Abstract
Objective
Since statins have pleiotropic effects on inflammation and coagulation that may interrupt delirium pathogenesis, we tested the hypotheses that statin exposure is associated with reduced delirium during critical illness whereas discontinuation of statin therapy is associated with increased delirium.
Design
Multicenter, prospective cohort study.
Setting
Medical and surgical intensive care units (ICUs) in two large tertiary care hospitals in the United States.
Patients
Patients with acute respiratory failure or shock.
Measurements and Main Results
We measured statin exposure prior to hospitalization and daily during the ICU stay, and we assessed patients for delirium twice daily using the Confusion Assessment Method for the ICU. Of 763 patients included, whose median [interquartile range] age was 61 [51–70] years and APACHE II was 25 [19–31], 257 (34%) were prehospital statin users and 197 (26%) were ICU statin users. Overall, 588 (77%) developed delirium. After adjusting for covariates, ICU statin use was associated with reduced delirium (p < 0.01). This association was modified by sepsis and study day; e.g., statin use was associated with reduced delirium among patients with sepsis on study day 1 (odds ratio [OR], 0.22; 95% confidence interval [CI], 0.10–0.49) but not among patients without sepsis on day 1 (OR, 0.92; 95% CI, 0.46–1.84) or among those with sepsis later, e.g., on day 13 (OR, 0.70; 95% CI, 0.35–1.41). Prehospital statin use was not associated with delirium (OR, 0.86; 95% CI, 0.44–1.66; p = 0.18), yet the longer a prehospital statin user’s statin was held in the ICU, the higher the odds of delirium (overall p < 0.001 with the OR depending on sepsis status and study day due to significant interactions).
Conclusions
In critically ill patients, ICU statin use was associated with reduced delirium, especially early during sepsis; discontinuation of a previously used statin was associated with increased delirium.
Keywords: delirium, cognition, hydroxymethylglutaryl-CoA reductase inhibitors, critical illness, intensive care units, sepsis
Every year up to 20 million patients receive mechanical ventilation in intensive care units (ICU) (1); 60% to 80% of these patients experience delirium (2), an acute brain dysfunction associated with prolonged hospitalization (3, 4), increased mortality (4–6), excess healthcare costs (7), and persistent cognitive impairment (2). Though ongoing research is examining interventions that may prevent and/or treat delirium during critical illness, no intervention studied to date has been proven consistently efficacious.
Statins, among the most commonly prescribed medications, are used to prevent and manage cardiovascular diseases by over 25% of Americans ≥45 years old (8). Statins have anti-inflammatory (9, 10), endothelial function-enhancing (11, 12), and anticoagulant effects (13, 14) that may interrupt the pathologic events thought to lead to delirium during critical illness. These pleiotropic effects make statins promising candidates for the treatment of delirium during critical illness (15) since they might interrupt the neuroinflammatory cascade hypothesized to contribute to delirium (16, 17). In addition, evidence suggests that treatment of acutely ill patients with statins can reduce inflammation (18–20) whereas abrupt cessation of statins may cause rebound inflammation (21), leading to adverse cardiovascular outcomes (22–25). Given that intensivists frequently discontinue these and other home medications during acute critical illness (26), data are needed to examine not only on whether statin use reduces delirium but also whether statin discontinuation increases delirium.
Before the current investigation was designed, three observational studies examined the association between statins and delirium among cardiac surgery patients (27–29), but none of these studies assessed critically ill patients at highest risk for delirium, and the investigations had inconsistent results. We therefore conducted a multicenter, prospective cohort study to test our a priori hypotheses that 1) statin exposure is associated with reduced delirium during critical illness, 2) discontinuation of chronic statin therapy is associated with increased delirium during critical illness, and 3) the association between statins and delirium is most pronounced during critical illness characterized by inflammation and deranged coagulation, namely sepsis.
MATERIALS AND METHODS
Study Design and Population
We conducted this prospective cohort study at two large, independent tertiary care medical centers in Nashville, Tennessee, USA: Vanderbilt University Medical Center and Saint Thomas Hospital. We sought to study the most severely ill patients and therefore considered only adults (≥18 years old) admitted to a medical or surgical ICU with acute respiratory failure or shock to be eligible for enrollment. A patient was considered in respiratory failure if they were receiving invasive mechanical ventilation, noninvasive positive pressure ventilation, continuous positive airway pressure, or supplemental oxygen via a non-rebreather mask or nasal cannula delivering heated high-flow oxygen at the time of enrollment. We considered a patient to be in cardiogenic shock if they were being treated with an intra-aortic balloon pump or any of the following medications administered for acute cardiac dysfunction at the time of enrollment: dopamine ≥7.5 mcg/kg/min, dobutamine ≥5 mcg/kg/min, norepinephrine ≥5 mcg/min, phenylephrine ≥75 mcg/min, epinephrine at any dose, milrinone at any dose (if used with another vasopressor), or vasopressin ≥0.03 mcg/min (if used with another vasopressor). A patient was considered in septic shock if suspected or proven infection was documented in the setting of hypotension being treated with any of the previously listed medications.
In order to study patients at low risk for preexisting cognitive impairment, we excluded those with significant recent ICU time (i.e., >5 total ICU days in the 30 days preceding the current ICU admission, any ICU days spent on mechanical ventilation in the two months preceding the current ICU admission, and/or >72 hours in the current ICU admission); severe dementia (identified by a Clinical Dementia Rating Scale score of 3) (30); ICU admission post-cardiopulmonary resuscitation with suspected anoxic injury; or cardiac bypass surgery <3 months before the current ICU admission. In addition, to prevent enrollment of patients for whom long-term follow-up—which was conducted as part of a concurrent investigation of the same study population (31)—would not be possible, we also excluded patients due to active substance abuse or psychotic disorder; recent suicidal gesture necessitating hospitalization; blindness, deafness, or inability to speak English; moribund status (i.e., not expected to survive >24 hours and/or withdrawing life support); incarceration; homelessness; or residence >200 miles from Nashville. Lastly, we excluded from these analyses patients who were enrolled but never assessable for delirium due to persistent coma.
We enrolled eligible patients after obtaining informed consent from the patient or their authorized surrogate. Patients for whom consent was initially obtained through a surrogate were asked to provide informed consent if they became competent during their hospitalization. The Vanderbilt University Medical Center and Saint Thomas Hospital Institutional Review Boards approved the study protocol.
