Abstract
Aim
To assess the real contribution of modern radiation therapy (RT) technology in the more common tumoral types in Central America, Caribbean and South America.
Background
RT is an essential tool in the management of cancer. RT can be either palliative or of curative intent. In general, for palliative radiotherapy, major technologies are not needed.
Materials and methods
We analyzed the contribution of RT technology based on published evidence for breast, lung, gastric, gallbladder, colorectal, prostate and cervix cancer in terms of disease control, survival or toxicity with especial focus on Latin America.
Results
Findings indicate that three dimensional conformal radiation therapy (3D RT) is the gold standard in most common type of cancer in the studied regions. Prostate cancer is probably the pathology that has more benefits when using new RT technology such as intensity modulated radiation therapy (IMRT) versus 3DRT in terms of toxicity and biochemical progression-free survival.
Conclusions
In light of the changes in technology, the ever-increasing access of developing countries to such technology, and its current coverage in Latin America, any efforts in this area should be aimed at improving the quality of the radiotherapy departments and centers that are already in place.
Keywords: Radiation therapy, Technology, Oncology
1. Background
The radiotherapy (RT) is an essential tool in the handling of cancer. From the discovery of the X-rays, the RT has evolved very much as for the comprehension of his functioning, justification and form of use, generating articles of high quality investigation. During the natural history of the cancer, about 50% of the patients will need RT during the course of his illness, this needs changes in percentage, depending on the tumor type that has the patient, his stage and form of presentation and of the population profile in which we are.1
If we analyze the region of Central America, Caribbean and South America, the cancer is the second cause of death,2 having 75% of the countries programs of alertness of cancer. However, only 25% have programs of screening of cervix cancer.2 In the zone of the Caribbean there is one radiotherapist and 1.4 machines of RT every 1.6 million persons versus 9 radiotherapists and 6.4 machines every 1.6 million persons in the developed countries.2 In 2008 in the region of Central America, Caribbean and South America there was an incidence of cancer of 1,011,000 of cases (4,89,000 men versus 5,22,000 women) and a mortality of 5,89,000 cases (3,02,000 men versus 2,87,000 women).2 The most common cancer incidences in men, in decreasing order, are prostate, lung, gastric and colorectal, being the higher mortality for lung cancer, followed by prostate, gastric and colorectal (Fig. 1). The most common incidences in women, in decreasing order, are breast, cervix, colorectal, gastric and lung, being the most higher mortality for breast cancer, continued of cervix, gastric, lung and colorectal cancer (Fig. 1).
Fig. 1.
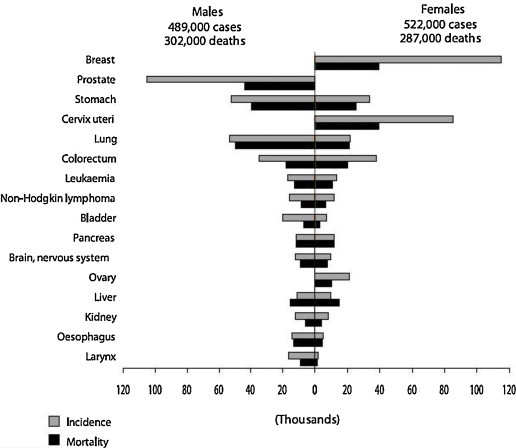
Incidence and mortality of cancer in Southern America.
For the particular case of Chile,3 the most common cancer incidences in men, in decreasing order, are prostate, gastric, lung, colon and gallbladder, being the higher mortality for gastric cancer, followed by prostate, lung, gallbladder and colon (Table 1). The most common incidences in women, in decreasing order, are breast cancer, gallbladder, cervix, gastric, colon and lung, being the higher mortality for gallbladder cancer, followed by breast, gastric, lung, cervix and colon (Table 1).
Table 1.
Estimated mortality from cancer in different locations in men and women (Chile 2003–2007).
| Men |
Women |
||||||
|---|---|---|---|---|---|---|---|
| Location | No. of cases/year | CI 95% | No. of deaths/year | Location | No. of cases/year | CI 95% | No. of deaths/year |
| Prostate | 4098 | 3919–4284 | 1538 | Breast | 3791 | 3587–4017 | 1128 |
| Gastric | 2388 | 2245–2520 | 2062 | Gallbladder | 1531 | 1417–1647 | 1344 |
| Lung | 1373 | 1276–1467 | 1475 | Cervix uteri | 1279 | 1167–1384 | 667 |
| Colon | 748 | 652–837 | 458 | Gastric | 1173 | 1072–1273 | 1025 |
| Gallbladder | 599 | 527–664 | 504 | Colon | 877 | 782–968 | 586 |
| Lung | 815 | 732–899 | 817 | ||||
RT can be either palliative or of curative intent. In general, for palliative radiotherapy, major technologies are not needed. The objective of this review is to assess the evidence-based contribution of the technology in the most common type of cancers in the region of Central America, Caribbean and South America, with emphasis on the Chilean population.
