Abstract
Studies have associated chronic low back pain (cLBP) with grey matter thinning. But these studies have not controlled for important clinical variables (such as a comorbid affective disorder, pain medication, age, or pain phenotype), which may reduce or eliminate these associations. We conducted cortical thickness and voxel-based morphometry (VBM) analyses in 14 cLBP patients with a discogenic component to their pain, not taking opioids or benzodiazepines, and not depressed or anxious. They were age and gender matched to 14 healthy controls (HCs).
An ROI-driven analysis (regions of interest) was conducted, using 18 clusters from a previous arterial spin labeling study demonstrating greater regional cerebral blood flow (rCBF) in these cLBP subjects than the HCs. Cortical thickness and VBM-based gray matter volume measurements were obtained from a structural MRI scan and group contrasts were calculated. MANOVA showed a trend toward cortical thickening in the right paracentral lobule in cLBP subjects (F(1,17)=3.667, p<0.067), and significant thickening in the right rostral middle frontal gyrus (F(1,17)=6.880, p<0.014). These clusters were non-significant after including age as a covariate (p<0.891; p<0.279). A whole-brain cortical thickness and VBM analysis also did not identify significant clusters of thinning or thickening.
Exploratory analyses identified group differences for correlations between age and cortical thickness of the right rostral middle frontal gyrus (cLBP: R=-0.03, p=0.9; HCs: R=-0.81, p<0.001), i.e., HCs demonstrated age-related thinning while cLBP patients did not. Our pilot results suggest that controlling for affect, age, and concurrent medications may reduce or eliminate some of the previously reported structural brain alterations in cLBP.
Keywords: Chronic Low Back Pain, Cortical Thickness, brain morphometry, clinical research methods
Introduction
A question under active investigation[1] is whether the experience of chronic pain, such as chronic low back pain (cLBP), is associated with patho-plastic changes in brain structure. Structural changes can be quantified with non-invasive imaging and morphometric measures, such as diminished cortical thickness or grey matter density. Previous studies in patients with chronic pain have identified regions of cortical thinning localized to the dorsal lateral prefrontal cortex (DLPFC), thalamus, basal ganglia, primary somatosensory cortex, posterior parietal cortex, and brain stem structures[2-7]. These regions have also been associated with functional Magnetic Resonance Imaging (fMRI) signal changes during processing of acute and chronic pain[1]. However, other structural MRI studies have examined the same brain regions and found no significant differences between subjects with chronic pain and healthy controls[8, 9].
Confounding factors, such as older age, severity of affective symptoms or concurrent use of opioids, have themselves been shown to strongly correlate with cortical thinning, as have pain intensity and duration[10-12]. A recent meta-analysis of this body of work concluded that many of these highly prevalent clinical factors and potential confounders in chronic pain populations were not adequately controlled for in prior studies, nor included as covariates in the statistical models.[13] These conclusions call into serious question whether there is indeed a relationship between chronic pain and cortical thickness.
In chronic low back pain, these potential clinical confounders may in fact account for the previously reported associations between cLBP and cortical structural changes. Furthermore, while it is useful to account for the use of medications and/or depression/anxiety symptoms through covariate regression analyses, this does not necessarily fully or adequately control for these potential effects. This is because covariate analyses are estimates of the effect of the covariate on the model, which is potentially confounded by many factors, such as the degree of shared and unshared variances with other predictors in the regression model[14]. A better control for these factors would be to not include patients with an affective disorder, or those taking benzodiazepines and/or opioids in the study sample, for example, even though that will necessarily limit the generalizability of the results. In addition, in studying brain morphometry, it is important to use as homogenous a clinical sample as possible to prevent the subtle but noticeable effects of clinical factors/comorbidities influencing the disease state and/or brain structure. This is particularly true in such a heterogeneous condition as chronic low back pain.[15]
The aim of this present study was to use a homogenous population of patients with chronic low back pain to study the possible associations of cLBP and brain morphometry versus an age-paired and gender-matched sample with no chronic pain. We used two measures of grey matter morphometry - cortical thickness and grey matter volume – in areas we previously found to preferentially process the experience of exacerbated cLBP within the same study sample[16]. We carefully controlled for medication use, comorbid affective disorders, and age to ascertain if previously reported changes still hold true under careful sample selection and clinical characterization. We hypothesized that in a cLBP sample without psychiatric comorbidity, and not taking opioids or benzodiazepines, chronic pain would not be positively associated with cortical thinning.
