Abstract
Streptococcus intermedius caused a liver abscess in a patient with chronic granulomatous disease (CGD). In contrast to typical staphylococcal abscesses in CGD, this abscess was liquid, easily drained, and resolved without surgery or steroids. This case and literature review provide insight into this organism’s pathogenesis, including in CGD.
Keywords: Chronic granulomatous disease, liver abscess, streptococcus intermedius
Introduction
Chronic granulomatous disease (CGD) is a phagocyte defect characterized by recurrent life-threatening bacterial and fungal infections due to defective NADPH oxidase activity, and impaired superoxide and downstream hydrogen peroxide (H2O2) production [1]. This defect can be inherited as an X-linked trait, affecting the NADPH oxidase gp91phox subunit (CYBB [cytochrome b-245, alpha polypeptide]), or as an autosomal recessive trait, affecting the remaining NADPH oxidase subunits (p22phox (CYBA [cytochrome b-245, beta polypeptide]), p47phox (NCF1 [neutrophil cytosolic factor 1]), p67phox (NCF2 [neutrophil cytosolic factor 2]), and p40phox (NCF4 [neutrophil cytosolic factor 4]) [2]. The recurrent infections in patients with CGD primarily affect the lung, skin, lymph nodes and liver, and are typically due to a rather narrow spectrum of bacteria and fungi, including Staphylococcus aureus, Serratia marcescens, Burkholderia cepacia complex, Nocardia species and Aspergillus species [3].
In contrast to patients with CGD, pyogenic liver abscesses in the general population are typically polymicrobial. Commonly isolated pathogens include members of the Streptococcus anginosus group (formerly known as the Streptococcus milleri group), Staphylococcus aureus, Streptococcus pyogenes, Escherichia coli, Klebsiella pneumoniae and Candida species [4].
We report a case of a liver abscess in a patient with CGD caused by Streptococcus intermedius, a member of the Streptococcus anginosus group. We also provide a review of reported cases of streptococcal infections in patients with CGD. Streptococcus intermedius is a usual suspect causing liver abscess in an unusual host, a finding that reinforces the importance of microbiologic identification of all liver abscesses in CGD.
Case Report
A 27 year-old Caucasian man presented with one week of fevers, rigors, diaphoresis, malaise and generalized fatigue while on prophylactic trimethoprim-sulfamethoxazole and levofloxacin. He was diagnosed with X-linked CGD (CYBB c.1234 G>C; p.G412R in the middle of exon 10) at three months because of perirectal abscesses. At one year he developed a Staphylococcus aureus liver abscess, and six months later a Burkholderia cepacia complex pneumonia. At age 20 a right upper lobe cavitary lesion was detected, which was eventually resected at 26 years. CGD colitis had been present for several years and was treated with mesalamine. He had no history of sick contacts, foreign travel, recent dental or surgical procedures and had been clinically well for one year prior to his current presentation.
He was febrile and diaphoretic (39.5 C), but otherwise well. Laboratory data on admission showed elevated liver enzymes (alanine aminotransferase 166 IU/L, aspartate aminotransferase 195 IU/L) and inflammatory markers (erythrocyte sedimentation rate 95 mm/h and c-reactive protein 272 mg/L). Computed axial tomography (CT) of the abdomen with intravenous contrast showed an abscess in the posterior right hepatic lobe with enlarged portocaval lymph nodes. Gadolinium-enhanced magnetic resonance imaging (MRI) of the abdomen confirmed a 6.5×5×4.5 cm abscess with subcentimeter satellite lesions communicating with the dominant abscess cavity (Fig. 1a). The abscess was biopsied and a drain placed percutaneously under CT guidance, releasing 90 ml of green mucopurulent fluid. Gram stain showed neutrophils with many gram-positive cocci in pairs and chains. Pinpoint alpha hemolytic colonies were seen after 48 h on sheep blood agar, with a distinctive butterscotch smell. One set of blood cultures grew gram-positive cocci in pairs and chains at 23 h. The API 20 Strep system (bioMerieux, Inc Hazelwood, MO) did not yield an acceptable identification score in conjunction with biochemical testing (catalase: negative, esculin: positive). Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) using a MALDI-TOF MicroFlex LT mass spectrometer (Bruker Daltonics, Billerica, MA) identified Streptococcus anginosus group with high score (~2.0). Partial sequencing of the 16S rRNA gene (~500 bp) showed 100% match to Streptococcus intermedius type strain.
