Summary
Background
Autoimmunity may be involved in sleep and neurodegenerative disorders. We aimed to describe a neurological syndrome with prominent sleep dysfunction and antibodies to a previously unknown neuronal antigen.
Methods
In this observational study, clinical and video-polysomnography (V- PSG) investigations identified a novel sleep disorder in three patients referred to the Sleep Unit of Hospital Clinic University of Barcelona for abnormal sleep behaviors and obstructive sleep apnea(OSA). They had antibodies against a neuronal surface antigen also present in five additional patients referred to our laboratory for antibody studies. These five patients had been evaluated with PSG and in two, the study was done or reviewed in our Sleep Unit. Two patients underwent postmortem brain examination. Immunoprecipitation and mass spectrometry were used to characterize the antigen and to develop a diagnostic test. Serum or CSF from 285 patients with neurodegenerative, sleep, or autoimmune disorders served as controls.
Findings
All eight patients (five women; range: 52–76 years, median 59) had abnormal sleep movements and behaviors and OSA confirmed by PSG. Six patients had a chronic evolution (range 2–12 years, median 5.5); in four the sleep disorder was the initial and most prominent feature, and in two it was preceded by gait instability, and followed by dysarthria, dysphagia, ataxia, or chorea. Two patients had a rapid evolution with disequilibrium, dysarthria, dysphagia, and central hypoventilation, and died two and six months after symptom onset. In 5/5 patients, the V-PSG reviewed in our Unit disclosed OSA, stridor, and abnormal sleep architecture with undifferentiated NREM sleep or poorly structured stage N2 with simple movements and finalistic behaviors, normalization of NREM sleep by the end of the night, and REM sleep behavior disorder. Four/4 patients carried the HLA-DRB1*1001 and HLA-DQB1*0501 alleles. All patients had antibodies (mainly IgG4) against IgLON5, member of a family of neuronal cell adhesion molecules. Only 1/285 controls (with progressive supranuclear palsy) had IgLON5 antibodies. Neuropathology showed neuronal loss and extensive deposits of hyperphosphorylated tau mainly involving the tegmentum of the brainstem and hypothalamus.
Interpretation
IgLON5-antibodies identify a unique NREM and REM parasomnia with sleep breathing dysfunction and pathological features suggesting a tauopathy.
Funding
Fondo de Investigaciones Sanitarias. Centros de Investigación Biomédica en Red de enfermedades neurodegenerativas (CIBERNED) and Respiratorias (CIBERES), Ministerio de Economía y Competitividad, Fundació la Marató TV3 and the National Institutes of Health.
Keywords: tauopathy, parasomnia, autoantibodies, IGLON5
Introduction
A number of relevant and well-characterized sleep disorders may occur in neurodegenerative diseases or autoimmune encephalitis.1–3 The immune system has been implicated in pathological processes of neurodegeneration but the exact mechanisms involved or whether they play a primary or secondary role are unclear.4 Some patients with neurodegenerative diseases develop antibodies against neuronal proteins, although the intracellular location of the target antigens suggests they are not pathogenic.5 In contrast, there is a category of rapidly progressive disorders, named autoimmune encephalitis, in which a disruption of sleep patterns can be prominent, the target antigens are known, and highly specific probably pathogenic antibodies against cell surface or synaptic proteins can be used as disease biomarkers.6 For example, patients with limbic encephalitis and antibodies against the protein, leucine rich glioma inactivated 1 (Lgi1) often develop rapid eye movement (REM) sleep behavior disorder (RBD),7 and patients with Morvan’s syndrome and antibodies against contactin-associated protein related 2 (Caspr2) develop severe insomnia with abnormal sleep-related motor activation (agrypnia excitata).8,9
In clinical practice, determination of antibodies to cell-surface or synaptic proteins is increasingly being considered in patients with rapidly progressing encephalitis of unclear etiology, but it is rarely assessed in patients with protracted neurological deficits suggestive of a neurodegenerative disease, including those with prominent sleep abnormalities. In such clinical scenario the potential detection of highly specific antibodies against a neuronal cell surface protein would provide a link between immune mechanisms, neurodegeneration, and sleep dysfunction.
We report here the clinical features and detailed video-polysomnography (V-PSG) of a novel sleep disorder identified in eight patients. In addition, we describe the target neuronal cell surface antigen recognized by antibodies present in all patients’ serum and cerebrospinal fluid (CSF), and the neuropathological findings in two cases.
Patients and methods
Identification of patients and controls
In 2010, a 59 year-old man (patient 1, Table 1) with an insidiously progressive atypical sleep disorder was studied in our multidisciplinary Sleep Disorders Unit (Hospital Clinic, University of Barcelona). Although the clinical features were different from Morvan’s syndrome, the presence of dysautonomic features led us to investigate his serum and CSF for antibodies to Caspr2. We did not find antibodies against Caspr2 or other neuronal surface antigens ( NMDA, GABAB, AMPA, mGluR1, or mGluR5 receptors, DPPX, and Lgi1)6 but the immunohistochemical study on frozen rat brain sections and cultured neurons identified an antibody against a novel neuronal cell surface protein which identity was unknown. While the characterization of the antigen was being performed, two additional patients (patients 2, 3, Table 1) with clinical and video-PSG features remarkably similar to the index patient were identified in the same Sleep Unit. In these two patients the recognition of the disorder was solely based on the characteristic pattern of sleep alterations, without other ancillary or immunological studies. This led to subsequently investigate their serum and CSF, showing the same pattern of antibody reactivity with rat brain and cell surface of cultured neurons as those of the index case. Then, we used our extensive collection of samples in the neuroimmunology center to identify patients with the same autoantibody and potentially the same disorder. Serum and CSF samples in the neuroimmunology center are from patients referred worldwide for study of neuronal antibodies. This search (masked of clinical information) identified five additional patients (one from our center and four from other institutions) with the same autoantibody. The clinical information, MRI, EEG, EMG, neuropsychological tests, and CSF studies of these patients were reviewed by the treating physicians, additional information obtained, and compared to that of our patients. After characterization of the antigen and development of a diagnostic cell-based assay (CBA) to detect the antibody, serum or CSF of 298 patients with neurodegenerative or sleep disorders, autoimmune encephalitis, and multiple sclerosis (see appendix)were used as controls to determine the specificity of the antibody.
Table 1.