Materials and Methods
Subjects
Twenty eight (28) subjects were studied, which included 14 cLBP subjects (46.9± 14.6 years) and 14 age-paired and gender-matched healthy controls (45.9± 12.9 years). CLBP subjects were selected consecutively from 2009-2011, as they responded to advertisements for the study. Subjects with cLBP were included if they were: (1) between the ages of 21–65 yr, (2) had ongoing chronic pain that averaged at least 3 on a 0–10 scale of pain intensity, (3) had no back surgery within the past year, (4) were not taking opioids or benzodiazepines, (5) had low back pain with intermittent radicular pain of at least 6 months' duration, (6) did not have sensory or motor deficits that precluded participation in the study procedures (i.e., Quebec Task Force Classification Grade III[17]), (7) were right handed, (8) had a significant discogenic component to their pain syndrome, confirmed by a clinical evaluation and a lumbar MRI, and (9) did not have a current co-morbid affective disorder, as assessed by the Hospital Anxiety and Depression Scale (HADS).
The lead investigator (ADW) determined eligibility at the first visit through a review of a history and physical examination and MRI findings confirming disc disease. Patients were included if this evaluation found that a source of pain was at least one degenerated, herniated, or torn lumbar disc with either a minimum grade III disc degeneration, abnormal morphology, or a hyperintense zone[18-20]. These criteria, used by the authors in previous studies[16, 21, 22], narrows the heterogeneity of subjects with cLBP. They exclude those with pain resulting from purely nonspecific or myofascial causes and include those with the commonly presenting mixed syndrome of low back pain with underlying disc pathology and possibly spinal stenosis or facet disease, and no sensory or motor deficits. Studies indicate that 40% of all cLBP may be discogenic in nature[23].
Age-paired and gender-matched healthy controls (HC) were also enrolled and evaluated with the same procedures as cLBP subjects in order to exclude chronic pain. Other inclusion/exclusion criteria were the same as noted above for cLBP subjects.
All study protocols were approved by the Partners Human Research Committee and informed consent was obtained from all subjects.
Data Acquisition
Subjects completed clinical measures of their pain and affect including the Brief Pain Inventory (BPI)[24], Neuropathic Pain Questionnaire (NPQ)[25], Oswestry Disability Index (ODI)[26], and Hospital Anxiety and Depression Scale (HADS)[27]. Average pain was calculated by averaging the baseline BPI average pain score obtained at the screening visit with the second study visit BPI average pain score 1 week later.
Description of Measures
The Brief Pain Inventory
The BPI is a 15 item questionnaire assessing pain location, intensity, relief, and quality, as well as pain-interference, and has been validated in cancer and non-cancer pain studies [28].
The Hospital Anxiety and Depression Scale (HADS)
The HADS is a self-report measure that does not include somatic items that may be attributable to medical illness, and thus it is more appropriate for screening in a chronic pain patient population. On each subscale a high score is >8[29]. Scores above these levels are highly correlated with a comorbid major depression or generalized anxiety disorder in patients with cLBP[29, 30]. Anxiety and depression subscale scores each had to be <9 for study inclusion[27]. The HADS best functions as a uni-dimensional measure of negative affect, while retaining excellent case-finding ability.[31, 32]
The Oswestry Disability Inventory (ODI)
The ODI is an extensively used 10-item scale to describe the level of physical function in patients with chronic low back pain [33].
The Neuropathic Pain Questionnaire (NPQ)
The NPQ is a validated, 12-item questionnaire that asks patients to rate the neuropathic qualities of their pain [34]. It was used to classify the pain syndrome as having a neuropathic symptom component or not.
Neuroimaging Methods and Cortical Thickness Analysis
A high-resolution MPRAGE scan was obtained for each subject using 3T Siemens TIM Trio MRI System (Siemens Medical, Erlangen, Germany), equipped with a 32-channel head coil (TR/TE 2300/3.39 ms, voxel size 1*1*1.33 mm).
Arterial Spin Labeling data was also used to identify significant clusters where exacerbated back pain caused greater cortical activation and increased regional cerebral blood flow (rCBF) in cLBP subjects than HCs. For a review of these methods and scan parameters, please see Wasan and Loggia et al 2011[16].
Cortical thickness measurements were obtained by first processing the structural T-1 weighted data using the FreeSurfer version 5.1 (http://surfer.nmr.mgh.harvard.edu) cortical reconstruction pipeline[35, 36]. Any inaccuracies in the reconstruction of white and pial surfaces of individual subjects were manually corrected before calculating cortical thickness following the instructions provided by the software developers. The cortical thickness measure was computed as the averaged distances between the pial and white matter surfaces at each point across the cortical mantle.
