Abstract
The development of high-affinity radiotracers for positron emission tomography has allowed for quantification of dopamine receptors in extrastriatal as well as striatal regions of brain. As these new radiotracers have distinctly different kinetic properties than their predecessors, it is important to examine the suitability of kinetic models to represent their uptake, distribution and in vivo washout. Using the simplified reference tissue model, we investigated the influence of reference region choice on striatal binding potential (BPND) of 18F-fallypride, a high-affinity dopamine D2/D3 receptor ligand. We compared visual cortex and a white matter region (superior longitudinal fasciculus) to the cerebellum, a commonly used reference tissue, in a PET-fallypride study of healthy and methamphetamine-dependent subjects. Compared to cerebellum, use of visual cortex produced significantly greater sample variance in BPND. Use of the white matter region was associated with BPND values and sample variance similar to those obtained with cerebellum, and a larger effect size for the group differences in striatal BPND between healthy and methamphetamine-dependent subjects. Our results do not support the use of visual cortex as a reference region in 18F-fallypride studies, and suggest that white matter may be a reasonable alternative to cerebellum as it displays similar statistical and kinetic properties.
Keywords: 18F-fallypride, PET, SRTM, reference region, cerebellum, dopamine
Introduction
Assessment of dopamine receptors as biomarkers of dopaminergic signaling in the brain is relevant to numerous neuropsychiatric conditions, such as Parkinson's disease, drug use disorders and schizophrenia. With development of high-affinity radiotracers, for dopamine receptors, it is now possible to quantify dopamine receptor binding using positron emission tomography (PET) in areas outside of the striatum, such as the thalamus, amygdala and cerebral cortex, where dopamine receptor densities are lower than in the striatum. The advent of new radiotracers with binding and kinetic properties that differ distinctly from those of earlier radiotracers, however, has made it important to evaluate the kinetic modeling methods used to describe the uptake, distribution and in vivo washout of these tracers.
The Reference Tissue Model (RTM) was developed for autoradiography and PET in order to simplify the standard quantification method for radiotracer kinetic analysis, which requires an arterial plasma input function based on radioactivity of the free radiotracer (not the protein-bound parent compound or metabolites) 1-3. This two-tissue compartment model (four parameters) was originally applied to, and validated for, tracers with relatively fast kinetics and modest receptor binding potentials (BPND: binding potential relative to non-displaceable uptake) in the range of 1-5 (e.g., 3H-diprenorphine and 11C-raclopride). While RTM produces large BPND values, it converges slowly (often not at all) and the standard errors of the parameters estimated in the model (R1, k2, and k3) tend to be large 4. A subsequent simplification of the RTM method; the Simplified Reference Tissue Model (SRTM) 4 was developed to address these problems. SRTM is a single-tissue compartmental model (3 parameters), applicable only to radiotracers that produce regional time-radioactivity curves (TACs) that can be fit satisfactorily to a single exponential form. SRTM was validated in human subjects for 11C-SCH23390 and 11C-raclopride, tracers that meet this requirement 4. However, a simulation study of the binding of 11C-WAY-100635 5, which has slower kinetics and higher affinity, showed that estimates of BPND obtained with SRTM can have substantial errors.
Reference tissue models have also been applied to 11C- and 18F-labeled receptor ligands that exhibit relatively slow kinetics and yield very high values of BPND (in the range 10-40). Striatal BPND values for these tracers in different laboratories vary substantially (Table 1). Although these differences may be influenced by definitions of the target and reference tissues, scanning duration and processing methods, it is unlikely that these factors explain the large ranges of published striatal BPND values. Differences across scanner models are likely due to the implementation of various software programs for scatter and attenuation correction during reconstruction. In order to help clarify the source of the variation in measurements across laboratories, we review the advantages of the RTM and SRTM in neuroreceptor imaging and the stringent requirements for their use, and we extend this discussion to an investigation of the influence of choice of reference region on BPND using 18F-fallypride.
Table 1.
Variation of reported 18F-fallypride BPND from reference-region methods with the cerebellum as a reference region in healthy subjects.
