Abstract
Objective
Two experiments were conducted to examine the effects of task importance on event-based prospective memory (PM) in separate samples of adults with HIV-associated Neurocognitive Disorders (HAND) and HIV-infected young adults with Substance Use Disorders (SUD).
Method
All participants completed three conditions of an ongoing lexical decision task: 1) without PM task requirements; 2) with PM task requirements that emphasized the importance of the ongoing task; and 3) with PM task requirements that emphasized the importance of the PM task.
Results
In both experiments, all HIV+ groups showed the expected increase in response costs to the ongoing task when the PM task’s importance was emphasized. In Experiment 1, individuals with HAND showed significantly lower PM accuracy as compared to HIV+ subjects without HAND when the importance of the ongoing task was emphasized, but improved significantly and no longer differed from HIV+ subjects without HAND when the PM task was emphasized. A similar pattern of findings emerged in Experiment 2, whereby HIV+ young adults with SUD (especially cannabis) showed significant improvements in PM accuracy when the PM task was emphasized.
Conclusions
Findings suggest that both HAND and SUD may increase the amount of cognitive attentional resources that need to be allocated to support PM performance in persons living with HIV infection.
Keywords: Infectious disease, Episodic memory, AIDS dementia complex, Marijuana abuse, Attention, Substance-related disorders
INTRODUCTION
Persons living with HIV disease commonly experience failures in prospective memory (PM; Carey et al., 2006), which involves the ability to successfully execute an intended action in response to specific cues that are based on time (e.g., attending a medical appointment at 3pm) or an event (e.g., take a medication after a meal). Although PM deficits correlate with executive dysfunction, retrospective memory impairment, and slowed information processing in HIV disease (e.g., Zogg et al., 2011), they are also separable from HIV-associated neurocognitive deficits as supported by evidence from biomarker (Woods et al., 2006), cognitive (Gupta et al., 2010), and real-world functioning (e.g., Woods et al., 2008) studies. HIV-associated PM deficits are of clinical concern, as they are associated with engagement in HIV transmission risk behaviors (e.g., Martin et al., 2007) and increase odds of functional dependence (e.g., Woods et al., 2008), including suboptimal adherence to combination antiretroviral therapies (cART; Woods et al., 2009). Thus, investigating the cognitive mechanisms and co-occurring conditions that modulate the expression of HIV-associated PM deficits is of both scientific and clinical value.
The perceived importance of a PM task is one interesting psychological mechanism with particular relevance to HIV clinical care. The idea that task importance may influence PM functioning was first described by Freud (1914), but formal empirical investigations did not emerge until the 1980s. For example, Kvavilashvili (1987) found that university students were significantly less likely to execute a prescribed intention when its importance was not emphasized, particularly when the ongoing task was highly demanding. By definition, PM involves a dual task condition in which one is performing an ongoing task (e.g., engaging in normal daily activities) while simultaneously monitoring the environment for the PM cue (e.g., a meal) that will trigger retrieval and execution of the intention (e.g., take a dose of a prescribed medication). Thus, the ongoing task and the many complex elements of the PM task (e.g., cue monitoring and detection) are in competition for limited cognitive resources (see Loft & Remington, 2013). Viewed through the lens of multiprocess theory (Einstein et al., 2005), which claims that PM processes vary along a continuum of spontaneous/automatic to strategic/executive, increasing the importance of the PM task should encourage the strategic allocation of cognitive resources away from the ongoing task and toward the PM task, thereby improving PM accuracy. For example, Kliegel and colleagues (2001; 2004), and Loft and colleagues (2007; 2008), have showed that emphasizing PM task importance in healthy adults increases the allocation of attentional resources to PM as evidenced by poorer performance (viz., costs) on the ongoing task. In turn, the increased resource allocation toward the PM task can improve PM accuracy, particularly for PM tasks that are strategically demanding, such as when the PM cue is based on time (Kliegel, Martin, McDaniel, & Einstein, 2001), or is not focal to the ongoing task (Kliegel, Martin, McDaniel, & Einstein, 2004).
Yet we know very little about the moderating effects of task importance in populations vulnerable to deficits in strategically demanding aspects of PM that may interfere with independent living. According to multiprocess theory, in clinical populations with diminished strategic PM resources, as in the case of HIV infection (e.g., Doyle et al., 2013), emphasizing the importance of the PM task should focus strategic processing on PM cue monitoring and thereby enhance PM accuracy. In 2007, Altgassen et al. reported that individuals with Parkinson’s disease performed worse on the PM task than healthy adults when the importance of ongoing task was emphasized, but not when the importance of the PM task was stressed. More recently, Hering and colleagues (2013) demonstrated that age-related PM deficits evident in a low PM importance condition were ameliorated in the high PM importance condition (cf. Smith & Hunt, 2014). Across this literature, the beneficial effects to PM accuracy of emphasizing the relative importance of the PM task were accompanied by increased performance cost to the ongoing task. When considered in the context of the multiprocess theory and the laboratory studies reviewed above, these data suggest that experimenter-driven allocation of additional attentional resources to PM cue monitoring by way of a task importance instruction manipulation can enhance PM performance in populations vulnerable to deficits in strategically demanding PM.
The present investigation describes two separate experiments designed to determine the role of task importance in the expression of PM deficits in persons living with HIV disease. We focused our experiments on two cardinal clinical aspects of HIV disease, namely HIV-associated neurocognitive disorders (HAND) and substance use disorders (SUD). In the first experiment, we examine the effects of PM task importance in adults with HAND, which are prevalent in the era of combination antiretroviral therapy (cART; Heaton et al., 2010), a major risk factor for real-world complications including non-adherence (e.g., Hinkin et al., 2002), and an important determinant of both time- and event-based PM deficits (e.g., Zogg et al., 2011; Morgan et al., 2012). A second experiment was conducted to investigate whether SUD modify the response to PM task importance among young adults infected with HIV. SUD are common in persons living with HIV disease, particularly so in young adults (Murphy et al., 2001; Rotheram-Borus, Murphy, Kennedy, Stanton, & Kuklinski, 2001), and are known to exacerbate HIV-associated neural injury (e.g., Chana et al., 2006), including deficits in episodic memory (e.g., Meyer et al., 2013) and executive functions (Scott et al., 2007). Indeed, a growing body of evidence is elucidating a relationship between SUD and PM impairment as evidenced on laboratory-based tasks (e.g., Iudicello et al., 2011; Weinborn et al., 2011), and is thought to be due at least in part to frontostriatal circuitry injury (Volkow, Fowler, Wang, & Goldstein, 2002). Furthermore, there is evidence that HIV-infected young adults with SUD are susceptible to suboptimal cART adherence (Murphy et al., 2001), and so identifying mechanisms that could improve PM is of clinical relevance. Across both of these experiments, we expected that emphasizing PM task importance would enhance allocation of attention resources to the PM task as shown by increased performance costs to the ongoing task and, in turn, improve PM accuracy in subjects with HAND and SUD risk as compared to their study counterparts. In other words, we hypothesized that there would be detrimental effects of HAND and SUD when the importance of the ongoing task was emphasized, but not when the importance of the PM task was emphasized.