Clinical features, treatment, and outcome
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | |
|---|---|---|---|---|---|---|---|---|
| Age at onset/ sex | 59/M | 53/M | 52/M | 69/F | 59/F | 58/F | 76/F | 65/F |
| Presentation | Sleep disorder | Sleep disorder | Sleep disorder | Sleep disorder | Gait instability | Gait instability | Gait instability | Gait instability |
| Disease duration | 4 years | 6 years | 5 years | 2 years | 12 years | 7 years | 6 months | 2 months |
|
Abnormal sleep behaviors |
Yesa | Yesa | Yesa | Yesb | Yesa | Yesb | Yesa | Yesb |
| Sleep apnea | Yesa | Yesa | Yesa | Yesb | Yesa | Yesb | Yesa | Yesb |
| Stridor | Yesa | Yesa | Yesa | Unknown | Yesa | Yesb | Yesa | Unknown |
|
Excessive day sleepiness |
Episodic but intense |
Mild | Episodic but intense |
Moderate | Mild | No | No | No |
|
Cognitive deterioration |
No (MMSE: 28) |
No (MMSE:30) |
No (MMSE:29) |
Yes (MMSE:21) |
Yesc (MMSE: ND) |
No (MMSE:28) |
No (MMSE: ND) |
No (MMSE: ND) |
|
Neuropsychological testing (years from onset of disorder) |
Yes (3)d | Yes (5)d | Yes (4)d | Yes (1)e | Yes (10)f | Yes (5)g | ND | ND |
| Gait instability | No | No | Mild | No | Severe | Severe | Severe | Severe |
| Parkinsonism | No | No | No | No | No | No | No | No |
| Chorea | No | No | Yes | Yes | Yes | Yes | No | No |
| Limb ataxia | No | No | No | No | Yes | Yes | No | Yes |
|
Abnormal ocular movements |
No | No | No | Limitation of vertical gaze |
Bilateral horizontal gaze paresis |
Vertical and horizontal nystagmus, |
Saccadic intrusions on pursuit |
Bilateral horizontal nystagmus |
| Bulbar symptoms | Mild dysphagia, vocal cord paresis |
Mild dysphagia |
Vocal cord paresis, mild dysarthria and dysphagia |
Dysarthria, central hypoventilation |
Dysphagia, dysarthria |
Dysphagia, dysarthria, central hypoventilation |
Dysphagia, dysarthria, vocal cord paresis, central hypoventilation |
Dysphagia, dysarthria, mandibular spasms, central hypoventilation |
| Dysautonomia | Hypersalivation and episodes of intense perspiration |
Enuresis | Urinary urgency and hesitance |
Syncopes and bradyarrhythmia |
Urinary urgency and incontinency |
Hypersalivation | No | Episodes of intense perspiration and drooling |
| Treatment | 3 cycles of iv steroids and Cy, 1 of iv Ig |
3 cycles of iv steroids, and Cy |
3 cycles of iv steroids, and Cy |
1 cycle of iv steroids |
1 cycle of iv plus oral steroids and rituximab |
3 cycles of iv Ig | 3 cycles of iv + oral steroids, 2 cycles of Cy |
1 cycle of iv Ig, steroids and rituximab |
| Outcome | No change | No change. Sudden death at home while asleep |
No change | No change. Sudden death at home |
No change. Sudden death during wakefulness |
No change. Died in the ICU |
No change. Sudden death at home while asleep |
Improved. Discharged from the ICU. Sudden death during wakefulness |
confirmed by centrally reviewed video-PSG
confirmed by PSG not centrally reviewed
cognitive deterioration started 10 years after onset of the disorder
neuropsychological evaluation (Auditory verbal learning test, Boston naming test, digit span, trail making A and B, Poppelreuter test, FAS word fluency test, and semantic fluency test) was normal (patient 1) or showed minor impairment of verbal episodic memory, decreased attention span, and deficit in visuospatial functions (patients 2 and 3)
neuropsychological evaluation (information on type of tests was not available) disclosed cognitive deterioration
the neuropsychological evaluation (block span test, digit span test, word pairs memory test, color word test, trail making test A + B, Colored progressive matrices (A/B), Regensburg word fluency test) disclosed impairment in non-verbal episodic memory, slowed cognitive speed and reduced selective attention
neuropsychological evaluation (ADAS test, Boston naming test, digit span, trail making A and B, Stroop test, Brixton test, Raven test, word fluency test, Buschke memory test) was normal.
Cy: cyclophosphamide; Ig: immunoglobulins; ICU: intensive care unit; iv: intravenous; MMSE: Mini-mental state examination; ND: Not done.
Brain tissue, serum, and CSF samples used in the study are deposited in the Neurological Tissue Bank and the Biobank of the Institutd’Investigacions Biomèdiques August Pi i Sunyer, Barcelona, Spain. Written informed consent for the storage and use of these samples for research was obtained from all patients. The eight patients or their relatives (five cases) gave written permission to be included in the study. Additional written consent was obtained from patients whose videos are included in the manuscript. The study was approved by the ethics committee of the Hospital Clínic, Barcelona, Spain.
Video-Polysomnographic studies
Four patients (patients 1–3 and 7)underwent V-PSG several nights throughout their clinical course at the Sleep Disorders Unit, Hospital Clínic, University of Barcelona, and one (patient 5) at the sleep laboratory in the University of Ulm, Germany, and the recording subsequently reviewed at Hospital Clinic. V-PSG were recorded as previously described (see appendix).7 Sleep stages and associated events (e.g., sleep apneas), were scored according to the 2007 American Academy of Sleep Medicine criteria.10 with the modifications previously proposed when the standard criteria could not be applied.11 The later include the following definitions: 1. We defined “undifferentiated NREM sleep” in those epochs with irregular slow theta EEG activity, clearly different from the awake alpha rhythm, lacking vertex sharp waves, K complexes, sleep spindles or delta slowing, and without definite and recurrent rapid eye movements, such as those typically seen in REM sleep. 2. We defined “poorly structured stage N2” in epochs with definite K complexes or spindles at 12–14 Hz, associated with either excessive EMG activity, movements or occasional bursts of rapid eye movements of lower amplitude than those typical seen in REM sleep in the same patient. 3. “RBD” was defined as increased EMG activity in the submentalis muscle and/or limbs associated with abnormal movements during in REM sleep. 4. “Vocalizations” were classified as simple (e.g, murmuring, whispering, groaning) and complex (e.g., talking, shouting, laughing, crying).5. “Motor events” were classified as jerks (sudden contractions of a single or several muscle groups), simple (movements like raising the arms, kicking and punching) and finalistic (elaborated movements following a complex pattern that clearly reminded an identifiable daytime activity such as eating, drinking or manipulating objects). Rapid periodic leg movements was defined as repetitive and stereotyped lower extremity movements separated by an interval of less than five seconds that did not fulfill criteria for periodic leg movements in sleep.12 6. “Stridor” was defined as a crowing harsh high pitched sound that occurred with breathing.
Laboratory studies for detection of IgLON5 antibodies and characterization of the antigen
The methods for immunohistochemistry on rat brain sections, immunofluorescence with cultures of neurons and Human Epithelial Kidney (HEK) 293 cells transfected with plasmids, and techniques of immunoprecipitation have been reported previously and are shown in detail in the Appendix.13–16 To further confirm the specificity of the antigen, HEK293 cells were transfected with plasmids containing IgLON1, 2, 3, 4, and 5 (green fluorescent protein (GFP)-tagged clones from Origene: RG213594, RG226879, RG207618, RG216034, RG225495) as described.17 To rule out the presence of other neuronal antibodies, serum diluted 1:200 was immunoabsorbed with live HEK293 cells expressing IgLON5 or cells transfected with plasmids without insert. After six rounds of immunoabsorption (sequential passing of serum into six plates with the indicated HEK cells), the serum was applied to sections of rat brain and live hippocampal neurons and the reactivity developed as described.16
Neuropathological examination
Neuropathological examination was done according to standardized protocols at the Neurological Tissue Bank of the IDIBAPS Biobank in two patients (patients 2 and 7 Table 1).18 Immunohistochemistry was performed applying a panel of primary antibodies (Table 1S) as described.18 To evaluate the expression of IgLON5 in the areas affected by tau pathology, sections from patient 7 and comparable areas from a patient with Alzheimer disease were incubated with a commercial antibody against IgLON5 (Abcam, Cambridge, UK) and the immunoreactivity visualized with the avidin-biotin immunoperoxidase method.
Role of the funding sources
The study sponsors had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to all the data in the study and the corresponding author had final responsibility for the decision to submit for publication.
Results
Clinical findings
The eight patients (five women; age range at disease onset: 52 to 76 years, median: 59) had a sleep disorder characterized by abnormal sleep movements and behaviors, and obstructive sleep apnea (OSA) identified by PSG. The three index patients had initial PSG without synchronized audiovisual recording in other hospitals, , and they were misdiagnosed with isolated OSA. The clinical features are described in detail in Table 1 and appendix. Six patients (patients 1–6, Table 1) had a chronic, slow evolution of the symptoms (median: 5.5 years from disease onset to death or last visit, range 2–12 years). In patients 1–4, the sleep disorder was the presenting and most prominent complaint during the evolution of the disease. All four patients had variable features of bulbar involvement and dysautonomia; patient 3 developed choreic movements in the four limbs, and patient 4 in the arms along with orofacialdyskinesias(Table 1).
Patients 5 and 6 started with frequent falls and slowly progressive gait instability, subsequently developing a similar sleep disorder as that of patients 1–4. Both patients also developed dysarthria, dysphagia, limb ataxia, and choreic movements in the limbs and face. During the last year of the disease, patient 6 developed several episodes of central hypoventilation that required multiple admissions in the intensive care unit. Patients 7 and 8 had a sub acute evolution characterized by the sleep disorder in association with severe disequilibrium, gait failure, dysarthria, dysphagia, vocal cord paresis, and central hypoventilation until their death two and six months after symptom onset. None of the eight patients developed parkinsonism or abnormal ocular movements as those reported in patients with progressive supranuclear palsy (PSP).19
Brain MRI, EEG, CSF, and EMG findings were unremarkable in all patients (EMG not done in patient 4). CSF hypocretin levels were normal in 3/3 patients(patients 2–3, and 7); from the other patients no CSF was available or the amount was insufficient. Human leukocyte antigen (HLA) typing was performed in four patients (patients 1–3, 7) and all showed the HLA-DQB1*0501 and HLA-DRB1*1001 alleles. HLA typing could not be done in the rest of the patients because by the time the HLA association was identified they were already death and DNA was not available. Based on the detection of antibodies against a neuronal surface antigen, all patients were treated with immunotherapy (Table 1). Only patient 8 improved; a few days after being discharged home, she died suddenly in the morning after awakening from sleep. The cause of death was unclear and no electrocardiographic abnormalities were detected during her stay in the hospital. Four additional patients died suddenly out of the hospital, two during wakefulness (patient 4 had presented previous episodes of syncope with bradyarrhythmia), and two during sleep (patient 7 after recent closure of the tracheostomy for an episode of central hypoventilation).
