Abstract
Aim
This study evaluates the acute toxicity outcome in patients treated with RapidArc for localized prostate cancer.
Background
Modern technologies allow the delivery of high doses to the prostate while lowering the dose to the neighbouring organs at risk. Whether this dosimetric advantage translates into clinical benefit is not well known.
Materials and methods
Between December 2009 and May 2012, 45 patients with primary prostate adenocarcinoma were treated using RapidArc. All patients received 1.8 Gy per fraction, the median dose to the prostate gland, seminal vesicles, pelvic lymph nodes and surgical bed was 80 Gy (range, 77.4–81 Gy), 50.4 Gy, 50.4 Gy and 77.4 Gy (range, 75.6–79.2 Gy), respectively.
Results
The time between the last session and the last treatment follow up was a median of 10 months (range, 3–24 months). The incidence of grade 3 acute gastrointestinal (GI) and genitourinary (GU) toxicity was 2.2% and 15.5%, respectively. Grade 2 acute GI and GU toxicity occurred in 30% and 27% of patients, respectively. No grade 4 acute GI and GU toxicity were observed. Older patients (>median) or patients with V60 higher than 35% had significantly higher rates of grade ≥2 acute GI toxicity compared with the younger ones.
Conclusions
RapidArc in the treatment of localized prostate cancer is tolerated well with no Grade >3 GI and GU toxicities. Older patients or patients with higher V60 had significantly higher rates of grade ≥2 acute GI toxicity. Further research is necessary to assess definitive late toxicity and tumour control outcome.
Keywords: Prostate cancer, RapidArc, Toxicity, Arc therapy
1. Background
Prostate Cancer is one of the most frequent tumours in men around the world. In the United States of America, prostate cancer is the number one non coetaneous cancer in men, and it is the second most common in Europe.1 The American Cancer Society estimates that in 2013 there will be 238,590 new cases diagnosed of prostate cancer in the United States and 29,720 men will die for it.2
External beam radiotherapy (EBRT) is a standard treatment modality for localized and locally advanced prostate cancer.3,4 The practice of primary EBRT for prostate cancer has changed dramatically over the past years. Modern technologies allow the delivery of high doses to the prostate while lowering the dose to the neighbouring organs at risk.5,6 Escalation of the radiation dose beyond 70 Gy has improved biochemical control in low, intermediate and high risk patients, but the rates of rectal toxicity also increased. Volumetric modulated arc therapy using RapidArc is a novel modality of radiotherapy delivery that allows the radiation dose to be delivered during gantry rotation. This technology improves dose conformity while significantly shortening treatment time; it delivers treatments two to eight times faster than other treatments. It has been made possible by a treatment planning algorithm that simultaneously changes three parameters during treatment: rotation speed of the gantry, shape of the treatment aperture using the movement of multileaf collimator leaves in both directions and delivery dose rate.7
However, even with IMRT, up to 50% of the patients treated with doses >70 Gy experience bladder of bowel symptoms during treatment.8 Clinical variables such as any pretreatment symptoms, androgen suppression, and prior transurethral resection of the prostate appeared to be important prognostic factors for radiation induced acute genitourinary (GU) and gastrointestinal (GI) toxicity.5 The use of modern radiation technology is needed to avoid excessive toxicity technology is needed to avoid excessive toxicity with higher doses, as has been shown in randomized trials.9
2. Aim
The purpose of this study was to evaluate the acute toxicity outcome in patients treated with RapidArc for localized prostate cancer, with the hypothesis that using RapidArc it is possible to reach local control by giving a standard dose to the target volume without increasing the risk of injury or toxicity in the organs at risk in patients with localized disease.
3. Materials and methods
3.1. Selection criteria
Between December 2009 and May 2012, 45 patients were treated for primary prostate cancer. Inclusion criteria were primary diagnosis of adenocarcinoma of the prostate (T1c-T4)10 and no prior history of radiotherapy. All patients were stratified by risk groups, based upon the current National Comprehensive Centre Network prognostic risk groupings, which include the low risk, intermediate risk and high risk.11 Pretreatment evaluation consisted of documented history and physical examination, including performance status, digital rectal examination and serum prostate specific antigen (PSA) values performed. Base line patient characteristics are shown in Table 1.