A region of interest (ROI) analysis was used to contrast changes in morphometric indices between cLBP subjects and HCs in regions previously identified to preferentially activate in cLBP subjects undergoing a measured low back pain exacerbation during the MRI scan session. A total of 18 clusters were used for comparison (Figure 1, for a list of ROI coordinates please see Wasan and Loggia et al, 2011 Table 2[16]). Cortical thickness measurements for each subject were extracted from the ROIs and analyzed in SPSS version 20 (Chicago, Illinois). Multivariate analysis of variance (MANOVA) was used to contrast cortical thickness measurements from each ROI by group and multivariate analysis of covariance (MANCOVA) was used to control for age and HADS scores as covariates in the model of ROI thickness and group (to control for between subject variability). For each ROI, from the cortical thickness measurement differences between groups, we also calculated the required sample size to determine if there are significant differences between groups, should the magnitude of those differences exist.
Figure 1.
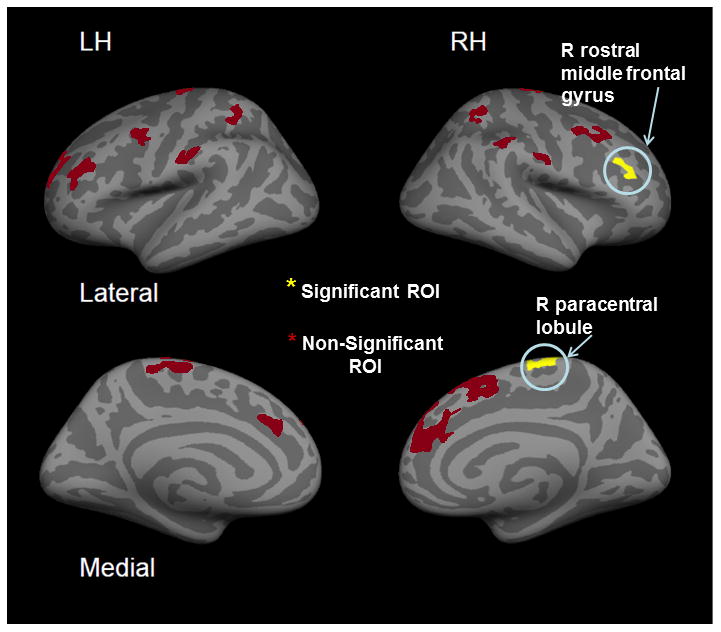
Regions of Interest (ROIs) from a previous ASL study where cluster activation in cLBP subjects vs. Healthy controls (cLBP > HCs) after exacerbated low back pain was significant.
Two regions showing cortical thickness cLBP subjects > HC are labeled.
Table 2. ROI Cortical Thickness Values.
| ROI Brain Region | cLBP Cortical Thickness Avg (SD) mm | HC Cortical Thickness Avg (SD) mm | F | Sig (P) | # Needed per Group for 80% PowerŦ |
|---|---|---|---|---|---|
| L superior parietal lobule | 2.50 (0.26) | 2.39 (0.26) | 1.34 | 0.26 | 90 |
| L secondary somatosensory cx | 2.60 (0.19) | 2.50 (0.34) | 0.99 | 0.33 | 121 |
| L superior frontal gyrus | 2.85 (0.39) | 2.75 (0.24) | 0.66 | 0.42 | 163 |
| L rostral middle frontal gyrus | 2.53 (0.23) | 2.51 (0.15) | 0.08 | 0.79 | 1098 |
| L superior parietal lobule | 2.27 (0.30) | 2.32 (0.31) | 0.19 | 0.67 | 586 |
| L paracentral gyrus | 2.39 (0.20) | 2.42 (0.28) | 0.08 | 0.79 | 1467 |
| L rostral middle frontal gyrus | 2.55 (0.30) | 2.59 (0.18) | 0.17 | 0.69 | 607 |
| L precentral gyrus | 2.71 (0.27) | 2.57 (0.42) | 1.04 | 0.32 | 106 |
| L caudal middle frontal gyrus | 2.53 (0.27) | 2.43 (0.38) | 0.62 | 0.44 | 177 |
| R insula | 3.37 (0.40) | 3.26 (0.24) | 0.77 | 0.39 | 147 |
| R superior frontal gyrus | 2.77 (0.18) | 2.72 (0.23) | 0.36 | 0.56 | 324 |
| R superior parietal lobule | 2.34 (0.28) | 2.25 (0.25) | 0.81 | 0.38 | 138 |
| R caudal middle frontal gyrus | 2.69 (0.12) | 2.62 (0.19) | 1.20 | 0.28 | 95 |
| R superior frontal gyrus | 2.95 (0.28) | 2.88 (0.23) | 0.60 | 0.45 | 185 |
| R paracentral gyrus | 2.36 (0.20) | 2.22 (0.16) | 3.67 | 0.07 | 31 |
| R rostral middle frontal gyrus* | 2.46 (0.29) | 2.21 (0.22) | 6.88 | 0.01 | 17 |
| R postcentral gyrus | 2.42 (0.31) | 2.39 (0.34) | 0.07 | 0.80 | 1616 |
| R supramarginal gyrus | 2.68 (0.30) | 2.74 (0.28) | 0.34 | 0.57 | 324 |
p< 0.01
= Number needed to find significant differences between groups, should they exist for these magnitude of differences between groups at each ROI, assuming 80% power, p=.05, and a 2-tailed independent samples T test
A follow-up whole brain cortical thickness analysis was also conducted between cLBP subjects and HCs. Group analyses were performed by resampling each subject's data to the FreeSurfer average atlas, distributed as a part of FreeSurfer. Cortical thickness maps were smoothed using a Gaussian kernel with a full width half maximum (FWHM) of 8mm. Vertex wise analyses of cortical thickness between groups were performed with an initial vertex-wise threshold was set at p<0.001 (uncorrected). Montecarlo simulations (5,000 sets) were run on the vertex-wise results to control for multiple comparisons using a vertex-level threshold of p<0.001 and cluster-level threshold of p<0.05.
