Abstract
Klebsiella pneumoniae is one of the most common pathogens in nosocomial infections and is becoming increasingly multidrug-resistant. However, the underlying molecular pathogenesis of this bacterium remains elusive, limiting the therapeutic options. Understanding the mechanism of its pathogenesis may facilitate the development of antibacterial therapeutics. Here, we show that Lyn, a pleiotropic Src tyrosine kinase, is involved in host defense against K. pneumoniae (Kp) by regulating phagocytosis process and simultaneously downregulating inflammatory responses. Using acute infection mouse models, we observed that lyn−/− mice were more susceptible to Kp with increased mortality and severe lung injury compared with wild-type mice. Kp infected-lyn−/− mice exhibited elevated inflammatory cytokines (IL-6 and TNF-α), and increased superoxide in the lung and other organs. In addition, the phosphorylation of p38 and NF-κB p65 subunit increased markedly in response to Kp infection in lyn−/− mice. We also demonstrated that the translocation of p65 from cytoplasm to nuclei increased in cultured murine lung epithelial cells by Lyn siRNA knockdown. Furthermore, lipid rafts clustered with activated Lyn and accumulated in the site of Kp invasion. Taken together, these findings revealed that Lyn may participate in host defense against Kp infection through the negative modulation of inflammatory cytokines.
Keywords: Bacterial pathogenesis, Bioluminescence, Cytokinesis, In vivo imaging, Infectious diseases
Introduction
K. pneumoniae (Kp) is a capsulated Gram-negative bacterium found in the normal flora of the mouth, skin, and intestine, and is also the third most commonly isolated microorganism in blood cultures from sepsis patients. Due to the emerging antibiotic resistance, Kp infection is fast becoming a major health threat [1]. The most common infection caused by Klebsiella bacteria outside the hospital is pneumonia, typically in the form of bronchopneumonia and also bronchitis, which have a high mortality rate of about 50% even under antimicrobial therapy [2]. However, the molecular mechanisms that underlie pathogenesis and determinants of the host defense against pulmonary Kp infections remain elusive. It is thought that multi-faceted factors are involved in Kp infection in a coordinated manner. Our recent studies have indicated that endocytosis regulating protein caveolin-1 is involved in inflammatory responses in Kp infected mice [3]. Another study indicates that the Src kinase Lyn may coordinate lipid rafts and impact cellular function of caveolin-1 [4]. Therefore, it is possible that Lyn is also involved in host defense against Kp infection.
Lyn is involved in monocyte-related phagocytosis through FcγR via the phosphorylation of tyrosines in immunoreceptor tyrosine-based activation motifs (ITAM) [5]. By contrast, immunoreceptor tyrosine-based inhibition motifs (ITIM) phosphorylation subsequently leads to recruitment and activation of phosphatases, such as SHIP-1 and SHP-1, which down-modulate signaling pathways, attenuating cell activity [6]. Ageing Lyn deficient mice may manifest a phenotype including splenomegaly and proliferation of myeloid progenitors/monocytes [7]. A complex and intertwined network may explain Lyn’s role as a pleiotropic player involved in a variety of cellular processes, including inflammatory responses. Lyn is shown to be associated with diseases caused by viral infection as the first direct link to infectious diseases [8]. Recently, we also showed that Lyn may participate in immune defense against Pseudomonas aeruginosa infection as this protein is highly expressed in both alveolar macrophages and epithelial cells [9]. Lyn is located on the inner leaflet of the plasma membrane and in the proximity of lipid rafts, and can thus be translocated into the activated membrane domains to transmit cellular signals for either facilitating phagocytosis or regulating inflammatory responses [10]. However, it is unknown whether Lyn in the host cells is involved in Kp infection.
Employing a murine model, we investigated the role of Lyn in host defense during Kp-induced acute pneumonia. We demonstrated that Lyn deficiency led to a more severe disease phenotype in mice and a heightened inflammatory cytokine response. Furthermore, our studies showed that Lyn, working with lipid rafts, may be crucial in clearing the invading bacteria which otherwise drive intensified inflammatory cytokine responses.
RESULTS
Kp infection caused severe disease and mortality rates in lyn−/− mice
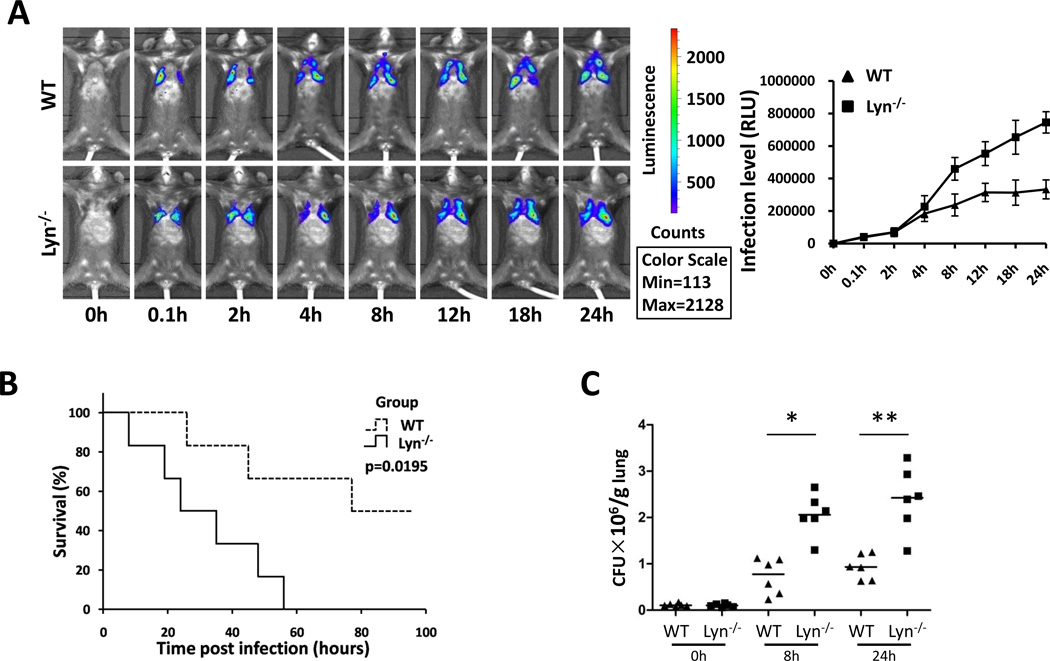
To assess the role of Lyn in Kp infection, we set out to use lyn−/− mice after backcrossing the mice to C57BL6 background for 7 generations. We intranasally instilled mice with Kp Xen-39 (ATCC43816, an engineered bacterium used for bioluminescence imaging) at 1×105 colony-forming unit (CFU in 50 µl PBS) per mouse (6 mice per group) [11]. Although the distribution of the bacteria in the lung were variable and somewhat influenced by breath, the results showed that lyn−/− mice exhibited wider dissemination of bioluminescence in the area of thoracic cavity after 4 h post infection with in vivo dynamic analysis using an IVIS XRII 200 biophotonic imager. However, dissemination areas in WT mice were more constrained than those in lyn−/− mice (Fig. 1A). Approximately 50% of lyn−/− mice died within 24 h post infection, and all lyn−/− mice had died at 56 h, whereas 50% of WT control mice remained alive at that time point (Fig. 1B). Lung homogenates were used to measure bacterial burdens. We found that lyn−/− mice manifested significantly increased CFU of Kp compared with WT mice both at 8 h and 24 h post infection (Fig. 1C). These increased bacterial loads may be responsible for severe pneumonia [3].
