Abstract
Objective
Perivascular adipose tissue (PVAT) expands during obesity, is highly inflamed, and correlates with coronary plaque burden and increased cardiovascular risk. We tested the hypothesis that PVAT contributes to the vascular response to wire injury and investigated the underlying mechanisms.
Approach and Results
We transplanted thoracic aortic PVAT from donor mice fed a high-fat diet (HFD) to the carotid arteries of recipient HFD-fed LDLR−/− mice. Two weeks after transplantation, wire injury was performed, and animals were sacrificed two weeks later. Immunohistochemistry was performed to quantify adventitial macrophage infiltration and neovascularization, and neointimal lesion composition and size. Transplanted PVAT accelerated neointimal hyperplasia, adventitial macrophage infiltration and adventitial angiogenesis. The majority of neointimal cells in PVAT-transplanted animals expressed α-smooth muscle actin, consistent with smooth muscle phenotype. Deletion of MCP-1 in PVAT substantially attenuated the effects of fat transplantation on neointimal hyperplasia and adventitial angiogenesis, but not adventitial macrophage infiltration. Conditioned medium from perivascular adipocytes induced potent monocyte chemotaxis in vitro and angiogenic responses in cultured endothelial cells.
Conclusions
These findings indicate that PVAT contributes to the vascular response to wire injury, in part through MCP-1-dependent mechanisms.
Keywords: Adipose tissue, Neointimal hyperplasia, Adventitial angiogenesis, Vasculopathy
Introduction
Obesity is associated with expansion of metabolically-active adipose tissues leading to local and systemic inflammation, insulin resistance, and dyslipidemia, all of which can contribute to cardiovascular disease1. In addition to these systemic effects, obesity is associated with expansion of perivascular adipose tissue (PVAT) immediately adjacent to the adventitia of large arteries. Emerging evidence suggests that PVAT may contribute to the pathogenesis of vascular disease2.
PVAT is anatomically co-localized with atherosclerotic lesions in humans, correlating with plaque burden and vascular calcification3,4. Also, inflammatory cell infiltration was reported to be markedly increased in PVAT surrounding atherosclerotic human aorta as compared with non-diseased aorta5. Moreover, inflammatory gene expression was shown to be upregulated5,6, and expression of adiponectin, an anti-inflammatory adipokine, was downregulated7,8, in PVAT surrounding diseased human coronary arteries. Thus, both the amount of PVAT and the degree of PVAT inflammation correlate with cardiovascular disease in humans.
Inflammation of human PVAT may in part relate to the unique properties of human perivascular adipocytes, which express higher levels of chemokines compared to adipocytes from other depots. In particular, expression of MCP-1 is approximately 10–40-fold higher in perivascular adipocytes (coronary artery) compared to corresponding subcutaneous and perirenal adipocytes derived from the same subjects9. Likewise, human perivascular adipocytes surrounding the radial artery secrete more MCP-1 than visceral or subcutaneous adipocytes derived from the same patients10. MCP-1 is best known for its role in recruiting monocytes/macrophages to the arterial wall, but it may also contribute to vascular smooth muscle cell (VSMC) proliferation, angiogenesis and other pathological processes11–15. In this regard, MCP-1 has been implicated in both atherosclerosis and wire injury-induced neointimal hyperplasia in rodent models11–15. However, the specific role of MCP-1 expression in PVAT in local vascular pathology has never been examined.
To examine the influence of PVAT on vascular disease, rodent models have been devised, each of which has inherent strengths and limitations. Several mouse models spontaneously lack PVAT, including the A-ZIP/F-1 mouse, the FAT-ATTAC mouse, and the SMPG knockout mouse16–18. Amongst these models, only the SMPG knockout mouse (generated by breeding PPARγ-floxed mice with SM22α-Cre mice) is selectively devoid of PVAT rather than generally lipodystrophic; however, loss of PPARγ expression in smooth muscle cells in the SMPG knockout mouse may complicate the vascular phenotype18. Surgical models have also been developed which involve removal of endogenous PVAT and/or transplantation of adipose tissue to the arterial wall19–21. Surgically removing endogenous PVAT is technically demanding and potentially can induce arterial injury. Likewise, transplanting PVAT poses major technical challenges given the minute size of this adipose depot in mice. In fact, prior publications have not included data on transplanted PVAT19–21; rather, the investigators transplanted subcutaneous or epididymal fat, which differs phenotypically from PVAT, to the vascular wall.
In this study, we devised a novel model enabling us to transplant PVAT harvested from the aorta to a region of the arterial tree of mice that is normally devoid of PVAT– the common carotid artery. We found that transplanted PVAT augmented injury-induced neointimal hyperplasia in the setting of HFD, while subcutaneous adipose tissue (SQAT) had no effect. The effects of PVAT transplantation were at least in part dependent on MCP-1 released locally by the adipose tissue. These findings demonstrate a causal relationship between PVAT and the development of local vasculopathy, and implicate MCP-1 secretion by PVAT as a mediator of this pathological process.
Materials and Methods
Materials and methods are available in the on-line only Supplement.
Results
To study the local effect of transplanted adipose tissue in vivo, PVAT was collected from the thoracic aorta of donor C57Bl/6J (wt) male animals fed a HFD for two weeks. Inguinal SQAT was harvested from the same group of donor mice and used as a control. PVAT from donor MCP-1−/− male mice on a C57Bl/6J background fed a HFD was also harvested for a second treatment group. We transplanted 2–3 mg of PVAT (wt or MCP-1−/−) or SQAT (wt) per mouse and executed wire injury of the left common carotid arteries of HFD-fed LDL receptor knockout (LDLR−/−) mice 14 days after transplantation22–25. To control for surgical manipulations, sham transplantation was performed on the contralateral right common carotid arteries and the left common carotid arteries of separate mice.
At the time of wire injury, sham transplanted segments were devoid of fat and showed no evidence of fibrosis or adhesions (Fig. 1A & 1B), while PVAT- (Fig. 1C & 1D) and SQAT- (not shown) transplanted segments demonstrated glistening fat that was incorporated into the carotid adventitia and exhibited grossly-visible neovessels. Histology of PVAT-transplanted arteries demonstrated a mixed population of white and brown adipocytes abutting the adventitia, with inflammatory cells dispersed throughout the adipose tissue (Figure 2). Assessment of gene expression demonstrated that in transplanted PVAT and SQAT, mRNA expression of adiponectin and leptin was similar to endogenous fat harvested from the corresponding depots of recipient mice, suggesting that adipose phenotype was unaffected by the transplantation procedure (Supplemental Figure 2). Comparing PVAT-transplanted animals to sham-transplanted animals, total cholesterol (1085±187 vs. 1368±457 mg/dL, respectively, p=0.38) and triglyceride levels (440±175 vs. 484±134 mg/dL, respectively, p=0.75) were unchanged. These findings indicate that the transplanted adipose tissue acquired sufficient nutritive blood flow within two weeks to maintain viability, and that the phenotype of adipose tissue and systemic lipid levels were not significantly affected by the transplantation procedure.
