Abstract
The stress system effectively restores the internal balance––or homeostasis––of living organisms in the face of random external or internal changes, the stressors. This highly complex system helps organisms to provide a series of neuroendocrine responses to stressors—the stress response—through coordinated activation of the hypothalamic–pituitary–adrenal (HPA) axis and the locus coeruleus/norepinephrine (LC/NE) autonomic nervous systems. In addition to stressors, life is influenced by daily light/dark changes due to the 24-h rotation of Earth. To adjust to these recurrent day/night cycles, the biological clock system employs the heterodimer of transcription factors CLOCK/BMAL1, along with a set of other transcription factors, to regulate the circadian pattern of gene expression. Interestingly, the stress system, through the HPA axis, communicates with the clock system; therefore, any uncoupling or dysregulation could potentially cause several disorders, such as metabolic, autoimmune, and mood disorders. In this review, we discuss the biological function of the two systems, their interactions, and the clinical implications of their dysregulation or uncoupling.
Keywords: HPA axis, circadian clock system, glucocorticoids, glucocorticoid receptor, acetylation
The HPA axis, glucocorticoids, and glucocorticoid receptor
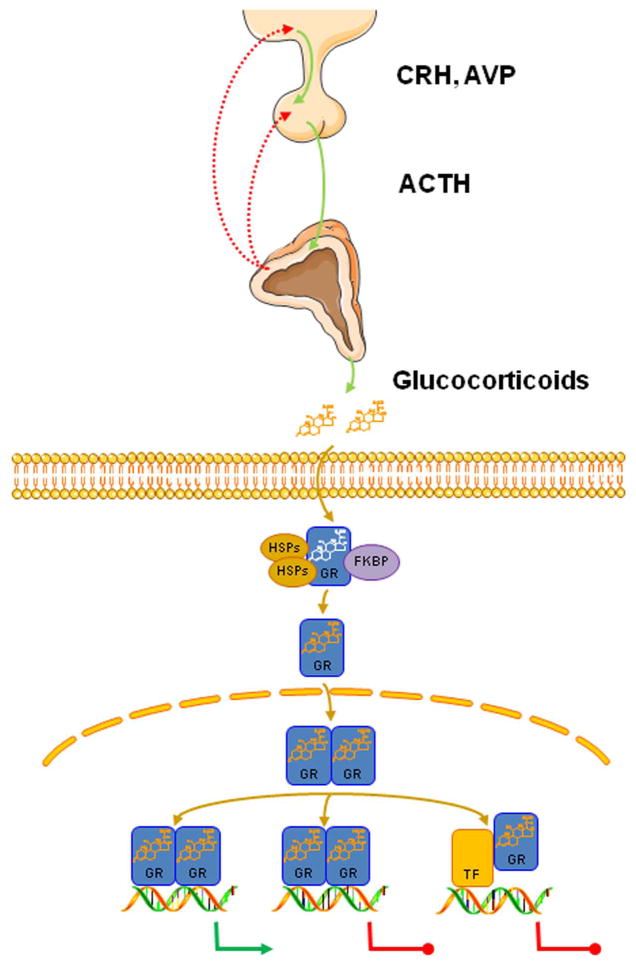
The hypothalamic–pituitary–adrenal (HPA) axis is essential for survival in mammals. This highly complex neuroendocrine axis functions synergistically with the locus coeruleus/norepinephrine (LC/NE) autonomic nervous systems to provide a stress response of the appropriate magnitude and duration in the face of random, unforeseen, internal, or external stressful stimuli.1 The HPA axis is composed of positive hormonal influences and negative feedback mechanisms, both of which strictly regulate the activity of the axis (Fig. 1). In response to stressors, neurons located in the paraventricular nuclei (PVN) of the hypothalamus are stimulated and secrete corticotropin-releasing hormone (CRH) and arginine vasopressin (AVP) into the hypophysial portal system, reaching the anterior lobe of the pituitary.1,2 CRH and AVP then bind to their cognate transmembrane receptors and trigger the synthesis and secretion of adrenocorticotropic hormone (ACTH) by the corticotroph cells of the anterior pituitary gland.3,4 In turn, the increased circulating ACTH concentrations activate the biosynthetic pathway of glucocorticoids (cortisol in humans and corticosterone in most rodents) in the zona fasciculata.5
Figure 1.
The neuroendocrine hypothalamic–pituitary–adrenal (HPA) axis. The HPA axis consists of the paraventricular nuclei (PVN) located in the hypothalamus, the pituitary gland, and the adrenal cortices. Upon stimulation, neurons of the PVN produce and release CRH and AVP into the hypophysial portal system. In turn, CRH and AVP induce the secretion of ACTH by the anterior pituitary gland. ACTH then reaches the zona fasciculata and stimulates the synthesis and release of glucocorticoids. In the target cell, glucocorticoids bind to the glucocorticoid receptor (GR), which dissociates from HSPs and immunophilins (such as FKBP), translocates to the nucleus, and interacts with the glucocorticoid response elements (GREs) of target genes as homo- or heterodimer, influencing the transcription rate of numerous genes in a positive or negative fashion. Alternatively, the GR can modulate gene expression by physically interacting with other transcription factors (NF-κB, AP-1, STAT5) influencing the expression of their target genes. CRH, corticotropin-releasing hormone; AVP, arginine vasopressin; ACTH, adrenocorticotropic hormone; GR, glucocorticoid receptor; HSP, heat shock proteins; FKBP, FK506-binding protein. Figure 1 was prepared using image vectors from Servier Medical Art (www.servier.com), licensed under the Creative Commons Attribution 3.0 Unported License (http://creativecommons.org/license/by/3.0/).