Figure 1. lyn−/− mice showed increased mortality rates and lung infection against Kp.
(A) Whole animal imaging (6 mice per group, the data are representative) of bioluminescence were obtained using IVIS XRII system at different time points. (B) Kaplan-Meier survival curves were obtained (p=0.0195; 95% confidence interval: 11.7–36.3, log rank test). (C) Bacterial burdens of Kp-infected lyn−/− mice and WT mice at 8 h and 24 h. *, p<0.05; **, p<0.01; Mann Whitney U test.
Lyn deficiency was associated with increased oxidation and severe lung injury during Kp infection
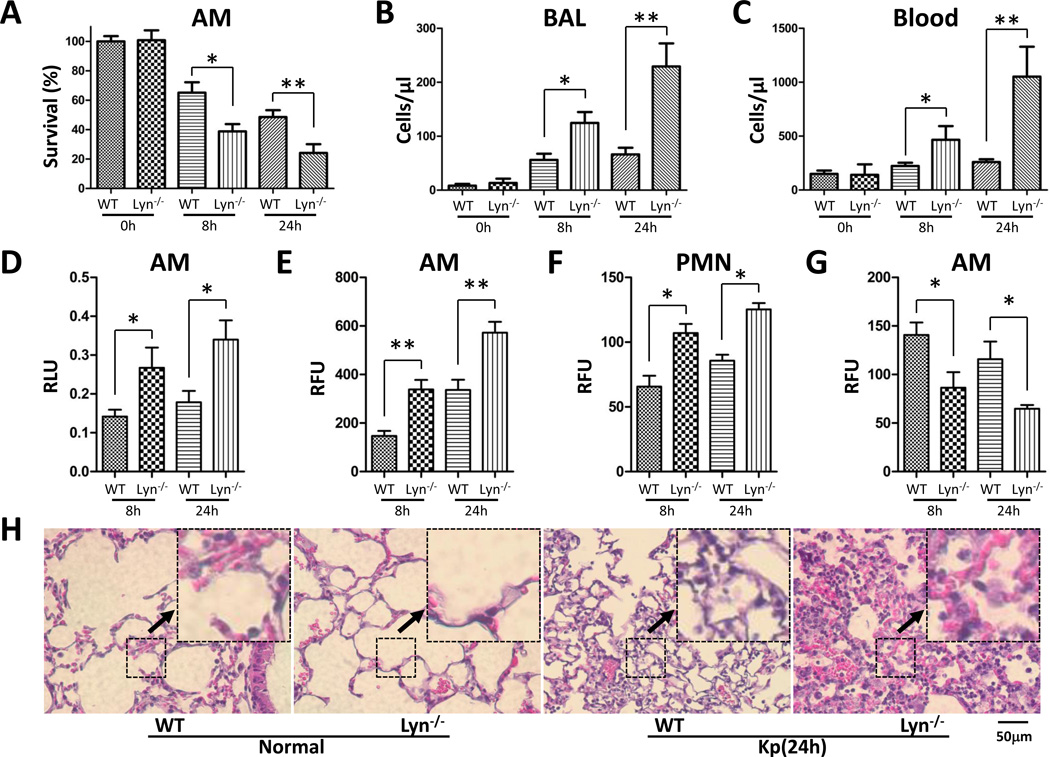
Because Kp Xen-39 was an engineered bacterium which was specifically used for IVIS imaging, we next chose the widely used Kp and Kp-GFP strain both in ATCC 43816 background to explore the pathogenesis mechanisms of Kp infection. Alveolar macrophages (AM) are a key immunity entity against Gram-negative bacteria infection. We retrieved AM cells from infected mice to evaluate their viability post infection. After culturing the lavaged AM for 24 h, we found that survival levels of AM decreased by approximately 2.0-fold in lyn−/− mice compared with WT mice both at 8 h and 24 h post infection, as assessed using an MTT assay. These results suggest that lyn deficiency contributes at least in part to impaired AM function against Kp infection (Fig. 2A). We also enumerated polymorphonuclear neutrophils (PMN) from blood vessels in the lungs and blood at 8 h and 24 h post infection [12]. PMN penetrations were higher both in the BAL fluid and blood of lyn−/− mice compared with WT mice, and further increased with time (Fig. 2B, C). These findings suggest that the increased PMN penetration in lyn−/− mice may also contribute to the increased lung damage and worsened disease.
Figure 2. Increased PMN and oxidation injury in lyn−/− mice following Kp infection.
(A) Viability was determined in Alveolar macrophages (AM) by MTT assay. (B and C) PMN infiltration in the BAL and blood was counted by Hema staining. (D) Superoxide production in AM cells detected using NBT assay. (E and F) Oxidative stress in AM cells was determined by H2DCF assay. (G) Mitochondrial potential as assessed by the JC-1 fluorescence assay. (H) Lung injury as assessed by histological analysis (20×, scale bar=50 µm, inset shows the typical tissue injury and inflammatory influx). The data are representative of three independent experiments in triplicate. Mean+SEM; *, p<0.05; **, p<0.01; one-way ANOVA (Tukey’s post hoc). RLU, relative luciferase units; RFU, relative fluorescence units.
Phagocyte-derived reactive oxygen species (ROS) is of crucial importance for host resistance to bacterial infection [13]. Untreated AM cells and PMNs were isolated from mice to determine oxidative stress. AMs of lyn−/− mice showed about 2-fold increase in oxidative stress at both 8 h and 24 h post infection compared with WT AMs, as determined by NBT assays (Fig. 2D). H2DCF-DA assay was also used to quantify superoxide in AMs and PMNs, which showed a similar increase in superoxide in lyn−/− mice (Fig. 2E, F). Excessive production of ROS may lead to a loss of cell function, and ultimately cell death. A decreased mitochondrial membrane potential was also observed in lyn−/− AMs using a JC-1 fluorescence assay (Fig. 2G). In mice, PMNs may migrate to the lung to clear bacteria through ROS and proteases, also contributing to increased superoxide release [14, 15]. Based on lung histology analysis at 24 h post infection, both WT mice and lyn−/− mice exhibited signs of pneumonia. However, more severe histological alterations (signs of inflammatory response and tissue damage) occurred in the lungs of lyn−/− mice (Fig. 2H), which may be related to the intense superoxide release.
lyn−/− mice exhibited increased infection dissemination
Another mortality factor may be bacterial dissemination from lungs to other organs, as also called septicemia [16]. We thus examined bacterial burdens in the liver, spleen, and kidney and found that although the magnitude of the bacteria loads is much lower than those in lungs, bacterial CFUs also increased significantly in the liver, spleen, and kidney of lyn−/− mice compared with the same organs of WT mice (Fig. S1A). Of note, our in vivo imaging analysis with a bioluminescence strain could not track the infection in liver or spleen, which may be due to overall less bacterial loads than those in the lung. These data suggest that the bacteria were spread from the original inoculation site (lungs) to other organs, which may be causally related to increased mortality. We next detected myeloperoxidase (MPO) activity of the lung and other organs to gain additional evidence of neutrophils penetration [17, 18]. As expected, MPO activity increased in the lung, liver, spleen, and kidney of lyn−/− mice (Fig. 3A, S1B). Increased MPO in these major organs suggests that oxidative stress may have resulted from systemic spread of the invading Kp bacteria. As increased oxidation can also cause tissue injury by oxidative degraded lipids, we used a thiobarbituric acid-reactive substrate assay to detect lipid peroxidation in the lung, liver, spleen, and kidney tissue [19]. To our no surprise, lipid peroxidation significantly increased in the organs of lyn−/− mice compared with that of WT mice (Fig. 3B, S1C). Notably, lipid peroxidation levels of the lung and spleen were even higher than those of the liver and kidney, respectively, suggesting that the lung and spleen might be the main targets. These data were consistent with the severity of lung injury determined by MPO and superoxide assays, indicating that increased lipid peroxidation in lyn−/− mice may also play a role in the progression of lung injury.
