Abstract
Background/Purpose
Gastroschisis is the most common congenital abdominal wall defect. Despite advances in the surgical closure of gastroschisis, consensus is lacking as to which method results in the best patient outcomes. The purpose of this meta-analysis was to compare short-term outcomes associated with primary fascial closure and staged repair with a silo in patients with gastroschisis.
Methods
We reviewed Medline citations, as well as the Cochrane Database of Systematic Reviews, between January 1, 1996 and June 1, 2012. Articles were identified using the search term “gastroschisis” and [(“treatment outcome” or “prognosis”) or randomized controlled trials]. Case reports, reviews, letters, abstracts only, non-English abstracts, and studies that did not address at least one of the outcomes of interest were excluded from the meta-analysis. Two independent reviewers identified relevant articles for final inclusion. A standard data collection form created by the authors was used to extract study information, including study design, patient characteristics, and reported patient outcomes. The data were analyzed using standard meta-analytic techniques.
Results
Twenty studies were included in the meta-analysis. In the five studies that selected closure method randomly or as a temporal shift in practice, silo was associated with better outcomes, with a significant reduction in ventilator days (p<0.0001), time to first feed (p=0.04), and infection rates (p=0.03). When all studies were included, primary closure was associated with improved outcomes.
Conclusions
Silo closure is associated with better clinical outcomes in the studies with the least selection bias. Larger prospective studies are needed to definitively determine the best closure technique.
Keywords: Gastroschisis, abdominal wall defects, primary fascial closure, spring-loaded silo, surgical silo
Gastroschisis is the most common abdominal wall defect in the newborn, and incidence is increasing worldwide, affecting 4–5/10,000 newborns (1, 2). Survival has dramatically improved to greater than 90% over the past 6 decades, due to improved techniques to close the abdominal wall defect and advances in neonatal care (3, 4, 5). Despite these advances, post-surgical care for gastroschisis remains challenging, and gastroschisis is the congenital defect with the longest ICU length of stay (LOS)(6). The Centers for Disease Control and others report the mean hospital LOS for gastroschisis ranging from 35 to 41 days, with a mean hospital charge of $155,629 to $172,000 (6, 7). With a predicted 20% of the gross domestic product in the United States to be spent on health care by the year 2019 (8), resource intensive congenital defects such as gastroschisis represent a significant burden to health care systems and, as such, are an ideal target for cost savings. Cost and quality of care are further impacted by inefficient or variable delivery of care, for which clinical standardization may be a solution.
Variation in the management of gastroschisis, including closure/coverage techniques, continues to be a crucial factor in both short- and long-term outcomes of infants with gastroschisis (9). Three surgical techniques have been described: primary fascial closure, staged repair with a silo, and most recently, “umbilical turban” or plastic closure. In 1943, the first successful primary fascial closure of an abdominal wall defect was reported (10, 11). Historically, patients failing primary closure received a custom-made silo sewn to the abdominal fascia, which enabled gradual reduction of the bowel into the abdomen.
By the mid-1970s, preformed silos were introduced, and by 1995 featured a spring-loading mechanism that inserts beneath the fascia, providing an alternate method of temporary closure (10, 12, 13). Spring loaded silos (SLS) or preformed silos have been used as both a salvage technique in patients failing primary closure or as an initial therapy to provide intestinal coverage with definitive closure at a later time. Placement of a SLS was welcomed as an alternative to a silo sutured to the fascia, as it can be placed at the bedside rather than in the OR, and requires minimal sedation, without the need for endotracheal intubation. Placement of a SLS has the theoretical benefit of allowing the bowel edema to resolve, allowing partial reduction of the intestine and reduced intra-abdominal pressures at the time of definitive fascial closure (12, 14). It is postulated that reduction of bowel in the setting of reduced intra-abdominal pressures improves splanchnic perfusion resulting in faster return of bowel function and reduced rates of infection and necrotizing enterocolitis (NEC) (14, 15, 16).
The umbilical turban is the most recent reported closure technique for gastroschisis (17). In this procedure, the intestinal contents are reduced into the abdomen and the umbilical cord is coiled over the defect and fixed into place with an adhesive dressing. Eventually the defect epithelizes and the umbilical cord desiccates and detaches, occasionally leaving patients with an umbilical hernia.
Multiple studies have attempted to address the impact of these closure methods on clinical outcomes, and none of the methods has emerged as a clearly superior choice. This meta-analysis sought to identify the best closure method for newborns with gastroschisis by comparing hospital outcomes in patients receiving either primary fascial closure or staged repair with a silo.
METHODS
The review was performed according to the PRISMA guidelines (Appendix 1).
Selection of Studies
We reviewed Medline citations between January 1, 1996 and June 1, 2012 and the Cochrane Database of Systematic Reviews database through June 1, 2012. Citations from DynaMed and other reviews were included. The search terms used were “gastroschisis” and [(“treatment outcome” or “prognosis”) or randomized controlled trials]. Reference lists from selected papers were further used to identify publications of interest. Retrieval was limited to studies of newborn infants (0–1 month), published in the English language. Case reports, reviews, letters, abstracts only, non-English abstracts, and studies that did not address at least one of the outcomes of interest were identified and excluded independently by two reviewers. Studies were also excluded from analysis when continuous outcomes were reported in a non-continuous manner or for which a minimum of number and p-value were not provided. Only studies that directly compared primary fascial closure to delayed closure using a silo (either SLS or sutured silos) were included. Though we had originally anticipated including turban closure in the analysis, too few studies directly compared turban closure to either of the other methods.