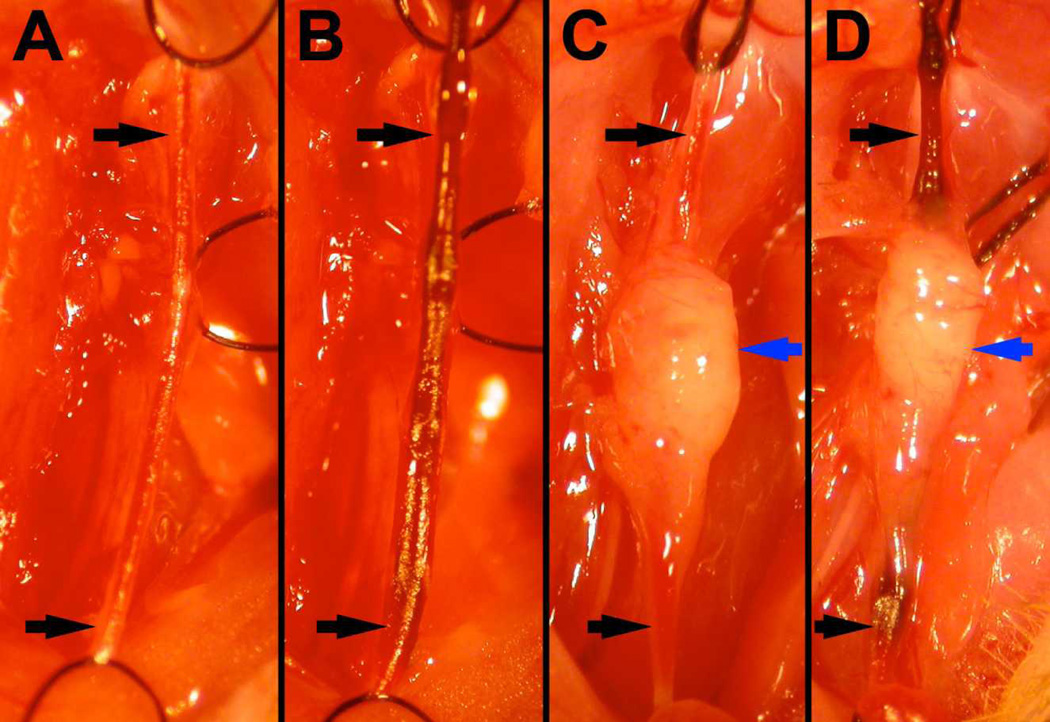
Figure 1.
Surgical images taken during wire injury procedure. Two weeks after either sham transplantation (A, B) or transplantation of 2–3mg of perivascular adipose tissue (C, D), wire injury was performed (B,D). The carotid artery (black arrows) was ligated with silk sutures proximally and distally, relative to the carotid bifurcation. Note that transplanted PVAT is healthy appearing, with incorporated vessels, at the time of wire injury (blue arrows, C & D).
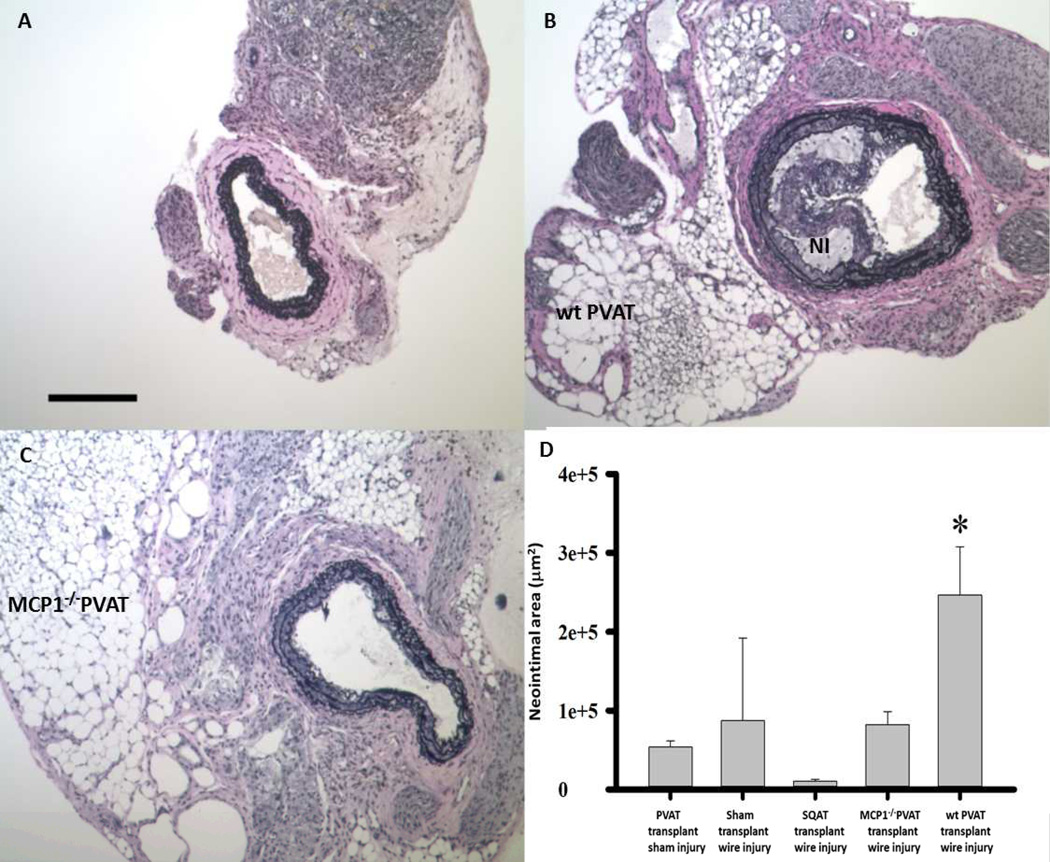
Figure 2.