A sensitivity analysis was conducted which included investigating the interaction of age and group on cortical thickness in the whole brain analysis as well. ROI thickness was correlated with age within each group, identifying Pearson correlations and P values. The age*group interaction was also examined among all ROIs and significant interactions were identified (p<0.05).
Voxel-Based Morphometry (VBM) Analysis
VBM is a measure of cortical density and volume[37]. VBM is comprised of a voxel-wise comparison of the local concentration of gray matter between two groups of subjects[37]. VBM analysis was conducted in SPM8[38] (http://www.fil.ion.ucl.ac.uk/spm) running in Matlab version 7.7 (The MathWorks, Natick, MA). First, structural T-1 weighted images were skull stripped using Brain Extraction Tool (BET)[39] and spatially normalized into the MNI standard space. Next all images were segmented into grey matter (GM), white matter (WM), cerebrospinal fluid (CSF), and non-brain-volumes. GM images were then averaged to produce the initial template brain. An iterative process of applying the inverse deformation from this initial template to individual brains was completed to create a new template – i.e. the final DARTEL template brain (Diffeomorphic Anatomical Registration using Exponentiated Lie algebra: DARTEL toolbox8[40]). Data were smoothed with a Gaussian kernel and FWHM of 8mm.
Voxel-wise general linear model (GLM) analysis was performed contrasting GM volumes from cLBP and HC subjects. Statistical maps were thresholded at an uncorrected voxel level (p < 0.001) and then corrected for multiple comparisons by false discovery rate (FDR, p<0.05).
Results
Clinical Characteristics
Demographic and clinical characteristics of 14 cLBP subjects and 14 age-paired and gender matched HCs are displayed in Table 1. No significant differences were identified between groups in age as determined by an independent samples t-test (t(26)=0.206, p<0.838). Significant differences were identified in HADS scores between groups (P<.01). However, all cLBP subjects and HCs scored below the threshold for likely having a co-morbid affective disorder (HADSanxiety< 9, and HADSdepression< 9).
Table 1. Demographics and Clinical Measures.
| Clinical Measure | cLBP Subjects (n=14) | Healthy Controls (n=14) |
|---|---|---|
| Age | 46.93 (±14.56) | 45.86 (±12.88) |
| Gender | 9F (64.29%) | 9F (64.29%) |
| HADS (total score) | 12.07 (±5.3) | 1.17 (±2.73)* |
| Avg Pain (0-10) | 5.21 (±1.77) | |
| Pain Duration (yrs) | 8.21 (±2.1) | |
| NPQ (% neuropathic pain) | 35.71% | |
| ODI (% disability) | 16.8% (±5.13) | 0 |
P<.01
= 1 standard deviation above and below the mean
cLBP = Chronic low back pain
HADS = Hospital Anxiety and Depression Scale
Avg Pain = Average pain on 11-point numerical rating scale (0-10)
NPQ = Neuropathic Pain Questionnaire
ODI – Oswestry Disability Index
Neuroimaging Findings
MANOVA of ROIs between cLBP subjects and HCs (Figure 1, Table 2) showed a cLBP subject cortical thickening trend in the right paracentral lobule, part of S1 - the primary somatosensory area, (F(1,17)=3.667, p<0.067) and significant thickening in the right rostral middle frontal gyrus, part of the DLPFC, (F(1,17)=6.880, p<0.014). However, these areas did not hold significance after including age as a covariate in the model (S1, p<0.891; DLPFC p<0.279). The follow-up whole-brain analysis as well did not identify significant differences in cortical thickness between groups after correcting for multiple comparisons. VBM also did not identify any significant differences in grey matter volume after controlling for multiple comparisons. Using the cortical thickness measurements, Table 2 also displays the sample sizes needed to find statistically significant differences in cortical thickness between groups for each ROI, should they exist for the magnitude of differences we found between groups. The numbers needed per group range from 17-1616. These statistics illustrate the extent to which our sample size of 14 per group may have failed to identify significant differences between groups. However, they also convey how similar the cortical thickness measurements are between groups.