| PET scanner | Reconstruction Algorithm | n | Age (years) mean ± SD | BPnd | |||
|---|---|---|---|---|---|---|---|
| Model | Target region | mean ± SD | |||||
| Vernaleken I et al. 29 | Siemens ECAT EXACT 922/47 | filtered back- projection | 10 | 24.4 ± 3.9 | SRTM | Putamen | 24.7 ± 3.6 |
| Kegeles LS et al.30 | Siemens ECAT EXACT HR+ | filtered back- projection | 22 | 26 ± 6 | SRTM | P. Putamen | 19.7 ± 2.1 |
| Landvogt C et al. 31 | Siemens ECAT EXACT | not described a | 21 | 30.5 ± 5.1 | SRTM | A. Putamen | 10.4 ± 1.7 |
| Cropley VL et al. 32 | GE Advance tomograph | filtered back- projection | 14 | 29 ± 8 | SRTM | Putamen | 19.8 ± 1.3 |
| Siessmeier T et al.33 | Siemens ECAT EXACT | not described a | 10 | 32.0 ± 9.5 | SRTM | Striatum | 23.3 ± 4.9 |
| Riccardi P et al.34 | GE Discovery LS PET scanner | not described a | 6 | 23 - 38 c | RTM 3 | Putamen | 39.7 ± 6.1 |
| Rominger A et al.35 | Siemens ECAT EXACT HR+ | filtered back- projection | 14 | 44.0 ± 11.6 | Logan 36 | Striatum | 16.8 ± 3.0 |
| MukherjeeJ et al. 37 | Siemens ECAT EXACT HR+ | ECAT v7.2 OSEM | 6 | 21-63 c | Logan | Putamen | 27.2 ± 6.1 |
| Present data | Siemens ECAT EXACT HR+ | ECAT v7.3 OSEM | 43 | 36.1 ± 8.4 | SRTM | Striatum | 18.8 ± 3.8 |
| Unpublished data b | Philips GEMINI TF | LOR-RAMLA | 13 | 29.8 ± 9.0 | SRTM | Striatum | 29.0 ± 5.0 |
The method of reconstruction algorithm was not descried in the article.
Another data set, which is different from present data set and unpublished, in our group
age range A. Putamen = Anterior Putamen, P. Putamen = Posterior Putamen
RTM and SRTM substitute an input function derived from measured activity in a reference tissue for an arterial plasma input function, obviating the need for arterial cannulation and metabolite assay. The reference-tissue measurement also is preferred to the arterial measurement because it is continuous, creating reference- and target- TACs with equal numbers of data points. Furthermore, assuming that all radioactivity in the brain represents the parent compound 6, 7, the reference-tissue measurement obviates the need for assay of radiolabeled peripheral metabolites and tracer bound to plasma protein, which contribute to uncertainty and bias in the arterial input function measurement. The requirements of RTM and SRTM are as follows:
1. The distribution volumes (VND) for the target and reference regions must be equal. Specifically, the condition VND=K1/k2=K1'/k2' must be satisfied, where the unprimed coefficients are the target-tissue rate parameters and the primed coefficients are those for the reference-tissue. Notably, studies with 18F-fallypride and 11C-FLB 457 have shown violation of this requirement when the reference region is the cerebellum 8.
2. The kinetics in the target region are such that the specifically bound and the free-/non-specific compartments are nearly indistinguishable. Accordingly, the target-tissue compartments must be “well-mixed” and act as a single tissue compartment; violations of this assumption with 11C-WAY-100635 produce biased BPND estimates from SRTM 9, 10.
3. The concentration of bound ligand in the reference tissue must be small compared to that of non-displaceable ligand. This requirement is particularly important for high-affinity tracers, since the ratio of bound to free ligand is ~k3/k4=B'max/KD. Thus, when KD is small (i.e., high-affinity ligands), a relatively small B'max (as in low receptor density areas) can lead to substantial contamination of the reference-tissue activity by specifically bound ligand. Studies using 11C-FLB 457 have shown that specific binding contributes to activity in the cerebellum and can lead to gross underestimation (by more than a factor 2) of striatal BPND 11, 12.
Several of the aforementioned requirements are of particular concern for the high-affinity dopamine D2/D3 receptor (D2/3R) radioligands 18F-fallypride and 11C-FLB 457. Both tracers have relatively slow kinetics, requiring prolonged scanning time (2-3 h scan duration) 13, 14. Due to rapid cerebellar clearance, the measured reference-tissue activity is small during much of the scan. The striatal activity reaches ~1 μCi/ml (decay-corrected to injection time) and remains relatively constant. The cerebellar activity (decay-corrected to injection time) falls to 0.01 - 0.02 (1-2%) of striatal activity by the end of the scan, to an absolute activity of < 10 nCi/cc, which is comparable in magnitude to activity due to scatter from the high-activity striatum.
The accuracy of BPND estimations using RTM and SRTM crucially depends on how well the reference-tissue TAC is measured. The reference-tissue TAC is substantially smaller than the target-tissue TAC during most of the scanning period, thereby having potentially large effects on the accuracy of the BPND estimation. Further, because reference-tissue activity is small, it is more vulnerable to bias produced by inaccuracies in random-, scatter- or attenuation-correction. Even accurate random- and scatter-correction degrades the statistical precision of a low-activity measurement; i.e., if the corrected activity for a region is given by AC = AM-AR-AS where AC is corrected activity, AM is measured activity, AR is activity due to random coincidences, and AS is activity due to scatter. The corresponding variance is given by VC = VM+VR+VS. Thus, precision of the reference-tissue TAC may be compromised when the total measured activity is small and consists substantially of activity from scatters and randoms. Finally, another source of measurement degradation in the reference-tissue TAC is reconstruction inaccuracy. Reconstruction errors occur relatively frequently in low-activity regions 15-17. When filtered-back-projection reconstruction is used, streak artifacts are particularly prominent in low-activity regions; and when iterative reconstruction is used, inaccuracy is greatest in low activity regions because of the positivity constraint 16, 17.