Fig. 1.
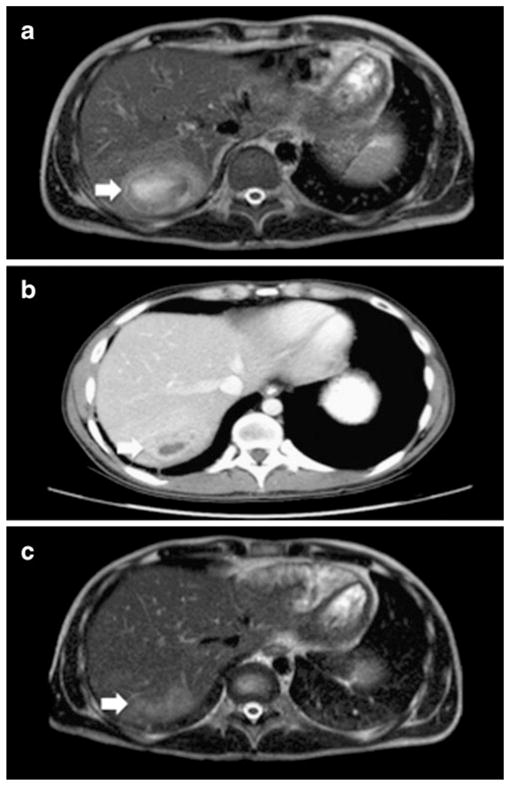
a MRI of the abdomen on admission. T2 heterogeneously hyperintense lesion measuring 6.5×5×4.5 cm within hepatic segment VIII, compatible with liver abscess. b CT scan of the abdomen two weeks after removal of percutaneous drain. c MRI of the abdomen one month after discharge. Residual 3.5×1.5 ×1.5 cm area of moderately increased T2 hyperintensity visible at the previous site of the abscess with mild enhancement but no central fluid collection
H2O2 production in the Streptococcus intermedius isolate was assessed using the method described by McGroarty et al. [5]. Streptococcus intermedius isolates from both the liver abscess and blood cultures were compared to Lactobacillus jensenii (positive control) [5] and Hemophilus paraphrophilus (negative control) isolates [6]. With this method, H2O2-producing colonies and their surroundings turn blue. In our case, only the Lactobacillus jensensii colonies developed a blue pigment, a finding that supports the previously reported lack of H2O2 production in Streptococcus intermedius isolates [7].
E-test® (Biomerieux Diagnostics, Marcy l’Etoile, France) showed that the isolate was susceptible to penicillin (minimal inhibitory concentrations in μg/ml [MIC]00.008), ceftriaxone (MIC00.125), meropenem (MIC00.016) and vancomycin (MIC01.0) but resistant to both clindamycin (MIC>256) and levofloxacin (MIC>32).
Following the sampling and initial drainage of the abscess, antibiotic coverage was initiated with vancomycin, clindamycin and meropenem. Five days later, antibiotics were narrowed to meropenem, and finally to penicillin G once speciation and sensitivities were confirmed.
The patient remained febrile for the first 7 days of his hospitalization with a maximum temperature of 41.3 C. Surveillance blood cultures were negative and follow up CT scan of the abdomen obtained two weeks after removal of the drain showed a significant decrease in size of the abscess, with inflammation tracking along the site of the hepatic drain (Fig. 1b). Given the patient’s history of CGD colitis, a colonoscopy was performed to evaluate for a potential source for the patient’s bacteremia and liver abscess. He had mild focal active colitis and several small poorly formed granulomata along with typical collections of pigmented macrophages in the rectum and ascending colon. The patient was discharged on an eight-week course of amoxicillin and probenecid. Liver MRI obtained one month after his discharge showed only a residual 3.5× 1.5×1.5 cm mildly enhancing lesion without central fluid collection (Fig. 1c), and subsequent studies have shown complete resolution.
