Abstract
Flowering time and plant height are important agronomic traits for crop production. In this study, we characterized a semi-dwarf and late flowering (dlf1) mutation of rice that has pleiotropic effects on these traits. The dlf1 mutation was caused by a T-DNA insertion and the cloned Dlf1 gene was found to encode a WRKY transcription factor (OsWRKY11). The dlf1 mutant contains a T-DNA insertion at the promoter region, leading to enhanced accumulation of Dlf1 transcripts, resulting in a semidominant mutation. The dlf1 mutation suppressed the transcription of Ehd2/RID1/OsId1 and its downstream flowering-time genes including Hd1, Ehd1 and Hd3a under both long-day (LD) and short-day (SD) conditions. Knock-down of Dlf1 expression exhibited early flowering at LD condition related to the wild-type plants. Accumulation of Dlf1 mRNA was observed in most tissues, and two splicing forms of Dlf1 cDNAs were obtained (OsWRKY11.1 and OsWRKY11.2). These two proteins showed transactivation activity in yeast cells. Dlf1 protein was found to be localized in the nucleus. Enhanced expression of OsWRKY11.2 or its 5′ truncated gene showed similar phenotypes to the dlf1 mutant, suggesting that it might function as a negative regulator. We conclude that Dlf1 acts as a transactivator to downregulate Ehd2/RID1/OsId1 in the signal transduction pathway of flowering and plays an important role in the regulation of plant height in rice.
Introduction
Increasing cereal output has been a fundamental goal to meet the soaring demand for food. Plant height, potential yield and flowering time are important traits for cereal production. Plant height, one of the main selection trait in rice (Oryza sativa) breeding, is controlled mostly by genes related to the synthesis and regulation of phytohormones, such as gibberellin (GA) and brassinolide [1]–[3]. Potential yield per rice plant is determined by grain weight, and numbers of grains per panicle and tillers per plant [4]–[6]. Flowering time of plants is controlled by both environmental and developmental factors, with photoperiod as an important environmental signal. Molecular genetic analysis in Arabidopsis thaliana, a long-day (LD) plant, has identified a set of key regulators functioning in the photoperiod-mediated flowering pathway. For example, the nuclear protein CONSTANS (CO) positively regulates the flowering activator FLOWERING LOCUS T (FT), which further interacts with the bZIP transcription factor FLOWERING LOCUS D (FD) to control flowering time in Arabidopsis [7]–[9]. SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1 (SOC1), encoding a MADS box transcription factor, is activated by CO through FT and repressed by FLC (FLOWERING LOCUS C) via direct binding to the promoter [10].
Analysis of natural variants and mutants in rice, a short-day (SD) plant, has revealed the existence of a genetic pathway similar to that in Arabidopsis in photoperiodic flowering. Heading date 1 (Hd1), Heading date 3a (Hd3a), Heading date 6 (Hd6) and OsGI in rice are orthologs of Arabidopsis CO, FT, the α-subunit of kinase CK2, and GIGANTEA (GI), respectively. OsGI, a gene involved in the circadian clock control, regulates Hd1 and Hd3a in photoperiodic flowering, which promotes flowering under SD conditions and suppresses it under LD conditions [11]–[14]. In addition, early heading date 1 (Ehd1), a B-type response regulator that is specific to floral induction in rice, regulates the expression of Hd3a, FTL1 and OsMADS14 [15]. Ehd1 functions upstream of Hd3a and RFT1 through the Hd1-independent pathway. Ehd2/RID1/OsId1 were isolated separately by three groups and the locus was found to encode a Cys2/His2-type zinc finger transcription factor orthologous to the INDETERMINATE 1 (ID1) gene in maize [16]–[18]. Loss-of-function ehd2/rid1/Osid1 mutants were never- or extremely late-flowering in comparison with wild-type plants under both SD and LD conditions. Functional analysis revealed that Ehd2/RID1/OsId1 acts as a master switch and promoter of phase transition mainly by regulating Ehd1 and the downstream genes. Further, a CCT (CO, CO-LIKE and TIMING OF CAB1)-domain protein encoding gene Ghd7, which was uncovered from a natural variant rice, suppresses the expression of Ehd1 and Hd3a genes under LD conditions but does not affect Hd3a expression under SD conditions [19]. Recently, Hd17/Ef7, an ortholog of Arabidopsis EARLY FLOWERING 3 (ELF3), has been characterized to promote rice flowering by repressing Ghd7 expression under both LD and SD conditions [20], [21]. In addition, Ehd3, which encodes a protein containing two plant homeodomain (PHD) finger motifs, functions also as a LD suppressor of Ghd7 [22]. The mutation in DTH8/Ghd8/Hd5 shows pleiotropic effects on grain number, plant height and heading date, and causes delayed flowering by down-regulation of Ehd1 under LD conditions [23]–[25]. On the other hand, OsMADS51 is a SD promoter functioning downstream of OsGI1 and upstream of Ehd1 [9], [26], whereas Ehd4, encoding a CCCH-zinc finger regulator, promotes rice flowering by stimulating the expression of Ehd1 and its downstream genes [27].
Much progress has been achieved in the genetic dissection of photoperiodic flowering of rice, but the molecular regulation is still largely unknown. In this study, we characterized a semi-dwarf and late flowering (dlf1) mutant and identified Dlf1 gene that encodes a WRKY transcription factor. Our results showed that overexpression of Dlf1 suppressed flowering by inhibiting the expression of Ehd2/RID1/OsId1 under both LD and SD conditions and influenced plant height in rice.
Results
Isolation and morphological characterization of the dlf1 mutant
A semi-dwarf and late flowering (dlf1) mutant was identified from a T1 population of T-DNA insertion lines of cultivar Oryza sativa L. japonica Zhonghua 11 (ZH11). The flowering time of dlf1 plants was delayed for about two weeks compared with wild-type plants in the experimental field in late May, 2003 in Hangzhou, China (Fig. 1A). DNA blot analysis showed that there was only one copy of T-DNA insertion in the mutant line (data not shown).
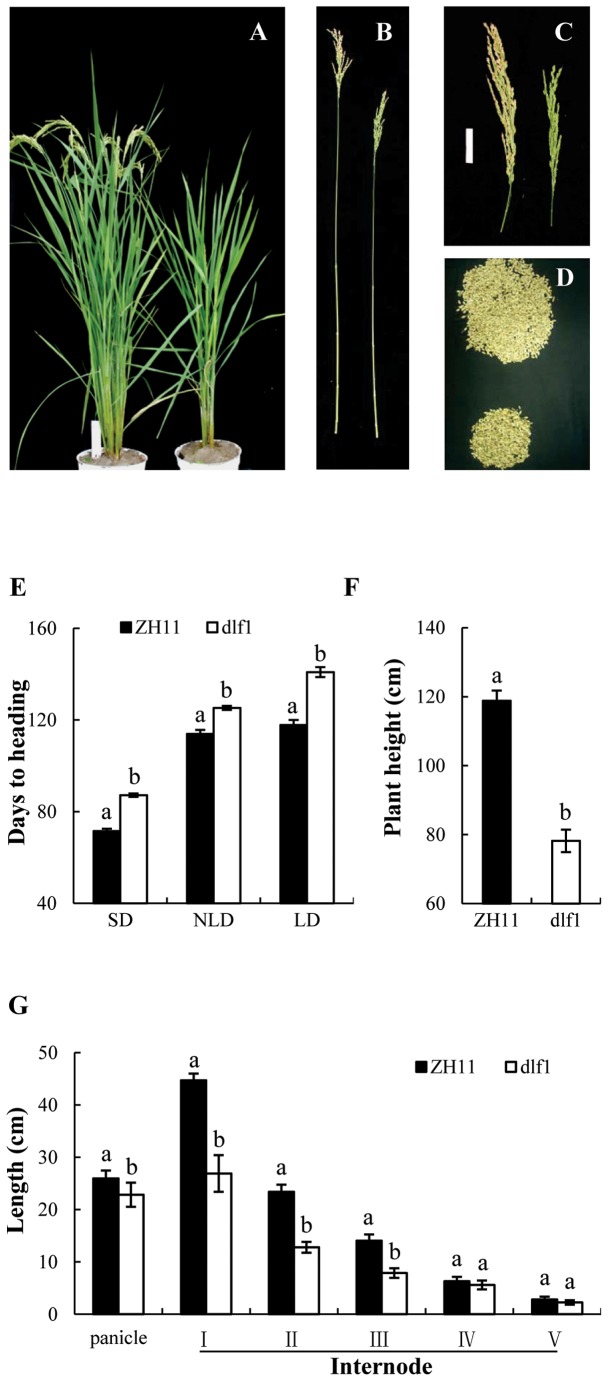
Figure 1. Phenotypes of Zhonghua 11 (ZH11) and dlf1 mutant.
(A) Photos of the wild-type ZH11 (left) and mutant dlf1 (right), taken when ZH11 reached maturity. (B) Photos of main culms of ZH11 (left) and dlf1 (right). (C) Main panicles of ZH11 (left) and dlf1 (right). (D) Grains from main panicles of ZH11 (top) and dlf1 (bottom). (E) Days to heading of ZH11 and dlf1 under SD, LD and NLD (natural LD) conditions. Data are represented as mean values ± standard derivation (SD) of 20 plants. (F) Plant heights of ZH11 and dlf1 under natural LD conditions. Values are means ± SD, n = 20. Experiment were performed three times, showing similar results. (G) Difference of internode lengths between matured ZH11 and dlf1 plants. The plants were grown in the experimental field under natural LD conditions. Values are means ± SD, n = 20. The same experiments were repeated three times, and the similar results were obtained. For SD and LD treatments, the plants were grown in greenhouse under natural light conditions and shaded at the time designated. a and b in figure indicate ranking by Duncan test at P<0.05, starting from a. b is significantly different from a.
