Summary
Foxp3+ T regulatory (Treg) cells prevent inflammatory disease but the mechanistic basis of suppression is not understood completely. Gene silencing by RNA interference can act in a cell-autonomous and non-cell-autonomous manner, providing mechanisms of intercellular regulation. Here, we demonstrate that non-cell-autonomous gene silencing, mediated by miRNA-containing exosomes, is a mechanism employed by Treg cells to suppress T-cell-mediated disease. Treg cells transferred microRNAs (miRNA) to various immune cells, including T helper 1 (Th1) cells, suppressing Th1 cell proliferation and cytokine secretion. Use of Dicer-deficient or Rab27a and Rab27b double-deficient Treg cells to disrupt miRNA biogenesis or the exosomal pathway, respectively, established a requirement for miRNAs and exosomes for Treg-cell-mediated suppression. Transcriptional analysis and miRNA inhibitor studies showed that exosome-mediated transfer of Let-7d from Treg cell to Th1 cells contributed to suppression and prevention of systemic disease. These studies reveal a mechanism of Treg-cell-mediated suppression mediated by miRNA-containing exosomes.
Highlights
-
•
Foxp3+ Treg-cell-derived exosomes contain distinct miRNAs
-
•
miRNAs and the exosomal pathway are required for proficient Treg cell function
-
•
Treg-cell-derived exosomes suppress Th1 cells in a Let-7d-dependent manner
The mechanisms through which T regulatory (Treg) cells prevent inflammation are not fully understood. Okoye et al. show that Treg cells release exosomes that transfer miRNAs to target T helper cells and suppress T-cell-mediated disease.
Introduction
Inflammation after infection, autoimmunity, or allergy is controlled to prevent immune-mediated pathologies (Nathan, 2002). T regulatory (Treg) cells prevent such inflammatory damage (Josefowicz et al., 2012; Sakaguchi, 2000; Shevach, 2000), but the precise mechanisms of suppression are incompletely understood. RNA interference (RNAi), pioneered in plants (Voinnet and Baulcombe, 1997) and worms (Fire et al., 1998), provides a mechanism of sequence-specific gene silencing, which functions in a cell-autonomous and non-cell-autonomous manner. Multivesicular bodies (MVBs) release intracellular vesicles formed from endosomal membrane, including exosomes, that have encapsulated cystolic contents (Théry, 2011). This exosomal pathway can sample miRNAs from donor cells and transfer miRNAs and other bioactive material between cells (Valadi et al., 2007), providing a vehicle for intercellular communication. Indeed, miRNA-containing exosomes have been isolated from various bodily fluids (Théry, 2011) and can regulate gene expression in various target cells (Kosaka et al., 2010).
Within the immune system, exosomes are released by a variety of innate (Théry et al., 2002; Valadi et al., 2007; Zitvogel et al., 1998) and adaptive (Bryniarski et al., 2013; Mittelbrunn et al., 2011; Raposo et al., 1996; Smyth et al., 2013) immune cells with thymus-derived exosomes capable of influencing lymphocyte differentiation (Wang et al., 2008). Furthermore, T-lymphocyte-derived exosomes can transfer miRNAs to dendritic cells, modulating gene expression in recipient cells (Mittelbrunn et al., 2011). In this study, we tested whether Treg cells release exosomes and whether miRNA-containing exosomes contribute to Treg-cell-mediated control of immune responses. In summary, we observed that Treg cells released a significant quantity of miRNA-containing exosomes and that miRNAs were transferred to Th1 cells in vitro and in vivo. Coculture and transcriptional analyses identified that Treg cells transferred Let-7d to Th1 cells, suppressing Th1 cell proliferation and IFN-γ secretion. More importantly, miRNA biogenesis and the ability of Treg cells to release exosomes were both required for Treg cells to suppress Th1 cell proliferation in vivo and prevent systemic disease.