While some of these problems for reference-tissue methods, such as violations of SRTM requirements, are specific for the tracer, others, such as contamination of the reference-tissue activity by scatter and reconstruction artifacts can vary among different possible reference tissues. Tissues further from high-activity regions, or from the radioactive blood pool, have less scatter contamination than closer regions. Reconstruction artifact varies across the field of view of the scanner because scatter and attenuation are better modeled in some parts of the brain than others 15. The most substantial bias in reconstruction is found in lower brain regions, including brain stem and cerebellum 15. Brain regions other than the cerebellum also have comparably small D2/3R densities 12, 18 and may serve as alternative choices of a reference region with fewer of the aforementioned problems in measurement error. Therefore, we sought to determine whether, on the basis of the statistical properties of the results, we could identify one or more reference tissues as superior to others. If these reference regions have comparable tracer kinetic properties but one offers advantages in susceptibility to bias by random-, scatter- or attenuation correction, then it is reasonable to consider its use as a reference tissue for PET studies with high-affinity D2/3R ligands. We tested the influence of the choice of reference region on striatal BPND using 18F-fallypride, comparing the visual cortex, and a white matter region (superior longitudinal fasciculus), to the cerebellum as reference regions for striatal BPND measurements with 18F-fallypride, a high-affinity D2/3R ligand (KD ~0.20 nM in vivo) 10.
Materials and Methods
Research Participants
Data used in this investigation were collected in a study of the effects of methamphetamine dependence on D2/3 receptor binding potential. Thirty-six methamphetamine-dependent (mean age = 34.2, SD = 9.5) and 43 age- and sex-matched control research participants (mean age = 36.1, SD = 8.4) provided written informed consent, and underwent PET scanning as approved by the UCLA Institutional Review Board. Some were re-scanned ~30 days later. Data from controls who were retested (n=16) were used to evaluate measurement reproducibility. A report on the effects of methamphetamine was published 19.
PET scanning
PET scanning was performed on a Siemens ECAT EXACT HR+ scanner (in-plane resolution FWHM = 4.6 mm, axial FWHM = 3.5 mm, axial FOV = 15.52 cm) in 3D mode. A 7-min transmission scan was performed using a rotating 68Ge/68Ga rod source. Dynamic data acquisition was initiated with a bolus injection of 18F-fallypride 19-21 (~185 MBq ± 5%, specific activity ≥ 37 GBq/μmol) and continued for 80 min. After a short break, another transmission scan was performed, and emission data collection continued for another 80 min. Data were reconstructed using ECAT v7.3 OSEM (3 iterations, 16 subsets) after corrections for decay, attenuation, and scatter 22, 23.
MRI scanning and definition of volumes of interest (VOIs)
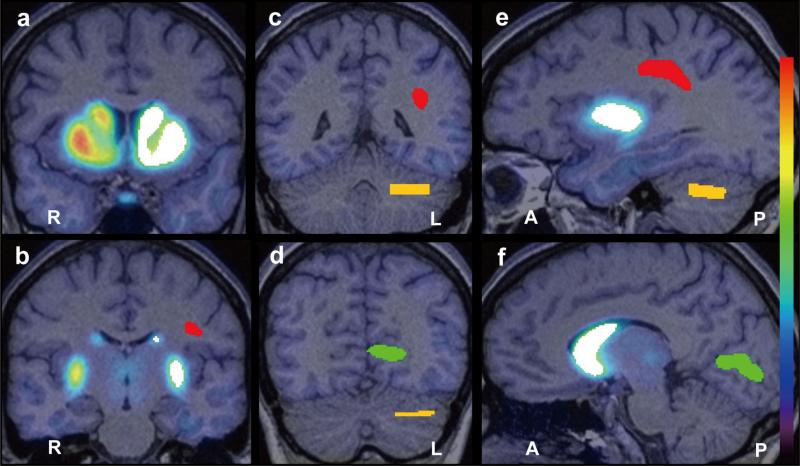
MRI scans were acquired at 1.5 T (Siemens Sonata) [sagittal T1-weighted 3D volumetric, MPRAGE sequence (TR/TE = 25/11 ms, NEX = 1, slice thickness = 1.2 mm contiguous, in-plane resolution = 1 × 1 mm2, runtime = 10 min)], and processed using the FMRIB Software Library (FSL; Oxford University). The striatum, cerebellum, visual cortex and white matter were defined as VOIs (Fig. 2). A whole-striatum VOI was created by combining bilateral caudate, putamen and nucleus accumbens VOIs, anatomically defined in native space using FSL FIRST. A cerebellum VOI was created by transforming a bilateral VOI drawn manually sampling the cerebellar hemispheres in MNI152 space to, to native space. For white matter and visual cortex VOIs, gray and white matter were first segmented using FSL FAST. Bilateral VOIs sampling the visual cortex (intracalcarine cortex) and white matter (superior longitudinal fasciculus) were selected from the Harvard-Oxford cortical structural atlas and JHU DTI-based white matter atlas, respectively (included in FSL). Bilateral VOIs were combined and transformed from MNI152 space into native space, and were masked with the native gray matter and white matter segmentation masks, respectively, in native space. (Fig. 2b - f).