Carotid arteries from LDLR−/− mice following wire injury. Compared to sham-transplanted control (A), transplanted wild-type (wt) PVAT resulted in a robust neointimal response (NI, panel B). Note that the transplanted adipocytes are healthy appearing and in intimate contact with the lamina adventitia. Transplanted MCP-1−/− PVAT was not associated with robust NI formation (C). scale bar = 200µm. (D) Transplanted PVAT increases cross-sectional area of neointimal lesion following wire injury. *p<0.05 PVAT + injury vs. all other groups. Data were analyzed by one-way analysis of variance (ANOVA) followed by pairwise multiple comparison procedures (Holm-Sidak method).
Fourteen days after wire injury, the mice were sacrificed, carotid arteries were harvested, and neointimal formation was examined in PVAT-transplanted versus sham-transplanted and SQAT-transplanted arteries. Histological analysis demonstrated that, compared to sham controls (Figure 2A), transplanted PVAT caused an increase in injury-induced neointimal area in LDLR−/− mice in the setting of HFD (NI, Fig. 2B). In contrast, transplanted PVAT from MCP-1−/− mice failed to induce a robust neointimal response (Figure 2C). Quantification analysis revealed that, at 14 days after injury, wt PVAT resulted in ~3 fold increase in neointimal formation compared to sham transplant control (Fig. 2D, Supplemental Figure 3). Transplanted SQAT was not statistically different in influencing neointimal hyperplasia compared to sham transplantation. Taken together, these data suggest that transplanted PVAT augments neointimal hyperplasia through a mechanism that is dependent on locally produced MCP-1.
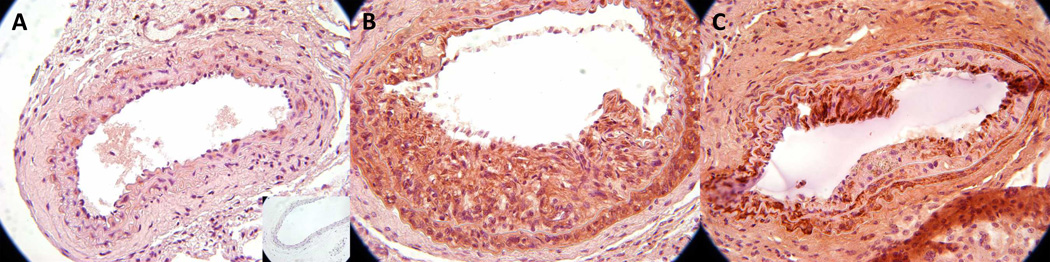
To determine the composition of the neointimal lesions in PVAT-transplanted mice, we performed immunostaining for α-smooth muscle actin (SMA, Figure 3). We found that compared to sham transplanted controls (Figure 3A), mice transplanted with wt PVAT exhibited a SMA-rich neointima, suggesting a predominant VSMC composition of the neointima (Figure 3B and Supplemental Figure 4). We also detected rare cells expressing the macrophage marker F4/80 dispersed throughout the neointima (Figure 4B, black arrow). Notably, mice transplanted with PVAT from MCP-1−/− mice exhibited marked reduction in S–positive neointimal staining (Figure 3C and Supplemental Figure 4), suggesting reduced accumulation of VSMC in the neointima.
Figure 3.
Composition of VSMC in neointima following PVAT transplantation and wire injury. SM actin staining in sham-transplanted control (A), transplanted wild-type (wt) PVAT (B) and transplanted MCP-1−/− PVAT (C) carotid arteries following wire injury. Note that the majority of NI cells in B and C stain positively for SMA.
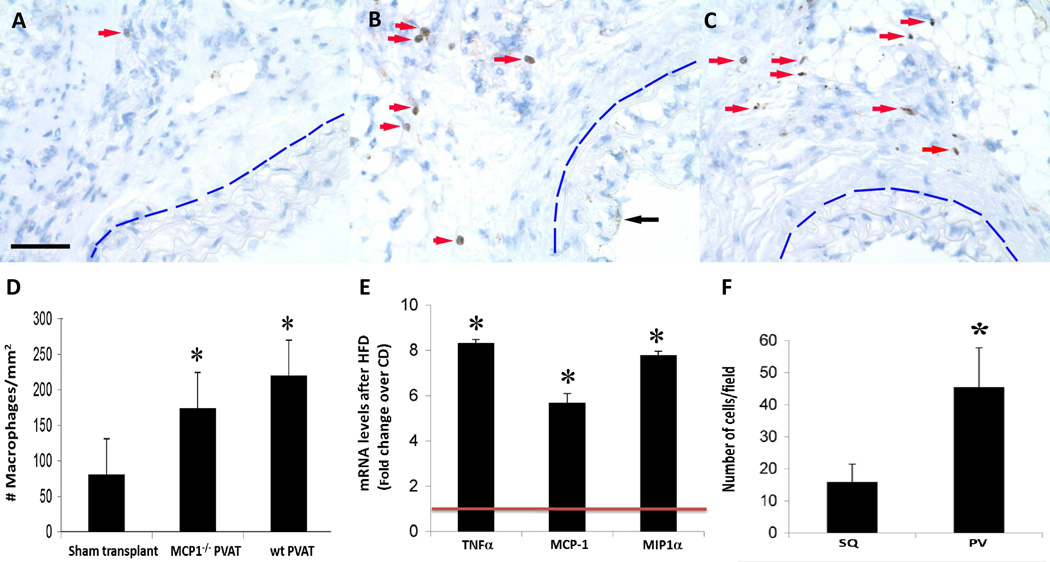
Figure 4.
Inflammation following PVAT transplantation and wire injury. F4/80 staining revealed few adventitial macrophages following wire injury surrounding sham-transplanted arteries (panel A, red arrow). In contrast, transplanted WT PVAT markedly increased the presence of adventitial macrophages surrounding injured arteries (panel B, red arrows); transplanted MCP-1−/− PVAT was associated with a similar increase in the infiltration of adventitial macrophages (panel C, red arrows). Note: In all panels, the blue line demarcates the external elastic lamina of the injured vessel; black arrow in B points to neointimal F4/80 staining. scale bar = 50 µm. Quantification of adventitial macrophages in all three groups is shown in (D): sham transplant: 81±12; MCP-1−/− PVAT: 174±48; wt PVAT: 220±108 cells/mm2 (all data expressed as mean ± standard deviation). *p<0.01 vs. sham transplant. (E) mRNA levels of inflammatory cytokines TNF-α, MCP-1 and MIP-1α in intact perivascular adipose tissue of C57Bl/6J mice fed a chow or HFD for 2 weeks. mRNA levels of selected genes were quantified by qRT-PCR after normalizing to the house-keeping gene RPLPO (ribosomal protein large O) according to previously described methods (9). Results are normalized to the expression in chow-fed animals (red line). *p<0.001 vs. chow from three independent experiments. Data in D and E were evaluated by one-way ANOVA followed by Student–Newman–Keuls testing. (F) Monocyte migration through endothelial monolayer toward conditioned medium from cultured subcutaneous (SQ) or perivascular (PV) murine adipocytes. Monocytes were stained with DAPI, captured in three random view fields (40×), and quantified using Image J. *p<0.001 vs. SQ. Data were compared by one-way ANOVA followed by a 2-tailed Student’s t test to evaluate levels of significance at 95% confidence.