2. Evidence based radiation oncology according to location
2.1. Breast cancer
The RT has a clear roll in the handling of in situ and infiltrative breast cancer.4
We know that the RT in the ductal in situ carcinoma, on having been added to the conservative surgery, diminishes the ipsilateral recurrence, with a benefit of 15.2% to 10 years (p < 0.00001),5 without a benefit in the overall survival or some increase of mortality for cardiac and pulmonary toxicity to 10 years (p = NS).5 For infiltrative breast carcinoma, the RT, on having been added to the conservative surgery, gives a benefit in overall survival to the specific deaths caused by breast cancer of 3.8% to 15 years (p = 0.0001).6 On having been added to the entire Mastectomy, in infiltrative breast carcinoma, in patients N (−), it gives a detriment in overall survival to the specific deaths caused by breast cancer of 3.6% to 15 years (p = 0.01) and an increase in the incidence of counter side cancers of breast in a 1.8% to 15 years (p = 0.002).7 But in patients N (+), the RT post entire mastectomy, in infiltrative breast cancer, gives a benefit in overall survival to the specific deaths caused by breast cancer of 5.4% to 15 years (p = 0.0002).7
The problem is that the RT in infiltrative breast cancer, produces an increase in the mortality in the deaths not related to breast cancer of 0.5% and 1.3% to 10 and 15 years respectively (p = 0.001),7 this attributed largely to the cardiac toxicity, which is major of the left side, but bearing in mind that this information collaborates to RT of ancient skill (Orthovoltaje, 2D), it reaches port significantly with major mortality to 10 and 15 years after the RT in the period of 1973–1982,8 then without major cardiac toxicity in the period of 1983–1992 and 1993–2001, which is when8 becomes massive the skill of 3D RT. Knowing that the new technologies as the IMRT do not contribute a benefit in survival in these patients, perhaps this roll this one in diminishing the toxicity. If we know that to homogenize the dose we have wedges, compensating skin, intensity modulated of forward planning or intensity modulated of inverse planning. But, more than the homogeneity of the dose, what more it is going to influence the late toxicity the mammary tissue it is going to be the size of the breast, the cosmetics pre RT, post operative infections and use of tobacco, which are independent factors to the technology.
2.2. Lung cancer
The RT has his role in lung cancer as much of small cells (SCLC) as non small cells lung cancer (NSCLC). In NSCLC, the cancer presents to itself in stages I in 24%, the II in 7%, the III in 31%, the IV in 38%.9 The standard of treatment in stage I and II is the surgery, be already lobectomy or pneumonectomy with mediastinal lymph node sampling, the RT has curative intensity in this case when the patient is medically unoperable. Since we know that the post operative RT for the stages I and II gives a detriment in the survival and for more advanced stages so much combined with chemotherapy (CT) or of exclusive form it does not contribute in survival,10 but when we realize 3D RT, dose escalated, without nodal elective irradiation of mediastinum,11–14 with restriction of dose to organ healthy lung, a benefit exists in the local control and survival in these patients, with a low index of pneumonitis, turning feasible the treatment. Another treatment that has showed excellent results in stage I is the stereotactic ablative RT (SBRT), reaching a local control for 2 years of 95% in patients T1-2 treated with 3 fractions of 20–21 Gy and a survival of 66.6%.15 But for this special skill of RT15 needs a rigorous planning, dosimetry, measurement, immobilization and advanced cross-check of. The good of this skill is that there would not be difference his result versus surgery,16 which turns out to be tantalizing at the time of realizing it, but has to still be proved in a Phase III study. For the stage III of lung, the combination of Surgery and RT + CT versus RT + CT has failed in showing benefit,17,18 but the RT + CT based on Cisplatin versus the exclusive RT, has showed benefit in the events free survival of 6% and 3.5% to 2 and 6 years respectively and overall survival of 4% and 2.2% to 2 and 5 years in the randomized controlled trials and meta analyses,19–23 all of them realized with conventional technology of 3D RT, transforming in the gold standard of treatment, where there still does not remain clear the roll of the new technologies, which must be proved inside Phase III studies, where at least they must show a descent in the toxicity, who understands esophagitis, nausea, vomiting, neutropenia of 8–32%24,25 for the conventional technology. In SCLC, the RT + CT is the standard of treatment in limited illness, including elective irradiation of the mediastinum, with a survival of 20–26% to 5 years,26 which is demonstrated in the later meta analyses with conventional technology of 3D RT and CT based on Cisplatin and Etoposide.27
2.3. Gastric cancer
The RT and CT, for patients loco regionally advanced (cT3–4, N0–1, M0), based on 5FU they have adjuvant roll in the handling of gastric cancer, considered standard from the publication of the INT 0116, with a benefit in the median of overall survival for the group of surgery plus RT + CT of 36 months versus 27 months for surgery alone (p = 0.005),28 benefited that it is supported in its update with 10 years of pursuit with a benefit in the median of overall survival for the group of surgery plus RT + QT of 35 months versus 27 months for surgery alone (p = 0.0046).29 This treatment is based on a 3D RT, retrospective Chilean studies show a comparable result,30,31 without the need for a major technology.
2.4. Gallbladder cancer
The roll of the RT in this field of the oncology still remains uncertain, although there exist works32 that show that when RT is added to 3D RT to the surgery, in patients loco regionally advanced (T2–3, N0–1, M0), the median survival increases from 8 to 14 months (p < 0.001), this is verified also by other authors33,34 than when the RT is added to the surgery, a benefit exists in the overall survival (HR = 0.78, 95% IC 0.70–0.87, p < 0.01), which is major in the patients with positive lymph nodes (HR = 0.65, 95% IC 0.54–0.79, p < 0.01). Also there exists a meta analyses35 that shows benefit for the adjuvant RT+CT versus surgery alone (OR, 0.33; 95% IQ, 0.14–0.81; p = 0.01) in R1 operated Gallbladder cancer, and an uncertain benefit for the operated patients R0 with positive nodes respectively (OR, 1.26; 95%CI, 0.88–1.79; p = 0.20). In the Chilean literature, where the treatment that there considers to be standard who consists of a simple cholecystectomy for initial tumors (that they invade up to the proper muscular layer) and then extended with node dissection when the tumor invades even subserous plus 3D RT + CT based on Fluoropyrimidines, national series,36 with loco regionally advanced patients, that receive the treatment, they reach 57% of overall survival to 5 years, without major need of technology.