Data Extraction
Abstracts for all articles were independently subjected to preliminary review by two investigators (JA, SK) to determine whether inclusion criteria were satisfied. A third reviewer (JT) resolved disagreement between reviewers. Data abstraction was conducted independently by the same two investigators from the studies that met inclusion criteria. A standard data collection form (Appendix 2) created by the authors (JA, JT, SK) was used to extract information about study quality, including overall and per-group sample sizes, selection of method to close gastroschisis, inclusion of patients receiving surgically sutured silos in the silo group, and inclusion of patients with complicated gastroschisis (atresia, volvulus, or perforation) in the analysis; patient characteristics, including gestational age and birth weight; ascertainment bias; and reported patient outcomes. Extracted data were cross-referenced between reviewers for accuracy. Discrepancies between reviewers were resolved by consensus.
Primary Outcomes
Outcomes of clinical interest were initially devised by the study authors; from this list, outcomes to be included in the analysis were selected based on whether data were reported in at least half of the studies selected, and included the following: total hospital LOS, total days on the ventilator, time to first feed (TFiF), time to full feeds (TFuF), parenteral nutrition duration (PND), infection rates (including sepsis, central line infections, pneumonia, and infections not otherwise specified), rates of necrotizing enterocolitis (NEC), and mortality rates.
Statistical Analysis
The data were analyzed using standard meta-analytic techniques (18). For each study, treatment effects were calculated using a fixed effect model to obtain weighted mean difference and 95% confidence interval for continuous outcomes, and odds ratio and 95% confidence interval for dichotomous outcomes. For studies reporting median and either range or interquartile range, median was entered as an estimate of the mean, and standard deviations were estimated, conservatively, as larger of [(max-median)/2 OR (median-min)/2] for range and as larger of [(Q3 – median)/.675 OR (median – Q1)/0.675] for interquartile range. To assess associations of gestational age and birth weight with estimated treatment effects, Pearson correlations were calculated for weighted mean differences for continuous outcomes and for log-transformed odds ratios for dichotomous outcomes.
In studies comparing primary closure to placement of a silo, rationale for selection of closure method differed and was categorized into three groups: 1) objective, based on randomization or temporal assignment due to shift in practice within the institution, 2) subjective, based on surgeon preference, or 3) silo placement after failed primary closure. To assess potential bias associated with subjective treatment allocation, sensitivity analyses were performed in which pooled estimates of treatment effect were calculated based only on studies that randomized closure method or in which closure method differed due to a temporal shift in practice within an institution. In pre-specified subgroup analyses, we qualitatively compared estimated treatment effects based on the following factors, which represent variation in study design: 1) inclusion of patients receiving surgically sutured silos in the silo group; and 2) inclusion of patients with complicated gastroschisis, including atresia, volvulus, or perforation, in the analysis. Heterogeneity was assessed by the I2 statistic, which is a measure of the proportion of variance across studies that can be attributed to heterogeneity rather than chance. All meta-analyses were performed using Comprehensive Meta Analysis software (19). The original source for all variables may be found in Appendix 3.
RESULTS
Studies Analyzed
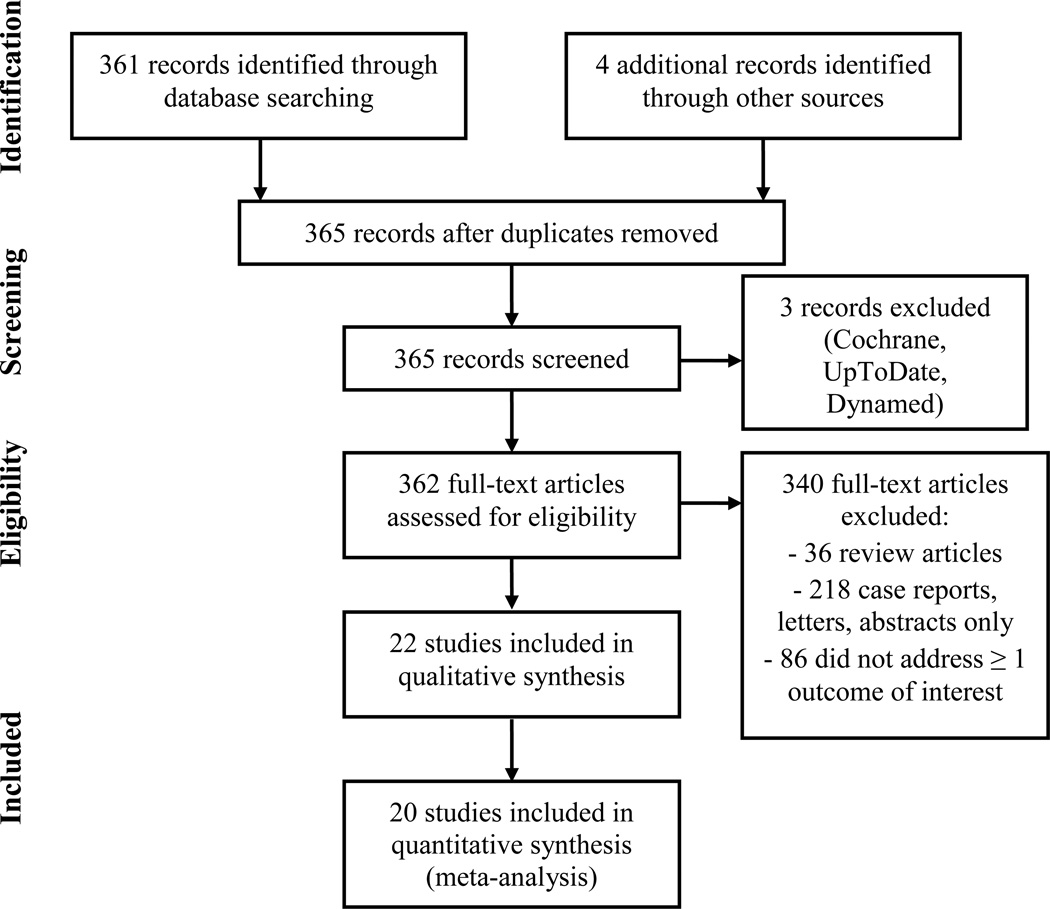
The literature search yielded 365 articles (Figure 1). Reviews, case reports, letters, and abstracts only (n=257) were excluded. After review of the remaining 108 abstracts and/or full text articles by two independent reviewers, studies that did not address gastroschisis repair methods and studies that did not address at least one of the outcomes of interest (n=88) were excluded. Twenty studies met inclusion criteria and were included in the meta-analysis: 1 randomized controlled trial, 3 prospective cohort studies, and 16 retrospective studies (Table 1).