In view of previous reports that HFD induces inflammation of adipose tissues, especially visceral adipose tissues, we surmised that inflammatory cell infiltration in the adjacent vascular adventitia would be enhanced in PVAT-transplanted arteries subjected to wire injury. Indeed, staining for F4/80 demonstrated increased infiltration of macrophages into the adventitia after PVAT transplantation (Fig. 4B, red arrows) as compared with sham transplantation (Fig. 4A) or SQAT transplantation (Fig. 4F). Staining for a second macrophage marker, Mac3, showed similar results, as well as clustering of macrophages near the border of PVAT with the adventitia, as has been demonstrated in atherosclerotic human aorta (Supplemental Figure 5). HFD upregulates MCP-1 expression in visceral adipose tissues, and this inflammatory mediator can contribute to recruitment of leukocytes and adipose inflammation26,27. Transplantation of MCP-1−/− PVAT, however, produced similar numbers of infiltrating adventitial macrophages compared to the wild-type PVAT (Fig. 4C & 4D, Supplemental Figure 5), suggesting that other chemokines from PVAT could compensate for MCP-1 gene deletion to promote macrophage recruitment to the adventitia. Consistent with this notion, we observed that, in addition to MCP-1, mRNA expression of TNF-α and MIP-1α in PVAT was approximately 6-fold increased following two weeks of HFD versus chow diet (Fig. 4E). Likewise, immunostaining for CD3 appeared similar in arteries transplanted with MCP-1−/− PVAT as compared to wt PVAT, suggesting that MCP-1 gene deletion did not significantly impact T cell recruitment (Supplemental Figure 6).
Tissue macrophages can be classified by their polarization state, broadly described as pro-inflammatory (“M1 polarized”) versus anti-inflammatory (“M2 polarized”). We performed limited experiments to examine the mRNA expression of iNOS, a prototypical marker of M1 polarization, and Ym1, a marker of M2 polarization. Expression of iNOS was decreased by 6-fold (p<0.01) in MCP-1−/− PVAT compared to wild-type PVAT, while the expression Ym1 was largely unchanged (p = NS). Interestingly, both of these markers were significantly downregulated in SQAT from the same animals, suggesting a depot-specific effect of MCP-1 gene deletion (Supplemental Figure 7).
We previously demonstrated that human perivascular adipocytes secrete considerably more MCP-1, IL-6 and IL-8 as compared with subcutaneous and perirenal adipocytes9, implying an enhanced chemoattractant property of PV adipocytes. To investigate the potential involvement of murine perivascular adipocytes in recruiting inflammatory cells, we analyzed the effects of conditioned medium from perivascular versus subcutaneous adipocytes on monocyte migration through an endothelial monolayer. Conditioned medium from murine perivascular adipocytes elicited a significantly greater monocyte migration compared to conditioned medium from subcutaneous adipocytes (Figure 4F). In a separate series of experiments, we conditioned medium for 4 hours at 37 degrees by incubating with 10 mg of intact, freshly harvested PVAT from either MCP-1−/− or wt mice fed a Western diet for 4 weeks. We found that MCP-1 deletion did not diminish monocyte migration using the same in vitro assay (Supplemental Figure 8). Taken together, these results suggest that perivascular adipocytes are more potent than subcutaneous adipocytes at eliciting monocyte chemotaxis, and that production of other chemokines in PVAT compensates for loss of MCP-1 to promote leukocyte recruitment.
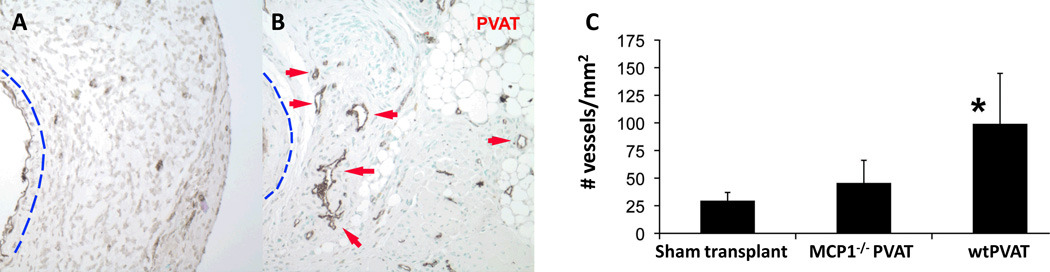
The grossly visible blood vessels coursing through PVAT (Fig 1) suggested enhanced neovascularization induced by the transplanted fat. This was confirmed by staining for the endothelial marker Factor VIIIa-related antigen, which showed markedly increased adventitial neovessel formation after PVAT transplantation as compared with sham transplantation (Fig. 5B, red arrows). Specifically, injured sham transplanted adventitia was characterized by small vascular structures with poorly-defined lumens, while PVAT transplantation was associated with an extensive adventitial vasculature between the transplanted PVAT and external elastic lamina of the artery. Interestingly, transplanted PVAT from MCP-1−/− mice failed to elicit a robust angiogenic response in the adventitia, and the change in neovessel density was not statistically significant compared to sham transplantation (Figure 5C).
Figure 5.
Adventitial neovascularization of injured carotid arteries. Endothelial cells were identified by staining for the Factor VIIIa-associated antigen. Wire injury following sham PVAT transplantation was associated with little evidence of adventitial neovessels (A). In sharp contrast, PVAT transplantation resulted in robust adventitial neovessels with well-defined lumens adjacent to the injured vessel (panel B, red arrows). Note: In panels A and B, the blue line demarcates the external elastic lamina of the injured vessel. PVAT transplantation significantly increased the density of neovessels within 150 µm of the IEL of injured carotid arteries from 30±8 to 100±50 vessels/mm2 (panel C, n=4, *p<0.01 vs. sham transplant, all data expressed as mean ± standard deviation). MCP-1−/− PVAT was not associated with a statistically significant increase in adventitial neovessel density (50±20 vessels/mm2). Data were compared by one-way ANOVA followed by a 2-tailed Student’s t test to evaluate levels of significance at 95% confidence.