Literature Review
A literature review of streptococcal infections in patients with CGD was performed using the Medline/PubMed database. Searches were performed using the terms “chronic granulomatous disease” and “streptococcus”. Our search yielded 5 reports of streptococcal infection in patients with CGD (Table I). We identified only two other cases of Streptococcus anginosus group pyogenic liver abscesses in patients with CGD. In one case, the organism was identified as Streptococcus intermedius [8], while in the other, the organism was simply indentified as belonging to the Streptococcus anginosus group [9]. In addition, one case of Streptococcus intermedius-brain abscess in a patient with CGD has also been reported [10]. All patients with Streptococcus anginosus group infections were treated with antibiotics and survived. Amongst the reported cases of streptococcal infections in patients with CGD, one patient died at 40 days of life of invasive group B streptococcus disease. The patient had surgery on an abscess of the anal margin, which grew out E. coli and Klebsiella pneumoniae, 20 days earlier [11].
Table I.
Streptococcal infections in patients with CGD
| Reference | Age/Gender | Genetics | Presentation | Pathogen | Treatment | Outcome |
|---|---|---|---|---|---|---|
| Present case | 27 y/male | X-linked | Liver abscess | Streptococcus intermedius | Penicillin G followed by amoxicillin and probenecid | Survived |
| [8] | 12 y/male | Not specified | Liver abscess | Streptococcus intermedius | Surgery and antibiotics | Survived |
| [10] | 20 y/male | X-linked | Brain abscess | Streptococcus intermedius | Carbapenem and granulocyte transfusion | Survived |
| [9] | 4 mo/female | Not specified | Liver abscess | Streptococcus anginosus group | Ceftazadime and clindamycin | Survived |
| [17] | 14 y/male | Autosomal recessive (not specified) | Mastoiditis and left postauricular abscess | Streptococcus viridans | Ceftriaxone | Survived |
| [18] | 46 y/female | Autosomal recessive (p47phox) | Cervical adenitis Liver abscess |
Streptococcus
viridans Streptococcus mitis |
Unspecified Percutaneous drainage and antibiotics |
Survived |
| [11] | 35 d/male | Not specified | Bacteremia meningitis | Group B Streptococcus | Ampicillin, gentamicin cefotaxime | Died |
Discussion
CGD patients are generally prone to infections by catalase-producing organisms, which are thought to degrade both microbial and host H2O2. Host-derived H2O2 in the phagolysosome is created by metabolism of the superoxide generated by the NADPH oxidase. Streptococcal infections are thought to be rare in CGD because these catalase-negative, H2O2-producing bacteria produce H2O2 which complements the superoxide production defect in CGD cells. However, Streptococcus intermedius is a H2O2 non-producing organism [7, 10], which may explain its pathogenicity in CGD. A similar situation has been described for Hemophilus paraphrophilus infection in patients with CGD [6].
Although streptococci are inherently catalase negative, the ability to produce H2O2 depends on the strain. Whiley et al., found that only 34 of 47 strains of Streptococcus anginosus produced H2O2, compared to 6 of 49 strains of Streptococcus constellatus, but none of the 61 strains of Streptococcus intermedius [7]. Interestingly, of the three subspecies listed, Streptococcus intermedius was especially associated with abscess formation, hematogenous spread and deep seated infections in the general population [12]. The reason for this predisposition to abscess formation is unclear, but may be related to toxic interactions with polymorphonuclear leukocytes [13]. Moreover, Streptococcus intermedius isolates from our patient did not produce H2O2. These findings confirm important biologic variation within the Streptococcus anginosus group, supporting the value of definitive speciation, such as by 16S rRNA sequencing.
