Summary
The centromedial amygdala (CeM), a subdivision of the central amygdala (CeA), is believed to be the main output station of the amygdala for fear expression. We provide evidence that the Tac2 gene, expressed by neurons specifically within the CeM, is required for modulating fear memories. Tac2 is colocalized with GAD65 and CaMKIIα but not with PKCd and Enk neurons in the CeM. Moreover, the Tac2 product, NkB, and its specific receptor, Nk3R, are also involved in the consolidation of fear memories. Increased Tac2 expression, through a stress-induced PTSD-like model, or following lentiviral CeA overexpression, are sufficient to enhance fear consolidation. This effect is blocked by the Nk3R antagonist, osanetant. Concordantly, silencing of Tac2-expressing neurons in CeA with DREADDs impairs fear consolidation. Together these studies provide a new understanding of the role of the Tac2 gene and CeM in fear processing and may provide novel approaches to intervention for fear-related disorders.
Introduction
Among learning and memory processes, fear memories are crucial in anxiety disorders such as Panic Disorder, Phobia, and Posttraumatic stress disorder (PTSD). PTSD occurs in some individuals after experiencing or witnessing extreme traumatic events. The symptoms of PTSD include re-experiencing memories of these traumatic events through intrusive thoughts, flashbacks, and nightmares (American Psychiatric Association, 2013). PTSD is also generally accompanied by hyperarousal symptoms. Moreover, persistent highly aversive memories related to the trauma, potentially over-consolidated memories, and the inability of these memories to be extinguished are all frequent characteristics of this disorder. Specifically relevant is the memory consolidation phase following emotional learning since it is required to stabilize the initial fear memory trace.
In order to decrease the prevalence of PTSD, it is necessary to identify biological and environmental risk and resilience markers (Norrholm and Ressler, 2009; Vermetten and Lanius, 2012), to find early interventions after trauma exposure (Kearns et al., 2012) and to treat the disorder when it is present and debilitating (Andero and Ressler, 2012; Hetrick et al., 2010). Notably, understanding molecular pathways mediating the initial fear consolidation event is particularly important to target the prevention of PTSD. The only FDA-approved pharmaceutical treatments for PTSD are selective serotonin reuptake inhibitors (SSRI) antidepressants, which have met with limited results in clinical trials (Hetrick et al., 2010). Even when antidepressants are combined with exposure-based psychotherapy, increased effectiveness has not always been demonstrated (Hetrick et al., 2010). Thus, more effective, targeted approaches to prevention and treatment are needed to normalize the functioning of areas key to fear processes such as the amygdala, the hippocampus or the medial prefrontal cortex (mPFC).
The tachykinins refer to two peptides encoded in rodents by the Tachykinin 1 (Tac1) and Tac2 (TAC3 in humans) genes which are involved in neurotransmission and neuromodulation in the central nervous system (Beaujouan et al., 2004). Tac1 encodes a precursor protein that produces two peptides, substance P (SP) and neurokinin A (NkA), whereas Tac2/TAC3 encodes neurokinin B (NkB). SP and NkA have been previously implicated in fear processes and PTSD (Dunlop et al., 2012). Unfortunately, clinical trials with pharmaceutical agents targeting the Tac1 pathway have not previously shown beneficial effects in PTSD treatment (Dunlop et al., 2012). A possible explanation for this lack of effect is that the SP and NkA receptors (Neurokinin 1 receptor, Nk1R, and Neurokinin 2 receptor, Nk2R) are widely expressed in the brain. So, when administering drugs that specifically target Nk1 and Nk2 they interact with multiple brain regions affecting multiple functions (Beaujouan et al., 2004). In contrast, the expression of Tac2, NkB, and its specific receptor, Neurokinin 3 receptor (Nk3R), are relatively restricted in rodents to brain regions that regulate emotion, such as the amygdala (Beaujouan et al., 2004; Duarte et al., 2006). Nk3R is a G-protein coupled tachykinin receptor that binds NkB with highest affinity (Gether, 2000; Khawaja and Rogers, 1996). Nk3R couples to the pertussis toxin-insensitive-G proteins Gq/G11, the activation of which results in the production of inositol triphosphate and diacylglycerol, and the activation of protein kinase C (Khawaja and Rogers, 1996). Additionally, it has been shown that TAC3 and Nk3R are expressed in the equivalent areas in rhesus monkeys and humans (Mileusnic et al., 1999; Nagano et al., 2006).
Here, beginning with an unbiased discovery approach, we show that the Tac2 gene is dynamically regulated during the consolidation of conditioned fear within the central amygdala (CeA). Additionally, Nk3R activation is required for normal consolidation of fear memory formation in mice. Furthermore, increased expression of the Tac2 gene, NkB peptide and activation of Nk3R may be involved in stress sensitization and over-consolidation of fear. In contrast, genetic silencing of Tac2-expressing neurons impairs fear consolidation. Blockade of this pathway may provide for a novel therapeutic approach for disorders with altered fear learning such as PTSD.
Results
Tac2 is involved in fear learning
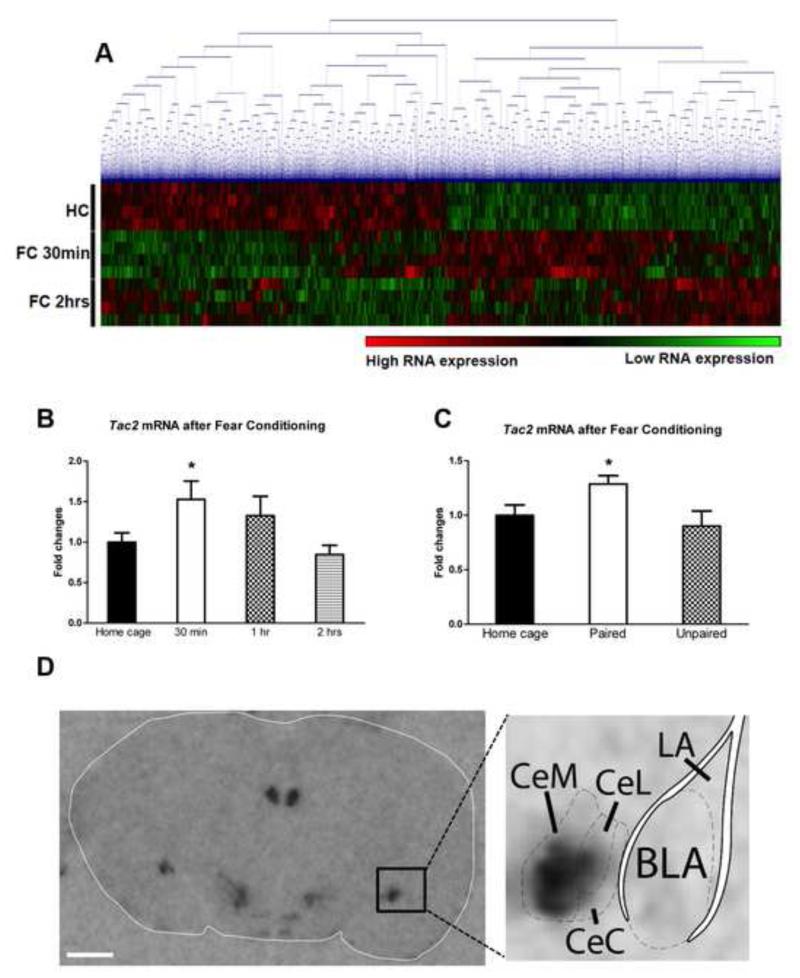
Using amygdala tissue punches from mice that had been sacrificed 30 minutes or 2 hours after auditory fear conditioning (FC) (CS, acoustic tone; US, electric footshocks, Figure S1A), we performed an mRNA microarray. Using average linkage hierarchical clustering, the microarray heat map shows differential gene regulation at 30 minutes and at 2 hours after fear learning, which is a critical period for consolidation of fear memories (Ressler et al., 2002), Figure 1A. FDR was calculated with SAM 4.01 using a standard 5% cutoff criteria. The cutoff criteria was set with an FDR at the 1.3 fold level for the 2hrs after fear conditioning (FC) group, since with the more conservative 1.5 fold cutoff used in the 30 min after FC group, no genes were initially identified. The criteria followed in Supplemental Table 1 and 2 for a Yes in the column “Specifically highly expressed in the amygdala”: 1) Very high expression in the amygdala (red color, Allen Brain Atlas) 2) No expression of the gene in the hippocampus nor PFC (other key areas related to emotional learning). Moreover, from the top candidates of this microarray, the only gene which is specifically highly expressed in the amygdala and belongs to a ‘druggable’ pathway with available agonists and antagonists which cross the blood-brain barrier and can be used systemically is Tac2 (See Table S1-2, Figure S1B and S2). Therefore we focused on understanding and manipulating the Tac2 pathway.