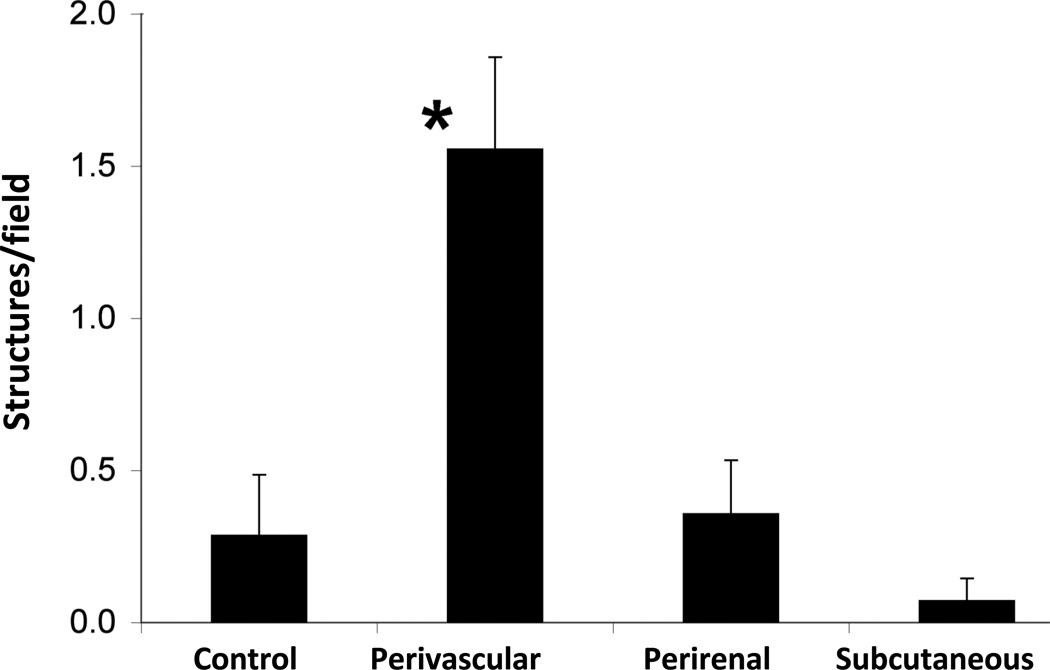
Adipocytes are powerful inducers of angiogenesis28. To compare the angiogenic potential of perivascular adipocytes with subcutaneous and visceral adipocytes, we performed an in vitro bioassay. Human perivascular preadipocytes were differentiated into mature adipocytes, as described previously9, and conditioned medium from the cultured cells was collected and applied to quiescent, subconfluent HCAEC. Medium from identically processed subcutaneous and perirenal adipocytes derived from the same subjects were used for comparison purposes. We found that conditioned medium from differentiated cultures of human PV adipocytes strongly induced HCAEC to elongate and form branching structures indicative of angiogenesis (Figure 6). The angiogenic effects of conditioned medium from perivascular adipocytes far exceeded those elicited by subcutaneous or perirenal adipocytes.
Figure 6.
Angiogenic effects of cultured perivascular adipocytes. Subconfluent human coronary artery endothelial cells (HCAEC) were treated with conditioned medium (CM) from primary cultures of differentiated human perivascular, subcutaneous or perirenal adipocytes. The number of branching structures, an indicator of angiogenesis, was quantified by blinded observers. *p<0.01 vs. all other groups. Data were compared by one-way ANOVA followed by a 2-tailed Student’s t test to evaluate levels of significance at 95% confidence.
VEGF is a powerful angiogenic factor that has been implicated in adventitial neovascularization following endothelial injury29–30 and is produced by adipocytes31. Therefore, we examined release of VEGF into the medium by perivascular adipocytes. As compared with subcutaneous adipocytes, perivascular adipocytes released approximately twice as much VEGF into the medium during incubation (94±35 vs 49±5 pg) for an equal number of cells.
Discussion
PVAT – the adipose tissue surrounding the great vessels - expands during obesity, is highly inflamed and correlates with coronary plaque burden and increased cardiovascular risk in humans. A consensus is emerging that PVAT is a cause of cardiovascular disease, but direct proof is lacking2. Here, we have established a model whereby a small quantity of PVAT is transplanted to the mouse carotid artery in order to test its effects on neointimal formation. In addition to being the first to assess the local effect of transplanted perivascular adipose tissue on the vasculature, our model is unique in that we transplanted merely 2–3 mg of PVAT, which is proportionate to the amount of endogenous PVAT surrounding conduit human arteries (Figure 1). Our data using this model suggest that PVAT can enhance the neointimal response to vascular injury through a mechanism involving MCP-1.
The focus in atherosclerosis research has traditionally revolved around the endothelium, the innermost component of the arterial wall. The deeper layers of the arterial wall, including the media and adventitia, have received far less attention yet are clearly important to the disease process. In particular, remodeling of the adventitia, and proliferation of adventitial vasa vasorum, has been detected very early in the course of experimental hyperlipidemia, preceding structural and functional changes in the endothelium32. Also, the adventitia has been identified as the major site of vascular inflammatory cell accumulation in hyperlipidemic, atherosclerosis-prone apoE deficient mice33. These and other studies implicate the vascular adventitia in the pathogenesis of vascular disease (e.g., “outside-in” process of lesion formation). The mechanisms that drive adventitial inflammation and angiogenesis, however, remain to be determined. We demonstrate adventitial inflammation, angiogenesis and increased neointimal formation in PVAT-transplanted carotid arteries following short-term high fat feeding and wire injury, suggesting a pathogenic role for PVAT in vascular disease. On the other hand, meticulous removal of all endogenous PVAT enhanced the neointimal response to wire injury in the femoral artery19, suggesting that the presence of some amount of PVAT is vasculoprotective in this blood vessel. Moreover, data in the SMPG knockout mouse suggest an atheroprotective effect of PVAT when animals are housed at a reduced temperature, presumably due to increased systemic metabolic activity from adaptive thermogenesis18.
Several studies have examined the impact of transplanted adipose tissue on local vascular biology. In two studies, endogenous PVAT was removed and replaced with subcutaneous or visceral fat prior to performing wire injury of the femoral artery19,21. In these studies, subcutaneous fat transplanted from mice fed a normal diet inhibited neointimal formation after injury, whereas subcutaneous fat transplanted from high fat-, high sucrose-fed mice had no effect19. Transplantation of visceral epididymal adipose tissue also diminished neointimal hyperplasia, and this protective effect was lost when the pro-inflammatory angiopoietin-like protein 2 gene was overexpressed in the transplanted adipose tissue21. In a third study, atherosclerosis was quantified following transplantation of visceral or subcutaneous fat to the carotid artery20. In this model, epididymal fat transplantation augmented atherosclerosis, while subcutaneous fat had minimal effect20. In the latter study, the investigators transplanted 60 mg of fat, which is disproportionate to the amount of PVAT that spontaneously forms around conduit arteries. Nevertheless, the augmentation of atherosclerosis observed in that study was clearly a local phenomenon, as vessels devoid of transplanted fat were unaffected. Therefore, studies to date have yielded conflicting results, suggesting that the influence of PVAT on vascular pathology is complex and dependent on the particular blood vessel and the experimental model employed.