Glucocorticoids are the final hormonal products of the HPA axis. These cholesterol-derived molecules are released into the periphery in an ultradian, circadian, and stress-related fashion and play a fundamental role in the maintenance of resting and stress-related homeostasis.6,7 Indeed, 15–20% of the human leukocyte transcriptome is influenced by glucocorticoids,8 and almost two thirds of them are induced, while the rest are suppressed.8 Through their genomic actions, glucocorticoids regulate cellular metabolism primarily through catabolic actions in the liver, muscle, and adipose tissue.8,9 Moreover, cardiovascular tone is maintained by the inhibitory actions of glucocorticoids in the cardiovascular system.8 Numerous functions of central nervous system (CNS) are also strongly influenced by glucocorticoids.8,9 Finally, multiple components regulating the quantity and quality of immune/inflammatory responses are well-recognized glucocorticoid targets, providing the basis for the wide use of glucocorticoids as potent anti-inflammatory/immunosuppressive drugs in the treatment of inflammatory diseases and cancer.10 In humans, glucocorticoids exert their pleiotropic functions mostly through a ubiquitously expressed intracellular and membrane-bound protein, the human glucocorticoid receptor (hGR), as well as via the human mineralocorticoid receptor (hMR), both of which belong to the steroid receptor family of the nuclear receptor superfamily of transcription factors.11
Although there are multiple hGR protein isoforms in the target cell, produced through complex transcription/translation mechanisms, there is only a single gene, the hGR gene NR3C1 (nuclear receptor subfamily 3, group C member 1), which is located on chromosome 5 and consists of 10 exons.12 The alternative splicing of exon 9 produces two distinct receptor isoforms with different properties with respect to localization, activity, and function.12,13 Thus, the α isoform (hGRα) contains 777 amino acids and has three functional domains: the N-terminal or immunogenic domain (NTD), the DNA-binding domain (DBD) that contains the characteristic and highly conserved motif of two zinc fingers, and the ligand-binding domain (LBD) of the receptor. Finally, a hinge region is located between the DBD and LBD, and provides the appropriate structural flexibility to the protein.12, 13
The hGRα isoform is the classic hGR and is ubiquitously expressed in every cell type, binds glucocorticoids, and mediates all genomic actions of these hormones.9, 11 On the other hand, the hGRβ is a 742–amino acid protein, which does not bind natural or synthetic glucocorticoids, and acts as a dominant negative regulator of hGRα-mediated transcriptional activity through several mechanisms.14–18 In addition to the two main receptor isoforms generated by alternative splicing at the transcriptional level, it was recently demonstrated that additional mechanisms during the translation process could give rise to functionally distinct receptor isoforms. Indeed, through ribosomal leaky scanning or ribosomal shunting, the hGRα mRNA was shown to be translated to eight different protein isoforms.19 Given that the translation process starts from the same 5′-terminus of hGRβ mRNA, it is possible that the above translation mechanisms could potentially generate eight additional different hGRβ proteins.9 Thus, the sixteen amino terminal hGRα and hGRβ protein isoforms may form 256 homo- or heterodimers to transduce the glucocorticoid signal.9
The glucocorticoid signaling pathway is activated upon the binding of the receptor to glucocorticoids (Fig. 1). The ligand-induced activation of the receptor causes conformational changes to the protein, resulting in the dissociation of the receptor from chaperon heat shock proteins and immunophilins.9,11 The activated hGRα translocates into the nucleus and binds, as a homo- or heterodimer, to the specific glucocorticoid response elements (GREs) within the promoter sequences of target genes, thereby inducing or repressing their transcription.9,11 Moreover, the ligand-activated hGRα can modulate the expression of numerous other genes by physically interacting with other transcription factors, including the activator protein-1 (AP-1), nuclear factor-κB (NF-κB), and signal transducers and activators of transcription (STATs) (Fig. 1).11
The heterodimer CLOCK/BMAL1
In an attempt to find novel partners that potentially alter hGRα actions in target cells, we performed a yeast two-hybrid screening using GR fragments as baits. Among other molecules, we identified the CLOCK transcription factor as an interacting molecule with the hGRα.20 CLOCK, the circadian locomotor output cycle kaput, forms a heterodimer with the brain–muscle–arnt–like protein 1 (BMAL1). These two transcription factors belong to the basic helix–loop–helix (bHLH)–PER–ARNT–SIM (PAS) superfamily of transcription factors. The heterodimer CLOCK/BMAL1, along with a set of other transcription factors, is responsible for the circadian oscillations of gene expression under the control of the biological clock system.21–24
The clock system is composed of a central clock and numerous peripheral clocks.21,22,25 While the central clock is located within the suprachiasmatic nuclei (SCN) of the hypothalamus, the peripheral clocks are ubiquitously expressed in all tissues. The central clock receives light/dark signals from the eyes through the retinohypothalamic tract and communicates with the peripheral clocks via poorly understood connections, effectively synchronizing its activity with those of the latter.21,22,25,26 The central and peripheral clocks use the same set of transcription factors, including CLOCK and BMAL1, to generate the circadian pattern of gene expression.25
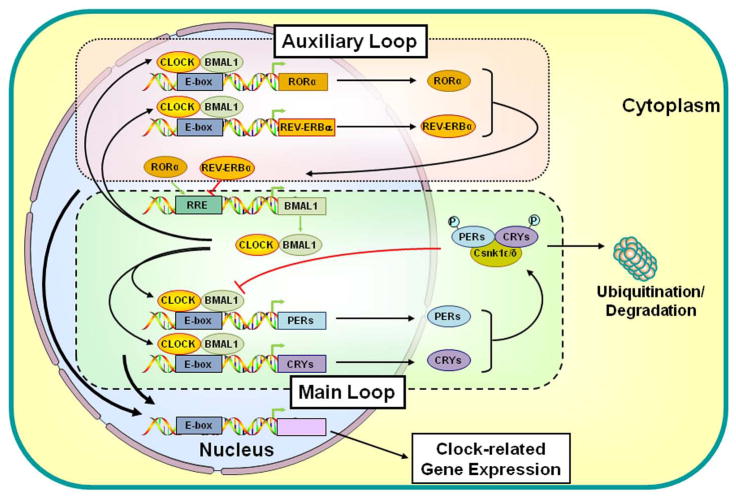
The molecular mechanisms underlying circadian oscillations of gene expression are mediated by transcription/translation feedback loops (Fig. 2). The principal or core feedback loop is mediated by the activated CLOCK/BMAL1 heterodimer, which regulates the expression of numerous target genes upon binding to cognate DNA sequences, the E-box response elements. Central among the target genes are other clock genes, such as Periods (Per1, Per2, and Per3) and Cryptochromes (Cry1 and Cry2), which, when expressed, interact with casein kinase 1ε and δ, forming a protein complex. PERs and CRYs then undergo phosphorylation and finally enter the nucleus, where they inhibit the transcriptional activity of the CLOCK/BMAL1 heterodimer through prevention of CLOCK/BMAL1 binding to the E-box response elements located in the Per and Cry promoters, or recruitment of chromatin modifier proteins, and/or repression of transcriptional termination and re-initiation. 21,25,27–29 Consequently, Per and Cry genes are downregulated, PER- and CRY-mediated inhibition is relieved, and the transcription/translation cycle starts again. In addition to this principal or core feedback loop described above, a secondary or auxiliary feedback loop exists to maintain the activity of the principal loop. Thus, the expression of other clock-related molecules, such as REV-ERBα, retinoic acid receptor–related orphan receptor α (RORα), DEC1, DEC2, and albumin gene D site–binding protein (DBP), are regulated by the heterodimer CLOCK/BMAL1. Finally, all these clock-related transcription factors regulate the transcription rates of many clock-responsive genes influencing a broad spectrum of physiologic functions, such as sleep/wakefulness, feeding, thermoregulation, energy expenditure, and glucose metabolism.21,22,30 Peripheral clocks located in organs also influence organs-specific activities under the synchronized day/night information, but in constant conditions as well (free run) (Fig. 2).