Figure 1. Differential regulation of Tac2 gene expression in the amygdala during cued-fear conditioning.
A) With average linkage hierarchical clustering of a RNA microarray there is a differential gene regulation 30 minutes and 2 hours after auditory fear conditioning (FC) when compared to home cage group (no FC). N=4 per group. B) Tac2 mRNA levels are rapidly up-regulated in the amygdala during fear consolidation 30 minutes after fear conditioning. *P ≤0.05 vs HC and 2 hrs. N=7-8 per group. C) Tac2 up-regulation occurs when the conditioned stimulus (acoustic tone) and the unconditioned stimulus (electric footshock) are paired but not when they are unpaired. *P ≤0.05 vs HC and unpaired. N=11-15 per group. Mean + SEM is shown. D) Tac2 expression by radioactive in situ hybridization in the amygdala is restricted to the central amygdala (CeA) with highest expression in the CeM amygdala. Scale bar = 1 mm. See also Figure S1 and Table S1 and S2.
Independent replication studies with additional fear conditioned mice show that Tac2 is rapidly up-regulated at 30 minutes after FC, returning to basal levels at 2 hours (ANOVA F3,28 = 5.014, P ≤ 0.01, Post-hoc *P ≤ 0.05 vs Home Cage (HC) and 2hrs, Figure 1B). Moreover, in an additional replication, Tac2 mRNA up-regulation only occurred when the conditioned and unconditioned stimuli are paired, but not when they are unpaired, suggesting that within this paradigm, Tac2 increased expression is specific to associative cued fear learning and independent of non-specific stress and/or contextual learning (ANOVA F2,36 = 3.93, P ≤ 0.05, Post-hoc *P ≤0.05 vs HC and unpaired, Figure 1C). See Figure S3 for detailed interactions of the Tac2 gene and Nk3R.
Tac2, NkB and Nk3R in the amygdala
Figure 1D and S1B show a radioactive in situ hybridization demonstrating that the areas where Tac2 gene is expressed are quite specific and limited within in the mouse brain: bed nucleus of the stria terminalis, hypothalamus, habenula, central amygdala (CeA), zona incerta and medial mammillary nucleus. Tac2 is highly expressed in the CeA within the amygdala with no expression in the basolateral amygdala (BLA) nor lateral amygdala (LA) (Figure 1D). The highest expression of Tac2 within the CeA occurs in the medial subdivision of the central amygdaloid nucleus (CeM), whereas lower expression is observed in the centro-lateral (CeL) and centro-central (CeC) amygdala.
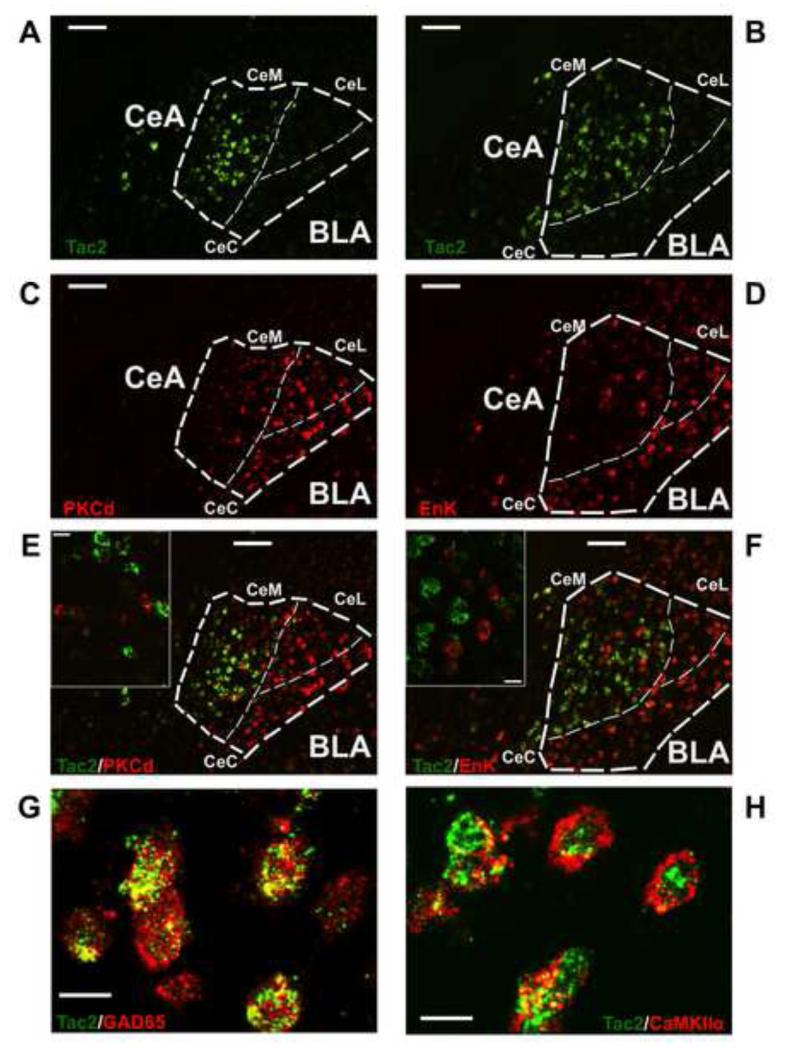
Recently, specific cell populations within the central nucleus have received attention for distinct roles in fear learning. For example, PKCd has been suggested to be part of a microcircuit in which the CeL amygdala neurons inhibit neuronal output to the CeM during the conditioned stimulus, which drives fear expression, called CeLoff units (Haubensak et al., 2010). Moreover, around 40% of Protein Kinase C Delta (PKCd) expressing neurons also express Enkephalin (Enk) in the CeL (Haubensak et al., 2010). Of note, Enk mRNA levels are increased after FC in the CeL (Petrovich et al., 2000). Since other neuronal populations have been previously related to fear processes in the CeA, we wished to examine if Tac2 mRNA colocalized with them suggesting functional interactions. Using double fluorescent in situ hybridization (FISH), we show that Tac2 gene expression is mostly not colocalized with PKCd nor Enk and is expressed primarily within the CeM (Figure 2A-F). Thus, given the lack of colocalization and regional and functional specificity of these cell populations, we have uncovered a subdivision-specific cell population that may be involved in the consolidation of fear memory. Additionally, the colocalization of Tac2 mRNA levels and the 65-kDa isoform of glutamic acid decarboxylase (GAD-65) peptide in the CeM (Figure 2G) may provide deeper understanding of the functions of gamma-aminobutyric acid (GABA) in fear learning. CaMKIIα, a well characterized neuronal population involved in synaptic plasticity, is also colocalized with Tac2 mRNA in the CeM (Figure 2H). Interestingly, GAD65 and CaMKII are associated with the consolidation of fear memories in the amygdala although little is known about the specific role of these peptides in each substructure (Bergado-Acosta et al., 2008; Lepicard et al., 2006).
Figure 2. Tac2 is colocalized with glutamate decarboxylase 65 (GAD65) and calmodulin-dependent protein kinase II α (CAMKIIα) but is not colocalized with Protein Kinase C Delta nor Enkephalin-expressing neurons in the CeM.
A and B) Tac2 mRNA expression in the CeA and BLA by non-radioactive fluorescent in situ hybridization (FISH). Scale bar = 100 μm. C) PKCd mRNA expression by FISH. Scale bar = 100 μm. D) Enk mRNA expression by FISH. Scale bar = 100 μm. E) Right, A and C merged showing different pattern of expression of Tac2 and PKCd in the CeA. Scale bar = 100 μm. Left, confocal image showing no colocalization of Tac2 and PKCd in the CeM. Scale bar = 15 μm. F) Right, B and D merged showing different pattern of expression of Tac2 and Enk. Scale bar = 100 μm. Left, confocal image showing no colocalization of Tac2 and Enk in the CeM. Scale bar = 15 μm. G) Confocal image showing colocalization of Tac2 mRNA expression and GAD65 peptide in the CeM. Scale bar = 15 μm. H) Confocal image showing colocalization of Tac2 mRNA expression and CaMKIIα peptide in the CeM. Scale bar = 15 μm. CeM = centro-medial amygdala, CeL = centro-lateral amygdala, CeC = centro-central amygdala, CeA = central amygdala, BLA = basolateral amygdala.