Figure 2.
An example of VOIs placed on the whole Striatum (white), Cerebellum (yellow), Visual Cortex (intracalcarine cortex; green) and White Matter (superior longitudinal fasciculus; red) within the left hemisphere is displayed in four coronal sections (a, b, c and d) and two sagittal sections (e and f) where a representative PET image is superimposed on the corresponding structural magnetic resonance image. The VOI volumes in this example are 20031mm3, 5692mm3, 6986mm3 and 5476mm3 for the whole striatum, cerebellum, visual cortex and white matter, respectively.
VOI = volume of interest, PET = positron emission tomography, L = left, R = right, A = anterior, P = posterior
PET image processing
Reconstructed PET data (160 min) were combined into 16 images, each representing an average of 10-min of dynamic data. After motion-correction using FSL FLIRT (six-parameter rigid-body transformation), the PET images were co-registered to the corresponding structural MRI data with the ART software package 24. Time-radioactivity data were extracted from the VOIs and imported into PMOD Kinetic Modeling Tool version 3.1 (PMOD Technologies Ltd.). BPND of 18F-fallypride to striatal D2/3Rs was calculated using SRTM with each of the three reference tissues using procedures described previously 4.
Data analysis and Statistical Analysis
Correlation of BPND values calculated using the cerebellum with those using the other reference regions was tested by linear regression analysis with Pearson's correlation test. The coefficient of variation (CV=standard deviation/mean) was used as an index to assess BPND variability, and Levene's test was used to assess the equality of variance between the three different samples. Differences between subject groups and between scan sessions were tested by independent and paired t tests, respectively. The effect size (Cohen's d) of the between-group difference in whole striatum BPND was calculated by dividing the difference in means, by the standard deviation.
Test-retest variability (VAR) was calculated as the ratio of the absolute value of the difference in BPND between the test and retest values to the mean of the test and retest BPND values, and the difference in VAR among the three reference regions was tested by one-way repeated measures ANOVA. Results were deemed statistically significant at p < 0.05.
Results
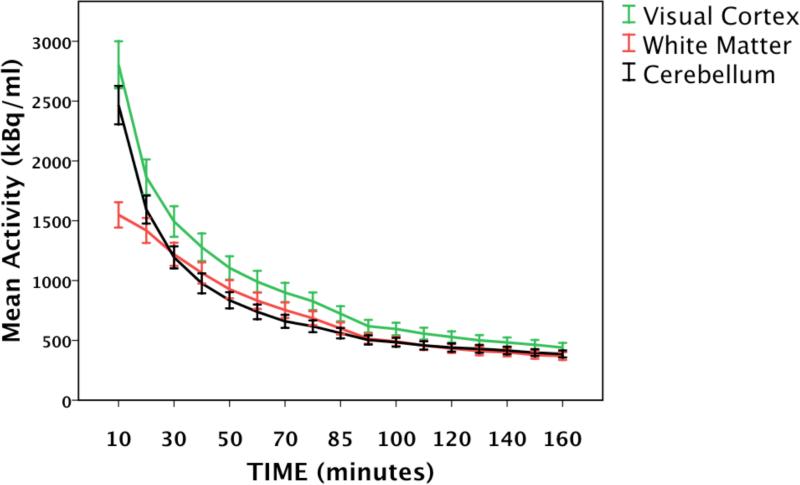
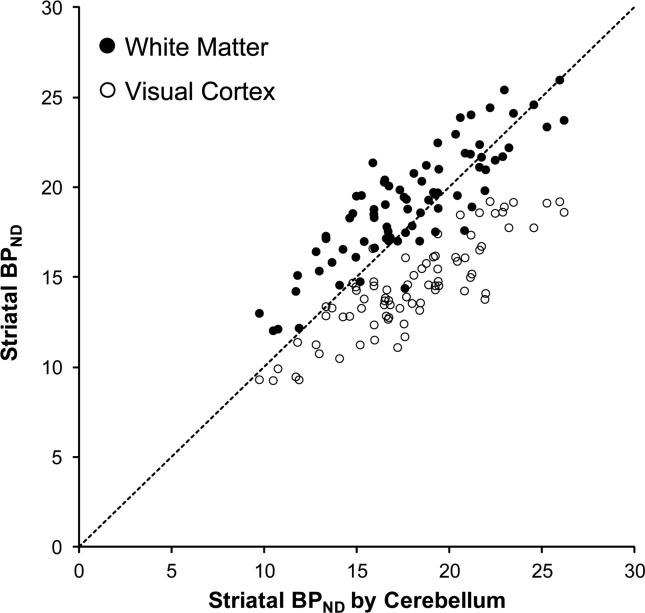
The TACs obtained from each of the three reference regions exhibited similar curve shapes, especially near the end of the scan session (Fig. 1). Notably, the peak activity for the white-matter region was lower than those of the other regions. Regardless of reference region used, BPND values obtained in this study were within the large range of published striatal BPND values using 18F-fallypride (Table 1). In healthy controls, BPND values determined with white matter as the reference region were 6.2% higher than BPND values determined with the cerebellum; BPND determined with visual cortex were 19.9% lower than with cerebellum. In methamphetamine-dependent individuals, BPND values determined with white matter as the reference region were 7.7% higher than BPND values calculated with the cerebellum; BPND determined with visual cortex were 18.1% lower than BPND values determined the cerebellum (Table 2). BPND values calculated using the cerebellum as the reference region were highly correlated with values obtained using white matter (r = 0.854, p < 0.001) and visual cortex (r = 0.858, p < 0.001) (Fig. 3; n=79, groups combined).