Figure 2.
Main and auxiliary transcriptional loops of the circadian clock system. The CLOCK/BMAL1 heterodimer binds to the E-box response elements and regulates the expression of Per and Cry genes. Accumulating proteins PERs and CRYs interact with casein kinase 1ε and δ, form a protein complex with the latter, undergo phosphorylation, and translocate into the nucleus where they inhibit CLOCK/BMAL1–mediated transcriptional activity. In addition to this main transcriptional loop, CLOCK/BMAL1 regulates the expression of other clock-related molecules, forming an auxiliary loop that maintains the activity of the main regulatory loop. Finally, these clock-related transcription factors regulate the expression of many clock-responsive genes influencing several physiologic functions. BMAL1, brain–muscle–arnt–like protein 1; CLOCK, circadian locomotor output cycle kaput; CRYs, cryptochromes; Csnk1ε/δ, casein kinase 1ε/δ; P, phosphate residue on the phosphorylated molecules; PERs, periods; RORα, retinoic acid receptor–related orphan nuclear receptor α. Modified from Ref. 25.
CLOCK/BMAL1, which participates in both the principal and auxiliary feedback loops discussed above, was found to physically interact with the LBD of hGRα and repress the hGRα-induced transcription of glucocorticoid-responsive genes, providing strong evidence for the interaction of the HPA axis and the clock system at the transcriptional level in peripheral tissues.20 The suppression of the hGRα transcriptional activity was associated with the acetyltransferase activity of CLOCK.31 Indeed, CLOCK acetylated lysines at amino acid positions 480, 492, 494, and 495 within the hinge region of the receptor, thereby reducing its binding to its cognate GREs.20 Furthermore, the part of the hinge region that undergoes acetylation by CLOCK contains common amino acid sequences with the nuclear localization signal 1 (NL1), which plays an important role in the cytoplasmic-to-nuclear translocation of the receptor following ligand-induced activation. Therefore, this CLOCK-mediated post-translational modification of hGRα seems to alter the nuclear translocation of the receptor, indicating that the acetylation of hGRα by CLOCK repressed the hGRα-induced transactivation of glucocorticoid target genes by several molecular mechanisms.20 These findings also suggest that CLOCK/BMAL1 behaves as a negative regulator of hGRα in peripheral target tissues and antagonizes the physiologic actions of fluctuating cortisol.20 The results discussed above were further confirmed by Han et al. who showed that the heterodimer of transcription factors CLOCK/BMAL1 decreased maximal GR transactivation of target genes, as well as the efficacy of the receptor to induce the expression of glucocorticoid-responsive genes. In contradistinction, the PER1/CRY1 complex reduced the maximal GR transactivation but not the efficacy of the receptor.32
Peripheral clock regulates hGRα transcriptional activity
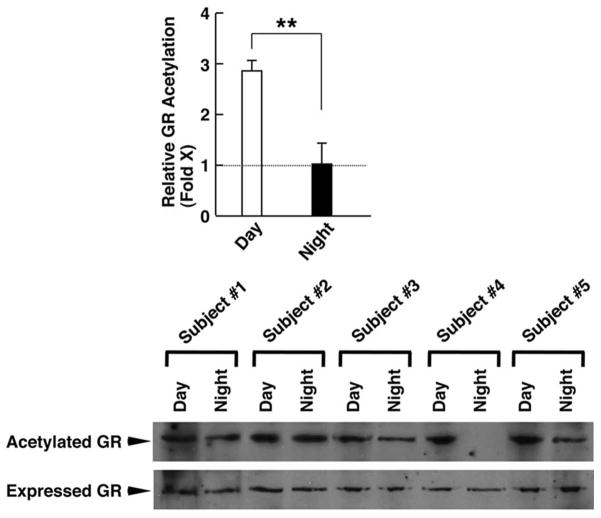
In addition to our in vitro studies, we also investigated the hGRα acetylation levels and mRNA expression of clock-related, as well as glucocorticoid-responsive, genes in humans in the early morning (8:00 am) and the late evening (8:00 pm).33 We demonstrated that the acetylation status of hGRα was higher in the morning and lower in the evening samples, and actually mirrored the diurnal fluctuations of circulating cortisol concentrations33 (Fig. 3A). The mRNA levels expressed from Clock and Bmal1 genes were higher in the morning samples compared to the evening samples. In contradistinction to these transcription factors, the mRNA levels of Per1, Cry1, and RORα were higher at night than in the morning (Fig. 3B).33 The increased expression of Clock and Bmal1 and the concurrent decreased expression of Per1 and Cry1 observed in the morning samples could provide a convincing explanation of the high acetylation status of hGRα. To our surprise, we found that the mRNA expression of some glucocorticoid target genes did not show the expected diurnal fluctuations, indicating that the acetylated hGRα may influence the transcription rate of target genes in a gene- and probably tissue-specific fashion, possibly due to different binding affinities with GREs or because of altered interaction with other molecules involved in the transcription process.33
Figure 3.