EXPERIMENT 1: HIV-ASSOCIATED NEUROCOGNITIVE DISORDERS
Method
Participants
The study in which these data were gathered was approved by the UC San Diego human research protections program. The study sample included 31 HIV- individuals, 15 HIV+ individuals diagnosed with HAND, and 35 HIV+ individuals not diagnosed with HAND, all of whom were recruited from local HIV clinics and community organizations. HAND was diagnosed according to Frascati criteria (Antinori et al., 2007) as determined by results from a comprehensive neuropsychological, medical, and psychiatric evaluation (for details, see Morgan et al., 2012). Exclusion criteria included a diagnosis of severe psychiatric (e.g., schizophrenia) or neurologic illness (e.g., seizure disorder, active opportunistic infection, stroke), or a verbal IQ estimate <70 (based on the Wechsler Test of Adult Reading, WTAR; Psychological Corporation, 2001). No study subjects met substance dependence criteria within one month of evaluation as determined by the Composite International Diagnostic Interview (CIDI version 2.1; World Health Organization, 1998). In addition, we excluded subjects who were urine toxicology positive for illicit substances on the day of evaluation (excluding marijuana or prescribed medications), or tested positive for alcohol via a Breathalyzer test. Of note, all individuals were enrolled in a NIH-funded R01 parent study that examines the combined effects of HIV and aging on PM, which utilized a discrepant age classification approach such that no individuals between the ages of 40 and 50 were enrolled in the parent study. The demographic, psychiatric, and medical characteristics of the study participants in Study 1 are provided in Table 1.
Table 1.
Demographic, Psychiatric, and HIV Disease Characteristics of the Study Samples in Experiment 1.
| Characteristics | HIV- (n=31) | HIV+ without HAND (n=35) |
HIV+ with HAND (n=15) |
p |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 44.4 (15.7) | 44.5 (13.2) | 51.1 (12.5) | .112 |
| Education (years) | 14.0 (3.4) | 13.6 (2.3) | 14.4 (2.6) | .162 |
| Estimated verbal IQa | 100.7 (13.4) | 104.0 (9.4) | 104.7 (9.4) | .602 |
| Sex (% male) | 61.3 | 85.7 | 100.0 | .005 |
| Ethnicity (% Caucasian) | 48.4 | 57.1 | 66.7 | .489 |
| Psychiatric | ||||
| Current MDD (%) | 9.7 | 17.1 | 20.0 | .572 |
| LT MDD (%) | 35.5 | 62.9 | 73.3 | .022 |
| LT Alcohol dependence (%) | 22.6 | 42.9 | 40.0 | .200 |
| LT Non-alcohol substance dependence (%) | 38.7 | 62.9 | 33.3 | .065 |
| Cannabis (%) | 12.9 | 17.1 | 20.0 | .806 |
| Cocaine (%) | 22.6 | 31.4 | 20.0 | .058 |
| Methamphetamine (%) | 16.1 | 51.4 | 26.7 | .008 |
| Opiates (%) | 19.4 | 14.3 | 6.7 | .521 |
| HIV Disease | ||||
| Estimated duration of infection (mos.)b | -- | 204 (53, 279) | 131 (56, 193) | .391 |
| Nadir CD4 T-cell count (cells/µl)b | -- | 200 (120, 366) | 141 (21, 420) | .472 |
| Current CD4 T-cell count (cells/µl)b | -- | 559 (396, 799) | 432 (254, 644) | .115 |
| Plasma HIV RNA (% detectable) | -- | 20.0 | 40.0 | .140 |
| cART (% prescribed) | -- | 77.1 | 100.0 | .043 |
Note.
Based on the WTAR. cART = combination antiretroviral therapy. MDD = Major Depressive Disorder. LT = lifetime.
Data are presented in medians and interquartile ranges.
Materials and Procedure
After providing written informed consent, study participants completed a neuropsychological, psychiatric, and medical evaluation (see Morgan et al., 2012).
The current study employed a computerized laboratory paradigm in which a PM task is embedded within an ongoing lexical decision task. For the lexical decision task, participants were required to decide, as quickly and as accurately as possible, whether presented letter strings were English words or non-words by pressing designated keys representing “yes” and “no.” A list of 150 medium frequency words (i.e., 20–50 occurrences per million), all of 4–6 letters in length, was obtained from the Sydney Morning Herald database (Dennis, 1995). Given that the task was originally developed for use within Australian samples, words that were uncommon in American English were excluded and replaced. A list of 150 non-words, also 4–6 letters in length, was obtained from the Macquarie University ARC non-word database (Rastle, Harrington, & Coltheart, 2002). On each lexical decision task trial, a fixation cross was presented on the computer screen followed by a presentation of the letter string. The letter string was then removed from the display and replaced by a fixation cross for the next trial after the participant responded, or after the maximum duration of 3000 ms, whichever occurred first.
The experiment consisted of three experimental blocks, each of which included 100 lexical decision trials. The first block presented was the baseline condition, in which no PM instruction was given and participants only performed the lexical decision task. The following two blocks, in which a PM task was required in addition to the ongoing lexical decision task, were randomized in their order of presentation. The assignment of words and non-words to the three blocks as well as the presentation order within blocks was randomized but yoked across the two PM conditions. Ten additional words that contained the syllable “tor” (e.g., “monitor,” “tortoise”) were selected from the Sydney Morning Herald database and used as PM cues; five were randomly assigned to each of the two PM blocks. Within each PM block, a PM cue was presented every 18–21 trials.
After participants completed ten practice lexical decision trials followed by the baseline lexical decision block, they were informed that the researcher had a secondary interest in their ability to remember to perform actions in the future. Specifically, they were told to press the “Q” key instead of the “yes” or “no” keys if they saw any word that contained the syllable “TOR.” Participants then received additional instructions for either the PM block in which the importance of the ongoing task was emphasized (“Please note that in this case, your performance on the word-nonword task is more important than looking for ‘TOR’ and remembering to press the ‘Q’ key. In other words, we would like you to put more effort into performing the word-nonword task”), or for the PM block in which the importance of the PM task was emphasized (“Please note that in this case, looking for ‘TOR’ and remembering to press the ‘Q’ key is more important than your performance on the word-nonword task. In other words, we would like you to put more effort into looking for ‘TOR’ and remembering to press the ‘Q’ key”). Before each PM block, participants were engaged in a paper-and-pencil distractor task for two minutes. Upon completion of each PM block, participants completed a recognition test for the five PM targets that had just been presented in that block.