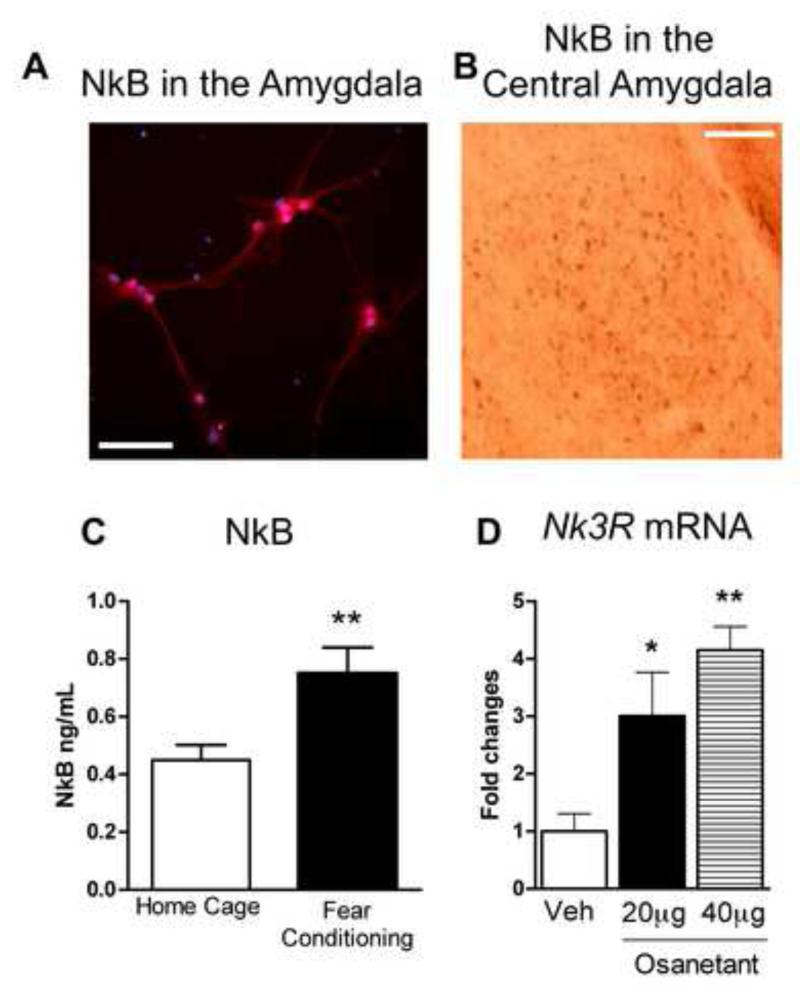
We also examined detection of the NkB peptide in amygdala cell culture, demonstrating that the peptide is highly present in both soma of neurons and dendrites (Figure 3A). Moreover, NkB peptide is also highly expressed in the CeA (Figure 3B). Interestingly, NkB is up-regulated in the amygdala 2 hours after FC (Student’s t test, t = −2.902, **P ≤ 0.01 Fear Conditioning vs Home Cage, Figure 3C). The Nk3R antagonist osanetant has already been used in humans in clinical trials for schizophrenia. Although it appears to have no beneficial effects in the treatment of schizophrenia, these studies show that it is well tolerated and safe in humans (Meltzer et al., 2004). Here, in amygdala cell culture, osanetant inactivates the Nk3R and leads to a compensatory increase in Nk3R expression as suggested by dose-dependent enhanced Nk3R mRNA levels (ANOVA, F3,5 = 10.014, P ≤ 0.05; Post-hoc *P ≤0.05 vs Veh, **P ≤0.01 vs Veh, Figure 3D).
Figure 3. Fear conditioning, expression of Neurokinin B and Neurokinin 3 receptor in the amygdala.
A) The Tac2 product, Neurokinin B (NkB) is detected by immunocytochemistry in mouse amygdala cell culture. NkB is highly expressed in the soma and in the dendrites. Red is NkB signal. Blue is neuronal nucleus, NeuN. Scale bar = 25 μm. B) Immunohistochemistry studies show high expression of NkB in the central amygdala (CeA). Scale bar = 125 μm. C) NkB is up-regulated at 2 hrs in the amygdala after fear conditioning. **P ≤0.01 vs Home Cage, N=6-8 per group. D) Amygdala cell culture with osanetant, a potent and specific neurokinin 3 (Nk3R) antagonist. Incubation with 20 μg and 40 μg of osanetant enhances Nk3R mRNA levels. This suggests that osanetant activates Nk3R and its downstream signaling in the amygdala. *P ≤0.05 vs Veh, **P ≤0.01 vs Veh, N=2 per group. Mean + SEM is shown.
Osanetant and emotional learning
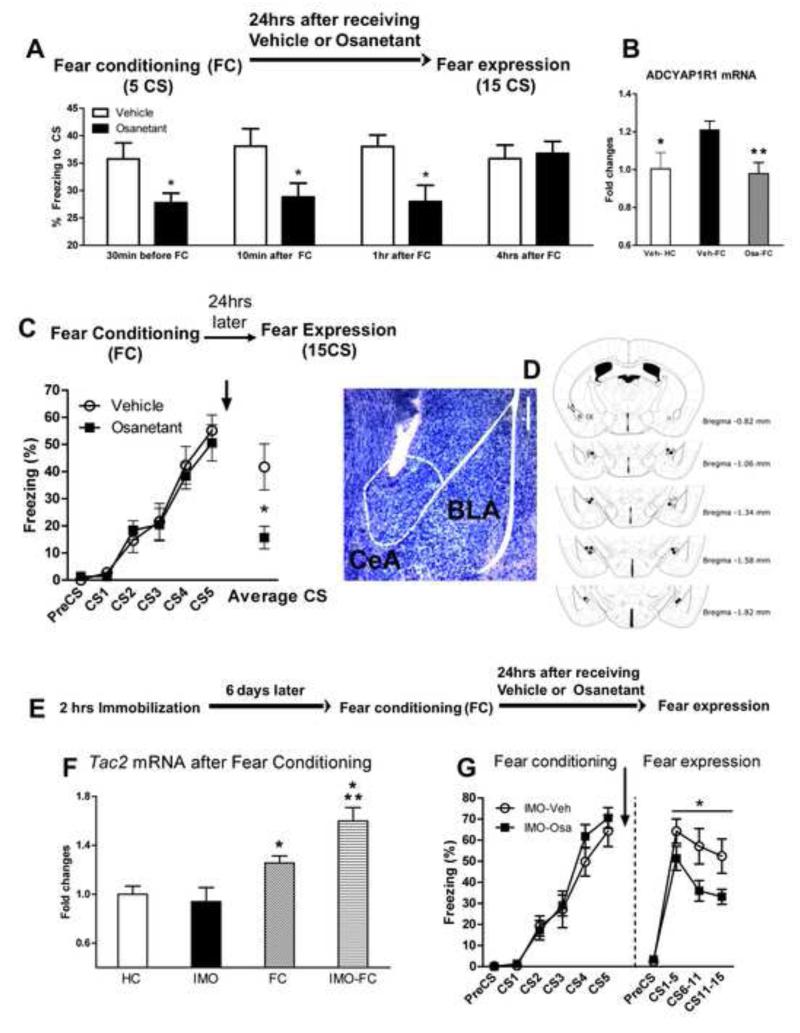
The above studies suggest that osanetant may be an ideal candidate to target the Nk3R in the amygdala in vivo, and we wished to examine its effects behaviorally. Osanetant given systemically, 30 minutes before open field, elevated plus maze and the conditioning chamber elicits no changes in anxiety-like behavior, locomotor activity or electric shock reactivity (Figure S4). Notably, when osanetant is dosed from 30 min before auditory FC up to 1 hour after training it does not affect fear acquisition, but impairs fear memory consolidation as shown by decreased freezing in the fear expression test (Figure 4A and S4G; Student’s t test, 30 min t = 3.042; 10 minutes after FC, t = 2.277; 1 hour after FC, t = 2.872; *P ≤ 0.05 vs vehicle).
Figure 4. A Nk3R antagonist impairs cued-fear memory consolidation when infused systemically, in the central amygdala and in a PTSD-like mouse model.