To determine whether the heading time of the dlf1 mutant differed under different photoperiod conditions, the mutant and wild-type plants were grown under SD conditions (10/14 h light/dark) and LD conditions (14/10 h light/dark). Heading time of the dlf1 mutant plants was 87.2±1.0 d, which was delayed ca. 16 d in comparison with wild-type (71.5±0.8 d) under SD conditions (Fig. 1E). Under LD conditions, heading time of the dlf1 mutant plants was 140.9±2.2 d and increased by ca. 23 d compared with ZH11 (117.8±2.2 d). Other phenotypes differed significantly under natural LD conditions, including homozygous dlf1 mutant lowered plant height (Figs. 1A and F) caused by reducing cell size (Fig. S1A), and decreased number of spikelets per panicle (Fig. S1B). The dlf1 mutant plants also had less 1000-grain weight (Fig. S1C) and showed leaf rolling phenotypes (Figs. S1D and E) under natural LD conditions.
Dlf1 encodes a WRKY transcription factor
Using the thermal asymmetric interlaced PCR (Tail-PCR) method, we isolated the genomic DNA flanking of the T-DNA insertion site from the dlf1 mutant. A BLAST search of the flanking sequence revealed that the T-DNA was inserted at 67 bp upstream of the initial ATG (the ‘A’ was defined as +1) of the predicted coding sequence of the gene OsWRKY11 (LOC_Os01g43650, AK108745; and named as Dlf1 in the present study) (Fig. 2A). The flanking sequence of the other T-DNA border was amplified and revealed a 42 bp deletion at the insertion site.
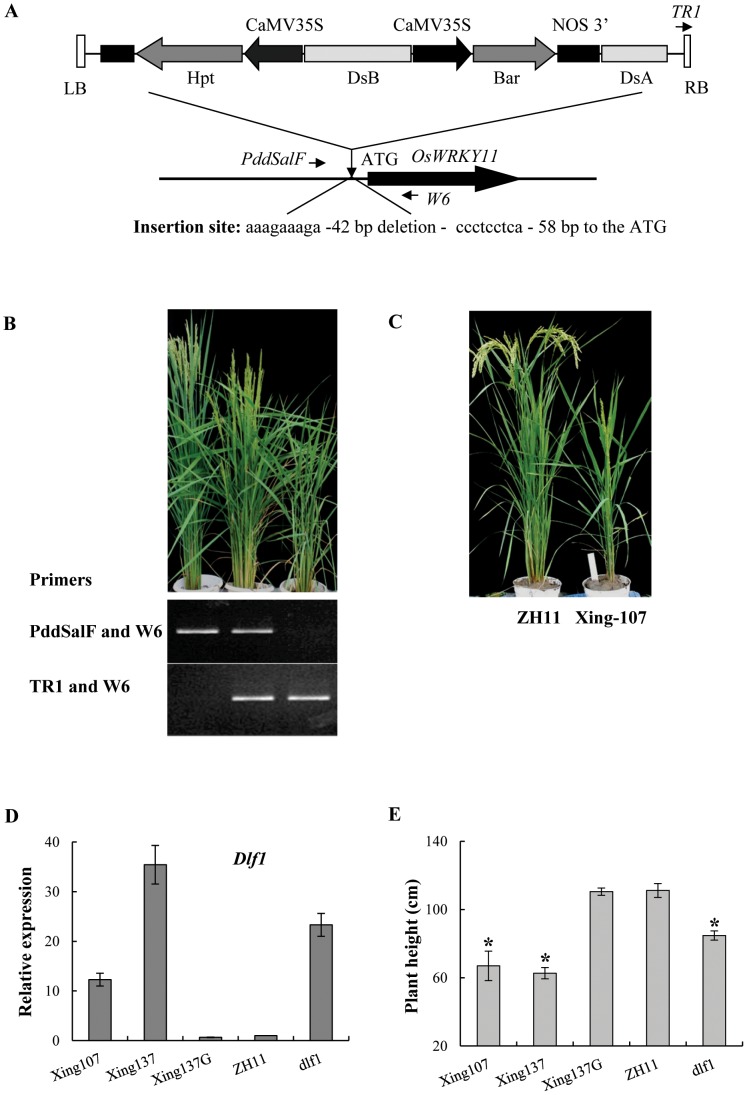
Figure 2. Molecular features of Dlf1.
(A) Structure of T-DNA insertion site. The perpendicular arrow head indicates the insertion site. The T-DNA was inserted into the upstream of OsWRKY11. Arrows indicate the primers used for analyzing the insertion site. LB and RB represent the left and right borders of T-DNA. (B) PCR genotyping Dlf1 segregants in F2 with primers as indicated in A (showed in italics). Primers PddSalF and W6 amplify a product from wild-type. PCR-positive plants with primers of TR1 and W6 indicate T-DNA insertion in the examined site and a homozygote for the insertion if without an amplification product from PddSalF and W6. (C) Photos of ZH11 (left) and Cp-Ins-Dlf1 transgenic T1 plant (named as Xing-, right), taken when ZH11 reached maturity. (D) Expression of Dlf1 in transgenic plants of T1 progenies under natural LD conditions. The first and second youngest leaves were sampled from 90-d-old plants for RNA isolation. Transcription levels were quantified by quantitative reverse-transcription PCR (qPCR) and the gene expression was normalized to rice ubiquitin gene (Ubq) for each sample. Transcription levels are expressed as ratio to the level of transcript in ZH11. (E) Plant heights. Xing-, the transgenic plants, the suffix G for segregated non-transgenic plants; ZH11, the control and dlf1, the mutant. The plants were grown in the experimental field under natural LD conditions. Values are shown as means ± SD of two independent experiments. Asterisks indicate significant difference between ZH11 and other lines (P<0.05, Duncan test).
To ascertain whether the dlf1 phenotypes are associated with the T-DNA insertion, genetic analysis was performed using two filial populations of reciprocal crosses between the dlf1 mutant and wild-type plants. The flowering time and the plant heights co-segregated in a manner of fit the 1∶2∶1 population (data not shown). Correspondingly, the genotypes of the T-DNA insertion in the same populations were determined by PCR and showed a tight linkage with the phenotypes (Fig. 2B). These results indicated that the Dlf1 is semi-dominant and the T-DNA insertion event upstream of the dlf1 gene is responsible for the mutant phenotype.
To examine the effect of the T-DNA insertion on the gene expression, the Dlf1 total expression level was assayed by quantitative real-time PCR (qPCR). The transcript levels of Dlf1 in rice leaves were enhanced significantly in the dlf1 mutant compared with the wild-type ZH11 (Figs. 2D). To verify that the mutant phenotypes were caused by the high expression of Dlf1 that was related to the T-DNA insertion, a fragment from the T-DNA insertion site to the end of the Dlf1 coding region (Cp-Ins-Dlf) were transformed into the wild-type background. Among 14 plants regenerated (named as Xing-), 10 showed variation in dwarfism, and late flowering phenotypes in the T0 progeny (data not shown). Two lines were used for further analysis in the T1 generation. The levels of Dlf1 expression were higher in dwarf plants compared with the wild-type and segregated non-transgenic plants (Figs. 2C, D and E). The dwarf plants also showed delayed flowering for about two weeks late under natural LD conditions, indicating that the increase of Dlf1 expression caused the dlf1 phenotypes.
Dlf1 is ubiquitously expressed and alternatively spliced in rice
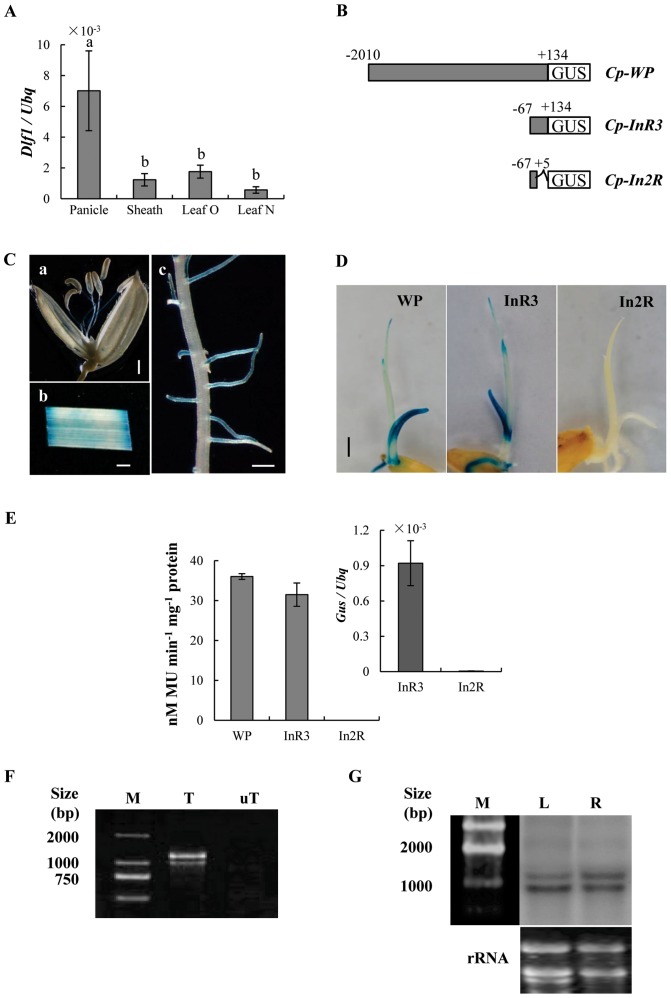
To examine the expression pattern of Dlf1, qPCR analysis was performed with total RNA prepared from leaves, sheaths, young panicles and roots of ZH11 wild-type plants grown under natural LD conditions. Dlf1 was expressed in all tissues examined (Fig. 3A). Further, we stained the transgenic plants harboring the Dlf1 promoter- driven Gus fusion gene (−2010 – +134 bp; Cp-WP:Gus, Fig. 3B). Gus staining was observed in leaves, roots and panicles (Fig. 3C), which confirmed the qPCR results. Since the plants harboring Cp-Ins-Dlf construct showed the dlf1 phenotypes, the fragment from the insertion site (−67 – +134 bp; Cp-InR3:Gus) of Dlf1 was also fused with Gus gene. Surprisingly, Cp-InR3:Gus plants exhibited the similar level of expression as the Cp-WP:Gus plants in the young seedlings (Figs. 3D and E) though the InR3 fragment was a region transcribed. However, the expression of Gus gene and Gus activity were low in the Cp-In2R:Gus (−67 – +5 bp) transgenic plants, which revealed that the deduced translation region in InR3 was required for the promoter activity in comparison with the In2R fragment.