Video-Polysomnographic studies
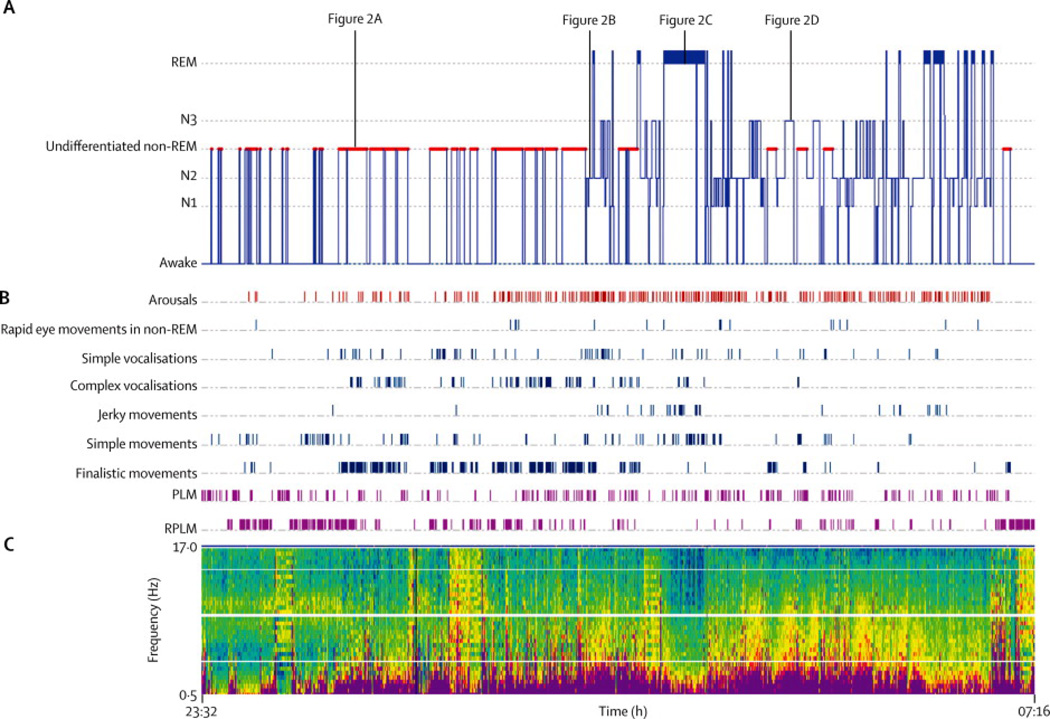
Five subjects (patients 1–3,5,7) underwent video-PSG (in total 19 studies) and their most prominent features are shown in Figures 1 and 2 and Tables 2 and 3. In four patients, sleep onset was accompanied by rapid periodic leg movements (Figure 2A, video segment 1). Initiation of sleep and reentering sleep after awakenings were abnormal in all five patients, either as an undifferentiated NREM sleep or poorly structured NREM sleep stage N2 sleep. In these NREM sleep stages there were frequent simple and complex vocalizations plus simple and finalistic movements resembling daytime activities such as eating, drinking or manipulating objects (Figure 2B, video segments 2–3). Normal relaxed N1 sleep was infrequent and well-structured N2 sleep with spindles and K complexes was rare. In all patients, clear periods of delta slowing typical of N3 sleep stage with frequent spindles and without vocalizations or movements were recorded (Figure 2D). Four patients showed RBD of mild intensity characterized by body and limb jerks and simple movements with absence of finalistic behaviors and vocalizations(Figure 2C, video segment 4). In the remaining patient REM sleep was not recorded.
Figure 1. Sleep recording in patient 1 with continuous positive airway pressure (index case).
A. Hypnogram; B. Arousals, dissociations and periodic movements; C. Density Spectral Array (DSA) showing the power spectrum of electroencephalographic frequencies (0–17 Hz) in electrode C3 referenced to electrode O2. Warmer colors indicate more dominant frequencies. Abbreviations: Rems: Rapid eye movements; RBD: REM sleep behavior disorder; PLM: Periodic limb movements; RPLM: Rapid periodic leg movements.
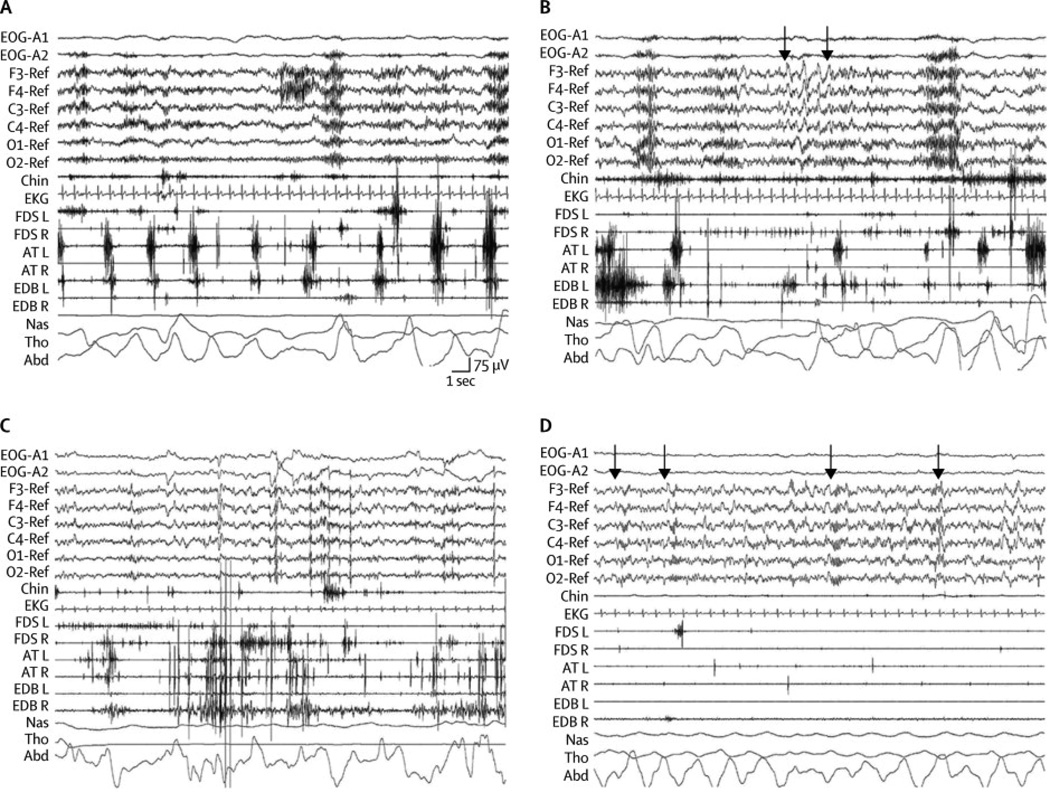
Figure 2. Polysomnograhic epochs illustrative of each sleep state in patient 1 (index case).
See also figure 1. A:Sleep onset characterized by undifferentiated NREM sleep with diffuse theta activity and rapid periodic leg movements that were particularly prominent at the left AT EMG channel; B:N2 sleep with chain of four consecutive K complexes (arrows) with frequent muscular phasic activity in EMG surface of the limbs that correlate with vocalizations and finalistic movements; C: REM sleep with typical rapid eye movements and EEG features with excessive phasic and tonic muscular activity and body jerks typical of REM sleep behavior disorder; D: N3 with diffuse delta activity and well defined sleep spindles at 13 Hz (arrows) without body/limb movements.