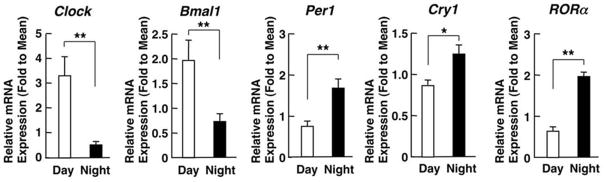
(A) Levels of acetylated hGRα in the morning and evening samples from healthy humans. Acetylation of hGRα is higher in the morning hours than during the evening. Bars represent mean ± S.E. values of relative GR acetylation; **P < 0.01. A representative Western blot image shows the higher levels of acetylated GRs in the samples obtained in the morning compared to the evening samples. (B) Circadian expression of clock-related genes. The mRNA expression of Clock and Bmal1 was higher in the morning than in the evening, whereas the negative regulators of the clock system (Per1, Cry1, and RORα) were expressed in higher levels in the evening than in the morning samples. Bars represent mean ± S.E. values of relative mRNA expression of the indicated genes; **P < 0.01. Adapted from Ref. 33.
Interactions between the clock system and the HPA axis
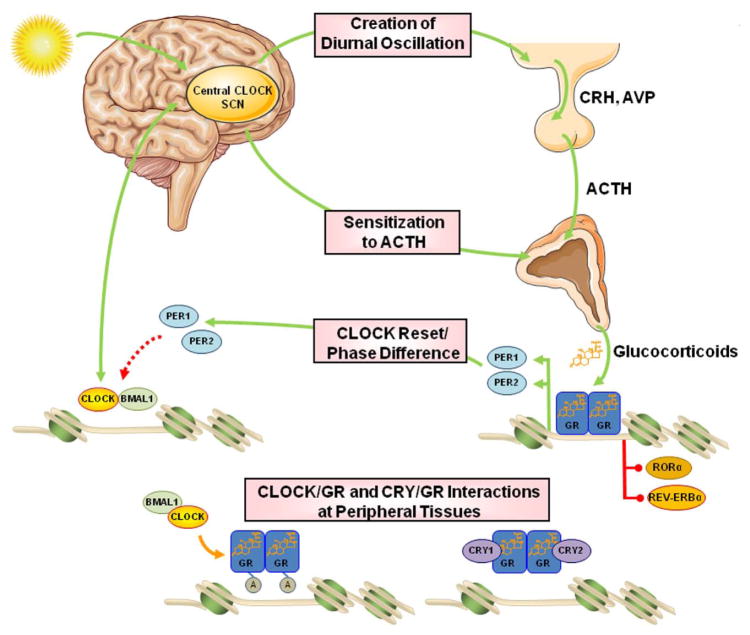
Accumulating evidence supports the concept that there is a strong interrelation between the clock system and the HPA axis at multiple levels (Fig. 4).25,34–41 The light-activated central clock controls the activity of the HPA axis through synapses between the SCN and the PVN,26,42 thereby providing the basis for the circadian release of glucocorticoids, which in humans reach their zenith concentrations early in the morning and their nadir concentrations late at night. Moreover, the central clock system seems to influence the secretion of glucocorticoids from the adrenal cortices by altering the sensitivity of the zona fasciculata to ACTH, an effect that has been attributed to the activation of the autonomic nervous system by the SCN and/or to the presence of an adrenal peripheral clock.26,42–45 Indeed, in mice, the adrenal glands have their own clock system, which confers rhythmic expression of several clock genes, as well as genes encoding molecules of the steroidogenic pathway and the ACTH signal transduction cascade in the zona glomerulosa and zona fasciculata.44,45 In peripheral target tissues of mice, Yang et al. demonstrated that GR expression oscillated in white and brown adipose tissues, whereas the expression of the receptor in liver and muscle was non-rhythmic, indicating that the molecular clock can control rhythmic expression of GRs in a tissue-specific fashion.46 In addition, it was recently shown that CRY1 and CRY2 interacted with the C-terminal domain of GRs in a ligand-dependent fashion, repressing the GR-mediated transactivation of target genes.47 However, this novel protein–protein interaction did not influence the ability of GRs to transrepress the expression of numerous inflammatory genes, suggesting that cryptochromes, upon interaction with the GR, could modulate the expression of a distinct group of GR target genes, especially those encoding proteins involved in the regulation of HPA axis activity, gluconeogenesis, and steroidogenesis.47 This CRY-dependent dissociation of the transactivating and transrepressing activities of the GR might ameliorate the metabolic side effects often observed during chronic administration of synthetic glucocorticoids by opting for the right timing of treatment or by co-administering glucocorticoids with compounds that stabilize cryptochromes in the liver.47
Figure 4.
Sites of interactions between the circadian clock system and the HPA axis. The central CLOCK, within the SCN, is entrained by light and is responsible for the regular diurnal secretion of CRH, AVP, ACTH, and, ultimately, glucocorticoids from the adrenal cortices. It also alters the adrenal sensitivity to ACTH, adding a second level of interaction. The peripherally expressed clocks receive regulatory information from the central clock through neural and humoral signals and also contribute to the rhythmic release of glucocorticoid hormones. On the other hand, glucocorticoids reset and phase delay the circadian rhythm of the peripheral but not the SCN clocks by regulating the expression of clock-related genes, such as Per1 and Per2, through genomic actions mediated by the activated GR. In addition to Per1 and Per2, which contain positive GREs, the Rev-ERBa and RORa genes carry functional negative GREs. Finally, the peripheral clocks regulate GR transcriptional activity in local tissues by CLOCK-mediated acetylation of multiple lysine residues in the hinge region of the receptor or by CRY/GR interaction, both resulting in reduced GR transcriptional activity. SCN, suprachiasmatic nuclei; CRH, corticotropin-releasing hormone; AVP, arginine vasopressin; ACTH, adrenocorticotropic hormone; GR, glucocorticoid receptor; CLOCK, circadian locomotor output cycle kaput; BMAL1, brain–muscle–arnt–like protein 1; PER, period; CRY, cryptochrome; RORα, retinoic acid receptor–related orphan nuclear receptor α. Modified from Ref. 25. Figure 4 was prepared using image vectors from Servier Medical Art (www.servier.com), licensed under the Creative Commons Attribution 3.0 Unported License (http://creativecommons.org/license/by/3.0/).