Results
Ongoing Task
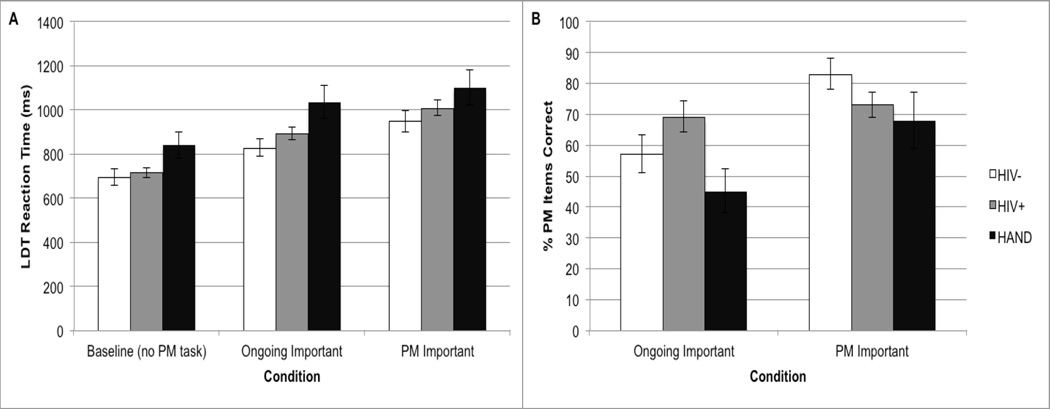
Analysis of Lexical decision response times were based on word trials only. We excluded incorrect lexical decisions, and response times greater than 3 SDs from each participant’s mean for each block (see Loft et al., 2007, 2008). We conducted a mixed effects analysis of variance (ANOVA) on accuracy and RT to the word trials of the ongoing lexical decision task. Block (baseline, ongoing task emphasis, and PM task emphasis) was the within-subjects factor and HAND group (i.e., HIV-, HIV+ without HAND, and HAND) the between-subjects factor. Lifetime diagnoses of MDD and SUD were included as covariates in this model. Lexical decision accuracy was near ceiling and we observed no significant main effects or interactions (Fs<1, see Table 2). For lexical decision RT, there was a main effect of Block (F[2, 75]=107.8, p<.001). Follow-up analyses showed that lexical decision RTs were significantly faster in the Baseline condition as compared to the Ongoing Task Emphasis condition (t(80)=13.2, p<.001), which in turn was faster than the PM Task Emphasis condition (t(80)=7.9, p<.001). These data indicate that the increased performance costs to the ongoing task under Ongoing Task Emphasis compared to baseline conditions, and under PM Task Emphasis compared to Ongoing Task Emphasis conditions, were both significant and comparable between the three groups. There was also a main effect of HAND group (F[2, 76]=3.9, p=.024), such that individuals with HAND were significantly slower than the HIV- and HIV+ subjects without HAND in both the baseline and Ongoing Task Emphasis conditions (ps<.05), but not in PM Task Emphasis condition (ps>.05) (see Figure 1). No main effects of either of the covariates were observed (ps>.10), and no interaction was found between HAND and Block (F<1, p>.10).
Table 2.
Proportions of Correct Lexical Decision Responses across the Study Groups in Experiments 1 and 2.
| Experiment 1 | Experiment 2 | ||||
|---|---|---|---|---|---|
| Condition | HIV- (n=31) |
HIV+ (n=35) |
HAND (n=15) |
No SUD Risk (n=25) |
SUD Risk (n=33) |
| Baseline | 94.6 | 95.8 | 95.7 | 89.1 | 88.6 |
| Ongoing Important | 94.7 | 95.0 | 92.6 | 88.3 | 84.9 |
| PM Important | 93.7 | 94.1 | 91.5 | 87.7 | 86.9 |
Figure 1.
Panel A displays the response times to the ongoing lexical decision task in the HIV- (n=31), HIV+ neurocognitively normal (n=35), and HAND (n=15) groups in Experiment 1 at baseline (i.e., no PM instructions), and in the two conditions in which either the relative importance of ongoing or the PM task was emphasized. Panel B displays the PM accuracy data across the HAND study groups and PM task importance conditions in Experiment 1. Standard errors are represented in the figure by the error bars on each column.
PM Accuracy
We also conducted a mixed effects ANOVA using PM accuracy, with PM Task Importance (Ongoing Task Emphasis and PM Task Emphasis) as the within-subjects factor and HAND group (HIV-, HIV+ without HAND, and HAND) as the between-subjects factor. Lifetime diagnoses of MDD and SUD were once again included as covariates. Results revealed no main effect of HAND group (F[2, 76]=1.9, p=.162); however, there was a main effect of PM Task Importance (F[1, 76]=18.5, p<.001) and a significant interaction between HAND and PM Task Importance (F[2, 76]=3.2, p<.048). Planned post-hoc examination of the interaction term showed significant omnibus effects of HAND group on PM accuracy in the Ongoing Task Emphasis condition (F[2, 78]=3.1, p<.049). This effect was driven by lower PM accuracy in HAND versus HIV+ (p=.018, Hedges g=.78), as the HAND group did not differ from the HIV- cohort (p=.230, g=.36) despite evidence of a small-to-medium effect size. There were no significant effects of HAND in the PM Task Emphasis condition (F[2, 76]=1.6, p=.210). PM accuracy improved significantly from the Ongoing Task Emphasis condition to the PM Task Emphasis block in both the HIV- (t(30)=4.3, p<.001, g=.91) and HAND (t(14)=2.9, p=.011, g=.71) groups; however, there was no evidence of PM accuracy improvement in the HIV+ subjects without HAND across task emphasis blocks (t(34)=.70, p=.490, g=.13).
Given that individuals who were urine toxicology positive for cannabis were included in the study sample, we examined the potential influence of this variable on ongoing task RT and PM accuracy. As only two HIV- participants produced positive urine toxicology screens, these potential effects were examined within the HIV+ without HAND group, the HIV+ with HAND group, and the HIV+ sample as a whole. These analyses showed no significant associations between a positive urine toxicology for cannabis and any of the study dependent variables (ps>.10).
EXPERIMENT 2: SUBSTANCE USE DISORDERS
Method
Participants
The human research protections programs at UC San Diego and Wayne State University approved this study. The study sample included 58 HIV-infected young adults enrolled in a multisite NIDA-funded study of PM in San Diego (n = 28) or Detroit (n = 30). All participants had documented HIV infection and were between the ages of 18 and 24 years of age. Exclusion criteria included a diagnosis of severe psychiatric (e.g., psychosis) or neurologic (e.g., seizure disorder, closed head injury with loss of consciousness more than 30 minutes) conditions. SUD status was determined by the Alcohol, Smoking, and Substance Involvement Screening Test (ASSIST version 3.0; WHO ASSIST Working Group, 2002), which is a brief instrument that queries subjects on substance use frequency, craving, and related problems over the three months prior to assessment. Participants were classified as likely having a substance use disorder (SUD) if they met criteria for “moderate” or “high” risk for at least one illicit substance (excluding alcohol and tobacco). A total of 33 (56.9%) HIV+ young adults met criteria for SUD, with cannabis and methamphetamine being the two most common substances of abuse. The demographic, psychiatric, and medical characteristics of the study participants in Experiment 2 are provided in Table 3.