Figure 1.
The averaged regional time activity curves for F18-fallypride in human brain in each of three reference regions; Visual Cortex (green; intracalcarine cortex), White Matter (red; superior longitudinal fasiculus) and the Cerebellum (black). Error bars: +/− 1 SEM.
Table 2.
Binding potential (BPND) values calculated using each of three reference regions in the healthy and methamphetamine-dependent participants.
| Reference region | ||||
|---|---|---|---|---|
| Cerebellum | White Matter | Visual Cortex | ||
| HC (n=43) | Striatal BPND | |||
| Mean (SD) | 18.85 (3.78) | 20.02 (3.29) | 15.09 (2.76) | |
| CV | 0.201 | 0.165 | 0.184 | |
| MA (n=36) | Striatal BPND | |||
| Mean (SD) | 16.75 (3.25) | 18.04 (2.53) | 13.71 (2.13) | |
| CV | 0.194 | 0.140 | 0.156 | |
| HC vs MA | t test* | |||
| t value | 2.61 | 2.94 | 2.43 | |
| p value | 0.010 | 0.004 | 0.017 | |
| Effect size | 0.60 | 0.68 | 0.57 | |
HC; healthy participants, MA; methamphetamine-dependent participants CV; coefficient of variation = standard deviation / mean Effect size = Difference in means / standard deviation
Independent t test of group difference in mean.
Figure 3.
Correlations of striatal BPND calculated using the Cerebellum with using White Matter (•: r = 0.854, p < 0.001, y = 0.72x + 6.2) or using Visual Cortex (○: r = 0.858, p < 0.001, y = 0.60× + 3.7) in all 79 (43 control and 36 methamphetamine-dependent) participants.
CV values obtained for BPND using white matter were 17.9%, and 27.8% lower than those calculated with the cerebellum in healthy control and methamphetamine-dependent subjects, respectively (Table 2). CV values for BPND using visual cortex were 8.4%, and 19.5% lower than those calculated with the cerebellum in healthy control and methamphetamine-dependent subjects, respectively Levene's test revealed inequality of variance among the three samples (Levene's statistic=4.85; p=0.009, n=79, groups combined); post-hoc tests reveal greater sample variance of BPND using visual cortex than cerebellum (p=0.002, n=79 groups combined) and a trend towards inequality of variance between BPND using cerebellum and white matter (p=0.116, n=79, groups combined). BPND obtained with each of the three reference regions showed a significant subject-group difference between healthy- and methamphetamine-dependent participants by independent t tests: white matter (p = 0.004), visual cortex (p = 0.017) and cerebellum (p = 0.010). The effect size of the group difference in BPND was largest with white matter (0.68), followed by cerebellum (0.60) and visual cortex (0.57) (Table 2).
In order to interpret the practical impact of the differences in observed effect size, we calculated the projected number of subjects needed per group (in an independent sample) to allow detection this group difference in BPND with 80% power, using the observed effect sizes. The estimated number of subjects per group was 45 when using the cerebellum as the reference region, 50 when using the visual cortex, and 36 needed to detect a group difference in BPND when using white matter.
In the test-retest subsample analysis, test-retest reproducibility among the three reference regions was similar as the BPND values did not differ significantly between sessions, irrespective of reference region (paired t test). Finally, test-retest variability (VAR) in BPND using the visual cortex was 12.8% lower than VAR (Table 3) in BPND using cerebellum, and VAR using white matter was similar to cerebellum (1% lower in white matter), but there was no significant difference in VAR among BPND data obtained with the three reference regions (one-way repeated measures ANOVA; p = 0.576).
Table 3.