The HPA axis strongly affects both the circadian rhythm and the activity of the clock system. Glucocorticoids were demonstrated to phase shift the expression of many clock-related genes, including Per1 and Per2 genes, in several peripheral organs of mice, such as the heart, liver, and kidney.48–50 The Per1 gene was shown to have GREs in its regulatory sequences, whereas GRs seem to influence the expression of Per2 through binding to an intronic domain of the latter.50 In addition to Per1 and Per2, the Rev-ERBa and RORa genes were added to the long list of glucocorticoid-responsive genes containing functional negative GREs.51,52 By changing the expression of these clock genes, glucocorticoids transiently override the peripheral clock system in influencing daily gene expression upon exposure to stressors, which is apparently beneficial for organisms to fight against. In addition, this glucocorticoid-mediated regulation of clock gene expression plays an important role in adjusting food intake–associated uncoupling between the central and peripheral clock.53 Indeed, when phasing between the central SCN clock and peripheral oscillators is uncoupled under time-restricted feeding,53 glucocorticoids are secreted in high concentrations in an attempt to slow down the uncoupling.54 This protective effect of glucocorticoids could be part of the stress response, since time-restricted feeding is a major stressor in living organisms.
Clinical implications of aberrant clock system and HPA axis coupling
Any dysregulation in either system or disruption of their molecular interrelation may lead to hypercortisolism in target tissues and provide the basis for the development of metabolic and immune diseases. Interestingly, any dysregulation in the clock system, as occurs in mice defective in clock-related genes55,56 or in carriers of specific single nucleotide polymorphisms within the Clock gene,57,58 causes disturbances in the molecular pathways regulating carbohydrate and/or lipid metabolism, ultimately leading to hyperglycemia, insulin resistance, aberrant accumulation of visceral fat, dyslipidemia, and hypertension, all known components of the metabolic syndrome. Similarly, patients treated with high doses of synthetic glucocorticoids or subjects under chronic stress often develop central obesity and diabetes mellitus.59,60 The common pathologic clinical manifestations seen in dysregulation of either system strongly support their interaction. However, whether disruption of the clock system dysregulates intermediary metabolism through activation of the HPA axis or whether these two regulatory systems independently control the same core metabolic cascades is still unknown. We speculate that both mechanisms contribute synergistically to the development of metabolic syndrome with its resultant cardiovascular consequences.
Typical examples of uncoupling between the clock system and the HPA axis are rotating shift workers,61, 62 whose nighttime activity/daytime sleep resets their clock, and subjects who are exposed to frequent jet lag due to traveling over time zones.63,64 These individuals are at increased risk for myocardial infarction and stroke. Moreover, subjects under chronic stress often present with clinical manifestations of the metabolic syndrome. Indeed, their HPA axis is highly activated by signals from higher brain centers, which results in a blunting of the decreases in circulating cortisol during the late evening.25
Immune function and responses are also tightly regulated by the clock system and the HPA axis. Several cytokines, including interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α fluctuate in a circadian fashion in humans, demonstrating their peak concentrations between midnight and early morning hours, which in part provides a convincing explanation of the flare of symptoms in the morning in patients with autoimmune diseases.65–67 Moreover, relative to day workers, rotating and night shift workers display a higher risk for common infections,68 multiple sclerosis,69 and other autoimmune disorders.70 Similarly, acute or chronic activation of the HPA axis strongly suppresses the immune reaction because of protein–protein interactions between the hGRα and other crucial transcription factors (NF-κB, AP-1) or due to genomic actions of the receptor, which suppress pro-inflammatory and induce anti-inflammatory genes.10 Thus, glucocorticoids are frequently used in the treatment of inflammatory and autoimmune diseases. To ameliorate the morning clinical manifestations, patients with these disorders are treated with prednisolone or other glucocorticoids with potent anti-inflammatory activity in the evening, a therapeutic strategy that leads to significant improvement of many symptoms observed in the morning.71,72 The successful therapeutic outcome associated with the administration of synthetic glucocorticoids during the evening hours may be supported, in part, by the increased sensitivity of immune cells to glucocorticoids due to less acetylation of hGR in the evening, which effectively suppresses the expression of inflammatory cytokines and inhibits the inflammatory reaction at this time of the day. However, it should be mentioned that the administration of glucocorticoids in the evening might increase the frequency of adverse side effects of these hormones, despite the low doses employed.73
In addition to metabolic and autoimmune diseases, night shift workers are at increased risk for mood disorders, such as depression and anxiety.74 Recent in vivo studies have demonstrated that an aberrant light cycle impaired mood and learning.75 Indeed, mice exposed to irregular light changes showed depressive behavior and impaired learning function.75 Although they maintained circadian corticosterone rhythmicity, they had increased corticosterone concentrations.75 Administration of antidepressants alleviated depression-like behavior and effectively restored learning function. The beneficial effects of antidepressants were attributed to the significant reduction of corticosterone concentrations, since the circadian rhythms of these mice did not show any alterations.75
Future directions
Despite the significant progress that has been made in research on the molecular mechanisms underlying the multilevel communication between the circadian clock and the stress response system, most of the physiologic and pathophysiologic aspects of this communication remain to be elucidated. Moreover, since both systems tend to maintain internal homeostasis, the CLOCK-mediated acetylation of hGRα must be followed by deacetylation of the receptor in the late evening hours. As occurs with several nuclear receptors, we speculate that Sirt1, a class III HDAC and a member of the sirtuin family, might deacetylate hGRα, closing the acetylation/deacetylation cycle of the receptor and restoring its transcriptional activity to normal levels.76,77 Further, HDAC2 is known to deacetylate GRs;78 thus, this molecule might inactivate the CLOCK effect on hGR as well. We hypothesize that the balance between acetylation and deacetylation of the hGRα promoted by CLOCK and possibly Sirt1 or HDAC2, respectively, might be critical for the maintenance of proper glucocorticoid signaling, ultimately increasing the chance for survival.
