Abstract
Biology needs a concept of individuality in order to distinguish organisms from parts of organisms and from groups of organisms, to count individuals and compare traits across taxa, and to distinguish growth from reproduction. Most of the proposed criteria for individuality were designed for ‘unitary’ or ‘paradigm’ organisms: contiguous, functionally and physiologically integrated, obligately sexually reproducing multicellular organisms with a germ line sequestered early in development. However, the vast majority of the diversity of life on Earth does not conform to all of these criteria. We consider the issue of individuality in the ‘minor’ multicellular taxa, which collectively span a large portion of the eukaryotic tree of life, reviewing their general features and focusing on a model species for each group. When the criteria designed for unitary organisms are applied to other groups, they often give conflicting answers or no answer at all to the question of whether or not a given unit is an individual. Complex life cycles, intimate bacterial symbioses, aggregative development, and strange genetic features complicate the picture. The great age of some of the groups considered shows that ‘intermediate’ forms, those with some but not all of the traits traditionally associated with individuality, cannot reasonably be considered ephemeral or assumed transitional. We discuss a handful of recent attempts to reconcile the many proposed criteria for individuality and to provide criteria that can be applied across all the domains of life. Finally, we argue that individuality should be defined without reference to any particular taxon and that understanding the emergence of new kinds of individuals requires recognizing individuality as a matter of degree.
Keywords: cellular differentiation, individuality, life history, major transitions, multicellularity, organisms, symbiosis
I. Introduction
Multicellular organisms have evolved from unicellular ancestors numerous times and in distantly related lineages (Bonner, 1998; Carroll, 2001; Grosberg & Strathmann, 2007). Multicellularity has evolved independently in at least 25 separate lineages, including bacteria, Archaea, and several lineages spanning the deepest divergences within the eukaryotes (Bonner, 1998; Carroll, 2001; Baldauf, 2003; Grosberg & Strathmann, 2007). Furthermore, this list includes only those multicellular lineages that have extant descendants; there may well have been others that left no surviving progeny. Only a handful of lineages, though, have evolved cellular differentiation, a prerequisite for large, complex body plans (Knoll, 2011).
Multicellular organisms are one level of the hierarchy of life, made up of the descendants of free-living (usually eukaryotic) cells and sometimes making up social or eusocial colonies. Eukaryotic cells are in turn descended from two or more free-living prokaryotic cells. The existence of this hierarchy is an outcome, not an initial condition, of the evolutionary process (Buss, 1987), and understanding the emergence of new levels of the hierarchy is a major goal of evolutionary biology. The events that generated new levels of the hierarchy have been called “major transitions in evolution” (Maynard Smith & Szathmáry, 1997) or “evolutionary transitions in individuality” (ETIs; Michod & Roze, 1997).
The result of any ETI is a new kind of individual, but this raises the question of what qualifies as an individual. In spite of suggestions to the contrary (e.g. Wilson, 2000), the question of what biological units qualify as individuals is not always merely semantic but has real consequences for some biological questions. For example, in order to make meaningful comparisons across species, it is imperative that we match apples with apples (Pepper & Herron, 2008). Furthermore, questions in population biology require resolution of the “demographer's dilemma,” identifying what units to count and to associate with measures of fitness (Clarke, 2012). The choice of what units to count affects how fitness is measured, and different choices can lead to different predictions about evolutionary dynamics and outcomes (Pedersen & Tuomi, 1995; Clarke, 2012). Indeed, any calculation of fitness commits the investigator to a particular notion of individuality, at least for the species in question (Ariew & Lewontin, 2004). Whether a given adaptive process should be considered individual selection or group selection hinges on the distinction between individuals and groups (Tuomi & Vuorisalo, 1989). Finally, being precise about what we mean by an individual organism affects our understanding of individuality as a derived trait, allowing us to frame meaningful questions about how it evolves (Queller & Strassmann, 2009; Clarke, 2010).
(1) Traditional criteria
Informally, ‘individual’ typically refers to entities with two notable features: they are separate from other such entities, making them easily countable, and they cannot be divided without losing their character. A number of criteria have been proposed for specifying how to recognize these two basic features in the biological world. For example, biological individuals (or organisms; we will treat these as synonyms in the context of individual multicellular organisms) are said to be spatially and temporally bounded (Hull, 1980), contiguous (Hull, 1980), and physiologically discrete (Buss, 1987). Further, individuals should be genetically unique and genetically homogeneous (Weismann, 1904), possibly because they pass through a single-cell bottleneck (Huxley, 1912), either in sexual or asexual reproduction. An individual should be functionally integrated and autonomous (Huxley, 1912), have boundaries maintained by an immune response (Burnet, 1969), and have a division of labour between reproductive (germ) and somatic cells (Buss, 1987). Individuals should serve as units of selection and share a common evolutionary fate (Janzen, 1977), and as a result they should come to bear adaptations (Queller, 1997). This is by no means an exhaustive list, and some of these criteria are overlapping or otherwise non-independent. See Clarke (2010) for a more comprehensive list.
(2) Recent syntheses
Several recent efforts have attempted to reconcile different criteria for individuality and to relate them more explicitly to evolutionary theory. In each of these recent syntheses, the authors grapple with individuality as it relates to the process of natural selection. The connection between the concepts of adaptation and individuality figures prominently. Most of the reasons for needing an individuality concept (outlined above) are addressed by an evolution-focused approach to understanding individuality. However, there is one notable exception. The issue of how to recognize individuals that are appropriate for broad-scale comparative purposes is often not discussed explicitly by those taking an evolutionary approach.
One recent synthesis proposes that organisms be recognized by high levels of cooperation and low levels of conflict among their component parts (Queller & Strassmann, 2009; Strassmann & Queller, 2010). The focus is on realized levels of cooperation and conflict, not potential sources of cooperation and conflict such as levels of genetic dissimilarity. The two factors are treated as independent and continuous, setting up a two-dimensional space with organisms occupying one quadrant. This view subsumes many of the traditional criteria as causes, results, or indicators of high cooperation and low conflict. Thus germ-soma specialization, policing, and single-cell bottlenecks are mechanisms that increase within-organism cooperation or reduce within-organism conflict. High cooperation and low conflict indicate that organisms are highly functionally integrated and allow them to function as “bundles of adaptation” (Strassmann & Queller, 2010, p. 605). Genetic homogeneity will often (but need not always) be associated with high cooperation and low conflict.
In another recent review, Folse & Roughgarden (2010) argue that the crucial criteria for organismality are alignment of fitness interests of the lower-level units so that little or no within-organism conflict occurs, interdependence of the parts due to germ-soma differentiation, and functional integration as evidence of adaptation. As in the view of Queller & Strassmann (2009; Strassmann & Queller, 2010), genetic homogeneity and unicellular bottlenecks are ways of preventing conflict (not necessarily the only ways). The relevance of germ-soma differentiation is that it exports fitness from the lower-level units to the higher-level unit (Michod, 1997; Michod et al., 2006). Adaptive functional integration at the organism level indicates that the organism is a unit of fitness. Folse & Roughgarden (2010, p. 451) envision these traits arising in order during ETIs, “…beginning with alignment of fitness by genetic relatedness, the export of fitness by germ-soma specialization, and, finally, functional organization by adaptation at the higher level.”
In the framework of Godfrey-Smith (2009), individuals are defined by their membership in Darwinian populations, those that are capable of adaptive evolution. At a minimum, such populations must possess Lewontin's (1970) criteria for evolution by natural selection: heritable variation in phenotypes that affect fitness. Traditional criteria for individuality are sometimes associated with the capacity for adaptive change, but it is the capacity itself that is central. Godfrey-Smith (2009) recognizes a continuum of Darwinian and Darwinian-like processes, from marginal cases that meet only the minimal criteria to “paradigm” cases that are capable of producing complex adaptations. Populations vary more or less continuously along several axes, including the amount of phenotypic variation present, the reliability of inheritance, the strength of intra-specific ecological interactions, the extent to which fitness depends on intrinsic features, and the smoothness of the adaptive landscape. Populations that possess all of these features in high degree have the potential for sustained and complex adaptive change, while those with low degrees of one or more criteria are at best capable of less interesting evolutionary outcomes. Other criteria may also be important for particular kinds of organisms. The most relevant of these types for our purposes is collective reproducers, those that are composed of parts that are themselves capable of reproduction. Collective reproducers have the potential for complex adaptive processes, and thus have a high degree of individuality, when they pass through a bottleneck during development, have a separate germ line, and are highly integrated. High degrees of these criteria indicate that Darwinian processes will be more powerful, and thus adaptations will tend to occur, at the level of the collective rather than of its components. The ideas that individuality means ‘membership in a Darwinian population’ and that the degree of individuality is related to the complexity of the outcome of a selective process can be understood in terms of evolvability, though Godfrey-Smith (2009) does not use that term (Sterelny, 2011). By this conception, the process of natural selection both depends on and can create aspects of individuality, making the issue of individuality a fundamental and subtle part of evolutionary theory.
(3) Aims
Most of the traditional criteria have been identified as such because they apply to ‘paradigm’ (Wilson, 1999) or ‘unitary’ (Santelices, 1999) organisms, those (such as vertebrates) for which their status as individuals is rarely in doubt. When we broaden our scope beyond these easy cases, though, cracks begin to show. Living things form colonies, clones, chimeras, coenobia, syncytia, symbioses, and other units that possess some but not all of the traditional criteria for individuality. In fact, when the diversity of life is considered, only a tiny minority of living things are found in units that possess all of these criteria (Buss, 1983, 1987; Folse & Roughgarden, 2010).
Our aim is to explore the cracks in the concept of individuality by considering how the various criteria apply to the “minor” multicellular eukaryotic taxa, those that include from a few to perhaps 10,000 known species. All of our chosen examples include at least some species with cellular differentiation. We consider only cases of spatial differentiation (i.e. two or more cell types in the same multicellular body, or thallus, at the same time) as opposed to temporal differentiation (i.e. organisms with cells that change over time, either because of an alternation of generations or due to environmental stimuli).
For each group, we focus on one or two ‘model’ species that have been especially well studied. We hope that, by attempting to apply the concept of individuality to diverse, real biological examples, we can reveal limitations that are not apparent when considering this concept in the abstract. We will see that unexpected complications arise, revealing that most concepts of individuality fail universally and unambiguously to distinguish individuals from groups of individuals or from parts of individuals. Finally, we evaluate some recent attempts to overcome these limitations by constructing concepts of individuality that can be applied across all taxa.
Aside from animals, land plants and fungi, cellular differentiation is found in at least some members of the red, green and brown algae, the cellular slime molds, and the ciliates. Differentiated multicellularity has thus arisen (often more than once) in five of the eight major groups of eukaryotes (sensu Baldauf, 2003): plants (red algae, green algae, land plants), Amoebozoa (cellular slime molds), Opisthokonts (animals, fungi), Alveoloates (ciliates), and Heterokonts (brown algae). In addition, there are several examples of differentiated multicellularity in prokaryotes (e.g. cyanobacteria, myxobacteria, biofilms), which we do not consider here (Meeks & Elhai, 2002; Webb, Givskov & Kjelleberg, 2003; Whitworth, 2008). Nearly every multicellular group has a unique associated vocabulary; because few readers will be familiar with all of them, we have tried when possible to avoid specialized jargon.
II. Plantae
The plants (sensu Baldauf, 2003) include the direct descendents of the primary endosymbiosis of a cyanobacterium approximately 1400-1600 million years ago (MYA) (Moreira, LeGuyader & Phillipe, 2000; Yoon et al., 2004; Bhattacharya et al., 2009). This monophyletic group includes the land plants and their closest relatives, the Charophytes (together referred to as Streptophyta; Bremer et al., 1987), the red algae (Rhodophyta), the green algae (Chlorophyta), and the unicellular glaucophyte algae. All other photosynthetic eukaryotes had their origins in secondary (or higher-order) endosymbiotic events, in which the engulfed partner was a unicellular eukaryotic (usually red or green) alga (Hackett et al., 2007).
(1) Green algae (Chlorophyta): “master colony formers”
The Chlorophyte green algae include the closest living relatives of the Streptophyta (Charophytes and land plants), from which they diverged approximately 900-1000 MYA (Hedges et al., 2004; Berbee & Taylor, 2007; Hackett et al., 2007; Bhattacharya et al., 2009). Chlorophyte growth forms are exceptionally diverse, ranging from unicells to large (up to 1 m) seaweeds with true tissues and including several lineages of motile multicellular colonies (van den Hoek, Mann & Jahns, 1995; Lewis & McCourt, 2004). Chlorophyte life histories typically include an alternation of haploid and diploid generations, and these may be isomorphic to strongly heteromorphic (Bold & Wynne, 1985; Lewis & McCourt, 2004).
In spite of many poorly resolved relationships, it is clear that the Chlorophyte algae include numerous origins of multicellularity (Mattox & Stewart, 1984; Lewis & McCourt, 2004). Of the four classes of Chlorophytes, three (Chlorophyceae, Trebouxiophyceae, and Ulvophyceae) include members that are multicellular during at least part of their life history. The unicellular Prasinophyceae are likely paraphyletic with respect to the other three classes (Karol et al., 2001; Lewis & McCourt, 2004). The Trebouxiophyceae are mostly unicellular but include filamentous forms such as Microthamnion and membranous thalli such as Prasiola (Lewis & McCourt, 2004).