Figure 3. Expression pattern of Dlf1.
(A) Expression of Dlf1 in different tissues was analyzed by qPCR. RNA was isolated from young panicles, sheathes, older leaves (leaf O) and younger leaves (leaf N). Tissue samples were collected at 4 h after dawn. Values are shown as means ± SD of two biological experiments. Values marked with different letters are significantly different (P<0.05, Duncan test). (B) Gus-fused constructs with different lengths of Dlf1 promoter. (C) Dlf1 promoter-driven Gus expression (Cp-WP) in (a) young panicles (b) leaves and (c) roots of three-week-old plants. (D) Gus histochemical staining of 6-day-old transgenic lines. (E) Gus enzyme activity was measured in six-day-old seedlings harboring different constructs. Transcription analysis of Gus gene in the transgenic plants. The gene expression was normalized to rice ubiquitin gene (Ubq) for each sample. Means and their standard deviations are shown from three independent experiments. (F) Different transcripts of Dlf1. Total RNAs were isolated from leaves of three-week-old ZH11 plants. PCR products were obtained by amplification using templates of reverse-transcribed RNA (T) and RNA (uT) and separated by electrophoresis. (G) Northern blot analysis of Dlf1 expression in leaves (L) and roots (R), using the total RNAs isolated from three-week-old plants. The probes used were the Dlf1 coding region. rRNA of ethidium bromide staining was used as the loading control. Bar = 1 mm (C) and 2 mm (D).
To obtain the Dlf1 cDNA, we amplified the open reading frame region from total RNA isolated from ZH11 using RT-PCR with the primers W1 and W5. Two amplified products were obtained (Fig. 3F), the longer one (assigned as OsWRKY11.1) encoded the deduced 379-aa Dlf1 protein and the shorter sequence (assigned as OsWRKY11.2) contained a 161 bp deletion in the third exon causing a premature stop of translation (Fig. S2). The deduced amino acid sequence of OsWRKY11.2 encodes a protein of 270 residues, which still contains the WRKY domain (aa 205–262). Northern blot analysis revealed two hybridization bands using the Dlf1 coding region, (Fig. 3G), confirming the existence of alternative splicing of the Dlf1 mRNAs.
Dlf1 has transcriptional activation activity in yeast
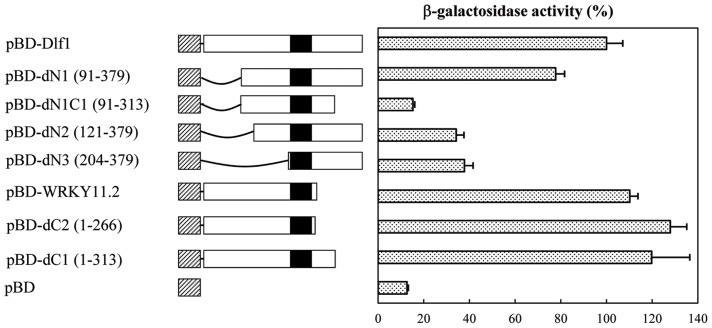
Dlf1 contains an acidic N-terminus that may function as a transcriptional activation domain. To investigate this possibility, the coding region of the Dlf1 full length cDNA and its truncated derivatives were fused in frame to the GAL4 DNA-binding domain in the pGBKT7 vector. The transactivation activity assay in yeast showed that the region of 91–120 aa was required for its transactivation (Fig. 4). We also determined the activation activity of OsWRKY11.2 (pBD-WRKY11.2), showing slight increase in activity compared with full length Dlf1 transcript (pBD-WRKY11.1). This result was confirmed by deletion of the 3′ terminal of full length transcript (pBD-dC2 construct).
Figure 4. Dlf1 is transactivator in yeast.
The full encoding sequence and deletion mutants of Dlf1 were fused in frame to the GAL4-binding domain (BD) in pBDKT7 to generate various vectors for yeast transformation. The constructed vectors were transformed into yeast AH109 strain, and grew on the selective medium at 30°C for 3 d. The β-galactosidase activity of transformants was determined using o-nitrophenyl β-D-galatopyranoside as a substrate. An empty vector pGBKT7 (pBD) was used as the negative control. The values from three independent experiments were shown as means ± SD. Slash boxes represent BD in pGBKT7 and the black boxes for the WRKY domain of Dlf1, whereas the white boxes represent the rest part of Dlf1, and the line indicates the deleted region. The numbers in the brackets are the start and end positions of each translation product of Dlf1 in the construct.
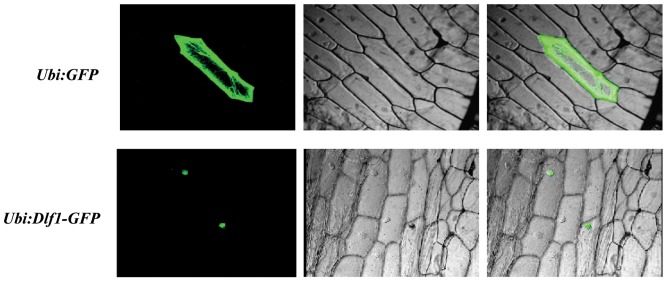
Nuclear localization signal (NLS) of Dlf1 was predicted using cNLS Mapper (http://nls-mapper.iab.keio.ac.jp/). A NLS (amino acid 151–181) was identified with a high score of 7.5. The OsWRKY11.2 protein also contains the sequence of nuclear localization signal. To confirm the subcellular localization of Dlf1, we fused OsWRKY11.1 with the enhanced green fluorescent protein (GFP) gene. The chimeric gene was put under the control of maize ubiquitin (Ubi) promoter. The resulting plasmid (Ubi:Dlf1-GFP) was then bombarded into onion epidermal cells. Localization of the WRKY11.1-GFP fusion protein was visualized exclusively in the nucleus (Fig. 5), whereas the control GFP (Ubi:GFP) was distributed both in the cytoplasm and the nucleus, indicating Dlf1 is a nuclear protein.
Figure 5. Nuclear localization of Dlf1-GFP fusion protein in onion epidermal cells.
Onion epidermal cells were transformed with plasmids expressing GFP (top panel), or the Dlf1-GFP fusion protein (bottom panel) by bombardment and examined after 24 h. GFP fluorescence (left panel) and differential interference contrast image (middle panel) were compared to show the subcellular localization of GFP (cytoplasmic and nuclear) and Dlf1-GFP (nuclear). The images of the right panel were merged for each.
The expression of Ehd2, Ehd1, Hd1 and Hd3a was repressed in dlf1 mutant
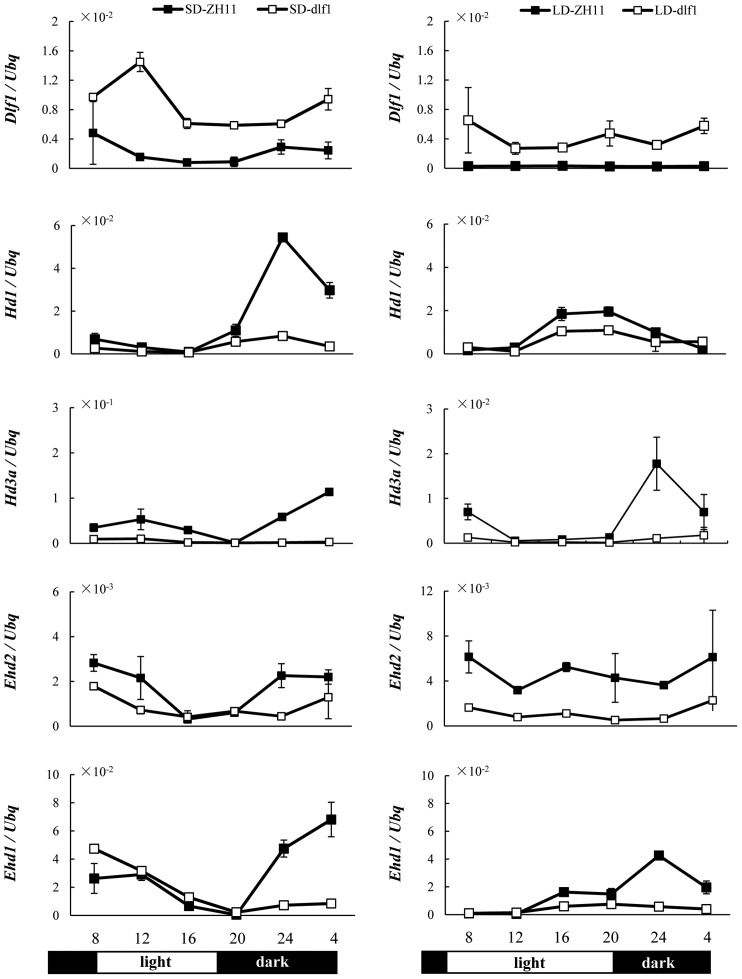
To determine whether the late flowering phenotype of the dlf1 mutant was due to the changes in flowering-related gene expression, qPCR analysis was performed in the wild-type and dlf1 plants. Leaf samples were collected from 40 or 90-d-old plants grown under SD or LD conditions. The developmental stage of the plants was about one month before flowering in ZH11. The total expression levels of Dlf1 in the mutant were higher than in the wild-type plants under both SD and LD conditions (Fig. 6). Moreover, Dlf1 mRNA accumulation showed diurnal changes in the wild-type and dlf1 mutant plants. Ehd2/RID1/OsId1 is a key regulator in the genetic network that controls photoperiodic flowering in rice, promoting floral transition by upregulating Ehd1 and then the downstream Hd3a [15]–[17]. Ehd2/RID1/OsId1 mRNA accumulation was reduced in the dlf1 mutant compared with wild-type plants under SD and LD conditions (Fig. 6). Subsequently, the levels of Hd3a and Ehd1 expression were decreased in the mutant plants. The expression of Hd1 was also suppressed in the dlf1 mutant under both SD and LD conditions (Fig. 6). However, the expression of photoperiod-related genes OsGI, Se5 and Ghd7 were not significantly affected in the dlf1 mutant (Fig. S3), indicating that Dlf1 specifically suppressed the expression of Ehd2 and downstream genes.