Test-retest reproducibility in 16 healthy participants
| Reference region | |||||
|---|---|---|---|---|---|
| Cerebellum | White Matter | Visual Cortex | |||
| HC (n=16) | Test | Striatal BPND | |||
| mean | 19.63 | 20.38 | 15.06 | ||
| CV | 0.205 | 0.176 | 0.201 | ||
| Retest | Striatal BPND | ||||
| mean | 18.86 | 19.45 | 14.63 | ||
| CV | 0.216 | 0.149 | 0.187 | ||
| Test vs Retest | t test* | ||||
| t value | 1.37 | 1.42 | 1.06 | ||
| p value | 0.19 | 0.17 | 0.30 | ||
| VAR (%) | |||||
| Mean ± SD | 9.93 ± 9.32 | 9.83 ± 11.88 | 8.65 ± 8.59 | ||
HC; healthy participants CV; coefficient of variation = standard deviation (SD) / mean
paired t test VAR (%); test-retest variability (%) = 100 × |scan 2 - scan 1| / (scan 2 + scan 1) / 2
Discussion
The primary objective of this study was to investigate the influence of reference- region choice on BPND using18F-fallypride and SRTM. We compared the visual cortex and the superior longitudinal fasciculus (a white matter region) with the cerebellum to determine if any region offered statistical or technical advantages over others. Using white matter as a reference region produced higher striatal BPND values compared to the other reference regions tested, consistent with reports of lower specific binding to D2/3 receptors in white matter than in cerebellum or visual cortex 12, 18. In this respect, white matter adheres more to the STRM requirements of very low specific binding in the reference region than the other reference regions examined in this study. Notably, using white matter yielded the most robust group difference (largest effect size) in BPND between healthy- and methamphetamine-dependent participants, consistent with prior reports of these differences 19, 25.
While other groups have noted that data obtained using 11C-FLB 457 and reference regions comprised mostly of grey matter exhibit TACs with markedly different shapes than those obtained using reference regions comprised of mostly white matter 12, 18F-fallypride TACs had similar shapes for all three reference tissues tested (Fig. 1), which suggests that the tracer kinetics are similar for 18F-fallypride among the reference regions examined. Higher BPND calculated using white matter might be explained by lower volume of distribution (K1′/k2′) of white matter compared to the target tissue. Although we are aware of no studies reporting K1′/k2′ of white matter using 18F-fallypride, the calculated total volume of distribution for 11C-FLB 457 in the cerebellum is highly correlated with that calculated in white matter 12. Although it should be noted that values of K1′/k2′ obtained for D2/3 receptor radioligands are both region- and tracer-specific 8, 26-28.
The present findings suggest that using the visual cortex as a reference region with SRTM produces BPND values for 18F-fallypride with higher sample variance than when the cerebellum is used. More sample variance in these data, compared to those obtained with white matter or cerebellum, may be attributed to poorer quality of the reference-region TAC measurement, possibly as a result of greater error in attenuation and scatter correction in the visual cortex when compared to the other reference regions. Attenuation and scatter correction are the most important correction processes of PET for quantitative analysis. These corrections, however, can be biased due to incorrect estimation of the attenuator. Because of poor counting statistics, bones near the lower parts of the skull are often misclassified as tissue on attenuation maps, and bias due to attenuation error in these regions is prominent. In particular, the largest negative bias from attenuation correction has occurred in the brain stem and cerebellum while the largest positive bias occurred in the occipital cortex and superior portion of the cerebellum 15.
On the basis of the data presented here, the visual cortex appears to offer little statistical or technical advantages over the cerebellum as a reference region for assessment of BPND for 18F-fallypride. However, our data support the use of the superior longitudinal fasciculus, a white-matter region, as a reference region in 18F-fallypride PET studies. While using white matter and the cerebellum produced comparable BPND values, with similar sample variance, the use of white matter resulted in a larger effect size for the group difference in BPND between healthy controls and methamphetamine-dependent individuals, and the required number of subjects needed to detect a group difference at ~80% power was approximately 20% fewer subjects when using white matter when compared to cerebellum. Therefore, white matter appears comparable in performance to the cerebellum as a reference region in studies using 18F-fallypride, and may offer slight statistical advantages in studies using high affinity D2/3 receptor radioligands. These findings have implications that could be extended to other high-affinity radiotracers with rapid cerebellar clearance and slow kinetics.
Although this work does not fully address limitations of reference-tissue models with high-affinity radioligands, investigating the effect of reference-region choice on BPND is an initial step towards identifying potential problems associated with modeling the kinetics of newly developed high-affinity ligands. Further investigations are advisable to assess the degree to which reference-region methods and other kinetic modeling techniques can be used accurately with high-affinity D2/3R ligands to estimate binding potential. As new radiotracers are developed for use with PET, it is necessary to re-evaluate the methods used to describe their binding kinetics accurately in vivo and to identify potential sources of error.
Acknowledgments
The research was supported by P20 DA022539, R01 DA015179, R01 DA020726 (EDL); M01 RR00865 (UCLA GCRC) and endowments from Marjorie Green Trust and the Thomas P. and Katherine K Pike Chair in Addiction Studies (EDL). CL Robertson was supported by T32 DA024635. We are grateful to Drs. Gerhard Hellemann and J David Jentsch for helpful comments.