2.5. Colorectal cancer
The rectal cancer born under the sigmoid reflection and it spreads up to the anal channel, separating it from the colon cancer, entity to which we will not refer in this article. The RT is the standard treatment in loco regionally advanced rectal cancer (cT3–4 or cTanyN1–2, M0), it is possible to base in diverse neoadjuvant or adjuvant schemes of treatment. The neoadjuvant treatment includes pre operative RT of short course, 25 Gy in 5 fractions followed by immediate surgery, or long course, 50.4 Gy in 25–28 fractions and then surgery in 4–8 weeks. The latter scheme must always go accompanied by CT based on fluoropyrimidines. The advantage of long versus the short one is that it leaves one major chance of downsizing including major pathological complete responses, improving the resection capability, although this did not translate into a higher sphincter preservation rate and no statistically significant differences were observed in disease free survival or overall survival.37,38 Nevertheless, the scheme of short course is equivalent to the long scheme in the tumors where the downsizing is not needed or that do not have commitment of the mesorectal fascia. When compares pre operative RT + CT followed by adjuvant CT versus post operative RT + QT, it has been demonstrated that the first one significantly reduces the risk of local recurrence, it has less sharp and long-term toxicity, allows a major preservation and sphincter function in low rectal tumors.39–41 Nevertheless, does not exist benefit in distance control and overall survival of a scheme versus other.39–41
The adjuvant treatment includes RT + CT followed by adjuvant CT for patients who did not receive the pre operatively treatment,42–46 based on the long scheme of treatment of 50.4 Gy in 25–28 concomitant fractions with CT based on fluoropyrimidines. The potential advantage of this treatment versus the preoperative treatment is to be provided with a better selection of the patients based on the pathological study. The disadvantages include increase of the toxicity of small bowel or of the scar after an abdomino perineal resection (APR) and potentially major radio resistance in the field for hypoxic cells in the post surgical area. In a small study of APR randomize to early RT + CT versus late RT + QT, and the early group achieved a better disease free survival to 10 years (63% versus 40%; p = 0.043), therefore it is necessary to give early post operative RT + CT to those who had indication of pre operative RT + CT.44 All the included schemes of treatment that include RT were realized based on 3D RT. It seems that further investigation with novel technology is needed in order to know the benefit of more conformal therapies especially in terms of toxicity.
2.6. Prostate cancer
The prostate cancer, considering this one in its curative stage (T1–4, N0, M0), according to 3 risk groups of metastatic disease, these factors get together with the examination of prostate palpation, PSA levels in blood and the Gleason score of the prostate biopsy; that of low risk (T1–2a, less PSA to 10, less or equal Gleason to 6), intermediate risk (T2b–2c, PSA 10–20, equal Gleason to 7), high risk (T3a–4, Gleason 8–10, PSA > 20). The contribution of the RT is present in these 3 risk groups. For the low risk group, the curative alternatives includes radical prostatectomy, external beam RT and brachytherapy with a cancer specific survival of 100% to 10 years46,47 For the intermediate risk group, the curative alternatives include radical prostatectomy, external beam RT plus neoadjuvant hormonal treatment (LHRHa) for 3–6 months.47,48 For the high risk group, the curative alternatives include radical prostatectomy, external beam RT plus neoadjuvant hormonal treatment (LHRHa) for 3–6 months and then adjuvant for 2 years.47,48 It is recommended post operative RT, that shows benefit in terms of PSA fail and benefit in survival, in patients of high risk, and salvataje RT in patients who present biochemical failure during its PSA monitoring, with a demonstrated survival benefit, but with a significant increase of the genitourinary, intestinal and sexual complications.47,48
As for the skills of RT, the IMRT versus 3D RT significantly reduces the gastro intestinal complications.49,50 This skill would allow a surer scaling of dose, where it has been proved that this one delivers a benefit in disease free survival of biochemical and clinical progression.51–54 As for the RT dose, the doses for low risk must be to 75.6–79.2 Gy in low risk group and on 81 Gy for intermediate and high risk.49,55,56 Another sure way of dose escalation, if it is that he does not get ready of IMRT but if of high dose rate brachytherapy of valuation, it is with hypofractionated 3D RT combined with the last one, which demonstrates a benefit in the disease free survival versus hypofractionated 3D RT alone, with similar genitourinary and gastro intestinal toxicity.57
2.7. Cervix cancer