Results
Treg Cells Release Exosomes
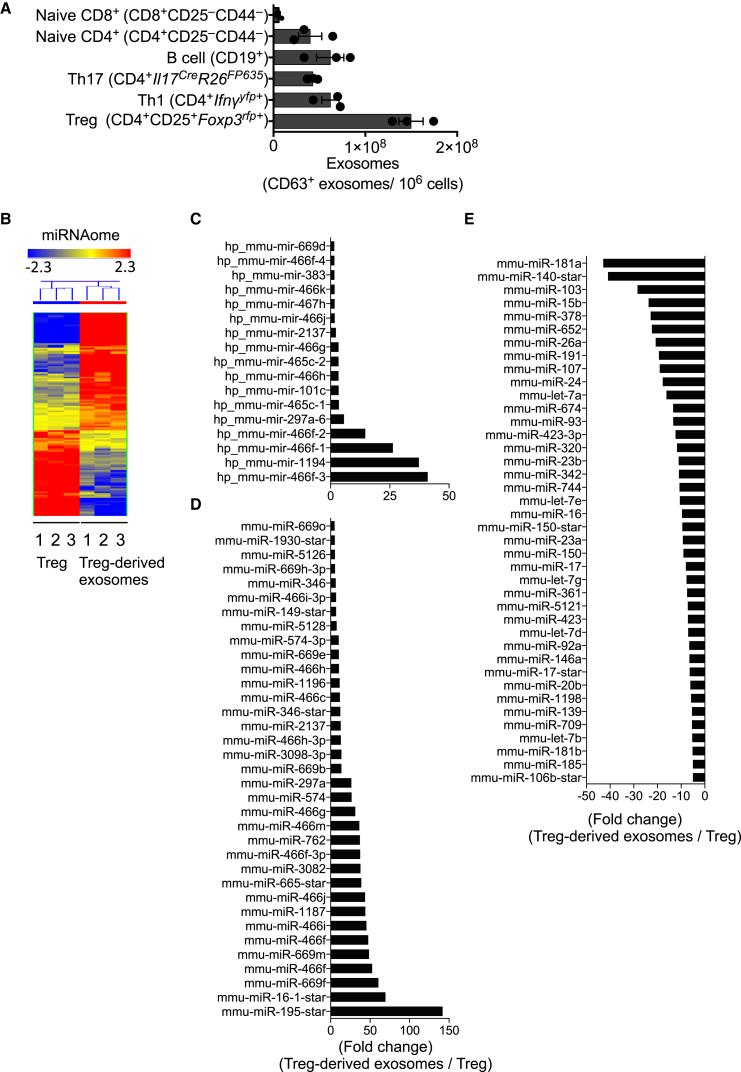
Exosome release was quantified from different lymphocytes, including CD4+ and CD8+ naive T cells, T helper 1 (Th1) and Th17 cells, Treg cells, and CD19+ B cells, using CD63 as an exosomal surface marker, which correlated with other exosome markers including CD9 and CD81 (Figures S1A–S1C available online). After activation of various T cells, with anti-CD3 and anti-CD28, or B cells, with CD40L and LPS, Treg cells released more CD63+ exosomes per cell than other lymphocytes, despite only a minor change in cell number (Figures 1A, S1D, and S1E). Factors that regulate Treg cells, including interleukin 2 (IL-2) (Boyman et al., 2006; Fontenot et al., 2005), Amphiregulin (Areg) (Zaiss et al., 2013), or all-trans retinoic acid (AT-RA) (Nolting et al., 2009), also regulated exosome release (Figures S1F and S1G) without any appreciable change in Treg cell number or metabolic activity over the culture period (Figure S1H). Despite 60% fewer exosomes released from naive T cells compared to Treg cells, exosome release from naive CD4+ T cells was also regulated by IL-2 (Figure S1G). Exosome release is regulated by changes in intracellular calcium (Ca2+) (Savina et al., 2003), hypoxia (King et al., 2012), and sphingolipid ceramide synthesis (Trajkovic et al., 2008). Similarly, exosome release by Treg cells was regulated by the calcium ionophore, monensin, hypoxia, and ceramide biosynthesis (Figure S1F).
Figure 1.
Treg Cells Produce More Exosomes than Other Lymphocytes that Carry Distinct miRNAs
(A) Exosomes were purified and quantified by CD63 ELISA from the supernatant of 106 stimulated primary lymphocytes.
(B) Total RNA was isolated from three biological replicates of Treg cells or purified Treg-cell-derived exosomes and used for miRNA microarrays.
(C–E) Premature (C) and mature upregulated (D) and mature downregulated (E) miRNAs in Treg-cell-derived exosomes are expressed relative to expression of premature and mature miRNAs in parental Treg cells. A representative of two or three experiments shown.
Error bars are SD.
miRNAs are essential for Treg-cell-autonomous functions (Liston et al., 2008; Zhou et al., 2008). However, miRNAs have also been observed in exosomes, released from Jurkat T cell lines (Mittelbrunn et al., 2011) and other T cell clones (Nolte-’t Hoen et al., 2012), raising the possibility that miRNAs may also function in a non-T-cell-autonomous manner. Using genome-wide miRNA analysis, we found that Treg-cell-derived exosomes contained both premature and mature miRNAs (Figures 1B–1E, Table S1). Furthermore, the composition of miRNAs in Treg-cell-derived exosomes was distinct from that of parental Treg cells. Of note, the most abundant miRNAs in Treg-cell-derived exosomes (miR-466 family [Druz et al., 2011], miR-195 [Yang et al., 2012], and miR-16 [Cai et al., 2012]) have either proapoptotic or antiproliferative properties. Microarray analysis of Th1- and Th2-cell-derived exosomes revealed a different suite of miRNAs to those identified in Treg-cell-derived exosomes (Figures S1I–S1K), suggesting that different T cells release different miRNAs. In addition to miRNAs, genome-wide mRNA analysis identified many mRNA transcripts enriched in Treg-cell-derived exosomes (Figure S2 and Table S2). Unbiased pathway analysis of mRNA transcripts in Treg-cell-derived exosomes identified a profile consistent with the suppression of T cells (Figure S2D), suggesting that Treg-cell-derived exosomes may be involved in T cell regulation.