Figure 1.
Details of included and excluded studies.
Table 1.
Characteristics of included studies.
| Study
author, year (reference) |
Study design | Number
of patients Total |
Outcomes included | Method
of closure selection |
Silo
group contains surgical silos |
Data
for uncomplicated gastroschisis only |
|
|---|---|---|---|---|---|---|---|
| Primary | Silo | ||||||
| Allotey, 2007 (20) | Retrospective, single-center review | 53 | TFiF, TFuF, PND, ventilator days, LOS, infection, NEC, mortality | Temporal | No | Yes | |
| 40 | 13 | ||||||
| Banyard, 2010 (21) | Retrospective, single-center review | 235 | TFiF, PND, ventilator days, LOS, NEC, mortality | Surgeon preference | Yes | No | |
| 188 | 47 | ||||||
| Bonnard, 2008 (22) | Retrospective, single-center, case-control | 22 | TFiF, TFuF, PND, ventilator days, LOS, infection, NEC, mortality | Surgeon preference | Yes | Yes | |
| 11 | 11 | ||||||
| Bradnock, 2011 (23) | Prospective, multi-center cohort study | 219 | TFuF, PND, LOS, infection, NEC | Surgeon preference | No | Yes | |
| 120 | 99 | ||||||
| Chiu, 2006 (24) | Retrospective, single-center review | 48 | TFuF, ventilator days, LOS, infection, NEC, mortality | Temporal | No | No | |
| 28 | 20 | ||||||
| Driver, 2000 (25) | Retrospective, single-center review | 90 | TFuF, PND, LOS | Primary failure | Yes | No | |
| 72 | 18 | ||||||
| Eggink, 2006 (26) | Retrospective, single-center review | 55 | TFiF, TFuF, ventilator days, infection | Surgeon preference | Yes | Yes | |
| 21 | 34 | ||||||
| Kandasamy, 2010 (27) | Retrospective, single-center review | 28 | TFiF, TFuF, PND, ventilator days, LOS, infection, NEC, mortality | Primary failure | Yes | Yes | |
| 22 | 6 | ||||||
| Kidd, 2003 (15) | Retrospective, single-center review | 118 | TFuF, ventilator days, LOS, infection, NEC, mortality | Primary failure | Yes | No | |
| 58 | 60 | ||||||
| Lobo, 2010 (28) | Retrospective, single-center review | 37 | TFiF, TFuF, PND, ventilator days, LOS, infection, NEC, mortality | Primary failure | No | No | |
| 10 | 27 | ||||||
| McNamara, 2010 (29) | Retrospective, single-center review | 14 | TFiF, PND, LOS | Surgeon preference | No | Yes: TFiF, PND No: LOS | |
| 5 | 9 | ||||||
| Minkes, 2000 (30) | Retrospective, single-center review | 43 | TFuF, ventilator days, LOS, NEC | Temporal | No | No | |
| 30 | 13 | ||||||
| Owen, 2006 (31) | Retrospective, single-center, case-control | 48 | TFiF, TFuF, PND, ventilator days, LOS, infection, NEC | Surgeon preference | No | Yes | |
| 27 | 21 | ||||||
| Owen, 2010 (32) | Prospective cohort study | 290 | Infection, NEC, mortality | Surgeon preference | No | Yes | |
| 170 | 120 | ||||||
| Pastor, 2008 (33) | Multicenter randomized controlled trial | 54 | PND, ventilator days, LOS, infection, NEC, mortality | Random | No | No | |
| 27 | 27 | ||||||
| Schlatter, 2003 (12) | Retrospective, single-center review | 65 | TFiF, TFuF, PND, ventilator days, LOS, infection, NEC, mortality | Temporal | No | No | |