Exposures, Outcome, and Covariates
We examined statin use, the exposure of interest, using two variables: prehospital use, assessed once per patient, and ICU use, assessed daily throughout the 30-day study period. We classified patients as prehospital statin users if a statin was included in their electronic admission medication list. We classified patients as ICU statin users each day that their hospital medication administration record showed a statin was administered. These records were also used to determine the duration that statins were discontinued or temporarily held among prehospital statin users.
The primary outcome was delirium during the ICU stay. Trained research personnel assessed patients for delirium twice daily by using the Confusion Assessment Method for the ICU (CAM-ICU), a widely validated instrument that has high sensitivity and specificity for diagnosing delirium in the ICU (32–35). If either CAM-ICU assessment during a day was positive, then the patient was considered delirious on that day. When a delirium assessment was missing for reasons other than death, coma, or hospital discharge, we used single imputation to assign mental status for that day, relying on mental status the day before and after the missing assessment as well as whether death or discharge occurred the day after the missing assessment. Of all patient-days during which a mental status assessment was expected, only 3% were missing and required imputation.
We selected covariates for all models a priori based on multidisciplinary expert clinical judgment and prior research indicating these variables may confound the association between statin use and delirium. Covariates collected at study enrollment included age, baseline cognitive impairment assessed by the Short Informant Questionnaire of Cognitive Decline in the Elderly (Short IQCODE) (36, 37), clinical or subclinical cerebrovascular disease assessed by the Framingham Stroke Risk profile (38), and the hepatic component of the Sequential Organ Failure Assessment (SOFA) score (39). The latter was assessed as an enrollment covariate rather than a daily covariate because bilirubin was available at ICU admission but not often measured on a daily basis.
Because previous studies have suggested a “healthy user” effect among statin users, we also collected numerous additional variables for inclusion in a propensity score utilized to adjust for a patient’s likelihood of being on a statin before the hospitalization. We constructed the propensity score to estimate the likelihood of being prescribed a statin prior to hospitalization by fitting a logistic regression model with prehospital statin use as the dichotomous outcome (user vs. non-user). Independent variables included age, gender, history of stroke, ischemic heart disease, chronic heart failure, cancer, liver disease, renal disease, chronic obstructive pulmonary disease, diabetes, peripheral vascular disease, psychiatric illness, depression, Framingham Stroke Risk Profile (38), Charlson Comorbidity Index (40), current cigarette smoking and alcohol abuse, activities of daily living (ADLs) (41), instrumental ADLs (42), baseline cognitive impairment (Short IQCODE score) (37), home use of antidepressant or antipsychotics, insurance status, race, weight, and years of education. The linear predictor from this logistic regression model for each patient, which indicates the patient’s probability of being a prehospital statin user based on the variables included in the propensity score model, was included in all other models as a patient’s propensity for prehospital statin use.
Covariates collected on a daily basis included total daily doses of sedatives and analgesics (including benzodiazepines in midazolam equivalents, propofol, dexmedetomidine, and opioids in fentanyl equivalents); mechanical ventilation status; steroid use; study day; severity of illness as measured by the cardiovascular and respiratory components of the SOFA score (39); and presence of sepsis. Rather than categorizing patients as septic or not for the entirety of their hospitalization, we accounted for the time-varying nature of sepsis (i.e., the syndrome may occur at any time during a hospitalization and similarly may resolve at any time) by determining sepsis as present or absent on a daily basis according to international consensus criteria. Specifically, sepsis was considered present on each study day during which the patient had two or more Systemic Inflammatory Response Syndrome criteria and received an antibiotic for suspected or proven infection (43) and absent on each study day during which the international consensus criteria were not present.
Statistical Analysis
Lacking preliminary data from a critically ill population, we did not perform a priori power calculations to guide sample size. Instead, we enrolled as many patients as possible during the enrollment period, which was limited to three years given the funding and personnel available to support the investigation.
To determine whether prehospital and ICU statin use were associated with delirium in the ICU, which was an outcome measured repeatedly (i.e., daily in the ICU), we used multiple logistic regression with a robust Huber-White sandwich estimator to accurately estimate variance-covariance structure among correlated repeated measures. Prehospital statin use and covariates measured at enrollment, including the propensity score for prehospital statin use, were included in the logistic regression model as fixed variables in all observations for a given patient, whereas daily ICU statin use and covariates measured on a daily basis (including sepsis) were included in the logistic regression model as time-varying variables that could change daily, i.e., in each observation. Delirium was a time-varying, binary (delirium vs. normal mental status) repeated outcome variable, which also could change on a daily basis, i.e., the logistic regression model accounted for fluctuating delirium status; when a patient could not be assessed for delirium on a particular day in the 30-day study period because of coma, death, or hospital discharge, the observation was excluded from analysis. To determine whether associations between prehospital statin or ICU statin use and delirium were modified by sepsis or changed over the course of the ICU stay, we assessed for interactions between sepsis, study day, and prehospital and ICU statin use by including cross-product terms (e.g., sepsis*ICU statin use) in the regression model.
To examine the association between duration of statin discontinuation and delirium among prehospital statin users, we again used multiple logistic regression with a robust Huber-White sandwich estimator; in this case, daily delirium status was the outcome, and the cumulative number of days a prehospital statin user had been off statins was the primary exposure variable. All other components of this model (including covariates and interaction terms) were similar to the previously described regression model.
Though we planned a priori based on biological plausibility to examine potential interactions between statin exposure (and discontinuation), sepsis, and study day in regard to their associations with delirium, we also considered the possibility that such interactions might be influenced by changes in the cohort over time, e.g., patients in the ICU with sepsis after three weeks are likely different than those with sepsis early in their ICU stay. Thus, we conducted post hoc sensitivity analyses using the same multiple logistic regression models described above but limiting the data included to those obtained during the first five days of the study period, a period chosen because the majority of patients in this cohort spent 5 or fewer days in the ICU.
In all models, drug covariates were transformed using their cubic root to improve model fit and reduce the influence of extreme outliers. Continuous variables were allowed to have a nonlinear association with the outcome using restricted cubic splines (except for dexmedetomidine use, which was too uncommon in this cohort to provide enough unique doses for splines). We used R version 2.15.1 for all statistical analyses (44).