The treatment of cervix cancer according to the FIGO clinical classification,58 which depends on the stage, includes surgery, RT and RT + CT. For the stage IA only needs surgery,59–61 and external beam RT plus brachytherapy is reserved in medically unoperable patients.62 For the stages IB and IIA can be treated with surgery with lymph node dissection or external beam RT plus brachytherapy, which are the same way effective59–61 Only a randomized study compares them, its overall survival and disease free survival to 5 years it is 83% and 74% respectively, taking into account that 66% of the patients of the surgical arm, with risk factors, received adjuvant RT, what increased the severe complications 28% for the surgical arm versus 12% of the RT arm.63 For stages IIB to IVa, the RT + CT plus brachytherapy is the gold standard treatment,58–60 with a local control of 88–95% IB, 70–80% IIB, 30–40 III and a 5 years survival higher than 80% IB, 65% IIB and 40% III.64,65 The treatment of external beam RT, includes the pelvis with dose of 45 Gy, in addition to the boost to the positive lymph nodes areas or positive surgical margins. In absence of positive lymph nodes, the top of the field must be even L4–L5 or up to the aortic fork.59–61 The form of external beam RT, has not been compared in randomized studies. Retrospective reviews show a low coverage of the lymph nodes based on the bony points of the 2D RT. The IMRT does not recommend to itself of given routine the motility of the organs, for what is surer to realize with 3D RT.59–61 For the treatment of positive lymph nodes and parametrial involvement, the ideal dose of external beam RT to sterilize them, preferred by most of the clinical ones, is around 60 Gy.59–61 It is not clear that the surgical debulking or dose escalation improves the survival in these patients, since Grigsby et al.66 concluded that these patients develop more often a distance failure, indiscriminately of the dose escalation or local surgery. For the treatment of CT, in 1999 the U.S. National Cancer Institute publishes an alert that the CT based on Cisplatin, added to RT, improves the survival in patients with cervix cancer stages IIB to IVa, based on 5 Randomized trials released simultaneously.67–71 This treatment would have a HR in favor of the CT arm for the risk of recurrence of 0.54–0.74, with an increase in the disease free survival of 8% to 5 years, 9% in the loco regional disease free survival and of 7% in the metastases disease free survival, advantage that spreads inclusive for the regimens that do not contain Cisplatin.72 The world CT standard is not yet definite, but the most used regimen is Cisplatin 40 mg/m2 weekly for 5–6 weeks, reserving other regimens in cases of intolerance to the Cisplatin or renal illness.72
In addition to the external beam RT, the ideal handling includes brachytherapy with dose of 80–90 bioequivalent Gy to low dose rate brachytherapy (LDR) to the point A or high risk clinical volume,59–61 this treatment must be carried out <50–55 days of initiated not to compromise the result.73 Difference does not exist in overall survival, local control, long-term toxicity between the low dose rate (LDR) and high dose rate (HDR) brachytherapy for stages I–III.59–61 It is possible to use interstitial brachytherapy in the cases with bulky illness, anatomical distortion, vaginal extension.74,75 Probably one of the best advances to date related to technology is the administration of MRI-3D guided brachytherapy which allows for not only target volumes reduction but also improve local control and overall survival versus the historical Vienna series.76 In cases in which one does not count with brachytherapy, it is possible to give the boost with external beam RT with dose of 64–75 Gy, trying to protect to the maximum nearby organs.59
3. Discussion
We know that in the region of Central America, Caribbean and South America cancer is the second leading cause of death,2 these regions belong to the axis of the developing countries, therefore, the strategy to fight cancer in our countries, as says the World Cancer Report 20082 should be based on surveillance, primary prevention, secondary prevention, proper diagnosis and treatment, whereas in the latter surgery and the possibility of access to RT and CT, and a palliative care management, whereas only 75% of countries in the area have cancer surveillance programs, only 25% have screening programs for cervix uteri cancer and in the Caribbean area there are 1 Radiotherapist and 1.4 Radiotherapy machines every 1.6 million people versus 6.4 Radiotherapists and 9 Radiotherapy machines every 1.6 million people in developed countries.2
Based on this brief review of the evidence and the contribution of technology to the most common tumor types in Central America, Caribbean and South America with a particular emphasis on the Chilean population, the findings in terms of disease control, survival, toxicity reduction, and better accuracy of the treatments can be summarizes at follows.
In situ breast cancer simply adding 3D RT adds a benefit in reducing ipsilateral recurrences,5 in infiltrating breast cancer simply adding 3D RT after conservative surgery confers a benefit in overall survival6 and 3D RT in post mastectomy patients with positive lymph nodes gives a survival benefit in specific deaths from breast cancer.7
In non small cell lung cancer, 3D RT dose escalation in stage I and II medically inoperable without elective nodal irradiation of mediastinum shows a benefit in local control and survival in these patients,11–14 and ablative SBRT for stage I, which have not yet results being compared to surgery in a Phase III study. For stage III, 3D RT plus CT based on cisplatin versus exclusive RT has shown benefit in event-free survival and overall survival in randomized trials and meta-analyzes.19–23 Small cell Lung cancer, 3D RT and CT is the standard treatment for limited disease, including elective mediastinal irradiation,26 which is demonstrated in the subsequent meta-analysis with conventional 3D RT and CT based on cisplatin and etoposide.27
In gastric cancer, 3D RT in conjunction with CT based on 5FU for loco regionally advanced patients (cT3–4, N0–1, M0) is considered standard since publication of INT 0116, with a benefit in median overall survival for the surgery group with RT + CT versus surgery alone,28 a benefit that is held in updating with 10 years of follow up.29 Where Chilean retrospective studies show comparable results.30,31