Table 1.
Patient characteristics.
| Characteristic | No. of patients (%) |
|---|---|
| Age (years) | |
| Median (range) | 67 (43–81) |
| Gleason | |
| <7 | 2 (4) |
| 7 | 21 (47) |
| >7 | 12 (48) |
| T stage | |
| T1 | 10 (22) |
| T2 | 16 (36) |
| T3 | 18 (40) |
| T4 | 1 (2) |
| PSA (ng/ml) | |
| <10 | 27 (60) |
| 10–19 | 9 (20) |
| >20 | 9 (20) |
| Risk | |
| Low | 2 (4) |
| Intermediate | 11 (24) |
| High | 32 (72) |
| Androgen deprivation | |
| No | 12 (48) |
| Yes | 33 (52) |
| Radiation dose (Gy) | |
| Median (range) | 80 (77.4–81) |
| PLNs irradiation | |
| No | 3 (7) |
| Yes | 42 (93) |
| Prostate planning tumour volume (cc) | |
| Median (range) | 95 (27–245) |
3.2. Treatment
All patients were treated using a Clinac iX, equipped with Millenium Multileaf Colimator (MLC120), On Board Imager, and RapidArc capabilities. Patients were immobilized in the supine position with the same immobilization device combifix and instructions were given regarding daily preparation, full bladder (instructed to drink a glass of water 30 min before treatment). The planning target volume (PTV) was defined by 5–10 mm margin from the prostate or surgical bed.
All the treatment plans consisted in two complete arcs, with 177 control points each. Optimization for the PTV, bladder, rectum femoral heads, penile bulb and bowels was done using Eclipse V8.6 (Varian Medical Systems) optimizator and the Analytical Anisotropic Algorithm (AAA) for dose calculation. Dose constraints used in the plan prescription are shown in Table 2. All treatment plans were verified by quality assurance process before treating the patient, using the gamma analysis criteria (DD = 3%, DTA = 2 mm, (<1) 94%). The average dose volume histograms for the PTV, bladder and rectum can been seen in Figs. 1–2.
Table 2.
Dose constraints used in the plan prescription.
| Organ | Dose constraint | |
|---|---|---|
| Small bowel (individual loops) | V15 | <120cc |
| Small bowel (peritoneal cavity) | V45 | <195cc |
| Rectum | V50 | <50% |
| Rectum | V60 | <35% |
| Rectum | V65 | <25% |
| Rectum | V70 | <20% |
| Rectum | V75 | <15% |
| Bladder | V60 | <50% |
| Bladder | V70 | <35% |
| Bladder | V75 | <25% |
| Bladder | V80 | <15% |
Fig. 1.
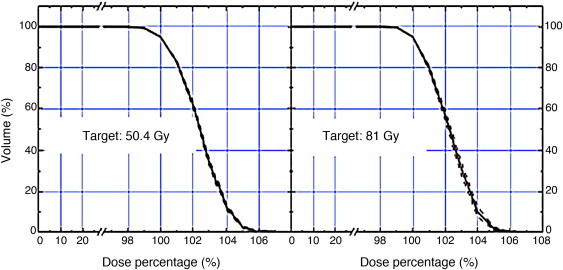
An example of a dose volume histogram for the pelvic lymph nodes (50.4 Gy) and the planning tumour (81 Gy) irradiation.
Fig. 2.
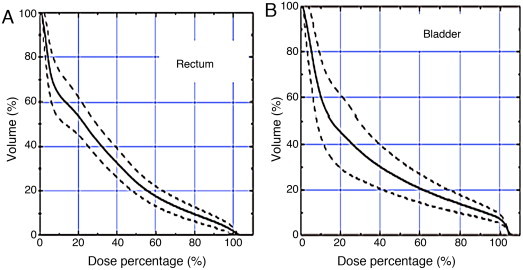
An example of a dose volume histogram for the (A) rectum and the (B) bladder.