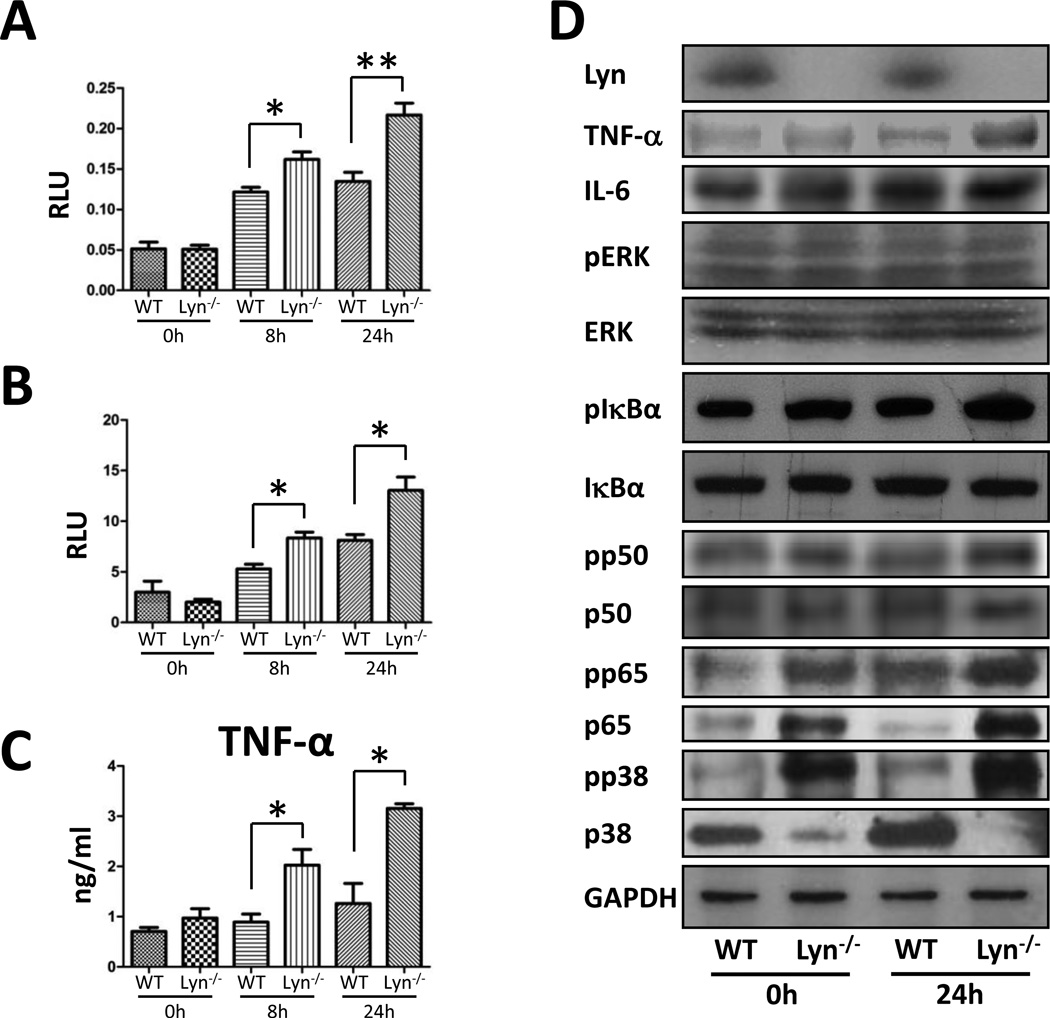
Figure 3. Lyn deficiency altered the inflammatory responses and induced activation of p38/NF-κB pathway.
(A) Increased MPO in lung of lyn−/− mice. (B) Increased lipid peroxidation in the lung of lyn−/− mice. (C) TNF-α in BAL fluid was assayed by ELISA (n=3 per group). (D) The p38/NF-κB pathway was evaluated by western blot. GAPDH was used as a loading control. Data are representative of three independent experiments. Mean+SEM; *, p<0.05; **, p<0.01; one-way ANOVA (Tukey’s post hoc). p65 phospho-p65; pp38, phospho-p38.
Lyn modulated Kp-induced inflammatory response via the p38/NF-κB pathway
To analyze whether Lyn deficiency impacts the inflammatory responses induced by Kp infection, various cytokines in BAL fluids were assessed by a standard ELISA [20]. The levels of TNF-α, IL-2, IL-4, and IL-22 increased significantly in the BAL fluids of lyn−/− mice compared with those of WT mice at both 8 h and 24 h post infection (Fig. 3C, S2A). However, the levels of IL-1β and IL-17 did not have significant changes upon Kp infection (data not shown), which may indicate that deficiency of Lyn selectively impacted the production of proinflammatory cytokines in Kp-infected mice.
To validate the inflammatory response data and directly measure the lung environment, we assayed mRNA and protein levels of cytokines by RT-PCR and Western blotting in lung tissue homogenates. Both mRNA and protein levels of TNF-α, IL-4, and IL-12A increased by more than 2-fold in lyn−/− mice compared with wild-type mice, consistent with the ELISA results (Fig. S2B, C). To further explore the molecular mechanism of Lyn in Kp infection, we examined inflammatory-relevant signaling proteins in lung homogenates. We found that Lyn deficiency resulted in slightly increased phosphorylation of p38 in uninfected mice, consistent with a previous finding that Lyn dysfunction may impair the p38 pathway [21]. Though Lyn deficiency results in lowered p38 expression, the phosphorylation of p38 (not ERK) increased by 1.79 fold in lyn−/− mice upon Kp infection compared with uninfected lyn−/− mice (Fig. 3D). These data implied an important role of p38 in cellular processes during this infection. NF-κB, a master transcription factor, is involved in a variety of cellular processes and is required for initiating transcription of cytokines, while p38 was previously shown to activate NF-κB in bacterial infections [22, 23]. To elucidate a potential role for NF-κB, we examined the protein expression and phosphorylation of NF-κB (p65/p50), and found that both p65 and pp65 (Ser 536) increased under Lyn deficiency, while p50 and pp50 (Ser 337) did not show significant changes (Fig. 3D), suggesting the specific involvement of p65 in this model. Lyn deficiency also results in higher phosphorylation of IκBα (Fig. 3D). These results collectively indicate that Lyn may act as an upstream regulator to impact the p38/NF-κB pathway.