Abbreviations: EEG: electroencephalogram; EMG: electromyogram; EOG: electrooculogram; Chin: electromyography of mentalis muscle; EKG: electrocardiogram; FDS: flexor digitorumsuperficialis muscle left (L) and right (R); EDB: extensor digitorumbrevis muscle left (L) and right (R); AT: anterior tibialis left (L) and right (R); NAS: nasal air flow; THO: thoracic respiratory movement; ABD: abdominal respiratory movement. Note the calibration mark for time / EEG voltage.
Table 2.
Type of sleep recordings and main findings
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | |
|---|---|---|---|---|---|---|---|---|
| Initial sleep study | ||||||||
|
Time from first symptom |
10 months | 6 months | 6 months | 6 months | 12 years | 4 years | 4 months | 1.5 months |
| Type of sleep study1 | PSG2 | Respiratory PG | PSG2 | PSG2 | Video-PSG | PSG2 | Video-PSG in ICU |
PSG2 in ICU |
|
Initial diagnosis and treatment |
Snoring with OSA 3 (AHI: 81) CPAP |
Snoring with OSA 3 (AHI: 59) CPAP |
Snoring with OSA 3 (AHI: 33) CPAP |
OSA (AHI: NA) CPAP not tolerated |
Stridor with OSA (AHI: 84) Died before starting CPAP |
Stridor and OSA (AHI: NA) CPAP not tolerated |
NREM parasomnia4 |
OSA (AHI: 97) CPAP |
| Sleep parameters and findings in a representative sleep study available for review at the Hospital Clinic | ||||||||
|
Type of sleep study and time from first symptom |
Video-PSG with CPAP at 12 cm H2O 18 months |
Video-PSG with CPAP at 7 cm H2O 42 months |
Video-PSG with CPAP at 9 cm H2O 12 months |
NA | Video-PSG 5 12 years |
NA | Video-PSG with tracheostomy 5 months |
NA |
| Total time in bed (min) | 464 | 446 | 478 | − | 303 | − | 423 | − |
| Total sleep time (min) | 315.5 | 318.5 | 342 | − | 254 | − | 371 | − |
| Sleep efficiency (%) | 68 | 71 | 72 | − | 84 | − | 88 | − |
| Sleep Latency (min) | 6 | 25 | 22 | − | 7 | − | 3.5 | − |
| Stage N1 (%) | 7.1 | 0.8 | 1.7 | − | 9.4 | − | 3.9 | − |
| Stage UN-NREM (%) | 44.1 | 28.9 | 30.6 | − | 12 | − | 0 | − |
| Stage PS-N2 (%) | 26.6 | 25.9 | 46.5 | − | 20.9 | − | 46.6 | − |
| Stage N3 (%) | 8.2 | 37 | 6.7 | − | 57.6 | − | 40.3 | − |
| Stage REM (%) | 13.9 | 7.3 | 14.6 | − | 0 | − | 9.1 | − |
| NREM parasomnia | Yes | Yes | Yes | − | Yes 5 | − | Yes | − |
| RPLM | Yes | Yes | Yes | − | Yes | − | No | − |
| RBD | Yes | Yes | Yes | − | REM sleep not recorded |
− | Yes | − |
| Stridor | No 6 | No 6 | No 6 | − | Yes | − | Yes 7 | − |
| AHI | 4.5 | 0.2 | 2.2 | − | 84 | − | 3 7 | − |
| Sleep breathing features in a Video-PSG without CPAP | ||||||||
| Stridor | Yes | Yes | Yes | Yes8 | ||||
| AHI | 37 | 20 | 44 | 23 | ||||
AHI: Number of apnea-hypopnea per hour of sleep; CPAP: Continuous positive airway pressure; ICU: Intensive Care Unit; NA: Not available; OSA: Obstructive sleep apnea; RBD: REM sleep behavior disorder;PS-N2: Poorly-structured N2; Respitatory-PG:Respiratory polygraphy; RPLM: rapid periodic leg movements; PSG: polysomnography; UN-NREM: Undifferentiated NREM sleep; Video-PSG: Synchronized full video-polysomnography
Initial sleep studies in all patients were not available for review at the Hospital Clínic except in patients 5 and 7.
PSG without video recording
Abnormal sleep movements were initially attributed to OSA in other sleep centers.
This study was performed in the ICU the day after ventilation weaning and sleep efficiency was low (26%) due to noise, light and continuous stimulation by ICU nurses. A NREM parasomnia was recorded. REM sleep was not recorded. Stridor and apneas were not observed.
Abnormal movements during sleep were observed by sleep technicians and recorded as movement artifacts during PSG in Ulm Sleep Lab but video could not be reviewed at the Hospital Clínic, where undifferentiated NREM sleep and poorly structured N2 sleep was noted. Movement artifacts were abundant during the undifferentiated NREM sleep and poorly structured N2.
With CPAP, stridor and obstructive apneas and hypopneas were absent.
When tracheostomy was closed, stridor was present during sleep. With open tracheostomy, stridor and apneas were absent.
Video-PSG performed without CPAP and before tracheostomy.
Table 3.
Summary of video-polysomnographic characteristics1
|
These features refer to the 5 patients with video-polysomnography reviewed at the Sleep Unit Disorders in the Hospital Clínic Barcelona.
Rapid periodic leg movements during wakefulness and sleep-initiation were absent in patient 7.
REM sleep behaviour disorder was present in four patients. In patient 5 REM sleep was not recorded.
Patients had inspiratory stridor and frequent obstructive sleep apneic events (apnea-hypopnea indices ranging from 20 to 84; normal ≤ 5) that were more evident during quiet N3 sleep stage (video segment 5). Stridor did not occur during wakefulness. Most events were obstructive hypopneas with moderate oxyhemoglobin desaturations (nadir ranging from 75% to 88%)and arousals. CPAP therapy in three patients and tracheostomy in one abolished stridor and OSA, but the abnormal NREM and REM movements and behaviors persisted.
Total sleep time and sleep efficiency were moderately reduced compared to normative values (Table 2).20 There was a characteristic distribution throughout the night of the sleep abnormalities (present in 14/19 V-PSG). Periods of undifferentiated NREM and poorly structured N2 with finalistic movements predominated and were of longer duration after onset of nocturnal sleep or following awakenings in the first half of the night. Despite the severity of the sleep architecture abnormalities, there were normal N3 periods that were more frequent and lasted longer in the second half of the night. REM sleep, always in the form of RBD, also had a similar tendency being more prominent in the second half of the night.
Antibody characterization
The serum of the eight patients, and the CSF (available from five) showed an identical pattern of reactivity with the neuropil of rat brain. Immunoreactivity was more intense in the molecular layer and synaptic buttons of the granular layer of the cerebellum (Figure 3). All samples labeled the membrane of live neurons in culture indicating the antigen was exposed on the cell surface (Figure 3). Immunocompetition assays showed that all samples blocked the reactivity of the biotinylated IgG of patient 1, strongly suggesting that the antibodies of the eight patients reacted with the same epitopes (not shown). Serum antibody titers (measured with serial dilutions of serum and brain tissue immunohistochemistry) ranged from1/5,000 to 1/40,000. Follow-up titers were obtained in five patients: in four, the titers decreased more than two-fold after immunotherapy, and they did not change during one year without treatment in the fifth patient. Analysis of IgG subclasses showed that in all patients the novel antibody was IgG4 (Figure 1S); four patients had additional mild IgG1 reactivity, one mild IgG2, and none IgM. None of the patients had antibodies to previously known cell surface or synaptic proteins including, NMDA, GABAB, AMPA, mGluR1, or mGluR5 receptors, DPPX, Lgi1 and Caspr2.