In gallbladder cancer the role of 3D RT remains uncertain, although there are studies32 showing that when adding 3D RT to surgery in patients loco regionally advanced (T2–3, N0–1, M0) increases the median survival, this will also checked by other authors33,34 that when adding RT to surgery, there is a benefit in overall survival, higher in the presence of positive lymph nodes. There is also a meta-analysis35 showing benefit for adjuvant RT + CT versus surgery alone in gallbladder cancer operated R1, and an uncertain benefit for patients with positive lymph nodes operated R0 respectively. In Chileans studies who received extended cholecystectomy for tumors invading to the subserosa with lymph node dissection, the addition of 3D RT plus fluoropyrimidine based CT, national series achieved a 57% overall survival at 5 years.36
For rectal cancer patients loco regionally advanced (cT3–4 or o cTanyN1–2, M0) 3D RT is an essential part of the treatment of this condition, when doing so may be preoperative neoadjuvant short course, 25 Gy in 5 fractions followed by immediate surgery, or long course, 50.4 Gy in 25–28 fractions Concomitant CT and then surgery in 4–8 weeks, with the latter potentially better to reduce the risk of local recurrence and improve survival.37–39 When comparing preoperative RT + CT followed by adjuvant CT versus post operative RT + CT, we see that both schemes have the same overall survival, but greater toxicity post operative regimen.39–41
In prostate cancer, in its curative stage, the RT indication is present in the 3 risk groups. About radiation mode, IMRT versus 3D RT significantly reduces gastrointestinal complications.49,50 This technique would allow a safer dose escalation, where it has been proven that this provides a benefit in clinical and biochemical progression-free survival.51–54 As the dose of RT, for low risk prostate cancer should be 75.6–79.2 Gy in conventional fractionation and 81 Gy for intermediate and high risk.49,55,56 In the case of not having IMRT, can be escalate dose with hypo fractionated 3D RT and HDR brachytherapy, which shows a benefit in recurrence-free survival versus hypo fractionated 3D RT alone, with similar urinary and gastrointestinal toxicity.57
In cervix cancer, the handling of RT and Brachytherapy encompasses all stages, for stage IA reserves external beam RT plus brachytherapy in medically inoperable patients62 for stage IIB–IVa, 3D RT with concurrent CT plus brachytherapy is the gold standard treatment,59–61 with a 88–95% local control IB, IIB 70–80%, 30–40 III and a 5-year survival greater than 80% IB, IIB 65% and 40% III,64,65 which can be improved with the use of MRI adaptive brachytherapy combined with 3DCRT.76 As for the type of external beam RT, retrospective reviews show a lower level of coverage to the lymph nodes based on bony points of 2D RT. The IMRT routine is not recommended due to the great motility of the organs, so that is safer realize it with 3D RT.59–61
4. Conclusions
In light of the changes in technology, the ever-increasing access of developing countries to such technology, and its current coverage in Latin America, any efforts in this area should be aimed at improving the quality of the radiotherapy departments and centers that are already in place.
Conflict of interest
The author Nicolas Isa do not have any conflict of interest.
Financial disclosures
None declared.
References
- 1.Lopez Guerra J.L., Isa N., Kim M.M. New perspectives in radiation oncology: young radiation oncologist point of view and challenges. Rep Pract Oncol Radiother. 2012;17:251–254. doi: 10.1016/j.rpor.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World cancer report 2008. ISBN 978 92 832 0423 7. http://www.iarc.fr/en/publications/pdfs-online/wcr/2008/.
- 3.Primer informe de registros poblacionales de cáncer Chile. Quinquenio 2003–2007. http://epi.minsal.cl/epi/0notransmisibles/cancer/INFORME%20RPC%20CHILE%202003-2007,%20UNIDAD%20VENT,%20DEPTO.EPIDEMIOLOGIA-MINSAL,13.04.2012.pdf.
- 4.Algara M., Arenas M., De las Peñas D. Radiation techniques used in patients with breast cancer: results of a survey in Spain. Rep Pract Oncol Radiother. 2012;17:122–128. doi: 10.1016/j.rpor.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Overview of the randomized trials of radiotherapy in ductal carcinoma in situ of the breast. J Natl Cancer Inst Monogr. 2010;41:162–177. doi: 10.1093/jncimonographs/lgq039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707–1710. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–2090. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 8.Darby S.C., McGale P., Taylor C.W. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300,000 women in US SEER cancer registries. Lancet Oncol. 2005;6:557–565. doi: 10.1016/S1470-2045(05)70251-5. [DOI] [PubMed] [Google Scholar]
- 9.Fry W.A., Menck H.R., Winchester D.P. The National Cancer Data Base report on lung cancer. Cancer. 1996;77(9):1947–1955. doi: 10.1002/(SICI)1097-0142(19960501)77:9<1947::AID-CNCR27>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 10.PORT Meta-analysis Trialists Group Postoperative radiotherapy in non-small-cell lung cancer: systematic review and meta-analysis of individual patient data from nine randomised controlled trials. Lancet. 1998;352:257–263. [PubMed] [Google Scholar]
- 11.Chen M., Hayman J.A., ten Haken R.K. Long-term results of high-dose conformal radiotherapy for patients with medically inoperable T1-3N0 non-small-cell lung cancer: is low incidence of regional failure due to incidental nodal irradiation? Int J Radiat Oncol Biol Phys. 2006;64:120–126. doi: 10.1016/j.ijrobp.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 12.Hayman J.A., Martel M.K., ten Haken R.K. Dose escalation in non-smallcell lung cancer using three-dimensional conformal radiation therapy: update of a phase I trial. J Clin Oncol. 2001;19:127–136. doi: 10.1200/JCO.2001.19.1.127. [DOI] [PubMed] [Google Scholar]