Figure 6. Dlf1 suppresses the expression of Hd3a, Hd1, Ehd1 and Ehd2.
Diurnal expression patterns of Dlf1, Hd1, Hd3a, Ehd2 and Ehd1 in wild-type ZH11 (filled squares) and dlf1 (open squares) plants under SD (10/14 h light/dark) and LD (14/10 h light/dark) conditions by qPCR analysis. The expression levels are relative to the ubiquitin (Ubq) mRNA. The plants were grown at conditions as described in Fig. 1. Values are shown as means ± SD of two independent experiments. The open and filled bars at the bottom represent the light and dark periods, respectively.
Transcription levels of Dlf1 and the flowering-related genes were also examined at different developmental stages under LD conditions every 20 days. The accumulation of Dlf1 mRNA slightly increased and reached a peak at 70 d after germination. Subsequently, the transcript level gradually decreased and remained at low levels even after flowering in the wild-type plants (Fig. 7). In the dlf1 mutant, Dlf1 transcript accumulated in a pattern quite similar to that of the wild type ZH11 plants, but the expression levels of Dlf1 were at least 10-fold higher throughout the experiment periods.
Figure 7. Expression of Dlf1 and other flowering-time genes during development.
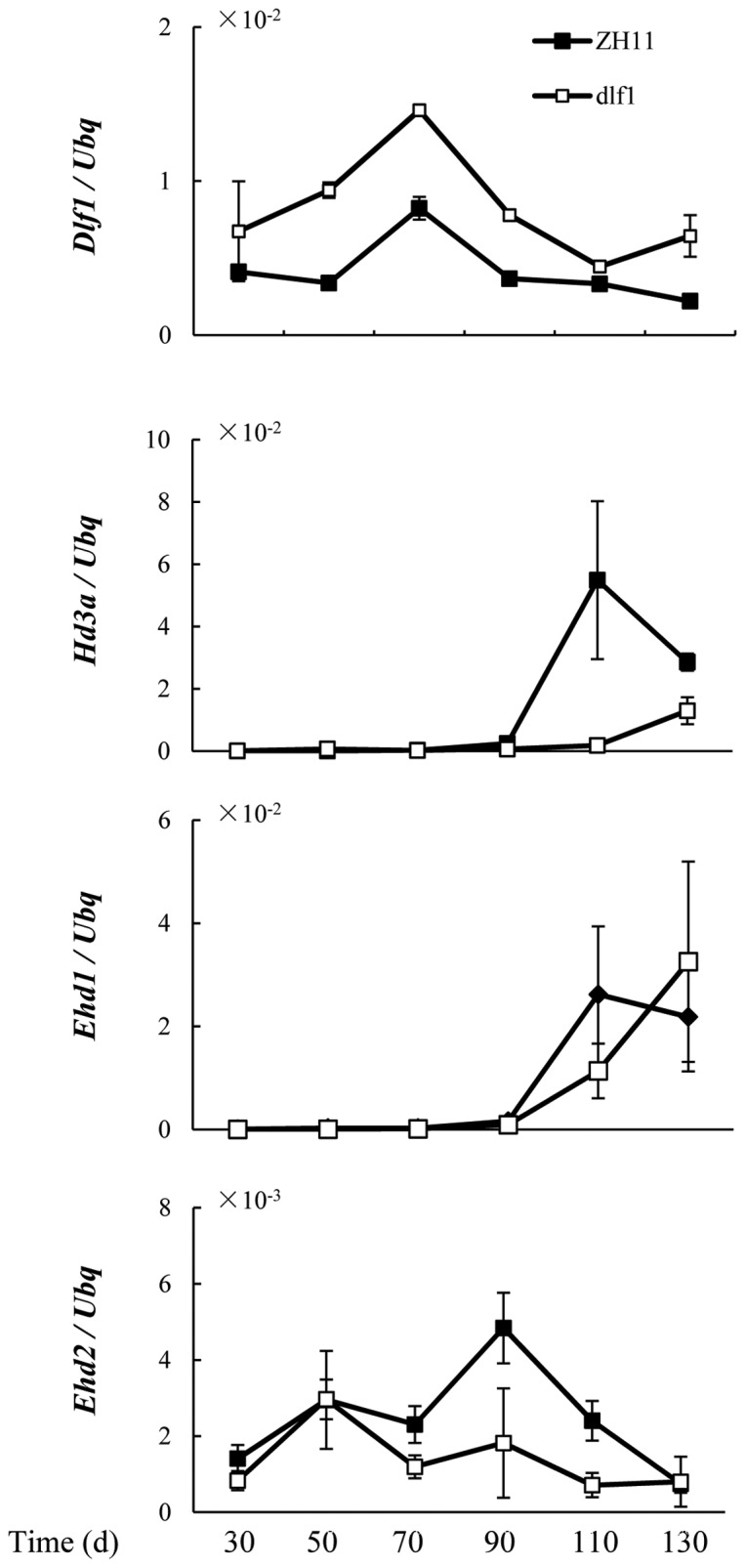
Changes of Dlf1, Hd3a, Ehd1 and Ehd2 transcription levels in wild-type (filled squares) and dlf1 (open squares) plants during development under LD conditions. The expression levels are relative to the ubiquitin (Ubq) mRNA. The values of Dlf1 expression in ZH11 were scaled up 10 times. The plants were grown at conditions as described in Fig. 1. Developing leaves were harvested 4 h after dawn. Values are shown as means ± SD of two independent experiments.
Decrease of Dlf1 expression showed early flowering under LD conditions
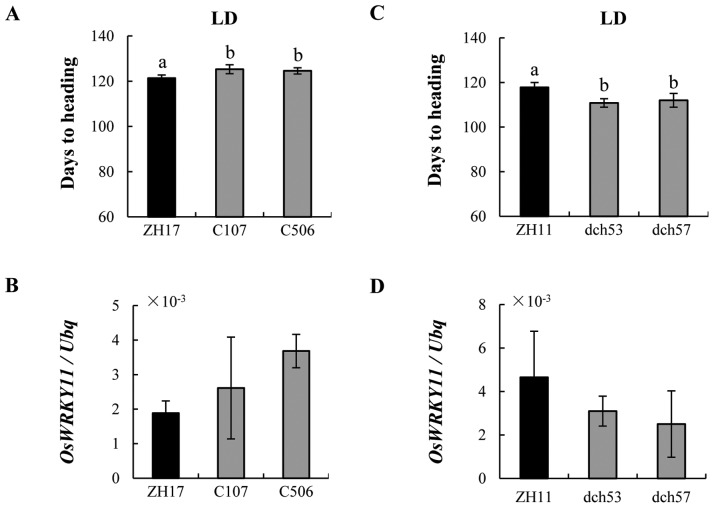
To further dissect the function of Dlf1, several constructs were generated to examine possible roles of the different transcripts by means of overexpression and RNAi. The full-length OsWRKY11.1 and the alternatively spliced OsWRKY11.2 transcript were put under control of the Ubi promoter, respectively, generating the Ubi:W11.1 and Ubi:W11.2 constructs for rice transformation. To knockdown Dlf1, a 276-bp fragment was used for the RNAi construct under the control of the CaMV35S promoter. More than 15 independent overexpressing transgenic lines were obtained for Ubi:W11.1 in the genetic background of ZH11 and ZH17, respectively. However, only two Ubi:W11.1 lines of ZH17 genetic background (named as C-) were found to increase OsWRKY11 expression level and to delay flowering under LD condition comparing with ZH17 plants (Figs. 8A and B). Most of the transgenic lines in ZH11 background (named as OE-) showed slight variations in plant height (Fig. S4B) and insignificant changes in total transcripts of OsWRKY11 (data not shown). Further, RNAi lines of dch53 and dch57 displayed suppressed transcription of OsWRKY11 and flowered earlier than the ZH11 controls under LD condition (Figs. 8C and D). These results collectively suggested that Dlf1 negatively regulate flowering in rice.