(a) Chlorophyceae
The Chlorophyceae include so many separate multicellular lineages that Kirk (1998, p. 22) called them “master colony formers.” Within the order Volvocales, Volvox carteri has been developed as a model for the evolution of multicellularity and cellular differentiation (Starr, 1969; Kirk, 1998, 2001). Volvox carteri is a transparent, motile spheroid with approximately 2000-4000 small, biflagellate somatic cells embedded near the surface and approximately 12-16 much larger reproductive cells just below (Starr, 1969; Kirk, 1998).
The V. carteri life cycle is described in Fig. 1. Haploid asexual spheroids reproduce by autocolony formation, in which each reproductive cell divides and develops into a miniature spheroid within the mother spheroid. Male and female strains are genetically distinct but morphologically identical in the asexual phase, but differences become apparent upon entry into the sexual phase. Sexual reproduction is triggered by a pheromone, the production of which can be caused by heat shock or by spontaneous mutation (Callahan & Huskey, 1980; Kirk & Kirk, 1986).
Fig. 1.
Life cycle of the Chlorophyte green alga Volvox carteri. Haploid asexual spheroids undergo asymmetric cell divisions early in development, producing small somatic cells and large reproductive cells (gonidia). Each gonidium develops into a juvenile spheroid, which is eventually released from the mother spheroid. Juveniles escape from the parental spheroid possessing all of the cells they will have as adults; continued growth occurs by increases in cell size and in the volume of extracellular matrix rather than by cell division (Starr, 1969). Spheroids that are exposed to a chemical sex inducer (S.I.) produce sexual (male or female, depending on the strain) offspring (Starr, 1970). Female spheroids appear similar to asexual spheroids, but instead of approximately 12-16 reproductive cells, they produce approximately 35-45 somewhat smaller eggs (Starr, 1969). Males are considerably smaller, up to 512 cells, with half of the cells somatic and half producing sperm packets of 64-128 biflagellate sperm (Starr, 1969). Sperm packets are released from the male spheroids, swim to female spheroids, penetrate their surface, and dissociate into individual sperm to fertilize the eggs (Starr, 1969). Fertilized eggs mature into thick-walled, desiccation-resistant, dormant spores, which germinate upon the return of optimal growth conditions (Starr, 1969). Zygote germination involves meiosis but produces only a single haploid germling, which develops into a small asexual spheroid, along with three polar bodies (Starr, 1969, 1975). Adapted from Kirk (2001), Nishii & Miller (2010).
The traditional criteria give conflicting answers regarding what constitutes an individual in V. carteri. Because many rounds of asexual reproduction can occur between subsequent events of sex induction and mating, many genetically identical spheroids typically descend from a single V. carteri zygote. Thus the spatially bounded, contiguous, physiologically discrete and autonomous units (the spheroids) will typically not be genetically unique. Rather the genetically unique units, and the largest genetically homogenous units, are the clonal descendants of a given zygote (i.e. a genet; Sarukhán & Harper, 1973). By different criteria, then, a particular V. carteri spheroid can be considered an individual or a part of an individual (the genet).
Volvox carteri is a recurring character in discussions of individuality, and it appears in all three of the recent syntheses we review here. Queller & Strassmann (2009; Strassmann & Queller, 2010) consider V. carteri spheroids individuals on the grounds that their component cells cooperate in a germ-soma division of labour, and that within-spheroid conflicts are rare. Similarly, Folse & Roughgarden (2010) cite the germ-soma division of labour, and the resulting functional integration of spheroids, as decisive. In Godfrey-Smith's (2009) framework, V. carteri spheroids have an intermediate degree of individuality, scoring high on germ-soma division of labour and passing through a single-cell bottleneck during development and having an intermediate level of functional integration.
(b) Ulvophyceae
In the Ulvophyceae, most species have multicellular thalli during at least some part of the life cycle. The closely related orders Ulvales and Ulotrichales may represent a single origin of multicellularity, and the terrestrial Trentopohliales at least one other (Lewis & McCourt, 2004). Membranous Ulvophytes, such as Monostroma and Ulva, typically have prostrate, filamentous rhizoids and an upright, membranous blade.
The Ulva mutabilis life cycle is described in Fig. 2. The diploid sporophytes and haploid gametophytes of U. mutabilis are isomorphic, with a small holdfast and a large (up to 30 cm), membranous blade (Løvlie, 1964). Early germlings of U. mutabilis sporophytes and gametophytes develop similarly (Fjeld, 1972). A motile propagule (gamete, zoospore or zygote) attaches to the substratum and begins dividing to form a filament consisting of a single row of cells. At the four- or eight-celled stage, the basal cell elongates and begins to differentiate into a primary rhizoid, the first component of the holdfast (Fjeld, 1972). Neighbouring cells form a hollow tube and enlarge to become giant stem cells, the most basal of which produce additional rhizoids, while the apical cells form a blade two cell layers thick (Fjeld, 1972). Normal development and differentiation require the presence of symbiotic bacteria, without which only slow-growing, undifferentiated “callus-like colonies” develop (Stratmann, Paputsoglu & Oertel, 1996).
Fig. 2.
Life cycle of the Ulvophyte green alga Ulva mutabilis. Diploid zygotes formed by the fusion of gametes develop into diploid sporophytes. Sporophytes produce haploid zoospores through meiosis, and zoospores develop into haploid gametophytes, which are morphologically indistinguishable from the sporophytes. Gametophytes are of one of two genetically determined mating types and produce gametes of the same mating type. Gametes can either fuse to form a zygote that germinates into a sporophyte or settle and develop parthenogenetically (Løvlie, 1964; Fjeld, 1972; Hoxmark & Nordby, 1974). Germlings originating as unfused gametes can either develop into gametophytes or double their chromosome number and develop as sporophytes (Föyn, 1958; Fjeld, 1972). Dashed arrows indicate meiosis. Adapted from Hoxmark & Nordby (1974).
As with Volvox carteri, the mixture of sexual and asexual (parthenogenetic) reproduction in the U. mutabilis life cycle means that the spatially bounded, contiguous, physiologically discrete units (the thalli) will often be neither genetically unique nor the largest genetically homogeneous units. Furthermore, the requirement for symbiotic bacteria in development calls into question whether the thallus by itself or only the combination of alga and bacteria should be considered the individual on the bases of functional integration and autonomy. Finally, the chromosome doubling that sometimes occurs during sporophyte development from unfused gametes (Föyn, 1958; Fjeld, 1972) means that a given thallus may not be genetically homogenous, at least in terms of ploidy.
Ulva mutabilis thalli, both sporophyte and gametophyte, have a germ-soma division of labour and pass through a single-cell bottleneck during development, and so they probably qualify as individuals by the criteria of high cooperation and low conflict (Queller & Strassmann, 2009; Strassmann & Queller, 2010). For similar reasons, U. mutabilis thalli likely qualify as individuals by the criteria of Folse & Roughgarden (2010). These two views diverge, though, in their application to U. mutabilis' symbiotic bacteria. Folse & Roughgarden (2010) do not address symbioses, but their treatment of chimeras (which they do not consider organisms) suggests that they would consider the potential conflict implied by the partners' distinct genomes to be decisive. Strassmann & Queller (2010), by contrast, allow for the possibility of “egalitarian organisms,” those composed of genetically distinct subunits (as opposed to “fraternal organisms” made up of like units, based on the distinction originally set forth in Queller, 1997). It is an open question, though, whether the degree of cooperation between U. mutabilis and its associated bacteria suffices to qualify the conglomerate as an individual.
Evaluating the degree of individuality of Ulva mutabilis thalli in the framework of Godfrey-Smith (2009) requires some speculation about biological details. The observation that U. mutabilis sporophytes preferentially settle on bacterial biofilms (Joint et al., 2000, 2002) suggests that the symbiosis is reestablished each generation, so the degree to which the bacterial portion passes through a bottleneck is difficult to say. Without their associated bacteria, U. mutabilis thalli lack any obvious cellular differentiation, including a germ-soma division of labour, and the degree of functional integration seems to be much higher with the bacteria than without. Thus it seems that the U. mutabilis thallus itself does well by the botttleneck criterion, while the combination of alga and bacteria does better by the criteria of functional integration and germ-soma division of labour. Bearing in mind that bottlenecks, germlines, and integration are indicators for the main criterion of capacity for adaptive change, Godfrey-Smith's (2009) framework leads us to conclude that both the symbiotic partnership and the alga alone possess some degree of individuality.
(2) Red algae (Rhodophyta): ancient origin of multicellularity
The red algae are an ancient and morphologically diverse group, most of which are multicellular. Growth forms range from single cells to large (up to 2 m) thalli with differentiation into true tissues (Coomans & Hommersand, 1990; Pueschel, 1990). Red algal life histories are complex and variable, with as many as three distinct phases, some haploid and some diploid, some isomorphic and some heteromorphic (Hawkes, 1990). Heteromorphic life-history phases can be dramatically so, and they may even be ecologically differentiated, living in very different habitats (Graham & Wilcox, 2000). Some are so different that they were originally described not only as separate species but as separate genera (Graham & Wilcox, 2000).
In spite of their diversity of morphology and life history, red algae are recognized as a monophyletic group (Van de Peer & De Wachter, 1997; Burger et al., 1999; Yoon et al., 2006). Recent molecular evidence unambiguously shows red algae as the sister group to green algae + Streptophytes (Moreira et al., 2000; Rodríguez-Ezpeleta et al., 2005). There is broad agreement on the composition of the major lineages of red algae, but little resolution of the relationships among these lineages (Saunders & Hommersand, 2004; Yoon et al., 2006). Until these relationships are resolved, it will be difficult to evaluate the possibility that multicellularity may have evolved more than once within the red algae or that some unicellular lineages may have evolved by reduction from multicellular ancestors (Garbary & Gabrielson, 1990).
One species of red alga, Porphyra yezoensis, has been developed as a developmental model (Kitade et al., 1998; Yamazaki, Nakanishi & Saga, 1998). The life cycle of P. yezoensis is described in Fig. 3 and includes a microscopic, filamentous diploid sporophyte and a macroscopic, foliose haploid gametophyte. The blade of a mature P. yezoensis gametophyte is only one cell thick but can be up to 1 m in length and may have as many as five cell types (Polne-Fuller & Gibor, 1984). Aside from the long, tapered cells of the holdfast, the thallus includes large vacuolated cells near the base, smaller vegetative cells making up most of the blade, and patches of male and female reproductive cells (Polne-Fuller & Gibor, 1984). Porphyra yezoensis gametophytes require symbiotic bacteria to develop normally: those grown in axenic cultures produce undifferentiated clumps, which can be rescued by the addition of two strains of marine bacteria (Mori et al., 2004; Yamazaki et al., 1998).
Fig. 3.
Life cycle of the red alga Porphyra yezoensis. The haploid, foliose gametophyte phase begins when a diploid spore settles and attaches to the substratum before undergoing meiosis. The resulting four-celled embryos are arranged linearly, with one end attached to the substratum (Wang et al., 2009). The cells present at this phase differ in their developmental fate, with the descendants of the most basal cell or two differentiating into the holdfast and those of the other two or three cells forming the blade (Polne-Fuller & Gibor, 1984). The gametophyte produces non-motile male and female gametes as well as haploid monospores that develop into new gametophytes. Fertilization produces diploid zygotes that divide mitotically to form carpospores, which develop into microscopic, filamentous diploid sporophytes. The diploid spores produced by sporophytes can either develop into new sporophytes (monospores) or germinate meiotically to produce gametophytes (conchospores). Dashed arrow indicates meiosis. Adapted from Polne-Fuller & Gibor (1984).
Gametophytes of P. yezoensis are chimeric in the sense that the thallus develops from all four products of meiosis (Ohme, Kunifuji & Miura, 1986). The genetic distinctness of those parts of the thallus derived from distinct meiotic products can be seen most dramatically in pigment mutants, which can develop into thalli in which the colour differs across the midline of the blade (Wang et al., 2009). In the closely related P. purpurea, a similar process can produce monoecious thalli with genetically distinct male and female halves (Mitman & van de Meer, 1994).
Identification of individuals in P. yezoensis involves some of the same difficulties as in Ulva mutabilis. The thalli possess many of the proposed criteria for individuality (e.g. spatial boundedness, contiguity, physiological discreteness, single-cell bottleneck, germ-soma differentiation), while the mitotic descendants of a zygote may include a large number of thalli that, as a group, are genetically unique and homogeneous. On the other hand, P. yezoensis gametophytes are not genetically homogeneous even within a single thallus (Ohme et al., 1986), meaning that a strict application of the genetic homogeneity criterion will mean that a P. yezoensis individual can consist of a part of a thallus (in the gametophyte) or a group of thalli (in the case of parthenogenetically reproducing sporophytes). Finally, as in Ulva mutabilis, the reliance on symbiotic bacteria calls into question whether P. yezoensis can qualify as an individual by the criteria of functional integration and autonomy, or whether only the combination of alga and bacteria so qualifies.