RESULTS
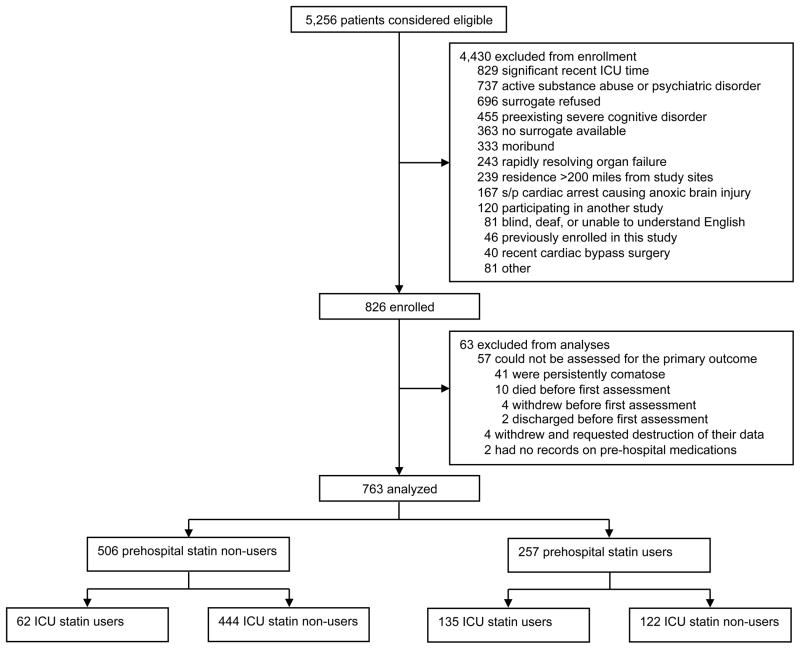
From March 2007 to May 2010, a total of 5,256 patients were screened; 4,430 of these were excluded, most often because of a recent ICU stay (Figure 1). Of the 826 patients enrolled, 63 were excluded from analyses, most due to persistent coma preventing assessment for delirium. Therefore, a total of 763 patients were included in this cohort; 257 (34%) of these were prehospital statin users. Among the 257 prehospital users, simvastatin (54%) was the most commonly used statin (median [IQR] dose, 40 [20–80] mg) with atorvastatin (20%), lovastatin (12%), rosuvastatin (8%), and pravastatin (6%) also used. Overall, these prehospital statins were discontinued for a median of 3 [0 to 13] days during the study period. In the ICU, simvastatin (40 [20–80] mg) was used by the large majority (78%) of the 197 patients who received an ICU statin; atorvastatin (14%), rosuvastatin (6%), and pravastatin (1%) were also used. Demographic data and clinical characteristics for the cohort are shown in Table 1. Statin users were generally older than non-users and had more cardiovascular disease, as indicated by higher Framingham Stroke Risk Profile scores and a higher proportion admitted with myocardial infarction and congestive heart failure. All groups had a high severity of illness (median [IQR] APACHE II score, 25 [19–31]). Though sepsis and/or ARDS were only documented as the admission diagnosis for one-third of patients, 418 (55%) patients met consensus criteria for sepsis on study day 1. A total of 588 (77%) patients experienced delirium during their ICU stay.
Figure 1.
Enrollment and Statin Exposure
Table 1.
Demographics and Clinical Characteristicsa
| Characteristics | Entire cohort N=763 | Non-statin users N=444 | ICU-only statin users N=62 | Prehospital & ICU statin users N=135 | Prehospital-only statin users N=122 |
|---|---|---|---|---|---|
| Age | 61 [51–70] | 57 [46–68] | 65 [55–72] | 67 [58–72] | 65 [58–73] |
| Female, N (%) | 372 (49) | 242 (45) | 24 (39) | 54 (40) | 52 (43) |
| Caucasian, N (%) | 686 (90) | 395 (89) | 59 (95) | 124 (92) | 108 (89) |
| Baseline Short IQCODEb | 3 [3–3.1] | 3 [3–3.1] | 3 [3–3.1] | 3 [3–3.1] | 3 [3–3.1] |
| Charlson Comorbidity Index | 2 [1–4] | 2 [1–4] | 1 [0–3] | 3 [1–4] | 3 [1–4] |
| Framingham Stroke Risk Profile score | 10 [6–14] | 8 [4–13] | 11 [7–16] | 14 [9–17] | 11 [8–16] |
| APACHE II score at ICU admission | 25 [19–31] | 25 [19–30] | 24 [21–29] | 25 [19–30] | 28 [21–34] |
| SOFA score at study enrollment | 10 [7–12] | 9 [7–12] | 10 [7–12] | 9 [7–11] | 9 [7–13] |
| ICU type, N (%) | |||||
| Medical | 507 (66) | 277 (62) | 52 (84) | 107 (79) | 71 (58) |
| Surgical | 256 (34) | 167 (38) | 10 (16) | 28 (21) | 51 (42) |
| ICU admission diagnoses, N (%) | |||||
| Sepsis/ARDS | 263 (34) | 174 (39) | 16 (26) | 33 (24) | 40 (33) |
| Surgeryc | 140 (18) | 89 (20) | 3 (5) | 17 (13) | 31 (25) |
| CHF/MI/arrhythmia | 126 (17) | 39 (8) | 35 (56) | 39 (29) | 13 (11) |
| Altered mental status | 90 (12) | 52 (12) | 1 (2) | 22 (16) | 15 (12) |
| COPD/asthma | 43 (6) | 20 (5) | 3 (5) | 14 (10) | 6 (5) |
| Otherd | 101 (13) | 70 (16) | 4 (6) | 10 (7) | 17 (14) |
| Mechanical ventilation at enrollment, N (%) | 667 (87) | 385 (87) | 58 (94) | 115 (85) | 109 (89) |
| ICU length of stay, days | 5.5 [2.9–11.9] | 5.0 [2.9–11.1] | 6.6 [3.0–14.6] | 3.8 [2.1–9.0] | 8.9 [4.1–14.8] |
| Delirium | |||||
| Prevalence during study period, N (%) | 595 (78) | 342 (77) | 53 (85) | 103 (76%) | 97 (80%) |
| Duration, days | 2 [1–6] | 2 [1–5] | 3 [1–6] | 2 [1–4] | 4 [1–8] |
Abbreviations: ARDS, acute respiratory distress syndrome; ADL, activities of daily living; APACHE II, Acute Physiology and Chronic Health Evaluation II; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; IQCODE, Informant Questionnaire of Cognitive Decline in the Elderly; IADL, instrumental activities of daily living; MI, myocardial infarction; ICU, intensive care unit; SOFA, Sequential Organ Failure Assessment.
Median [interquartile range] unless otherwise noted.
Patients with a Short IQCODE score ≥3.3 were considered to have preexisting cognitive impairment of mild to moderate severity. We excluded patients from study enrollment with dementia that prevented them from living independently and/or with a Clinical Dementia Rating (CDR) score of 3 or more, indicative of severe dementia.
Excluding cardiac and neurological surgery.