The results obtained in the current study are not strictly comparable to any of the prior publications, since it is the only study in which authentic PVAT was transplanted. PVAT exhibits a distinct phenotype as compared with subcutaneous and epididymal adipose tissues, which must be taken into account when interpreting results from this study in the context of prior publications. To add to the complexity, PVAT in rodents and perhaps humans is comprised of both brown and white adipose tissue, depending on the anatomic location34,35. The divergent metabolic and inflammatory state of brown versus white adipose tissues may be an important factor in determining how PVAT locally influences vascular pathophysiology in rodent models and in humans.
PVAT differs from both subcutaneous and epididymal adipose tissues, both under basal conditions and after dietary manipulations, which may relate in part to the unique biochemical and molecular properties of perivascular adipocytes9,36. One of the most striking differences between perivascular adipocytes and adipocytes derived from other depots is increased expression of chemokines, especially MCP-1 (10–40 fold increased as compared with subcutaneous and perirenal adipocytes). Our results with PVAT transplanted from MCP-1−/− mice clearly implicate MCP-1 secretion by PVAT in the vascular response to wire injury. Based on these findings, we expected that neointimal proliferation associated with PVAT transplantation would consist of largely of inflammatory cells. Rather, we observed that most of the cells stained positively for α-smooth muscle actin, with relatively few inflammatory cells scattered throughout the neointima. Moreover, while PVAT transplantation triggered pronounced adventitial macrophage infiltration, it was not significantly diminished by ablation of MCP-1 expression in the PVAT. It is possible that polarization of the macrophages was affected by MCP-1 gene deletion in PVAT, based on the limited data with iNOS and Ym1 gene expression. Thus, phenotypic changes in macrophages could have indirectly contributed to the reduction in neointimal remodeling observed in the mice transplanted with MCP-1−/− PVAT. Given the small amount of available tissue, however, we were not able to specifically isolate macrophages from the PVAT tissue, nor were we able to assess expression of other genes related to macrophage polarization. Nevertheless, taken together with the immunostaining data for α-smooth muscle actin, these findings suggest that the effects of MCP-1 expression by PVAT on the vascular response to wire injury are largely independent of monocyte/macrophage recruitment.
Besides functioning as a chemokine to recruit inflammatory cells, MCP-1 has been demonstrated to elicit diverse effects on the vascular wall, including stimulation of VSMC proliferation and migration37,38. For example, incubation of human VSMC with MCP-1 stimulated an increase in proliferating nuclear cell antigen and cyclin A expression, and a two-fold increase in cell numbers39. Mechanistically, MCP-1-induced VSMC proliferation was found to be independent of NFκβ activation and mediated via phosphotidylinositol 3-kinase activation37. Whether MCP-1 expressed in PVAT acts on VSMC to enhance the neointimal response to injury remains to be determined. Also, it is conceivable that the MCP-1 might act on progenitor cells in the PVAT depot or the vascular adventitia, triggering their migration to the neointima and differentiation into a VSMC phenotype40. Interestingly, progenitor cells isolated from the aortic adventitia and transferred to the adventitial side of vein grafts implanted into hyperlipidemic mice were demonstrated to migrate to the intima, providing support for such a mechanism40.
We also observed that PVAT transplantation markedly stimulated adventitial angiogenesis, a finding which may shed light into the mechanisms of adventitial vasa vasorum proliferation following short-term high fat feeding32. In vitro studies demonstrated that perivascular adipocytes, as compared with their subcutaneous and perirenal counterparts, release higher quantities of soluble factors, such as VEGF, that induce pro-angiogenic responses in endothelial cells. Interestingly, the adventitial angiogenesis was significantly attenuated by transplanting PVAT from MCP-1−/− mice. A previous report suggested that MCP-1 can induce angiogenesis via upregulation of VEGF gene expression41. Further studies will be required to determine if this mechanism underlies the potent angiogenic effects of PVAT.
In summary, we report here that transplantation of 2–3 mg of PVAT to the carotid artery is sufficient to enhance vascular responses to wire injury in HFD-fed, hyperlipidemic mice, leading to accumulation of a VSMC rich neointima and prominent adventitial inflammation and angiogenesis. The pathogenic effects of PVAT transplantation in this model are mediated in part by expression of MCP-1.
Supplementary Material
Significance.
Perivascular adipose tissue (PVAT) surrounds most conduit arteries, and the amount and inflammatory state of PVAT correlates with the presence of vascular disease in humans. However, the mechanisms whereby PVAT interacts with the blood vessel wall to regulate vascular disease are poorly understood. We devised a novel model of adipose tissue transplantation to the common carotid artery, which is normally devoid of PVAT. We found that transplanted PVAT augmented injury-induced neointimal hyperplasia in the setting of high fat diet, while subcutaneous adipose tissue had no effect. The effects of PVAT transplantation were in part dependent on MCP-1 released locally by the adipose tissue, contributing to both accumulation of neointimal smooth muscle cells and adventitial angiogenesis. These findings provide new insight into the mechanisms whereby PVAT and MCP-1 regulate vascular disease.
Acknowledgements
sources of funding: this work was supported by NIH grants HL076684 and HL112640 (to N.L.W.), HL086555 (to Y.T.), DK74932 (to D.Y.H), and HL105675 (to A.L.B.)