Acknowledgments
This work was supported by (1) the European Union (European Social Fund, ESF) and Greek national funds through the operational program “Education and Lifelong Learning” of the National Strategic Reference Framework (NSRF) Research Funding Program THALIS, University of Athens (UOA), Athens, Greece; and (2) the intramural program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Maryland.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995;332:1351–1362. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- 2.Chrousos GP, et al. Corticotropin releasing factor: basic studies and clinical applications. Prog Neuropsychopharmacol Biol Psychiatry. 1985;9:349–359. doi: 10.1016/0278-5846(85)90187-3. [DOI] [PubMed] [Google Scholar]
- 3.Calogero AE, et al. Regulation of rat hypothalamic corticotropin-releasing hormone secretion in vitro: potential clinical implications. Adv Exp Med Biol. 1988;245:167–181. doi: 10.1007/978-1-4899-2064-5_13. [DOI] [PubMed] [Google Scholar]
- 4.Smith MA, et al. Corticotropin-releasing hormone: from endocrinology to psychobiology. Horm Res. 1989;31:66–71. doi: 10.1159/000181089. [DOI] [PubMed] [Google Scholar]
- 5.Bornstein SR, Chrousos GP. Clinical review 104: Adrenocorticotropin (ACTH)- and non-ACTH-mediated regulation of the adrenal cortex: neural and immune inputs. J Clin Endocrinol Metab. 1999;84:1729–1736. doi: 10.1210/jcem.84.5.5631. [DOI] [PubMed] [Google Scholar]
- 6.Chrousos GP, Charmandari E, Kino T. Glucocorticoid action networks—an introduction to systems biology. J Clin Endocrinol Metab. 2004;89:563–4. doi: 10.1210/jc.2003-032026. [DOI] [PubMed] [Google Scholar]
- 7.Chrousos GP. The glucocorticoid receptor gene, longevity, and the complex disorders of Western societies. Am J Med. 2004;117:204–7. doi: 10.1016/j.amjmed.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Galon J, et al. Gene profiling reveals unknown enhancing and suppressive actions of glucocorticoids on immune cells. FASEB J. 2002;16:61–71. doi: 10.1096/fj.01-0245com. [DOI] [PubMed] [Google Scholar]
- 9.Chrousos GP, Kino T. Intracellular glucocorticoid signaling: a formerly simple system turns stochastic. Sci STKE. 2005:pe48. doi: 10.1126/stke.3042005pe48. [DOI] [PubMed] [Google Scholar]
- 10.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids – new mechanisms for old drugs. N Engl J Med. 2005;353:1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 11.Nicolaides NC, et al. The human glucocorticoid receptor: Molecular basis of biologic function. Steroids. 2010;75:1–12. doi: 10.1016/j.steroids.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou J, Cidlowski JA. The human glucocorticoid receptor: one gene, multiple proteins and diverse responses. Steroids. 2005;70:407–17. doi: 10.1016/j.steroids.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Duma D, Jewell CM, Cidlowski JA. Multiple glucocorticoid receptor isoforms and mechanisms of post-translational modification. J Steroid Biochem Mol Biol. 2006;102:11–21. doi: 10.1016/j.jsbmb.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 14.Kino T, Chrousos GP. Glucocorticoid and mineralocorticoid receptors and associated diseases. Essays Biochem. 2004;40:137–155. doi: 10.1042/bse0400137. [DOI] [PubMed] [Google Scholar]
- 15.Bamberger CM, et al. Glucocorticoid receptor β, a potential endogenous inhibitor of glucocorticoid action in humans. J Clin Invest. 1995;95:2435–2441. doi: 10.1172/JCI117943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charmandari E, et al. The human glucocorticoid receptor (hGR) β isoform suppresses the transcriptional activity of hGRα by interfering with formation of active coactivator complexes. Mol Endocrinol. 2005;19:52–64. doi: 10.1210/me.2004-0112. [DOI] [PubMed] [Google Scholar]
- 17.Yudt MR, et al. Molecular origins for the dominant negative function of human glucocorticoid receptor β. Mol Cell Biol. 2003;23:4319–4330. doi: 10.1128/MCB.23.12.4319-4330.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kino T, Su YA, Chrousos GP. Human glucocorticoid receptor isoform beta: recent understanding of its potential implications in physiology and pathophysiology. Cell Mol Life Sci. 2009;66:3435–3448. doi: 10.1007/s00018-009-0098-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu NZ, Cidlowski JA. Translational regulatory mechanisms generate N-terminal glucocorticoid receptor isoforms with unique transcriptional target genes. Mol Cell. 2005;18:331–342. doi: 10.1016/j.molcel.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 20.Nader N, et al. Circadian rhythm transcription factor CLOCK regulates the transcriptional activity of the glucocorticoid receptor by acetylating its hinge region lysine cluster: potential physiological implications. FASEB J. 2009;23:1572–1583. doi: 10.1096/fj.08-117697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi JS, et al. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet. 2008;9:764–775. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15:R271–R277. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- 23.Hastings M, et al. Circadian clocks: regulators of endocrine and metabolic rhythms. J Endocrinol. 2007;195:187–198. doi: 10.1677/JOE-07-0378. [DOI] [PubMed] [Google Scholar]
- 24.Cermakian N, Sassone-Corsi P. Multilevel regulation of the circadian clock. Nat Rev Mol Cell Biol. 2000;1:59–67. doi: 10.1038/35036078. [DOI] [PubMed] [Google Scholar]