| 39 | 26 | ||||||
| Schmidt, 2011 (34) | Prospective, single-center cohort study | 45 | TFiF, PND, LOS, infection, NEC | Primary fail | Yes | No | |
| 24 | 21 | ||||||
| Singh, 2003 (35) | Retrospective, multicenter review | 181 | PND, ventilator days, LOS, infection, mortality | Primary fail | No | No | |
| 151 | 30 | ||||||
| Tsai, 2010 (36) | Retrospective, single-center review | 44 | TFuF, ventilator days, LOS, infection, NEC, mortality | Surgeon preference | Yes | Yes: TFuF, ventilator days, LOS, mortality No: infection, NEC | |
| 32 | 12 | ||||||
| Weil, 2012 (37) | Retrospective, single-center review | 190 | TFuF, ventilator days, LOS, infection, mortality | Surgeon preference | No | No | |
| 43 | 147 | ||||||
LOS = length of stay; NEC = necrotizing enterocolitis; TFiF = time to first feed; TFuF = time to full feeds; primary fail = silo after failure of primary closure
Results of Meta-Analyses
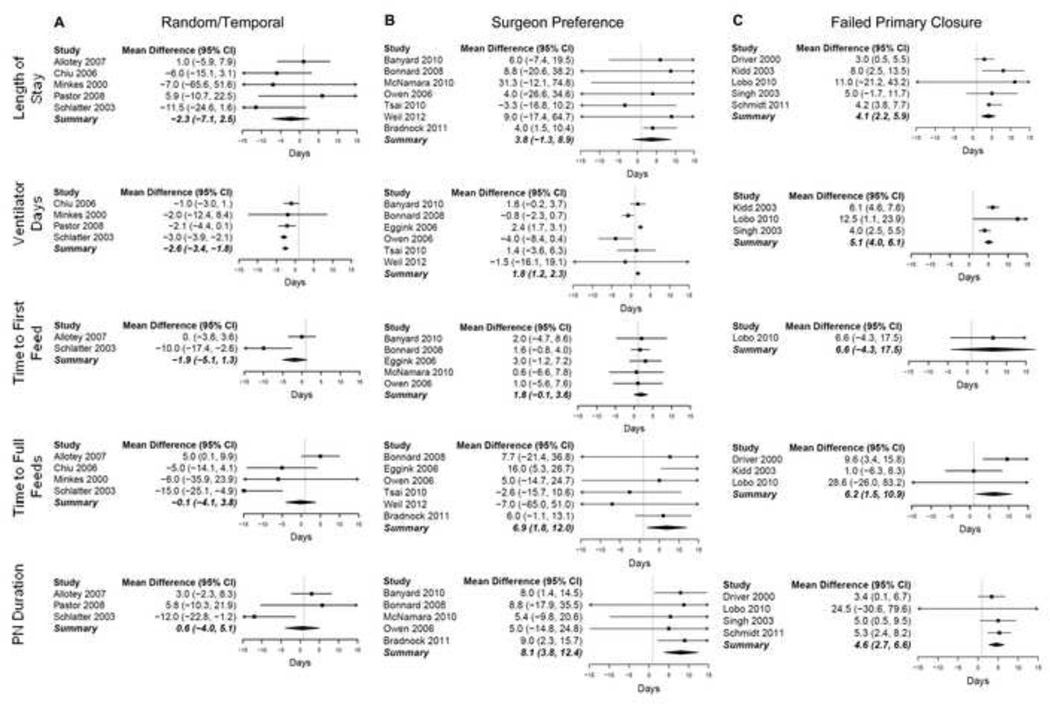
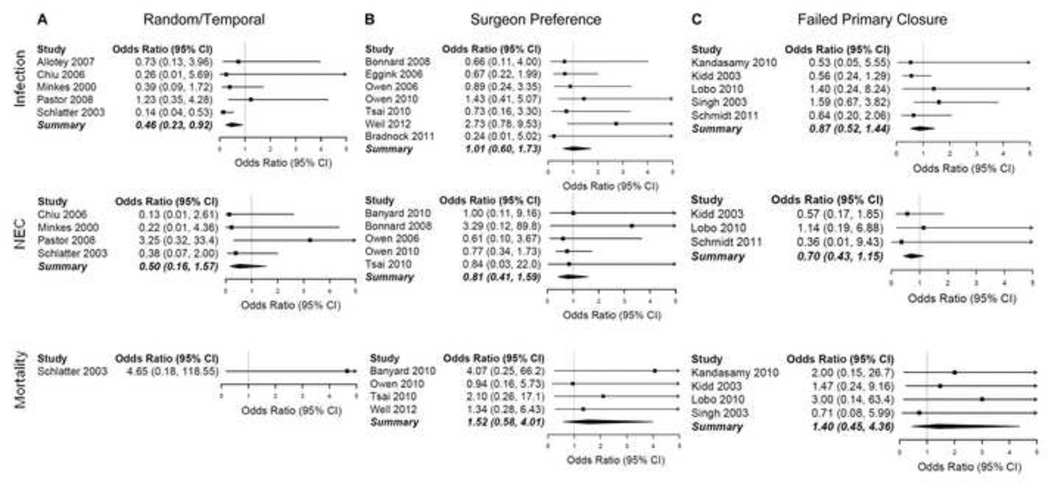
Estimates of weighted mean differences and odds ratios for individual studies are shown in Figures 2 and 3, respectively. In studies with less biased manners of selecting closure method (n=5), silo closure was associated with shorter TFiF, fewer ventilator days, and lower infection rates (Table 2, random/temporal group). Ventilator days were reduced by 2.6 days (95% CI: 1.8–3.4 days; P<0.001; I2=14%); TFiF was decreased by 1.9 days (95% CI: −1.3–5.1 days; P<0.001; I2=82%), and infection rates were reduced by 14% (OR: 0.46; 95% CI: 0.23–0.92; P=0.03; I2=32%). However, when studies judged to be prone to ascertainment bias in their selection of closure technique were included, primary closure was associated with shorter LOS, PND, and ventilator days (Table 2, overall group). In this overall analysis, primary closure and silo closure did not differ for any of the other outcomes studied.
Figure 2.
Forest plots for continuous variables. (A) Mean differences for studies with random or temporal allocation of patients to study group. (B) Mean differences for studies using surgeon preference to assign patients to study group. (C) Mean differences for studies in which silo closure was only used after failure of primary closure. Mean differences falling to the left of the line favor silo closure; those falling to the right of the line favor primary fascial closure.
Figure 3.
Forest plots for dichotomous variables. (A) Odds ratios for studies with random or temporal allocation of patients to study group. (B) Odds ratios for studies using surgeon preference to assign patients to study group. (C) Odds ratios for studies in which silo closure was only used after failure of primary closure. Odds ratios falling to the left of the line favor silo closure; those falling to the right of the line favor primary fascial closure.
Table 2.
