. 2005;47:287–293. doi: 10.1590/s0036-46652005000500009. [DOI] [PubMed] [Google Scholar]
- 126.Candia N, Parra GI, Chirico M, et al. Acute diarrhea in Paraguayan children population: detection of rotavirus electropherotypes. Acta Virol. 2003;47:137–140. [PubMed] [Google Scholar]
- 127.Carneiro NB, Diniz-Santos DR, Fagundes SQ, et al. Clinical and epidemiological aspects of children hospitalized with severe rotavirus-associated gastroenteritis in Salvador, BA, Brazil. Braz J Infect Dis. 2005;9:525–528. doi: 10.1590/s1413-86702005000600013. [DOI] [PubMed] [Google Scholar]
- 128.Castello AA, Arguelles MH, Rota RP, et al. Detection and characterization of group C rotavirus in Buenos Aires, Argentina, 1997–2003. J Med Virol. 2009;81:1109–1116. doi: 10.1002/jmv.21453. [DOI] [PubMed] [Google Scholar]
- 129.da Rosa e Silva ML, Naveca FG, Pires de Carvalho I. Epidemiological aspects of rotavirus infections in Minas Gerais, Brazil. Braz J Infect Dis. 2001;5:215–222. doi: 10.1590/s1413-86702001000400007. [DOI] [PubMed] [Google Scholar]
- 130.Gonzalez FS, Sordo ME, Rowensztein G, et al. Rotavirus diarrhea. Impact in a pediatric hospital of Buenos Aires. Medicina (B Aires) 1999;59:321–326. [PubMed] [Google Scholar]