Abbreviations
- BPND
Binding potential relative to non-displaceable uptake
- D2/3R
D2/D3 dopamine receptor subtype
- TAC
Time-activity curve
- SRTM
Simplified reference tissue model
References
- 1.Cunningham VJ, Jones T. Spectral analysis of dynamic PET studies. J Cereb Blood Flow Metab. 1993;13:15–23. doi: 10.1038/jcbfm.1993.5. [DOI] [PubMed] [Google Scholar]
- 2.Hume SP, Myers R, Bloomfield PM, et al. Quantitation of carbon-11-labeled raclopride in rat striatum using positron emission tomography. Synapse. 1992;12:47–54. doi: 10.1002/syn.890120106. [DOI] [PubMed] [Google Scholar]
- 3.Lammertsma AA, Bench CJ, Hume SP, et al. Comparison of methods for analysis of clinical [11C]raclopride studies. J Cereb Blood Flow Metab. 1996;16:42–52. doi: 10.1097/00004647-199601000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996;4:153–158. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- 5.Slifstein M, Laruelle M. Effects of statistical noise on graphic analysis of PET neuroreceptor studies. J Nucl Med. 2000;41:2083–2088. [PubMed] [Google Scholar]
- 6.Osman S, Lundkvist C, Pike VW, et al. Characterisation of the appearance of radioactive metabolites in monkey and human plasma from the 5-HT1A receptor radioligand, [carbonyl-11C]WAY-100635--explanation of high signal contrast in PET and an aid to biomathematical modelling. Nucl Med Biol. 1998;25:215–223. doi: 10.1016/s0969-8051(97)00206-0. [DOI] [PubMed] [Google Scholar]
- 7.Mukherjee J, Yang ZY, Das MK, Brown T. Fluorinated benzamide neuroleptics--III. Development of (S)-N-[(1-allyl-2-pyrrolidinyl)methyl]-5-(3-[18F]fluoropropyl)-2, 3-dimethoxybenzamide as an improved dopamine D-2 receptor tracer. Nucl Med Biol. 1995;22:283–296. doi: 10.1016/0969-8051(94)00117-3. [DOI] [PubMed] [Google Scholar]
- 8.Christian BT, Narayanan T, Shi B, et al. Measuring the in vivo binding parameters of [18F]-fallypride in monkeys using a PET multiple-injection protocol. J Cereb Blood Flow Metab. 2004;24:309–322. doi: 10.1097/01.WCB.0000105020.93708.DD. [DOI] [PubMed] [Google Scholar]
- 9.Oikonen V, Allonen T, Nagren K, et al. Quantification of [Carbonyl-(11)C]WAY-100635 binding: considerations on the cerebellum. Nucl Med Biol. 2000;27:483–486. doi: 10.1016/s0969-8051(00)00116-5. [DOI] [PubMed] [Google Scholar]
- 10.Slifstein M, Hwang DR, Huang Y, et al. In vivo affinity of [18F]fallypride for striatal and extrastriatal dopamine D2 receptors in nonhuman primates. Psychopharmacology (Berl) 2004;175:274–286. doi: 10.1007/s00213-004-1830-x. [DOI] [PubMed] [Google Scholar]
- 11.Asselin MC, Montgomery AJ, Grasby PM, Hume SP. Quantification of PET studies with the very high-affinity dopamine D2/D3 receptor ligand [11C]FLB 457: re-evaluation of the validity of using a cerebellar reference region. J Cereb Blood Flow Metab. 2007;27:378–392. doi: 10.1038/sj.jcbfm.9600340. [DOI] [PubMed] [Google Scholar]
- 12.Narendran R, Mason NS, Chen CM, et al. Evaluation of dopamine D(2)/(3) specific binding in the cerebellum for the positron emission tomography radiotracer [(1)(1)C]FLB457: implications for measuring cortical dopamine release. Synapse. 2011;65:991–997. doi: 10.1002/syn.20926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vandehey NT, Moirano JM, Converse AK, et al. High-affinity dopamine D2/D3 PET radioligands 18F-fallypride and 11C-FLB457: a comparison of kinetics in extrastriatal regions using a multiple-injection protocol. J Cereb Blood Flow Metab. 2010;30:994–1007. doi: 10.1038/jcbfm.2009.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vernaleken I, Peters L, Raptis M, et al. The applicability of SRTM in [(18)F]fallypride PET investigations: impact of scan durations. J Cereb Blood Flow Metab. 2011;31:1958–1966. doi: 10.1038/jcbfm.2011.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Son YD, Kim HK, Kim ST, et al. Analysis of biased PET images caused by inaccurate attenuation coefficients. J Nucl Med. 2010;51:753–760. doi: 10.2967/jnumed.109.070326. [DOI] [PubMed] [Google Scholar]
- 16.Belanger MJ, Mann JJ, Parsey RV. OS-EM and FBP reconstructions at low count rates: effect on 3D PET studies of [11C] WAY-100635. Neuroimage. 2004;21:244–250. doi: 10.1016/j.neuroimage.2003.08.035. [DOI] [PubMed] [Google Scholar]
- 17.Reilhac A, Tomei S, Buvat I, et al. Simulation-based evaluation of OSEM iterative reconstruction methods in dynamic brain PET studies. Neuroimage. 2008;39:359–368. doi: 10.1016/j.neuroimage.2007.07.038. [DOI] [PubMed] [Google Scholar]