Meta-analysis results by subgroups and overall analysis.
| Study Group Based on Selection
of Closure Method Closure method favored (p-value) (Number of studies) |
Study Group Based on Type of Silo Included Closure method favored (p-value) (Number of studies) |
Study Group Based on Inclusion of Complicated Gastroschisis Closure method favored (p-value) (Number of studies) |
Overall | |||||
|---|---|---|---|---|---|---|---|---|
| OUTCOME | Random/Temporal | Surgeon Preference |
Failed Primary Closure |
Spring-loaded silo only |
Spring-loaded and surgical silos |
Uncomplicated gastroschisis only |
Complicated gastroschisis included |
|
| Length of stay | Silo (p=0.28) (5) | Primary (p=0.17) (7) | Primary (p<0.0001) (5) | Primary (p=0.37) (11) | Primary (p<0.0001) (6) | Primary (p=0.37) (5) | Primary (p=0.004) (12) | Primary (p=0.004) (17) |
| Ventilator days | Silo (p<0.0001) (4) | Primary (p=0.04) (6) | Primary (p<0.0001) (3) | Silo (p=0.51) (8) | Primary (p<0.0001) (5) | Primary (p=0.06) (4) | Primary (p=0.001) (9) | Primary (p<0.0001) (13) |
| Time to first feed | Silo (p=0.04) (2) | Primary (p=0.13) (5) | Primary (p<0.0001) (2) | Silo (p=0.42) (5) | Primary (p=0.002) (4) | Primary (p=0.16) (5) | Primary (p=0.22) (4) | Primary (p=0.07) (9) |
| Time to full feeds | Silo (p=0.14) (4) | Primary (p=0.04) (6) | Primary (p=0.03) (3) | Primary (p=0.66) (8) | Primary (p=0.007) (5) | Primary (p=0.003) (6) | Silo (p=0.87) (7) | Primary (p=0.06) (13) |
| Parenteral nutrition duration | Silo (p=0.69) (3) | Primary (p<0.0001) (5) | Primary (p<0.0001) (4) | Primary (p=0.005) (8) | Primary (p<0.0001) (4) | Primary (p=0.003) (5) | Primary (p<0.0001) (7) | Primary (p<0.0001) (12) |
| Infection | Silo (p=0.03) (5) | Primary (p=0.96) (7) | Silo (p=0.59) (5) | Silo (p=0.83) (11) | Silo (p=0.06) (6) | Silo (p=0.44) (7) | Silo (p=0.27) (10) | Silo (p=0.18) (17) |
| Necrotizing enterocolitis | Silo (p=0.24) (4) | Silo (0.54) (5) | Silo (p=0.40) (3) | Silo (p=0.23) (7) | Silo (p=0.47) (5) | Silo (p=0.53) (3) | Silo (p=0.18) (9) | Silo (p=0.16) (12) |
| Mortality | Primary (p=0.35) (1) | Primary (p=0.40) (4) | Primary (p=0.56) (4) | Primary (p=0.60) (5) | Primary (p=0.22) (4) | Primary (p=0.55) (3) | Primary (p=0.29) (6) | Primary (p=0.23) (9) |
Bold text indicates statistically significant result (p<0.05).
We next stratified the studies by whether the silo group comprised only SLS or included surgically sutured silos and SLS. Of the 8 studies that combined SLS and surgically sutured silos, 4 reported proportions for each type of silo (20, 21, 23, 26). There were 135 patients in the silo groups across these 4 studies, of which 101 (74.8%) were SLS and 34 (15.2%) were surgically placed (data not shown). When surgically placed silos were included in the study group, primary closure was associated with significantly shorter LOS, TFiF, TFuF, PND, and ventilator days. PND was significantly shorter in the primary closure group when only SLS were included in the silo group (Table 2).
Studies were also stratified by whether complicated gastroschisis (for example, gastroschisis associated with bowel atresia or perforation), was included in the analysis. In studies that included patients with complicated gastroschisis, primary closure was associated with significantly shorter LOS, PND, and ventilator days. However, in studies with only uncomplicated gastroschisis patients, primary closure was associated with significantly shorter TFuF and PND (Table 2).
Data for birth weight and gestational age by type of closure were provided for 16 studies, and neither birth weight nor gestational age was strongly associated with study effects (Table 3). However, the following variables were moderately (0.3>r>0.7) associated: birth weight and gestational age with ventilator days and TFiF; gestational age with NEC and mortality; and birth weight with TFuF and PND.
Table 3.
Associations of study outcomes with gestational age and birth weight.
| Outcome | n | Effect Size Measur e |
Gestation al Age (r) |
Birth Weight (r) |
|---|---|---|---|---|
| Length of stay | 14 | std mean | 0.04 | −0.02 |
| Ventilator days | 11 | std mean | −0.43 | −0.40 |
| Time to first feed | 9 | std mean | −0.42 | −0.68 |
| Time to full feed | 10 | std mean | −0.02 | −0.38 |
| Parenteral nutrition duration | 10 | std mean | −0.12 | 0.50 |
| Infection | 12 | log(odds) | −0.23 | −0.07 |
| Necrotizing enterocolitis | 9 | log(odds) | −0.39 | 0.11 |
| Mortality | 6 | log(odds) | 0.49 | 0.30 |
r = Pearson’s correlation; std mean = standardized (weighted) mean difference; log(odds) = log-transformed odds ratios
DISCUSSION
This meta-analysis demonstrates that in studies with less selection bias, silo closure is associated with better outcomes for patients with gastroschisis. The results also highlight the importance of assessing bias prior to drawing conclusions, as primary closure appears better when more biased studies are included in the analysis.
In studies based on treatment allocation via randomization or temporal shifts in practice, selection bias was reduced, favoring silo closure with significantly reduced ventilator days, infection, and TFiF. The study by Schlatter appeared to more strongly favor silo closure than the other studies (Figure 2) (12). This effect is most evident when evaluating ventilator days, where other studies in this selection group did not demonstrate a significant difference in ventilator days based on closure method. The authors attribute their improved outcomes with SLS closure to several factors, including avoidance of anesthesia leading to fewer ventilator days, lower intra-abdominal pressures reducing complications, and use of more PICC lines placed later in the SLS group (as opposed to Broviac lines placed on the first day of life with primary closure) leading to fewer infectious complications (12). Of note, the only prospective randomized controlled study did not demonstrate a significant difference in outcomes based on closure method (33). This study had small numbers in each arm (n=27) that were similar in terms of gestational age, weight, sex, Apgar scores, prenatal diagnosis, and mode of delivery.