- 13.Bradley J., Graham M.V., Winter K. Toxicity and outcome results of RTOG 9311, A phase I-II dose-escalation study using three-dimensional conformal radiotherapy in patients with inoperable non-smallcell lung carcinoma. Int J Radiat Oncol Biol Phys. 2005;61:318–328. doi: 10.1016/j.ijrobp.2004.06.260. [DOI] [PubMed] [Google Scholar]
- 14.Rosenzweig K.E., Fox J.L., Yorke E. Results of a phase I dose-escalation study using three-dimensional conformal radiotherapy in the treatment of inoperable nonsmall cell lung carcinoma. Cancer. 2005;103:2118–2120. doi: 10.1002/cncr.21007. [DOI] [PubMed] [Google Scholar]
- 15.Timmerman R., Paulus R., Galvin J. Toxicity analysis of RTOG 0236 using SBRT to treat medically inoperable early stage lung cancer patients. Int J Radiat Oncol Biol Phys. 2007;69:86. [Google Scholar]
- 16.Denlinger C., Bradley J.D., El Naqa I.M. Propensity matched comparison of surgery vs. stereotactic body radiation therapy in early stage lung cancer. Presented at the 89th annual meeting of the American Association for Thoracic Surgery; Boston, MA, May 9–13; 2009. [Google Scholar]
- 17.Albain K.S., Swann R.S., Rusch V.W. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet. 2009;1:379–386. doi: 10.1016/S0140-6736(09)60737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Meerbeeck J.P., Kramer G.W.P., Van Schil P.E.Y. Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIa non-small cell lung cancer. J Natl Cancer Inst. 2007;99:442–450. doi: 10.1093/jnci/djk093. [DOI] [PubMed] [Google Scholar]
- 19.Dillman R.O., Seagren S.L., Propert K.J. A randomized trial of induction chemotherapy plus high-dose radiation versus radiation alone in stage III non-small cell lung cancer. N Engl J Med. 1990;323:940–945. doi: 10.1056/NEJM199010043231403. [DOI] [PubMed] [Google Scholar]
- 20.Sause W., Kolesar P., Taylor S. Final results of phase III trial in regionally advanced unresectable non-small cell lung cancer: Radiation Therapy Oncology Group, Eastern Cooperative Oncology Group, and Southwest Oncology Group. Chest. 2000;117:358–364. doi: 10.1378/chest.117.2.358. [DOI] [PubMed] [Google Scholar]
- 21.Non-Small Cell Lung Cancer Collaborative Group Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomized trials. Brit Med J. 1995;311:899–909. [PMC free article] [PubMed] [Google Scholar]
- 22.Auperin A., Le Pechoux C., Pignon J.P. Concomitant radio-chemotherapy based on plating compounds in patients with locally advanced non-small cell lung cancer (NSCLC): A meta-analysis of individual data from 1764 patients. Ann Oncol. 2006;17:473–483. doi: 10.1093/annonc/mdj117. [DOI] [PubMed] [Google Scholar]
- 23.Le C.T., Arriagada R., Tarayre M. Significant effect of adjuvant chemotherapy on survival in locally advanced non-small-cell lung carcinoma. J Natl Cancer Inst. 1992;84:58. doi: 10.1093/jnci/84.1.58. [DOI] [PubMed] [Google Scholar]
- 24.Furuse K., Fukuoka M., Nishiyaki Y. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small cell lung cancer. J Clin Oncol. 1999;17:2692–2700. doi: 10.1200/JCO.1999.17.9.2692. [DOI] [PubMed] [Google Scholar]
- 25.Pierre F., Maurice P., Gilles R. A randomized phase III Trial of sequential chemoradiotherapy versus concurrent chemoradiotherapy in locally advanced non-small cell lung cancer (NSCLC) (GLOT-GFPC NPC95-01) Pro. Am Soc Clin Oncol. 2001;20:312A. [Google Scholar]
- 26.Turrisi A.T., Kim K., Blum R. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med. 1999;340:265–271. doi: 10.1056/NEJM199901283400403. [DOI] [PubMed] [Google Scholar]
- 27.De Ruysscher D., Pijls-Johannesma M., Bentzen S. Time between the first day of chemotherapy and the last day of chest radiation is the most important predictor of survival in limited-disease small cell lung cancer. J Clin Oncol. 2006;24:1057–1060. doi: 10.1200/JCO.2005.02.9793. [DOI] [PubMed] [Google Scholar]
- 28.Macdonald J.S., Smalley S.R., Benedetti J. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–730. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 29.Smalley S.R., Benedetti J.K., Haller D.G. Updated analysis of SWOG-directed intergroup study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J Clin Oncol. 2012;30(19):2327–2333. doi: 10.1200/JCO.2011.36.7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baeza M., Gianinni O., Rivera R. Adjuvant radiochemotherapy in the treatment of completely resected, locally advanced gastric cancer. Int J Radiat Oncol Biol Phys. 2001;50(3):645–650. doi: 10.1016/s0360-3016(01)01467-5. [DOI] [PubMed] [Google Scholar]
- 31.Isa N., Solís J. Radioquimioterapia adyuvante en cáncer gástrico localmente avanzado completamente resecado de alto riesgo: experiencia inicial Hospital Carlos Van Buren. Rev Chil Cancerol Hematol. 2011 [Google Scholar]
- 32.Mojica P., Smith D., Ellenhorn J. Adjuvant radiation therapy is associated with improved survival for gallbladder carcinoma with regional metastatic disease. J Surg Oncol. 2007;96:8–13. doi: 10.1002/jso.20831. [DOI] [PubMed] [Google Scholar]
- 33.Zaydfudim V., Feurer I.D., Wright J.K. The impact of tumor extent (T stage) and lymph node involvement (N stage) on survival after surgical resection for gallbladder adenocarcinoma. HPB (Oxford) 2008;10:420–427. doi: 10.1080/13651820802320057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang S.J., Fuller C.D., Kim J.S. Prediction model for estimating the survival benefit of adjuvant radiotherapy for gallbladder cancer. J Clin Oncol. 2008;26:2112–2117. doi: 10.1200/JCO.2007.14.7934. [DOI] [PubMed] [Google Scholar]
- 35.Horgan A., Amir E., Walter T. Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis. J Clin Oncol. 2012;30:1934–1940. doi: 10.1200/JCO.2011.40.5381. [DOI] [PubMed] [Google Scholar]
- 36.González M.E., Giannini O.H., González P. Adjuvant radio-chemotherapy after extended or simple cholecystectomy in gallbladder cancer. Clin Transl Oncol. 2011;13(7):480–484. doi: 10.1007/s12094-011-0685-y. [DOI] [PubMed] [Google Scholar]