Footnotes
disclosures: none
References
- 1.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–880. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 2.Verhagen SN, Visseren FL. Perivascular adipose tissue as a cause of atherosclerosis. Atherosclerosis. 2011;214:3–10. doi: 10.1016/j.atherosclerosis.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 3.Lehman SJ, Massaro JM, Schlett CL, O'Donnell CJ, Hoffmann U, Fox CS. Peri-aortic fat, cardiovascular disease risk factors, and aortic calcification: the Framingham Heart Study. Atherosclerosis. 2010;210:656–661. doi: 10.1016/j.atherosclerosis.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahabadi AA, Reinsch N, Lehmann N, Altenbernd J, Kalsch H, Seibel RM, Erbel R, Mohlenkamp S. Association of pericoronary fat volume with atherosclerotic plaque burden in the underlying coronary artery: a segment analysis. Atherosclerosis. 2010;211:195–199. doi: 10.1016/j.atherosclerosis.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Henrichot E, Juge-Aubry CE, Pernin A, Pache JC, Velebit V, Dayer JM, Meda P, Chizzolini C, Meier CA. Production of chemokines by perivascular adipose tissue: a role in the pathogenesis of atherosclerosis? Arterioscler Thromb Vasc Biol. 2005;25:2594–2599. doi: 10.1161/01.ATV.0000188508.40052.35. [DOI] [PubMed] [Google Scholar]
- 6.Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, Sarov-Blat L, O'Brien S, Keiper EA, Johnson AG, Martin J, Goldstein BJ, Shi Y. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–2466. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 7.Baker AR, Silva NF, Quinn DW, Harte AL, Pagano D, Bonser RS, Kumar S, McTernan PG. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc Diabetol. 2006;5:1. doi: 10.1186/1475-2840-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iacobellis G, Pistilli D, Gucciardo M, Leonetti F, Miraldi F, Brancaccio G, Gallo P, di Gioia CR. Adiponectin expression in human epicardial adipose tissue in vivo is lower in patients with coronary artery disease. Cytokine. 2005;29:251–255. doi: 10.1016/j.cyto.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Chatterjee TK, Stoll LL, Denning GM, Harrelson A, Blomkalns AL, Idelman G, Rothenberg FG, Neltner B, Romig-Martin SA, Dickson EW, Rudich S, Weintraub NL. Proinflammatory phenotype of perivascular adipocytes: influence of high-fat feeding. Circ Res. 2009;104:541–549. doi: 10.1161/CIRCRESAHA.108.182998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rittig K, Dolderer JH, Balletshofer B, Machann J, Schick F, Meile T, Küper M, Stock UA, Staiger H, Machicao F, Schaller HE, Königsrainer A, Häring HU, Siegel-Axel DI. The secretion pattern of perivascular fat cells is different from that of subcutaneous and visceral fat cells. Diabetologia. 2012;55:1514–1525. doi: 10.1007/s00125-012-2481-9. [DOI] [PubMed] [Google Scholar]
- 11.Aiello RJ, Bourassa P-AK, Lindsey S, Weng W, Natoli E, Rollins BJ, Milos PM. Monocyte chemoattractant protein-1 accelerates atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 1999;19:1518–1525. doi: 10.1161/01.atv.19.6.1518. [DOI] [PubMed] [Google Scholar]
- 12.Furukawa Y, Matsumori A, Ohashi N, Shioi T, Ono K, Harada A, Matsushima K, Sasayama S. Anti-monocyte chemoattractant protein-1/monocyte chemotactic and activating factor antibody inhibits neointimal hyperplasia in injured rat carotid arteries. Circ Res. 1999;84:306–314. doi: 10.1161/01.res.84.3.306. [DOI] [PubMed] [Google Scholar]
- 13.Roque M, Kim WJ, Gazdoin M, Malik A, Reis ED, Fallon JT, Badimon JJ, Charo IF, Taubman MB. CCR2 deficiency decreases intimal hyperplasia after arterial injury. Arterioscler Thromb Vasc Biol. 2002;22:554–559. doi: 10.1161/hq0402.105720. [DOI] [PubMed] [Google Scholar]
- 14.Arderiu G, Pena E, Aledo R, Juan-Babot O, Badimon L. Tissue factor regulates microvessel formation and stabilization by induction of chemokine (C-C motif) ligand 2 expression. Arterioscler Thromb Vasc Biol. 2011;31:2607–2615. doi: 10.1161/ATVBAHA.111.233536. [DOI] [PubMed] [Google Scholar]
- 15.Schober A. Chemokines in vascular dysfunction and remodeling. Arterioscler Thromb Vasc Biol. 2008;28:1950–1959. doi: 10.1161/ATVBAHA.107.161224. [DOI] [PubMed] [Google Scholar]
- 16.Moitra J1, Mason MM, Olive M, Krylov D, Gavrilova O, Marcus-Samuels B, Feigenbaum L, Lee E, Aoyama T, Eckhaus M, Reitman ML, Vinson C. Life without white fat: a transgenic mouse. Genes Dev. 1998;12:3168–3181. doi: 10.1101/gad.12.20.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pajvani UB1, Trujillo ME, Combs TP, Iyengar P, Jelicks L, Roth KA, Kitsis RN, Scherer PE. Fat apoptosis through targeted activation of caspase 8: a new mouse model of inducible and reversible lipoatrophy. Nat Med. 2005;11:797–803. doi: 10.1038/nm1262. [DOI] [PubMed] [Google Scholar]
- 18.Chang L1, Villacorta L, Li R, Hamblin M, Xu W, Dou C, Zhang J, Wu J, Zeng R, Chen YE. Loss of perivascular adipose tissue on peroxisome proliferator-activated receptor-γ deletion in smooth muscle cells impairs intravascular thermoregulation and enhances atherosclerosis. Circulation. 2012;126:1067–1078. doi: 10.1161/CIRCULATIONAHA.112.104489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takaoka M, Nagata D, Kihara S, Shimomura I, Kimura Y, Tabata Y, Saito Y, Nagai R, Sata M. Periadventitial adipose tissue plays a critical role in vascular remodeling. Circ Res. 2009;105:906–911. doi: 10.1161/CIRCRESAHA.109.199653. [DOI] [PubMed] [Google Scholar]
- 20.Ohman MK, Luo W, Wang H, Guo C, Abdallah W, Russo HM, Eitzman DT. Perivascular visceral adipose tissue induces atherosclerosis in apolipoprotein E deficient mice. Atherosclerosis. 2011;219:33–39. doi: 10.1016/j.atherosclerosis.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tian Z, Miyata K, Tazume H, Sakaguchi H, Kadomatsu T, Horio E, Takahashi O, Komohara Y, Araki K, Hirata Y, Tabata M, Takanashi S, Takeya M, Hao H, Shimabukuro M, Sata M, Kawasuji M, Oike Y. Perivascular adipose tissue-secreted angiopoietin-like protein 2 (Angptl2) accelerates neointimal hyperplasia after endovascular injury. J Mol Cell Cardiol. 2013;57C:1–12. doi: 10.1016/j.yjmcc.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Manka D, Collins RG, Ley K, Beaudet AL, Sarembock IJ. Absence of p-selectin, but not intercellular adhesion molecule-1, attenuates neointimal growth after arterial injury in apolipoprotein e-deficient mice. Circulation. 2001;103:1000–1005. doi: 10.1161/01.cir.103.7.1000. [DOI] [PubMed] [Google Scholar]