miRNAs Are Transferred from Treg Cells to Conventional T Effector Cells In Vitro and In Vivo
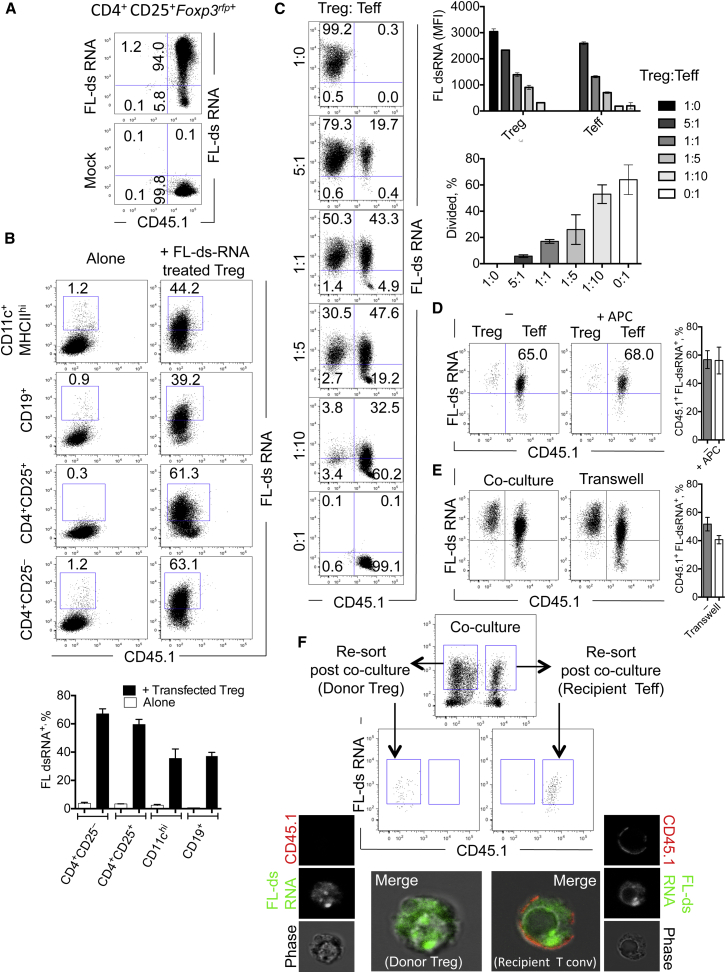
To test whther RNA was transferred between immune cells, we developed a flow-cytometry-based system using Treg cells transfected with fluorescent oligonucleotide duplexes (FL-dsRNA) (Figure 2A), allowing us to track Treg-cell-derived dsRNA in single cells. When we cocultured Treg cells with other leukocytes, Treg cells transferred FL-dsRNA to ∼60% of cocultured naive T cells (CD3+CD4+CD25–) or other Treg cells (CD3+CD4+Foxp3rfp+CD25+) and to ∼40% of cocultured DCs (CD11c+MHCIIhi) or B cells (CD3–CD19+) (Figure 2B). Using an in vitro suppression assay with a decreasing number of FL-dsRNA-transfected Treg cells to conventional T effector (Teff) cells, we observed a dose-dependent transfer of FL-dsRNA to Teff cells (Figure 2C), with the amount of FL-dsRNA transferred correlated with the decrease in target cell proliferation. These data suggest that transfer of RNA from Treg cells to conventional T cells may be involved in the suppression of conventional T cells.
Figure 2.
Treg Cells Transfer RNA to Various Leukocyte Populations In Vitro
(A) Primary Treg cells (CD4+CD25+Foxp3rfp+) were transfected with fluorescently labeled double stranded RNA (100 nM, FL-dsRNA), rested overnight, washed, and analyzed for transfection efficiency.
(B) Splenic dendritic cells (CD11c+MHCII+), B cells (CD19+CD4–CD8–CD25–CD44–), Treg cells (CD4+CD25+), and naive T cells (CD4+CD25–CD44–) were cultured alone (left) or with an equal number of FL-ds RNA-transfected Treg cells (right). The percentage of FL-ds RNA+ nontransfected cocultured cells were quantified after 48 hr of coculture.
(C) FL-dsRNA-transfected Treg cells were cocultured with naive conventional T cells (CD4+CD25–CD44–) at the indicated ratios on anti-CD3 (1 μg/ml)- and anti-CD28 (5 μg/ml)-coated plates for 3 days. The proliferation of conventional T cells and MFI of either transfected Treg cells and nontransfected conventional T cells were quantified by FACS.
(D) FL-dsRNA-transfected Treg cells were cocultured with naive conventional T cells (CD4+CD25–CD44–) in the presence or absence of APCs (CD11c+MHCII+), as indicated, for 24 hr.
(E) FL-dsRNA-transfected Treg cells were cultured with naive conventional T cells (CD4+CD25–CD44–) separated by a 0.4 μm filter in a transwell assay system.