Streptococcus anginosus infections often follow dental or gastrointestinal procedures. We hypothesize that our patient’s CGD colitis may have been a risk factor for bacterial seeding from the gastrointestinal tract to the liver. However, given the considerable prevalence of colitis in patients with CGD, this would not explain why Streptococcus anginosus is seen so infrequently in CGD.
The course and response to treatment in this case was very different than that of CGD patients with the more typical staphylococcal liver abscesses [14]. Staphylococcus aureus is the most common pathogen in CGD liver abscesses, which are typically difficult to treat. They usually require surgical resection, in addition to antibiotics, or systemic corticosteroids for complete resolution [15]. Our patient’s response to antibiotics and drainage alone was more rapid and less complicated than commonly seen with staphylococcal liver abscess in CGD. Moreover, our patient developed a Streptococcus intermedius liver abscess while on prophylactic levofloxacin. Although there is little data on the susceptibility of Streptococcus intermedius to quinolones, a study involving 180 clinical isolates of Streptococcus anginosus (29 of them Streptococcus intermedius) revealed 17.3% resistance of Streptococcus intermedius to ciprofloxacin [16]. Thus, it is possible that prophylactic levofloxacin may have predisposed our patient to a resistant strain of this organism.
This Streptococcus intermedius liver abscess in a patient with CGD is an example of a common pathogen causing disease in an uncommon host. Speciation within the Streptococcus anginosus group can lead to a better understanding of its pathogenesis in CGD and suggests that microorganisms other than Staphylococcus aureus should be considered in liver abscesses in patients with CGD.
Acknowledgments
Funding This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD 20892. The views expressed in this article are those of the authors and do not reflect the official policy of the U.S. Government.
Footnotes
Conflicts of Interest The authors declare that they have no conflict of interest.
Contributor Information
E. Liana Falcone, Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, 10 Center Drive, CRC, Rm B3 4141, MSC 1684, Bethesda, MD 20892-1684, USA.
Stephan Hanses, Department of Medicine, George Washington University Hospital, Washington, DC 20037, USA.
Frida Stock, Microbiology Service, Department of Laboratory Medicine, Clinical Center, National Institutes of Health, Bethesda, MD 20892, USA.
Steven M. Holland, Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, 10 Center Drive, CRC, Rm B3 4141, MSC 1684, Bethesda, MD 20892-1684, USA
Adrian M. Zelazny, Microbiology Service, Department of Laboratory Medicine, Clinical Center, National Institutes of Health, Bethesda, MD 20892, USA
Gulbu Uzel, Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, 10 Center Drive. CRC, MSC 1899, Bethesda, MD 20892-1899, USA.
References
- 1.Segal BH, Leto TL, Gallin JI, Malech HL, Holland SM. Genetic, biochemical, and clinical features of chronic granulomatous disease. Medicine (Baltimore) 2000;79:170–200. doi: 10.1097/00005792-200005000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Matute JD, Arias AA, Wright NA, Wrobel I, Waterhouse CC, Li XJ, et al. A new genetic subgroup of chronic granulomatous disease with autosomal recessive mutations in p40 phox and selective defects in neutrophil NADPH oxidase activity. Blood. 2009;114:3309–15. doi: 10.1182/blood-2009-07-231498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Towbin AJ, Chaves I. Chronic granulomatous disease. Pediatr Radiol. 2010;40:657–68. doi: 10.1007/s00247-009-1503-3. quiz 792–653. [DOI] [PubMed] [Google Scholar]