- 25.Nader N, Chrousos GP, Kino T. Interactions of the circadian CLOCK system and the HPA axis. Trends Endocrinol Metab. 2010;21:277–86. doi: 10.1016/j.tem.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalsbeek A, et al. SCN outputs and the hypothalamic balance of life. J Biol Rhythms. 2006;21:458–469. doi: 10.1177/0748730406293854. [DOI] [PubMed] [Google Scholar]
- 27.Kondratov RV, et al. Post-translational regulation of circadian transcriptional CLOCK(NPAS2)/BMAL1 complex by CRYPTOCHROMES. Cell Cycle. 2006;5:890–895. doi: 10.4161/cc.5.8.2684. [DOI] [PubMed] [Google Scholar]
- 28.Kiyohara YB, et al. The BMAL1 C terminus regulates the circadian transcription feedback loop. Proc Natl Acad Sci U S A. 2006;103:10074–10079. doi: 10.1073/pnas.0601416103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Padmanabhan K, et al. Feedback regulation of transcriptional termination by the mammalian circadian clock PERIOD complex. Science. 2012;337:599–602. doi: 10.1126/science.1221592. [DOI] [PubMed] [Google Scholar]
- 30.Ripperger JA, Schibler U. Rhythmic CLOCK-BMAL1 binding to multiple E-box motifs drives circadian Dbp transcription and chromatin transitions. Nat Genet. 2006;38:369–374. doi: 10.1038/ng1738. [DOI] [PubMed] [Google Scholar]
- 31.Doi M, et al. Circadian regulator CLOCK is a histone acetyltransferase. Cell. 2006;125:497–508. doi: 10.1016/j.cell.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 32.Han DH, et al. Modulation of glucocorticoid receptor induction properties by core circadian clock proteins. Mol Cell Endocrinol. 2014;383:170–180. doi: 10.1016/j.mce.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 33.Charmandari E, et al. Peripheral CLOCK Regulates Target-Tissue Glucocorticoid Receptor Transcriptional Activity in a Circadian Fashion in Man. PLoS ONE. 2011;6:e25612. doi: 10.1371/journal.pone.0025612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kino T, Chrousos GP. Circadian CLOCK-mediated regulation of target-tissue sensitivity to glucocorticoids: implications for cardiometabolic diseases. Endocr Dev. 2011;20:116–26. doi: 10.1159/000321232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kino T, Chrousos GP. Acetylation-mediated epigenetic regulation of glucocorticoid receptor activity: circadian rhythm-associated alterations of glucocorticoid actions in target tissues. Mol Cell Endocrinol. 2011;336:23–30. doi: 10.1016/j.mce.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ota T, et al. Circadian clock signals in the adrenal cortex. Mol Cell Endocrinol. 2012;349:30–37. doi: 10.1016/j.mce.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 37.Kalsbeek A, et al. Circadian rhythms in the hypothalamo-pituitary-adrenal (HPA) axis. Mol Cell Endocrinol. 2012;349:20–29. doi: 10.1016/j.mce.2011.06.042. [DOI] [PubMed] [Google Scholar]
- 38.Barclay JL, Tsang AH, Oster H. Interaction of central and peripheral clocks in physiological regulation. Prog Brain Res. 2012;199:163–181. doi: 10.1016/B978-0-444-59427-3.00030-7. [DOI] [PubMed] [Google Scholar]
- 39.Son GH, Chung S, Kim K. The adrenal peripheral clock: Glucocorticoid and the circadian timing system. Front Neuroendocrinol. 2011;32:451–465. doi: 10.1016/j.yfrne.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 40.Dickmeis T, Foulkes NS. Glucocorticoids and circadian clock control of cell proliferation: At the interface between three dynamic systems. Mol Cell Endocrinol. 2011;331:11–22. doi: 10.1016/j.mce.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 41.Dickmeis T. Glucocorticoids and the circadian clock. J Endocrinol. 2009;200:3–22. doi: 10.1677/JOE-08-0415. [DOI] [PubMed] [Google Scholar]
- 42.Ulrich-Lai YM, et al. Adrenal splanchnic innervations contributes to the diurnal rhythm of plasma corticosterone in rats by modulating adrenal sensitivity to ACTH. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1128–R1135. doi: 10.1152/ajpregu.00042.2003. [DOI] [PubMed] [Google Scholar]
- 43.Ishida A, et al. Light activates the adrenal gland: timing of gene expression and glucocorticoid release. Cell Metab. 2005;2:297–307. doi: 10.1016/j.cmet.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 44.Oster H, et al. The circadian rhythm of glucocorticoids is regulated by a gating mechanism residing in the adrenal cortical clock. Cell Metab. 2006;4:163–173. doi: 10.1016/j.cmet.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 45.Son GH, et al. Adrenal peripheral clock controls the autonomous circadian rhythm of glucocorticoid by causing rhythmic steroid production. Proc Natl Acad Sci U S A. 2008;105:20970–20975. doi: 10.1073/pnas.0806962106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang X, et al. Nuclear Receptor Expression Links the Circadian Clock to Metabolism. Cell. 2006;126:801–810. doi: 10.1016/j.cell.2006.06.050. [DOI] [PubMed] [Google Scholar]
- 47.Lamia KA, et al. Cryptochromes mediate rhythmic repression of the glucocorticoid receptor. Nature. 2011;480:552–556. doi: 10.1038/nature10700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Balsalobre A, et al. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289:2344–2347. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- 49.Yamamoto T, et al. Acute physical stress elevates mouse period1 mRNA expression in mouse peripheral tissues via a glucocorticoid-responsive element. J Biol Chem. 2005;280:42036–42043. doi: 10.1074/jbc.M509600200. [DOI] [PubMed] [Google Scholar]