Figure 8. Alternation of Dlf1 expression changes the heading day of rice.
(A) The heading time of Ubi:W11.1 transgenic lines in T2 progenies (named as C-) and the ZH17 control under LD conditions. (B) Expression of total OsWRKY11 (including the transferred and endogenous genes) in the Ubi:W11.1 transgenic and ZH17 plants. (C) The heading day of ZH11 and the RNAi lines in T2 progenies (named as dch-) under LD condition. (D) Expression of total OsWRKY11 in the RNAi transgenic and ZH11 control plants. Leaves of the first and second youngest were sampled from 90-d old plants. Values are shown as means ± SD of two independent experiments. a and b in figure indicate ranking by Duncan test at P<0.05, starting from a. b is significantly different from a.
The C-terminus of Dlf1 plays a role in the regulation of its expression level
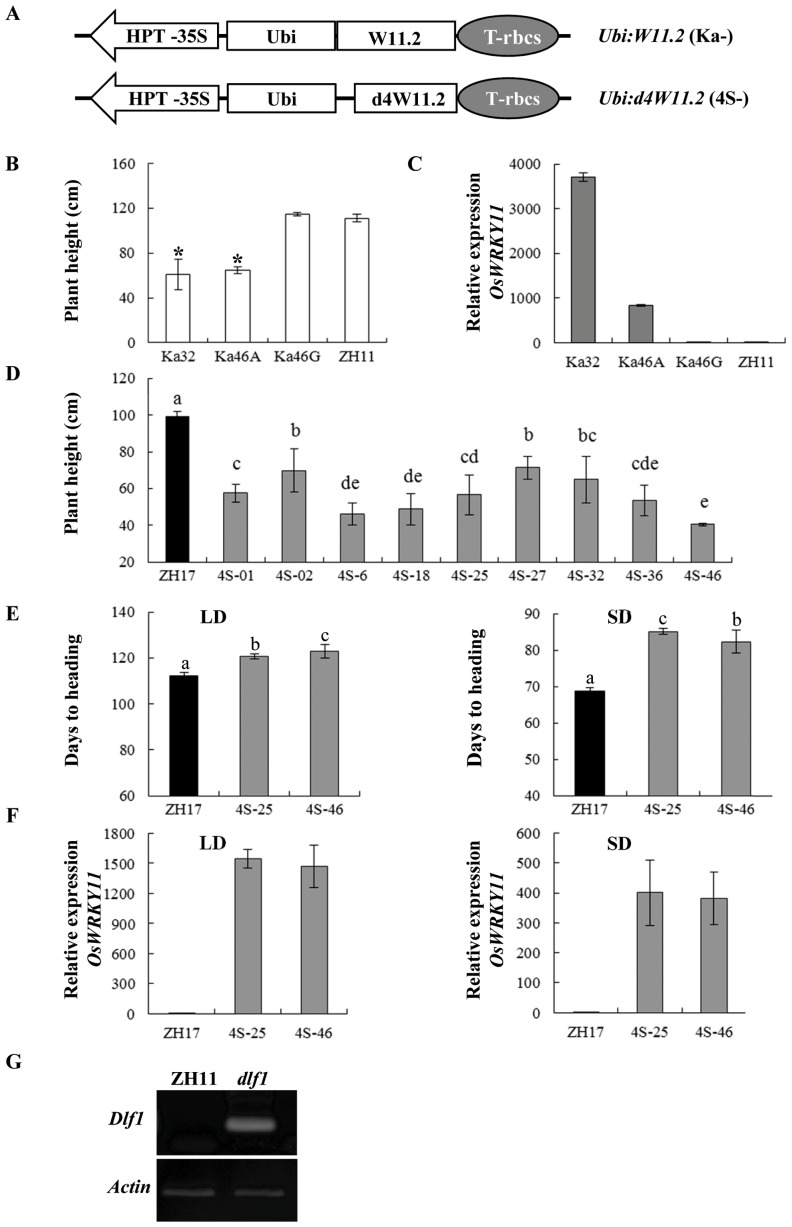
In contrast to Ubi:W11.1 construct, most of the Ubi:W11.2 transgenic lines (named as Ka-) exhibited severe dwarfism (Fig. 9B) and delayed flowering of about 2–3 weeks compared with ZH11 under natural LD conditions. The expression of total OsWRKY11 in transgenic plants was much higher than in ZH11 control and the segregated non-transgenic plants (Fig. 9C), suggesting that the C-terminus of Dlf1 plays a role in controlling its expression level.
Figure 9. High level expression of the OsWRKY11.2 leads to dwarfism and late flowering.
(A) Schematic diagram of Ubi:W11.2 (Ka-) and Ubi:d4W11.2 (4S-, with 37-aa deletion at the N-terminus of W11.2) constructs. (B) and (D) Plant heights of those transformed with Ubi:W11.2 or Ubi:d4W11.2 in ZH11 or ZH17 genetic background, respectively. (C) Expression of total OsWRKY11 (including the transferred and endogenous genes) in ZH11 and the Ubi:W11.2 transgenic lines of T1 progenies under natural LD conditions. The first and second youngest leaves were sampled from 90-d-old plants for RNA isolation. Transcription levels were quantified by qPCR and the gene expression was normalized to rice ubiquitin gene (Ubq) for each sample. Transcription levels are expressed as ratio to the level of transcript in ZH11. The suffix A for dwarf and G for segregated non-transgenic plants. (E) Days to heading of the Ubi:d4W11.2 plants of T2 progenies under SD and LD conditions (the same treatments as in Fig. 6). (F) Expression of total OsWRKY11 in ZH17 and the Ubi:d4W11.2 transgenic plants of T2 progenies under both LD and SD conditions (the same treatments as in Fig. 6). Transcription levels are expressed as ratio to the level of transcript in ZH17. (G) Analysis of the possible degraded mRNA of Dlf1 using the RNA ligase-mediated amplification of 5′ cDNA ends (RLM-RACE). Total RNAs were isolated from the dlf1 mutant (dlf1) and the wild-type (ZH11) of three-week-old plants. Two rounds of PCR were performed: 1) by using an outer primer from the adaptor and a Dlf1-specific reverse primer W5; and 2) by using the inner primer from the adaptor and a Dlf1 gene primer W4 for 30 cycles. Actin gene was used as an internal standard. Asterisks indicate significant difference between ZH11 and the overexpression lines (P<0.05, Duncan test). a, b, c, d, and e indicate ranking by Duncan test at P<0.05, starting from a. Different letters indicate significantly difference from each other.
Due to the promoter activity of InR3 fragment, transgenic plants were also obtained with the constructs excluding the N-terminal 37 aa of Dlf1 (assigned as Ubi:d4W11.1 and Ubi:d4W11.2). The Ubi:d4W11.2 transgenic lines (named as 4S-) presented dwarf and late flowering phenotypes under both LD and SD conditions (Figs. 9D and E), whereas the Ubi:d4W11.1 plants had no such phenotype (data not shown). Analysis of the expression level of the transgenic plants revealed that the accumulation of OsWRKY11 total mRNA was increased over 400-fold in the Ubi:d4W11.2 progenies (Fig. 9F). These results suggest that the C-terminal region of Dlf1 influenced its expression and high level of expression of truncated OsWRKY11.2 might function in a similar manner as OsWRKY11.2.
To get a clue of the effect on Dlf1 expression, we examined the possible degraded mRNA of Dlf1 using the RNA ligase-mediated amplification of 5′ cDNA ends (RLM-RACE) [28]. A stronger PCR band was obtained in the dlf1 mutant than the ZH11 (Fig. 9G). Sequencing verified the degraded positions at 1093 and 1099 bp of Dlf1. These observations support the idea that RNA processing proteins or microRNAs may regulate the expression of Dlf1, if the level of Dlf1 mRNA containing the 3′ end reached a limit.
Discussion
In this study, we demonstrated that the Dlf1 gene had pleiotropic effects on a variety of traits, including flowering time, plant height, grain number and leaf rolling. Yield, plant height and heading date are the most important agronomic traits in rice, and a number of genes have been isolated that control each of these traits. For example, Gn1a, a gene for cytokinin oxidase/dehydrogenase, regulates the number of grains per panicle [5], and a RING-type E3 ligase (GW2) controls grain width and weight [6]. The height of a rice plant is regulated by the gibberellin-insensitive gene Dwarf 1, encoding the alpha-subunit of GTP-binding protein, and the brassinosteroid biosynthesis gene Dwarf 11 [1], [3]. Ghd7, encoding a CCT-domain protein, was shown to have multiple effects on grain number, heading date and plant height [19]. DTH8/Ghd8/Hd5, encoding a HAP3 subunit of the CCAAT-box binding protein, is also reported to suppress rice flowering under LD conditions and regulate plant height and yield potential [23], [24]. Our data indicate that the rice WRKY transcription factor Dlf1 also widely affects rice development. Dlf1 regulates plant height by altering cell size in the internodes, similar to the effect of DTH8 but different from that of Ghd7 (Fig. S1) [19], [23]. The phenotypes of short internode length and leaf rolling in the dlf1 mutant are supported by a recent report of OsWRKY11 transgenics, which is controlled by the promoter of heat shock-inducible HSP101 gene [29]. Among the four transgenic rice plants reported, three had bent leaves or dwarf phenotype, and two had significantly enhanced heat and drought tolerance under heat induction conditions.