To examine the relationship between clinical factors and cortical thickness, a linear regression analysis was used. Gender, pain intensity, the duration of pain, the presence of neuropathic pain, HADS scores, or functional ratings (ODI scores) were not significant univariate predictors of cortical thickness among the ROIs or the whole brain measurements. However, a dichotomous trend in the correlation of age and cortical thickness of the right rostral middle frontal gyrus(DLPFC) between cLBP subjects (R=-0.027, p< 0.928) and HCs (R=-0.805, p< 0.001) was identified (Figure 2). This indicates that the healthy controls had thinning with age in this area, while the cLBP patients did not.
Figure 2.
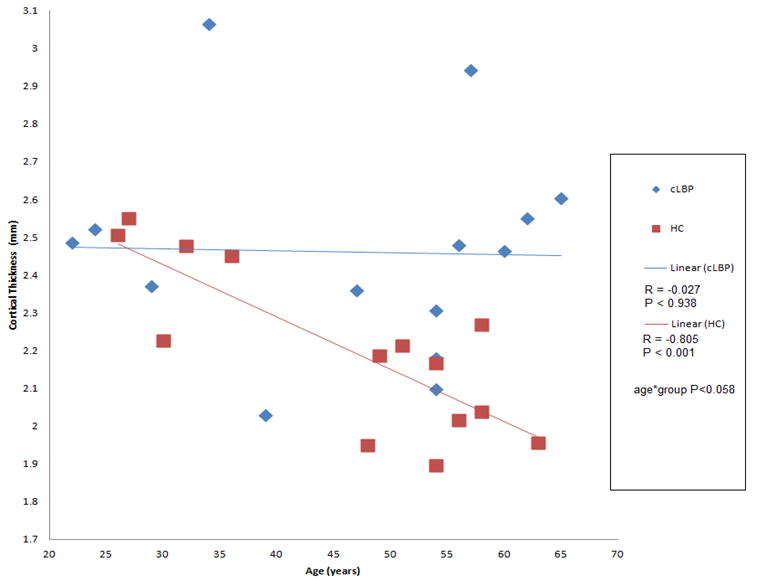
Correlations of age and right rostral middle frontal gyrus thickness are plotted by group.
A trending interaction between group and age is observed.
HCs have a strong negative correlation between thickness and age while cLBP subjects have no correlation.
Discussion
This study investigated the impact of simultaneously controlling for known predictive factors of morphometric change in a cLBP subject sample with a discogenic pain component and comparing them to healthy controls. We used multiple methods of grey matter quantification to identify potential differences in regions we previously found to be more active in cLBP, when LBP was worsened. These include region of interest, whole brain, and VBM approaches. In contrast to most studies of cLBP cortical thickness, the present study found that cLBP subjects demonstrated cortical thickening in pain processing regions – significant at the level of the DLPFC and trending in S1 (leg somatotopic region, paracentral lobule). These regions did not retain significance when age was included as a covariate of no-interest in the statistical model. However, a dichotomous trend was found in the DLFPC, indicating that the cLBP cohort did not have expected age-related thinning as seen in the healthy controls. VBM analysis did not show state-related changes (either thinning or thickening). Thus, in a carefully phenotyped population with chronic low back pain, our primary result was that we could not replicate previously reported findings of cortical thinning found in more diverse clinical cohorts[2-7], despite the use of multiple methods to investigate these findings.
Similar results have been reported in other chronic pain populations. In a matched study of fibromyalgia patients with and without affective disorder and healthy controls, cortical thickness measurements were obtained with negative affect scores as a covariate[8]. It was found that patients with high negative affect showed thinning in the right insula. However, those without an affective disorder did not significantly differ from healthy controls, suggesting that an affective disorder and not a pain condition may be associated with cortical thinning. In addition, age is another strong predictor of cortical thickness that is associated with decreases in brain volume.[12] One study has identified interactions between age and group (patients with temporomandibular disorder (TMD) vs. healthy controls) in the pre-motor area and dorsal striatum, where chronic pain subjects showed increased cortical density with age rather than the expected cortical thinning that is observed in healthy controls[41]. These findings comport with our results and highlight the importance of including age in the statistical model. If age is not included as a covariate, the interaction between disease state and thickness cannot be well characterized.