(F) CD4+CD25hiCD45.2+ Treg cells were transfected with FL-dsRNA and cocultured with CD4+CD25–CD44–CD45.1+ conventional T cells for 24 hr. After 24 hr of coculture, FL-dsRNA+ Treg cells or conventional T cells were resorted and analyzed by confocal microscopy to identify the cellular location of the FL-dsRNA+. A representative of three experiments shown.
Error bars are SD.
RNA can be transferred from T cells to antigen-presenting cells (APCs) (Mittelbrunn et al., 2011) and between various APC populations (Montecalvo et al., 2012). However, addition of APCs to Treg cell and conventional T cell cocultures had no impact on RNA transfer to conventional T cells (Figure 2D). HIV-1 virus can be transferred between T cells in nanotubules (Sowinski et al., 2008) in a cell-contact-dependent manner. However, when Treg cells and conventional T cells were cultured in a transwell system, physically separating Treg cell and conventional T cells beyond the reach of nanotubes, RNA transfer was only slightly reduced (Figure 2E), suggesting transfer of RNA by an extracellular microvesicle-related mechanism.
To determine whether FL-dsRNA was being transferred into the cytoplasm of recipient conventional T cells from donor Treg cells, rather than simply decorating the surface of recipient cells, we cocultured Treg cells and conventional T cells and then FACS purified each population after coculture. Using anti-CD45.1 membrane stain on the recipient cell, we verified that the FL-dsRNA was indeed delivered inside the recipient cell (Figure 2F).
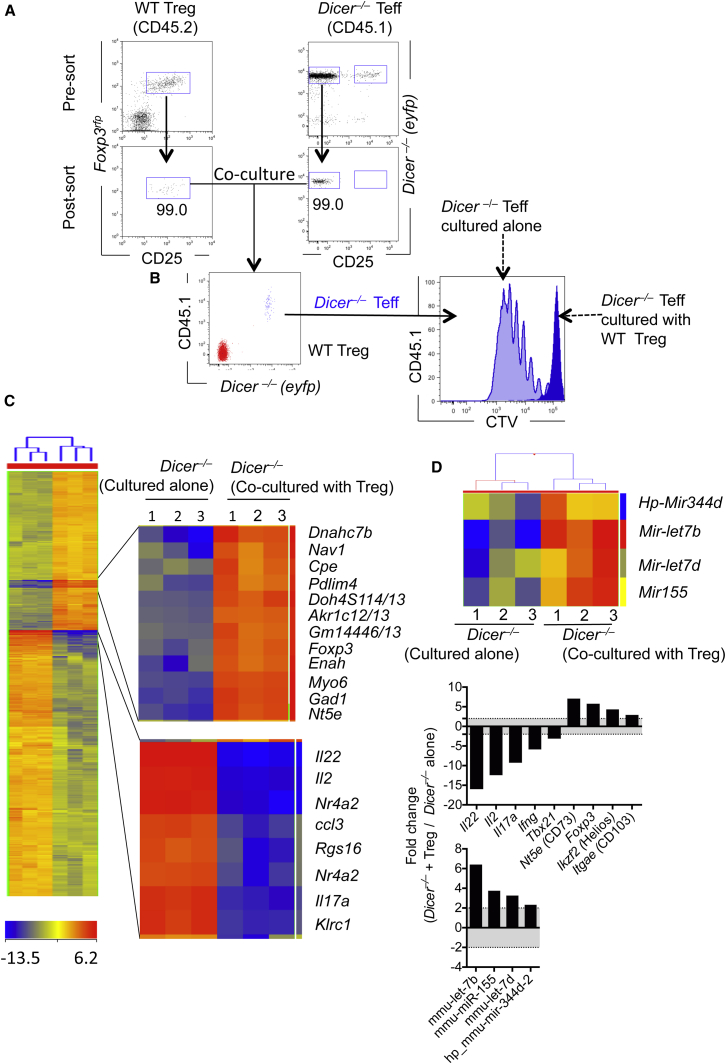
Next, we took advantage of the dependency on Dicer for mature miRNA processing (Hutvágner et al., 2001) to formally test whether miRNAs were transferred from Treg cells to conventional T cells in vitro. Treg cells from miRNA-sufficient CD45.2 WT mice were cocultured with miRNA-deficient conventional T cells from congenic CD45.1+Cd4CreDicerfl/flR26eyfp (Dicer–/–) mice, which were labeled with cell trace violet (Figure 3A). This system allowed us to (1) accurately discriminate and separate each population, (2) assess proliferation of Dicer–/– conventional T cells, and (3) repurify Dicer–/– conventional T cells after coculture to identify the presence of any acquired miRNAs. As expected, Treg cells suppressed (Figure 3B) and transcriptionally altered (Figure 3C) Dicer–/– T cells. Several inflammatory cytokines (Il22, Il2, il17a, Ifng) were downregulated in “suppressed” Dicer–/– T cells, with the concomitant increase in several regulatory genes (Cd73, Foxp3, Ikzf2 [encoding Helios], and Cd103) (Figure 3C, Table S3), relative to Dicer–/– T cells cultured alone. Concurrently, Dicer–/– conventional T cells acquired three mature (miR-155, Let-7b, and Let-7d) and one pre-miRNA (Hp_miR-344d-2) from Treg cells (Figures 3D and S2E). Using a Dicer-sufficient (WT) congenic system with CD45.2+ WT Treg cells and CD45.1+ WT conventional T cells, we also observed an increase in miR-155, Let-7d, and Let-7b in Dicer-sufficient WT conventional T cells, when cocultured with WT Treg cells (Figure S2F), further supporting the observation that miRNAs were transferred between cells. Finally, using CD45.2+ mir155–/– conventional T cells as recipient cells, cocultured with CD45.1+ mir155+/+ (WT) Treg cells, we confirmed the transfer of miR-155 from Treg to mir155–/– conventional T cells (Figure S2G). Taken together these data support the notion that Treg cells transfer specific miRNAs to conventional T cells, suppressing effector gene expression and proliferation.