Including cirrhosis/hepatic failure, gastrointestinal bleeding, malignancy, metabolic disarray, hemoptysis, pulmonary embolism, renal failure, and seizure/status epilepticus.
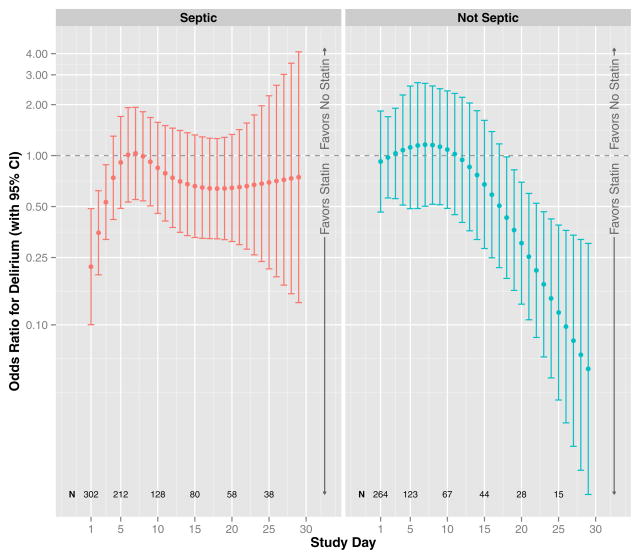
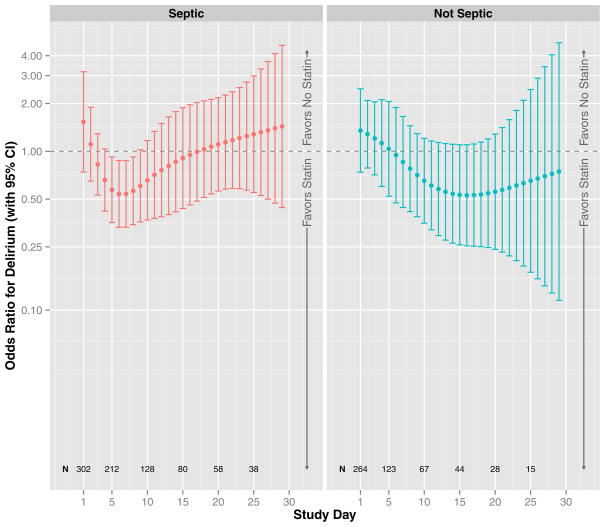
After adjusting for covariates, including prehospital statin use and daily severity of illness per organ-specific SOFA scores, ICU statin use was associated with reduced delirium (overall p for ICU statin use < 0.01, Figure 2). This association was significantly modified by sepsis (interaction p for sepsis*ICU statin use = 0.02) and by study day (interaction p for study day*ICU statin use < 0.01), which confirmed our a priori hypothesis and indicated that a single summary measure of the association between ICU statin use and delirium in the entire cohort (i.e., a single odds ratio) would be incomplete, ignoring these significant interactions with sepsis and study day. Figure 2 therefore presents the associations between ICU statin use and delirium according to the presence/absence of sepsis and study day. For example, on study day 1, statin use was associated with a 78% reduced odds of delirium among patients with sepsis (odds ratio, 0.22; 95% confidence interval, 0.10–0.49), whereas statin use was not associated with reduced delirium on study day 1 among patients without sepsis (odds ratio, 0.92; 95% confidence interval, 0.46–1.84) or among those with sepsis later in the study, e.g., on study day 13 (odds ratio, 0.70; 95% confidence interval, 0.35–1.41). In general, ICU statin use was associated with reduced delirium during days of sepsis early in the ICU stay and with reduced delirium during days without sepsis late in the ICU period. Prehospital statin use, alternatively, had no association with delirium, regardless of sepsis or study day (overall odds ratio, 0.86; 95% confidence interval, 0.44–1.66; p = 0.18, Figure 3).
Figure 2. ICU Statin Use and Delirium.
The odds ratio for delirium that was associated with ICU statin use during the 30-day study, after adjusting for covariates, is indicated by the dark circles (with corresponding 95% confidence intervals indicated by vertical lines). Results are shown according to the study day (indicated by position along the x-axis) and the presence or absence of severe sepsis (left and right panels, respectively); N displays the number of patients in the cohort on specific study days, which changes over time due to discharge, death, and changing sepsis status. After adjusting for age, baseline cognitive function, Framingham Stroke Risk Profile, propensity score for prehospital statin use, severity of illness, sepsis, mechanical ventilation, sedative and analgesic doses, steroid use, and study day, ICU statin use was associated with reduced delirium (p < 0.01), with the association significantly modified by sepsis (interaction p = 0.02) and by study day (interaction p < 0.01). Specifically, ICU statin use was associated with reduced delirium during days of sepsis early in the ICU stay and with reduced delirium during days without sepsis late in the ICU stay.
Figure 3. Prehospital Statin Use and Delirium.
The odds ratio for delirium that was associated with prehospital statin use, after adjusting for covariates, is indicated by the dark circles (with corresponding 95% confidence intervals indicated by vertical lines); results are shown according to the study day (indicated by position along the x-axis) and the presence or absence of severe sepsis (left and right panels, respectively); N displays the number of patients in the cohort on specific study days, which changes over time due to discharge, death, and changing sepsis status. After adjusting for age, baseline cognitive function, Framingham Stroke Risk Profile, propensity score of prehospital statin use, severity of illness, sepsis, mechanical ventilation, sedative and analgesic doses, steroid use, and study day, prehospital statin use was not associated with delirium (p = 0.21).
Results from the sensitivity analysis, which examined data from the first 5 study days, similarly showed that ICU statin use was associated with reduced delirium (overall p for ICU statin use < 0.01). This association, as shown in Table 2, was again significantly modified by sepsis (interaction p for sepsis*ICU statin use = 0.02), such that ICU statin use was associated with reduced delirium among patients with sepsis but not among those without sepsis. In contrast, the association was not modified by study day (interaction p for study day*ICU statin use = 0.80) in the 5-day analysis.
Table 2.
Associations between Statin Use or Discontinuation and Delirium during Study Days 1–5 (Sensitivity Analyses Results)a,b
| Exposure | Association with Delirium | |
|---|---|---|
| Odds Ratio | 95% Confidence Interval | |
| Statin Usea | ||
| In the ICUb | ||
| With sepsisc | 0.45 | 0.22 to 0.94 |
| Without sepsisc | 0.92 | 0.42 to 2.05 |
| Prehospital | ||
| All patientsd | 0.85 | 0.44 to 1.66 |
| Statin Discontinuatione | ||
| All patientsd | 2.24 | 1.17 to 4.26 |
Abbreviations: CI, confidence interval.