Fifteen patients received treatment to the surgical bed. All patients received 1.8 Gy per fraction, the median dose to the prostate gland, seminal vesicles, pelvic lymph nodes and surgical bed was 80 Gy (range, 77.4–81 Gy), 50.4 Gy, 50.4 Gy and 77.4 Gy (range, 75.6–79.2 Gy), respectively. The prescribed dose covered at least 95% of the PTV and the common organ at risk dose constraints were used.12
3.3. Patient care
All patients during the course of radiotherapy were seen every Monday (weekly) and more often if needed for clinical evaluation and disease management. After completion of the therapy, we evaluated every patient at approximately two weeks and then every 3 months. The evaluations consisted of a history and physical examination. Serum PSA values were performed every three months for the first year after treatment, every 4 months for the second year, and every 6 months for the following years. Any additional studies were obtained at the discretion of the treating physician.
3.4. Statistical methods
All data analyses were done using the SPSS (version 19.0) statistical software. The primary endpoint was the occurrence of any grade ≥2 acute GU and GI toxicity within 3 months of RT, scored using the Radiation Therapy Oncology Group (RTOG) scoring system.13 Secondary endpoints were biochemical failure-free survival defined by the American Society for Therapeutic Radiology and Oncology (ASTRO) and the Phoenix (nadir + 2) definition, including any clinical failure defined as local, regional, or distant relapse. Potential risk factors for toxicity were assessed in a univariate logistic regression analysis. Because of the possible confounding effect of clinical factors on toxicity, associations found to be significant in the univariate analysis were adjusted by patient (age), tumour (diagnosis PSA, gleason, T stage, N stage, risk, planning tumour volume [PTV]),10 treatment (indication [definitive, adjuvant, salvage], treatment time, radiation dose, pelvic lymph nodes treatment), and dosimetric factors. Multivariate Cox regression was performed to adjust for factors significant on univariate analysis, as well as any other factors that might have confounded the univariate analysis. Quantitative variables were evaluated by using the median as the cut-off.
4. Results
The median age at diagnosis was 65 (range, 43–81 years) and the median follow up was 10 months (range, 0–24 months). Four patients had biochemical recurrence, two cases with bone metastasis confirmed by images and two cases unknown.
4.1. Toxicity data
The incidence of grade 3 acute gastrointestinal and genitourinary toxicity was 2% (n = 1) and 15% (n = 7), respectively. Grade 1 and 2 acute gastrointestinal toxicity occurred in 31.1% (n = 15) and 30% (n = 14), respectively. Grade 1 and 2 acute genitourinary toxicity occurred in 18 and 12 patients, respectively. No grade 4 acute GI and GU toxicity were observed (Table 3).
Table 3.
Acute complications for all patients according Radiation Therapy Oncology Group scale.
| Acute genitourinary toxicity |
Acute gastrointestinal toxicity |
|
|---|---|---|
| No patients (%) | No patients (%) | |
| Grade 0 | 15 (33) | 8 (17) |
| Grade 1 | 15 (33) | 18 (40) |
| Grade 2 | 14 (31) | 12 (27) |
| Grade 3 | 1 (2) | 7 (15) |
4.2. Prognostic factors
Several factors including dosimetric parameters were evaluated as predictors of acute toxicity in a univariate analysis (see statistical subsection). In the multivariate analysis, older patients (>median) had significantly higher rates of grade ≥2 acute GI toxicity compared with the younger ones (P = 0.03). In addition, the rectum V60 showed a significant association with grade ≥2 acute GI toxicity in the univariate and multivariate analyses (P = 0.02 and P = 0.02, respectively; Table 4). Patients treated with a higher rectum V60 (>median) had significantly higher rates of grade ≥2 acute GI toxicity compared with those treated with a lower rectum V60. Median V60 was 12% (range, 2.4–38). No other factor was independently associated with acute GI and GU toxicity.
Table 4.