Figure 3.
Treg Cells Transfer miR-155, Let-7b, and Let-7d to T Cells In Vitro
(A) FACS-purified Dicer–/– cell-trace violet (CTV)-labeled conventional T cells (CD45.1+Cd4CreDicerfl/flR26eyfp+CD4+CD25–) were cultured alone or with primary Treg cells (CD45.2+CD4+CD25+Foxp3rfp) for 3 days on anti-CD3 (1 μg/ml)- and anti-CD28 (5 μg/ml)-coated plates.
(B) Proliferation of Dicer–/– conventional Teff cells was assessed and FACS sorted.
(C and D) RNA was extracted from three biological replicates of CD45.1+Cd4CreDicerfl/flR26eyfpCD4+CD25 Teff cells after being cultured alone or with WT Treg cells. Heatmaps of differentially expressed genes used for mRNA (C) or miRNA (D) analysis. Graph on right highlights expression of inflammatory and regulatory genes in CD45.1+Dicer–/– conventional T cells cocultured with WT Treg cells, expressed relative to CD45.1+Dicer–/– conventional T cells cultured alone. A representative of three experiments shown, with three biological replicates used in the microarray analysis.
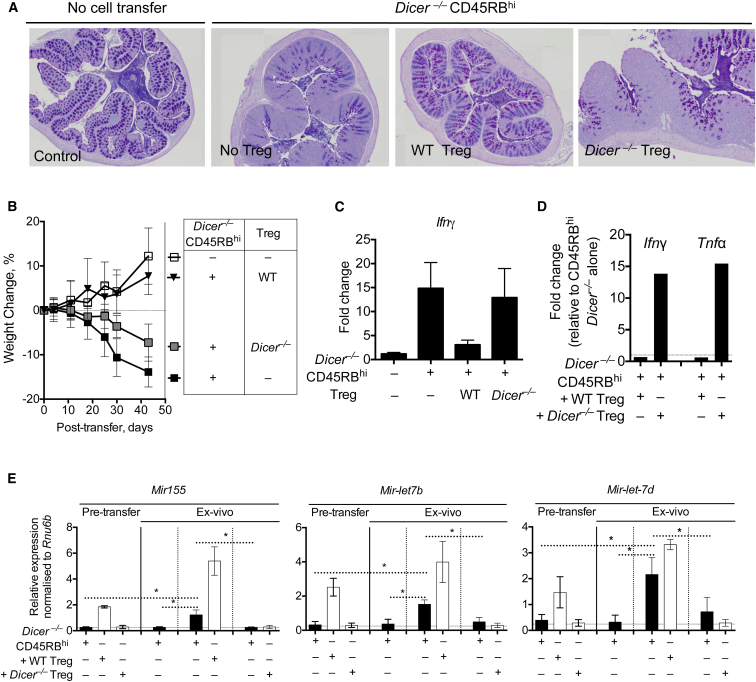
The adoptive transfer of Treg-cell-depleted CD4+CD45RBhi T cells into T-cell-deficient mice leads to systemic inflammation (Powrie et al., 1994), which can be prevented by the cotransfer of Treg cells (Figures S3A–S3E). Despite the loss of miRNAs, Dicer–/– CD45RBhi T cells also caused a similar wasting disease with systemic inflammation (Figures 4A and 4B) and IFN-γ production (Figures 4C, 4D, and S3F–S3H), which could be prevented by cotransfer of WT, but not Dicer–/–, Treg cells. Because Dicer–/– CD45RBhi cells retained pathogenicity and sensitivity to Treg-cell-mediated control, we were able to test whether miRNAs were transferred to Dicer–/– CD45RBhi cells in vivo. After 5 weeks, “pathogenic” CD4+Dicer–/– YFP+ (Dicer–/– CD45RBhi cells transferred alone) or “regulated” CD4+Dicer–/– YFP+ (Dicer–/– CD45RBhi cells cotransferred with WT Treg cells) were recovered ex vivo to determine whether Dicer–/– cells acquired miRNAs in vivo (Figure S4A). Consistent with a suppressed state, regulated CD4+Dicer–/– YFP+ cells had reduced Ifng and Tnf expression (Figure 4D), compared to pathogenic CD4+Dicer–/– YFP+ cells. miRNA analysis of CD4+Dicer–/– CD45RBhi cells pretransfer and pathogenic and regulated CD4+Dicer–/– YFP+ cells isolated ex vivo confirmed our in vitro observations (Figure 3) and identified the presence of miR-155, Let-7b, and Let-7d in regulated CD4+Dicer–/– YFP+ cells, when WT Treg cells had been cotransferred (Figure 4E). In contrast, miR-155, Let-7b, and Let-7d weres not observed in pathogenic CD4+Dicer–/– YFP+ cells, when no Treg cells were transferred, suggesting that WT Treg cells either supported or directly transferred miRNAs to Dicer–/– cells. Relative to a housekeeping small RNA, RNU6B, regulated CD4+Dicer–/– YFP+ cells had almost as much miR-155, Let-7b, and Let-7d as WT Treg cells pretransfer, suggesting that a large amount of RNA was being transferred. Of note, WT Treg cells recovered ex vivo had elevated expression of miR-155, Let-7b, and Let-7d compared to WT Treg cells pretransfer (Figures 4E and S4B), suggesting that activated Treg cells also increase transcription of these miRNAs.