Results show the odds ratio for delirium associated with use of a statin in the ICU or during the prehospital period (with no statin exposure being the reference comparator), after adjusting for age, baseline cognitive function, Framingham Stroke Risk profile, propensity score for prehospital statin use, severity of illness, sepsis, mechanical ventilation, sedative and analgesic doses, steroid use, and study day.
During study days 1–5, the association between ICU statin use and delirium was not significantly modified by study day (interaction p for study day*ICU statin use = 0.96), all interaction terms were retained in the model because of the significant interaction between sepsis and ICU statin use (see footnote c). Thus, the odds ratios (and corresponding confidence intervals) displayed are specific to study day 3 (the median study day in this sensitivity analysis) but do not significantly different from the results specific to other study days.
During study days 1–5, the association between ICU statin use and delirium was significantly modified by sepsis (interaction p for sepsis*ICU statin use = 0.02); the odds ratios (and corresponding confidence intervals) are therefore shown according to whether sepsis was present or absent. Sepsis status was determined on a daily basis (i.e., patients could be septic on one day and non-septic on the next) according to international consensus criteria (40).
During study days 1–5, neither the association between prehospital statin use and delirium nor between statin discontinuation and delirium were modified by sepsis (interaction p = 0.46 and 0.22, respectively); the odds ratios (and corresponding confidence intervals) are therefore shown for all patients rather than according to whether sepsis was present or absent.
Results show the odds ratio for delirium associated with 3 days of statin discontinuation (with 0 days being the reference comparator), after adjusting for age, baseline cognitive function, Framingham Stroke Risk profile, severity of illness, sepsis, mechanical ventilation, sedative and analgesic doses, steroid use, and study day.
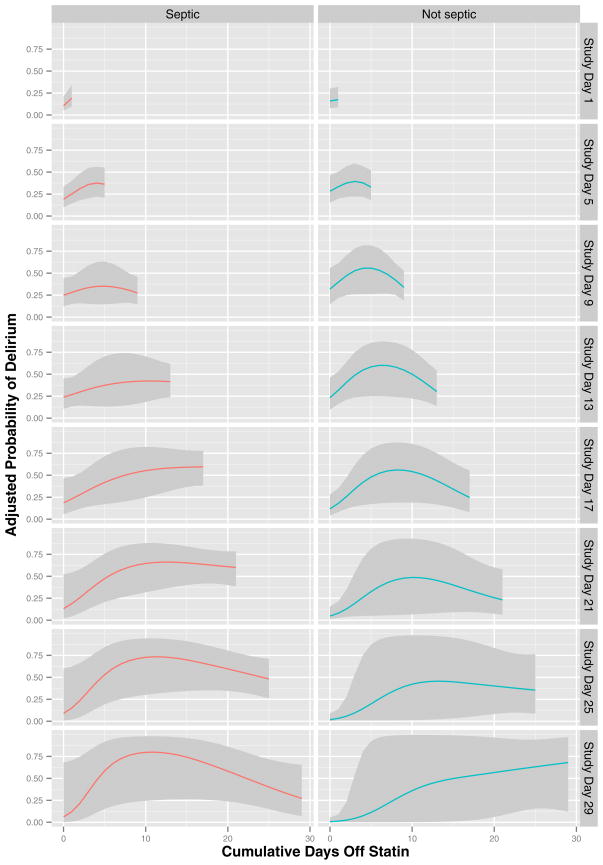
Statin discontinuation was associated with increased delirium among prehospital statin users (overall p for duration of statin discontinuation < 0.001), an association that was modified by sepsis and study day (both interaction p < 0.001) in the analyses of all 30 days of data. In general, the probability of delirium increased the longer a prehospital statin user remained off a statin, but the slope and strength of this association depended on sepsis and study day (Figure 4). Similarly, as shown in Table 2, duration of statin discontinuation was associated with increased delirium among prehospital statin users in the sensitivity analysis restricted to the first 5 study days (overall p for duration of statin discontinuation = 0.047). During this period, however, neither sepsis nor study day significantly modified the association between statin discontinuation and delirium (overall p for all interaction terms = 0.22).
Figure 4. Duration of Statin Discontinuation and Delirium.
The probability of delirium that was associated with the duration of statin discontinuation, after adjusting for covariates, is indicated by the colored lines (with corresponding 95% confidence intervals indicated by gray ribbons); results are shown according to study day (indicated by position along the x-axis) and the presence or absence of sepsis (left and right panels, respectively). After adjusting for age, baseline cognitive function, Framingham Stroke Risk Profile, propensity score for prehospital statin use, severity of illness, sepsis, mechanical ventilation, sedative and analgesic doses, steroid use, and study day, longer durations of statin discontinuation among prehospital statin users were associated with increased delirium (p < 0.001), with the association significantly modified by sepsis and by study day (both interaction p < 0.001).
DISCUSSION
This is the first multicenter, prospective cohort study to identify an association between statin use or discontinuation and delirium during critical illness. We found that statin use in the ICU was associated with reduced delirium early during the ICU stay in the setting of sepsis and late in the ICU stay when patients were not septic. In addition, discontinuation of statins during the ICU stay among prehospital statin users was associated with increased delirium. These results call into question the common practice of holding prehospital statin therapy during a patient’s ICU stay and indicate that statins may have efficacy in the prevention or treatment of delirium during critical illness, both of which are questions that must be addressed by randomized placebo-controlled trials.
Our results are consistent with and extend those recently published by Page et al. (45), who examined the association between ICU statin exposure and delirium-free days in a single-center, prospective cohort study of 470 medical and surgical ICU patients in the United Kingdom; receipt of a statin in their study was independently associated with a 2.28-fold increase in the odds of being delirium free the following day. In our investigation, not only was ICU statin exposure associated with reductions in delirium, but discontinuation in the ICU of a previously administered statin was associated with increased delirium.
Our study and that of Page et al. (45) differ significantly from earlier observational studies (27–29) that investigated statins and postoperative delirium, primarily because we studied critically ill patients with a high severity of illness whereas prior studies examined elective cardiac surgery patients. In a large, retrospective cohort study, Redelmeier et al. (27) found that statins were associated with an increased odds of delirium after elective cardiac surgery. Delirium, however, was likely underdiagnosed in this trial since it was identified using ICD-9 codes. In contrast, Katznelson et al. (28) diagnosed delirium in their prospective cohort study using a validated delirium tool (the CAM-ICU) and found that preoperative statin use was associated with a reduction in delirium after cardiac surgery. In the largest study to date, Mariscalco and colleagues (29) retrospectively examined 4,569 patients, over 90% of whom underwent elective cardiac surgery. Using propensity matching for likelihood of preoperative statin use, Mariscalco et al. found no association between delirium and preoperative statin administration.