The T-DNA insertion site in the dlf1 mutant was 67 bp upstream of the predicted translational starting site of OsWRKY11 (Fig. 2A). However, the Dlf1 expression was significantly increased in comparison with the wild-type plants. Transgenic plants harboring the genomic DNA of Dlf1, starting from the T-DNA insertion site to the end of Dlf1 coding region, recapitulated the dlf1 phenotypes (Fig. 2C), suggesting that the region has promoter function. This is confirmed by fusion with the Gus reporter gene (Fig. 3B, D and E). However, the Gus activities of Cp-WP:Gus and Cp-InR3:Gus were at a similar level, inconsistent with a higher level of expression Dlf1 in the dlf1 mutant than that of the wild-type, implying a suppressor element existed outside of the WP fragment used in the experiment. We also generated Dlf1 overexpressing and RNAi transgenic lines. Unexpectedly, most of the OsWRKY11.1 transgenic plants did not show morphological differences to controls (Fig. S4). Nevertheless, two RNAi lines with decrease in OsWRKY11 expression showed early flowering under LD conditions (Fig. 8C and D). On the other hand, the accumulation of total OsWRKY11 mRNA (including the endogenous and transferred gene) was extremely high in the lines harboring the Ubi:W11.2 construct, which is 109 aa shorter than Ubi:W11.1 in the C-terminus (Fig. S2). These results suggested that the C-terminus of Dlf1 was involved in controlling accumulation level of its mRNA. This notion is further supported by comparison of the transgenic plants containing Ubi:d4W11.1 or Ubi:d4W11.2 constructs. Interestingly, most of the Ubi:W11.2 and Ubi:d4W11.2 transgenic plants exhibited dwarf and late flowering phenotypes, similar to the dlf1 mutant (Fig. 9). Likely, OsWRKY11.2 retained the transactivation activity (Fig. 4) and the sequence of nuclear localization signal (position 179–187 aa). The results suggested that the high level of OsWRKY11.2, or its N-terminus-truncated protein might function as a negative regulator. This information also implies that different splicesomes of Dlf1 might work together to regulate downstream gene expression, although further study is required to test the existence of alternative splicing in planta.
RNA processing plays an important role in control of plant flowering time. FLOWERING LOCUS C (FLC), a repressor of the transition to flowering in Arabidopsis, functions to delay flowering by down-regulating expression of genes promoting the floral state. Processing of FLC mRNA is regulated by autonomous pathway components of FCA and FY, which encode a pre-mRNA processing protein and a homolog of the yeast RNA 3′-processing factor Pfs2p, respectively [30], [31]. FCA expression is also regulated through alternative processing of its pre-mRNA into four different transcripts, in which only the fully spliced FCA transcripts can promote flowering [30]. Furthermore, FCA negatively regulates its own expression by increasing cleavage and polyadenylation within intron 3, thus limiting the production of active FCA protein to keep the balance of pathways controlling flowering time. They also found that active FCA can be overexpressed only when the cis-element within intron 3 required for the negative feedback is removed [32], [33]. As mentioned above, the accumulation of OsWRKY11.1 mRNA was not much increased in the overexpressing plants. An explanation is that the C-terminal part of OsWRKY11.1 interacts with a protein involved in regulation of the OsWRKY11.1 mRNA level. When the OsWRKY11.1 protein reaches a threshold level it will activate the protein–protein interaction and decrease accumulation of the OsWRKY11.1 transcript. This or another interaction might also possibly involve in the alternative splicing of Dlf1.
Rice is a facultative SD plant which flowers under LD conditions. As a counterpart of the GI–CO–FT signaling pathway in Arabidopsis, the rice orthologous proteins consist of OsGI–Hd1–Hd3a. The clock-associated protein OsGI upregulates Hd1 expression and in turn Hd1 induces the expression of Hd3a during SD conditions in rice [9], [12], [13]. The expression of Hd3a is also induced by the Ehd1 activator, which is an evolutionarily unique gene in rice with no counterpart in Arabidopsis [15]. Ehd2/RID1/OsId1 was found to promote flowering under both SD and LD conditions by upregulating Ehd1 expression [16]–[18]. Since Ehd2/RID1/OsId1 expression was suppressed under both SD and LD conditions in the dlf1 mutant (Fig. 6), the late flowering phenotype of the mutant is easily explained by loss of the promoting action of Ehd2/RID1/OsId1 (Fig. 9).
Dlf1 was expressed in leaves, roots and panicles (Fig. 3A). Expression of Dlf1 in leaves is consistent with the role of genes in flowering-time regulation, such as Ehd2 and Ghd7 [16], [19]. Ehd2/RID1/OsId1 is considered the master switch for the transition from vegetative to reproductive phase, a crucial process in higher plants. We found that increased Dlf1 expression delayed the phase transition and initiation of floral induction, leading to late flowering in the dlf1 mutant. This was further supported by studies of gene expression in the whole developmental process, which showed that Ehd2/RID1/OsId1 is suppressed in the dlf1 mutant with a higher level of Dlf1 mRNA accumulation under LD conditions (Fig. 7). Diurnal expression of Dlf1 was observed under both LD and SD conditions; however, the expression of photoperiod-related genes OsGI and Se5 was not significantly changed between the mutant and wild-type plants (Fig. 6; Fig. S3), suggesting that Dlf1 is unlikely to be upstream of these genes in the pathways of photoperiodic flowering in rice. WRKY proteins are a super family of plant transcription factors, which are characterized by binding specifically with W-box (a core sequence of TGAC). Genetic and molecular analyses have revealed that WRKY genes play roles in diverse biotic and abiotic stresses, as well as in development [34]–[36]. It is commonly observed that a single transcription factor may regulate multiple plant processes and that some may work in a redundant manner. AtWRKY6 is reported to be associated with senescence- and defense-related processes, and was shown to respond to low Pi stress in Arabidopsis [37], [38]. Recently, Roboni et al. [39] have shown that GI plays a key role in photoperiodic cues and drought escape response via ABA-dependent activation of florigens and SOC1. The gi mutants exhibit hyper tolerance to oxidative stress, enhanced salt tolerance, and elevated starch content, highlighting the importance of carbohydrate metabolism in the regulation of flowering [40]–[43]. Meanwhile, SOC1 and FLC are mediated in crosstalk between cold response and flowering time regulation [44].
Our data combined with others indicate that Dlf1 play important roles on plant height, heading date, yield potential and responses to abiotic stress [29]. The CCT-domain protein Ghd7 and DTH8 protein have been demonstrated to have pleiotropic effects on heading time, height and yield potential [19], [23]. Further investigation of their relationship should help illuminate the complexities of these important agronomic traits, as well as aid in manipulation of the traits for yield improvement.
Materials and Methods
Plant materials and growth conditions
Rice seeds of the wild-type (Oryza sativa L. japonica, Zhonghua 11 or Zhonghua 17, ZH11 or ZH17), mutant and transgenic progenies were sterilized and germinated on half-strength Murashige and Skoog medium for 7 d and then transferred to soil in a greenhouse. For flowering time measurements, plants were grown either in 10/14 h light/dark for SD or 14/10 h light/dark for LD. Rice flowering time was measured in days from germination until emergence of the first panicle. For diurnal expression analyses, young leaves were harvested from wild-type ZH11 and the dlf1 mutant of 40-d-old (SD) or 90-d-old (LD) plants at 4-h intervals for a total of 24 h. To analyze gene expression during development, the first and second youngest leaves from three plants were harvested from 30, 50, 70, 90, 110 and 130-d-old plants at 4 h after dawn under LD condition. The rice plants examined under natural field conditions were sown at late April and transplanted at early June in the experimental field of China Agricultural University, Beijing (39°54′N, 116°23′E), China.
Genotyping of mutant plants and Tail-PCR
For genotyping analysis, the dlf1 segregating population was assayed by PCR using the primers of PddSalF, TR1 and W6 (Table S1). The primer pair of TR1 and W6 was used for amplification of the T-DNA insertion. Thermal asymmetric interlaced PCR (Tail-PCR) method was used to isolate genomic fragment flanking of the T-DNA insertion site from the dlf1 mutant plant. The primers TR1, TR2, TR3, AD1, AD2 and AD3 are shown in Table S1.
Vector construction and transformation
The full-length coding region of Dlf1 was obtained by PCR amplification using the primers W1 and W5, along with a shorter product (assigned as OsWRKY11.2). The OsWRKY11.1 and OsWRKY11.2 cDNAs were put under the control of a maize ubiquitin promoter in a modified pCambia 1301 vector to generate Ubi:W11.1 and Ubi:W11.2 for overexpression [34]. Similarly, the PCR products, amplified with the primer pairs of d40BIF/W10H3r and d40BIF/W10SH3r were used to construct Ubi:d4W11.1 and Ubi:d4W11.2 vectors, with deletions of the 5′-ends in comparison with OsWRKY11.1 and OsWRKY11.2, respectively. Each overexpressing construct contained a Flag tag in the 5′-end of the gene. The Dlf1 fragment of 276 bp (from −39 to +237 bp) was used to generate the Dlf1-RNAi plasmid using procedures similar to the previous description [34]. The hairpin structure was put under the control of the CaMV35S promoter (Cam35S:dsW11). For promoter constructs, the PCR products were fused to β-glucuronidase (Gus) reporter gene as following: the Dlf1 promoter from −2010 to +134 bp as Cp-WP:Gus, −67 – +134 bp as Cp-InR3:Gus, and −67 – +5 bp as Cp-In2R:Gus. For complementation, the genomic DNA fragment from −67 to the end of Dlf1 was put into a modified pCambia 1301 vector, designed as Cp-Ins-Dlf. All vectors were verified by sequencing and transformed into rice through Agrobacterium-mediated transformation [35]. The transgenic lines obtained were determined by PCR amplifications.