Figure 4.
Dicer–/– Treg Cells Fail to Suppress Systemic Inflammation and Transfer miR-155, Let7-b, and Let-7d to Conventional T Cells In Vivo
Analysis of disease in mice after transfer of Dicer–/– CD4+CD45RBhi T cells with or without cotransfer of WT and Dicer–/– Treg (CD4+CD25hi) cells.
(A) Histopathology of colon sections stained with AB-PAS 5 weeks after cell transfer.
(B) Weight loss after Dicer–/– CD45RBhi cell transfer alone or with WT or Dicer–/– Treg cells.
(C) Expression of Ifng in the colon of mice 5 weeks after cell transfer.
(D) Expression of Ifng and Tnf in ex vivo recovered Dicer–/– conventional T cells (CD4+CD25– eYFP+Dicer–/–) 5 weeks after cell transfer, as shown in Figure S4, from mice that received either WT (Dicer–/– + WT Treg cells) or Dicer–/– (Dicer–/– + Dicer–/– Treg cells) CD4+CD25hi Treg cells, mRNA expressed relative to Dicer–/– CD45RBhi cell transfer alone. A representative of three experiments shown.
(E) Expression of miR-155, Let-7b, Let-7d in eYFP+Dicer–/–CD45Rbhi cells, WT Treg cells, and Dicer–/– Treg cells before transfer (left three bars) or in ex vivo recovered, FACS-purified Dicer–/– effector T cell (CD4+CD25– eYFP+Dicer–/–) 5 weeks after cell transfer, from mice that received either Dicer–/– effector T cells alone, Dicer–/– effector T cells with WT Treg cells, or Dicer–/– conventional T cells with Dicer–/– Treg cells. Dicer–/– cells (black bars) or Treg cells (white bars). miRNA expression relative to Rnu6b. A representative of three experiments shown. ∗p < 0.05.
Error bars are SD.
Within miRNA-sufficient Rag2–/– hosts, it was conceivable that the regulated CD4+Dicer–/– YFP+ cells acquired miRNAs from non-Treg cells. We therefore used an additional control of Dicer–/– Treg cells, cotransferred with Dicer–/– CD45RBhi cells. Dicer–/– Treg cells failed to suppress disease. Furthermore, Dicer–/–CD4+YFP+ cells from mice that were cotransferred with Dicer–/– Treg cells did not have measurable miR-155, Let-7b, or Let-7d (Figure 4E). These data demonstrate that Treg-cell-mediated suppression is accompanied by the transfer of these three, and possibly other, miRNAs from Treg cells.