Lyn facilitated the phagocytosis of Kp in cooperation with lipid rafts in vitro
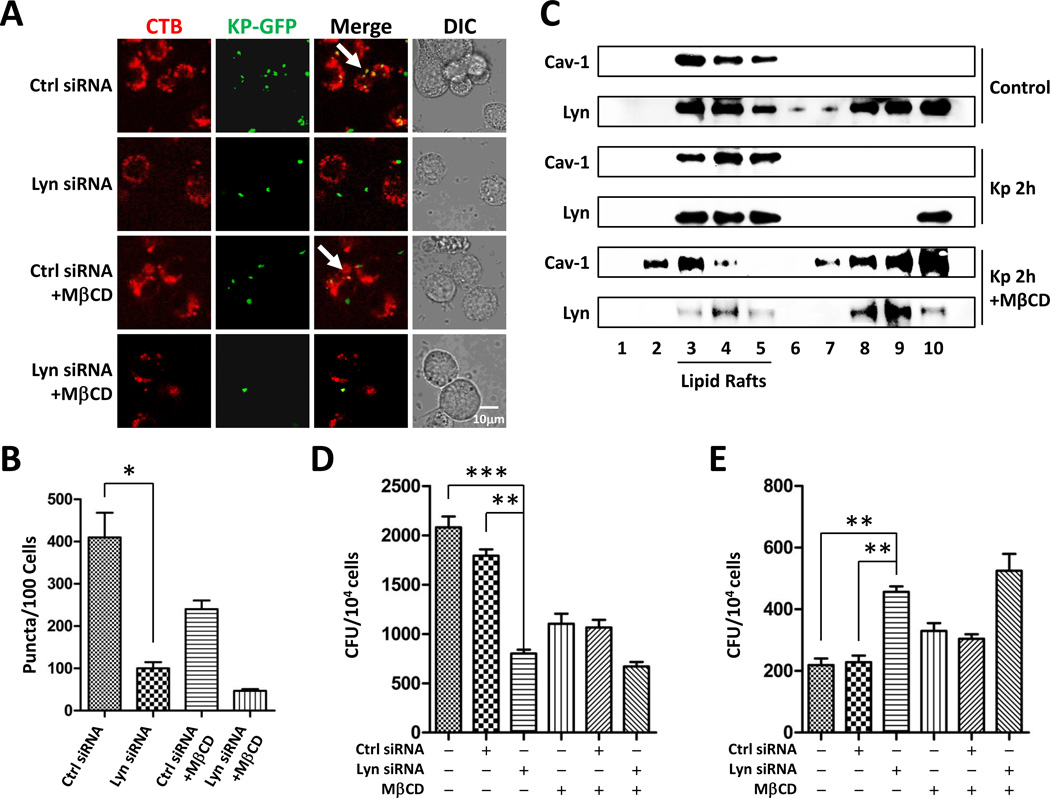
Almost immediately after microbial attacks, AM cells in the lungs begin to migrate to the infection sites and exert their phagocytic actions. When activated by the products of invading microorganisms and host inflammatory mediators, the first effect is rapid accumulation of these cells. All the in vivo data strongly indicate that Lyn had a function during Kp infection, and our prior finding suggests that Lyn and lipid rafts are both involved in P. aeruginosa infection [9]. To further define the interrelationship between Lyn and rafts in Kp infection, we performed a morphological study of lipid rafts by knocking down Lyn in both alveolar macrophages (MH-S) and primary macrophages in vitro. Cells were infected with 10:1 of m.o.i. (bacteria to cells) and stained with lipid raft marker rhodamine-labeled cholera toxin B chain (CTB-red) [24]. Confocal microscope demonstrated that the internalized Kp co-localized with lipid rafts. Lyn-deficiency interfered with the formation of raft aggregates and similarly cholesterol chelator (MβCD at 10 mM, 30 min pretreatment) diminished the co-localization between Lyn and Kp. Importantly, combining Lyn-deficiency and MβCD almost completely abolished lipid raft aggregation, resulting in less bacterial internalization (Fig. 4A, B, S3A, B) in Lyn siRNA transfected MH-S cells as well as primary AM cells. To convincingly determine the role of lipid rafts, we performed classical raft isolation assay to identify the signal proteins involved raft aggregation as described previously [25]. Lipid rafts were isolated from MH-S cell lysates by sucrose density gradient centrifugation in detergent containing buffer, and fractions of 1.1 ml were collected and analyzed by SDS-PAGE from top to bottom after concentrated by ammonium sulfate precipitation. Fractions 3, 4 and 5 were identified as raft fractions by the presence of Cav-1 (caveolar rafts). Kp-infected samples showed a shift of Lyn from the detergent-soluble fractions (8, 9, and 10) to the raft fractions (3, 4, and 5) as compared with uninfected controls (Fig. 4C). With 10 mM MβCD to block lipid raft, Lyn was pushed away from raft to nonraft fractions which clearly indicates that the role of Lyn is associated with raft platform. Phagocytosis usually occurs at the initial time upon infection in macrophages. We determined whether phagocytic function is dependent on lipid rafts, and found that either Lyn siRNA transfection or MβCD pretreatment decreased phagocytosis in MH-S cells (Fig. 4D). Moreover, clearance usually starts after internalization and may take longer times to finish. Similar results were found in primary macrophages (Fig. S3C, D). Our studies suggest that Lyn may have two critical roles in these processes. The first is the mediation of phagocytosis and the second is the regulation of the intracellular response including bacterial clearance. Bacterial clearance was also inhibited in Lyn siRNA-transfected cells (Fig. 4E). These results indicate that Lyn in cooperation with lipid rafts potently influences phagocytosis and bacterial clearance.
Figure 4. Decreased lipid raft function upon Lyn siRNA interference.
(A) Confocal microscopy image showing Kp internalization with lipid raft staining using CTB (scale bar=10 µm). (B) Mean lipid raft counts of MH-S cells. (C) Raft association with Lyn upon Kp infection shown by western blot. Data are representative of three independent experiments. (D) Phagocytosis ability was measured in MH-S cells following Kp infection at m.o.i. of 10:1 (bacteria: cells) for 1 h, and data was evaluated by CFU. (E) Bacterial clearance was evaluated in MH-S cells following Kp infection overnight at m.o.i. of 10:1. The data are representative of three independent experiments in triplicate. Mean+SEM; *, p<0.05; **, p<0.01; ***, p<0.001; one-way ANOVA (Tukey’s post hoc).
ROS overproduction impaired host defense against Kp infection in vitro
Both in vivo and in vitro data suggested that Lyn deficiency impaired phagocyte-mediated oxidative response against Kp infection. However, the underlying mechanism remains unknown. We have noted enhanced NADPH-dependent ROS generation in lyn−/− AMs may be counterproductive upon Kp infection. Thus we used diphenyleneiodonium (DPI) as ROS inhibitor to determine whether ROS played a role in host defense or inflammatory responses during Lyn deficiency. MH-S cells and PMNs were infected with 10:1 of m.o.i. Kp with DPI pretreatment (5 µM) for 30 min. DPI effectively decreased part of ROS generation in MH-S cells and PMNs (Fig. 5A, S4A). Despite some untoward effects, DPI successfully relieved some cell lethality upon Kp infection when Lyn was knocked down (Fig. 5B, S4B). Besides, TNF-α and IL-6 release was partially inhibited by DPI, which was mesured by ELISA in MH-S cells (Fig. 5C, D). To clarify the possible involvement of p38 with Lyn deficiency during Kp infection, we examined pp38 and found that DPI addition effectively inhibited p38 phosphorylation (Fig. S4C). These inhibitor-based data indicate that ROS acts as a double-edged sword, which may contribute to tissue damage with Lyn deficiency through affecting p38 upon Kp infection.
Figure 5. ROS plays complex roles in host defense against Kp infection in vitro.
(A) ROS generation was determined by H2DCF-DA assay. (B) The cell viability index was measured by MTT assay. (C, D) Cytokine levels in MH-S cells culture supernatant were assayed by ELISA. The data are representative of three independent experiments in triplicate. Mean+SEM; *, p<0.05; **, p<0.01; ***, p<0.001; one-way ANOVA (Tukey’s post hoc).