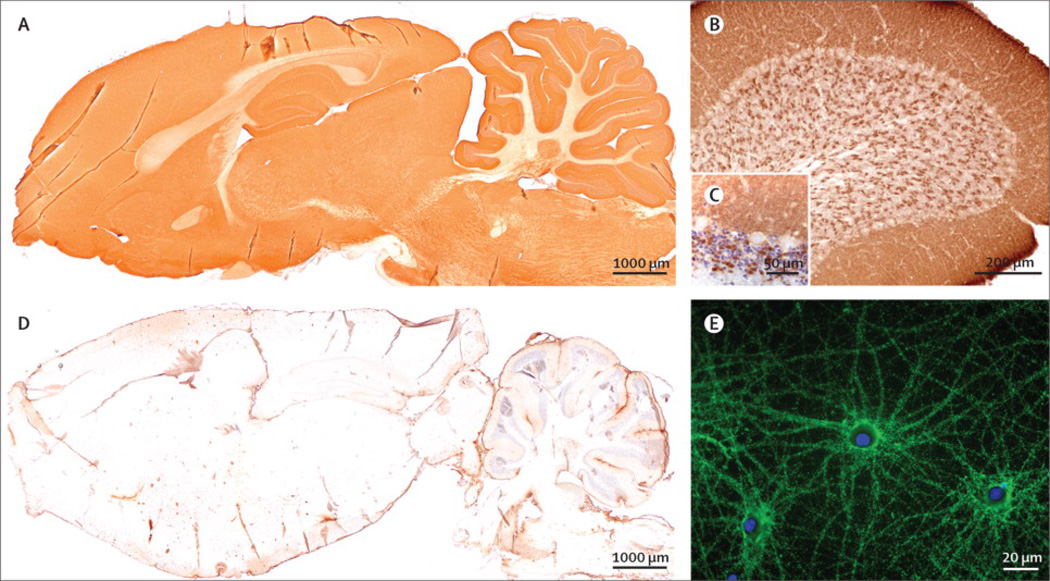
Figure 3. Reactivity of patient’s antibodies with rat brain and cultures of hippocampal neurons.
(A) Sagittal section of rat brain immunostained with a patient’s CSF: there is a diffuse staining of the neuropil, which is not seen when rat brain sections are incubated with a control CSF (B). Immunoreactivity was particularly robust in the cerebellum (C) where there was diffuse staining of the molecular layer and synaptic glomerula of the granular cell layer (D, counterstained with hematoxylin). (E) Culture of rat hippocampal neurons incubated (non-permeabilized) with a patient’s serum showing intense reactivity with a cell surface antigen. Scale bars in A and B=1000 µm, C=200 µm, D=50 µm and E=20 µm
Identification of IgLON family member 5 as the target antigen
Two independent immunoprecipitations using live cultured neurons and serum of two patients, followed by peptide characterization with mass spectrometry revealed seven peptides containing 29% of the IgLON5 protein sequence, and 3 peptides containing 14% of the same protein (Figure 2S). To confirm the specificity of patients’ antibodies for IgLON5, HEK293 cells transfected with plasmids coding each of the five members of the IgLON family were used in a cell-based assay (CBA). The serum and CSF of the eight patients only reacted with cells transfected with the GFP-tagged IgLON5 (Figure 4). Immunoblots of HEK293 cells transfected with IgLON5 showed reactivity with a commercial IgLON5 antibody but were negative with patients’ antibodies suggesting that the latter were directed against conformational epitopes (Figure 3S).
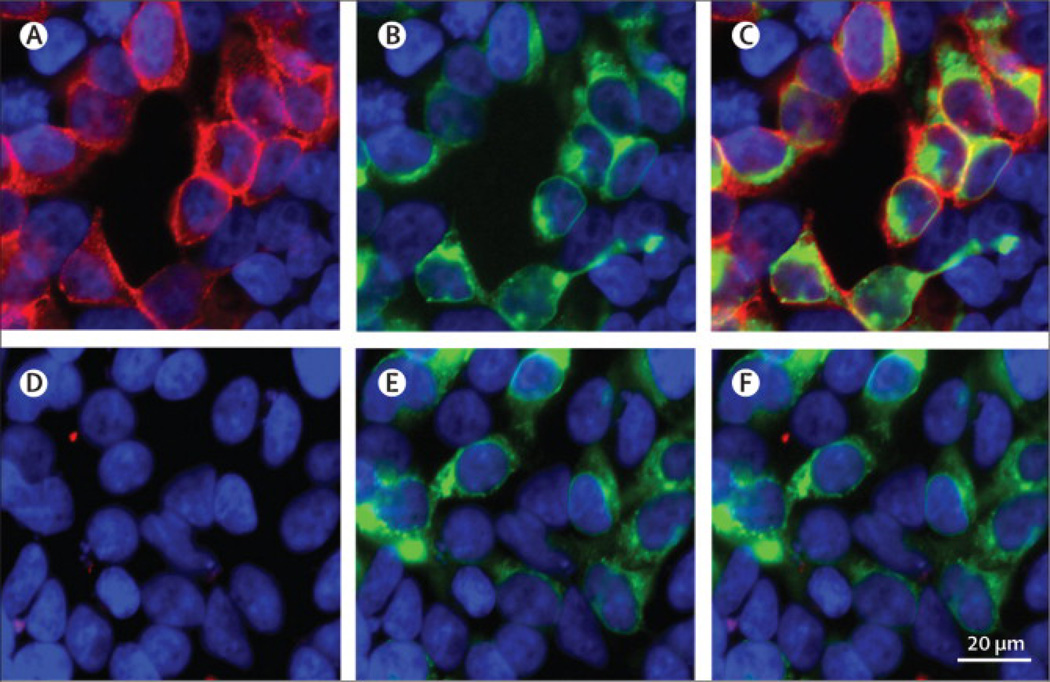
Figure 4. Detection of IgLON5 antibodies using a HEK293 cell based assay.
HEK293 cells were transfected to express green fluorescent protein (GFP)-tagged IgLON5 and incubated live, not permeabilized, with a patient’s (A–C) or control (D–F) serum. Patient’s serum, but not control serum, stained the cell surface of cells (red) that specifically express IgLON5, as demonstrated by the GFP fluorescence (green). Both reactivities are shown merged in C. Nuclei counterstained with DAPI. Scale bar=20 µm
Analysis of serum or CSF of the 298 controls using the IgLON5 CBA identified only one patient with serum, but not CSF, antibodies. This patient is currently being followed in our Movement Disorders Unit of Hospital Clinic, University of Barcelona. He is an 81 year-old man who fulfills clinical criteria of PSP.19 The only unusual feature of this patient is the prolonged course of the disease of nearly 20 years. He has not developed sleep disturbances, and he is HLA- DQB1*0501 and HLA-DRB1*1001 negative.
To determine if in addition to IgLON5 antibodies there were other antibodies that could associate with clinical features identified in only some patients, we immunoabsorbed the serum of three patients (# 1, 5, 7), who differed in some symptoms (except for the sleep disorder) and clinical evolution, with HEK293 cells expressing IgLON5 or cells transfected with plasmids without insert. The absorption with IgLON5 abrogated the reactivity of the three sera with rat brain and hippocampal neurons indicating that the antibodies were directed only against IgLON5 (Figure 4S).
Neuropathological examination
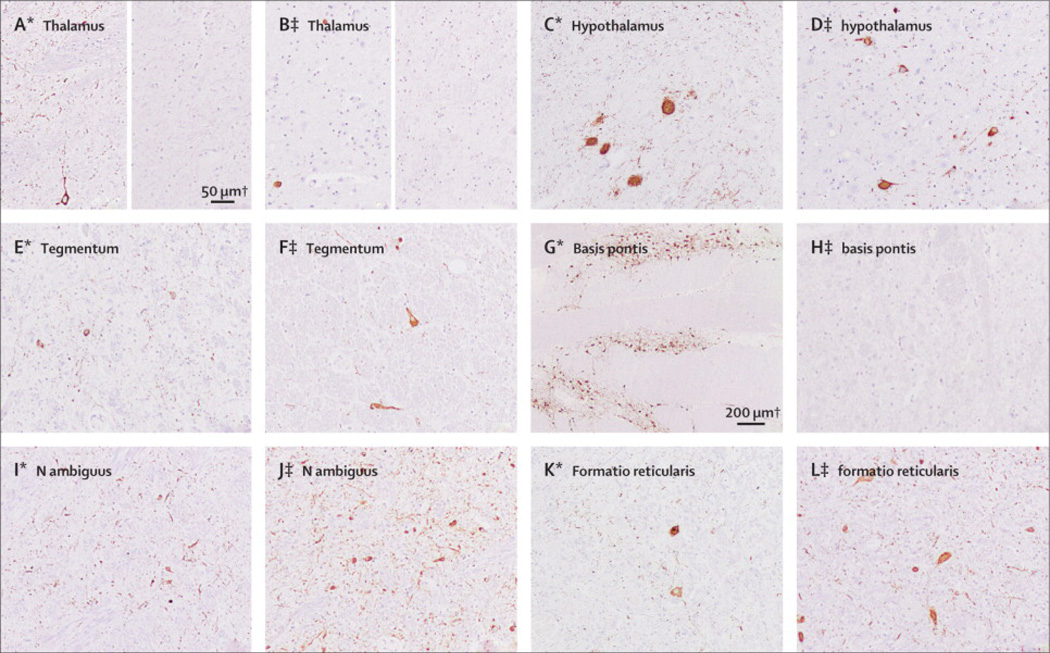
A detailed description of the post-mortem brain examination of both patients (patients 2 and 7) is given in the appendix. In brief, there was a widespread accumulation of abnormal, hyperphosphorylated tauptotein in neurons, much beyond of that expected for “normal” ageing.21 Neuronal tau deposits were predominant inhypothalamus, prehypothalamic region, and tegmentum of brainstem including laterodorsal tegmental area, periaqueductal gray matter, the region of the pedunculopontine nucleus, magnocellular nuclei, and nucleus ambiguus. Neuronal loss, astrogliosis and microglial activation was variable in these areas, being globally mild in Patient 2 with predominant involvement of ventral parts of dorsomedial thalamus, hypothalamic nuclei, periaquaeductal gray matter, pontine tegmental area, and mild involvement of formatioreticularis and magnocellular nuclei of medulla oblongata. Neuronal loss was more severe in Patient 7, with preferential involvement of zonaincerta, hypothalamus, periaqueductal gray matter, mesencephalic and pontinetegmentum, medulla oblongata, and particularly n. ambiguus and magnocellular nuclei (Table 2S, Figures 5 and 5S).