Regarding the symbiosis between algae and bacteria, most of the discussion of Ulva mutabilis applies to P. yezoensis as well, but the chimerism of the P. yezoensis gametophyte is different in important ways from that of the U. mutabilis parthenosporophyte. The chromosome doubling that takes place during the development of U. mutabilis parthenosporophytes leads to a thallus that is genetically heterogeneous only in terms of ploidy; the diploid portion presumably has the same complement of alleles as the haploid portion, but with twice as many copies. The fitness interests of the haploid and diploid portions are aligned and the resulting potential for conflict is low, presenting no problems for the frameworks of Queller & Strassmann (2009; Strassmann & Queller, 2010) and Folse & Roughgarden (2010). In P. yezoensis gametophytes, though, the portions of the thallus descended from the four products of meiosis will likely bear different alleles, separating their fitness interests and creating the potential for conflict (though it is an open question whether such conflict is realized). Although P. yezoensis gametophytes develop from a single cell, this does not have the usual effect of restricting genetic heterogeneity, and so Godfrey-Smith's (2009) bottleneck criterion is only poorly approximated. Similarly, the existence of a germ line (four germ lines, really) may limit conflicts within the four genetically distinct portions of the thallus, but it probably does not prevent conflicts among these lineages. The degree of functional integration depends on the degree to which these potential conflicts are realized.
III. Heterokonta
(1) Brown algae (Phaeophyta): convergent origin of multicellularity
The brown algae represent a striking parallel to the distantly related land plants. Although they diverged from the plant lineage over a billion years ago (Hackett et al., 2007; Bhattacharya et al., 2009), aspects of their morphology, life history and development are often surprisingly similar (Niklas, 2000). All brown algae are multicellular, ranging from simple filaments and crustose forms to large, plantlike wracks and kelps, the thalli of which can be up to 50 m long (Clayton, 1990; van den Hoek et al., 1995). Most brown algae have a biphasic life history, with a diploid sporophyte and a haploid gametophyte. The phases can be isomorphic to strongly heteromorphic, and the sporophyte is usually the dominant phase in heteromorphic species (Clayton, 1990). As in the red algae, life-history phases can be so different that they are sometimes described as separate species and even separate genera (van den Hoek et al., 1995).
Brown algae form a monophyletic group within the heterokonts, so-called because swimming cells have two unequal flagella, one of which is covered with mastigonemes, short bristles extending laterally from the main shaft (de Reviers, 2002). Photosynthetic heterokonts are thought to have originated with the secondary endosymbiosis of an autotrophic eukaryote, probably a red alga, resulting in chloroplasts with four membranes (van den Hoek et al., 1995; Cavalier-Smith, 1998).
The small, filamentous species Ectocarpus siliculosus, in the order Ectocarpales, is emerging as a model organism for the brown algae (Peters et al., 2004; Charrier et al., 2008; Cock et al., 2010; Heesch et al., 2010). The E. siliculosus life cycle is described in Fig. 4 and includes diploid sporophytes and separate male and female haploid gametophytes, as well as haploid parthenosporophytes that develop from unfertilized gametes (Müller, 1967; Charrier et al., 2008). The life cycle is described as “slightly heteromorphic” (Le Bail et al., 2008). Sporophytes and gametophytes both include upright, branched filaments, but sporophytes of both kinds (haploid and diploid) include a branched prostrate structure not found in the gametophyte (Müller, 1967; Charrier et al., 2008). All types of thalli have specialized reproductive structures on the upright filaments (Müller, 1967; Charrier et al., 2008).
Fig. 4.
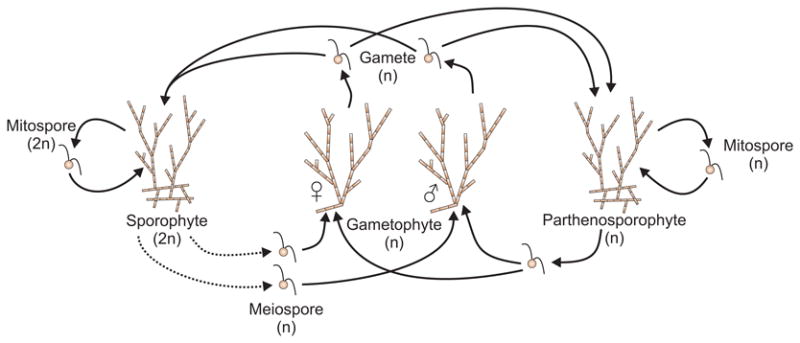
Life cycle of the brown alga Ectocarpus siliculosus. Filamentous diploid sporophytes produce mitospores that develop into new sporophytes and haploid meiospores that develop into gametophytes. Gametophytes produce either male or female gametes, which can fuse to produce a sporophyte or develop mitotically into a haploid parthenosporophyte. Parthenosporophytes produce mitospores that develop into new parthenosporophytes and spores that develop into gametophytes. Diploid sporophytes and parthenosporophytes are morphologically indistinguishable, but both share a branched prostrate structure absent from the gametophyte (Müller, 1967; Charrier et al., 2008). Dashed arrows indicate meiosis. Adapted from Charrier et al. (2008).
In E. siliculosus, both the sporophyte and the gametophyte can reproduce asexually, leading to difficulties similar to those in the algae previously reviewed. As in these other cases, a thallus may be bounded, discrete, and contiguous, while a group of thalli (the genet) is genetically unique and homogeneous.
IV. Amoebozoa
(1) Social amoebae: dispersal through aggregation and cooperation
The delicate and varied fruiting bodies formed by cellular slime molds have long captured the attention of those interested in multicellular development. Cellular slime molds are also known as social amoebae or Dictyostelia, and they are a clade within the primarily unicellular supergroup Amoebozoa (Baldauf & Doolittle, 1997; Baldauf, 2008). Unlike most multicellular organisms, a slime mold fruiting body develops from the aggregation and differentiation of previously independent cells.
Close relatives of the cellular slime molds (Dictyostelia) include plasmodial slime molds (Myxogastria) and Protostelia, all of which have fruiting bodies with a cellulosic stalk supporting one or more spores (Baldauf & Doolittle, 1997; Baldauf et al., 2000; Olive, 1975). Protostelid species are characterized by fruiting bodies with a single (or very few) spores (Olive & Stoianovitch, 1960) and are thought to be important “intermediates” between species that do not form fruiting bodies and those with multicellular fruiting bodies. Whereas Dictyostelids form fruiting bodies with differentiated cells, Myxogastrids form large, multinucleated plasmodia, which break up to form undifferentiated multi-celled fruiting bodies (Glöckner et al., 2008; Marwan, Sujatha & Starostzik, 2005; Olive, 1975). Cellular slime molds with and without cellular differentiation are phylogenetically interspersed, so it is not clear whether cellular differentiation arose multiple times or only once, with subsequent losses (Schaap et al., 2006; Shadwick et al., 2009; Fiore-Donno et al., 2010).
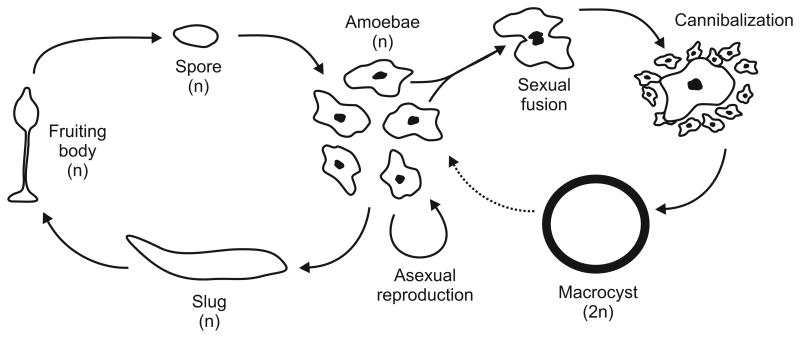
The life cycle of the most-studied social amoeba species, Dictyostelium discoideum, is described in Fig. 5. Multicellular development is triggered by starvation, which induces amoeboid cells to aggregate and form a motile “slug.” The slug develops into a fruiting body, often after migrating to a suitable location. Most of the cells in the slug form spores at the top of the fruiting body, but a subset are incorporated into the stalk. Since the stalk cells die without reproducing, the spore-stalk cell type distinction is a form of germ-soma differentiation. Dictyostelium discoideum can also undergo a sexual cycle, although this has not been extensively studied.
Fig. 5.
Life cycle of the social amoeba Dictyostelium discoideum. Haploid unicellular amoebae emerge from spores, feed independently on bacteria in the soil and leaf litter, and reproduce by binary fission. As food supplies dwindle, multicellular development begins, with independent cells aggregating in response to secreted pulses of cyclic AMP (Kessin, 2001; Konijn et al., 1967). The aggregated cells form a mound of up to 105 cells, and the aggregated cells sometimes migrate as a “slug” (Shaulsky & Kessin, 2007). Ultimately, the aggregate develops into a fruiting body, with about 20% of the cells forming the support structure (stalk) and the remainder forming a mass of spores atop the stalk. The fruiting body places the thick-walled spores in a favourable position for being dispersed by arthropods or annelids (Bonner, 2008). Once the spores encounter good conditions, unicellular haploid amoebae hatch and again begin the independent trophic phase of the asexual life cycle. Three genetically determined mating types are known (Erdos, Raper & Vogen, 1973; Bloomfield et al., 2010), and haploid amoebae of different mating types may fuse to from a diploid zygote or macrocyst (Blaskovics & Raper, 1957; Nickerson & Raper, 1973; Saga, Okada & Yanagisawa, 1983). The macrocyst attracts and cannibalizes other haploid amoebae, which contribute to the formation of a resistant cellulose wall (O'Day, 1979; Filosa & Dengler, 1972). After a period of dormancy, the macrocyst germinates meoitically to produce haploid amoebae (Filosa & Dengler, 1972; Wallace & Raper, 1979). Dashed arrow indicates meiosis. Adapted from Strassmann & Queller (2011a).
Multicellular development by aggregation allows for the possibility of chimeric (genetically heterogeneous) multicellular bodies. In nature, D. discoideum fruiting bodies are often clonal or nearly clonal, and in the laboratory, they demonstrate the ability to recognize and aggregate preferentially with kin (Ostrowski et al., 2008; Flowers et al., 2010; Strassmann & Queller, 2011b). Kin discrimination may allow for the avoidance of much conflict during development in modern-day Dictyostelia. Nevertheless, there are indications that conflict among cells may have been important during the evolution of developmental mechanisms. Clones that over-represent themselves in the spore population of chimeras have been identified, and the functionality (mobility) of chimeric slugs is less than that of clonal slugs of the same size (Foster et al., 2002; Strassmann & Queller, 2011b). Differentiation into spore rather than stalk may be a competitive process, with the losing cells making the most of their bad situation by forming the stalk (Castillo, Queller & Strassmann, 2011).
Like Volvox carteri, Dictyostelium discoideum is a frequent subject in discussions of individuality. In both the unicellular and the multicellular phases of the D. discoideum life cycle, different units possess the various criteria proposed for individuality. In the unicellular phase, the mitotic descendants of one product of meiosis may be genetically unique and homogeneous, while contiguity, physiological discreteness and functional integration occur in the single cells. The multicellular slug may be genetically homogeneous (or not) and genetically unique (or not) depending on the structure of the population of amoebae. Unless the population of amoebae is highly structured, the resulting slug will be genetically unique but not genetically homogeneous. The slug meets the criteria of contiguity, boundedness, discreteness, autonomy, and functional integration, and it develops into a fruiting body with germ-soma differentiation. Depending on the genetic composition, the realized levels of cooperation, conflict, and internal policing may vary.
Dictyostelium discoideum figures prominently in the discussions of Folse & Roughgarden (2010) and, not surprisingly, Queller & Strassmann (2009; Strassmann & Queller, 2010). In spite of its usual genetic heterogeneity and lack of a single-cell bottleneck, Queller & Strassmann (2009; Strassmann & Queller, 2010) consider the D. discoideum slug an individual, emphasizing the normally low levels of realized conflict among cells. Folse & Roughgarden (2010) reach the opposite conclusion, acknowledging D. discoideum's functional integration and germ-soma specialization but ultimately deciding that the conflict implied by genetic heterogeneity means that a D. discoideum slug is better interpreted as a social group. Godfrey-Smith (2009) (2009, Fig. 5.1) assigns D. discoideum an intermediate degree of individuality, intermediate in terms of germ-soma specialization and functional integration but with no bottleneck.
V. Alveolata
(1) Ciliates (Ciliophora): aggregative and clonal development
Ciliates (phylum Ciliophora) are heterotrophs that may be found in virtually any aquatic environment. Two distinct forms of multicellularity have evolved in this group. In the peritrich ciliates (class Oligohymenophorea: subclass Peritrichia), two genera (Zoothamnium and Apocarchesium) form branching colonies with multiple cell types. The distantly related species Sorogena stoianovitchae (class: Colpodea) follows a life cycle similar to the cellular slime molds; indeed, it was initially thought to be related to this group (Olive, 1975).