Of note, our sample size (n=28) was similar to many previous studies of cortical thickness in patients with chronic pain.[13] Using the cortical thickness measurement differences between groups, we also performed a power analysis to address issues of being under powered as well as to illustrate the similarities in thickness measurements between groups (i.e., non-inferiority, not equivalence). We have demonstrated that for the vast majority of the ROI's (except S1 and the DLPFC, which were not significant when controlling for age), the required sample sizes are quite large (>100 per group) to determine that the magnitude of cortical thickness differences we found between groups are statistically significant, should they indeed exist.
While the cortical thickening results in the cLBP patients are subtle findings, they are useful to discuss. The exact mechanisms of possible cortical thickening are not well understood in pain conditions, and one possible explanation for this thickening is activation and proliferation of glial cells, including astrocytes and microglia, in brain areas showing increased activation and regional cerebral blood flow (rCBF) in subjects suffering from cLBP. Reactive gliosis can occur in association with a variety of pathological events, including excitotoxocity resulting from excess release of glutamate[42]. In cLBP, S1 is repeatedly stimulated due to nerve inflammation or nerve injury located at the site of disc degeneration. Nociceptive afference to S1 results from potential tissue damage even during benign behaviors. Overtime, S1 neurons may undergo damage due to the increased glutamatergic signaling coming from ascending sensory inputs. While neuronal damage is often associated with cortical thinning, glial cells may proliferate in areas of inflammation (particularly in S1 for chronic pain conditions), leading to glial scarring, astrocytosis, and increased cortical thickness.
This reactive gliosis hypothesis may also hold true for the thickening findings related to the DLPFC. This area is implicated in working memory and attention[43]. Studies have associated cLBP with decreased performance on attention demanding and working memory tasks through detailed neuropsychological testing when compared to age and education matched healthy controls[44]. The constant pain signaling is a highly salient event that directs attention to the experience of pain. Consequently, the DLPFC area may also become hyper-activated with resultant excitotoxicity and increased astrocyte growth. This may also explain our findings in cLBP, where, in contrast to HCs, age-related thinning was not found in DLPFC. With possible glial cell proliferation in cLBP, the DLPFC may hold a stable thickness rather than thinning over time.
While this study was careful in its characterization and selection of subjects, it still holds some important limitations. The power of the study would be greatly enhanced if the sample size were increased. Hence, our results are best thought of as pilot findings, since a larger sample size would be required to definitely dispel the notion that chronic low back pain is intrinsically associated with cortical thinning. Our findings suggest that there is more to the morphometry of cLBP than cortical thinning, but carefully planned follow up studies will be needed to test this theory of cortical thickening. It would also be important to include a sample of patients who have cLBP and depression/anxiety as another arm in a future study and compare the differences between cortical thickness in depressed and non-depressed cLBP patients and to then collapse these two groups into one cohort and compare against healthy controls. When the pain sample is more heterogeneous we may expect to find cortical thinning, however this thinning may be better attributed to a comorbid affective disorder rather than cLBP in and of itself. Another follow-up study could examine reactive gliosis and neuroinflammation through the effects of anti-inflammatory medications and novel glial cell inhibitors on cortical thickness. Such a study may provide molecular evidence for gliosis in vivo.
The present study provides groundwork for future studies to be conducted investigating morphometric changes in a homogenous pain population. In addition to the reported methodological advances in phenotyping patients with chronic pain, morphometric brain change in cLBP may include more nuanced changes than cortical thinning as reported by previous studies. Cortical thickening may have important implications regarding neuroinflammation and provide a new avenue to pursue novel treatment therapies for chronic pain. Our pilot results suggest that controlling for affect, age, and concurrent medications may reduce or eliminate some of the previously reported structural brain alterations in cLBP.