Figure 5. Distribution of tau pathology.
Panels A1-F1 correspond to patient 2, and panels A2-F2 correspond to patient 7. Moderate amounts of AT8 immunoreactive neuropil threads and neurofibrillary tangles are detected in hypothalamic nuclei (B1, A2. posterior hypothalamic nucleus; example of score ++) and anterior thalamus (A1, B2: left figure), but are completely absent in lateral and posterior thalamic neurons of both cases (A1, B2, right figure; example of score 0). While the pontinetegmentum is mildly (D2; example of score +) and moderately (C1) affected in Patients 7 and 2, respectively, neurons of n. propii of basis pontis show extensive Tau-pathology mainly in form of pretangles(D1; example of score +++), which is not observed in patient 7 (C2). In contrast, prominent pathology in n. ambiguus is detected in patient 7 (F2; example of score +++), and less in case 2 (E1) and to a lesser extent in magnocellular nuclei of formatioreticularis in both cases (F1, E2).
Tau aggregates were exclusively neuronal, in form of pretangles, tangles and neuropil threads (Figure 6S), with presence of 3-repeat and 4-repeat tau isoforms in patient 7 and predominance of 4-repeat tau isoforms in patient 2 (not shown). Neuronal loss and gliosis correlated better with the presence of neurofibrillary tangles than with pretangles. There were no tau-positive grains and no glial tau pathology, neither in astrocytes (tufted or thorn shaped astrocytes, bush-like astrocytes or astrocytic plaques), nor in oligodendrocytes (coiled bodies, globular glial inclusions). No inflammatory infiltrates or concomitant abnormal protein deposits of beta-amyloid, or alpha-synuclein, were detected. IgLON5 immunoreactivity was not decreased in the affected brainstem regions of patient 7 compared with those of a patient with Alzheimer disease used as control (Figure 7S).
Discussion
We report eight patients with a neurological disorder characterized by a unique NREM and REM parasomnia with sleep breathing dysfunction, variable features of gait instability and brainstem symptoms, and autoantibodies against IgLON5, a neuronal cell adhesion protein. Several findings establish this disorder as a new entity, 1) the characteristic sleep disorder identified in the first three patients by investigators in the Sleep Disorders Unit (masked of information regarding autoantibodies), and in the other five patients by investigators in the Neuroimmunology Laboratory (masked of clinical information)after searching for cases with antibodies similar to those found in the three index patients, 2) the demonstration that patients’ antibodies recognized the same neuronal cell surface antigen (IgLON5), 3) the identification that all patients’ IgLON5 antibodies were mainly IgG4, an isotype considered involved in anti-inflammatory responses,22 although in some disorders they can be pathogenic,23 4) the demonstration in 4/4 patients of the same HLA DRB1*1001 and DQB1*0501 alleles which are uncommon among the Spanish population,24 and 5) the presence of similar neuropathology in two patients, suggesting a novel tauopathy with predominant brainstem and hypothalamic involvement.
In addition to IgLON5 antibodies, the main clinical signature of this disorder is the V-PSG features characterized by disrupted sleep architecture and abnormal motor activation that involves both NREM and REM sleep stages with several findings that differ from those described in “agrypnia excitata”.8 First, the total sleep time was only moderately reduced; second, there were clear periods of normal NREM sleep; and third, the finalistic movements were associated with a different PSG pattern and never invaded daytime wakefulness. Moreover, the presence of periods of NREM and REM sleep (associated with RBD) differentiates our patients from those with “status dissociatus”. This term defines an extreme form of wakefulness/sleep state dissociation in which patients have sleep-related motor agitation with continuous limb movement and vocalizations and a PSG without identifiable sleep stages, specifically lack of K complexes and sleep spindles.25 A previous case report with dementia and a sleep disorder defined as “Stages 1–2 NREM sleep behavior disorder” had some similarities with the findings of our patients, including the presence of finalistic behaviors in stages N1-N2 NREM sleep and RBD; however, the patient did not have identifiable N3.26 We did not record sleepwalking or sleep terror episodes in any of the V-PSG performed in our patients, and together with the severe distortion of sleep architecture the diagnosis of overlap parasomnia is unlikely.27 Another finding not described in any of the parasomnias mentioned above is the presence of OSA and stridor. The three patients investigated in our Sleep Unit had been previously studied in other centers and misdiagnosed of isolated OSA. The reason for this diagnosis was that the stridor that accompanied the agitated nocturnal behavior was confused with snoring and audiovisual recordings were not available. In contrast to isolated OSA, the abnormal behaviours of our patients did not improve with CPAP therapy.28
Neuropathological examination showed neuronal loss and gliosis associated with an atypical tauopathy mainly involving the tegmentum of brainstem and hypothalamus. Part, but not all, of the subcortical distribution pattern is observed in other tauopathies, such as progressive supranuclear palsy or corticobasal degeneration. However, the absence of glial pathology, grains or globular glial inclusions, prevents to classify the pathology of our patients within any of the currently known tauopathies.29,30
Two cases with clinical and neuropathological features similar to our patients were previously reported as possible PSP and atypical Alzheimer disease respectively.31,32 The first patient was a 60 year-old woman who developed nocturnal agitation, continuous respiratory groan, gait instability, chorea, progressive dysphagia, and episodes of respiratory insufficiency that required thracheostomy.31 The second patient was a 77 year-old woman with a six year history of progressive dysphagia, breathing problems, stridor, paralysis of the vocal cords that required tracheostomy, and central hypoventilation. 32
The diffuse involvement of the brainstem nuclei in our patients probably explains the sleep breathing disorder, gait instability, and bulbar symptoms.33 The hypothalamus has an important role in the regulation of sleep onset and organization of sleep; therefore, the hypothalamic abnormalities in our patients likely contributed to the difficulties in the proper order of entering sleep.34 A striking finding was the presence of abnormal finalistic behaviors during NREM sleep. Similar behaviors have been associated with thalamic degeneration,8 but our patients did not have substantial involvement of the thalamus. The similarities between these behaviors and those performed during routine awake activities suggest the occurrence of an abnormally intense sleep-related hippocampal reactivation of awake motor patterns.35,36 Damage of the hippocampus or its afferents from the tegmentum of the brainstem together with a non-specific motor hyperactivation would potentially result in the complex finalistic behaviors characteristic of this parasomnia. Finally, the RBD would be related to the involvement of the nucleus magnocellularis considering the relative preservation of the coeruleus/subcoeruleus complex.37
The target antigen of all eight patients’ antibodies is IgLON5, the latest identified member of the IgGLON family (Table 2S) which is part of the immunoglobulin superfamily of cell adhesion molecules.38 IgLON proteins are highly glycosylated, contain three immunoglobulin-like domains, and are attached to the plasma membrane through a glycosylphosphatidyl inositol (GPI) anchor.38 The family of IgLON proteins has important functions in neuronal path finding and synaptic formation during brain development.39 Autoimmunity against other members of the immunoglobulin superfamily has been mainly reported in multiple sclerosis and other demyelinating syndromes. Antibodies against neurofascin and contactin-2-specific T cells are believed to play a role in the axonal and gray matter degeneration in multiple sclerosis.40
Our study suggests an intriguing link between the presence of IgLON5 antibodies and a tauopathy. If IgLON5 antibodies are a primary or secondary event in the pathophysiology of the disorder is currently unclear. The IGLON5 gene was identified during the sequencing of human chromosome 19 and it is mostly expressed in the nervous system (www.ebi.ac.uk/gxa/experiment/E-GEOD-803/ENSG00000142549?ef=).41 Although the exact role of IgLON5 is unknown, our study suggests that it could be involved in the physiology of tau. Alternatively, the development of IgLON5 antibodies could be a consequence of the neurodegenerative process. The chronic clinical course in six patients, the lack of response to immunotherapy despite decrease of the antibody levels, and the neuropathological features would favor a primary neurodegenerative disorder. On the other hand, the frequency of HLA-DRB1*1001 and DQB1*0501 among the Spanish population is 1.6% and 14.4%,24 arguing that the association noted in all four patients with these alleles represents a genetic susceptibility for autoimmune disease.