(a) Peritrich ciliates
Colonial peritrichs are filter feeders that form colonies reminiscent of arborescent bryozoans, with inverted bell-shaped cells (zooids) connected to a substratum by a stalk. Colonies grow by binary fission of zooids, after which one or both daughter cells secretes its own stalk, creating the branching pattern observed in colonies. A bundle of contractile filaments that runs through the stalk, the spasmoneme, can contract, causing the stalk to coil, pulling the zooid away from a potential threat. In Zoothamnium, the spasmonemes of all cells are continuous throughout the colony, so that the entire colony may contract if one zooid is touched. In the other colonial peritrichs, the spasmonemes of zooids are not continuous, so that individual zooids retract independently.
In peritrich species with cellular differentiation, zooids are differentiated into actively dividing terminal zooids, larger macrozooids that produce dispersing cells, and smaller microzooids specialized for feeding (Ji & Song, 2004; Li et al., 2008; Ji & Kusuoka, 2009; Norf & Foissner, 2010). Colonies are founded by telotrochs, motile cells that do not feed but are able to redifferentiate into feeding zooids shortly after attaching to a suitable site (Viljoen & As, 1987). Cellular differentiation is present in several species of Zoothamnium and two species of Apocarchesium (Ji & Song, 2004; Li et al., 2008; Ji & Kusuoka, 2009; Norf & Foissner, 2010). Cellular differentiation may have evolved once in peritrich ciliates and been lost subsequently in some lineages, or it may have evolved separately in Zoothamnium and in Apocarchesium (Li et al., 2008; Sun et al., 2011).
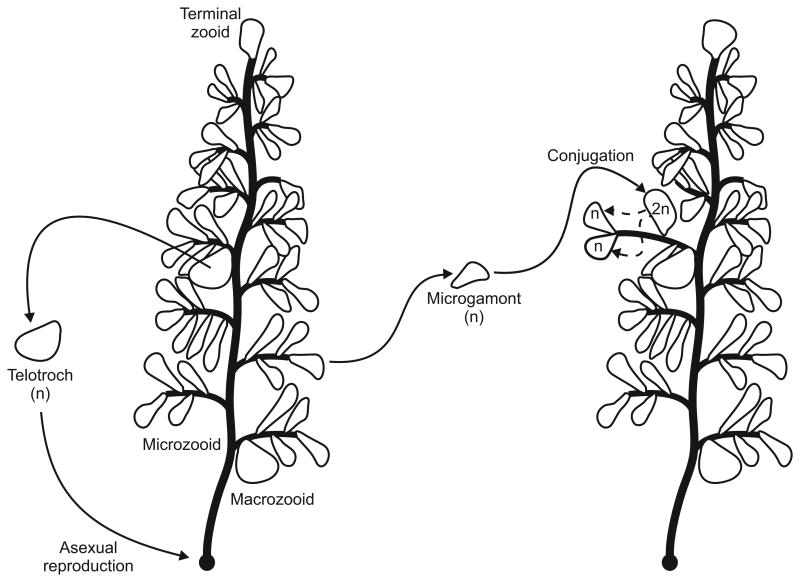
The largest species of Zoothamnium, Z. niveum, forms feather-like colonies up to 1.5 cm long, with a central stalk and alternating branches (Rinke et al., 2006, Bauer-Nebelsick, Bardele & Ott, 1996b). The Z. niveum life cycle is described in Fig. 6. Colonies of Z. niveum are always found in association with a bacterial ectosymbiont, a single species of sulphide-oxidizing gammaproteobacteria (Bauer-Nebelsick, Bardele & Ott, 1996a,b; Rinke et al., 2006). The symbiotic association is apparently obligate, as Z. niveum cannot survive without the bacteria (Bauer-Nebelsick et al., 1996b). The bacteria cover the colony in a monolayer and are morphologically differentiated over the surface of the colony, rod-shaped on the macrozooids, terminal zooids and branches and more coccoid on the oral side of microzooids (Bauer-Nebelsick et al., 1996a).
Fig. 6.
Life cycle of the peritrich ciliate Zoothamnium niveum. Multicellular development begins when a haploid disperser, or telotroch, settles and attaches to a substratum and then begins to secrete a stalk (Summers, 1938b). The first cell division is asymmetric, resulting in a larger product that becomes the apical zooid and a smaller product that becomes the terminal zooid of the first branch (Fauré-Fremiet, 1930; Summers, 1938b). Each division of the apical cell produces an apical zooid and a new branch, while branches grow by continued divisions of their respective terminal zooids (Summers, 1938b). Subapical zooids cease dividing and become feeding microzooids, some of which differentiate into macrozooids and disperse as telotrochs (Summers, 1938b). Actively dividing zooids sometimes differentiate into “microgamonts,” free-swimming cells capable of conjugation, the sexual process in ciliates (Summers, 1938a,b). Gamonts swim to another colony and fuse with one of the actively dividing cells, which resumes division and normal development after a delay of several days (Summers, 1938a). Dashed arrow indicates meiosis. Some details of the Z. niveum life cycle are not available, so this account is partly based on Z. alternans, which is morphologically similar (but smaller) and closely related (Clamp & Williams, 2006). Adapted from Rinke et al. (2007).
The symbiosis between Z. niveum and its associated bacteria creates difficulties similar to those in Ulva mutabilis and Porphyra yezoensis. Zoothamnium niveum takes the relationship to a new level, though, in two ways. First, the requirement of bacterial symbionts for Z. niveum to survive at all further raises the question of whether or not the eukaryotic portion of a colony can be considered an individual at all by the criteria of functional integration and autonomy. In addition, the morphological differentiation of the bacteria across the surface of a Z. niveum colony suggests a high degree of functional integration not only for the ciliate but for the bacteria as well.
Aside from issues related to the bacterial symbionts, Zoothamnium niveum presents other problems we have already seen. The mixture of sexual and asexual reproduction means that the contiguous and autonomous units (the colonies) are not always genetically unique. Finally, conjugation can cause the descendant cells (zooids) to differ genetically from others in the colony so that colonies do not meet the criterion of genetic homogeneity (Summers, 1938a,b).
(b) Sorogena
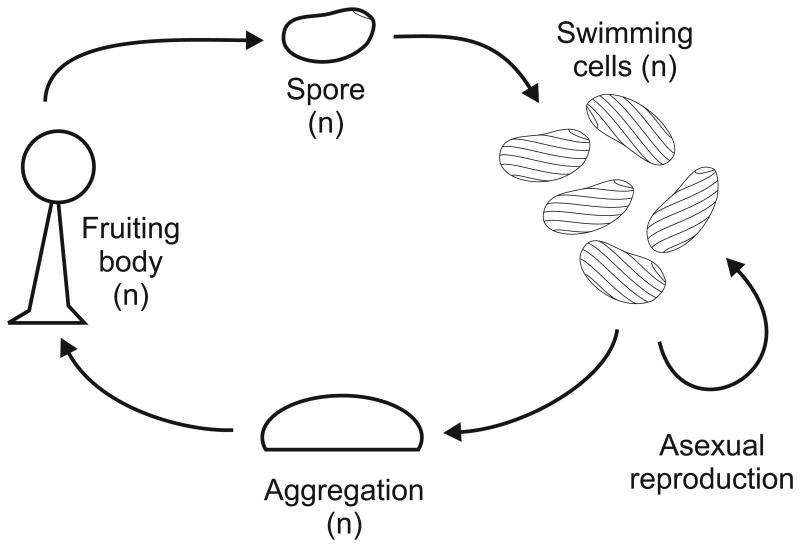
The life cycle of the bacteriovorus S. stoianovitchae (described in Fig. 7) is similar to that of the cellular slime molds, with free-living cells aggregating into a fruiting body, or sorocarp. Sex has not been observed in Sorogena stoianovitchae, and in fact only one of the approximately 200 species of Colpodea, the class that includes Sorogena, has been shown to have sex (Dunthorn & Katz, 2010).
Fig. 7.
Life cycle of the sorocarp-forming ciliate Sorogena stoianovichae. Free-swimming haploid cells reproduce asexually by mitosis. Upon starvation, free-living S. stoianovitchae cells begin to aggregate beneath the surface of the water during continuous dark. Subsequently, apparently due to light stimulation, the aggregate becomes more compact and cell-to-cell adhesion occurs. Cells produce a mucoid matrix, and the aggregate begins to rise as the sheath material absorbs water and expands upwards. Cells in the fruiting body, or sorocarp, undergo encystment as sheath elongation ceases, thus completing development (Sugimoto & Endoh, 2006). No sexual cycle is known. Adapted from Sugimoto & Endoh (2008).
Sorocarp formation appears to be a recent innovation in S. stoianovitchae, as this species is very closely related to Platyophrya vorax, an asocial predatory ciliate (Lasek-Nesselquist & Katz, 2001). Comparatively little is known about the relationship between ancestral traits and the adaptations that were required for sorocarp formation. It is likely that pathways involved in encystment under environmental stress in other members of class Colpodea have been co-opted for novel function, for example in the formation of the stalk material (Sugimoto & Endoh, 2008).
In Sorogena stoianovitchae sorocarps, stalk formation occurs through the secretion of an extracellular, mucoid matrix that expands as it absorbs water. While sorocarp formation does not require ‘stalk cells’ (as in Dictyostelium discoideum and the prokaryote Myxococcus xanthus), a subpopulation of cells may remain at the root of the stalk, presumably resulting in zero direct fitness (Olive, 1978; Sugimoto & Endoh, 2008). The rest of the cells undergo encystment in preparation for dispersal (Olive, 1978). It is not clear whether the cells that remain in the base constitute a separate cell type or an arbitrary subpopulation.
The problems with Sorogena stoianovitchae individuality are similar to those of the other aggregative developer reviewed here, Dictyostelium discoideum. As in the social amoebae, many free-living ciliates may share a genotype, meaning that the genetic criteria will identify a group of cells as the individual while physical and physiological criteria indicate that the cells are individuals in their own right. Further, the genetic structure of the fruiting bodies depends on the population structure of the free-living cells, so that criteria such as genetic uniqueness and homogeneity become contingent on environmental conditions.
VI. Discussion
The question of what constitutes an individual or organism has been dismissed on the grounds that “…the most important questions about organisms do not depend on this concept.” (Wilson, 2000, p. S301). Leaving aside the issue of which biological questions are the most important, we agree that many such questions can be addressed without explicitly defining the individual. There are, however, some important biological questions for which it is important to think carefully about what we mean by individuality. For example, we might ask what features are common to the emergence of new kinds of individuals across taxa and at different levels of biological organization (Maynard Smith & Szathmáry, 1997; Michod & Roze, 1997). If so, we must know which cases to include, requiring us to be clear about when such a transition has occurred. For instance, whether or not we should include the evolution of sex depends on whether the mated pair is a group of individuals or an individual in its own right (Michod, 2011). Similarly, if we wish to assess the claim that group selection is common in nature (e.g. Eldakar & Wilson, 2011), we must distinguish whether the putative targets of selection are groups of individuals or individuals in their own right. As a final example, describing and explaining how various biological processes scale with body size also requires consideration of the relevant “individual body” across diverse forms of life (West, Brown & Enquist, 1997; DeLong et al., 2010; Agutter & Tuszynski, 2011). Far from being questions that “only philosophers love” (Queller & Strassmann, 2009, p. 3143), these are topics of considerable interest to, and active research among, biologists.
Having presented several concrete examples, it is worth taking a step back to consider the problem of individuality in the abstract. What conceptual work should we ask of a properly constructed definition of an individual organism? In other words, once we have settled on a set of criteria and judged whether these are met by a particular biological unit, what other questions should automatically be answered? The answer may depend on the specific biological question, though some general uses of the concept of an “individual” can be identified. First, a definition of “individual” can be used to distinguish organisms from parts of organisms and from groups of organisms (Pepper & Herron, 2008). Second, it can be used unambiguously to distinguish growth from reproduction (Godfrey-Smith, 2009). Finally, it can be used to identify the relevant units to count in studies of population biology in order to measure fitness (Clarke, 2010). Clearly these are not orthogonal; they may in fact be different ways of saying the same thing. If we can distinguish wholes from parts and from groups, we will know what to count in studies of population biology, and will we have no trouble recognizing when reproduction has taken place. On the other hand, it is not necessarily the case that one definition of “individual” suffices equally well for each of these tasks, particularly as they relate to a specific biological question. A set of criteria that is useful for distinguishing parts from whole organisms in, for example, a study of metabolic scaling may prove to be different from criteria that are helpful in determining the extent to which those same units are fitness-bearing. In principle, at least, it is possible to have legitimate but incompatible notions of “individuality” for different types of biological questions.
We did not set out to cherry-pick bizarre and pathological examples, but only to select the best-studied representative of each of the taxonomic groups we reviewed. Nevertheless, each of the model species on which we have focused reveals one or more limitations of the paradigmatic conception of an individual organism. In each of these cases, different biological units meet some but not all of the traditional criteria listed in the introduction. By different criteria, then, the same unit (e.g. a particular multicellular body or thallus) can qualify as a part of an organism, an organism in its own right, or a group of organisms.