Acknowledgments
Funding: NIH/NIDA 1K23DA020681-01A1
References
- 1.Davis KD, Moayedi M. Central Mechanisms of Pain Revealed Through Functional and Structural MRI. J Neuroimmune Pharmacol. 2012;24:24. doi: 10.1007/s11481-012-9386-8. [DOI] [PubMed] [Google Scholar]
- 2.Apkarian AV, Sosa Y, Sonty S, Levy RM, Harden RN, Parrish TB, Gitelman DR. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. 2004;24:10410–5. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodriguez-Raecke R, Niemeier A, Ihle K, Ruether W, May A. Brain gray matter decrease in chronic pain is the consequence and not the cause of pain. J Neurosci. 2009;29:13746–50. doi: 10.1523/JNEUROSCI.3687-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seminowicz DA, Labus JS, Bueller JA, Tillisch K, Naliboff BD, Bushnell MC, Mayer EA. Regional gray matter density changes in brains of patients with irritable bowel syndrome. Gastroenterology. 2010;139:48–57.e2. doi: 10.1053/j.gastro.2010.03.049. Epub 2010 Mar 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valet M, Gundel H, Sprenger T, Sorg C, Muhlau M, Zimmer C, Henningsen P, Tolle TR. Patients with pain disorder show gray-matter loss in pain-processing structures: a voxel-based morphometric study. Psychosom Med. 2009;71:49–56. doi: 10.1097/PSY.0b013e31818d1e02. Epub 2008 Dec 10. [DOI] [PubMed] [Google Scholar]
- 6.Buckalew N, Haut MW, Morrow L, Weiner D. Chronic pain is associated with brain volume loss in older adults: preliminary evidence. Pain Med. 2008;9:240–8. doi: 10.1111/j.1526-4637.2008.00412.x. [DOI] [PubMed] [Google Scholar]
- 7.Seminowicz DA, Wideman TH, Naso L, Hatami-Khoroushahi Z, Fallatah S, Ware MA, Jarzem P, Bushnell MC, Shir Y, Ouellet JA, Stone LS. Effective treatment of chronic low back pain in humans reverses abnormal brain anatomy and function. J Neurosci. 2011;31:7540–50. doi: 10.1523/JNEUROSCI.5280-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsu MC, Harris RE, Sundgren PC, Welsh RC, Fernandes CR, Clauw DJ, Williams DA. No consistent difference in gray matter volume between individuals with fibromyalgia and age-matched healthy subjects when controlling for affective disorder. Pain. 2009;143:262–7. doi: 10.1016/j.pain.2009.03.017. Epub 2009 Apr 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matharu MS, Good CD, May A, Bahra A, Goadsby PJ. No change in the structure of the brain in migraine: a voxel-based morphometric study. Eur J Neurol. 2003;10:53–7. doi: 10.1046/j.1468-1331.2003.00510.x. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt-Wilcke T, Leinisch E, Ganssbauer S, Draganski B, Bogdahn U, Altmeppen J, May A. Affective components and intensity of pain correlate with structural differences in gray matter in chronic back pain patients. Pain. 2006;125:89–97. doi: 10.1016/j.pain.2006.05.004. Epub 2006 Jun 5. [DOI] [PubMed] [Google Scholar]
- 11.Younger JW, Chu LF, D'Arcy NT, Trott KE, Jastrzab LE, Mackey SC. Prescription opioid analgesics rapidly change the human brain. Pain. 2011;152:1803–10. doi: 10.1016/j.pain.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 13.Smallwood RF, Laird AR, Ramage AE, Parkinson AL, Lewis J, Clauw DJ, Williams DA, Schmidt-Wilcke T, Farrell MJ, Eickhoff SB, Robin DA. Structural brain anomalies and chronic pain: a quantitative meta-analysis of gray matter volume. J Pain. 2013;14:663–75. doi: 10.1016/j.jpain.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tabachnick BG, Fidell LS. Limitations to analysis of covariance. In: Tabachnick BG, Fidell LS, editors. Using Multivariate Statistics. Boston: Pearson Education; 2007. pp. 200–203. [Google Scholar]
- 15.Fourney DR, Andersson G, Arnold PM, Dettori J, Cahana A, Fehlings MG, Norvell D, Samartzis D, Chapman JR. Chronic low back pain: a heterogeneous condition with challenges for an evidence-based approach. Spine (Phila Pa 1976) 2011;36:S1–9. doi: 10.1097/BRS.0b013e31822f0a0d. [DOI] [PubMed] [Google Scholar]
- 16.Wasan AD, Loggia ML, Chen LQ, Napadow V, Kong J, Gollub RL. Neural correlates of chronic low back pain measured by arterial spin labeling. Anesthesiology. 2011;115:364–74. doi: 10.1097/ALN.0b013e318220e880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loisel P, Vachon B, Lemaire J, Durand MJ, Poitras S, Stock S, Tremblay C. Discriminative and predictive validity assessment of the quebec task force classification. Spine (Phila Pa. 1976;27:851–7. doi: 10.1097/00007632-200204150-00013. [DOI] [PubMed] [Google Scholar]
- 18.Aprill C, Bogduk N. High-intensity zone: a diagnostic sign of painful lumbar disc on magnetic resonance imaging. Br J Radiol. 1992;65:361–9. doi: 10.1259/0007-1285-65-773-361. [DOI] [PubMed] [Google Scholar]