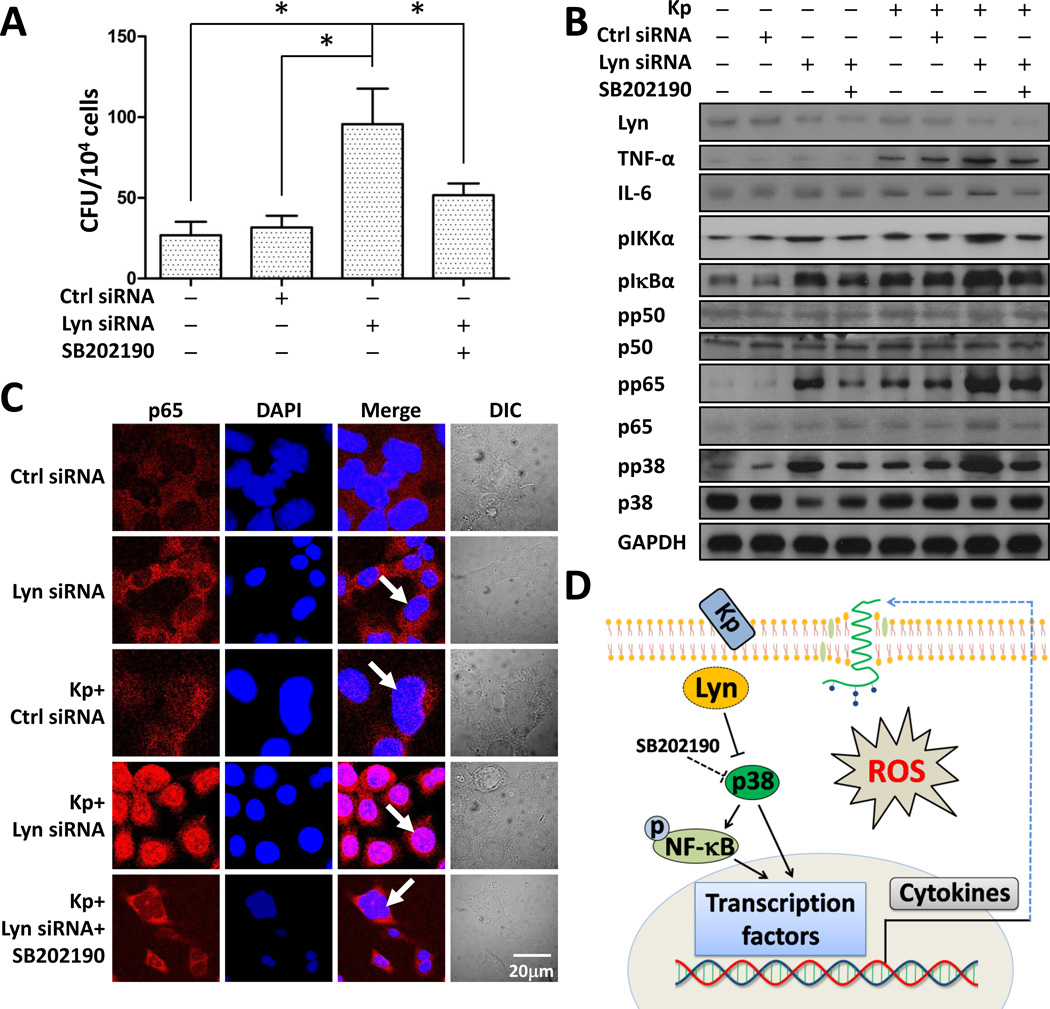
p38/NF-κB pathway was activated by Lyn siRNA in MLE-12 cells upon Kp infection
Alveolar epithelial cells may not uptake bacteria as efficiently as macrophages but they do ingest bacteria and participate in immune defense. To further validate our animal data in Lyn deficiency, in vitro models with MLE-12 cells were used to assess the underlying mechanisms since these cells have been widely used for analyzing murine lung epithelial function [26]. Consistent with the mouse data, bacterial burdens increased in Lyn siRNA-transfected cells compared with WT or control siRNA groups (Fig. 6A). To further dissect the role of p38 in the Lyn/NF-κB pathway, we pre-treated cells with p38 inhibitor SB202190 for 30 min. Using CFU and western blotting analysis, we found that inhibiting p38 activity significantly decreased bacterial burdens (Fig. 6A, S5A) and cytokine release (Fig. 6B), and thus decreasing the inflammatory response. These results suggest that augmented p38 affects Kp infection. Furthermore, we found that Lyn siRNA transfection increased the phosphorylation of p38, p65, IKKα and IκBα (Fig. 6B) upon Kp infection. Also, the phosphorylation of p38, p65, IKKα and IκBα was inhibited by adding SB202190, indicating that NF-κB is a downstream factor of the p38 pathway (Fig. 6B). These results suggest that Lyn can regulate NF-κB mediated inflammatory responses by regulating p38 activity. Next, we used p38 siRNA interference and ERK siRNA interference as comparison to further determine whether the specificity of cytokine signaling was p38 dependent. Without Lyn siRNA interference, knocking down p38 markedly decreased Kp-induced p65 phosphorylation and TNF-α release, and the phosphorylation of IκBα and IKKα was also inhibited (Fig. S5B). By contrast, siRNA interference of ERK1/2 did not affect inflammatory response upon Kp infection. Taken together, these data suggest that Lyn deficiency was involved in the p38/NF-κB axis in Kp infection. Inactive NF-κB is normally retained in the cytosol of MLE-12 cells [27], whereas activated NF-κB is translocated to the nucleus to trigger cytokine transcription [28]. To further prove our hypothesis, immunostaining of MLE-12 cells was used to determine the localization of NF-κB. As expected, p65 was translocated to the nucleus in the Lyn siRNA group upon Kp infection but not in the contorl siRNA group. Further, the translocation could be inhibited by pretreatment with SB202190, which indicates that Lyn plays a role in p38 inhibition, thus impacting the NF-κB activity (Fig. 6C). A similar result was found in p50 (Fig. S5C). In conclusion, both in vivo and in vitro results indicate that Lyn is an overall critical factor in inflammatory responses, and could cooperate with lipid rafts in regulating p38. Then p38 modulates NF-κB activity and thereby impacting Kp infection. Fig. 6D presents a schematic model of this cell signaling process, illustrating that Lyn and rafts may affect the cell function from membrane signals to cytoplasm to nuclear regulation and affecting physiological outcomes in infectious context.
Figure 6. Increased nuclear translocation of NF-κB in Lyn siRNA MLE-12 cells.
(A) MLE-12 cells were infected with Kp at m.o.i. of 10:1 for 1 h followed by polymyxin B treatment for 1 h (100 µg/ml) (n=3 per group). Finally, cells were lysed to perform CFU. One-way ANOVA (Tukey’s post hoc); *, p<0.05. (B) Cells were treated with p38 inhibitor, SB202190 (16 nM) for 30 min before infection, western blotting was used to determine the p38/NF-κB pathway and phosphorylation of signaling proteins. GAPDH was used as a loading control. (C) Confocal microscopy results showed the translocation of p65 using immune staining (arrow indicates the nuclear translocation, scale bar=20 µm). Data are representative of three independent experiments. (D) A schematic diagram showing how Lyn cooperates with lipid rafts and regulates the p38/NF-κB pathway to modulate the inflammatory response to Kp infection.
Discussion
In this study, we showed that Kp infection in lyn−/− mice resulted in a severe disease phenotype. We also demonstrated that Lyn deficiency significantly decreased bacterial clearance, heightened pro-inflammatory cytokines, and increased ROS release in animals. Previous studies have demonstrated that, Lyn in cooperation with lipid rafts and TLR2, could be activated by phosphorylation, which in turn significantly impacted internalization of P. aeruginosa to alveolar epithelial cells or bone marrow-derived mast cells [14, 15, 29]. Lyn has been reported to be engaged in phagocytosis of IgG-coated particles (through FcγR) in J774 cells [30]. Mechanistically, we demonstrated that the Lyn/p38/NF-κB circuit was responsible for the dysregulated inflammatory response. Furthermore, we found that Lyn was in cooperation with lipid rafts in phagocytosis of Kp, bringing in the significance of Lyn as it is normally localized in a region around raft domains. Thus, Lyn may directly participate in immune response against Kp infection.