(1) Limitations of the traditional criteria
The alternation of generations that occurs in most life cycles poses problems for several concepts of individuality. The view of an individual as the mitotic descendants of a fertilized zygote runs into problems when not all of the phases of a life cycle are derived mitotically from a fertilized zygote. In Volvox carteri, for example, there are no mitotic descendants of a diploid zygote (mitosis only occurs in the haploid phase). In Ectocarpus siliculosus, only the sporophyte develops mitotically from a zygote, yet there seems to be no justification for considering the sporophyte but not the gametophyte an individual organism simply because the gametophyte develops from a haploid propagule. Even in animals, the zygote view is only tenable because the haploid phase of the life cycle is unicellular, and as a result we do not usually think of animals as having an alternation of haploid and diploid generations (e.g. we rarely refer to human gametes as “haploid people”). In fact, this situation merely represents one end of a continuum that ranges from haploid and diploid phases being fully isomorphic as in Ulva mutabilis, to “slightly heteromorphic” as in Ectocarpus siliculosus, to dramatically heteromorphic as in Porphyra yezoensis, to the haploid (animals) or diploid (Volvox carteri, Dictyostelium discoideum) phase being reduced to a single cell.
As has been recognized for clonally reproducing plants and animals, a life cycle that includes asexual reproduction will produce contiguous, autonomous or semi-autonomous units (ramets; Stout, 1929) that are different from the genetically unique units (genets; Sarukhán & Harper, 1973). Thus, although Volvox carteri spheroids, asexually produced seaweed thalli, and Zoothamnium niveum colonies are physiologically and functionally integrated and develop from a single cell, they will not usually be genetically unique.
Conversely, several of the multicellular units are not (or not always) genetically homogeneous. The species with aggregative development (Dictyostelium discoideum, Sorogena stoianovitchae) will only be genetically homogeneous when there is a high level of spatial genetic structure or if they have especially strong kin-recognition mechanisms. Even among the clonal developers, though, two or more genotypes are often present in the same multicellular body or thallus. The cases of Ulva mutabilis, Porphyra yezoensis, and Zoothamnium niveum show that even a single-cell bottleneck is not always enough to guarantee genetic homogeneity.
Functional specialization of lower-level units is often taken as a hallmark of individuality at the higher level. For multicellular organisms, this requirement is often specified as a division of labour between reproductive and somatic cells (e.g. Buss, 1983, 1987; Michod, 2003; Michod & Nedelcu, 2003; Solari, Nedelcu & Michod, 2004). Such differentiation is considered a prerequisite for complex multicellular forms, because without cell specialization in distinct components of fitness (reproduction and survival), conflicts among cells destroy the integrity of the group (e.g. Michod, 2003; Solari et al., 2004). As with the single-cell bottleneck, though, the examples of Ulva mutabilis and Porphyra yezoensis show that a germ-soma division of labour is not always sufficient to prevent genetic heterogeneity, and thus it may not be sufficient to prevent conflicts among cells.
(2) Continuous variation
Our discussion to this point has assumed that the criteria under consideration are dichotomous, but intermediate cases exist for all of them. This raises the question of what degree of a particular criterion is required in order to consider something an individual organism.
Perfect genetic homogeneity, for example, is probably only ever met in very small multicellular organisms (Otto & Hastings, 1998; Pineda-Krch & Lehtilä, 2004). The cells of larger organisms will inevitably diverge due to mutation during growth and development, and this divergence “becomes steadily greater to the extent that life is long” (Godfrey-Smith, 2009, p. 76). Ulva mutabilis, Porphyra yezoensis, Zoothamnium niveum, Sorogena stoianovitchae and Dictyostelium discoideum provide further examples for which the physically contiguous units (thalli of the seaweeds, fruiting bodies of the aggregates) can occupy various positions along this continuum. Similarly, unicellular propagules occupy one end of a near continuum of possible propagule sizes (Roze & Michod, 2001; Godfrey-Smith, 2009). Physiological integration can vary across a broad range (Tuomi & Vuorisalo, 1989; Pepper & Herron, 2008). Even germ-soma differentiation has intermediate stages, as in some species of the Volvox relative Eudorina in which a subset of the cells act as soma under some environmental conditions but as reproductive cells in others (Nozaki et al., 1989).
(3) Symbioses
In some of the taxa reviewed here, a further complication arises as the result of some very tightly integrated symbioses. In these cases, the concept of individuality is perhaps better understood within the broader concept of the ‘holobiont.’ This term refers to a community of symbionts, for example, the coral animal, its endosymbiotic algae (zooxanthellae), and the associated biofilm of bacteria (Rohwer et al., 2002). Many species may fit this description to varying degrees, as symbioses are common and vary from loose affiliations to intimate, obligate relationships.
The holobiont concept seems particularly apt for Ulva mutabilis and Porphyra yezoensis, both of which form obligate associations with ectosymbiotic bacteria. The symbionts are so integrated into the development of these algae that without them the algae are incapable of normal development and form only undifferentiated clumps. In Zoothamnium niveum, not only is the symbiotic association with bacteria obligate, but the bacterial cells visibly differentiate in a spatially predictable pattern, introducing a new type of cellular differentiation if we consider the bacteria to be part of the holobiont. The view of individuals as autonomous units probably requires considering bacteria to be part of the holobiont, since the eukaryotic portion of the holobiont cannot survive (Zoothamnium niveum), at least in its normal form (Ulva mutabilis, Porphyra yezoensis), without the prokaryotic portion.
In the cases of Ulva mutabilis and Porphyra yezoensis, the relationship with symbiotic bacteria affects a trait that all of the recent syntheses agree is important for individuality, the division of labour between reproductive and somatic functions. In the case of Zoothamnium niveum, the affected trait is life itself, without which all discussions of biological individuality are moot. Nevertheless, a critic of the holobiont view might argue that the symbiotic bacteria in each case are merely a part of the external environment and that these cases are no different in principle from (for example) the requirement of a trace element for normal development. The crucial difference is that the external environmental factor in these symbioses has the capacity for adaptive evolution including coevolution when fitness interests are, to some degree, tied to those of the other partner.
(4) Recent syntheses
The attempts by Queller & Strassmann (2009; Strassmann & Queller, 2010), Folse & Roughgarden (2010), and Godfrey-Smith (2009) to define individuals in evolutionary terms share the strength that they are not defined in reference to any particular taxon. As McShea (1996) points out in reference to complexity, a definition that is derived by listing the traits of a particular taxonomic group can yield only trivial answers about how those traits correlate with others. Only by defining individuality in a taxon-independent way can we ask meaningful questions about how it evolves. Furthermore, all of the aforementioned authors recognize that the relevant criteria can vary continuously. Something like this is necessary for studying ETIs, where it is more useful to imagine a lineage gradually increasing in individuality than to try to draw a line above which the groups in question do, and below which they do not, qualify as individuals.
The approach of Godfrey-Smith (2009) is particularly relevant for understanding the emergence of new kinds of individuals. Since the necessary conditions for natural selection can occur at more than one level, this criterion does not always identify a particular level as that of the individual. In fact, the possibility of partial degrees of individuality coexisting at different levels is a central theme of Godfrey-Smith's (2009) book. Clarke (2012) proposes that we embrace this conclusion as a messy consequence of biological reality. Many useful categories have fuzzy boundaries, and “individual” may be an example. Along similar lines, Michod (2011, p.186) sees the volvocine algae in the genus Gonium as “partially integrated units of evolution and adaptation” that are “partway but not yet fully emerged as an evolutionary individual”. In Michod's view, evolutionary individuals meet Lewontin's (1970) conditions but also “possess properties that restrict within-group selection and enhance between group selection” (Michod, 2011, p. 184).
The set of criteria proposed by Folse & Roughgarden (2010) – alignment of fitness interests, germ-soma differentiation and functional integration – seems tractable and unambiguous when applied to the ‘fraternal’ transitions: multicellularity, eusociality, and the like (Queller, 1997). Yet it provides little guidance for the ‘egalitarian’ transitions, such as the endosymbiotic events that led to mitochondria and chloroplasts (Queller, 1997). Egalitarian transitions do not involve a reproductive division of labour, and so these criteria say nothing about, for example, when an endosymbiont and its host become one organism. In our examples, this shortcoming is most obvious in the cases of intimate bacterial symbioses: Ulva mutabilis, Porphyra yezoensis, and Zoothamnium niveum.
The criteria of Queller & Strassmann (2009; Strassmann & Queller, 2010) do appear to be universally applicable, although they are arguably more difficult to measure. Queller & Strassmann (2009; Strassmann & Queller, 2010) identify a single level of organization as organismal (for any particular case) by specifying that it is the highest level that meets their criteria. By contrast, Godfrey-Smith (2009) allows for multiple levels to share partial degrees of individuality. The view of Queller & Strassmann (2009; Strassmann & Queller, 2010) thus unambiguously distinguishes wholes from parts and groups, while Godfrey-Smith's (2009) view lends itself well to scenarios involving multilevel selection. Both of these views are also contingent on extrinsic factors: cooperation, conflict, and heritable variation in fitness may all be affected by the abiotic environment as well as by interactions with con- and heterospecifics. This may lead to the counterintuitive result that the status of a given biological unit as a part, whole or group (or its degree of individuality) can change over short time spans without any evolutionary change on the part of the unit in question.
(5) Implications
Although the taxa we have reviewed are phylogenetically diverse, our focus has in some sense been quite restricted. We selected all of our case studies from what should arguably have been the easiest set: multicellular eukaryotes with cellular differentiation. In so doing, we have neglected many potentially problematic cases, such as intracellular endosymbionts and organelles, colonial invertebrates, eusocial animals, facultatively sexual unicells, and coenocytic organisms. We neglected prokaryotes altogether, including those with cellular differentiation (such as some filamentous cyanobacteria). We also did not consider borderline cases between life and nonlife. Even within this restricted set of examples, we found myriad complications restricting the applicability of the most common criteria for individuality. Some of these difficulties only become obvious in light of detailed life histories, and so it seems likely that entirely new complications would arise in a similar investigation focusing on some of the cases that we have not considered (or that have yet to be discovered).
Clearly criteria that are designed for a particular set of organisms do not always translate well to other groups (Hutchings & Booth, 2004). This is not just a problem of fuzzy boundaries or of conflicting answers, but that some criteria fail to provide an answer for some groups. For example, the genet view developed for plants, of an individual as the mitotic descendants of a zygote, is not relevant for taxa in which the zygote never undergoes mitosis or in which there is no zygote at all. In our putative holobionts (Porphyra yezoensis, Ulva mutabilis, and Zoothamnium niveum) the criterion of spatial boundedness gives no guidance, since the multicellular unit is spatially bounded with or without the ectosymbiotic bacteria. In both cases the problem is not that the proposed criteria give the wrong answer, but rather that they give no answer at all, providing no guidance in distinguishing organisms from parts or from groups. These failures demonstrate the futility of basing our concept of individuality on one or a few taxa. Any such concept will inevitably work for only a subset of life's diversity.
Pepper & Herron (2008) raised the question of why all of the various criteria that characterize unitary or paradigm organisms so often coincide in the same biological units (the “organism syndrome”). On the contrary, when the full diversity of life is considered, such a situation turns out to be quite rare (although it may be worthwhile asking why these criteria ever coincide). Arguably only a subset of one branch of the Opisthokonts (the Metazoans) meets all of the proposed criteria (Buss, 1987). The impression that unitary organisms are common is an artifact of our (understandable) obsession with our own phylum (Buss, 1987). This argues strongly in favour of recognizing intermediate degrees of individuality in evolutionary studies. If this investigation has shown us anything, it is that the line between parts and wholes is often blurry. Elements of individuality are often partitioned among different biological units within a given species.
The ubiquity of intermediate levels of organization also has implications for the suggestion that extant taxa displaying some but not all of the symptoms of the organism syndrome may be partway through an ETI (Pepper & Herron, 2008). Although precise dates are not often available, it is clear that most of the groups reviewed here have been in these “intermediate” states for long periods of time. The brown algae (Berney & Pawlowski, 2006; Brown & Sorhannus, 2010) and the volvocine green algae (Herron et al., 2009) each diverged from unicellular ancestors on the order of 200 MYA. At least one lineage of ulvophyte green algae had cellular specialization by 700-800 MYA (Butterfield, Knoll & Swett, 1988), and the corresponding date in the red algae is 1200 MYA (Butterfield, Knoll & Swett, 1990). Thus, if it is true that these groups are in the middle of a transition in individuality, it is a very slow transition. It is not necessarily true that everything that is ‘partway through’ a transition will eventually emerge as a unitary individual. Intermediate levels of organization, with some but not all of the traits of organisms, appear to be quite stable over evolutionary time. Folse & Roughgarden (2010) point this out in reference to a partial case of germ-soma differentiation (Ayre & Grosberg, 2005), but the same logic could be applied to nearly any criterion or set of criteria.