A) Osanetant impairs cued-fear memory when given from 30 minutes to up to 1 hour after fear acquisition. The figure shows the time spent in freezing behavior during the fear expression test when the CS is presented. *P ≤.05 vs Veh. N=4-12 per group. B) Osanetant given intraperitoneally 30 minutes before fear conditioning impaired the enhancement of mRNA levels of the Pac1 receptor (Adcyap1r1), *P ≤0.05 vs Veh-FC; **P ≤0.01 vs Veh-FC. N= 9-12 per group. The PACAP-PAC1R pathway is associated with PTSD, fear conditioning and stress C) Osanetant bilaterally injected into the central amygdala immediately after fear conditioning causes impaired fear memory consolidation as shown by lower freezing in the cued-fear expression test *P ≤ 0.05, N= 3-9 mice per group. D) Histological verification of osanetant infusion sites. Left, toluidine blue staining showing an example of the tip of the cannula in the CeA. Scale bar = 250 μm. Right, the dots indicate the lowest point of the injector tip. E) Timeline of the experiment. F) Cued-fear conditioning enhances Tac2 levels 30 minutes after fear conditioning in naïve mice but more robustly in mice with a previous exposure to immobilization to a wooden board (IMO), a PTSD-like model. N=12-15 per group. *P ≤0.05 vs HC, **P ≤0.01 vs IMO. G) Osanetant was given immediately after FC and impaired fear memory consolidation in mice which had been previously exposed to a traumatic stress as shown by reduced freezing in the fear expression test, *P ≤0.05. N= 8 per group. Mean + or ± SEM is shown. Veh = vehicle; Osa = osanetant.
Recently, the PACAP-PAC1R pathway has been associated with PTSD in humans as well as in animal models (Ressler et al., 2011, (Stevens et al., 2014). These prior data showed that expression of the ADCYAP1R1 gene (encoding the PAC1 receptor) is increased following FC. Here we found that osanetant given before FC also normalizes the levels of ADCYAP1R1 mRNA levels in the amygdala (ANOVA F2,31 = 5.541, P ≤0.01; Post-hoc *P ≤0.05 vs Veh-FC; **P ≤0.01 vs Veh-FC, Figure 4B). These data suggest that inhibition of the Tac2/NKB/Nk3R pathway may prevent activation of a stress-related gene pathway previously associated with PTSD. Concordantly, bilateral infusion of osanetant in the CeA also impairs fear memory consolidation, suggesting that CeA-NK3R are required for the formation for emotional memories (Student’s t test; t = 2.268, *P ≤ 0.05 vs vehicle, Figure 4C and D).
We have shown in previous studies that mice exposed for 2 hours to a severe one-time stressor, immobilization to a wooden board (IMO), present long-term PTSD-like symptoms: impaired fear extinction and spatial memory, and enhanced anxiety-like behaviors (Andero et al., 2013; Andero et al., 2011). Additionally, IMO in rats elicits alterations of the hypothalamic-pituitary-adrenal (HPA) axis which may be similar to the process initiating PTSD in humans (Armario et al., 2008). Notably, Tac2 mRNA levels were more robustly up-regulated in IMO treated mice than in naïve mice after FC, consistent with enhanced Tac2-dependent fear processing (ANOVA F3,53 = 6.242, P ≤ 0.001, Post-hoc *P ≤0.05 vs HC, **P ≤0.01 vs IMO, Figure 4F). Additionally, osanetant given systemically after FC impaired memory consolidation in IMO treated mice, as shown by decreased freezing in the fear expression test (ANOVA repeated measures F1,13 = 6.072, *P ≤0.05, Figure 4G). This suggests that Nk3R antagonism reduces enhanced fear memory consolidation in a PTSD-like model.
Tac2 overexpression and blockade by osanetant
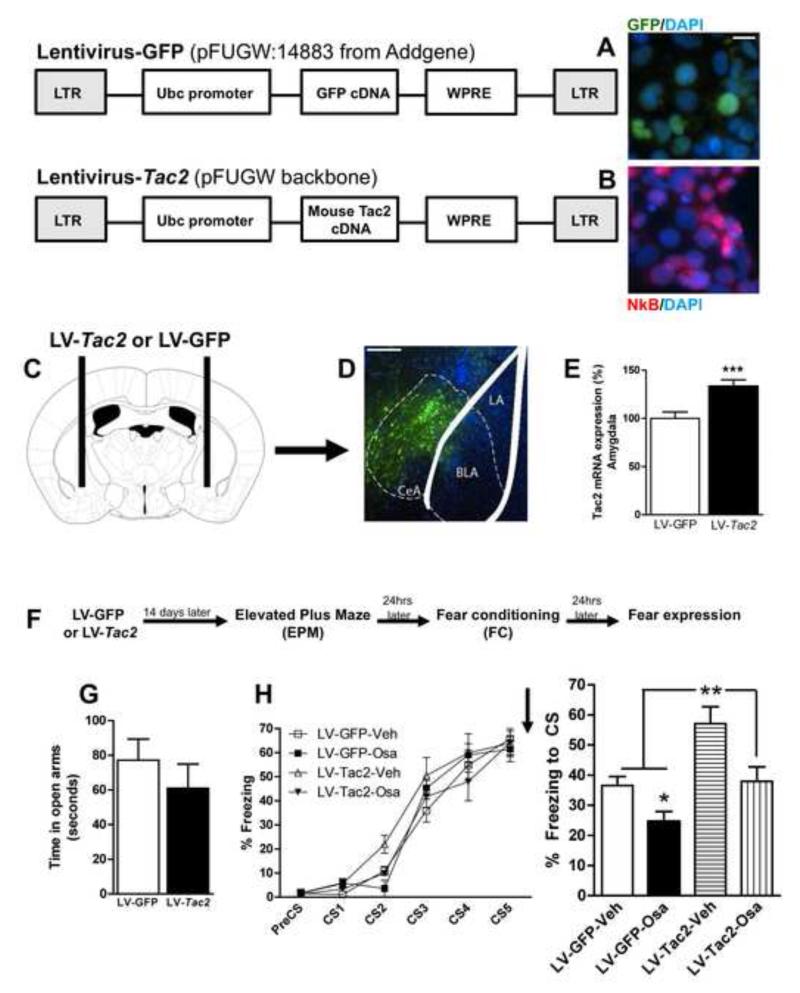
We next developed a viral vector to overexpress the Tac2 gene in an inducible fashion within the brain, the lentivirus-Tac2 (LV-Tac2). We first tested its functional expression by infecting HEK 293 cells with the LV-Tac2 compared to control LV-GFP lentiviruses, demonstrating that the NkB peptide was robustly expressed (Figure 5A and B). We then examined the behavioral effects of Tac2 overexpression in mice. LV-Tac2 or LV-GFP were bilaterally infused in the CeA (Figure 5C and D) and 14 days later Tac2 was found to be overexpressed by 42%, as determined by mRNA levels with in situ hybridization, compared to mice that had received LV-GFP (Student’s t test; t = −3841, ***P ≤ 0.001 vs LV-GFP, Figure 5E). Mice infected with the LV-Tac2 or LV-GFP received systemic osanetant or vehicle immediately after FC, and then fear expression was tested 24 hours later. Specific CeA-Tac2 overexpression elicited a significant enhancement of fear memory consolidation (ANOVA F3,20 = 8.512 P ≤ 0.05; Post-hoc **P ≤ 0.05 vs LV-GFP-Veh, Figure 5I). Interestingly, we found that Tac2 overexpression in the CeA did not induce changes in anxiety-like behavior nor fear acquisition (Figures 5G and H). Replicating our previous findings, osanetant impaired fear memory consolidation when given to mice with the control LV-GFP (Post-hoc, *P ≤ 0.05 vs LV-GFP-Veh, Figure 5I). Additionally, the enhanced fear memory consolidation caused by CeA-Tac2 overexpression was reversed by osanetant (Post-hoc, **P ≤ 0.01 vs LV-Tac2-Osa and LV-GFP-Osa Figure 5I). See Figure S5 for a graphical representation of the Tac2-LV overexpression.
Figure 5. Tac2 overexpression in the central amygdala is sufficient to enhance fear memory consolidation and it is blocked by an Nk3R antagonist.
A) The lentivirus GFP-FUGW induces GFP expression but not Neurokinin B (NkB) in Hek293 cells. Scale bar = 10 μm. B) The lentivirus Tac2-FUGW induces NkB expression in Hek293 cells. DAPI staining (blue) indicates the cellular nuclei. NkB staining (red) is contrasted with GFP fluorescence (green). C) Tac2-FUGW or GFP-FUGW were bilaterally infused in the central amygdala and mice were left undisturbed for 14 days. D-E) The lentivius Tac2-FUGW causes a 42% overexpression of Tac2 in the central amygdala. ***P ≤0.001 vs LV-GFP. N= 9-15 per group. Scale bar = 250 μm. F) Timeline of the experiment. G) Tac2 overexpression in the central amygdala does not alter anxiety-like behavior evaluated by the time spent in the open arms in the elevated plus maze. N= 9-15. H) Left, the lentiviruses GFP-FUGW and Tac2-FUGW causes no changes in fear conditioning. Osanetant or vehicle were given systemically immediately after fear acquisition. Right) Tac2 overexpression enhances fear memory consolidation (LV-Tac2-Veh) and osanetant impairs this effect (LV-Tac2-Osa). N= 3-8 per group. *P≤0.05 vs LV-GFP-Veh, **P ≤0.01 vs all other groups. Mean + or ± SEM is shown. See also Figure S5.