The major finding of our study is that Lyn deficiency could accelerate and intensify cytokine responses (TNF-α, IL-22, and IL-6) in mice infected by Kp, which is observed both in the lungs and BAL fluid. Although IL-2, IL-4, and IL-12A which targets T cells are not the typical proinflammatory cytokines, in this model TNF-α is regulated directly by NF-κB, which was increased in the lyn−/− mice. Others showed that IL-1β and IL-17 are important in the host responses to Kp [31]; however, we have not found significant changes in our model, which may be due to selective impact of Lyn deficiency on the production of proinflammatory cytokines in Kp-infected mice. The strong inflammatory responses may also contribute to higher ROS production as seen in the AM cells and neutrophils after Kp infection as we confirmed by determination of the superoxide dynamics with the ROS inhibitor DPI. Our data indicate that Lyn activity may be associated with ROS levels, influencing both cytokine production and cellular viability (Fig. 6 A–D), which is consistent with previous reports [32, 33]. ROS also acted like a double-edged sword and played a harmful role in host defense against Kp infection. Moreover, the wide-range bacterial dissemination into other organs may be the possible cause of mortality in these mice, a possibility which is supported by the increased MPO activity in the lung and other organs [3, 17]. Despite its importance in bacterial clearance, ROS accumulation may cause lung injury when produced excessively. Remarkably, many bacteria also have oxidation-sensing mechanisms to respond to and counteract the oxidation-mediated host response [34]. Our results also showed that lipid peroxidation increased markedly in some Kp-infected organs of lyn−/− mice as compared with those of WT mice [19].
The loss of Lyn leads to a spontaneous activation of p38/NF-κB in the absence of infection. However, there is no alteration of the lung tissue and the phenotype in resting conditions as we used mice within 3 months age, thus our data of the inflammatory responses were fully valid. p38, an important MAPK in cellular responses to external stress signals, may have anti-inflammatory effects in preclinical disease models, primarily through the inhibition of the expression of inflammatory mediators [35, 36]. To delve into the molecular mechanism in the infection progression, we probed the p38 and ERK1/2 pathway along with several other signaling proteins. Interestingly, even though ERK1/2 was previously reported to be associated with Lyn deficiency, its activity did not have significant changes upon Kp infection. However, the p38 activity was markedly activated with Lyn deficiency. NF-κB is a key transcription factor for various proinflammatory mediators, such as chemokines, cytokines, and adhesion molecules [21, 23, 35]. Here we found an increase in TNF-α release upon Kp infection by Lyn deficiency. The pro-inflammatory cytokine TNF-α triggers a signaling cascade, converging on the activation of the transcription factor NF-κB, which forms the basis for numerous physiological and pathological processes. Published data demonstrated that both the MyD88 and TRIF pathways downstream of TLR2/4 are critical for MAPK and NF-κB activation, cytokine production and neutrophils recruitment during Klebsiella infection, with the MyD88 pathway playing an earlier and somewhat greater role in these events. Also, Lyn is found to be able to control both MyD88- and TRIF-dependent signaling pathways downstream of TLR4 [37]. NF-κB involves the interaction of the ligand with its receptor at the cell surface (TNFR), which then recruits a protein called TRADD (TNF Receptor-Associated Death Domain). This protein binds to TRAF2 (TNF Receptor-Associated Factor-2), which activates RIP (Receptor-Interacting Protein). RIP interacts with MEKK (Mitogen-Activated Protein Kinase Kinase) and NIK (NF-κB-Inducing Kinase) to phosphorylate and activate IKK (IκBα kinase complex). Here NF-κB is actually impacted by Lyn and Lyn knockdown increased both the expression and phosphorylation of NF-κB by affecting the IKK complex which phosphorylates IκBα, leading to ubiquitination. The degradation of IκBα by the proteosome resulted in the translocation of NF-κB to the nucleus [38, 39]. Coincidentally, NF-κB nuclear translocation and IκBα phosphorylation were found to be suppressed by adding p38 inhibitor SB202190 in Lyn siRNA transfected cells upon Kp infection, consistent with the above observations. Our data also showed that raft aggregates decreased significantly in Lyn siRNA-transfected cells. In addition, perturbation of lipid rafts drastically reduced bacterial internalization in AM cells, indicating impairment in phagocytosis. These results strongly suggest that Lyn played an essential role in immune defense against Kp invasion by cooperation with lipid rafts, and that Lyn deficiency could lead to more severe dysfunction of innate immunity, impairing host defense against Kp infection. These results collectively demonstrate two important functions of Lyn. The first is its role in bacterial clearance by AM phagocytosis. The second is down-regulating proinflammatory responses (cytokine production) to minimize tissue injuries by epithelial cell-mediated inflammatory through direct influence on p38, through which Lyn regulates NF-κB and initiates protective immune defense during Kp infection.
The previous work has primarily focused on illustrating the role of Lyn in regulating cellular stress responses and tumorigenesis [40], with limited observations in infectious diseases. Lyn deficiency may affect bacterial adhesion to host cells and could differentially affect the extent of MyD88 and TRIF pathway activation. Although additional studies may be needed, to our knowledge, this is the first to define infection patterns in vivo with a Xen-39 bioluminecence strain in lyn−/− mice, with prior studies investigating this strain of infection with peptide radio-labeling [41] or in other mice [42]. Altogether, our observations demonstrated that bacterial clearance activity is impaired in lyn−/− mice during Kp infection. Thus our study reveals Lyn as a new target and may open new avenues for therapeutic strategies.
In summary, we found Lyn is required for full resistance to Kp infection and its deficiency contributes to elevated inflammatory cytokine responses, which resulted in a severe susceptibility to this infection. This is the first finding that demonstrates the corporation of Lyn-lipid rafts and anti-inflammatory function upon Kp infection, consistent with a recent report which analyzed the role of Lyn signaling in Francisella tularensis infection [43]. Further, we discovered that the phosphorylation and nuclear translocation of NF-κB are required for cytokine responses after IκBα phosphorylation, while inhibiting p38 reversed IκBα phosphorylation and diminished NF-κB activation. p38 is required for initiating the inflammatory response and is upstream of NF-κB since the activation and nuclear translocation of NF-κB is dependent on p38. The extents and levels of some inflammatory cytokines may not be as dramatically high despite being significant. Thus further studies of the molecular mechanism for inflammatory regulation will be useful for unraveling the novel circuit and developing therapeutically relevant regulators. Nonetheless, our studies revealed that the Lyn/p38/NF-κB axis may be one of the critical regulators in this particular dysregulated cytokine response.
Supplementary Material
Acknowledgments
We thank S. Rolling of UND imaging core for help with confocal imaging. This project was supported by Flight Attendant Medical Research Institute (FAMRI, Grant #103007), National 973 Basic Research Program of China (2011CB910703, 2012CB518900), the National Science and Technology Major Project (2012ZX09501001-003) and Chinese NSFC (81072022, 81172173), NIH AI101973-01, and AI097532-01A1 to M. W.