Biologists looking for broad-scale commonalities across a wide array of life forms would do well to consider carefully the question of biological individuality. Without an explicit concept of individuality, our basis for recognizing and applying the general principles that underpin biological diversity is limited. This survey of “minor” multicellular taxa has highlighted the difficulty of applying traditional individuality criteria to diverse life cycles and ecologies across the tree of life. The examples described here provide test cases against which candidate criteria can be measured, and a truly universal concept of individuality should be able to handle all of these cases along with others we have not considered. The more recent concepts of individuality we have reviewed have implications, such as that individuality comes in degrees and may be contingent on environmental conditions, that conflict with our intuitions. Only such taxon-independent concepts, though, have the potential to provide meaningful answers to certain types of biological questions. The conflict may be an indication not of any systemic problem with the concepts themselves but rather of intuitions based on the minority of life's diversity that makes up the majority of our day-to-day experience.
VII. Conclusions
The question of what constitutes an individual is not merely semantic but has real consequences for some biological questions.
Most proposed criteria for individuality were identified with vertebrates in mind, and these criteria often give conflicting answers when applied to other taxa.
For the majority of life on Earth, the various proposed criteria for individuality do not coincide in the same biological units.
Some taxa have intimate and obligate symbioses with bacteria, which may require extending the concept of individuality to the holobiont (the symbiotic community).
Several taxon-independent concepts of individuality have recently been proposed, and these avoid some of the failings of previously proposed criteria.
Intermediate states exist for all of the proposed criteria for individuality, and so fuzzy boundaries may be unavoidable.
Some taxa possessing some but not all of the proposed criteria for individuality are ancient, and such examples should not be assumed to be “on their way” to becoming full individuals.
Understanding the emergence of new kinds of individuals requires the recognition that individuality is a matter of degree.
Although recently devised, taxon-independent concepts of individuality have counterintuitive implications (e.g. contingency, partial degrees of individuality), this may be less a problem with these approaches than a mismatch between our intuitions and the reality of biological diversity.
Acknowledgments
We thank Ellen Clarke, Chandni Kher, Richard Michod, Aurora Nedelcu, Akira Peters, and Will Ratcliff for comments on early drafts of the manuscript. This work was supported by NSF IOS-1010669 and NIH 3T32GM084905-01A1S1 to W.W.D. M.D.H. was supported by a NASA Astrobiology Institute Postdoctoral Fellowship.
References
- Agutter PS, Tuszynski JA. Analytic theories of allometric scaling. Journal of Experimental Biology. 2011;214:1055–1062. doi: 10.1242/jeb.054502. [DOI] [PubMed] [Google Scholar]
- Ariew A, Lewontin RC. The confusions of fitness. British Journal for the Philosopy of Science. 2004;55:347–363. [Google Scholar]
- Ayre DJ, Grosberg RK. Behind anemone lines: factors affecting division of labour in the social cnidarian Anthopleura elegantissima. Animal Behaviour. 2005;70:97–110. [Google Scholar]
- Baldauf SL. The deep roots of eukaryotes. Science. 2003;300:1703–1706. doi: 10.1126/science.1085544. [DOI] [PubMed] [Google Scholar]
- Baldauf SL. An overview of the phylogeny and diversity of eukaryotes. Journal of Systematic Evolution. 2008;46:263–273. [Google Scholar]
- Baldauf SL, Doolittle WF. Origin and evolution of the slime molds (Mycetozoa) Proceedings of the National Academy of Sciences of the United States of America. 1997;94:12007–12012. doi: 10.1073/pnas.94.22.12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldauf SL, Roger AJ, Wenk-Siefert I, Doolittle WF. A kingdom-level phylogeny of eukaryotes based on combined protein data. Science. 2000;290:972–977. doi: 10.1126/science.290.5493.972. [DOI] [PubMed] [Google Scholar]
- Bauer-Nebelsick M, Bardele CF, Ott JA. Electron microscopic studies on Zoothamnium niveum (Hemprich & Ehrenberg, 1831) Ehrenberg 1838 (Oligohymenophora, Peritrichida), a ciliate with ectosymbiotic, chemoautotrophic bacteria. European Journal of Protistology. 1996a;32:202–215. [Google Scholar]
- Bauer-Nebelsick M, Bardele CF, Ott JA. Redescription of Zoothamnium niveum (Hemprich & Ehrenberg, 1831) Ehrenberg, 1838 (Oligohymenophora, Peritrichida), a ciliate with ectosymbiotic, chemoautotrophic bacteria. European Journal of Protistology. 1996b;32:18–30. [Google Scholar]
- Berbee ML, Taylor JW. Rhynie chert: a window into a lost world of complex plant-fungus interactions. The New Phytologist. 2007;174:475–479. doi: 10.1111/j.1469-8137.2007.02080.x. [DOI] [PubMed] [Google Scholar]
- Berney C, Pawlowski J. A molecular time-scale for eukaryote evolution recalibrated with the continuous microfossil record. Proceedings of the Royal Society of London B. 2006;273:1867–1872. doi: 10.1098/rspb.2006.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya D, Yoon HS, Hedges SB, Hackett JD. The Tme Tree of Life. Oxford University Press; New York: 2009. Eukaryotes (Eukaryota). In Hedges, SB and Kumar, S (eds) pp. 116–120. [Google Scholar]
- Blaskovics JC, Raper KB. Encystment stages of Dictyostelium. Biological Bulletin. 1957;113:58–88. [Google Scholar]
- Bloomfield G, Skelton J, Ivens A, Tanaka Y, Kay RR. Sex determination in the social amoeba Dictyostelium discoideum. Science. 2010;330:1533–1536. doi: 10.1126/science.1197423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bold HC, Wynne MJ. Introduction to the Algae, Second Edition. Prentice-Hall, Inc.; Englewood Cliffs, NJ: 1985. [Google Scholar]
- Bonner JT. The origins of multicellularity. Integrative Biology. 1998;1:27–36. [Google Scholar]
- Bonner JT. The Social Amoebae: The Biology of Cellular Slime Molds. Princeton University Press; Princeton, NJ: 2008. [Google Scholar]
- Bremer K, Humphries CJ, Mishler BD, Churchill SP. On cladistic relationships in green plants. Taxon. 1987;36:339–349. [Google Scholar]
- Brown JW, Sorhannus U. A molecular genetic timescale for the diversification of autotrophic stramenopiles (Ochrophyta): substantive underestimation of putative fossil ages. PLoS One. 2010;5:e12759. doi: 10.1371/journal.pone.0012759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger G, Saint-Louis D, Gray MW, Lang BF. Complete sequence of the mitochondrial DNA of the red alga Porphyra purpurea: cyanobacterial introns and shared ancestry of red and green algae. The Plant Cell. 1999;11:1675–1694. doi: 10.1105/tpc.11.9.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnet FM. Self and Not-Self: Cellular Immunology. Cambridge University Press; Cambridge, UK: 1969. [Google Scholar]
- Buss LW. Evolution, development, and the units of selection. Proceedings of the National Academy of Sciences of the United States of America. 1983;80:1387–1391. doi: 10.1073/pnas.80.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss LW. The Evolution of Individuality. Princeton: Princeton University Press; 1987. [Google Scholar]
- Butterfield NJ, Knoll AH, Swett K. Exceptional preservation of fossils in an Upper Proterozoic shale. Nature. 1988;334:424–427. doi: 10.1038/334424a0. [DOI] [PubMed] [Google Scholar]
- Butterfield NJ, Knoll AH, Swett K. A bangiophyte red alga from the Proterozoic of arctic Canada. Science. 1990;250:104–107. doi: 10.1126/science.11538072. [DOI] [PubMed] [Google Scholar]
- Callahan A, Huskey R. Genetic control of sexual development in Volvox. Developmental Biology. 1980;80:419–435. doi: 10.1016/0012-1606(80)90416-9. [DOI] [PubMed] [Google Scholar]
- Carroll SB. Chance and necessity: the evolution of morphological complexity and diversity. Nature. 2001;409:1102–1109. doi: 10.1038/35059227. [DOI] [PubMed] [Google Scholar]
- Castillo DI, Queller DC, Strassmann JE. Cell condition, competition, and chimerism in the social amoeba Dictyostelium discoideum. Ethology Ecology & Evolution. 2011;23:262–273. [Google Scholar]
- Cavalier-Smith T. A revised six-kingdom system of life. Biological Reviews of the Cambridge Philosophical Society. 1998;73:203–266. doi: 10.1017/s0006323198005167. [DOI] [PubMed] [Google Scholar]
- Charrier B, Coelho SM, Le Bail A, Tonon T, Michel G, Potin P, Kloareg B, Boyen C, Peters AF, Cock JM. Development and physiology of the brown alga Ectocarpus siliculosus: two centuries of research. The New Phytologist. 2008;177:319–332. doi: 10.1111/j.1469-8137.2007.02304.x. [DOI] [PubMed] [Google Scholar]
- Clamp JC, Williams D. A molecular phylogenetic investigation of Zoothamnium (Ciliophora, Peritrichia, Sessilida) Journal of Eukaryotic Microbiology. 2006;53:494–498. doi: 10.1111/j.1550-7408.2006.00132.x. [DOI] [PubMed] [Google Scholar]
- Clarke E. The problem of biological individuality. Biological Theory. 2010;5:312–325. [Google Scholar]
- Clarke E. Plant individuality: a solution to the demographer's dilemma. Biology and Philosophy. 2012;27:321–361. [Google Scholar]
- Clayton MN. Phylum Phaeophyta. In: Margulis L, Corliss JO, Melkonian M, Chapman DJ, editors. Handbook of Protoctista. Jones and Bartlett Publishers; Boston, MA: 1990. pp. 698–714. [Google Scholar]
- Cock JM, Sterck L, Rouzé P, Scornet D, Allen AE, Amoutzias G, Anthouard V, Artiguenave F, Aury JM, Badger JH, Beszteri B, Billiau K, Bonnet E, Bothwell JHF, Bowler C, Boyen C, Brownlee C, Carrano CJ, Charrier B, Cho GY, Coelho SM, Collén J, Corre E, Da Silva C, Delage L, Delaroque N, Dittami SM, Doulbeau S, Elias M, Farnham G, Gachon CMM, Gschloessl B, Heesch S, Jabbari K, Jubin C, Kawai H, Kimura K, Kloareg B, Küpper FC, Lang D, Le Bail A, Leblanc C, Lerouge P, Lohr M, Lopez PJ, Martens C, Maumus F, Michel G, Miranda-Saavedra D, Morales J, Moreau H, Motomura T, Nagasato C, Napoli CA, Nelson DR, Nyvall-Collén P, Peters AF, Pommier C, Potin P, Poulain J, Quesneville H, Read B, Rensing SA, Ritter A, Rousvoal S, Samanta M, Samson G, Schroeder DC, Ségurens B, Strittmatter M, Tonon T, Tregear J, Valentin K, von Dassow P, Yamagishi T, Van de Peer Y, Wincker P. The Ectocarpus genome and the independent evolution of multicellularity in the brown algae. Nature. 2010;465:617–621. doi: 10.1038/nature09016. [DOI] [PubMed] [Google Scholar]
- Coomans RJ, Hommersand MH. Vegetative growth and organization. In: Cole KM, Sheath RG, editors. Biology of the Red Algae. Cambridge University Press; New York: 1990. pp. 275–304. [Google Scholar]
- de Reviers B. Biologie et Phylogénie des Agues, Tome 1. Belin; Paris: 2002. [Google Scholar]
- DeLong JP, Okie JG, Moses ME, Sibly RM, Brown JH. Shifts in metabolic scaling, production, and efficiency across major evolutionary transitions of life. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:12941–12945. doi: 10.1073/pnas.1007783107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunthorn M, Katz LA. Secretive ciliates and putative asexuality in microbial eukaryotes. Trends in Microbiology. 2010;18:183–188. doi: 10.1016/j.tim.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Eldakar OT, Wilson DS. Eight criticisms not to make about group selection. Evolution. 2011;65:1523–1526. doi: 10.1111/j.1558-5646.2011.01290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdos GW, Raper KB, Vogen LK. Mating types and macrocyst formation in Dictyostelium discoideum. Proceedings of the National Academy of Sciences of the United States of America. 1973;70:1828–1830. doi: 10.1073/pnas.70.6.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauré-Fremiet E. Growth and differentiation of the colonies of Zoothamnium alternans (Clap. and Lachm.) Biological Bulletin. 1930;58:28–51. [Google Scholar]