Table 3.
Demographic, Psychiatric, and HIV Disease Characteristics of the Study Samples in Experiment 2.
| Characteristics | No SUD (n=25) | SUD (n=33) | p |
|---|---|---|---|
| Demographics | |||
| Age (years) | 22.6 (1.2) | 22.6 (1.4) | .702 |
| Education (years) | 12.4 (1.1) | 11.7 (1.5) | .077 |
| Estimated verbal IQa | 87.1 (11.7) | 90.2 (14.6) | .300 |
| Sex (% male) | 76.0 | 93.9 | .050 |
| Ethnicity (% Caucasian) | 8.0 | 15.2 | .408 |
| Psychiatric | |||
| Brief Symptom Inventory (%)b | 28.0 | 30.3 | .849 |
| HIV Disease | |||
| Estimated duration of infection (mos.)d | 31 (17, 53) | 35 (18, 52) | .797 |
| Nadir CD4 T-cell count (cells/µl)d | 363 (242, 447) | 282 (222, 415) | .350 |
| Current CD4 T-cell count (cells/µl)d | 576 (451, 716) | 488 (361, 644) | .146 |
| Plasma HIV RNA (% detectable) | 68.0 | 51.5 | .207 |
| cART (% prescribed) | 80.0 | 72.7 | .522 |
Note. Data are presented in means and standard deviations unless otherwise noted.
Based on the WTAR.
% of individuals with elevated global scores. cART = combination antiretroviral therapy. MDD = Major Depressive Disorder. LT = lifetime.
Data are presented in medians and interquartile ranges.
Materials and Procedure
After providing written informed consent, study participants completed a brief neurocognitive and psychiatric evaluation that included the identical PM task importance paradigm described in Experiment 1.
Results
Ongoing Task
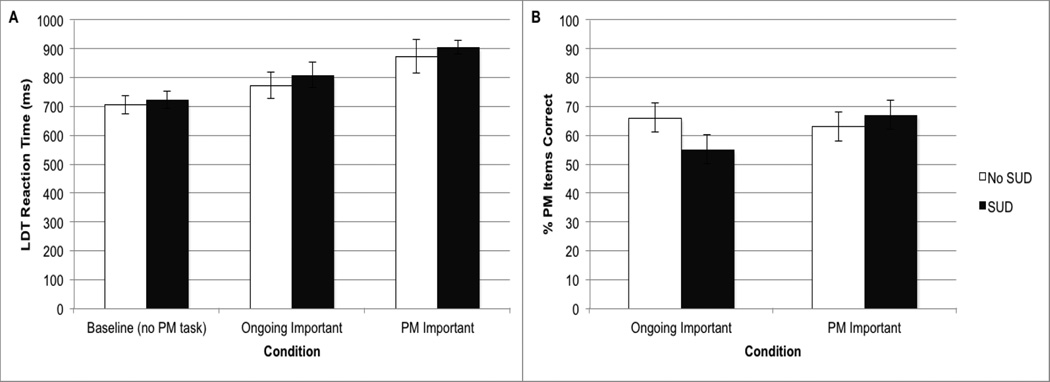
Paralleling our analytic approach in Experiment 1, we conducted a mixed effects ANOVA on RT to the word trials from the ongoing lexical decision task. Block (Baseline, Ongoing Task Emphasis, and PM Task Emphasis) was the within-subjects factor and SUD group (SUD, No SUD) the between-subjects factor. Education, gender, and tobacco risk as measured by the ASSIST were included as covariates. Ongoing task accuracy was near ceiling and we observed no significant main effects or interactions (Fs<1, see Table 2). For ongoing task RT, there was a significant within-subjects effect of Block (F[2, 52]=10.3, p<.001). As displayed in Figure 2, follow-up analyses showed that lexical decision RTs were significantly faster in the baseline condition versus the Ongoing Task Emphasis condition (t(57)=3.1, p=.004), which was also faster than the PM Task Emphasis condition (t(57)=4.0, p<.001).There was no significant main effects of SUD group and no interaction between SUD group and Block (Fs<1, ps>.10). Overall, this data indicate that the increased performance costs to the ongoing task under Ongoing Task Emphasis compared to baseline conditions, and under PM Task Emphasis compared to Ongoing Task Emphasis conditions, were both significant and comparable between the SUD group and the No SUD group.
Figure 2.
Panel A displays the response times to the ongoing lexical decision task in the HIV+ subjects with (n=33) and without (n=25) risk of substance use disorders (SUD) in Experiment 2 at baseline (i.e., no PM instructions), in the two conditions in which either the relative importance of ongoing or the PM task was emphasized. Panel B displays the PM accuracy data across the SUD study groups and PM task importance conditions in Experiment 2. Standard errors are represented in the figure by the error bars on each column.
PM Accuracy
We conducted a mixed effects ANOVA on PM accuracy, with PM Task Importance (Ongoing Task Emphasis and PM Task Emphasis) as the within-subjects factor and SUD group (SUD and No SUD) as the between-subjects factor. Education, gender, and tobacco use were again entered as covariates. There was no main effect of SUD group (F<1, p>.10). Consistent with Study 1, there was a main effect of PM Task Importance (F[1, 53]=4.6, p=.036), which was modified by a significant interaction with SUD group (F[2, 76]=11.2, p=.002). Planned analysis of the interaction term showed that the SUD group was marginally less accurate in PM than the No SUD group under Ongoing Task Emphasis conditions (F[1, 66]=3.1, p=.082, g=.4), which was driven by a significant association with problematic cannabis use as measured by the correlation with the continuous score from the ASSIST (r=−0.40, p=.020). All other individual substances of abuse, including alcohol, were not significantly associated with PM accuracy in the Ongoing Task Emphasis condition (ps>.10). In contrast, there were no significant effects of SUD group in the PM Task Emphasis condition (F<1). PM accuracy improved significantly from the Ongoing Task Emphasis condition to the PM Task Emphasis block in the SUD group (t(32)=2.6, p=.016, g=.44), but not in the No SUD group (t(24)=-.46, p=.651, g=−0.11).