Silencing of Tac2-expressing cells and emotional learning
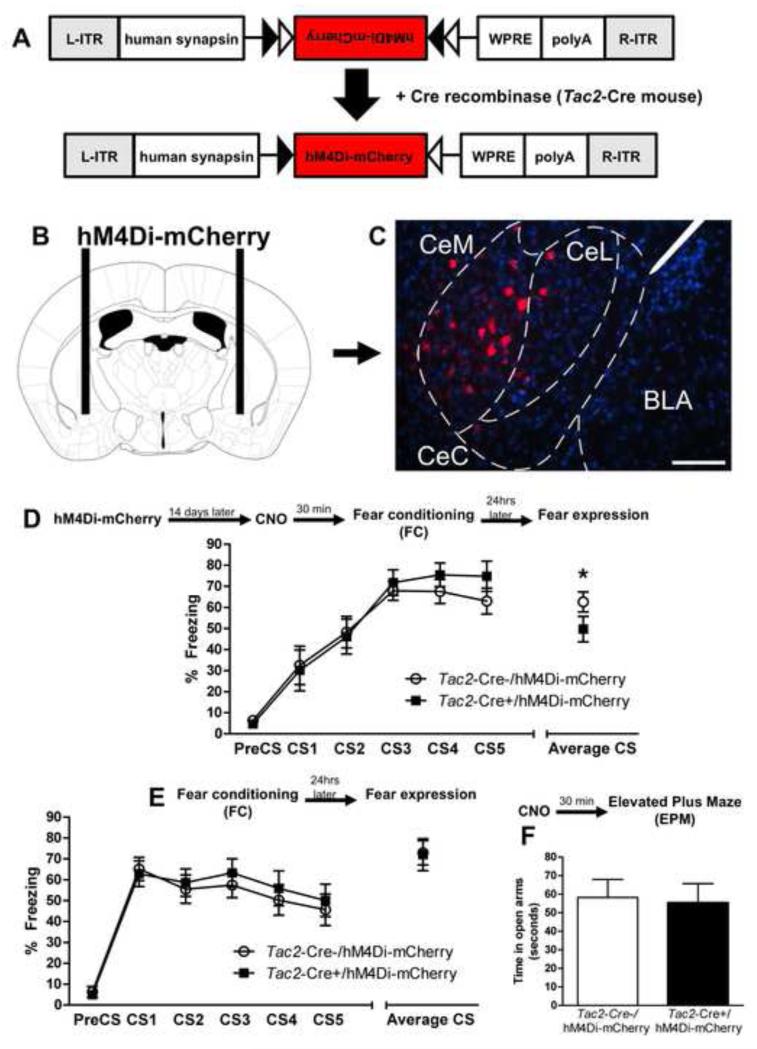
To further understand the role of the Tac2 gene, we temporarily silenced the activity of neurons expressing this gene in the CeA during fear learning using designer receptors exclusively activated by designer drugs (DREADD) technology. The B6.129-Tac2tm1.1(cre)Qima/J (Tac2-cre) (Mar et al., 2012) mice were infected with a DREADD Gi-coupled receptor via the pAAV-hSyn-double floxed hM4D-mCherry virus (hM4Di-mCherry AAV) (Figure 6A). This elicited specific expression of the mCherry reporter in Tac2 cells within the CeA, but not any other area of the brain, suggesting the insertion of the DREADD receptor on the plasma membrane (Figure 6B and 6C, (Krashes et al., 2011). 14 days later, clozapine-N-oxide (CNO), which binds to the inserted receptor but otherwise is pharmacologically inert, was given systemically 30 minutes before FC in both groups, Tac2-Cre-/hM4Di-mCherry and Tac2-Cre+/hM4Di-mCherry. CNO had no effect on fear acquisition as shown by equivalent amount of freezing in both groups (Figure 6D). However, when animals were tested for fear expression, 24hrs later in the absence of CNO, the Tac2-Cre+/hM4Di-mCherry mice presented less freezing, suggesting impaired fear memory consolidation (Student’s t test, t = 3.257, *P ≤ 0.05 Tac2-Cre-/hM4Di-mCherry vs Tac2-Cre+/hM4Di-mCherry, Figure 6D). This demonstrates that the animals expressing Tac2-Cre+/hM4Di-mCherry and inducible Gi to temporally silence the activity of Tac2-expressing neurons exhibit significantly less fear consolidation when tested for fear learning. Mice were then retrained with a different CS and a different context in the same FC apparatus, as in previous experiments but without dosing CNO. Tac2-Cre-/hM4Di-mCherry and Tac2-Cre+/hM4Di-mCherry mice showed similar amount of freezing in the FC and fear expression test (Figure 6E). This suggests that when Tac2-expressing neurons are not silenced there is normal fear memory consolidation in both Tac2-Cre-/hM4Di-mCherry and Tac2-Cre+/hM4Di-mCherry groups. Moreover, when given CNO, these two groups presented equivalent levels of anxiety-like behavior and pain sensitivity (Figure 6F and Figure S6).
Figure 6. Inducible silencing of Tac2-expressing neurons in the CeA with Gi-DREADD decreases conditioned fear.
A) Design of hM4Di-mCherry AAV and Tac2-cre mice. B and C) Tac2-Cre- or Tac2-Cre+ mice were infected with the hM4Di-mCherry AAV in the CeA. The Gi receptor was inserted only on the Tac2-Cre cells of Tac2-Cre+ mice, CeM = centro-medial amygdala, CeL = centro-lateral amygdala. Scale bar = 125 μm. D) CNO was given systemically 30 minutes prior to fear conditioning to Tac2-Cre-/hM4Di-mCherry and Tac2-Cre+/hM4Di-mCherry. Temporal silencing of the Tac2-expressing neurons in the Tac2-Cre+/hM4Di-mCherry group did not affect freezing during fear acquisition. However, when mice were tested the day after for fear expression, without CNO, Tac2-Cre+/hM4Di-mCherry mice showed less conditioned fear. *P ≤0.05 Tac2-Cre-/hM4Di-mCherry vs Tac2-Cre+/hM4Di-mCherry, N=10-11 per group. E) Tac2-Cre-/hM4Di-mCherry and Tac2-Cre+/hM4Di-mCherry mice were retrained to a different acoustic tone (CS) without receiving CNO. Both groups equally acquired fear learning and showed similar levels of fear memory consolidation. F) CNO given 30 minutes before the elevated plus maze showed no effect on Tac2-Cre-/hM4Di-mCherry nor Tac2-Cre+/hM4Di-mCherry in anxiety-like behavior. See also Figure S6.
Discussion
Previous reports have shown that Nk3R is associated with memory processes in hippocampus-dependent tasks in rodents (de Souza Silva et al., 2013; Siuciak et al., 2007; Zlomuzica et al., 2008). Here, we provide novel evidence that Tac2-NkB-Nk3R signaling within the CeA is required for the modulation of fear memory consolidation. Other studies have shown that the CeA is required for the acquisition, consolidation and expression of fear memories (Wilensky et al., 2006). Here we show novel mechanisms that may also be involved in those processes within the CeA. To the best of our knowledge, this is the first evidence that a neuronal population specifically highly expressed in the CeM, Tac2 and its product NkB peptide, are required for the modulation of fear memory consolidation affecting neither unconditioned fear nor anxiety-like behavior. All that is known about NkB release is from in vitro experiments, where it is suggested that NkB release is potassium-evoked and calcium-dependent, fulfilling the criterion of a neurotransmitter or a neuromodulator (Lindefors et al., 1985). More studies about this topic would be desirable to further understand the mechanisms of the Tac2/NkB/Nk3R pathway.
Our findings also suggest that CeA-Tac2 lentiviral overexpression enhances fear memory consolidation but Nk3R antagonism prevents it. This shows that Nk3R antagonism within the CeA is able to normalize dysregulated functioning induced by the Tac2 gene. We believe that it is possible that osanetant given systemically or intracranially within the CeA may be acting in the Nk3R in all areas of CeA and not only in the CeM (Smith and Flynn, 2000; Yip and Chahl, 1997). This impairment of fear memory consolidation by Nk3R antagonism is consistent with previous reports where Nk3R activation with senktide in the hippocampus and cortex leads to enhanced post-synaptic depolarization and long-term-potentiation (LTP) in slice physiology studies, and this effect was blocked by NK3R antagonism (Gallopin et al., 2006; Rekling, 2004). However, specific electrophysiological experiments in the amygdala should be performed in the future to study if activation or blockade of the Nk3R in this structure is involved in LTP or other types of activity-dependent plasticity.