- Filosa M, Dengler R. Ultrastructure of macrocyst formation in the cellular slime mold, Dictyostelium mucoroides: extensive phagocytosis of amoebae by a specialized cell. Developmental Biology. 1972;29:1–16. doi: 10.1016/0012-1606(72)90038-3. [DOI] [PubMed] [Google Scholar]
- Fiore-Donno AM, Nikolaev SI, Nelson M, Pawlowski J, Cavalier-Smith T, Baldauf SL. Deep phylogeny and evolution of slime moulds (Mycetozoa) Protist. 2010;161:55–70. doi: 10.1016/j.protis.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Fjeld A. Genetic control of cellular differentiation in Ulva mutabilis. Gene effects in early development. Developmental Biology. 1972;28:326–343. doi: 10.1016/0012-1606(72)90017-6. [DOI] [PubMed] [Google Scholar]
- Flowers JM, Li SI, Stathos A, Saxer G, Ostrowski EA, Queller DC, Strassmann JE, Purugganan MD. Variation, sex, and social cooperation: molecular population genetics of the social amoeba Dictyostelium discoideum. PLoS Genetics. 2010;6:e1001013. doi: 10.1371/journal.pgen.1001013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folse HJ, Roughgarden J. What is an individual organism? A multilevel selection perspective. Quarterly Review of Biology. 2010;85:447–472. doi: 10.1086/656905. [DOI] [PubMed] [Google Scholar]
- Foster KR, Fortunato A, Strassmann JE, Queller DC. The costs and benefits of being a chimera. Proceedings of the Royal Society of London B. 2002;269:2357–2362. doi: 10.1098/rspb.2002.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Föyn B. Über die Sexualität und den Generationswechsel von Ulva mutabilis (N.S.) Archiv für Protistenkunde. 1958;102:473–480. [Google Scholar]
- Garbary DJ, Gabrielson PW. Taxonomy and evolution. In: Cole KM, Sheath RG, editors. Biology of the Red Algae. Cambridge University Press; New York: 1990. pp. 477–498. [Google Scholar]
- Glöckner G, Golderer G, Werner-Felmayer G, Meyer S, Marwan W. A first glimpse at the transcriptome of Physarum polycephalum. BMC Genomics. 2008;9:6. doi: 10.1186/1471-2164-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey-Smith P. Darwinian Populations and Natural Selection. Oxford; Oxford University Press; 2009. [Google Scholar]
- Graham LE, Wilcox LW. Algae. Prentice Hall; Upper Saddle River, NJ: 2000. [Google Scholar]
- Grosberg RK, Strathmann RR. The evolution of multicellularity: a minor major transition? Annual Review of Ecology, Evolution, and Systematics. 2007;38:621–654. [Google Scholar]
- Hackett JD, Yoon HS, Butterfield NJ, Sanderson MJ, Bhattacharya D. Plastid endosymbiosis: sources and timing of the major events In Falkowski, P and Knoll, A (eds) Evolution of Primary Producers in the Sea. Elsevier Academic Press; Burlington, MA: 2007. pp. 109–132. [Google Scholar]
- Hawkes MW. Reproductive strategies. In: Cole KM, Sheath RG, editors. Biology of the Red Algae. Cambridge University Press; New York: 1990. pp. 455–476. [Google Scholar]
- Hedges SB, Blair JE, Venturi ML, Shoe JL. A molecular timescale of eukaryote evolution and the rise of complex multicellular life. BMC Evolutionary Biology. 2004;4:2. doi: 10.1186/1471-2148-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heesch S, Cho GY, Peters AF, Le Corguillé G, Falentin C, Boutet G, Cöedel S, Jubin C, Samson G, Corre E, Coelho SM, Cock JM. A sequence-tagged genetic map for the brown alga Ectocarpus siliculosus provides large-scale assembly of the genome sequence. The New Phytologist. 2010;188:42–51. doi: 10.1111/j.1469-8137.2010.03273.x. [DOI] [PubMed] [Google Scholar]
- Herron MD, Hackett JD, Aylward FO, Michod RE. Triassic origin and early radiation of multicellular volvocine algae. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:3254–3258. doi: 10.1073/pnas.0811205106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoxmark RC, Nordby Ø. Haploid meiosis as a regular phenomenon in the life cycle of Ulva mutabilis. Hereditas. 1974;76:239–250. doi: 10.1111/j.1601-5223.1974.tb01342.x. [DOI] [PubMed] [Google Scholar]
- Hull DL. Individuality and selection. Annual Review of Ecology and Systematics. 1980;11:311–332. [Google Scholar]
- Hutchings MJ, Booth D. Much ado about nothing… so far? Journal of Evolutionary Biology. 2004;17:1184–1186. doi: 10.1111/j.1420-9101.2004.00812.x. [DOI] [PubMed] [Google Scholar]
- Huxley JS. The Individual in the Animal Kingdom. Cambridge University Press; Cambridge, UK: 1912. [Google Scholar]
- Janzen DH. What are dandelions and aphids? The American Naturalist. 1977;111:586–589. [Google Scholar]
- Ji D, Kusuoka Y. A description of Apocarchesium rosettum n. gen., n. sp. and a redescription of Ophrydium eichornii Ehrenberg, 1838, two freshwater peritrichous ciliates from Japan. European Journal of Protistology. 2009;45:21–28. doi: 10.1016/j.ejop.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Ji D, Song W. Notes on a new marine peritrichous ciliate (Ciliophora: Peritrichida), Zoothamnopsis sinica sp. n. from North China, with reconsideration of Zoothamnium maximum Song, 1986. Acta Protozoologica. 2004;43:61–71. [Google Scholar]
- Joint I, Callow ME, Callow JA, Clarke KR. The attachment of Enteromorpha zoospores to a bacterial biofilm assemblage. Biofouling. 2000;16:37–41. [Google Scholar]
- Joint I, Tait K, Callow ME, Callow JA, Milton D, Williams P, Cámara M. Cell-to-cell communication across the prokaryote-eukaryote boundary. Science. 2002;298:1207. doi: 10.1126/science.1077075. [DOI] [PubMed] [Google Scholar]
- Karol KG, McCourt G, RMCimino MT, Delwiche CF. The closest living relatives of land plants. Science. 2001;294:2351–2353. doi: 10.1126/science.1065156. [DOI] [PubMed] [Google Scholar]
- Kessin RH. Dictyostelium: Evolution, Cell Biology, and the Development of Multicellularity. Cambridge University Press; Cambridge, UK: 2001. [Google Scholar]
- Kirk DL. Volvox: Molecular-Genetic Origins of Multicellularity and Cellular Differentiation. Cambridge University Press; Cambridge, UK: 1998. [Google Scholar]
- Kirk DL. Germ-soma differentiation in Volvox. Developmental Biology. 2001;238:213–223. doi: 10.1006/dbio.2001.0402. [DOI] [PubMed] [Google Scholar]
- Kirk DL, Kirk MM. Heat shock elicits production of sexual inducer in Volvox. Science. 1986;231:51–54. doi: 10.1126/science.3941891. [DOI] [PubMed] [Google Scholar]
- Kitade Y, Taguchi G, Shin JA, Saga N. Porphyra monospore system (Bangiales, Rhodophyta): A model for the developmental biology of marine plants. Phycological Research. 1998;46:17–20. [Google Scholar]
- Knoll AH. The multiple origins of complex multicellularity. Annual Review of Earth and Planetary Sciences. 2011;39:217–239. [Google Scholar]
- Konijn TM, Van De Meene J, Bonner JT, Barkley DS. The acrasin activity of adenosine-3′,5′-cyclic phosphate. Proceedings of the National Academy of Sciences of the United States of America. 1967;58:1152–1154. doi: 10.1073/pnas.58.3.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasek-Nesselquist E, Katz LA. Phylogenetic position of Sorogena stoianovitchae and relationships within the class Colpodea (Ciliophora) based on SSU rDNA sequences. Journal of Eukaryotic Microbiology. 2001;48:604–607. doi: 10.1111/j.1550-7408.2001.tb00197.x. [DOI] [PubMed] [Google Scholar]
- Le Bail A, Billoud B, Maisonneuve C, Peters AF, Cock JM, Charrier B. Early development pattern of the brown alga Ectocarpus siliculosus (Ectocarpales, Phaeophyceae) sporophyte. Journal of Phycology. 2008;44:1269–1281. doi: 10.1111/j.1529-8817.2008.00582.x. [DOI] [PubMed] [Google Scholar]
- Lewis LA, McCourt RM. Green algae and the origin of land plants. American Journal of Botany. 2004;91:1535–1556. doi: 10.3732/ajb.91.10.1535. [DOI] [PubMed] [Google Scholar]
- Lewontin RC. The units of selection. Annual Review of Ecology and Systematics. 1970;1:1–18. [Google Scholar]
- Li L, Song W, Warren A, Shin MK, Chen Z, Ji D, Sun P. Reconsideration of the phylogenetic positions of five peritrich genera, Vorticella, Pseudovorticella, Zoothamnopsis, Zoothamnium, and Epicarchesium (Ciliophora, Peritrichia, Sessilida), based on small subunit rRNA gene sequences. Journal of Eukaryotic Microbiology. 2008;55:448–456. doi: 10.1111/j.1550-7408.2008.00351.x. [DOI] [PubMed] [Google Scholar]
- Løvlie A. Genetic control of division rate and morphogenesis in Ulva mutabilis Foeyn. Comptes-rendus des travaux du Laboratoire Carlsberg. 1964;34:77–168. [PubMed] [Google Scholar]
- Marwan W, Sujatha A, Starostzik C. Reconstructing the regulatory network controlling commitment and sporulation in Physarum polycephalum based on hierarchical Petri Net modelling and simulation. Journal of Theoretical Biology. 2005;236:349–365. doi: 10.1016/j.jtbi.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Mattox KR, Stewart KD. Classification of the green algae: a concept based on comparative cytology. In: Irvine DEG, John DM, editors. The Systematics of Green Algae. Academic Press; London: 1984. pp. 29–72. [Google Scholar]
- Maynard Smith J, Szathmáry E. The Major Transitions in Evolution. Oxford University Press; Oxford: 1997. [Google Scholar]
- McShea DW. Perspective: metazoan complexity and evolution: is there a trend? Evolution. 1996;50:477–492. doi: 10.1111/j.1558-5646.1996.tb03861.x. [DOI] [PubMed] [Google Scholar]
- Meeks JC, Elhai J. Regulation of cellular differentiation in filamentous cyanobacteria in free-living and plant-associated symbiotic growth states. Microbiology and Molecular Biology Reviews. 2002;66:94–121. doi: 10.1128/MMBR.66.1.94-121.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michod RE. Evolution of the individual. The American Naturalist. 1997;150:S5–S21. doi: 10.1086/286047. [DOI] [PubMed] [Google Scholar]
- Michod RE. Cooperation and conflict mediation during the origin of multicellularity. In: Hammerstein P, editor. Genetic and Cultural Evolution of Cooperation. MIT Press; Cambridge, MA: 2003. pp. 261–307. [Google Scholar]
- Michod RE. Evolutionary transitions in individuality: multicellularity and sex. In: Calcott B, Sterelny K, editors. The Major Transitions in Evolution Revisited. MIT Press; Cambridge, MA: 2011. pp. 167–197. [Google Scholar]
- Michod RE, Nedelcu AM. On the reorganization of fitness during evolutionary transitions in individuality. Integrative and Comparative Biology. 2003;43:64–73. doi: 10.1093/icb/43.1.64. [DOI] [PubMed] [Google Scholar]
- Michod RE, Roze D. Transitions in individuality. Proceedings of the Royal Society of London B. 1997;264:853–857. doi: 10.1098/rspb.1997.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michod RE, Viossat Y, Solari CA, Hurand M, Nedelcu AM. Life-history evolution and the origin of multicellularity. Journal of Theoretical Biology. 2006;239:257–272. doi: 10.1016/j.jtbi.2005.08.043. [DOI] [PubMed] [Google Scholar]
- Mitman GG, van de Meer JP. Meiosis, blade development, and sex determination in Porphyra purpurea (Rhodophyta) Journal of Phycology. 1994;30:147–159. [Google Scholar]
- Moreira D, LeGuyader H, Phillipe H. The origin of red algae and the evolution of chloroplasts. Nature. 2000;405:69–72. doi: 10.1038/35011054. [DOI] [PubMed] [Google Scholar]
- Mori S, Yamazaki A, Matsuyama Serisawa K, Fukuda S, Mizuta H, Saga N. Effect of two symbiotic bacteria for growth of Porphyra yezoensis (Rhodophyta, Bangiales) in axenic culture. Suisanzoshoku. 2004;52:239–244. [Google Scholar]
- Müller DG. Generationswechsel, Kernphasenwechsel und Sexualität der Braunalge Ectocarpus siliculosus im Kulturversuch. Planta. 1967;75:39–54. doi: 10.1007/BF00380838. [DOI] [PubMed] [Google Scholar]
- Nickerson AW, Raper KB. Macrocysts in the life cycle of the Dictyosteliaceae. I. Formation of the macrocysts. American Journal of Botany. 1973;60:190–197. [Google Scholar]
- Niklas KJ. The evolution of plant body plans - A biomechanical perspective. Annals of Botany. 2000;85:411–438. [Google Scholar]