- 19.Fardon DF, Milette PC. Nomenclature and classification of lumbar disc pathology. Recommendations of the Combined task Forces of the North American Spine Society, American Society of Spine Radiology, and American Society of Neuroradiology. Spine (Phila Pa. 2001;26:E93–E113. doi: 10.1097/00007632-200103010-00006. [DOI] [PubMed] [Google Scholar]
- 20.Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa. 2001;26:1873–8. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 21.Jamison RN, Ross EL, Michna E, Chen LQ, Holcomb C, Wasan AD. Substance misuse treatment for high-risk chronic pain patients on opioid therapy: a randomized trial. Pain. 2010;150:390–400. doi: 10.1016/j.pain.2010.02.033. Epub 2010 Mar 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wasan AD, Davar G, Jamison R. The association between negative affect and opioid analgesia in patients with discogenic low back pain. Pain. 2005;117:450–61. doi: 10.1016/j.pain.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 23.Derby R, Aprill CN, Lee JE, DePalma MJ, Baker RM. Comparison of four different analgesic discogram protocols comparing the incidence of reported pain relief following local anesthetic injection into concordantly painful lumbar intervertebral discs. Pain Med. 2012;13:1547–53. doi: 10.1111/j.1526-4637.2012.01499.x. [DOI] [PubMed] [Google Scholar]
- 24.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–38. [PubMed] [Google Scholar]
- 25.Krause SJ, Backonja MM. Development of a neuropathic pain questionnaire. Clin J Pain. 2003;19:306–14. doi: 10.1097/00002508-200309000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine (Phila Pa. 1976;25:2940–52. doi: 10.1097/00007632-200011150-00017. discussion 2952. [DOI] [PubMed] [Google Scholar]
- 27.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52:69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 28.Tan G, Jensen M, Thornby J, Shanti B. Validation of the Brief Pain Inventory for Chronic Nonmalignant Pain. J Pain. 2004;5:133–37. doi: 10.1016/j.jpain.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Bjelland I, Dahl AA, Huag TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52:69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 30.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. ACTA Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 31.Cosco TD, Doyle F, Ward M, McGee H. Latent structure of the Hospital Anxiety And Depression Scale: a 10-year systematic review. J Psychosom Res. 2012;72:180–4. doi: 10.1016/j.jpsychores.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 32.Doyle F, Cosco T, Conroy R. Why the HADS is still important: Reply to Coyne & van Sonderen. J Psychosom Res. 2012;73:74. doi: 10.1016/j.jpsychores.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 33.Fairbank JCT, Pynsent PB. The Oswestry Disability Index. Spine. 2000;25:2940–53. doi: 10.1097/00007632-200011150-00017. [DOI] [PubMed] [Google Scholar]
- 34.Krause SJ, Backonja MM. The Development of a Neuropathic Pain Questionnaire. Clin J Pain. 2003;19:315–316. doi: 10.1097/00002508-200309000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–5. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–94. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 37.Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11:805–21. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 38.SPM. Statistical Parameter Mapping. [Accessed 8/1/13, 2013]; [Web Page]. Available at http://www.fil.ion.ucl.ac.uk/spm/
- 39.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–55. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. Epub 2007 Jul 18. [DOI] [PubMed] [Google Scholar]
- 41.Moayedi M, Weissman-Fogel I, Salomons TV, Crawley AP, Goldberg MB, Freeman BV, Tenenbaum HC, Davis KD. Abnormal gray matter aging in chronic pain patients. Brain Res. 2012;1456:82–93. doi: 10.1016/j.brainres.2012.03.040. Epub 2012 Mar 27. [DOI] [PubMed] [Google Scholar]
- 42.Buffo A, Rite I, Tripathi P, Lepier A, Colak D, Horn AP, Mori T, Gotz M. Origin and progeny of reactive gliosis: A source of multipotent cells in the injured brain. Proc Natl Acad Sci U S A. 2008;105:3581–6. doi: 10.1073/pnas.0709002105. Epub 2008 Feb 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–83. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- 44.Weiner DK, Rudy TE, Morrow L, Slaboda J, Lieber S. The relationship between pain, neuropsychological performance, and physical function in community-dwelling older adults with chronic low back pain. Pain Med. 2006;7:60–70. doi: 10.1111/j.1526-4637.2006.00091.x. [DOI] [PubMed] [Google Scholar]