In agreement with these prior findings, specific and temporal pharmacogenetic silencing of Tac2-expressing neurons in the CeA with DREADDs leads to impaired fear memory consolidation. Of note, no effects in fear acquisition, pain sensitivity nor anxiety-like behavior were detected when over-expressing Tac2, inhibiting Tac2-expressing neurons, or with the Nk3R antagonist, which is consistent with previous findings suggesting that the Tac2-NkB-Nk3R pathway is not directly involved in these processes, although it may modulate them (Ebner et al., 2009; Mar et al., 2012; Siuciak et al., 2007). Interestingly, in our fear paradigm, the Tac2 gene within the amygdala is involved in auditory (CS+US paired) but not stress nor contextual memories (US only). However, this does not preclude the hypothesis that different fear paradigms might reveal a role of Tac2 in the amygdala in contextual fear conditioning.
Thus, we believe that enhanced Tac2 gene expression in our fear models enhanced NkB production in the amygdala, binding to Nk3R and promoting fear memory consolidation. This up-regulation of Tac2 mRNA levels primarily within the CeM suggests several possible non-mutually exclusive scenarios. The first is that the Tac2 gene synthesizes NkB in the CeM amygdala, acting on local Nk3R within the CeM specifically. The second is that Tac2 mRNA and/or NkB are transported from the CeM to other nuclei within the amygdala such as CeL, CeC or BLA where they bind to the Nk3R. Our data also suggests that amygdala cell culture with osanetant increases Nk3R mRNA levels. The most likely interpretation is that osanetant antagonizes amygdala Nk3R and due to its decreased availability, Nk3R mRNA is increased to synthesize more Nk3R in a compensatory manner. The current data provide intriguing support for a specific role of Tac2 gene, via NkB activation of Nk3R in fear consolidation within the CeA.
CeLon neurons essentially serve as disinhibitory cells for CeLoff neurons. Both CeLon and CeLoff neurons release GABA when activated. CeLon neurons send projections to CeLoff neurons, and, therefore, the increased firing of CeLon neurons during CS presentation results in inhibition of CeLoff neurons (normally inhibiting the CeM), and, thus, in disinhibition of CeM, promoting conditioned fear responses (Ciocchi et al., 2010). Specifically, CeLoff neurons largely overlap with PKCd+ neurons (Haubensak et al., 2010). Moreover, we also show that Tac2 gene is not colocalized with Enk in the CeM. Enk is co-localized with PKCd in the CeL (Haubensak et al., 2010) and specific CeA-Enk deletion decreases fear expression during FC without affecting fear memory consolidation (Poulin et al., 2013). Thus, the Tac2-CeM neuronal population appears to be independent of, and complementary to, other previously described neuronal populations involved in FC. The GAD65 peptide, abundantly found at nerve terminals and synapses, plays a key role in GABA neurotransmission (Pinal and Tobin, 1998). Additionally, CaMKII is a well-known marker for synaptic plasticity. Thus, the colocalization of Tac2 mRNA levels and GAD65 and CaMKIIα peptides in the CeM suggest that Tac2 gene may have a role in neurotransmission within the GAD65 and CaMKIIα expressing neurons, in agreement with our data that suggest that this CeM population may be critically involved in fear memory consolidation.
Finally, one of the most interesting aspects of our data is the potential use of the Nk3R antagonist osanetant as a pharmacological agent to block fear memory consolidation shortly after exposure to a trauma. Additionally we found that osanetant prevented the up-regulation of the Adcyap1r1 gene, which encodes the PAC1 receptor. The PACAP-PAC1R pathway is involved in PTSD, fear conditioning, amygdala excitatory neurotransmission and stress (Almli et al., 2013; Cho et al., 2012; Hashimoto et al., 2011; Ressler et al., 2011; Uddin et al., 2013). All this could be relevant in PTSD prevention since it has previously been found that osanetant is safe in humans, although additional preclinical studies, such as those described herein are needed first to establish the mechanisms involved. This gives our findings an exciting potential approach to translation to human patients. Although other molecular pathways have previously been associated with PTSD we believe that there will be a number of different mechanisms identified that eventually will synergistically be used to target emotional memory modulation.
In summary, these studies provide a new understanding of the role of the Tac2 gene and the CeM in fear processing and provide novel approaches to intervention for fear-related disorders.
Experimental procedures
Procedures are described in detail in Supplemental Experimental Procedures.
Mice
Amygdala cell culture experiments were performed with male wild-type (WT) C57BL/6J p21 mice. All other experiments were performed on adult WT C57BL/6J or B6.129-Tac2tm1.1(cre)Qima/J (Tac2-Cre) (Mar et al., 2012) from Jackson Labs (Stock # 018938), male mice that were group-housed in a temperature-controlled vivarium, with ad libitum access to food and water. Animals were maintained on a 12-hour/12-hour light/dark cycle, with all behavioral procedures being performed during the light cycle. All procedures used were approved by the Institutional Animal Care and Use Committee of Emory University and in compliance with National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals.
Immobilization to wooden board (IMO)
Mice were exposed once for 2 hours to IMO, which was performed as previously described (Andero et al., 2013; Andero et al., 2011).
mRNA extraction and microarray
Total mRNA was isolated and purified from the tissue with the RNeasy Mini Kit (catalog 74106, Qiagen). Illumina MouseWG-6 v2 Expression BeadChip microarray (Illumina Inc.) was assayed for 45,281 transcripts as previously described (Andero et al., 2013). FDR was calculated with SAM 4.01 using a 5% cutoff for the FDR rate. The heat maps were created with Genesis 1.4.0 (Sturn et al., 2002). The pathway analysis was generated through the use of IPA (Ingenuity® Systems, www.ingenuity.com). The microarray data are publicly available in the Gene Expression Omnibus database under accession number GSE57465.
Behavioral experiments
Elevated plus maze, open field, cued-fear conditioning and fear expression tests were performed as previously described (Andero et al., 2013; Andero et al., 2011). The CS were 30 seconds 0.6 Khz tone and the US were 1 mA 500 ms electric footshocks. Retraining of mice was performed with a 12 kHz tone.
Complementary DNA synthesis and qPCR
Total mRNA was reverse-transcribed with the RT2 First Strand Kit (catalog 330401, Qiagen). The primers used for the qPCR were TaqMan Tac2 Mm01160362_m1, ADCYAP1R1 Mm01326453_m1 and NK3R Mm00445346_m1 from Applied Biosystems. The qPCR was performed and analyzed as previously described (Andero et al., 2013).
Radioactive in situ hybridization
Tissue was fixed in 4% paraformaldehyde, pretreated, hybridized with 36SUTP labeled cRNA riboprobes prepared from linearized constructs for antisense sequence of Tac2 (T7 RNA polymerase) as previously described (Rattiner et al., 2004).
Fluorescent in situ hybridization (FISH)
cRNA riboprobes prepared from linearized constructs for antisense sequences of Tac2, PKCd and Enkephalin (T7 RNA polymerase) as previously described (Jasnow et al., 2013). The Tac2 riboprobe was labeled with fluorescein and the PKCd and Enkephalin with digoxigenin. Signals were amplified with the TSA Plus Fluorescein Fluorescence System or TSA Plus Cy5 Fluorescence System (PerkinElmer) following each series of primary antibodies. Sections were then stained with DAPI (1:1000), washed, and coverslipped with Mowiol mounting medium (Jasnow et al., 2013).
Amygdala cell culture
Amygdala primary cell culture was performed as previously described (Mou et al., 2011).
Immunohistochemistry
Pep2/ProNkB, IS-39 ab (1:500) was the antibody used to detect NkB. The procedure was followed as previously described (Kallo et al., 2012). The procedure for detecting Gad65 (AB5082, Chemicon, 1:500) and CaMkIIα (Cell Signalling Solutions, 1:250) was similar as previously described (Jasnow et al., 2013) after performing the Tac2 FISH. After the ISH and IHC sections were stained with DAPI (1:1000) washed, and coverslipped with Mowiol mounting medium.
Immunocytochemistry
Immunocytochemistry was performed as previously described (Mou et al., 2011). The antibody used was Pep2/ProNkB IHC (IS-39 ab, 1:500) (Kallo et al., 2012) and DAPI or NeuN (1:1000).