- 50.So AY, et al. Glucocorticoid regulation of the circadian clock modulates glucose homeostasis. Proc Natl Acad Sci USA. 2009;106:17582–17587. doi: 10.1073/pnas.0909733106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Surjit M, et al. Widespread negative response elements mediate direct repression by agonist-liganded glucocorticoid receptor. Cell. 2011;145:224–241. doi: 10.1016/j.cell.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 52.Torra IP, et al. Circadian and glucocorticoid regulation of Reverbalpha expression in liver. Endocrinology. 2000;141:3799–3806. doi: 10.1210/endo.141.10.7708. [DOI] [PubMed] [Google Scholar]
- 53.Damiola F, et al. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Le Minh N, et al. Glucocorticoid hormones inhibit food-induced phase-shifting of peripheral circadian oscillators. EMBO J. 2001;20:7128–7136. doi: 10.1093/emboj/20.24.7128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rudic RD, et al. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2004;2:e377. doi: 10.1371/journal.pbio.0020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Turek FW, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sookoian S, et al. Genetic variants of Clock transcription factor are associated with individual susceptibility to obesity. Am J Clin Nutr. 2008;87:1606–1615. doi: 10.1093/ajcn/87.6.1606. [DOI] [PubMed] [Google Scholar]
- 58.Scott EM, et al. Association between polymorphisms in the Clock gene, obesity and the metabolic syndrome in man. Int J Obes (Lond) 2008;32:658–662. doi: 10.1038/sj.ijo.0803778. [DOI] [PubMed] [Google Scholar]
- 59.Kino T, Chrousos GP. Glucocorticoid effect on gene expression. In: Steckler T, et al., editors. Handbook on Stress and the Brain. Elsevier BV; 2005. pp. 295–312. [Google Scholar]
- 60.Chrousos GP. Glucocorticoid therapy. In: Felig P, Frohman LA, editors. Endocrinology and Metabolism. 4. McGraw-Hill; 2001. pp. 609–632. [Google Scholar]
- 61.Fujino Y, et al. A prospective cohort study of shift work and risk of ischemic heart disease in Japanese male workers. Am J Epidemiol. 2006;164:128–135. doi: 10.1093/aje/kwj185. [DOI] [PubMed] [Google Scholar]
- 62.Sookoian S, et al. Effects of rotating shift work on biomarkers of metabolic syndrome and inflammation. J Intern Med. 2007;261:285–292. doi: 10.1111/j.1365-2796.2007.01766.x. [DOI] [PubMed] [Google Scholar]
- 63.Ekstrand K, et al. Cardiovascular risk factors in commercial flight aircrew officers compared with those in the general population. Angiology. 1996;47:1089–1094. doi: 10.1177/000331979604701109. [DOI] [PubMed] [Google Scholar]
- 64.Scheer FA, et al. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA. 2009;106:4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Straub RH, Cutolo M. Circadian rhythms in rheumatoid arthritis: implications for pathophysiology and therapeutic management. Arthritis Rheum. 2007;56:399–408. doi: 10.1002/art.22368. [DOI] [PubMed] [Google Scholar]
- 66.Spies CM, et al. More night than day--circadian rhythms in polymyalgia rheumatica and ankylosing spondylitis. J Rheumatol. 2010;37:894–9. doi: 10.3899/jrheum.091283. [DOI] [PubMed] [Google Scholar]
- 67.Crofford LJ, et al. Circadian relationships between interleukin (IL)-6 and hypothalamicpituitary- adrenal axis hormones: failure of IL-6 to cause sustained hypercortisolism in patients with early untreated rheumatoid arthritis. J Clin Endocrinol Metab. 1997;82:1279–83. doi: 10.1210/jcem.82.4.3852. [DOI] [PubMed] [Google Scholar]
- 68.Mohren DC, et al. Prevalence of common infections among employees in different work schedules. J Occup Environ Med. 2002;44:1003–1011. doi: 10.1097/00043764-200211000-00005. [DOI] [PubMed] [Google Scholar]
- 69.Hedstrom AK, et al. Shift work at young age is associated with increased risk for multiple sclerosis. Ann Neurol. 2011;70:733–741. doi: 10.1002/ana.22597. [DOI] [PubMed] [Google Scholar]
- 70.Magrini A, et al. Shift work and autoimmune thyroid disorders. Int J Immunopathol Pharmacol. 2006;19:31–36. [PubMed] [Google Scholar]
- 71.Buttgereit F, et al. Efficacy of modified-release versus standard prednisone to reduce duration of morning stiffness of the joints in rheumatoid arthritis (CAPRA-1): a double-blind, randomized controlled trial. Lancet. 2008;371:205–14. doi: 10.1016/S0140-6736(08)60132-4. [DOI] [PubMed] [Google Scholar]
- 72.Arvidson NG, et al. The timing of glucocorticoid administration in rheumatoid arthritis. Ann Rheum Dis. 1997;56:27–31. doi: 10.1136/ard.56.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kino T, Charmandari E, Chrousos GP. Glucocorticoid receptor: implications for rheumatic diseases. Clin Exp Rheumatol. 2011;29:S32–41. [PMC free article] [PubMed] [Google Scholar]
- 74.Foster RG, Wulff K. The rhythm of rest and excess. Nat Rev Neurosci. 2005;6:407–414. doi: 10.1038/nrn1670. [DOI] [PubMed] [Google Scholar]
- 75.LeGates T, et al. Aberrant light directly impairs mood and learning through melanopsin-expressing neurons. Nature. 2012;491:594–598. doi: 10.1038/nature11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li X, et al. SIRT1 deacetylates and positively regulates the nuclear receptor LXR. Mol Cell. 2007;28:91–106. doi: 10.1016/j.molcel.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 77.Amat R, et al. SIRT1 is involved in glucocorticoid-mediated control of uncoupling protein-3 gene transcription. J Biol Chem. 2007;282:34066–34076. doi: 10.1074/jbc.M707114200. [DOI] [PubMed] [Google Scholar]
- 78.Ito K, et al. Histone deacetylase 2-mediated deacetylation of the glucocorticoid receptor enables NF-kappaB suppression. J Exp Med. 2006;203:7–13. doi: 10.1084/jem.20050466. [DOI] [PMC free article] [PubMed] [Google Scholar]