GENERAL DISCUSSION
HIV-associated deficits in the strategic aspects of PM are common (e.g., Doyle et al., 2013; Morgan et al., 2012) and greatly increase the risk of poorer real-world outcomes, including non-adherence to cART (Woods et al., 2009). In two complementary experiments, we demonstrated that PM task importance plays a key role in the expression of event-based PM in adults with HIV-associated Neurocognitive Disorders (HAND) and HIV+ young adults with substance use disorders (SUD). Specifically, HAND and SUD were each associated with relative deficits in non-focal event-based PM when the importance of the ongoing task was emphasized, but not when the importance of the PM task was emphasized. These HAND and SUD effects were independent of clinic demographic factors, including education, psychiatric comorbidity, and HIV disease severity, which were either comparable between groups or were covaried in the statistical models. As the HAND group was comprised entirely of men, we were unable to covary for this demographic factor; however we are unaware of any studies showing gender effects in the expression of PM task importance or HIV-associated PM deficits. In both experiments, the shifting of importance away from the ongoing task and toward the PM task was associated with a parallel increase in attentional resources apportioned to the PM task requirement by all groups of participants, and an accompanied improvement in PM accuracy for HIV+ individuals with HAND and SUD, and for HIV- individuals. Thus, these experiments align with prior findings from younger healthy subjects (e.g., Loft & Yeo, 2007), older adults (Hering et al., 2013) and Parkinson’s disease (Altgassen et al., 2007) in suggesting that supporting strategic PM cue monitoring by allocating increased attentional resources to the PM task can improve PM in persons living with HIV infection. Across these two experiments, emphasizing the importance of the PM task successfully decreased study participants’ focus on the ongoing task as indicated by slowed response times (but no gross changes in accuracy, which was generally at ceiling) to the lexical decision task when the PM instruction was emphasized. This effect of importance on the ongoing task was broadly comparable across all study groups, suggesting that individuals with and without HAND and SUD in Experiments 1 and 2, respectively, were able to increase allocation of attentional resources to the PM task to a similar extent when the importance of the PM task was emphasized. This cognitive resource allocation shift away from the ongoing task in response to PM task importance instructions in HIV disease is commensurate with prior data from healthy younger (e.g., Loft & Yeo, 2007) and older (Hering et al., 2013) adults, although prior findings vary by the relative demands of the ongoing task (e.g., Kliegel et al., 2001). In the only prior clinical study of PM task importance (Altgassen et al., 2007), patients with Parkinson’s disease did not show decrements in the ongoing working memory task accuracy when the importance was directed to the PM task, but this study did not report the more sensitive measure of response times. All told, data from the current experiments suggest that individuals living with HIV infection are successfully able to shift additional attentional resources from the ongoing task to the PM task when PM task importance is emphasized.
Of clinical relevance, the shift of attention away from the ongoing task afforded significant improvements in PM accuracy. In Experiment 1, HAND was associated with substantially lower PM accuracy as compared to neurocognitively normal HIV+ subjects when the ongoing task was emphasized. Indeed, HAND has reliably been associated with deficits in strategically demanding (i.e., non-focal) event-based PM (e.g., Zogg et al., 2011). However, when the PM task was emphasized the HAND group improved their PM accuracy significantly; in fact, the previously observed between-group effects of HAND were no longer evident. Interpretation of this null finding is somewhat tempered by the small sample of subjects with HAND, which may have increased our risk of Type II error in detecting the small effect size. Regardless, it is clear that the task importance manipulation dampened the effect of HAND on PM as evidenced by a medium-to-large effect size for PM accuracy across conditions, which suggests that individuals with HAND can more successfully execute PM task requirements when the resource demands of the ongoing task are intentionally limited. Emphasizing the importance of the PM task relative to the ongoing task in HAND can be conceptualized as bolstering strategic processing by way of allocating attention to PM cue monitoring, according to the assumptions of multiprocess theory (Einstein et al., 2005). Moreover, this finding is commensurate with prior studies from our group showing that automatic/spontaneous event-based PM processes can be spared in vulnerable subpopulations of HIV disease (e.g., encoding in older adults; Woods, Dawson, Weber, Grant, & The HNRC Group, 2010).
We observed a similar pattern of PM accuracy decrement and improvement across the task importance manipulations among young adults with SUD in Experiment 2. When the importance of the ongoing task was emphasized, HIV+ SUD showed marginally lower PM accuracy as compared to their non-SUD counterparts, which was driven by young adults with cannabis use disorders. To our knowledge, this is the first study to demonstrate adverse effects of cannabis use on PM in young adults infected with HIV. Such data are consistent with studies showing that cannabis use is associated with elevated PM failures in daily life (e.g., Bartholomew et al., 2010) and in the laboratory (e.g., Fisk & Montgomery, 2008; Montgomery, Seddon, Fisk, Murphy, & Jansari, 2012; cf. Cuttler, McLaughlin, & Graf, 2012) among seronegatives. Frequent cannabis use is also associated with worse procedural learning (Gonzalez, Schuster, Vassileva, & Martin, 2011) and mild retrospective episodic memory deficits (e.g., Cristiani, Pukay-Martin, & Bornstein, 2004) in adults with HIV disease. Limitations of this study include the absence of a demographically similar seronegative comparison group, and the relatively limited characterization of SUD, which was measured by the ASSIST. The ASSIST serves as a brief screening instrument to identify those who engage in problematic substance use, and thus are likely to meet diagnostic criteria for dependence. As such, the current study was unable to base SUD group categorization off formal, interview-based diagnostic criteria for substance use disorders per DSM. Additionally, other important and relevant substance use characteristics, such as age of onset and lifetime amount used, were not obtained in the current study. Future studies on the effects of PM in younger HIV-infected substance users would benefit from more comprehensive assessment substance use diagnoses and parameters based on structured clinical interviews.
Emphasizing the importance of the PM task and thereby shifting subjects’ attention away from the ongoing task not only significantly improved PM accuracy in the HIV+ SUD group, it also fully ameliorated the SUD group effects on PM. Paralleling the findings observed with HAND in Experiment 1, these data indicate that the PM accuracy of HIV+ young adults with SUD is greatly benefitted when individuals allocate additional attentional resources to support PM cue monitoring. Taken together, findings from these experiments suggest that HIV-infected persons with HAND and/or problematic cannabis use may have clinical vulnerabilities in PM cue monitoring that are amenable to improvement with strategic supports. It remains to be determined whether importance manipulations can successfully improve PM accuracy in other vulnerable subpopulations of the HIV epidemic, perhaps most notably older adults, who are at high risk for deficits in the strategic aspects of PM (Woods et al., 2010).
It is interesting to note that the PM task importance manipulation did not improve PM accuracy in HIV+ subjects without HAND or SUD. This despite the fact that both HIV+ comparison groups demonstrated the expected increase in response costs to the ongoing task in the PM emphasis condition that were comparable to the HAND and SUD groups. One possibility is that the “importance” manipulation used in this study, while powerful enough to improve PM accuracy in HAND and SUD subjects with PM cue monitoring deficits, was too weak to enhance PM in less vulnerable HIV+ subjects. In other words, simply instructing some HIV+ participants to allocate more attentional resources to the PM task may not be sufficiently motivating as an importance manipulation for individuals not in need of such a “boost.” If this were the case however, it is not clear why the PM performance of the seronegative participants in Experiment 1, and in prior studies (Loft et al., 2007, 2008), significantly benefitted from the same PM task emphasis instruction. Nonetheless, future studies with more salient incentives (e.g., monetary; McCauley, McDaniel, Pedroza, Chapman, & Levin, 2009) may be needed to amplify the PM task importance effect in these HIV+ individuals.