- 18.Hall H, Farde L, Halldin C, et al. Autoradiographic localization of extrastriatal D2-dopamine receptors in the human brain using [125I]epidepride. Synapse. 1996;23:115–123. doi: 10.1002/(SICI)1098-2396(199606)23:2<115::AID-SYN7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 19.Lee B, London ED, Poldrack RA, et al. Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J Neurosci. 2009;29:14734–14740. doi: 10.1523/JNEUROSCI.3765-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown AK, Mandelkern MA, Farahi J, et al. Sex differences in striatal dopamine D2/D3 receptor availability in smokers and non-smokers. Int J Neuropsychopharmacol. :1–6. doi: 10.1017/S1461145711001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishibashi K, Berman SM, Paz-Filho G, et al. Dopamine D2/D3 receptor availability in genetically leptin-deficient patients after long-term leptin replacement. Mol Psychiatry. 2012;17:352–353. doi: 10.1038/mp.2011.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brix G, Zaers J, Adam LE, et al. Performance evaluation of a whole-body PET scanner using the NEMA protocol. National Electrical Manufacturers Association. J Nucl Med. 1997;38:1614–1623. [PubMed] [Google Scholar]
- 23.Watson CC. New, Faster, Image-Based Scatter Correction for 3D PET IEEE Trans Nuc Sci. 2000;47:1587–1594. [Google Scholar]
- 24.Ardekani BA, Braun M, Hutton BF, et al. A fully automatic multimodality image registration algorithm. J Comput Assist Tomogr. 1995;19:615–623. doi: 10.1097/00004728-199507000-00022. [DOI] [PubMed] [Google Scholar]
- 25.Volkow ND, Chang L, Wang GJ, et al. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- 26.Olsson H, Halldin C, Swahn CG, Farde L. Quantification of [11C]FLB 457 binding to extrastriatal dopamine receptors in the human brain. J Cereb Blood Flow Metab. 1999;19:1164–1173. doi: 10.1097/00004647-199910000-00013. [DOI] [PubMed] [Google Scholar]
- 27.Ito H, Sudo Y, Suhara T, et al. Error analysis for quantification of [(11)C]FLB 457 binding to extrastriatal D(2) dopamine receptors in the human brain. Neuroimage. 2001;13:531–539. doi: 10.1006/nimg.2000.0717. [DOI] [PubMed] [Google Scholar]
- 28.Ginovart N, Willeit M, Rusjan P, et al. Positron emission tomography quantification of [11C]-(+)-PHNO binding in the human brain. J Cereb Blood Flow Metab. 2007;27:857–871. doi: 10.1038/sj.jcbfm.9600411. [DOI] [PubMed] [Google Scholar]
- 29.Vernaleken I, Klomp M, Moeller O, et al. Vulnerability to psychotogenic effects of ketamine is associated with elevated D2/3-receptor availability. Int J Neuropsychopharmacol. 2012:1–10. doi: 10.1017/S1461145712000764. [DOI] [PubMed] [Google Scholar]
- 30.Kegeles LS, Slifstein M, Xu X, et al. Striatal and extrastriatal dopamine D2/D3 receptors in schizophrenia evaluated with [18F]fallypride positron emission tomography. Biol Psychiatry. 2010;68:634–641. doi: 10.1016/j.biopsych.2010.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Landvogt C, Buchholz HG, Bernedo V, et al. Alteration of dopamine D2/D3 receptor binding in patients with juvenile myoclonic epilepsy. Epilepsia. 2010;51:1699–1706. doi: 10.1111/j.1528-1167.2010.02569.x. [DOI] [PubMed] [Google Scholar]
- 32.Cropley VL, Innis RB, Nathan PJ, et al. Small effect of dopamine release and no effect of dopamine depletion on [18F]fallypride binding in healthy humans. Synapse. 2008;62:399–408. doi: 10.1002/syn.20506. [DOI] [PubMed] [Google Scholar]
- 33.Siessmeier T, Zhou Y, Buchholz HG, et al. Parametric mapping of binding in human brain of D2 receptor ligands of different affinities. J Nucl Med. 2005;46:964–972. [PubMed] [Google Scholar]
- 34.Riccardi P, Baldwin R, Salomon R, et al. Estimation of baseline dopamine D2 receptor occupancy in striatum and extrastriatal regions in humans with positron emission tomography with [18F] fallypride. Biol Psychiatry. 2008;63:241–244. doi: 10.1016/j.biopsych.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 35.Rominger A, Cumming P, Xiong G, et al. [18F]Fallypride PET measurement of striatal and extrastriatal dopamine D 2/3 receptor availability in recently abstinent alcoholics. Addict Biol. 2011;17:490–503. doi: 10.1111/j.1369-1600.2011.00355.x. [DOI] [PubMed] [Google Scholar]
- 36.Logan J, Fowler JS, Volkow ND, et al. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16:834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- 37.Mukherjee J, Christian BT, Dunigan KA, et al. Brain imaging of 18F-fallypride in normal volunteers: blood analysis, distribution, test-retest studies, and preliminary assessment of sensitivity to aging effects on dopamine D-2/D-3 receptors. Synapse. 2002;46:170–188. doi: 10.1002/syn.10128. [DOI] [PubMed] [Google Scholar]