ELISA
Purchased from Mybiosource, Mouse Neurokinin B ELISA Kit (NKB), Catalogue #MBS744693. Inter-assay CV%: 7.5-8.6, Intra-assay CV%: 8.2-9.5, Spike Recovery: 95-103%. Procedure was followed as indicated by the manufacturer.
Production of Recombinant Viral Vectors
Mixture for transfection was 250ug of FUGW or FUW-Tac2 + 187.5ug of pCMVdelta 8.9 + 75ug of pV-SVG + 12ml of ddH2O + 12.5ml of 0.5M Ca2Cl + 25 ml of 2x HeBS to total volume 50ml, this solution was vortexed a few seconds and incubated for 20 min at room temperature. Procedure was followed as previously described (Huang et al., 2013). The pAAV-hSyn-double floxed hM4D-mCherry (hM4Di-mCherry AAV) was purchased from UNC Gene Therapy Center, NC, USA.
Surgery and injection of virus
Mice were anesthetized and placed in a stereotaxic frame. CeA coordinates were as follows: anteroposterior, −1.34mm; dorsoventral, −4.4mm; mediolateral, – 2.4mm relative to bregma. For the LV-Tac2 experiments the animals received bilateral intra-CeA amygdala injections of lentiviral vectors expressing Tac2-FUW or FUGW (GFP) in 1% BSA in phosphate buffered saline (PBS). 0.5 μl of virus/side. 1 μl of virus/side of the pAAV-hSyn-double floxed hM4D-mCherry (hM4Di-mCherry AAV) was injected in the CeA of Tac2-Cre- and Tac2-Cre+ mice. For all experiments the rate of injection was 0.1 μl/min and the needle was left in place for 10 min following injection and the skin was closed using a 6-0 Vicryl suture.
Drugs administration
The Nk3R antagonist osanetant (Axon Medchem, Axon 1533) was dissolved in physiological saline and 0.1% Tween 20 which was also the vehicle. Intraperitoneally (i.p.) dose was 5 mg/kg for systemic administration and 0.5 μl with 625 ng dose per side for the intra-CeA studies. Cannulation of the mice was performed as previously described (Andero et al., 2013). Clozapine-N-oxide (CNO, Sigma Aldrich C0832) was given i.p. at 1mg/kg (Krashes et al., 2011).
Statistics
Statistics were performed with IBM SPSS Statistics 19.0. Detection of outliers was performed and, when necessary, removed from analyses. ANOVA followed by post-hoc analyses were appropriate, repeated-measures ANOVA or Student’s t test (two-tailed) for independent samples was tested. The results are presented as means ± or + SEM, and statistical significance was set at P ≤ 0.05.
Supplementary Material
Highlights.
-
-
Tac2 is associated with memory consolidation in naïve mice and a PTSD-like model.
-
-
Antagonism of the Tac2 receptor restores normal and dysregulated fear consolidation.
-
-
Tac2 colocalizes with GAD65 and CaMKIIα but not with PKCd nor Enk neurons in the CeM.
-
-
Tac2 neurons are required for the modulation of fear memory consolidation in the CeA.
Acknowledgements
The authors would like to thank for their help to: Greg Doho (Microarray, Emory Cancer Genomics Shared Resource), Xinping Huang (Lentivirus, Viral Vector Core, Emory University), Oskar Laur (Cloning, Custom Cloning Core Facility, Emory University), Noreen Khan and Robert Bruner (Behavior), Liping Mou (Cell culture), Georgette Gafford (ISH and FISH), Aaron Jasnow (ISH), Takehito Sawamura (Stereotaxic surgery), Mallory Bowers and Joanna Dabrowska (Confocal microscope), Dennis Choi (Comments on the data), Philippe Cioffi (Donation of the NkB antibody IS-39). This research project was supported in part by the Viral Vector Core of the Emory Neuroscience NINDS Core Facilities grant, P30NS055077. This project was also funded by the Office of Research Infrastructure Programs/OD P51OD011132 (formerly NCRR P51RR000165). This work was also supported by these sources of funding: RA and KJR 1R21MH101492-01 and KJR 1R01MH096764, Burroughs Wellcome Fund and HHMI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental information includes Supplemental Experimental Procedures, six figures and two tables.
The authors declare no conflict of interests.
References
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM5) American Psychiatric Association; Washington, DC: 2013. [Google Scholar]
- Almli LM, Mercer KB, Kerley K, Feng H, Bradley B, Conneely KN, Ressler KJ. ADCYAP1R1 genotype associates with post-traumatic stress symptoms in highly traumatized African-American females. American journal of medical genetics Part B, Neuropsychiatric genetics: the official publication of the International Society of Psychiatric Genetics. 2013;162B:262–272. doi: 10.1002/ajmg.b.32145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andero R, Brothers SP, Jovanovic T, Chen YT, Salah-Uddin H, Cameron M, Bannister TD, Almli L, Stevens JS, Bradley B, et al. Amygdala-dependent fear is regulated by Oprl1 in mice and humans with PTSD. Science translational medicine. 2013;5:188ra173. doi: 10.1126/scitranslmed.3005656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andero R, Heldt SA, Ye K, Liu X, Armario A, Ressler KJ. Effect of 7,8-dihydroxyflavone, a small-molecule TrkB agonist, on emotional learning. The American journal of psychiatry. 2011;168:163–172. doi: 10.1176/appi.ajp.2010.10030326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andero R, Ressler KJ. Fear extinction and BDNF: translating animal models of PTSD to the clinic. Genes, brain, and behavior. 2012;11:503–512. doi: 10.1111/j.1601-183X.2012.00801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armario A, Escorihuela RM, Nadal R. Long-term neuroendocrine and behavioural effects of a single exposure to stress in adult animals. Neurosci Biobehav Rev. 2008;32:1121–1135. doi: 10.1016/j.neubiorev.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Beaujouan JC, Torrens Y, Saffroy M, Kemel ML, Glowinski J. A 25 year adventure in the field of tachykinins. Peptides. 2004;25:339–357. doi: 10.1016/j.peptides.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Bergado-Acosta JR, Sangha S, Narayanan RT, Obata K, Pape HC, Stork O. Critical role of the 65-kDa isoform of glutamic acid decarboxylase in consolidation and generalization of Pavlovian fear memory. Learning & memory. 2008;15:163–171. doi: 10.1101/lm.705408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JH, Zushida K, Shumyatsky GP, Carlezon WA, Jr., Meloni EG, Bolshakov VY. Pituitary adenylate cyclase-activating polypeptide induces postsynaptically expressed potentiation in the intra-amygdala circuit. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:14165–14177. doi: 10.1523/JNEUROSCI.1402-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocchi S, Herry C, Grenier F, Wolff SB, Letzkus JJ, Vlachos I, Ehrlich I, Sprengel R, Deisseroth K, Stadler MB, et al. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature. 2010;468:277–282. doi: 10.1038/nature09559. [DOI] [PubMed] [Google Scholar]
- de Souza Silva MA, Lenz B, Rotter A, Biermann T, Peters O, Ramirez A, Jessen F, Maier W, Hull M, Schroder J, et al. Neurokinin3 receptor as a target to predict and improve learning and memory in the aged organism. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:15097–15102. doi: 10.1073/pnas.1306884110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte CR, Schutz B, Zimmer A. Incongruent pattern of neurokinin B expression in rat and mouse brains. Cell and tissue research. 2006;323:43–51. doi: 10.1007/s00441-005-0027-x. [DOI] [PubMed] [Google Scholar]
- Dunlop BW, Mansson E, Gerardi M. Pharmacological innovations for posttraumatic stress disorder and medication-enhanced psychotherapy. Current pharmaceutical design 18. 2012:5645–5658. doi: 10.2174/138161212803530899. [DOI] [PubMed] [Google Scholar]
- Ebner K, Sartori SB, Singewald N. Tachykinin receptors as therapeutic targets in stress-related disorders. Current pharmaceutical design. 2009;15:1647–1674. doi: 10.2174/138161209788168074. [DOI] [PubMed] [Google Scholar]
- Gallopin T, Geoffroy H, Rossier J, Lambolez B. Cortical sources of CRF, NKB, and CCK and their effects on pyramidal cells in the neocortex. Cerebral cortex. 2006;16:1440–1452. doi: 10.1093/cercor/bhj081. [DOI] [PubMed] [Google Scholar]