Abbreviations
- Kp
Klebsiella pneumoniae
- ITIM
immunoreceptor tyrosine-based inhibition motifs
- AM
alveolar macrophage
- m.o.i.
multiplicity of infection
- MPO
myeloperoxidase
- NBT
nitro blue tetrazolium
- ANOVA
analysis of variance
- PMN
polymorphonuclear neutrophil
- ROS
reactive oxygen species
- CFU
colony-forming unit
- RLU
relative luciferase units
- RFU
relative fluorescence units
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Thornton MM, Chung-Esaki HM, Irvin CB, Bortz DM, Solomon MJ, Younger JG. Multicellularity and Antibiotic Resistance in Klebsiella pneumoniae Grown Under Bloodstream-Mimicking Fluid Dynamic Conditions. J Infect Dis. 2012;206:588–595. doi: 10.1093/infdis/jis397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chuang TY, Lin CJ, Chi CL, Liu AY, Lee SW, Lin TL, Wang JT, Hsueh PR. Rapidly fatal bacteremic pneumonia caused by Klebsiella pneumoniae with K1 hypermucoviscosity phenotype in a previously healthy young man receiving levofloxacin treatment. J Microbiol Immunol Infect. 2009;42:439–441. [PubMed] [Google Scholar]
- 3.Guo Q, Shen N, Yuan K, Li J, Wu H, Zeng Y, Fox J, 3rd, Bansal AK, Singh BB, Gao H, Wu M. Caveolin-1 plays a critical role in host immunity against Klebsiella pneumoniae by regulating STAT5 and Akt activity. Eur J Immunol. 2012;42:1500–1511. doi: 10.1002/eji.201142051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muller G, Frick W. Signalling via caveolin: involvement in the cross-talk between phosphoinositolglycans and insulin. Cell Mol Life Sci. 1999;56:945–970. doi: 10.1007/s000180050485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lannutti BJ, Drachman JG. Lyn tyrosine kinase regulates thrombopoietin-induced proliferation of hematopoietic cell lines and primary megakaryocytic progenitors. Blood. 2004;103:3736–3743. doi: 10.1182/blood-2003-10-3566. [DOI] [PubMed] [Google Scholar]
- 6.Cornall RJ, Cyster JG, Hibbs ML, Dunn AR, Otipoby KL, Clark EA, Goodnow CC. Polygenic autoimmune traits: Lyn, CD22, and SHP-1 are limiting elements of a biochemical pathway regulating BCR signaling and selection. Immunity. 1998;8:497–508. doi: 10.1016/s1074-7613(00)80554-3. [DOI] [PubMed] [Google Scholar]
- 7.Harder KW, Parsons LM, Armes J, Evans N, Kountouri N, Clark R, Quilici C, Grail D, Hodgson GS, Dunn AR, Hibbs ML. Gain- and loss-of-function Lyn mutant mice define a critical inhibitory role for Lyn in the myeloid lineage. Immunity. 2001;15:603–615. doi: 10.1016/s1074-7613(01)00208-4. [DOI] [PubMed] [Google Scholar]
- 8.Krishnan V, Zeichner SL. Host cell gene expression during human immunodeficiency virus type 1 latency and reactivation and effects of targeting genes that are differentially expressed in viral latency. J Virol. 2004;78:9458–9473. doi: 10.1128/JVI.78.17.9458-9473.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kannan S, Audet A, Huang H, Chen LJ, Wu M. Cholesterol-Rich Membrane Rafts and Lyn Are Involved in Phagocytosis during Pseudomonas aeruginosa Infection. J Immunol. 2008;180:2396–2408. doi: 10.4049/jimmunol.180.4.2396. [DOI] [PubMed] [Google Scholar]
- 10.Kannan S, Huang H, Seeger D, Audet A, Chen Y, Huang C, Gao H, Li S, Wu M. Alveolar epithelial type II cells activate alveolar macrophages and mitigate P. Aeruginosa infection. PLoS One. 2009;4:e4891. doi: 10.1371/journal.pone.0004891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan K, Huang C, Fox J, Gaid M, Weaver A, Li GP, Singh BB, Gao H, Wu M. Elevated inflammatory response in Caveolin-1 deficient mice with P. aeruginosa infection is mediated by STAT3 and NF-{kappa}B. J Biol Chem. 2011;286:21814–21825. doi: 10.1074/jbc.M111.237628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reino DC, Palange D, Feketeova E, Bonitz RP, Xu da Z, Lu Q, Sheth SU, Pena G, Ulloa L, De Maio A, Feinman R, Deitch EA. Activation of toll-like receptor 4 is necessary for trauma hemorrhagic shock-induced gut injury and polymorphonuclear neutrophil priming. Shock. 2012;38:107–114. doi: 10.1097/SHK.0b013e318257123a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adachi R, Suzuki K. Lyn, one of the Src-family tyrosine kinases expressed in phagocytes, plays an important role in beta2 integrin-signalling pathways in opsonized zymosan-activated macrophage-like U937 cells. Cell Biochem Funct. 2007;25:323–333. doi: 10.1002/cbf.1393. [DOI] [PubMed] [Google Scholar]
- 14.Tang H, Cao W, Kasturi SP, Ravindran R, Nakaya HI, Kundu K, Murthy N, Kepler TB, Malissen B, Pulendran B. The T helper type 2 response to cysteine proteases requires dendritic cell-basophil cooperation via ROS-mediated signaling. Nat Immunol. 2010;11:608–617. doi: 10.1038/ni.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Semple JW, Kim M, Hou J, McVey M, Lee YJ, Tabuchi A, Kuebler WM, Chai ZW, Lazarus AH. Intravenous immunoglobulin prevents murine antibody-mediated acute lung injury at the level of neutrophil reactive oxygen species (ROS) production. PLoS One. 2012;7:e31357. doi: 10.1371/journal.pone.0031357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez RJ, Weening EH, Frothingham R, Sempowski GD, Miller VL. Bioluminescence imaging to track bacterial dissemination of Yersinia pestis using different routes of infection in mice. BMC Microbiol. 2012;12:147. doi: 10.1186/1471-2180-12-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fietz S, Bondzio A, Moschos A, Hertsch B, Einspanier R. Measurement of equine myeloperoxidase (MPO) activity in synovial fluid by a modified MPO assay and evaluation of joint diseases - an initial case study. Res Vet Sci. 2008;84:347–353. doi: 10.1016/j.rvsc.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Franck T, Grulke S, Deby-Dupont G, Deby C, Duvivier H, Peters F, Serteyn D. Development of an enzyme-linked immunosorbent assay for specific equine neutrophil myeloperoxidase measurement in blood. J Vet Diagn Invest. 2005;17:412–419. doi: 10.1177/104063870501700502. [DOI] [PubMed] [Google Scholar]
- 19.Meerang M, Nair J, Sirankapracha P, Thephinlap C, Srichairatanakool S, Arab K, Kalpravidh R, Vadolas J, Fucharoen S, Bartsch H. Accumulation of lipid peroxidation-derived DNA lesions in iron-overloaded thalassemic mouse livers: comparison with levels in the lymphocytes of thalassemia patients. Int J Cancer. 2009;125:759–766. doi: 10.1002/ijc.24412. [DOI] [PubMed] [Google Scholar]
- 20.Regueiro V, Moranta D, Frank CG, Larrarte E, Margareto J, March C, Garmendia J, Bengoechea JA. Klebsiella pneumoniae subverts the activation of inflammatory responses in a NOD1-dependent manner. Cell Microbiol. 2011;13:135–153. doi: 10.1111/j.1462-5822.2010.01526.x. [DOI] [PubMed] [Google Scholar]