Multivariate analysis of factors significantly associated with ≥2 grade 2 acute gastrointestinal toxicity.
| Variable | Acute gastrointestinal toxicity grade ≤2 |
||
|---|---|---|---|
| OR | 95% CI | P value | |
| Age (years) | |||
| ≤median | 1.0 | ||
| ≥median | 4.2 | 1.1–15.4 | 0.03 |
| Rectum V60 (%) | |||
| ≤median | 1.0 | ||
| ≥median | 4.9 | 1.3–19.1 | 0.02 |
5. Discussion
This is the first study in Guatemala that reports toxicity of prostate cancer using a modern RT technology (RapidArc) for treatment. The purpose of this study was to evaluate the toxicity outcome in patients treated with RapidArc for localized prostate cancer, with the hypothesis that using RapidArc it is possible to reach local control by giving a standard dose to the target volume without increasing the risk of injury or toxicity in the organs at risk in patients with localized disease. In our study, older patients (>median) had significantly higher rates of grade ≥2 acute GI toxicity compared with the younger ones (P = 0.003). In addition, the rectum V60 showed a significant association with grade ≥2 acute GI toxicity (P = 0.02). No other factor was independently associated with acute GU or GU toxicity.
Most recent studies have reported that patients treated with RapidArc less frequently experienced acute toxicities and trended towards less GU toxicities as compared with patients treated with other techniques. Decreased acute toxicity for patients treated with modulated RT is likely a function of both enhanced daily imaging technology and visceral wall sparing.14–18
Aizer et al. reported that patients with larger prostates (>50cc) were more likely to develop genitourinary toxicity, an association that remained significant after multivariate analysis (P = 0.006).19 Patients with larger prostates should therefore be aware about the increased risk of incurring such toxicity when undergoing External Beam Radiotherapy for prostate cancer, particularly if other studies confirm the relationship among prostate size, bladder dose, and acute genitourinary toxicity.20 Another important fact not taken into consideration was bladder filling. Jain et al. reported that bladder filling appeared to be a dominant factor which predicted for acute toxicity following the use of IMRT.21
Mark et al. reported that men treated with RT to an intact prostate had significant grade ≥2 acute toxicity in contrast to men treated with post-prostatectomy RT to similar dose with a greater percentage of bladder in-field due to surgical repositioning, but there was no difference in late toxicity.22 These findings suggest that acute urinary toxicity may be due to RT-related inflammation of the prostate and prostatic urethra, and not RT injury to the bladder. Despite having modern technology, other factors may influence patient response to therapy.
We acknowledge several limitations of our study. First, the study is a retrospective single-centre experience of a small and heterogeneous (i.e. definitive vs. adjuvant treatment) group of prostate patients who had been treated with RapidArc, rather than being treated prospectively on a well-defined treatment protocol; the follow-up is still short, hence the long-term efficacy and toxicity are yet to be determined. Second, we recognize that factors such as the radiation dose as well as the percentage of bladder/rectum irradiated may have influenced the onset of acute toxicity.
We conclude that, although the follow-up time is relatively short, clinical outcome of Rapid in prostate cancer reducing rectum and bladder dose have so far been successful. GI and GU toxicities were tolerable without any grade >3 side effect. In addition, older patients or patients with higher V60 had significantly higher rates of grade ≥2 acute GI toxicity. Further research is necessary to assess definitive late toxicity and tumour control outcome.
Conflict of interest
None declared.
Financial disclosure
None declared.
References
- 1.Bray F., Sankila R., Ferlay J., Parkin D.M. Estimates of cancer incidence and mortality in Europe in 1995. Eur J Cancer. 2002;38:99–166. doi: 10.1016/s0959-8049(01)00350-1. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A., Siegel R., Ward E. Cancer statistics. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Teh B.S., Mai W.Y., Uhl B.M. Intensity-Modulated Radiation Therapy (IMRT) for prostate cancer with the use of a rectal balloon for prostate immobilization: acute toxicity and dose volume analysis. Int J Radiat Oncol Biol Phys. 2001;49:705–712. doi: 10.1016/s0360-3016(00)01428-0. [DOI] [PubMed] [Google Scholar]