- 23.Schober A, Manka D, von Hundelshausen P, Huo Y, Hanrath P, Sarembock IJ, Ley K, Weber C. Deposition of platelet RANTES triggering monocyte recruitment requires P-selectin and is involved in neointima formation after arterial injury. Circulation. 2002;106:1523–1529. doi: 10.1161/01.cir.0000028590.02477.6f. [DOI] [PubMed] [Google Scholar]
- 24.Manka D, Forlow SB, Sanders JM, Hurwitz D, Bennett DK, Green SA, Ley K, Sarembock IJ. Critical role of platelet P-selectin in the response to arterial injury in apolipoprotein-E-deficient mice. Arterioscler Thromb Vasc Biol. 2004;24:1124–1129. doi: 10.1161/01.ATV.0000127619.04687.f4. [DOI] [PubMed] [Google Scholar]
- 25.Moore ZW, Hui DY. Apolipoprotein E inhibition of vascular hyperplasia and neointima formation requires inducible nitric oxide synthase. J Lipid Res. 2005;46:2083–2090. doi: 10.1194/jlr.M500177-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sartipy P, Loskutoff DJ. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc Natl Acad Sci U S A. 2003;100:7265–7270. doi: 10.1073/pnas.1133870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fukumura D1, Ushiyama A, Duda DG, Xu L, Tam J, Krishna V, Chatterjee K, Garkavtsev I, Jain RK. Paracrine regulation of angiogenesis and adipocyte differentiation during in vivo adipogenesis. Circ Res. 2003;93:e88–e97. doi: 10.1161/01.RES.0000099243.20096.FA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhardwaj S, Roy H, Heikura T, Yla-Herttuala S. VEGF-A, VEGF-D and VEGF-D(DeltaNDeltaC) induced intimal hyperplasia in carotid arteries. Eur J Clin Invest. 2005;35:669–676. doi: 10.1111/j.1365-2362.2005.01555.x. [DOI] [PubMed] [Google Scholar]
- 30.Ohtani K, Egashira K, Hiasa K, Zhao Q, Kitamoto S, Ishibashi M, Usui M, Inoue S, Yonemitsu Y, Sueishi K, Sata M, Shibuya M, Sunagawa K. Blockade of vascular endothelial growth factor suppresses experimental restenosis after intraluminal injury by inhibiting recruitment of monocyte lineage cells. Circulation. 2004;110:2444–2452. doi: 10.1161/01.CIR.0000145123.85083.66. [DOI] [PubMed] [Google Scholar]
- 31.Zhang QX, Magovern CJ, Mack CA, Budenbender KT, Ko W, Rosengart TK. Vascular endothelial growth factor is the major angiogenic factor in omentum: mechanism of the omentum-mediated angiogenesis. J Surg Res. 1997;67:147–154. doi: 10.1006/jsre.1996.4983. [DOI] [PubMed] [Google Scholar]
- 32.Herrmann J, Lerman LO, Rodriguez-Porcel M, Holmes DR, Jr, Richardson DM, Ritman EL, Lerman A. Coronary vasa vasorum neovascularization precedes epicardial endothelial dysfunction in experimental hypercholesterolemia. Cardiovasc Res. 2001;51:762–766. doi: 10.1016/s0008-6363(01)00347-9. [DOI] [PubMed] [Google Scholar]
- 33.Moos MP, John N, Grabner R, Nossmann S, Gunther B, Vollandt R, Funk CD, Kaiser B, Habenicht AJ. The lamina adventitia is the major site of immune cell accumulation in standard chow-fed apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2005;25:2386–2391. doi: 10.1161/01.ATV.0000187470.31662.fe. [DOI] [PubMed] [Google Scholar]
- 34.Fitzgibbons TP, Kogan S, Aouadi M, Hendricks GM, Straubhaar J, Czech MP. Similarity of mouse perivascular and brown adipose tissues and their resistance to diet-induced inflammation. Am J Physiol Heart Circ Physiol. 2011;301:H1425–H1437. doi: 10.1152/ajpheart.00376.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Padilla J, Jenkins NT, Vieira-Potter VJ, Laughlin MH. Divergent Phenotype of Rat Thoracic and Abdominal Perivascular Adipose Tissues. Am J Physiol Regul Integr Comp Physiol. 2013;304:R543–R552. doi: 10.1152/ajpregu.00567.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chatterjee TK, Aronow BJ, Tong WS, Manka D, Tang Y, Bogdanov VY, Unruh D, Blomkalns AL, Piegore MG, Jr, Weintraub DS, Rudich SM, Kuhel DG, Hui DY, Weintraub NL. Human coronary artery perivascular adipocytes overexpress genes responsible for regulating vascular morphology, inflammation, and hemostasis. Physiol Genomics. 2013;45:697–709. doi: 10.1152/physiolgenomics.00042.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Porreca E, Di Febbo C, Reale M, Castellani ML, Baccante G, Barbacane R, Conti P, Cuccurullo F, Poggi A. Monocyte chemotactic protein 1 (MCP-1) is a mitogen for cultured rat vascular smooth muscle cells. J Vasc Res. 1997;34:58–65. doi: 10.1159/000159202. [DOI] [PubMed] [Google Scholar]
- 38.Selzman CH, Miller SA, Zimmerman MA, Gamboni-Robertson F, Harken AH, Banerjee A. Monocyte chemotactic protein-1 directly induces human vascular smooth muscle proliferation. Am J Physiol Heart Circ Physiol. 2002;283:H1455–H1461. doi: 10.1152/ajpheart.00188.2002. [DOI] [PubMed] [Google Scholar]
- 39.Selzman CH, Miller SA, Zimmerman MA, Gamboni-Robertson F, Harken AH, Banerjee A. Monocyte chemotactic protein-1 directly induces human vascular smooth muscle proliferation. Am J Physiol Heart Circ Physiol. 2002;283:H1455–H1461. doi: 10.1152/ajpheart.00188.2002. [DOI] [PubMed] [Google Scholar]
- 40.Hu Y, Zhang Z, Torsney E, Afzal AR, Davison F, Metzler B, Xu Q. Abundant progenitor cells in the adventitia contribute to atherosclerosis of vein grafts in ApoE-deficient mice. J Clin Invest. 2004;113:1258–1265. doi: 10.1172/JCI19628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hong KH, Ryu J, Han KH. Monocyte chemoattractant protein-1-induced angiogenesis is mediated by vascular endothelial growth factor-A. Blood. 2005;105:1405–1407. doi: 10.1182/blood-2004-08-3178. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.