- 4.Huang CJ, Pitt HA, Lipsett PA, Osterman FA, Jr, Lillemoe KD, Cameron JL, et al. Pyogenic hepatic abscess. Changing trends over 42 years. Ann Surg. 1996;223:600–7. doi: 10.1097/00000658-199605000-00016. discussion 607–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGroarty JA, Tomeczek L, Pond DG, Reid G, Bruce AW. Hydrogen peroxide production by Lactobacillus species: correlation with susceptibility to the spermicidal compound nonoxynol-9. J Infect Dis. 1992;165:1142–4. doi: 10.1093/infdis/165.6.1142. [DOI] [PubMed] [Google Scholar]
- 6.Kottilil S, Malech HL, Gill VJ, Holland SM. Infections with Haemophilus species in chronic granulomatous disease: insights into the interaction of bacterial catalase and H2O2 production. Clin Immunol. 2003;106:226–30. doi: 10.1016/s1521-6616(02)00048-7. [DOI] [PubMed] [Google Scholar]
- 7.Whiley RA, Fraser H, Hardie JM, Beighton D. Phenotypic differentiation of Streptococcus intermedius, Streptococcus constellatus, and Streptococcus anginosus strains within the “Streptococcus milleri group”. J Clin Microbiol. 1990;28:1497–501. doi: 10.1128/jcm.28.7.1497-1501.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakhleh RE, Glock M, Snover DC. Hepatic pathology of chronic granulomatous disease of childhood. Arch Pathol Lab Med. 1992;116:71–5. [PubMed] [Google Scholar]
- 9.Spiegel R, Miron D, Horovitz Y. Pyogenic liver abscess in children. Harefuah. 1997;133:613–5. 663. [PubMed] [Google Scholar]
- 10.Nagatomo T, Ohga S, Saito M, Takada H, Sasaki Y, Okada K, et al. Streptococcus intermedius-brain abscess in chronic granulomatous disease. Eur J Pediatr. 1999;158:872–3. doi: 10.1007/s004310051231. [DOI] [PubMed] [Google Scholar]
- 11.Garel D, Devictor D, Tchernia G, Pham T, Dommergues JP. Streptococcus group B tardive meningitis revealing chronic septic granulomatosis. Ann Pediatr (Paris) 1989;36:35–7. [PubMed] [Google Scholar]
- 12.Claridge JE, 3rd, Attorri S, Musher DM, Hebert J, Dunbar S. Streptococcus intermedius, Streptococcus constellatus, and Streptococcus anginosus (“Streptococcus milleri group”) are of different clinical importance and are not equally associated with abscess. Clin Infect Dis. 2001;32:1511–5. doi: 10.1086/320163. [DOI] [PubMed] [Google Scholar]
- 13.Wanahita A, Goldsmith EA, Musher DM, Clarridge JE, 3rd, Rubio J, Krishnan B, et al. Interaction between human polymorphonuclear leukocytes and Streptococcus milleri group bacteria. J Infect Dis. 2002;185:85–90. doi: 10.1086/338145. [DOI] [PubMed] [Google Scholar]
- 14.Lublin M, Bartlett DL, Danforth DN, Kauffman H, Gallin JI, Malech HL, et al. Hepatic abscess in patients with chronic granulomatous disease. Ann Surg. 2002;235:383–91. doi: 10.1097/00000658-200203000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leiding JW, Freeman AF, Marciano BE, Anderson VL, Uzel G, Malech HL, et al. Corticosteroid Therapy for Liver Abscess in Chronic Granulomatous Disease. Clin Infect Dis. 2011 doi: 10.1093/cid/cir896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Limia A, Jimenez ML, Alarcon T, Lopez-Brea M. Five-year analysis of antimicrobial susceptibility of the Streptococcus milleri group. Eur J Clin Microbiol Infect Dis. 1999;18:440–4. doi: 10.1007/s100960050315. [DOI] [PubMed] [Google Scholar]
- 17.Wilson R, Bhuta T, Haydon R. Multiple simultaneous complications of acute otitis media in a child diagnosed with chronic granulomatous disease: a case report. Ear Nose Throat J. 2008;87:271–2. 279. [PubMed] [Google Scholar]
- 18.Lo R, Rae J, Noack D, Curnutte JT, Avila PC. Recurrent streptococcal hepatic abscesses in a 46-year-old woman. Ann Allergy Asthma Immunol. 2005;95:325–9. doi: 10.1016/S1081-1206(10)61149-0. [DOI] [PubMed] [Google Scholar]