Our study has limitations that are inherent to the retrospective evaluation of some patients, and the small number of cases and autopsy studies which preclude defining the full spectrum of symptoms and pathological signature. However, the characterization of IgLON5 autoimmunity provides a useful biomarker of this disorder. An important practical implication is that testing for IgLON5 antibodies should be considered in patients who develop NREM sleep dysfunction with simple and finalistic behaviors, along with RBD, OSA, and stridor, preceded or followed by gait disequilibrium, chorea and brainstem dysfunction.
Research in context
Systematic review
References for this study were identified through searches of PubMed for articles published in English December 01, 2013 with the search terms (alone or in combination): “tauopathy”, “brainstem”, “IgLON family”, “parasomnia”, “sleep disorders”, and “autoantibodies”. Articles were also identified by searches of the authors’ files. We did not find any study on the function of IgLON5 protein or a previous description of the sleep disorder or neuropathology identified in our patients. A previous case report had some similarities with the findings of our patients, including the presence of finalistic behaviors in stages N1-N2 NREM sleep and RBD; however, the patient did not have identifiable N3.25 Two cases with clinical and neuropathological features suggesting those of our patients were previously reported as possible PSP and atypical Alzheimer disease respectively. The neuropathological studies of the two patients showed a neuronal tauopathy mainly involving the brainstem.31,32
Interpretation
This study describes a new antibody against a surface antigen, called IgLON5, found in eight patients with a characteristic sleep disorder previously not described and defined by NREM and REM parasomnia and obstructive sleep apnea with stridor. These clinical and immunological features were not previously described. Our study raises an intriguing link between the presence of an antibody against a surface neuronal antigen, whose function is unknown, and a tauopathy. It is currently unclear if IgLON5 antibodies are a primary or secondary event in the pathophysiology of the disorder. The lack of response to immunotherapy despite decrease of the antibody levels and the neuropathological features would favor a primary neurodegenerative disorder. However, the sharing of the same haplotypes and the presence of an antibody that may interact with the surface antigen in vivo indicate that the disorder could be immune mediated. Overall, the findings of this study are important because they provide clear guidelines to identify patients with a novel tauopathy and a unique sleep disorder not previously characterized. The identification of IgLON5 antibodies is important to confirm the diagnosis and clarify in the future the full clinical spectrum of this syndrome.
Supplementary Material
Segment 1 (patient 1). Rapid periodic leg movements during undifferentiated NREM sleep.
Segment 2 (patient 1). Finalistic movements mimicking eating during poorly structured N2 sleep.
Segment 3 (patient 3). Finalistic movements. The patient works as a TV antenna installer and it seems that he is acting as manipulating some wires.
Segment 4 (patient 3). Jerks during REM sleep.
Segment 5 (patient 3). During N3 sleep without CPAP mask, stridor is present.
Acknowledgements
We thank Dr. Chao-Xing Yuan, from the Proteomics and Systems Biology Core, University of Pennsylvania, for his help in the proteomic analysis and patients and brain donors for their contribution to research. Supported in part by grants (11/01780, JD; PI12/00611, FG, LS, LB) from the Fondo Investigaciones Sanitarias, Spain. Centros de Investigación Biomédica en Red de enfermedades neurodegenerativas (CIBERNED) (YC, CG, AI, JS) and Respiratorias (CIBERES) (CE), Spain; Fundació la Marató TV3 (JD), Spain and the National Institutes of Health RO1NS077851 (JD), USA. EG is supported by a grant of the Ministerio de Economía y Competitividad, Spain.
None of the funding sources had any influence on the collection, analysis, and interpretation of the data, or in the writing process.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: JD receives royalties from licensing fees to Euroimmun for a patent for the use of NMDAR as autoantibody test. FG and JD have filed a patent application (#14154384.3) for the use of IgLON5 antibodies as diagnostic test. The rest of the authors declare that they have no conflicts of interest.
Authors’ contributions: LS, CG, and EG: literature search, figures, study design, data collection, data analysis, data interpretation, writing, critical approval final manuscript. LB, JL, ET-V, AC, BG, YC, CE, IV, AI: data collection and interpretation, revising and critical approval final manuscript. JS: data collection, writing, and critical approval final manuscript; JD: data analysis, data interpretation, critical approval final manuscript, funding; FG: figures, study design, data collection, data analysis, data interpretation, writing, critical approval final manuscript, funding.
Reference list
- 1.Cornelius JR, Pittock SJ, McKeon A, et al. Sleep manifestations of voltage-gated potassium channel complex autoimmunity. Arch Neurol. 2011;68:733–738. doi: 10.1001/archneurol.2011.106. [DOI] [PubMed] [Google Scholar]
- 2.Iranzo A, Tolosa E, Gelpi E, et al. Neurodegenerative disease status and post-mortem pathology in idiopathic rapid-eye-movement sleep behaviour disorder: an observational cohort study. Lancet Neurol. 2013;12:443–453. doi: 10.1016/S1474-4422(13)70056-5. [DOI] [PubMed] [Google Scholar]
- 3.Boeve BF, Silber MH, Ferman TJ, et al. Clinicopathologic correlations in 172 cases of rapid eye movement sleep behavior disorder with or without a coexisting neurologic disorder. Sleep Med. 2013;14:754–762. doi: 10.1016/j.sleep.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Czirr E, Wyss-Coray T. The immunology of neurodegeneration. J Clin Invest. 2012;122:1156–1163. doi: 10.1172/JCI58656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gold M, Pul R, Bach JP, Stangel M, Dodel R. Pathogenic and physiological autoantibodies in the central nervous system. Immunol Rev. 2012;248:68–86. doi: 10.1111/j.1600-065X.2012.01128.x. [DOI] [PubMed] [Google Scholar]
- 6.Lancaster E, Dalmau J. Neuronal autoantigens--pathogenesis, associated disorders and antibody testing. Nat Rev Neurol. 2012;8:380–390. doi: 10.1038/nrneurol.2012.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iranzo A, Graus F, Clover L, et al. Rapid eye movement sleep behavior disorder and potassium channel antibody-associated limbic encephalitis. Ann Neurol. 2006;59:178–181. doi: 10.1002/ana.20693. [DOI] [PubMed] [Google Scholar]
- 8.Montagna P, Lugaresi E. Agrypnia Excitata: a generalized overactivity syndrome and a useful concept in the neurophysiopathology of sleep. Clin Neurophysiol. 2002;113:552–560. doi: 10.1016/s1388-2457(02)00022-6. [DOI] [PubMed] [Google Scholar]
- 9.Irani SR, Pettingill P, Kleopa KA, et al. Morvan syndrome: clinical and serological observations in 29 cases. Ann Neurol. 2012;72:241–255. doi: 10.1002/ana.23577. [DOI] [PubMed] [Google Scholar]