- 37.Ceelen W., Fierens K., Van Nieuwenhove Y. Preoperative chemoradiation versus radiation alone for stage II and III resectable rectal cancer: a systematic review and meta-analysis. Int J Cancer. 2009;124:2966–2972. doi: 10.1002/ijc.24247. [DOI] [PubMed] [Google Scholar]
- 38.Valentini V., Van Stiphout R.G., Lammering G. Nomograms for predicting local recurrence, distant metastases, and overall survival for patients with locally advanced rectal cancer on the basis of European randomized clinical trials. J Clin Oncol. 2011;29:3163–3172. doi: 10.1200/JCO.2010.33.1595. [DOI] [PubMed] [Google Scholar]
- 39.Sauer R., Becker H., Hohenberger W. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 40.Roh M.S., Colangelo L.H., O’Connell M.J. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol. 2009;27:5124–5130. doi: 10.1200/JCO.2009.22.0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frykholm G.J., Glimelius B., Pahlman L. Preoperative or postoperative irradiation in adenocarcinoma of the rectum: final treatment results of a randomized trial and an evaluation of late secondary effects. Dis Colon Rectum. 1993;36:564–572. doi: 10.1007/BF02049863. [DOI] [PubMed] [Google Scholar]
- 42.Gastrointestinal Tumor Study Group Prolongation of the disease-free interval in surgically treated rectal carcinoma. N Engl J Med. 1985;312:1465–1472. doi: 10.1056/NEJM198506063122301. [DOI] [PubMed] [Google Scholar]
- 43.O’Connell M.J., Martenson J.A., Wieand H.S. Improving adjuvant therapy for rectal cancer by combining protracted-infusion fluorouracil with radiation therapy after curative surgery. N Engl J Med. 1994;331:502–507. doi: 10.1056/NEJM199408253310803. [DOI] [PubMed] [Google Scholar]
- 44.Kim T.W., Lee J.H., Ahn J.H. Randomized trial of postoperative adjuvant therapy in stage II and III rectal cancer to define the optimal sequence of chemotherapy and radiotherapy: 10-year follow-up. Int J Radiat Oncol Biol Phys. 2011;81:1025–1031. doi: 10.1016/j.ijrobp.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 45.Tveit K.M., Guldvog I., Hagen S. Randomized controlled trial of postoperative radiotherapy and short-term time-scheduled 5-fluorouracil against surgery alone in the treatment of Dukes B and C rectal cancer, Norwegian Adjuvant Rectal Cancer Project Group. Br J Surg. 1997;84:1130–1135. [PubMed] [Google Scholar]
- 46.Krook J.E., Moertel C.G., Gunderson L.L. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med. 1991;324:709–715. doi: 10.1056/NEJM199103143241101. [DOI] [PubMed] [Google Scholar]
- 47.Horwich A., Parker C., Bangma C. Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl. 5):v129–v133. doi: 10.1093/annonc/mdq174. [DOI] [PubMed] [Google Scholar]
- 48.NCCN Guideline. Prostate Cancer. Version 1; 2013. http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.
- 49.Zelefsky M.J., Levin E.J., Hunt M. Incidence of late rectal and urinary toxicities after three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70(4):1124–1129. doi: 10.1016/j.ijrobp.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 50.Jani A.B., Su A., Correa D., Gratzle J. Comparison of late gastrointestinal and genitourinary toxicity of prostate cancer patients undergoing intensity-modulated versus conventional radiotherapy using localized fields. Prostate Cancer Prostatic Dis. 2007;10(1):82–86. doi: 10.1038/sj.pcan.4500910. [DOI] [PubMed] [Google Scholar]
- 51.Peeters S.T., Heemsbergen W.D., Koper P.C. Dose-response in radiotherapy for localized prostate cancer: results of the Dutch multicenter randomized phase III trial comparing 68 Gy of radiotherapy with 78 Gy. J Clin Oncol. 2006;24(13):1990–1996. doi: 10.1200/JCO.2005.05.2530. [DOI] [PubMed] [Google Scholar]
- 52.Pollack A., Zagars G.K., Starkschall G. Prostate cancer radiation dose response: results of the M, D. Anderson phase III randomized trial. Int J Radiat Oncol Biol Phys. 2002;53(5):1097–1105. doi: 10.1016/s0360-3016(02)02829-8. [DOI] [PubMed] [Google Scholar]
- 53.Zietman A.L., DeSilvio M.L., Slater J.D. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. J Am Med Assoc. 2005;294(10):1233–1239. doi: 10.1001/jama.294.10.1233. [DOI] [PubMed] [Google Scholar]
- 54.Kuban D.A., Tucker S.L., Dong L. Long-term results of the M, D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70(1):67–74. doi: 10.1016/j.ijrobp.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 55.Xu N., Rossi P.J., Jani A.B. Toxicity analysis of dose escalation from 75.6 gy to 81.0 gy in prostate cancer. Am J Clin Oncol. 2011;34(1):11–15. doi: 10.1097/COC.0b013e3181cae8c6. [DOI] [PubMed] [Google Scholar]
- 56.Eade T.N., Hanlon A.L., Horwitz E.M. What dose of external-beam radiation is high enough for prostate cancer? Int J Radiat Oncol Biol Phys. 2007;68(3):682–689. doi: 10.1016/j.ijrobp.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoskin P., Rojas A.M., Bownes P. Randomised trial of external beam radiotherapy alone or combined with high-dose-rate brachytherapy boost for localised prostate cancer. Radiother Oncol. 2012;103:217–222. doi: 10.1016/j.radonc.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 58.Pecorelli S., Zigliani L., Odicino F. Revised FIGO staging for carcinoma of the cervix. Int J Gynaecol Obstet. 2009;105:107–108. doi: 10.1016/j.ijgo.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 59.Gaffney D., Erickson-Wittmann B., Jhingran Y. ACR appropriateness criteria_ on advanced cervical cancer expert panel on radiation oncology – gynecology. Int J Radiat Oncol Biol Phys. 2011;81(3):609–614. doi: 10.1016/j.ijrobp.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 60.Colombo N., Carinelli S., Colombo A. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl. 7):vii27–vii32. doi: 10.1093/annonc/mds268. [DOI] [PubMed] [Google Scholar]
- 61.NC CN Guideline. Prostate Cancer. Version 2; 2013. http://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf.