- 21.Vega MI, Huerta-Yepaz S, Garban H, Jazirehi A, Emmanouilides C, Bonavida B. Rituximab inhibits p38 MAPK activity in 2F7 B NHL and decreases IL-10 transcription: pivotal role of p38 MAPK in drug resistance. Oncogene. 2004;23:3530–3540. doi: 10.1038/sj.onc.1207336. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi M, Suzuki E, Takeda R, Oba S, Nishimatsu H, Kimura K, Nagano T, Nagai R, Hirata Y. Angiotensin II and tumor necrosis factor-alpha synergistically promote monocyte chemoattractant protein-1 expression: roles of NF-kappaB, p38, and reactive oxygen species. Am J Physiol Heart Circ Physiol. 2008;294:H2879–H2888. doi: 10.1152/ajpheart.91406.2007. [DOI] [PubMed] [Google Scholar]
- 23.Wijayanti N, Huber S, Samoylenko A, Kietzmann T, Immenschuh S. Role of NF-kappaB and p38 MAP kinase signaling pathways in the lipopolysaccharide-dependent activation of heme oxygenase-1 gene expression. Antioxid Redox Signal. 2004;6:802–810. doi: 10.1089/ars.2004.6.802. [DOI] [PubMed] [Google Scholar]
- 24.Popik W, Alce TM, Au WC. Human immunodeficiency virus type 1 uses lipid raft-colocalized CD4 and chemokine receptors for productive entry into CD4(+) T cells. J Virol. 2002;76:4709–4722. doi: 10.1128/JVI.76.10.4709-4722.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kannan S, Audet A, Huang H, Chen LJ, Wu M. Cholesterol-rich membrane rafts and Lyn are involved in phagocytosis during Pseudomonas aeruginosa infection. J Immunol. 2008;180:2396–2408. doi: 10.4049/jimmunol.180.4.2396. [DOI] [PubMed] [Google Scholar]
- 26.Hahn PY, Evans SE, Kottom TJ, Standing JE, Pagano RE, Limper AH. Pneumocystis carinii cell wall beta-glucan induces release of macrophage inflammatory protein-2 from alveolar epithelial cells via a lactosylceramide-mediated mechanism. J Biol Chem. 2003;278:2043–2050. doi: 10.1074/jbc.M209715200. [DOI] [PubMed] [Google Scholar]
- 27.Sugahara K, Mizutani A, Yamamoto H. Effect Of Ethyl Pyruvate On Nuclear Translocation Of NF-kB In Cultured Lung Epithelial Cells. Am. J. Respir. Crit. Care Med. 2010;181:A3613. doi: 10.1016/j.pupt.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Arita Y, Ito T, Oono T, Kawabe K, Hisano T, Takayanagi R. Lysophosphatidic acid induced nuclear translocation of nuclear factor-kB in Panc-1 cells by mobilizing cytosolic free calcium. World. J. Gastroenterol. 2008;14:4473–4479. doi: 10.3748/wjg.14.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kannan S, Audet A, Knittel J, Mullegama S, Gao GF, Wu M. Src kinase Lyn is crucial for Pseudomonas aeruginosa internalization into lung cells. Eur J Immunol. 2006;36:1739–1752. doi: 10.1002/eji.200635973. [DOI] [PubMed] [Google Scholar]
- 30.Strzelecka-Kiliszek A, Kwiatkowska K, Sobota A. Lyn and Syk kinases are sequentially engaged in phagocytosis mediated by Fc gamma R. J Immunol. 2002;169:6787–6794. doi: 10.4049/jimmunol.169.12.6787. [DOI] [PubMed] [Google Scholar]
- 31.Ye P, Garvey PB, Zhang P, Nelson S, Bagby G, Summer WR, Schwarzenberger P, Shellito JE, Kolls JK. Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am J Respir Cell Mol Biol. 2001;25:335–340. doi: 10.1165/ajrcmb.25.3.4424. [DOI] [PubMed] [Google Scholar]
- 32.Yan SR, Novak MJ. Src-family kinase-p53/ Lyn p56 plays an important role in TNF-alpha-stimulated production of O2- by human neutrophils adherent to fibrinogen. Inflammation. 1999;23:167–178. doi: 10.1023/a:1020245129632. [DOI] [PubMed] [Google Scholar]
- 33.Paul R, Obermaier B, Van Ziffle J, Angele B, Pfister HW, Lowell CA, Koedel U. Myeloid Src kinases regulate phagocytosis and oxidative burst in pneumococcal meningitis by activating NADPH oxidase. J Leukoc Biol. 2008;84:1141–1150. doi: 10.1189/jlb.0208118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yi C, Jia G, Hou G, Dai Q, Zhang W, Zheng G, Jian X, Yang CG, Cui Q, He C. Iron-catalysed oxidation intermediates captured in a DNA repair dioxygenase. Nature. 2010;468:330–333. doi: 10.1038/nature09497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu HS, Pan CE, Liu QG, Yang W, Liu XM. Effect of NF-kappaB and p38 MAPK in activated monocytes/macrophages on pro-inflammatory cytokines of rats with acute pancreatitis. World J Gastroenterol. 2003;9:2513–2518. doi: 10.3748/wjg.v9.i11.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kannan S, Pang H, Foster D, Rao Z, Wu M. Human 8-oxoguanine DNA glycosylase links MAPK activation to resistance to hyperoxia in lung epithelial cells. Cell Death Differ. 2006;13:311–323. doi: 10.1038/sj.cdd.4401736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keck S, Freudenberg M, Huber M. Activation of murine macrophages via TLR2 and TLR4 is negatively regulated by a Lyn/PI3K module and promoted by SHIP1. J Immunol. 2010;184:5809–5818. doi: 10.4049/jimmunol.0901423. [DOI] [PubMed] [Google Scholar]
- 38.MacEwan DJ. TNF ligands and receptors--a matter of life and death. Br J Pharmacol. 2002;135:855–875. doi: 10.1038/sj.bjp.0704549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ganeff C, Remouchamps C, Boutaffala L, Benezech C, Galopin G, Vandepaer S, Bouillenne F, Ormenese S, Chariot A, Schneider P, Caamano J, Piette J, Dejardin E. Induction of the alternative NF-kappaB pathway by lymphotoxin alphabeta (LTalphabeta) relies on internalization of LTbeta receptor. Mol Cell Biol. 2011;31:4319–4334. doi: 10.1128/MCB.05033-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamanashi Y, Fukui Y, Wongsasant B, Kinoshita Y, Ichimori Y, Toyoshima K, Yamamoto T. Activation of Src-like protein-tyrosine kinase Lyn and its association with phosphatidylinositol 3-kinase upon B-cell antigen receptor-mediated signaling. Proc Natl Acad Sci U S A. 1992;89:1118–1122. doi: 10.1073/pnas.89.3.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Das SS, Britton KE, Solanki KK, Wareham DW. Technetium-99m labelled antimicrobial peptides discriminate between bacterial infections and sterile inflammations. Eur J Nucl Med. 2000;27:1865–1868. doi: 10.1007/s002590000377. [DOI] [PubMed] [Google Scholar]
- 42.Georgel P, Radosavljevic M, Macquin C, Bahram S. The non-conventional MHC class I MR1 molecule controls infection by Klebsiella pneumoniae in mice. Mol Immunol. 2011;48:769–775. doi: 10.1016/j.molimm.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 43.Dai S, Rajaram MV, Curry HM, Leander R, Schlesinger LS. Fine Tuning Inflammation at the Front Door: Macrophage Complement Receptor 3-mediates Phagocytosis and Immune Suppression for Francisella tularensis. PLoS Pathog. 2013;9:e1003114. doi: 10.1371/journal.ppat.1003114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.