- 10.Iber C, Ancoli-Israel S, Chesson A, Quan SF for the American Academy of Sleep Medicine. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 11.Santamaria J, Högl B, Trenkwalder C, Bliwise D. Scoring sleep in neurological patients: the need for specific considerations. Sleep. 2011;34:1283–1284. doi: 10.5665/SLEEP.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zucconi M, Ferri R, Allen R, et al. The official World Association of Sleep Medicine (WASM) standards for recording and scoring periodic leg movements in sleep (PLMS) and wakefulness (PLMW) developed in collaboration with a task force from the International Restless Legs Syndrome Study Group (IRLSSG) Sleep Med. 2006;7:175–183. doi: 10.1016/j.sleep.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Ances BM, Vitaliani R, Taylor RA, Liebeskind DS, Voloschin A, Houghton DJ, et al. Treatment-responsive limbic encephalitis identified by neuropil antibodies: MRI and PET correlates. Brain. 2005;128:1764–1777. doi: 10.1093/brain/awh526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernal F, Shams’ili S, Rojas I, et al. Anti-Tr antibodies as markers of paraneoplastic cerebellar degeneration and Hodgkin’s disease. Neurology. 2003;60:230–234. doi: 10.1212/01.wnl.0000041495.87539.98. [DOI] [PubMed] [Google Scholar]
- 15.Buchhalter JR, Dichter MA. Electrophysiological comparison of pyramidal and stellate nonpyramidal neurons in dissociated cell culture of rat hippocampus. Brain Res Bull. 1991;26:333–338. doi: 10.1016/0361-9230(91)90003-3. [DOI] [PubMed] [Google Scholar]
- 16.Lancaster E, Lai M, Peng X, et al. Antibodies to the GABA(B) receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol. 2010;9:67–76. doi: 10.1016/S1474-4422(09)70324-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091–1098. doi: 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gelpi E, Lladó A, Clarimón J, et al. Phenotypic variability within the inclusion body spectrum of basophilic inclusion body disease and neuronal intermediate filament inclusion disease in frontotemporal lobar degenerations with FUS-positive inclusions. J Neuropathol Exp Neurol. 2012;71:795–805. doi: 10.1097/NEN.0b013e318266efb1. [DOI] [PubMed] [Google Scholar]
- 19.Litvan I, Agid Y, Calne D, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996;47:1–9. doi: 10.1212/wnl.47.1.1. [DOI] [PubMed] [Google Scholar]
- 20.Walsleben JA, Kapur VK, Newman AB, et al. Sleep and reported daytime sleepiness in normal subjects: the Sleep Heart Health Study. Sleep. 2004;27:293–298. doi: 10.1093/sleep/27.2.293. [DOI] [PubMed] [Google Scholar]
- 21.Nelson PT, Alafuzoff I, Bigio EH, et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol. 2012;71:362–381. doi: 10.1097/NEN.0b013e31825018f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nirula A, Glaser SM, Kalled SL, Taylor FR. What is IgG4? A review of the biology of a unique immunoglobulin subtype. Curr Opin Rheumatol. 2011;23:119–124. doi: 10.1097/BOR.0b013e3283412fd4. [DOI] [PubMed] [Google Scholar]
- 23.Klooster R, Plomp JJ, Huijbers MG, et al. Muscle-specific kinase myasthenia gravis IgG4 autoantibodies cause severe neuromuscular junction dysfunction in mice. Brain. 2012;135:1081–1101. doi: 10.1093/brain/aws025. [DOI] [PubMed] [Google Scholar]
- 24.Balas A, García-Sánchez F, Vicario JL. Allelic and haplotypic HLA frequency distribution in Spanish hematopoietic patients. Implications for unrelated donor searching. Tissue Antigens. 2011;77:45–53. doi: 10.1111/j.1399-0039.2010.01578.x. [DOI] [PubMed] [Google Scholar]
- 25.Mahowald MW, Schenck CH. Status dissociatus: a perspective on states of being. Sleep. 1991;14:69–79. doi: 10.1093/sleep/14.1.69. [DOI] [PubMed] [Google Scholar]
- 26.Arnulf I, Mabrouk T, Mohamed K, Konofal E, Derenne JP, Couratier P. Stages 1–2 non-rapid eye movement sleep behavior disorder associated with dementia: a new parasomnia? Mov Disord. 2005;20:1223–1228. doi: 10.1002/mds.20517. [DOI] [PubMed] [Google Scholar]
- 27.Schenck CH, Boyd JL, Mahowald MW. A parasomnia overlap disorder involving sleepwalking, sleep terrors, and REM sleep behavior disorder in 33 polysomnographically confirmed cases. Sleep. 1997;20:972–981. doi: 10.1093/sleep/20.11.972. [DOI] [PubMed] [Google Scholar]
- 28.Iranzo A, Santamaría J. Severe obstructive sleep apnea/hypopnea mimicking REM sleep behavior disorder. Sleep. 2005;28:203–206. doi: 10.1093/sleep/28.2.203. [DOI] [PubMed] [Google Scholar]
- 29.Ludolph AC, Kassubek J, Landwehrmeyer BG, et al. Tauopathies with parkinsonism: clinical spectrum, neuropathologic basis, biological markers, and treatment options. Eur J Neurol. 2009;16:297–309. doi: 10.1111/j.1468-1331.2008.02513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spillantini MG, Goedert M. Tau pathology and neurodegeneration. Lancet Neurol. 2013;12:609–622. doi: 10.1016/S1474-4422(13)70090-5. [DOI] [PubMed] [Google Scholar]
- 31.Kaphan E, Pellissier JF, Rey M, Robert D, Auphan M, Ali Chérif A. Esophageal achalasia, sleep disorders and chorea in a tauopathy without ophthalmoplegia, parkinsonian syndrome, nor dementia (progressive supranuclear palsy?): clinicopathological study. Rev Neurol(Paris) 2008;164:377–383. doi: 10.1016/j.neurol.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 32.Pretnar-Oblak J, Zaletel M, Hajnsek TM, Meglic B, Hocevar-Boltezar I, Popovic M. Isolated bulbar paralysis in a patient with medullar tau pathology: a case report. J Neurol Neurosurg Psychiatry. 2010;81:847–849. doi: 10.1136/jnnp.2008.169029. [DOI] [PubMed] [Google Scholar]
- 33.Dutschmann M, Menuet C, Stettner GM, et al. Upper airway dysfunction of Tau-P301L mice correlates with tauopathy in midbrain and ponto-medullary brainstem nuclei. J Neurosci. 2010;30:1810–1821. doi: 10.1523/JNEUROSCI.5261-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep state switching. Neuron. 2010;68:1023–1042. doi: 10.1016/j.neuron.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- 36.Louie K, Wilson MA. Temporally structured replay of awake hippocampal ensemble activity during rapid eye movement sleep. Neuron. 2001;29:145–156. doi: 10.1016/s0896-6273(01)00186-6. [DOI] [PubMed] [Google Scholar]
- 37.Luppi PH, Clement O, Sapin E, et al. The neuronal network responsble for paradoxical sleep and its dysfunctions causing narcolepsy and rapid eye movement (REM) behavior disorder. Sleep Med Rev. 2011;15:153–163. doi: 10.1016/j.smrv.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 38.Karagogeos D. Neural GPI-anchored cell adhesion molecules. Front Biosci. 2003;8:s1304–s1320. doi: 10.2741/1214. [DOI] [PubMed] [Google Scholar]
- 39.Hashimoto T, Yamada M, Maekawa S, Nakashima T, Miyata S. IgLON cell adhesion molecule Kilon is a crucial modulator for synapse number in hippocampal neurons. Brain Res. 2008;1224:1–11. doi: 10.1016/j.brainres.2008.05.069. [DOI] [PubMed] [Google Scholar]
- 40.Derfuss T, Linington C, Hohlfeld R, Meinl E. Axo-glial antigens as targets in multiple sclerosis: implications for axonal and grey matter injury. J Mol Med. 2010;88:753–761. doi: 10.1007/s00109-010-0632-3. [DOI] [PubMed] [Google Scholar]
- 41.Grimwood J, Gordon LA, Olsen A, et al. The DNA sequence and biology of human chromosome 19. Nature. 2004;428:529–535. doi: 10.1038/nature02399. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Segment 1 (patient 1). Rapid periodic leg movements during undifferentiated NREM sleep.
Segment 2 (patient 1). Finalistic movements mimicking eating during poorly structured N2 sleep.
Segment 3 (patient 3). Finalistic movements. The patient works as a TV antenna installer and it seems that he is acting as manipulating some wires.
Segment 4 (patient 3). Jerks during REM sleep.
Segment 5 (patient 3). During N3 sleep without CPAP mask, stridor is present.