Treg-Cell-Mediated Suppression Is Rab27 Dependent
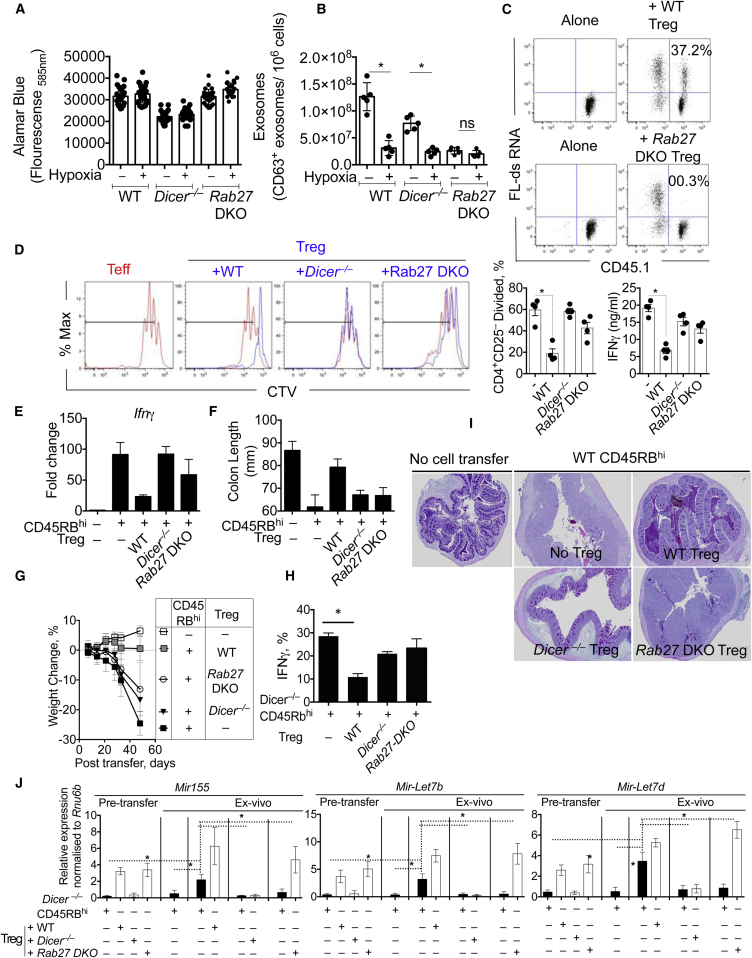
Exosome release requires Rab27a and Rab27b for docking multivesicular endosomes (MVE) to Rab27 effectors on the plasma membrane (Fukuda, 2013; Ostrowski et al., 2010; Singh et al., 2013). To test the role of Rab27 and exosome release, we purified Treg cells from Rab27ashen/ashenRab27b–/– double knockout mice (Rab27-DKO) and stimulated these cells, as above. Compared to WT and Dicer–/– Treg cells, Rab27-DKO Treg cells failed to release exosomes with no change in cell viability (Figures 5A and 5B), confirming the requirement of Rab27 for exosome release from Treg cells. Under hypoxic conditions, exosome release was significantly curtailed in WT and Dicer–/– Treg cells (Figure 5B). Rab27-DKO Treg cells also failed to transfer FL-dsRNA from Treg cells to conventional Teff cells (Figure 5C), indicating that a Rab27-regulated exosomal pathway was responsible for transferring RNA between T cells. Furthermore, when cocultured with Th1 cells, Rab27-DKO Treg cells failed to suppress Th1 cells, similar to Dicer–/– Treg cells (Figure 5D; Liston et al., 2008; Muljo et al., 2005). These data demonstrate that Rab27 is essential for (1) exosome release from Treg cells, (2) RNA transfer from Treg cells to other T cells, and (3) proficient Treg-cell-mediated suppression in vitro.
Figure 5.
The GTPase Rab27 Is Needed for Treg Cell Exosome Secretion and Is Essential for Treg Cell Function In Vitro and In Vivo
Analysis of Treg cell function in vitro and in vivo after transfer of CD4+CD45RBhi T cells with or without transfer of CD4+CD25hi WT, Dicer–/–, or Rab27-DKO Treg cells.
(A) WT, Dicer–/–, or Rab27-DKO Treg cells were stimulated on anti-CD3 (1 μg/ml)- and anti-CD28 (5 μg/ml)-coated plates for 3 days. Cell viability/proliferation was determined by Alamar blue fluorescence intensity in culture supernatants.
(B) Exosomes were purified from the supernatant of stimulated WT, Dicer–/–, or Rab27-DKO Treg cells, as in Figure 1.
(C) CD45.2+ WT Treg and CD45.2+Rab27-DKO Treg cells were transfected with FL-dsRNA and cocultured naive WT CD45.1+ conventional Teff cells (CD4+CD25–CD44–) on anti-CD3 (1 μg/ml)- and anti-CD28 (5 μg/ml)-coated plates. After 24 hr, cells were stained for CD45.1 and analyzed for FL-dsRNA by FACS.
(D) Proliferation of violet-labeled in vitro generated Th1 cells cultured alone (red trace in all four plots) or in presence of WT, Dicer–/–, or Rab27-DKO Treg cells (blue trace) for 3 days. Summarized in bar chart showing the percentage of divided cells in the presence of the indicated Treg cell population. IFN-γ measured in the supernatant of cocultured cells.
(E) Rag2–/– mice were given CD4+CD45RBhi T cells with or without transfer of CD4+CD25hi WT, Dicer–/–, or Rab27-DKO Treg cells. Expression of Ifng in the colon of mice 5 weeks after cell transfer.
(F) Colon length was measured 5 weeks after cell transfer.
(G) Weekly weight measurements after cell transfer, as in (E).
(H) Percentage of IFN-γ+ cells in the mesenteric lymph nodes 5 weeks after cell transfer.
(I) Histopathology of large intestine 5 weeks after cell transfer.
(J) Rag2–/– mice were given Dicer–/– CD4+CD45RBhi T cells with or without transfer of CD4+CD25hi WT, Dicer–/–, or Rab27-DKO Treg cells. Expression of miR-155, Let-7b, Let-7d in eYFP+Dicer–/–CD45Rbhi cells, WT Treg cells, Dicer–/– Treg cells, and Rab27-DKO Treg cells before transfer (left four bars) or in ex vivo recovered, FACS-purified Dicer–/– conventional T cells (CD4+CD25– eYFP+Dicer–/–) 5 weeks after cell transfer, from mice that received either Dicer–/– effector T cells alone, Dicer–/– conventional T cells with WT Treg, Dicer–/– effector T cells with Dicer–/– Treg cells, or Dicer–/– conventional T cells with Rab27-DKO Treg cells. Dicer–/– cells (black bars) or Treg cells (white bars). miRNA expression relative to Rnu6b. A representative of at least three experiments shown. ∗p < 0.05.