- Nishii I, Miller SM. Volvox: Simple steps to developmental complexity? Current Opinion in Plant Biology. 2010;13:646–653. doi: 10.1016/j.pbi.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Norf H, Foissner W. A new flagship peritrich (Ciliophora, Peritrichida) from the River Rhine, Germany: Apocarchesium arndti n. sp. Journal of Eukaryotic Microbiology. 2010;57:250–264. doi: 10.1111/j.1550-7408.2010.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki H, Kuroiwa H, Mita T, Kuroiwa T. Pleodorina japonica sp. nov. (Volvocales, Chlorophyta) with bacteria-like endosymbionts. Phycologia. 1989;28:252–267. [Google Scholar]
- O'Day DH. Aggregation during sexual development in Dictyostelium discoideum. Can Journal of Microbiology. 1979;25:1416–1426. doi: 10.1139/m79-221. [DOI] [PubMed] [Google Scholar]
- Ohme M, Kunifuji Y, Miura A. Cross experiments in the color mutants of Porphyra yezoensis Ueda. Japanese Journal of Phycology. 1986;34:101–106. [Google Scholar]
- Olive LS. The Mycetozoans. Academic Press; New York: 1975. [Google Scholar]
- Olive LS. Sorocarp development by a newly discovered ciliate. Science. 1978;202:530. doi: 10.1126/science.202.4367.530. [DOI] [PubMed] [Google Scholar]
- Olive LS, Stoianovitch C. Two new members of the Acrasiales. Bulletin of the Torrey Botanical Club. 1960;87:1–20. [Google Scholar]
- Ostrowski EA, Katoh M, Shaulsky G, Queller DC, Strassmann JE. Kin discrimination increases with genetic distance in a social amoeba. PLoS Biology. 2008;6:e287. doi: 10.1371/journal.pbio.0060287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto S, Hastings I. Mutation and selection within the individual. Genetica. 1998;102-103:507–52. [PubMed] [Google Scholar]
- Pedersen B, Tuomi J. Hierarchical selection and fitness in modular and clonal organisms. Oikos. 1995;73:167–180. [Google Scholar]
- Pepper JW, Herron MD. Does biology need an organism concept? Biological Reviews of the Cambridge Philosophical Society. 2008;83:621–627. doi: 10.1111/j.1469-185X.2008.00057.x. [DOI] [PubMed] [Google Scholar]
- Peters AF, Marie D, Scornet D, Kloareg B, Cock JM. Proposal of Ectocarpus siliculosus (Ectocarpales, Phaeophyceae) as a model organism for brown algal genetics and genomics. Journal of Phycology. 2004;40:1079–1088. [Google Scholar]
- Pineda-Krch M, Lehtilä K. Costs and benefits of genetic heterogeneity within organisms. Journal of Evolutionary Biology. 2004;17:1167–1177. doi: 10.1111/j.1420-9101.2004.00808.x. [DOI] [PubMed] [Google Scholar]
- Polne-Fuller M, Gibor A. Developmental studies in Porphyra. I. Blade differentiation in Porphyra perforata as expressed by morphology, enzymatic digestion, and protoplast regeneration. Journal of Phycology. 1984;20:609–616. [Google Scholar]
- Pueschel CM. Cell structure. In: Cole KM, Sheath RG, editors. Biology of the Red Algae. Cambridge University Press; New York: 1990. pp. 477–498. [Google Scholar]
- Queller DC. Cooperators since life began. Quarterly Review of Biology. 1997;72:184–188. [Google Scholar]
- Queller DC, Strassmann JE. Beyond society: the evolution of organismality. Philosophical Transactions of the Royal Society B. 2009;364:3143–3155. doi: 10.1098/rstb.2009.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinke C, Lee R, Katz S, Bright M. The effects of sulphide on growth and behaviour of the thiotrophic Zoothamnium niveum symbiosis. Proceedings of the Royal Society B. 2007;274:2259–2269. doi: 10.1098/rspb.2007.0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinke C, Schmitz-Esser S, Stoecker K, Nussbaumer AD, Molnár DA, Vanura K, Wagner M, Horn M, Ott JA, Bright M. “Candidatus Thiobios zoothamnicoli,” an ectosymbiotic bacterium covering the giant marine ciliate Zoothamnium niveum. Applied and Environmental Microbiology. 2006;72:2014–2021. doi: 10.1128/AEM.72.3.2014-2021.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Ezpeleta N, Brinkmann H, Burey SC, Roure B, Burger G, Löffelhardt W, Bohnert HJ, Philippe H, Lang BF. Monophyly of primary photosynthetic eukaryotes: green plants, red algae, and glaucophytes. Current Biology. 2005;15:1325–1530. doi: 10.1016/j.cub.2005.06.040. [DOI] [PubMed] [Google Scholar]
- Rohwer F, Seguritan V, Azam F, Knowlton N. Diversity and distribution of coral-associated bacteria. Marine Ecology Progress Series. 2002;243:1–10. [Google Scholar]
- Roze D, Michod RE. Mutation, multilevel selection, and the evolution of propagule size during the origin of multicellularity. The American Naturalist. 2001;158:638–654. doi: 10.1086/323590. [DOI] [PubMed] [Google Scholar]
- Saga Y, Okada H, Yanagisawa K. Macrocyst development in Dictyostelium discoideum II. Mating-type-specific cell fusion and acquisition of fusion-competence. Journal of Cell Science. 1983;60:157–168. doi: 10.1242/jcs.60.1.157. [DOI] [PubMed] [Google Scholar]
- Santelices B. How many kinds of individual are there? Trends in Ecology and Evolution. 1999;14:152–155. doi: 10.1016/s0169-5347(98)01519-5. [DOI] [PubMed] [Google Scholar]
- Sarukhán J, Harper JL. Studies on plant demography: Ranunculus repens L., R. bulbosus L. and R. acris L.: I. Population flux and survivorship. Journal of Ecology. 1973;61:675–716. [Google Scholar]
- Saunders GW, Hommersand MH. Assessing red algal supraordinal diversity and taxonomy in the context of contemporary systematic data. American Journal of Botany. 2004;91:1494–1507. doi: 10.3732/ajb.91.10.1494. [DOI] [PubMed] [Google Scholar]
- Schaap P, Winckler T, Nelson M, Alvarez-Curto E, Elgie B, Hagiwara H, Cavender J, Milano-Curto A, Rozen DE, Dingermann T, Mutzel R, Baldauf SL. Molecular phylogeny and evolution of morphology in the social amoebas. Science. 2006;314:661–663. doi: 10.1126/science.1130670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadwick LL, Spiegel FW, Shadwick JDL, Brown MW, Silberman JD. Eumycetozoa = Amoebozoa?: SSUrDNA phylogeny of protosteloid slime molds and its significance for the amoebozoan supergroup. PLoS One. 2009;4:e6754. doi: 10.1371/journal.pone.0006754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaulsky G, Kessin R. The cold war of the social amoebae. Current Biology. 2007;17:R684–R692. doi: 10.1016/j.cub.2007.06.024. [DOI] [PubMed] [Google Scholar]
- Solari CA, Nedelcu AM, Michod RE. Fitness and complexity in Volvocalean green algae. In: Lipson H, Antonsson EK, Koza JR, editors. Computational Synthesis, From Basic Building Blocks to High Level Functionality. AAAI Press; Stanford, CA: 2004. pp. 218–225. [Google Scholar]
- Starr RC. Structure, reproduction and differentiation in Volvox carteri f. nagariensis Iyengar, strains HK9 & 10. Archiv für Protistenkunde. 1969;111:204–222. [Google Scholar]
- Starr RC. Control of differentiation in Volvox. Developmental Biology. 1970;29:S59–S100. doi: 10.1016/b978-0-12-395534-0.50009-1. [DOI] [PubMed] [Google Scholar]
- Starr RC. Meiosis in Volvox carteri f. nagariensis. Archiv für Protistenkunde. 1975;117:187–191. [Google Scholar]
- Sterelny K. Darwinian spaces: Peter Godfrey-Smith on selection and evolution. Biology and Philosophy. 2011;26:489–500. [Google Scholar]
- Stout AB. The clon in plant life. Journal of the New York Botanical Garden. 1929;30:25–37. [Google Scholar]
- Strassmann JE, Queller DC. The social organism: congresses, parties, and committees. Evolution. 2010;64:605–616. doi: 10.1111/j.1558-5646.2009.00929.x. [DOI] [PubMed] [Google Scholar]
- Strassmann JE, Queller DC. Evolution of cooperation and control of cheating in a social microbe. Proceedings of the National Academy of Sciences of the United States of America. 2011a;108:10855–10862. doi: 10.1073/pnas.1102451108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassmann JE, Queller DC. How social evolution theory impacts our understanding of development in the social amoeba Dictyostelium. Development, Growth & Differentiation. 2011b;53:597–607. doi: 10.1111/j.1440-169X.2011.01272.x. [DOI] [PubMed] [Google Scholar]
- Stratmann J, Paputsoglu G, Oertel W. Differentiation of Ulva mutabilis (Chlorophyta) gametangia and gamete release are controlled by extracellular inhibitors. Journal of Phycology. 1996;32:1009–1021. [Google Scholar]
- Sugimoto H, Endoh H. Analysis of fruiting body development in the aggregative ciliate Sorogena stoianovitchae (Ciliophora, Colpodea) Journal of Eukaryotic Microbiology. 2006;53:96–102. doi: 10.1111/j.1550-7408.2005.00077.x. [DOI] [PubMed] [Google Scholar]
- Sugimoto H, Endoh H. Differentially expressed genes during fruiting body development in the aggregative ciliate Sorogena stoianovitchae (Ciliophora: Colpodea) Journal of Eukaryotic Microbiology. 2008;55:110–116. doi: 10.1111/j.1550-7408.2008.00312.x. [DOI] [PubMed] [Google Scholar]
- Summers FM. Form regulation in Zoothamnium alternans. Biological Bulletin. 1938a;74:130–154. [Google Scholar]
- Summers FM. Some aspects of normal development in the colonial ciliate Zoothamnium alternans. Biological Bulletin. 1938b;74:117–129. [Google Scholar]
- Sun P, Clamp JC, Xu D, Kusuoka Y, Hori M. Molecular phylogeny of the family Vorticellidae (Ciliophora, Peritrichia) using combined datasets with a special emphasis on the three morphologically similar genera Carchesium, Epicarchesium and Apocarchesium. International Journal of Systematic and Evolutionary Microbiology. 2011;61:1001–1010. doi: 10.1099/ijs.0.020255-0. [DOI] [PubMed] [Google Scholar]
- Tuomi J, Vuorisalo T. What are the units of selection in modular organisms? Oikos. 1989;54:227–233. doi: 10.1016/0169-5347(89)90075-X. [DOI] [PubMed] [Google Scholar]
- Van de Peer Y, De Wachter R. Evolutionary relationships among the eukaryotic crown taxa taking into account site-to-site rate variation in 18S rRNA. Journal of Molecular Evolution. 1997;45:619–630. doi: 10.1007/pl00006266. [DOI] [PubMed] [Google Scholar]
- van den Hoek C, Mann DG, Jahns HM. Algae: An Introduction to Phycology. Cambridge University Press; New York: 1995. [Google Scholar]
- Viljoen S, As JG. Notes on the morphology and asexual reproductive processes of sessile peritrichs. Hydrobiologia. 1987;154:75–86. [Google Scholar]
- Wallace MA, Raper KB. Genetic exchanges in the macrocysts of Dictyostelium discoideum. Journal of General Microbiology. 1979;113:327–337. doi: 10.1099/00221287-113-2-327. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhu J, Zhou W, Jiang P, Qin S, Xu P. Early development patterns and morphogenesis of blades in four species of Porphyra (Bangiales, Rhodophyta) Journal of Applied Phycology. 2009;22:297–303. [Google Scholar]
- Webb JS, Givskov M, Kjelleberg S. Bacterial biofilms: prokaryotic adventures in multicellularity. Current Opinion in Microbiology. 2003;6:578–585. doi: 10.1016/j.mib.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Weismann A. The Evolution Theory. Edward Arnold; London: 1904. [Google Scholar]
- West GB, Brown JH, Enquist BJ. A general model for the origin of allometric scaling laws in biology. Science. 1997;276:122–126. doi: 10.1126/science.276.5309.122. [DOI] [PubMed] [Google Scholar]
- Whitworth DE, editor. Myxobacteria: Multicellularity and Differentiation. American Society for Microbiology; Washington, DC: 2008. [Google Scholar]
- Wilson J. Biological Individuality: The Identity and Persistence of Living Entities. Cambridge University Press; Cambridge, UK: 1999. [Google Scholar]
- Wilson JA. Ontological butchery: organism concepts and biological generalizations. Philosophy of Science. 2000;67:S301–S311. [Google Scholar]
- Yamazaki A, Nakanishi K, Saga N. Axenic tissue culture and morphogenesis in Porphyra yezoensis (Bangiales, Rhodophyta) Journal of Phycology. 1998;34:1082–1087. [Google Scholar]
- Yoon HS, Hackett JD, Ciniglia C, Pinto G, Bhattacharya D. A molecular timeline for the origin of photosynthetic eukaryotes. Molecular Biology and Evolution. 2004;21:809–818. doi: 10.1093/molbev/msh075. [DOI] [PubMed] [Google Scholar]
- Yoon HS, Müller KM, Sheath RG, Ott FD, Bhattacharya D. Defining the major lineages of red algae (Rhodophyta) Journal of Phycology. 2006;42:482–492. [Google Scholar]