- 62.Small W., Jr., Strauss J.B., Jhingran A. ACR appropriateness criteria® definitive therapy for early-stage cervical cancer. Am J Clin Oncol. 2012;35(4):399–405. doi: 10.1097/COC.0b013e3182610537. [DOI] [PubMed] [Google Scholar]
- 63.Landoni F., Maneo A., Colombo A. Randomised study of radical surgery versus radiotherapy for stage IB-IIA cervical cancer. Lancet. 1997;350:535–540. doi: 10.1016/S0140-6736(97)02250-2. [DOI] [PubMed] [Google Scholar]
- 64.Barillot I., Horiot J.C., Pigneux J. Carcinoma of the intact uterine cervix treated with radiotherapy alone: a French cooperative study: update and multivariate analysis of prognostics factors. Int J Radiat Oncol Biol Phys. 1997;38:969–978. doi: 10.1016/s0360-3016(97)00145-4. [DOI] [PubMed] [Google Scholar]
- 65.Perez C.A., Grigsby P.W., Chao K.S. Tumor size, irradiation dose, and long term outcome of carcinoma of uterine cervix. Int J Radiat Oncol Biol Phys. 1998;41:307–317. doi: 10.1016/s0360-3016(98)00067-4. [DOI] [PubMed] [Google Scholar]
- 66.Grigsby P.W., Singh A.K., Siegel B.A. Lymph node control in cervical cancer. Int J Radiat Oncol Biol Phys. 2004;59:706–712. doi: 10.1016/j.ijrobp.2003.12.038. [DOI] [PubMed] [Google Scholar]
- 67.Keys H.M., Bundy B.N., Stehman F.B. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med. 1999;340:1154–1161. doi: 10.1056/NEJM199904153401503. [DOI] [PubMed] [Google Scholar]
- 68.Morris M., Eifel P.J., Lu J. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340:1137–1143. doi: 10.1056/NEJM199904153401501. [DOI] [PubMed] [Google Scholar]
- 69.Peters W.A., III, Liu P.Y., Barrett R.J., II Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18:1606–1613. doi: 10.1200/JCO.2000.18.8.1606. [DOI] [PubMed] [Google Scholar]
- 70.Rose P.G., Blessing J.A., Gershenson D.M. Paclitaxel and cisplatin as first-line therapy in recurrent or advanced squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. J Clin Oncol. 1999;17:2676–2680. doi: 10.1200/JCO.1999.17.9.2676. [DOI] [PubMed] [Google Scholar]
- 71.Whitney C.W., Sause W., Bundy B.N. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: a Gynecologic Oncology Group and Southwest Oncology Group study. J Clin Oncol. 1999;17:1339–1348. doi: 10.1200/JCO.1999.17.5.1339. [DOI] [PubMed] [Google Scholar]
- 72.Chemoradiotherapy for Cervical Cancer Meta-Analysis Collaboration Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: a systematic review and meta-analysis of individual patient data from 18 randomized trials. J Clin Oncol. 2008;26:5802–5812. doi: 10.1200/JCO.2008.16.4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pearcey R., Brundage M., Drouin P. Phase III trial comparing radical radiotherapy with and without cisplatin chemotherapy in patients with advanced squamous cell cancer of the cervix. J Clin Oncol. 2002;20:966–972. doi: 10.1200/JCO.2002.20.4.966. [DOI] [PubMed] [Google Scholar]
- 74.Demanes D.J., Rodriguez R.R., Bendre D.D. High dose rate transperineal interstitial brachytherapy for cervical cancer: high pelvic control and low complication rates. Int J Radiat Oncol Biol Phys. 1999;45:105–112. doi: 10.1016/s0360-3016(99)00124-8. [DOI] [PubMed] [Google Scholar]
- 75.Syed A.M., Puthawala A.A., Abdelaziz N.N. Long-term results of low-dose-rate interstitial-intracavitary brachytherapy in the treatment of carcinoma of the cervix. Int J Radiat Oncol Biol Phys. 2002;54:67–78. doi: 10.1016/s0360-3016(02)02900-0. [DOI] [PubMed] [Google Scholar]
- 76.Pötter R., Georg P., Dimopoulos J.C. Clinical outcome of protocol based image (MRI) guided adaptive brachytherapy combined with 3D conformal radiotherapy with or without chemotherapy in patients with locally advanced cervical cancer. Radiother Oncol. 2011;100(1):116–123. doi: 10.1016/j.radonc.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]