Data from animal and human studies suggest neuroinflammation is central in the pathophysiology of delirium during critical illness (46–48). Severe sepsis, the prototype for critical illness arising from systemic inflammation, is known to involve neuroinflammation as a response to circulating cytokines and chemokines (46, 49). This inflammation may cause neuronal damage through microglia activation (47), edema (49), microvascular thrombosis and alterations in blood flow (50), and/or apoptosis (51), manifesting clinically as delirium and likely leading to cognitive deficits that may persist for years after critical illness.
Statins are known to have numerous pleiotropic effects that may benefit the brain during critical illness, including anti-inflammatory (9, 10), endothelial function-enhancing (11, 12), and anticoagulant effects (13, 14). If these effects, which are well documented in animal and human studies, mitigate neuronal injury during critical illness, statins may reduce delirium. Alternatively, other effects of statins may contribute to their association with delirium. Statins might improve neuronal function, for example, via effects on N-methyl-D-aspartate (NMDA)-mediated glutamate excitotoxicity (52, 53) or endothelial function (11, 12). Randomized trials, including an ongoing phase 2 trial of simvastatin in mechanically ventilated ICU patients (ISRCTN89079989), could measure a broad array of markers of inflammation and coagulation as well as NMDA receptors and endothelial function, which has been associated with delirium during critical illness (54), so that the results of those trials can be understood mechanistically.
Our finding that discontinuation of a prehospital statin during an acute critical illness is associated with increased delirium is not surprising given results of studies performed in other settings (21, 23, 24). When statins were held during acute myocardial infarction, rebound inflammation was noted within 3–5 days (21). In another study, neurological deterioration among patients with acute stroke was more likely when statins were discontinued during the first three days of admission (24). In light of these investigations and our own results, the common practice of holding statins at ICU admission that were administered in the outpatient setting (26) should be examined carefully in a randomized trial.
Strengths of our investigation included the prospective cohort design, large sample size, granular classification of statin exposure, comprehensive adjustment for potential confounders (e.g., via use of a propensity score for prehospital statin use, organ-specific SOFA scores measured on each ICU day, and numerous other covariates), and accurate and thorough ascertainment of outcome using delirium assessments performed twice daily by trained research personnel using a valid and reliable delirium assessment tool (32, 33).
Limitations included lack of data on patients’ adherence to the prehospital prescription of statins, which might have led to misclassification biasing results regarding prehospital statin use and delirium though misclassification of ICU statin use was highly unlikely; results generalizable to patients without preexisting cognitive impairment since patients at high risk for preexisting cognitive impairment were excluded; and as with any observational study, the possibility of bias due to unmeasured confounders. To minimize bias, we used multivariable regression to adjust for a large number of potential confounders, including a propensity score based on factors known to be associated with patients’ compliance with statins (55–57) as well as multiple daily measures of illness severity, but the possibility of bias due to unmeasured confounders—e.g., factors of illness severity that may be associated with discontinuation of statins but are not reflected in the SOFA score or concurrent therapies such as reperfusion therapy in the setting of acute coronary syndrome—cannot be entirely eliminated. Changes over time in the study population, for example, due to death and discharge may have biased results from days later in the study period, but sensitivity analyses using data from the first 5 study days yielded similar results to those obtained from the entire dataset. Nevertheless, these results (especially those generated late in the study period when confidence intervals were wide due to a reduced sample size) should be interpreted with caution such that statins cannot be recommended as therapy for the prevention or treatment of delirium until randomized, placebo-controlled trials are conducted to determine whether statins are efficacious in the management of delirium in the ICU. Future studies are also warranted to determine the effect of statins on long-term cognitive outcomes.
CONCLUSIONS
This investigation found that statin use in the ICU was associated with reduced delirium during critical illness. Additionally, delirium was increased when previously used statins were discontinued during an acute critical illness. Delirium is well documented to be an important independent predictor of poor outcomes in multiple healthcare settings, yet it is most prevalent and detrimental in the ICU. Statins, therefore, may have a major public health impact on the epidemiology and outcomes of critical illness if they are found to reduce delirium and its associated long-term outcomes in randomized clinical trials, which are now needed to elucidate the effects of statin use and discontinuation on the brain during critical illness.
Acknowledgments
Funding/Support: This study was supported by National Institutes of Health (AG027472). In addition, Dr. Hughes receives support from the Foundation for Anesthesia Education and Research, and Drs. Pandharipande, Vasilevskis, Han, Jackson, Ely, and Girard receive support from the National Institutes of Health (HL111111, AG040157, AG032355, AG031322, AG027472, and AG035117). Drs. Pandharipande and Ely also receive support from the VA Clinical Science Research and Development Service (VA Career Development Award and VA Merit Review Award, respectively), Dr. Vasilevskis receives support from the Veterans Affairs Clinical Research Training Center of Excellence, and Drs. Vasilevskis, Ely and Girard receive support from the Veterans Affairs Tennessee Valley Geriatric Research, Education and Clinical Center (GRECC).
Footnotes
Author Disclosures: Dr. Pandharipande has received honoraria from Hospira, Inc. and Orion Pharma. Dr. Girard has received honoraria from Hospira, Inc. Dr. Ely has received honoraria from Pfizer, Inc., Eli Lilly and Company, Hospira, Inc., and Abbott Laboratories. All the other authors report no financial conflicts of interest.
Copyright Form Disclosures
Dr. Girard lectured for Hospira and received support for article research from NIH. His institution received grant support from NIH. Dr. Morandi received support for article research from NIH. His institution received grant support from NIH. Dr. Hughes received support for article research from NIH and the Foundation for Anesthesia Education and Research. His institution received grant support from NIH and FAER (Salary support for research from NIH and Foundation for Anesthesia Education and Research). Dr. Thompson received support for article research from NIH. Her institution received grant support from NIH. Dr. Pandharipande lectured for Hospira Inc. and Orion Pharma (research presentations) and received support for article research from NIH. His institution received grant support from NIH and Hospira (in collaboration with NIH grant). Dr. Shintani received support for article research from NIH. Her institution received grant support from NIH. Dr. Vasilevskis received support for article research from NIH. His institution received grant support from NIH 5K23AG040157-02. Dr. Han received support for article research from NIH. His institution received grant support from NIH. Dr. Jackson received support for article research from NIH. His institution received grant support from NIH. Dr. Laskowitz received support for article research from NIH. Dr. Bernard received support for article research from NIH. His institution received grant support from NIH. Dr. Ely lectured for Pfizer, Inc., Eli Lilly and Company, Hospira Inc., and Abbott Laboratories (for research presentations) and received support for article research from NIH. His institution received grant support from NIH.