Selection of the method of closure can clearly bias the conclusions of the study, which is highlighted in studies that compared primary closure to patients receiving a silo after failure of primary closure (Table 1) (15, 25, 28, 34, 35). Patients receiving a silo because primary closure has failed most likely represent a patient population prone to worse clinical outcomes (38). The association of primary closure with better outcomes in this study population may reflect this bias. The impact of this bias was the major contributor to the overall measured benefit of primary closure compared to silo closure.
Studies also favored primary closure when surgeon’s preference was used as the closure selection method, with a significant reduction in PND, TFuF, and ventilator days. Through experience, surgeons may have identified which patients have fascial defects more amenable to primary closure; when primary closure is perceived to be likely to fail, surgeons may be more prone to choose closure with SLS prior to attempting primary fascial closure. If such selection bias results in silo placement in patients with bowel wall edema or in infants too small to accommodate primary closure, clinical outcomes for patients receiving a silo may be negatively impacted. The studies by Bradnock et al (23) and Eggink et al (26) feature surgeon preference for selection of closure method and strongly impact the conclusions for this selection subgroup. The Eggink study reported a marked association of primary closure with better outcomes, including significantly shorter TFiF and TFuF, and a trend towards significantly fewer ventilator days in the primary closure group, even when complicated gastroschisis cases were excluded. In keeping with our assumptions, the authors of this study point to selection of a “sicker” population in patients receiving a silo as a possible explanation for better outcomes with primary closure. A similar study by Choi et al compared sutureless ward reduction (turban closure, without initial silo placement) to SLS placement and found that those patients undergoing sutureless reduction tended to have shorter TFiF, TFuF, PND, and LOS than those undergoing SLS placement (39). Again, these results are likely impacted by the method of selection, which was a combination of surgeon preference and silo after failure of sutureless reduction.
Studies were stratified to assess potential differences based on whether the silo group comprised solely SLS or also included surgically sutured silos. LOS, TFiF, TFuF, PND, and ventilator days were all improved in patients receiving primary closure compared to silo closure when surgically sutured silos were included in the silo group. Placement of a surgically sutured silo indicates a failed primary closure, implying that this cohort of patients is more complex. Thus, the benefit ascribed to primary closure may be incorrectly interpreted.
Similarly, studies were separated by whether patients with complicated gastroschisis were included in the analysis. When complicated gastroschisis patients were included, primary closure was associated with significantly shorter LOS, PND, and ventilator days. Patients who were primarily closed likely represented patients with simple gastroschisis and improved outcomes. In studies that excluded complicated gastroschisis, TFuF and PND were reduced in patients undergoing primary closure; these results were, again, influenced largely by the Bradnock and Eggink studies (23, 26), which feature surgeon preference as the method of closure selection. In addition, patients with complicated gastroschisis have prolonged hospital stays regardless of closure method, as the length of stay in complicated gastroschisis is often based on the patient’s intestinal pathology (40).
There are several limitations inherent in this meta-analysis. First, we identified the following three major variations in study design: 1) manner of selection of gastroschisis closure method, 2) inclusion of patients receiving surgically sutured silos in the silo group, and 3) inclusion of patients with complicated gastroschisis (including atresia, volvulus, and perforation). Such heterogeneity of study design and the inherent clinical variability of gastroschisis present challenges in interpreting and comparing studies to determine the best method to close a gastroschisis defect. As detailed previously, criteria for selection of closure method likely introduced selection bias into the closure decision in many studies. Grouping random and temporal methods of closure selection into the same group is imperfect, as outcomes after the adoption of SLS closure compared to controls may be confounded by unrelated advances in neonatal care. Second, definitions of silo closure differ between studies such that some studies include surgical silos in the silo group, while others include only spring-loaded silos. Such misclassification may also be present for other variables in these studies. Third, the current literature in gastroschisis closure methods is relatively small and comprises many retrospective reviews with few prospective or randomized trials. Given the paucity of large, randomized trials, controlling for covariates becomes difficult and one study can have a dominating influence on the outcome results. Fourth, due to the small number of studies, both overall and particularly within certain arms of the meta-analysis, we felt that it was not advisable to further sub-stratify studies into more specific groups (for example, a “gold standard” group with objective method of closure selection, uncomplicated gastroschisis only, and SLS only). Such analyses would enable a clearer understanding of the complex factors that may be associated with differences in outcomes of gastroschisis closure if more studies with these populations were available.
CONCLUSION
Silo closure is associated with better clinical outcomes in the studies with the least selection bias. This is especially relevant to the group of patients for whom primary closure is not readily achievable but whose presentation is not so complex as to immediately warrant delayed closure; for these patients, the evidence suggests that silo closure may be preferable. This analysis highlights the heterogeneity of the available literature on closure methods for gastroschisis and the impact of study design on reported outcomes. Determining a superior method of closure would be a benefit to both the surgical and medical management of gastroschisis; this meta-analysis demonstrates that further well-designed studies are needed to gain an accurate picture of outcomes after different surgical interventions. The randomized controlled trial registered in January 2012 will be an important step toward answering this important question (41).