Another, perhaps more likely, possibility for this curious null result is a “glass ceiling” effect in the HIV+ groups, whereby the enhanced monitoring afforded by emphasizing the PM task does not improve PM performance deficits driven by other component processes, such as PM cue-intention encoding (Altgassen et al., 2007) and cue detection (e.g., Doyle et al., 2013). In addition, the response cost evidence for the shift of attentional resources away from ongoing task may not have necessarily directly translated into effective PM cue monitoring. That is, while HIV+ participants demonstrated equivalent costs under PM importance emphasis conditions (an indicator of overall attention allocation to the PM task), it does not imply that they used qualitatively similar attentional processes as the other groups. Furthermore, sustained PM cue monitoring can absorb considerable cognitive resources and may be susceptible to lapses in attentional control – which is a neurocognitive hallmark of HIV infection (Morgan et al., 2011) – impairing PM cue detection.
The finding that HIV+ individuals with HAND and SUD benefit from emphasizing the importance of PM may have implications for designing interventions to improve PM and real-world functions in HIV disease. For example, McCauley and colleagues (2009) reported that increased monetary rewards for successful task performance improved naturalistic PM in subjects with both mild and severe traumatic brain injury. PM response to the monetary rewards was associated with the microstructural integrity (i.e., fractional anistropy) of frontolimbic white matter bundles (McCauley et al., 2011), which are also affected in HIV disease (see Ellis, Calero, & Stockin, 2009). Given that interventions based on cash payments also demonstrate promise for HIV prevention (e.g., Pettifour, MacPhail, Nguyen, & Rosenberg, 2012), incentive-based programs are increasingly being developed for reducing HIV risk transmission behaviors (e.g., Operario, Kuo, Sosa-Rubí, & Gálarraga, 2013). This is relevant because HIV transmission risk behaviors are strongly associated with strategically demanding PM among persons with SUD (Martin et al., 2007; Weinborn et al., 2013). Similar incentive-based interventions are being considered to improve adherence to cART (e.g., Farber et al., 2013), which is also reliant upon PM (Woods et al., 2009). A recent study demonstrated that endorsing normative beliefs about the importance of ART adherence was associated with greater likelihood of being adherent (Brown, Littlewood, & Vanable, 2013). Consideration of PM functioning as a possible moderator of the effectiveness of these incentive-based interventions is therefore warranted across the lifespan in persons infected with HIV.
Table 4.
Substance Use characteristics of the Study Samples in Experiment 2.
| Substance Use Characteristics | No SUD (n=25) | SUD (n=33) | p |
|---|---|---|---|
| Total SIS | 0.4 (1.2) | 21.4 (14.5) | <.001 |
| Alcohol SIS | 7.2 (7.5) | 9.9 (9.9) | <.305 |
| Alcohol - Low (%) | 72.0 | 69.7 | |
| Alcohol - Moderate (%) | 28.0 | 18.2 | |
| Alcohol - High (%) | 0.0 | 12.1 | |
| Tobacco SIS | 3.1 (6.7) | 13.7 (7.6) | <.001 |
| Tobacco - Low (%) | 80.0 | 15.2 | |
| Tobacco - Moderate (%) | 16.0 | 84.8 | |
| Tobacco - High (%) | 4.0 | 0.0 | |
| Cannabis SIS | 0.3 (0.8) | 12.8 (6.6) | <.001 |
| Cannabis - Low (%) | 100.0 | 0.0 | |
| Cannabis - Moderate (%) | 0.0 | 97.0 | |
| Cannabis - High (%) | 0.0 | 3.0 | |
| Cocaine SIS | 0.1 (0.4) | 1.0 (2.3) | .054 |
| Cocaine - Low (%) | 100.0 | 87.9 | |
| Cocaine - Moderate (%) | 0.0 | 12.1 | |
| Cocaine - High (%) | 0.0 | 0.0 | |
| Amphetamine SIS | 0.1 (0.4) | 4.0 (8.4) | .005 |
| Amphetamine - Low (%) | 100.0 | 69.7 | |
| Amphetamine - Moderate (%) | 0.0 | 27.3 | |
| Amphetamine - High (%) | 0.0 | 3.0 | |
| Inhalant SIS | 0.0 (0.0) | 0.5 (2.1) | 0.214 |
| Inhalant - Low (%) | 100.0 | 93.9 | |
| Inhalant - Moderate (%) | 0.0 | 6.1 | |
| Inhalant - High (%) | 0.0 | 0.0 | |
| Sedative SIS | 0.0 (0.0) | 1.2 (2.6) | .015 |
| Sedative - Low (%) | 100.0 | 84.8 | |
| Sedative - Moderate (%) | 0.0 | 15.2 | |
| Sedative - High (%) | 0.0 | 0.0 | |
| Hallucinogen SIS | 0.0 (0.0) | 0.2 (1.1) | .214 |
| Hallucinogen - Low (%) | 100.0 | 97.0 | |
| Hallucinogen - Moderate (%) | 0.0 | 3.0 | |
| Hallucinogen - High (%) | 0.0 | 0.0 | |
| Opioid SIS | 0.0 (0.0) | 1.3 (3.3) | .044 |
| Opioid - Low (%) | 100.0 | 84.8 | |
| Opioid - Moderate (%) | 0.0 | 15.2 | |
| Opioid - High (%) | 0.0 | 0.0 | |
| Other SIS | 0.0 (0.0) | 0.3 (1.2) | .125 |
| Other - Low (%) | 100.0 | 97.0 | |
| Other - Moderate (%) | 0.0 | 3.0 | |
| Other - High (%) | 0.0 | 0.0 | |
| Polysubstance (%) | 0.0 | 36.4 | <.001 |
Note. Data are presented in means and standard deviations unless otherwise noted. SIS = substance involvement score. Low = raw score of <11 for alcohol or <4 for all other substances. Moderate = raw score between 11–26 for alcohol and 4–26 for all other substances. High = raw score of >26 for all substances. Polysubstance = percent of individuals who met criteria for at least moderate substance involvement in two or more substances.
ACKNOWLEDGEMENTS
This research was supported by National Institutes of Health grants R01-MH073419, R01-DA034497, T32-DA31098, L30-DA032120, P30-MH062512, P50-DA026306, as well as and Discovery Grant DP-12010311 from the Australian Research Council. The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government.
The authors thank Marizela V. Cameron and P. Katie Riggs for their assistance with study coordination, Yanqi Ryan Li for his help with the construction of the PM task, and Donald Franklin and Stephanie Corkran for their assistance with data compilation.