References
- 1.Adhikari NK, Fowler RA, Bhagwanjee S, et al. Critical care and the global burden of critical illness in adults. Lancet. 2010;376:1339–1346. doi: 10.1016/S0140-6736(10)60446-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Girard TD, Jackson JC, Pandharipande PP, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010;38:1513–1520. doi: 10.1097/CCM.0b013e3181e47be1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomason JW, Shintani A, Peterson JF, et al. Intensive care unit delirium is an independent predictor of longer hospital stay: a prospective analysis of 261 non-ventilated patients. Crit Care. 2005;9:R375–R381. doi: 10.1186/cc3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shehabi Y, Riker RR, Bokesch PM, et al. Delirium duration and mortality in lightly sedated, mechanically ventilated intensive care patients. Crit Care Med. 2010;38:2311–2318. doi: 10.1097/CCM.0b013e3181f85759. [DOI] [PubMed] [Google Scholar]
- 5.Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 6.Pisani MA, Kong SY, Kasl SV, et al. Days of delirium are associated with 1-year mortality in an older intensive care unit population. Am J Respir Crit Care Med. 2009;180:1092–1097. doi: 10.1164/rccm.200904-0537OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milbrandt EB, Deppen S, Harrison PL, et al. Costs associated with delirium in mechanically ventilated patients. Crit Care Med. 2004;32:955–962. doi: 10.1097/01.ccm.0000119429.16055.92. [DOI] [PubMed] [Google Scholar]
- 8.National Center for Health Statistics. Health, United States, 2010: With Special Feature on Death and Dying. Hyattsville, MD: 2011. [PubMed] [Google Scholar]
- 9.Rosenson RS, Tangney CC, Casey LC. Inhibition of proinflammatory cytokine production by pravastatin. Lancet. 1999;353:983–984. doi: 10.1016/S0140-6736(98)05917-0. [DOI] [PubMed] [Google Scholar]
- 10.Dichtl W, Dulak J, Frick M, et al. HMG-CoA reductase inhibitors regulate inflammatory transcription factors in human endothelial and vascular smooth muscle cells. Arteriosclerosis, thrombosis, and vascular biology. 2003;23:58–63. doi: 10.1161/01.atv.0000043456.48735.20. [DOI] [PubMed] [Google Scholar]
- 11.Pleiner J, Schaller G, Mittermayer F, et al. Simvastatin prevents vascular hyporeactivity during inflammation. Circulation. 2004;110:3349–3354. doi: 10.1161/01.CIR.0000147774.90396.ED. [DOI] [PubMed] [Google Scholar]
- 12.McGown CC, Brown NJ, Hellewell PG, et al. Beneficial microvascular and anti-inflammatory effects of pravastatin during sepsis involve nitric oxide synthase III. Br J Anaesth. 2010;104:183–190. doi: 10.1093/bja/aep361. [DOI] [PubMed] [Google Scholar]
- 13.Bourcier T, Libby P. HMG CoA reductase inhibitors reduce plasminogen activator inhibitor-1 expression by human vascular smooth muscle and endothelial cells. Arteriosclerosis, thrombosis, and vascular biology. 2000;20:556–562. doi: 10.1161/01.atv.20.2.556. [DOI] [PubMed] [Google Scholar]
- 14.Steiner S, Speidl WS, Pleiner J, et al. Simvastatin blunts endotoxin-induced tissue factor in vivo. Circulation. 2005;111:1841–1846. doi: 10.1161/01.CIR.0000158665.27783.0C. [DOI] [PubMed] [Google Scholar]
- 15.Morandi A, Hughes CG, Girard TD, et al. Statins and brain dysfunction: a hypothesis to reduce the burden of cognitive impairment in patients who are critically ill. Chest. 2011;140:580–585. doi: 10.1378/chest.10-3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maclullich AM, Ferguson KJ, Miller T, et al. Unravelling the pathophysiology of delirium: a focus on the role of aberrant stress responses. J Psychosom Res. 2008;65:229–238. doi: 10.1016/j.jpsychores.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gunther ML, Morandi A, Ely EW. Pathophysiology of delirium in the intensive care unit. Crit Care Clin. 2008;24:45–65. doi: 10.1016/j.ccc.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Shyamsundar M, McKeown ST, O’Kane CM, et al. Simvastatin decreases lipopolysaccharide-induced pulmonary inflammation in healthy volunteers. Am J Respir Crit Care Med. 2009;179:1107–1114. doi: 10.1164/rccm.200810-1584OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Craig TR, Duffy MJ, Shyamsundar M, et al. A randomized clinical trial of hydroxymethylglutaryl- coenzyme a reductase inhibition for acute lung injury (The HARP Study) Am J Respir Crit Care Med. 2011;183:620–626. doi: 10.1164/rccm.201003-0423OC. [DOI] [PubMed] [Google Scholar]
- 20.Kruger P, Bailey M, Bellomo R, et al. A multicenter randomized trial of atorvastatin therapy in intensive care patients with severe sepsis. Am J Respir Crit Care Med. 2013;187:743–750. doi: 10.1164/rccm.201209-1718OC. [DOI] [PubMed] [Google Scholar]
- 21.Sposito AC, Carvalho LS, Cintra RM, et al. Rebound inflammatory response during the acute phase of myocardial infarction after simvastatin withdrawal. Atherosclerosis. 2009;207:191–194. doi: 10.1016/j.atherosclerosis.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 22.Heeschen C, Hamm CW, Laufs U, et al. Withdrawal of statins increases event rates in patients with acute coronary syndromes. Circulation. 2002;105:1446–1452. doi: 10.1161/01.cir.0000012530.68333.c8. [DOI] [PubMed] [Google Scholar]
- 23.Tseng MY, Czosnyka M, Richards H, et al. Effects of acute treatment with pravastatin on cerebral vasospasm, autoregulation, and delayed ischemic deficits after aneurysmal subarachnoid hemorrhage: a phase II randomized placebo-controlled trial. Stroke. 2005;36:1627–1632. doi: 10.1161/01.STR.0000176743.67564.5d. [DOI] [PubMed] [Google Scholar]
- 24.Blanco M, Nombela F, Castellanos M, et al. Statin treatment withdrawal in ischemic stroke: a controlled randomized study. Neurology. 2007;69:904–910. doi: 10.1212/01.wnl.0000269789.09277.47. [DOI] [PubMed] [Google Scholar]
- 25.Le Manach Y, Godet G, Coriat P, et al. The impact of postoperative discontinuation or continuation of chronic statin therapy on cardiac outcome after major vascular surgery. Anesth Analg. 2007;104:1326–1333. doi: 10.1213/01.ane.0000263029.72643.10. table of contents. [DOI] [PubMed] [Google Scholar]