Supplementary Material
Acknowledgements
We thank Susan Klawansky, MLS, medical librarian at Seattle Children’s Hospital, for her outstanding support in the literature search process. We also thank Laina Mercer, MS, for her biostatistical support. The following members of the Clinical Effectiveness Team of Seattle Children’s Hospital and the Gastroschisis Guideline Committee made significant contributions to the initial literature review and background for the manuscript: Linda Wallen, MD; Shilpi Chabra, MD; Edith Cheng, MD; Suzanne Peterson, MD; Lani Wolfe, ARNP; Kate Drummond, MS, MPA; and Steve Esmali, MBA, MHSA.
Funding
The Seattle Children’s Core for Biomedical Statistics is supported by the Center for Clinical and Translational Research at Seattle Children’s Research Institute and grant UL1RR025014 from the NIH National Center for Research Resources.
Abbreviations
- LOS
Length of stay
- NEC
Necrotizing enterocolitis
- PICC
Percutaneously-inserted central catheter
- PND
Parenteral nutrition duration
- SLS
Spring-loaded silo
- TFiF
Time to first feed
- TFuF
Time to full feeds
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosure
None
Conflicts of interest
There are no conflicts of interest to disclose.
- Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data.
- Drafting the article or revising it critically for important intellectual content; and
- Final approval of the version to be published.
References
- 1.Mortellaro VE, St Peter SD, Fike FB, et al. Review of the evidence on the closure of abdominal wall defects. Pediatr Surg Int. 2011;27(4):391–397. doi: 10.1007/s00383-010-2803-2. [DOI] [PubMed] [Google Scholar]
- 2.Holland AJ, Walker K, Badawi N. Gastroschisis: An update. Pediatr Surg Int. 2010;26(9):871–878. doi: 10.1007/s00383-010-2679-1. [DOI] [PubMed] [Google Scholar]
- 3.Abdullah F, Arnold MA, Nabaweesi R, et al. Gastroschisis in the united states 1988–2003: Analysis and risk categorization of 4344 patients. J Perinatol. 2007;27(1):50–55. doi: 10.1038/sj.jp.7211616. [DOI] [PubMed] [Google Scholar]
- 4.Fillingham A, Rankin J. Prevalence, prenatal diagnosis and survival of gastroschisis. Prenat Diagn. 2008;28(13):1232–1237. doi: 10.1002/pd.2153. [DOI] [PubMed] [Google Scholar]
- 5.Marven S, Owen A. Contemporary postnatal surgical management strategies for congenital abdominal wall defects. Semin Pediatr Surg. 2008;17(4):222–235. doi: 10.1053/j.sempedsurg.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention (CDC) Hospital stays, hospital charges, and in-hospital deaths among infants with selected birth defects—United States, 2003. MMWR Morb Mortal Wkly Rep. 2007;56(2):25–29. [PubMed] [Google Scholar]
- 7.Lao OB, Larison C, Garrison MM, et al. Outcomes in neonates with gastroschisis in U.S. children's hospitals. Am J Perinatol. 2010;27(1):97–101. doi: 10.1055/s-0029-1241729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sisko AM, Truffer CJ, Keehan SP, et al. National health spending projections: The estimated impact of reform through 2019. Health Aff (Millwood) 2010;29(10):1933–1941. doi: 10.1377/hlthaff.2010.0788. [DOI] [PubMed] [Google Scholar]
- 9.Aldrink JH, Caniano DA, Nwomeh BC. Variability in gastroschisis management: A survey of north american pediatric surgery training programs. J Surg Res. 2011 doi: 10.1016/j.jss.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Chabra S. Management of gastroschisis: Prenatal, perinatal, and neonatal. Neoreviews. 2006;7:e419. [Google Scholar]
- 11.Watkins DE. Gastroschisis. Virginia Medical Monthly. 1943;78:43. [Google Scholar]
- 12.Schlatter M, Norris K, Uitvlugt N, et al. Improved outcomes in the treatment of gastroschisis using a preformed silo and delayed repair approach. J Pediatr Surg. 2003;38(3):459–464. doi: 10.1053/jpsu.2003.50079. [DOI] [PubMed] [Google Scholar]
- 13.Shermeta DW, Haller JA., Jr A new preformed transparent silo for the management of gastroschisis. J Pediatr Surg. 1975;10(6):973–975. doi: 10.1016/s0022-3468(75)80108-4. [DOI] [PubMed] [Google Scholar]
- 14.McGuigan RM, Mullenix PS, Vegunta R, et al. Splanchnic perfusion pressure: A better predictor of safe primary closure than intraabdominal pressure in neonatal gastroschisis. J Pediatr Surg. 2006;41(5):901–904. doi: 10.1016/j.jpedsurg.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Kidd JN, Jr, Jackson RJ, Smith SD, et al. Evolution of staged versus primary closure of gastroschisis. Ann Surg. 2003;237(6):759–764. doi: 10.1097/01.SLA.0000071568.95915.DC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lacey SR, Bruce J, Brooks SP, et al. The relative merits of various methods of indirect measurement of intra-abdominal pressure as a guide to closure of abdominal wall defects. Journal of Pediatric Surgery. 1987;22:1207. doi: 10.1016/s0022-3468(87)80739-x. [DOI] [PubMed] [Google Scholar]
- 17.Sandler A, Lawrence J, Meehan J, et al. A "plastic" sutureless abdominal wall closure in gastroschisis. J Pediatr Surg. 2004;39(5):738–741. doi: 10.1016/j.jpedsurg.2004.01.040. [DOI] [PubMed] [Google Scholar]
- 18.Hedges LV, Olkin I. Statistical methods for meta-analysis. Orlando, FL: Academic Press; 1985. [Google Scholar]