Transactivation activity assay
The coding region of Dlf1 was amplified with the primers W10EI and W10BgSal (Table S3). The PCR product was fused to the GAL4 DNA binding-domain vector pGBKT7 (pBD, Clotech) to generate the plasmid pBD–Dlf1. Similarly, a fragment of Dlf1 encoding amino acids 91–379 (pBD–dN1), 121–379 (pBD–dN2), 204–379 (pBD–dN3), 91–313 (pBD–dN1C1), the first 313 amino acids (pBD–dC1), the first 266 amino acids (pBD–dC2), or OsWRKY11.2 (pBD-WRKY11.2) were fused to the GAL4 DNA binding-domain. These constructs or empty vector pBD were individually transformed into yeast cells of AH109 strain and grown on SD–Trp selective medium at 30°C for 3 d. An assay of β-galactosidase activity was performed with transformed cell lines grown in liquid SD-Trp medium using o -nitrophenyl β-D-galactopyranoside as a substrate, according to the manufacturer's protocol.
Subcellular localization of Dlf1
The coding sequence of Dlf1 was amplified and fused in frame to the upstream of green fluorescent protein (GFP) gene to generate the CamUbi:Dlf1-GFP construct. The resultant and the control CamUbi:GFP vectors were transformed into onion (Allium cepa) inner epidermal cells by bombardment using the PDS-1000/He system (Bio-Rad) with DNA-coated gold particles. The transformed cells were cultured on 1/2 MS medium at 28°C for 2 d and observed under a confocal microscope (Bio-Rad MRC 1024).
Gus assay and histochemical staining
Gus activity assay and histochemical staining were performed as described [45] and photographed using a Nikon SMZ 1000 stereoscope with a Nikon digital sight DS-SM camera or Nikon camera.
RNA gel-blot
Trizol reagent was used to extract the RNA from rice tissues. Total RNA was fractionated in 1.5% agarose containing formaldehyde, blotted onto Hybond-N+ nylon membrane and probed with the coding sequence of Dlf1. Hybridization was performed as previously described [34] and the membrane was autoradiographed by using a phosphoimaging system (Amersham Pharmacia Biotech, UK).
Real-time quantitative RT-PCR
Total RNA was isolated from different rice tissues using Trizol reagent following the manufacturer's procedures. DNaseI-treated RNAs were reverse transcribed with oligo (dT) and random primers. Real-time quantitative RT-PCR (qPCR) was performed in a final volume of 20 µL, including 10 µL SYBR Premix EX Taq (Takara), 2 µL of the diluted first-strand cDNA as templates and 0.2 µM of each primer. The reactions were carried out with an ABI PRISM 7000 or ABI StepOne in the following program: 95°C for 2 min, 40 cycles of 95°C for 5 s, 60°C for 20 s, and 72°C for 31 s. Every experiment was repeated more than twice. The primers of Ehd2, Hd1, Ehd1, Hd3a, Ubq, Ghd7, OsGI, Se5 FTL6 and Dlf1 are listed in Table S2. The level of ubiquitin (Ubq) expression was used to normalize the expression ratio of each gene.
RLM-RACE PCR
The RNA ligase-mediated amplification of 5′ cDNA ends (RLM-RACE) was performed according to the manufacturer's manual with modifications. Two µg total RNA was directly ligated to a RNA adaptor using T4 RNA ligase and transcribed by random primers. PCR was performed by using an outer primer from the adaptor and a Dlf1-specific reverse primer W5 at conditions of 95°C for 3 min (1 cycle), 95°C for 30 s, 55°C for 30 s, 72°C for 1 min (20 cycles), then 72°C for 10 min (1 cycle). A second round of nested PCR was amplified by using the inner primer and a Dlf1 gene primer W4 for 30 cycles. Actin gene was used as an internal standard. The RACE PCR products were sequenced to identify the mRNA degraded ends.
Supporting Information
Phenotypes of Zhonghua 11 (ZH11) and dlf1 mutant. (A) Longitudinal section of the stems approximately 2 cm above the upper-most nodes from the tiller culms of plants. (B) Number of spikelets per panicle. Values are means ± SD, n = 20. (C) 1000-grain weight. Values are means ± SD, n = 10. (D) Leaf rolling. (E) Transverse sections of the middle part of the first leaf from tillering plants. The plants were grown in the experimental field under natural LD conditions.
(PDF)
Multiple sequence alignment of OsWRKY11 cDNAs and proteins. (A) The full-length cDNA of Dlf1 (OsWRKY11.1) and the alternatively spliced transcript OsWRKY11.2 were aligned using the CLUSTAL W program. (B) The alignment of Dlf1 and OsWRKY11.2 proteins using CLUSTAL W program.
(PDF)
Expression of OsGI , Ghd7, Se5 , and FTL6 . Diurnal expression patterns of OsGI, Ghd7, Se5, and FTL6 in the ZH11 control (filled circle) and the mutant dlf1 (open circle) plants under SD (10 h light/14 h dark) and LD (14 h light/10 h dark) conditions by qPCR analysis. The expression levels are relative to the ubiquitin (Ubq) mRNA. Values are shown as means ± SD of two independent experiments. The open and filled bars at the bottom represent the light and dark periods, respectively.
(PDF)
Heights of Ubi:W11.1 transgenic plants. (A) Schematic diagram of Ubi:W11.1 construct (OE-). (B) Plant heights of some Ubi:W11.1 lines in T2 progenies and the ZH11 control. Values are means ± SD.
(PDF)
Primers of Dlf1 for genotype, expression, and vector construction.
(DOCX)
Primers of photoperiod- and flowering-time-related genes for real-time RT-PCR.
(DOCX)
Primers for transactivation activity.
(DOCX)
Acknowledgments
The authors thank Professors Yiji Xia (Hong Kong Baptist University) and Jun Fan (China Agricultural University) for critical reading of the manuscript.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was financially supported by National Nature Science of Foundation (31171833 and 31272025), the Program for Changjiang Scholars and Innovative Research Team in University (IRT042), and the 111 project (B13006). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Ashikari M, Wu J, Yano M, Sasaki T, Yoshimura A (1999) Rice gibberellin-insensitive dwarf mutant gene Dwarf 1 encodes the α-subunit of GTP-binding protein. Proc Natl Acad Sci USA 96: 10284–10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Itoh H, Tatsumi T, Sakamoto T, Otomo K, Toyomasu T, et al. (2004) A rice semi-dwarf gene, Tan-Ginbozu (D35), encodes the gibberellin biosynthesis enzyme, ent-kaurene oxidase. Plant Mol Biol 54: 533–547. [DOI] [PubMed] [Google Scholar]
- 3. Tanabe S, Ashikari M, Fujioka S, Takatsuto S, Yoshida S, et al. (2005) A novel cytochrome P450 is implicated in brassinosteroid biosynthesis via the characterization of a rice dwarf mutant, dwarf11, with reduced seed length. Plant Cell 17: 776–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li X, Qian Q, Fu Z, Wang Y, Xiong G, et al. (2003) Control of tillering in rice. Nature 422: 618–621. [DOI] [PubMed] [Google Scholar]
- 5. Ashikari M, Sakakibara H, Lin S, Yamamoto T, Takashi T, et al. (2005) Cytokinin oxidase regulates rice grain production. Science 309: 741–745. [DOI] [PubMed] [Google Scholar]
- 6. Song XJ, Huang W, Shi M, Zhu MZ, Lin HX (2007) A QTL for rice grain width and weight encodes a previously unknown RING-type E3 ubiquitin ligase. Nat Genet 39: 623–630. [DOI] [PubMed] [Google Scholar]
- 7. Corbesier L, Vincent C, Jang S, Fornara F, Fan Q, et al. (2007) FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis . Sci Signaling 316: 1030–1036. [DOI] [PubMed] [Google Scholar]
- 8. Kobayashi Y, Weigel D (2007) Move on up, it's time for change-mobile signals controlling photoperiod-dependent flowering. Genes Dev 21: 2371–2384. [DOI] [PubMed] [Google Scholar]
- 9. Tsuji H, Taoka KI, Shimamoto K (2011) Regulation of flowering in rice: two florigen genes, a complex gene network, and natural variation. Curr Opin Plant Biol 14: 45–52. [DOI] [PubMed] [Google Scholar]
- 10. Lee J, Lee I (2010) Regulation and function of SOC1, a flowering pathway integrator. J Exp Bot 61: 2247–2254. [DOI] [PubMed] [Google Scholar]
- 11. Yano M, Katayose Y, Ashikari M, Yamanouchi U, Monna L, et al. (2000) Hd1, a major photoperiod sensitivity quantitative trait locus in rice, is closely related to the Arabidopsis flowering time gene CONSTANS. Plant Cell 12: 2473–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Izawa T, Oikawa T, Sugiyama N, Tanisaka T, Yano M, et al. (2002) Phytochrome mediates the external light signal to repress FT orthologs in photoperiodic flowering of rice. Genes Dev. 16: 2006–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hayama R, Yokoi S, Tamaki S, Yano M, Shimamoto K (2003) Adaptation of photoperiodic control pathways produces short-day flowering in rice. Nature 422: 719–722. [DOI] [PubMed] [Google Scholar]