- Gether U. Uncovering molecular mechanisms involved in activation of G protein-coupled receptors. Endocrine reviews. 2000;21:90–113. doi: 10.1210/edrv.21.1.0390. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Shintani N, Tanida M, Hayata A, Hashimoto R, Baba A. PACAP is implicated in the stress axes. Current pharmaceutical design. 2011;17:985–989. doi: 10.2174/138161211795589382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, Biag J, Dong HW, Deisseroth K, Callaway EM, et al. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature. 2010;468:270–276. doi: 10.1038/nature09553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetrick SE, Purcell R, Garner B, Parslow R. Combined pharmacotherapy and psychological therapies for post traumatic stress disorder (PTSD) The Cochrane database of systematic reviews. 2010:CD007316. doi: 10.1002/14651858.CD007316.pub2. [DOI] [PubMed] [Google Scholar]
- Huang X, Hartley AV, Yin Y, Herskowitz JH, Lah JJ, Ressler KJ. AAV2 production with optimized N/P ratio and PEI-mediated transfection results in low toxicity and high titer for in vitro and in vivo applications. Journal of virological methods. 2013;193:270–277. doi: 10.1016/j.jviromet.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasnow AM, Ehrlich DE, Choi DC, Dabrowska J, Bowers ME, McCullough KM, Rainnie DG, Ressler KJ. Thy1-expressing neurons in the basolateral amygdala may mediate fear inhibition. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33:10396–10404. doi: 10.1523/JNEUROSCI.5539-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallo I, Vida B, Deli L, Molnar CS, Hrabovszky E, Caraty A, Ciofi P, Coen CW, Liposits Z. Co-localisation of kisspeptin with galanin or neurokinin B in afferents to mouse GnRH neurones. Journal of neuroendocrinology. 2012;24:464–476. doi: 10.1111/j.1365-2826.2011.02262.x. [DOI] [PubMed] [Google Scholar]
- Kearns MC, Ressler KJ, Zatzick D, Rothbaum BO. Early interventions for PTSD: a review. Depression and anxiety. 2012;29:833–842. doi: 10.1002/da.21997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khawaja AM, Rogers DF. Tachykinins: receptor to effector. The international journal of biochemistry & cell biology. 1996;28:721–738. doi: 10.1016/1357-2725(96)00017-9. [DOI] [PubMed] [Google Scholar]
- Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, Lowell BB. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. The Journal of clinical investigation. 2011;121:1424–1428. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepicard EM, Mizuno K, Antunes-Martins A, von Hertzen LS, Giese KP. An endogenous inhibitor of calcium/calmodulin-dependent kinase II is up-regulated during consolidation of fear memory. The European journal of neuroscience. 2006;23:3063–3070. doi: 10.1111/j.1460-9568.2006.04830.x. [DOI] [PubMed] [Google Scholar]
- Lindefors N, Brodin E, Theodorsson-Norheim E, Ungerstedt U. Calcium-dependent potassium-stimulated release of neurokinin A and neurokinin B from rat brain regions in vitro. Neuropeptides. 1985;6:453–461. doi: 10.1016/0143-4179(85)90144-1. [DOI] [PubMed] [Google Scholar]
- Mar L, Yang FC, Ma Q. Genetic marking and characterization of Tac2-expressing neurons in the central and peripheral nervous system. Molecular brain. 2012;5:3. doi: 10.1186/1756-6606-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer HY, Arvanitis L, Bauer D, Rein W, Meta-Trial Study G. Placebo-controlled evaluation of four novel compounds for the treatment of schizophrenia and schizoaffective disorder. The American journal of psychiatry. 2004;161:975–984. doi: 10.1176/appi.ajp.161.6.975. [DOI] [PubMed] [Google Scholar]
- Mileusnic D, Lee JM, Magnuson DJ, Hejna MJ, Krause JE, Lorens JB, Lorens SA. Neurokinin-3 receptor distribution in rat and human brain: an immunohistochemical study. Neuroscience. 1999;89:1269–1290. doi: 10.1016/s0306-4522(98)00349-2. [DOI] [PubMed] [Google Scholar]
- Mou L, Heldt SA, Ressler KJ. Rapid brain-derived neurotrophic factor-dependent sequestration of amygdala and hippocampal GABA(A) receptors via different tyrosine receptor kinase B-mediated phosphorylation pathways. Neuroscience. 2011;176:72–85. doi: 10.1016/j.neuroscience.2010.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano M, Saitow F, Haneda E, Konishi S, Hayashi M, Suzuki H. Distribution and pharmacological characterization of primate NK-1 and NK-3 tachykinin receptors in the central nervous system of the rhesus monkey. British journal of pharmacology. 2006;147:316–323. doi: 10.1038/sj.bjp.0706561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Ressler KJ. Genetics of anxiety and trauma-related disorders. Neuroscience. 2009;164:272–287. doi: 10.1016/j.neuroscience.2009.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Scicli AP, Thompson RF, Swanson LW. Associative fear conditioning of enkephalin mRNA levels in central amygdalar neurons. Behavioral neuroscience. 2000;114:681–686. doi: 10.1037//0735-7044.114.4.681. [DOI] [PubMed] [Google Scholar]
- Pinal CS, Tobin AJ. Uniqueness and redundancy in GABA production. Perspectives on developmental neurobiology. 1998;5:109–118. [PubMed] [Google Scholar]
- Poulin JF, Berube P, Laforest S, Drolet G. Enkephalin knockdown in the central amygdala nucleus reduces unconditioned fear and anxiety. The European journal of neuroscience. 2013;37:1357–1367. doi: 10.1111/ejn.12134. [DOI] [PubMed] [Google Scholar]
- Rattiner LM, Davis M, Ressler KJ. Differential regulation of brain-derived neurotrophic factor transcripts during the consolidation of fear learning. Learn Mem. 2004;11:727–731. doi: 10.1101/lm.83304. [DOI] [PubMed] [Google Scholar]
- Rekling JC. NK-3 receptor activation depolarizes and induces an after-depolarization in pyramidal neurons in gerbil cingulate cortex. Brain research bulletin. 2004;63:85–90. doi: 10.1016/j.brainresbull.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, Norrholm SD, Kilaru V, Smith AK, Myers AJ, et al. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature. 2011;470:492–497. doi: 10.1038/nature09856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Paschall G, Zhou XL, Davis M. Regulation of synaptic plasticity genes during consolidation of fear conditioning. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002;22:7892–7902. doi: 10.1523/JNEUROSCI.22-18-07892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siuciak JA, McCarthy SA, Martin AN, Chapin DS, Stock J, Nadeau DM, Kantesaria S, Bryce-Pritt D, McLean S. Disruption of the neurokinin-3 receptor (NK3) in mice leads to cognitive deficits. Psychopharmacology. 2007;194:185–195. doi: 10.1007/s00213-007-0828-6. [DOI] [PubMed] [Google Scholar]
- Smith ME, Flynn FW. Distribution of Fos-like immunoreactivity within the rat brain following intraventricular injection of the selective NK(3) receptor agonist senktide. The Journal of comparative neurology. 2000;426:413–428. doi: 10.1002/1096-9861(20001023)426:3<413::aid-cne6>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Stevens JS, Almli LM, Fani N, Gutman DA, Bradley B, Norrholm SD, Reiser E, Ely TD, Dhanani R, Glover EM, et al. PACAP receptor gene polymorphism impacts fear responses in the amygdala and hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:3158–3163. doi: 10.1073/pnas.1318954111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturn A, Quackenbush J, Trajanoski Z. Genesis: cluster analysis of microarray data. Bioinformatics. 2002;18:207–208. doi: 10.1093/bioinformatics/18.1.207. [DOI] [PubMed] [Google Scholar]
- Uddin M, Chang SC, Zhang C, Ressler K, Mercer KB, Galea S, Keyes KM, McLaughlin KA, Wildman DE, Aiello AE, Koenen KC. Adcyap1r1 genotype, posttraumatic stress disorder, and depression among women exposed to childhood maltreatment. Depression and anxiety. 2013;30:251–258. doi: 10.1002/da.22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermetten E, Lanius RA. Biological and clinical framework for posttraumatic stress disorder. Handbook of clinical neurology. 2012;106:291–342. doi: 10.1016/B978-0-444-52002-9.00018-8. [DOI] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26:12387–12396. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip J, Chahl LA. Localization of Fos-like immunoreactivity induced by the NK3 tachykinin receptor agonist, senktide, in the guinea-pig brain. British journal of pharmacology. 1997;122:715–725. doi: 10.1038/sj.bjp.0701416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlomuzica A, Dere E, Huston JP, de Souza Silva MA. NK(3) receptor agonism promotes episodic-like memory in mice. Neurobiology of learning and memory. 2008;90:420–425. doi: 10.1016/j.nlm.2008.04.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.