Footnotes
The authors report no conflicts of interest.
REFERENCES
- Altgassen M, Zöllig J, Kopp U, Mackinlay R, Kliegel M. Patients with Parkinson’s disease can successfully remember to execute delayed intentions. Journal of the International Neuropsychological Society. 2007;13:888–892. doi: 10.1017/S1355617707071068. [DOI] [PubMed] [Google Scholar]
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomew J, Holroyd S, Heffernan TM. Does cannabis use affect prospective memory in young adults? Journal of Psychopharmacology. 2010;24:241–246. doi: 10.1177/0269881109106909. [DOI] [PubMed] [Google Scholar]
- Brown JL, Littlewood RA, Vanable PA. Social-cognitive correlates of antiretroviral therapy adherence among HIV-infected individuals receiving infectious disease care in a medium-sized northeastern US city. AIDS Care. 2013;25:1149–1158. doi: 10.1080/09540121.2012.752566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Rippeth JD, Heaton RK, Grant I The HNRC Group. Prospective memory in HIV-1 infection. Journal of Clinical and Experimental Neuropsychology. 2006;28:536–548. doi: 10.1080/13803390590949494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chana G, Everall IP, Crews L, Langford D, Adame A, Grant I The HNRC Group. Cognitive deficits and degeneration of interneurons in HIV+ methamphetamine users. Neurology. 2006;67:1486–1489. doi: 10.1212/01.wnl.0000240066.02404.e6. [DOI] [PubMed] [Google Scholar]
- Cristiani SA, Pukay-Martin ND, Bornstein RA. Marijuana use and cognitive function in HIV-infected people. The Journal of Neuropsychiatry and Clinical Neurosciences. 2004;16:330–335. doi: 10.1176/jnp.16.3.330. [DOI] [PubMed] [Google Scholar]
- Cuttler C, McLaughlin RJ, Graf P. Mechanisms underlying the link between cannabis use and prospective memory. PLoS One. 2012;7:e36820. doi: 10.1371/journal.pone.0036820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis S. The Sydney Morning Herald word database. 4. Vol. 1. Noetica: Open Forum; 1995. Retrieved from http://psy.uq.edu.au/CogPsych/Noetica. [Google Scholar]
- Doyle KL, Loft S, Morgan EE, Weber E, Cushman C, Johnston E The HNRP Group. Prospective memory in HIV-associated neurocognitive disorders (HAND): The neuropsychological dynamics of time monitoring. Journal of Clinical and Experimental Neuropsychology. 2013;35:359–372. doi: 10.1080/13803395.2013.776010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einstein GO, McDaniel MA, Thomas R, Mayfield S, Shank H, Morrisette N, Breneiser J. Multiple processes in prospective memory retrieval: Factors determining monitoring versus spontaneous retrieval. Journal of Experimental Psychology. 2005;134:327–342. doi: 10.1037/0096-3445.134.3.327. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Calero P, Stockin MD. HIV infection and the central nervous system: A primer. Neuropsychology Review. 2009;19:144–151. doi: 10.1007/s11065-009-9094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber S, Tate J, Frank C, Ardito D, Kozal M, Justice AC, Braithwaite SR. A study of financial incentives to reduce plasma HIV RNA among patients in care. AIDS and Behavior. 2013;17:2293–2300. doi: 10.1007/s10461-013-0416-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisk JE, Montgomery C. Real-world memory and executive processes in cannabis users and non-users. Journal of Psychopharmacology. 2008;22:727–736. doi: 10.1177/0269881107084000. [DOI] [PubMed] [Google Scholar]
- Freud S. In: Psychopathology of everyday life. Trans A.A. Brill., editor. New York, NY: The Macmillan Company. (Original work published in 1901; 1914. [Google Scholar]
- Gonzalaz R, Schuster RM, Vassileva J, Martin EM. Impact of HIV and a history of marijuana dependence on procedural learning among individuals with a history of substance dependence. Journal of Clinical and Experimental Neuropsychology. 2011;33:735–752. doi: 10.1080/13803395.2011.553584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Woods SP, Weber E, Dawson MS, Grant I The HNRC Group. Is prospective memory a dissociable cognitive function in HIV infection? Journal of Clinical and Experimental Neuropsychology. 2010;32:898–908. doi: 10.1080/13803391003596470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Woods SP, Ake C, Vaida F CHARTER Group. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heise L, Lutz B, Ranganathan M, Watts C. Cash transfers for HIV prevention: Considering their potential. Journal of the International AIDS Society. 2013;16:18615. doi: 10.7448/IAS.16.1.18615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hering A, Phillips LH, Kliegel M. Importance effects on age differences in performance in event-based prospective memory. Gerontology. 2014;60:73–78. doi: 10.1159/000355057. [DOI] [PubMed] [Google Scholar]
- Hinkin CH, Castellon SA, Durvasula RS, Hardy DJ, Lam MN, Mason KI, Stefaniak M. Medication adherence among HIV+ adults: Effects of cognitive dysfunction and regimen complexity. Neurology. 2002;59:1944–1950. doi: 10.1212/01.wnl.0000038347.48137.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iudicello JE, Weber E, Grant I, Weinborn M, Woods SP The HNRC Group. Misremembering future intentions in methamphetamine dependent individuals. The Clinical Neuropsychologist. 2011;25:269–286. doi: 10.1080/13854046.2010.546812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliegel M, Martin M, McDaniel MA, Einstein GO. Varying the importance of a prospective memory task: Differential effects across time- and event-based prospective memory. Memory. 2001;9:1–11. doi: 10.1080/09658210042000003. [DOI] [PubMed] [Google Scholar]
- Kliegel M, Martin M, McDaniel MA, Einstein GO. Importance effects on performance in event-based prospective memory tasks. Memory. 2004;12:553–561. doi: 10.1080/09658210344000099. [DOI] [PubMed] [Google Scholar]
- Kvavilashvili L. Remembering intention as a distinct form of memory. British Journal of Psychology. 1987;78:507–518. [Google Scholar]
- Loft S, Remington RW. Wait a second: Brief delays in responding reduce focality effects in event-based prospective memory. The Quarterly Journal of Experimental Psychology. 2013;66:1432–1447. doi: 10.1080/17470218.2012.750677. [DOI] [PubMed] [Google Scholar]
- Loft S, Yeo G. An investigation into the resource requirements of event-based prospective memory. Memory & Cognition. 2007;35:263–274. doi: 10.3758/bf03193447. [DOI] [PubMed] [Google Scholar]
- Loft S, Kearney R, Remington R. Is task interference in event-based prospective memory dependent on cue presentation? Memory & Cognition. 2008;36:139–148. doi: 10.3758/mc.36.1.139. [DOI] [PubMed] [Google Scholar]
- Martin EM, Nixon H, Pitrak DL, Weddington W, Rains NA, Nunnally G, Bechara A. Characteristics of prospective memory deficits in HIV-seropositive substance-dependent individuals: Preliminary observations. Journal of Clinical and Experimental Neuropsychology. 2007;29:496–504. doi: 10.1080/13803390600800970. [DOI] [PubMed] [Google Scholar]