- 26.Bell CM, Brener SS, Gunraj N, et al. Association of ICU or hospital admission with unintentional discontinuation of medications for chronic diseases. JAMA. 2011;306:840–847. doi: 10.1001/jama.2011.1206. [DOI] [PubMed] [Google Scholar]
- 27.Redelmeier DA, Thiruchelvam D, Daneman N. Delirium after elective surgery among elderly patients taking statins. CMAJ. 2008;179:645–652. doi: 10.1503/cmaj.080443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katznelson R, Djaiani GN, Borger MA, et al. Preoperative use of statins is associated with reduced early delirium rates after cardiac surgery. Anesthesiology. 2009;110:67–73. doi: 10.1097/ALN.0b013e318190b4d9. [DOI] [PubMed] [Google Scholar]
- 29.Mariscalco G, Cottini M, Zanobini M, et al. Preoperative statin therapy is not associated with a decrease in the incidence of delirium after cardiac operations. Ann Thorac Surg. 2012;93:1439–1447. doi: 10.1016/j.athoracsur.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 30.Hughes CP, Berg L, Danziger WL, et al. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 31.Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369:1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286:2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 33.Ely EW, Margolin R, Francis J, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) Crit Care Med. 2001;29:1370–1379. doi: 10.1097/00003246-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 34.Guenther U, Popp J, Koecher L, et al. Validity and reliability of the CAM-ICU Flowsheet to diagnose delirium in surgical ICU patients. J Crit Care. 2010;25:144–151. doi: 10.1016/j.jcrc.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 35.Luetz A, Heymann A, Radtke FM, et al. Different assessment tools for intensive care unit delirium: which score to use? Crit Care Med. 2010;38:409–418. doi: 10.1097/CCM.0b013e3181cabb42. [DOI] [PubMed] [Google Scholar]
- 36.Jorm AF. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychol Med. 1994;24:145–153. doi: 10.1017/s003329170002691x. [DOI] [PubMed] [Google Scholar]
- 37.Jorm AF, Scott R, Cullen JS, et al. Performance of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) as a screening test for dementia. Psychol Med. 1991;21:785–790. doi: 10.1017/s0033291700022418. [DOI] [PubMed] [Google Scholar]
- 38.D’Agostino RB, Wolf PA, Belanger AJ, et al. Stroke risk profile: adjustment for antihypertensive medication. The Framingham Study Stroke. 1994;25:40–43. doi: 10.1161/01.str.25.1.40. [DOI] [PubMed] [Google Scholar]
- 39.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 40.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 41.Katz S, Ford AB, Moskowitz RW, et al. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychological function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 42.Pfeffer RI, Kurosaki TT, Harrah CH, Jr, et al. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37:323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 43.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 44.Ihaka R, Gentleman R. R: a language for data analysis and graphics. J Comput Graph Stat. 1996;5:299–314. [Google Scholar]
- 45.Page VJ, Davis D, Zhao XB, et al. Statin use and risk of delirium in the critically ill. Am J Respir Crit Care Med. 2014 Jan 13; doi: 10.1164/rccm.201306-1150OC. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Semmler A, Hermann S, Mormann F, et al. Sepsis causes neuroinflammation and concomitant decrease of cerebral metabolism. J Neuroinflammation. 2008;5:38. doi: 10.1186/1742-2094-5-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Gool WA, van de BD, Eikelenboom P. Systemic infection and delirium: when cytokines and acetylcholine collide. Lancet. 2010;375:773–775. doi: 10.1016/S0140-6736(09)61158-2. [DOI] [PubMed] [Google Scholar]
- 48.Girard TD, Ware LB, Bernard GR, et al. Associations of markers of inflammation and coagulation with delirium during critical illness. Intensive Care Med. 2012;38:1965–1973. doi: 10.1007/s00134-012-2678-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Papadopoulos MC, Lamb FJ, Moss RF, et al. Faecal peritonitis causes oedema and neuronal injury in pig cerebral cortex. Clin Sci (Lond) 1999;96:461–466. [PubMed] [Google Scholar]
- 50.Pfister D, Siegemund M, Dell-Kuster S, et al. Cerebral perfusion in sepsis-associated delirium. Crit Care. 2008;12:R63. doi: 10.1186/cc6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Semmler A, Okulla T, Sastre M, et al. Systemic inflammation induces apoptosis with variable vulnerability of different brain regions. J Chem Neuroanat. 2007;30:144–157. doi: 10.1016/j.jchemneu.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 52.Zacco A, Togo J, Spence K, et al. 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors protect cortical neurons from excitotoxicity. J Neurosci. 2003;23:11104–11111. doi: 10.1523/JNEUROSCI.23-35-11104.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ponce J, de la Ossa NP, Hurtado O, et al. Simvastatin reduces the association of NMDA receptors to lipid rafts: a cholesterol-mediated effect in neuroprotection. Stroke. 2008;39:1269–1275. doi: 10.1161/STROKEAHA.107.498923. [DOI] [PubMed] [Google Scholar]
- 54.Hughes CG, Morandi A, Girard TD, et al. Association between endothelial dysfunction and acute brain dysfunction during critical illness. Anesthesiology. 2013;118:631–639. doi: 10.1097/ALN.0b013e31827bd193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Avorn J, Monette J, Lacour A, et al. Persistence of use of lipid-lowering medications: a cross-national study. JAMA. 1998;279:1458–1462. doi: 10.1001/jama.279.18.1458. [DOI] [PubMed] [Google Scholar]
- 56.Blackburn DF, Dobson RT, Blackburn JL, et al. Adherence to statins, beta-blockers and angiotensin-converting enzyme inhibitors following a first cardiovascular event: a retrospective cohort study. Can J Cardiol. 2005;21:485–488. [PubMed] [Google Scholar]
- 57.Sung JC, Nichol MB, Venturini F, et al. Factors affecting patient compliance with antihyperlipidemic medications in an HMO population. Am J Manag Care. 1998;4:1421–1430. [PubMed] [Google Scholar]