- 19.Borenstein M, Hedges L, Higgins J, et al. Comprehensive Meta-analysis Version 2. Englewood, NJ: Biostat; 2005. [Google Scholar]
- 20.Allotey J, Davenport M, Njere I, et al. Benefit of preformed silos in the management of gastroschisis. Pediatr Surg Int. 2007;23(11):1065–1069. doi: 10.1007/s00383-007-2004-9. [DOI] [PubMed] [Google Scholar]
- 21.Banyard D, Ramones T, Phillips SE, et al. Method to our madness: An 18-year retrospective analysis on gastroschisis closure. J Pediatr Surg. 2010;45(3):579–584. doi: 10.1016/j.jpedsurg.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Bonnard A, Zamakhshary M, de Silva N, et al. Non-operative management of gastroschisis: A case-matched study. Pediatr Surg Int. 2008;24(7):767–771. doi: 10.1007/s00383-008-2153-5. [DOI] [PubMed] [Google Scholar]
- 23.Bradnock TJ, Marven S, Owen A, et al. BAPS-CASS. Gastroschisis: one year outcomes from national cohort study. BMJ. 2011;343:1–9. doi: 10.1136/bmj.d6749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiu B, Lopoo J, Hoover JD, et al. Closing arguments for gastroschisis: Management with silo reduction. J Perinat Med. 2006;34(3):243–245. doi: 10.1515/JPM.2006.043. [DOI] [PubMed] [Google Scholar]
- 25.Driver CP, Bruce J, Bianchi A, et al. The contemporary outcome of gastroschisis. J Pediatr Surg. 2000;35(12):1719–1723. doi: 10.1053/jpsu.2000.19221. [DOI] [PubMed] [Google Scholar]
- 26.Eggink BH, Richardson CJ, Malloy MH, et al. Outcome of gastroschisis: A 20-year case review of infants with gastroschisis born in galveston, texas. J Pediatr Surg. 2006;41(6):1103–1108. doi: 10.1016/j.jpedsurg.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 27.Kandasamy Y, Whitehall J, Gill A, et al. Surgical management of gastroschisis in north queensland from 1988 to 2007. J Paediatr Child Health. 2010;46(1–2):40–44. doi: 10.1111/j.1440-1754.2009.01615.x. [DOI] [PubMed] [Google Scholar]
- 28.Lobo JD, Kim AC, Davis RP, et al. No free ride? the hidden costs of delayed operative management using a spring-loaded silo for gastroschisis. J Pediatr Surg. 2010;45(7):1426–1432. doi: 10.1016/j.jpedsurg.2010.02.047. [DOI] [PubMed] [Google Scholar]
- 29.McNamara WF, Hartin CW, Escobar MA, et al. Outcome differences between gastroschisis repair methods. J Surg Res. 2011;165(1):19–24. doi: 10.1016/j.jss.2010.05.054. [DOI] [PubMed] [Google Scholar]
- 30.Minkes RK, Langer JC, Mazziotti MV, et al. Routine insertion of a silastic spring-loaded silo for infants with gastroschisis. J Pediatr Surg. 2000;35(6):843–846. doi: 10.1053/jpsu.2000.6858. [DOI] [PubMed] [Google Scholar]
- 31.Owen A, Marven S, Jackson L, et al. Experience of bedside preformed silo staged reduction and closure for gastroschisis. J Pediatr Surg. 2006;41(11):1830–1835. doi: 10.1016/j.jpedsurg.2006.06.048. [DOI] [PubMed] [Google Scholar]
- 32.Owen A, Marven S, Johnson P, et al. Gastroschisis: A national cohort study to describe contemporary surgical strategies and outcomes. J Pediatr Surg. 2010;45(9):1808–1816. doi: 10.1016/j.jpedsurg.2010.01.036. [DOI] [PubMed] [Google Scholar]
- 33.Pastor AC, Phillips JD, Fenton SJ, et al. Routine use of a SILASTIC spring-loaded silo for infants with gastroschisis: A multicenter randomized controlled trial. J Pediatr Surg. 2008;43(10):1807–1812. doi: 10.1016/j.jpedsurg.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt AF, Gonçalves A, Bustorff-Silva JM, et al. Does staged closure have a worse prognosis in gastroschisis? Clinics(Sao Paulo) 2011;66(4):563–566. doi: 10.1590/S1807-59322011000400007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh SJ, Fraser A, Leditschke JF, et al. Gastroschisis: Determinants of neonatal outcome. Pediatr Surg Int. 2003;19(4):260–265. doi: 10.1007/s00383-002-0886-0. [DOI] [PubMed] [Google Scholar]
- 36.Tsai MH, Huang HR, Chu SM, et al. Clinical features of newborns with gastroschisis and outcomes of different initial interventions: Primary closure versus staged repair. Pediatr neonatol. 2010;51(6):320–325. doi: 10.1016/S1875-9572(10)60062-9. [DOI] [PubMed] [Google Scholar]
- 37.Weil BR, Leys CM, Rescorla FJ. The jury is still out: changes in gastroschisis management over the last decade are associated with both benefits and shortcomings. J Pediatr Surg. 2012;47(1):119–124. doi: 10.1016/j.jpedsurg.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 38.Christison-Lagay ER, Kelleher CM, Langer JC. Neonatal abdominal wall defects. Semin Fetal Neonatal Med. 2011;16(3):164–172. doi: 10.1016/j.siny.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 39.Choi WW, McBride CA, Bourke C, et al. Long-term review of sutureless ward reduction in neonates with gastroschisis in the neonatal unit. J Pediatr Surg. 2012;47(8):1516–1520. doi: 10.1016/j.jpedsurg.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 40.Jager LC, Heij HA. Factors determining outcome in gastroschisis: Clinical experience over 18 years. Pediatr Surg Int. 2007;23(8):731–736. doi: 10.1007/s00383-007-1960-4. [DOI] [PubMed] [Google Scholar]
- 41.St. Peter S. Bedside Silo Versus Attempted Operative Closure for Gastroschisis. Clinicaltrials.gov, identifier number NCT01506531.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.