- 14. Hayama R, Coupland G (2004) The molecular basis of diversity in the photoperiodic flowering responses of Arabidopsis and rice. Plant Physiol 135: 677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Doi K, Izawa T, Fuse T, Yamanouchi U, Kubo T, et al. (2004) Ehd1, a B-type response regulator in rice, confers short-day promotion of flowering and controls FT-like gene expression independently of Hd1 . Genes Dev 18: 926–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Matsubara K, Yamanouchi U, Wang ZX, Minobe Y, Izawa T, et al. (2008) Ehd2, a rice ortholog of the maize INDETERMINATE1 gene, promotes flowering by up-regulating Ehd1 . Plant Physiol 148: 1425–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Park SJ, Kim SL, Lee S, Je BI, Piao HL, et al. (2008) Rice Indeterminate 1 (OsId1) is necessary for the expression of Ehd1 (Early heading date 1) regardless of photoperiod. Plant J 56: 1018–1029. [DOI] [PubMed] [Google Scholar]
- 18. Wu C, You C, Li C, Long T, Chen G, et al. (2008) RID1, encoding a Cys2/His2-type zinc finger transcription factor, acts as a master switch from vegetative to floral development in rice. Proc Natl Acad Sci USA 105: 12915–12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xue W, Xing Y, Weng X, Zhao Y, Tang W, et al. (2008) Natural variation in Ghd7 is an important regulator of heading date and yield potential in rice. Nat Genet 40: 761–767. [DOI] [PubMed] [Google Scholar]
- 20. Matsubara K, Ogiso-Tanaka E, Hori K, Ebana K, Ando T, et al. (2012) Natural variation in Hd17, a homolog of Arabidopsis ELF3 that is involved in rice photoperiodic flowering. Plant Cell Physiol 53: 709–716. [DOI] [PubMed] [Google Scholar]
- 21. Saito H, Ogiso-Tanaka E, Okumoto Y, Yoshitake Y, Izumi H, et al. (2012) Ef7 encodes an ELF3-like protein and promotes rice flowering by negatively regulating the floral repressor gene Ghd7 under both short- and long-day conditions. Plant Cell Physiol 53: 717–728. [DOI] [PubMed] [Google Scholar]
- 22. Matsubara K, Yamanouchi U, Nonoue Y, Sugimoto K, Wang ZX, et al. (2011) Ehd3, encoding a plant homeodomain finger-containing protein, is a critical promoter of rice flowering. Plant J 66: 603–612. [DOI] [PubMed] [Google Scholar]
- 23. Wei X, Xu J, Guo H, Jiang L, Chen S, et al. (2010) DTH8 suppresses flowering in rice, influencing plant height and yield potential simultaneously. Plant Physiol 153: 1747–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yan WH, Wang P, Chen HX, Zhou HJ, Li QP, et al. (2011) A major QTL, Ghd8, plays pleiotropic roles in regulating grain productivity, plant height, and heading date in rice. Mol Plant 4: 319–330. [DOI] [PubMed] [Google Scholar]
- 25. Fujino K, Utako Yamanouchi U, Yano M (2013) Roles of the Hd5 gene controlling heading date for adaptation to the northern limits of rice cultivation. Theor Appl Gen 126: 611–618. [DOI] [PubMed] [Google Scholar]
- 26. Kim SL, Lee S, Kim HJ, Nam HG, An G (2007) OsMADS51 is a short-day flowering promoter that functions upstream of Ehd1, OsMADS14, and Hd3a. Plant Physiol 145: 1484–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gao H, Zheng XM, Fei G, Chen J, Jin M, et al. (2013) Ehd4 encodes a novel and Oryza-genus-specific regulator of photoperiodic flowering in rice. PLoS Genet 9: e1003281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Franco-Zorrilla JM, Del Toro FJ, Godoy M, Pérez-Pérez J, López-Vidriero I, et al. (2009) Genome-wide identification of small RNA targets based on target enrichment and microarray hybridizations. Plant J 59: 840–850. [DOI] [PubMed] [Google Scholar]
- 29. Wu X, Shiroto Y, Kishitani S, Ito Y, Toriyama K (2009) Enhanced heat and drought tolerance in transgenic rice seedlings overexpressing OsWRKY11 under the control of HSP101 promoter. Plant Cell Rep 28: 21–30. [DOI] [PubMed] [Google Scholar]
- 30. Quesada V, Macknight R, Dean C, Simpson GG (2003) Autoregulation of FCA pre-mRNA processing controls Arabidopsis flowering time. EMBO J 22: 3142–3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Simpson GG, Dijkwel PP, Quesada V, Henderson I, Dean C (2003) FY is an RNA 3′ end-processing factor that interacts with FCA to control the Arabidopsis floral transition. Cell 113: 777–787. [DOI] [PubMed] [Google Scholar]
- 32. Macknight R, Bancroft I, Page T, Lister C, Schmidt R, et al. (1997) FCA, a gene controlling flowering time in Arabidopsis, encodes a protein containing RNA-binding domains. Cell 89: 737–745. [DOI] [PubMed] [Google Scholar]
- 33. Macknight R, Duroux M, Laurie R, Dijkwel P, Simpson G, et al. (2002) Functional significance of the alternative transcript processing of the Arabidopsis floral promoter FCA. Plant Cell 14: 877–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang H, Hao J, Chen X, Hao Z, Wang X, et al. (2007) Overexpression of rice WRKY89 enhances ultraviolet B tolerance and disease resistance in rice plants. Plant Mol Biol 65: 799–815. [DOI] [PubMed] [Google Scholar]
- 35. Zhang J, Peng Y, Guo Z (2008) Constitutive expression of pathogen-inducible OsWRKY31 enhances disease resistance and affects root growth and auxin response in transgenic rice plants. Cell Res 18: 508–521. [DOI] [PubMed] [Google Scholar]
- 36. Pandey SP, Somssich IE (2009) The role of WRKY transcription factors in plant immunity. Plant Physiol 150: 1648–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Robatzek S, Somssich IE (2001) A new member of the Arabidopsis WRKY transcription factor family, AtWRKY6, is associated with both senescence- and defence- related processes. Plant J 28: 123–133. [DOI] [PubMed] [Google Scholar]
- 38. Chen YF, Li LQ, Xu Q, Kong YH, Wang H, et al. (2009) The WRKY6 transcription factor modulates PHOSPHATE1 expression in response to low Pi stress in Arabidopsis . Plant Cell 21: 3554–3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Riboni M, Galbiati M, Tonelli C, Conti L (2013) GIGANTEA enables drought escape response via abscisic acid-dependent activation of the florigens and suppressor of overexpression of CONSTANS1. Plant Physiol 162: 1706–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Eimert K, Wang SM, Lue W, Chen J (1995) Monogenic recessive mutations causing both late floral initiation and excess starch accumulation in Arabidopsis . Plant Cell 7: 1703–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kurepa J, Smalle J, Van Montagu M, Inzé D (1998) Effects of sucrose supply on growth and paraquat tolerance of the late-flowering gi-3 mutant. Plant Growth Regul 26: 91–96. [Google Scholar]
- 42. Kim WY, Ali Z, Park HJ, Park SJ, Cha JY, et al. (2013) Release of SOS2 kinase from sequestration with GIGANTEA determines salt tolerance in Arabidopsis . Nature Comm 4: 1352–1364. [DOI] [PubMed] [Google Scholar]
- 43. Wahl V, Ponnu J, Schlereth A, Arrivault S, Langenecker T, et al. (2013) Regulation of flowering by trehalose-6- phosphate signaling in Arabidopsis thaliana . Science 339: 704–707. [DOI] [PubMed] [Google Scholar]
- 44. Seo E, Lee H, Jeon J, Park H, Kim J, et al. (2009) Crosstalk between cold response and flowering in Arabidopsis is mediated through the flowering-time gene SOC1 and its upstream negative regulator FLC . Plant Cell 21: 3185–3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: beta-glucuronidaseas a sensitive and versatile gene fusion marker in higher plants. EMBO J 6: 3901–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phenotypes of Zhonghua 11 (ZH11) and dlf1 mutant. (A) Longitudinal section of the stems approximately 2 cm above the upper-most nodes from the tiller culms of plants. (B) Number of spikelets per panicle. Values are means ± SD, n = 20. (C) 1000-grain weight. Values are means ± SD, n = 10. (D) Leaf rolling. (E) Transverse sections of the middle part of the first leaf from tillering plants. The plants were grown in the experimental field under natural LD conditions.
(PDF)
Multiple sequence alignment of OsWRKY11 cDNAs and proteins. (A) The full-length cDNA of Dlf1 (OsWRKY11.1) and the alternatively spliced transcript OsWRKY11.2 were aligned using the CLUSTAL W program. (B) The alignment of Dlf1 and OsWRKY11.2 proteins using CLUSTAL W program.
(PDF)
Expression of OsGI , Ghd7, Se5 , and FTL6 . Diurnal expression patterns of OsGI, Ghd7, Se5, and FTL6 in the ZH11 control (filled circle) and the mutant dlf1 (open circle) plants under SD (10 h light/14 h dark) and LD (14 h light/10 h dark) conditions by qPCR analysis. The expression levels are relative to the ubiquitin (Ubq) mRNA. Values are shown as means ± SD of two independent experiments. The open and filled bars at the bottom represent the light and dark periods, respectively.
(PDF)
Heights of Ubi:W11.1 transgenic plants. (A) Schematic diagram of Ubi:W11.1 construct (OE-). (B) Plant heights of some Ubi:W11.1 lines in T2 progenies and the ZH11 control. Values are means ± SD.
(PDF)
Primers of Dlf1 for genotype, expression, and vector construction.
(DOCX)
Primers of photoperiod- and flowering-time-related genes for real-time RT-PCR.
(DOCX)
Primers for transactivation activity.
(DOCX)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.