- McCauley SR, McDaniel MA, Pedroza C, Chapman SB, Levin HS. Incentive effects on event-based prospective memory performance in children and adolescents with traumatic brain injury. Neuropsychology. 2009;23:201–209. doi: 10.1037/a0014192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley SR, Wilde EA, Bigler ED, Chu Z, Yallampalli R, Oni MB, Levin HS. Diffusion tensor imaging of incentive effects in prospective memory after pediatric traumatic brain injury. Journal of Neurotrauma. 2011;28:503–516. doi: 10.1089/neu.2010.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer VJ, Rubin LH, Martin E, Weber KM, Cohen MH, Golub ET, Maki PM. HIV and recent illicit drug use interact to affect verbal memory in women. Journal of Acquired Immune Deficiency Syndromes. 2013;63:67–76. doi: 10.1097/QAI.0b013e318289565c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery C, Seddon AL, Fisk JE, Murphy PN, Jansari A. Cannabis-related deficits in real-world memory. Human Psychopharmacology. 2012;27:217–225. doi: 10.1002/hup.1273. [DOI] [PubMed] [Google Scholar]
- Morgan EE, Weber E, Rooney AS, Grant I, Woods SP The HNRP Group. Longer ongoing task delay intervals exacerbate prospective memory deficits in HIV-associated neurocognitive disorders (HAND) Journal of Clinical and Experimental Neuropsychology. 2012;34:416–427. doi: 10.1080/13803395.2012.654764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan EE, Woods SP, Delano-Wood L, Bondi MW, Grant I The HNRP Group. Intraindividual variability in HIV infection: Evidence for greater neurocognitive dispersion in older HIV seropositive adults. Neuropsychology. 2011;25:645–654. doi: 10.1037/a0023792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DA, Wilson CM, Durako SJ, Muenz LR, Belzer M The Adolescent Medicine HIV/AIDS Research Network. Antiretroviral medication adherence among the REACH HIV-infected adolescent cohort in the USA. AIDS Care. 2001;13:27–40. doi: 10.1080/09540120020018161. [DOI] [PubMed] [Google Scholar]
- Operario D, Kuo C, Sosa-Rubí SG, Gálarraga O. Conditional economic incentives for reducing HIV risk behaviors: Integration of psychology and behavioral economics. Health Psychology. 2013;32:932–940. doi: 10.1037/a0032760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettifour A, MacPhail C, Nguyen N, Rosenberg M. Can money prevent the spread of HIV? A review of cash payments for HIV prevention. AIDS and Behavior. 2012;16:1729–1738. doi: 10.1007/s10461-012-0240-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirogovsky E, Woods SP, Filoteo JV, Gilbert PE. Prospective memory deficits are associated with poorer everyday functioning in Parkinson’s disease. Journal of the International Neuropsychological Society. 2012;18:986–995. doi: 10.1017/S1355617712000781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psychological Corporation. Wechsler Test of Adult Reading. San Antonio, TX: Psychological Corporation; 2001. [Google Scholar]
- Rastle K, Harrington J, Coltheart M. 358,534 nonwords: The ARC Nonword Database. The Quarterly Journal of Experimental Psychology. 2002;55:1339–1362. doi: 10.1080/02724980244000099. [DOI] [PubMed] [Google Scholar]
- Rotheram-Borus MJ, Murphy DA, Kennedy M, Stanton A, Kuklinski M. Health and risk behaviors over time among youth living with HIV. Journal of Adolescence. 2001;24:791–802. doi: 10.1006/jado.2001.0432. [DOI] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, Grant I. Neurocognitive effects of methamphetamine: A critical review and meta-analysis. Neuropsychology Review. 2007;17:275–297. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- Smith RE, Hunt RR. Prospective memory in young and older adults: The effects of task importance and ongoing task load. Aging, Neuropsychology, and Cognition. 2014;21:411–431. doi: 10.1080/13825585.2013.827150. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Goldstein RZ. Role of dopamine, the frontal cortex and memory circuits in drug addiction: Insight from imaging studies. Neurobiology of Learning and Memory. 2002;78:610–624. doi: 10.1006/nlme.2002.4099. [DOI] [PubMed] [Google Scholar]
- Weinborn M, Moyle J, Bucks RS, Stritzke W, Leighton A, Woods SP. Time-based prospective memory predicts engagement in risk behaviors among substance users: Results from clinical and nonclinical samples. Journal of the International Neuropsychological Society. 2013;19:284–294. doi: 10.1017/S1355617712001361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinborn M, Woods SP, O’Toole S, Kellogg EJ, Moyle J. Prospective memory in substance abusers at treatment entry: Associations with education, neuropsychological functioning, and everyday memory lapses. Archives of Clinical Neuropsychology. 2011;26:746–755. doi: 10.1093/arclin/acr071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO ASSIST Working Group. The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): Development, reliability and feasibility. Addiction. 2002;97:1183–1194. doi: 10.1046/j.1360-0443.2002.00185.x. [DOI] [PubMed] [Google Scholar]
- Woods SP, Dawson MS, Weber E, Gibson S, Grant I, Atkinson JH The HNRP Group. Timing is everything: Antiretroviral nonadherence is associated with impairment in time-based prospective memory. Journal of the International Neuropsychological Society. 2009;15:42–52. doi: 10.1017/S1355617708090012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Dawson MS, Weber E, Grant I The HNRP Group. The semantic relatedness of cue-intention pairings influences event-based prospective memory failures in older adults with HIV infection. Journal of Clinical and Experimental Neuropsychology. 2010;32:398–407. doi: 10.1080/13803390903130737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Iudicello JE, Moran LM, Carey CL, Dawson MS, Grant I The HNRP Group. HIV-associated prospective memory impairment increases risk of dependence in everyday functioning. Neuropsychology. 2008;22:110–117. doi: 10.1037/0894-4105.22.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Morgan EE, Marquie-Beck J, Carey CL, Grant I, Letendre SL The HNRP Group. Markers of macrophage activation and axonal injury are associated with prospective memory in HIV-1 disease. Cognitive and Behavioral Neurology. 2006;19:217–221. doi: 10.1097/01.wnn.0000213916.10514.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Composite International Diagnostic Interview (CIDI, Version 2.1) Geneva, Switzerland: World Health Organization; 1998. [Google Scholar]
- Zogg JB, Woods SP, Weber E, Doyle K, Grant I The HNRP Group. Are time- and event-based prospective memory comparably affected in HIV infection? Archives of Clinical Neuropsychology. 2011;26:250–259. doi: 10.1093/arclin/acr020. [DOI] [PMC free article] [PubMed] [Google Scholar]