- 4.De Langhe S., De Ruyck K., Ost P. Acute radiation-induced nocturia in prostate cancer patients is associated with pretreatment symptoms, radical prostatectomy, and genetic markers in the TGFBI gene. Int L Radiat Oncol Biol. 2012;85:393–399. doi: 10.1016/j.ijrobp.2012.02.061. [DOI] [PubMed] [Google Scholar]
- 5.Lopez Guerra J.L., Isa N., Matute R. Hypofractionated helical tomotherapy using 2.5–2.6 Gy daily fractions for localized prostate cancer. Clin Transl Oncol. 2013;15:271–277. doi: 10.1007/s12094-012-0907-y. [DOI] [PubMed] [Google Scholar]
- 6.Lopez Guerra J.L., Isa N., Kim M.M., Bourgier C., Marsiglia H. New perspectives in radiation oncology: young radiation oncologist point of view and challenges. Rep Pract Oncol Radiother. 2012;17:251–254. doi: 10.1016/j.rpor.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dominello M., Ku K., Chen G. Volumentric modulated arc therapy decreases incidence of acute rectal toxicity in the treatment of low and intermediate risk prostate cancer. Int J Radiat Oncol Biol Phys. 2012;84:S365. [Google Scholar]
- 8.Lips I.M., Dehnad H., van Gils C.H., Boeken Kruger A.E., van der Heide U.A., van Vulpen M. High-dose intensity modulated radiotherapy for prostate cancer using daily fiducial markers-based position verification: acute and late toxicity in 331 patients. Radiat Oncol. 2008;3:15. doi: 10.1186/1748-717X-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dearnaley D.P., Khoo V.S., Norman A.R. Comparison of radiation side effects of conformal and conventional radiotherapy in prostate cancer: a randomized trial. Lancet. 1999;353:267–272. doi: 10.1016/S0140-6736(98)05180-0. [DOI] [PubMed] [Google Scholar]
- 10.American Joint Committee on Cancer. http://www.cancerstaging.org/.
- 11.NCCN Clinical Practice Guidelines in Oncology. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- 12.Marks L.B., Yorke E.D., Jackson A. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys. 2010;76:S10–S19. doi: 10.1016/j.ijrobp.2009.07.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cox. J.D., Stetz J., Pajak T.F. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research ant Treatment of Cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31:1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 14.Otto K. Volumetric modulated arc therapy: IMRT in a single gantry arc. Med Phys. 2008;35:310–317. doi: 10.1118/1.2818738. [DOI] [PubMed] [Google Scholar]
- 15.Palma D., Vollans E., James K. Volumetric modulated arc therapy for delivery of prostate radiotherapy: comparison with intensity-modulated radiotherapy and three-dimensional condormal radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72:996–1001. doi: 10.1016/j.ijrobp.2008.02.047. [DOI] [PubMed] [Google Scholar]
- 16.Pinkawa M., Fischedick K., Asadpuor B. Toxicity prole with a large prostate volume after external beam radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:83–89. doi: 10.1016/j.ijrobp.2007.05.051. [DOI] [PubMed] [Google Scholar]
- 17.Cuthbert D., Catton C., Lindsay P., Jiang H., Craig T. Dose conformality and acute toxicity analysis in patients with prostate adenocarcinoma treated with Volumetric Modulated Arc Therapy (VMAT) versus conventional Intensity Modulated Radiation Therapy (IMRT) Int J Radiat Oncol Biol Phys. 2012;84:S401. [Google Scholar]
- 18.Iyengar P., Levy L.B., Choi S., Lee A.K., Kuban D.A. Toxicity associated with postoperative radiation therapy for prostate cancer. Am J Clin Oncol. 2011;34:611–618. doi: 10.1097/COC.0b013e3181f946dc. [DOI] [PubMed] [Google Scholar]
- 19.Aizer A.A., Anderson N.S., Oh S.C. The impact of pretreatment prostate volume on severe acute genitourinary toxicity in prostate cancer patients treated with intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2011;79:379–384. doi: 10.1016/j.ijrobp.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 20.Doyle L.A., Studenski M., Harvey A. Dosimetric comparison of VMAT, IMRT and proton therapy for post-prostatectomy radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2010;78:S806. [Google Scholar]
- 21.Jain S., Loblaw D.A., Morton G.C. The effect of radiation thechnique in bladder filling on the acute toxicity of pelvic radiotherapy for localized high risk prostate cancer. Radiother Oncol. 2012;105:193–197. doi: 10.1016/j.radonc.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 22.Mark R.H., Hunt D., Shipley W.U. Acute and late urinary toxicity after radiation therapy in men with or without an intact prostate gland: a secondary analysis of RTOG 9408 and 9601 suggesting this toxicity in not due to bladder injury. Int J Radiat Oncol Biol. 2012;84:725–732. [Google Scholar]