Error bars are SD.
Beyond a failure to release exosomes, purified splenic Rab27-DKO Treg cells were very similar to WT Treg cells, with a comparable frequency (Figure S5A), transcriptional profile (Figures S5B, qRT-PCR, and S5C, microarray), and intracellular and surface Treg cell marker and tetraspanin expression profile (Figure S5D), indicating that Rab27 was not required for Treg cell development or many other features of Treg cells. Furthermore, the deletion of Rab27 did not impact the ability of Rab27-DKO Th cells to secrete cytokines (Figure S5E), migrate, and mediate disease when transferred into Rag2–/– hosts in vivo (Figures S5F–S5H), indicating that Rab27a and Rab27b, unlike SNAREs and Rho GTPases (Alonso and Millán, 2001), are primarily required for the exosomal pathway.
To test whether Rab27-dependent exosome release contributed to Treg cell function in vivo, we transferred WT or Rab27-DKO Treg cells with WT CD45RBhi T cells into Rag2–/– hosts. Similar to miRNA-ablated Dicer–/– Treg cells, Rab27-DKO Treg cells failed to prevent disease with significantly elevated Ifng expression (Figure 5E), colon shortening (Figure 5F), and weight loss (Figure 5G) with IFN-γ+ T cell recruitment (Figure 5H), despite a similar frequency of Treg cells observed (Figure S6A). Failure of Rab27-DKO Treg cells to control effector T cells also led to significant colonic and systemic inflammation and Ifng expression (Figures 5I, S6B, and S6C), demonstrating that Rab27-dependent exosome release was essential for Treg cell function in vitro and in vivo.
Using Dicer–/– CD4+CD45RBhi effector cells cotransferred with WT, Dicer–/–, or Rab27-DKO Treg cells, we identified increased amounts of miR-155, Let-7b, and Let-7d in ex vivo regulated CD4+Dicer–/– YFP+ cells that were cotransferred with WT, but not Dicer–/– or Rab27-DKO, Treg cells (Figure 5J). These data support and extend our in vitro and in vivo observations, indicating that both Dicer and Rab27 sufficiency in Treg cells is required for the transfer of miRNAs, suppression of effector T cells, and prevention of disease. Of note, Rab27-DKO mice suffered from mild pulmonary inflammation with increased granulocyte influx and abnormal airway epithelium (Bolasco et al., 2011). Upon further examination, aged Rab27-DKO mice had inflammatory foci in the lung, liver, and colon, which may be due to compromised Treg-cell-mediated immune regulation (Figure S6D).
Let-7d in Treg-Cell-Derived Exosomes Contributes to Suppression of Th1 Cells
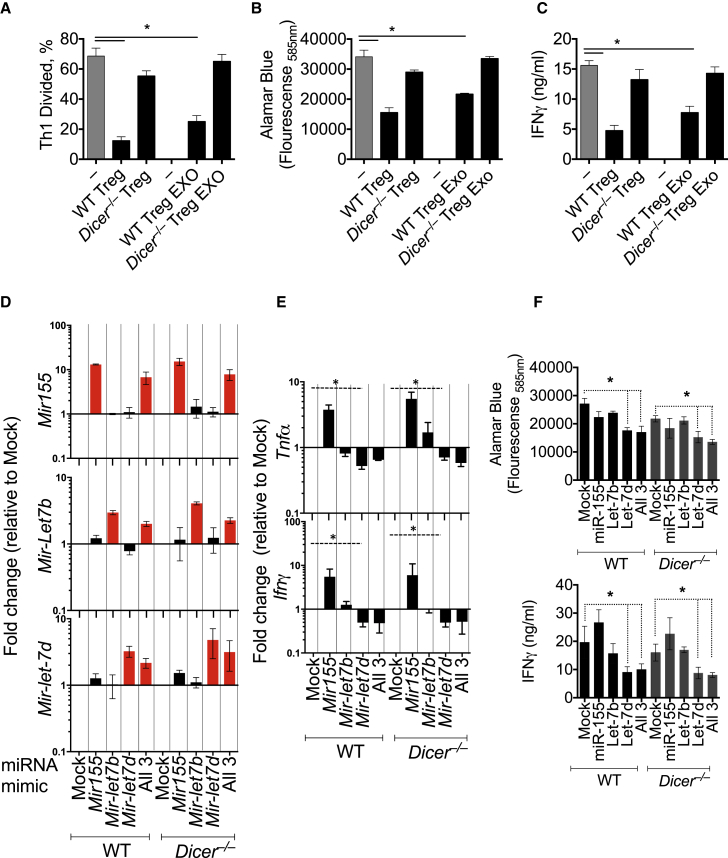
To determine whether miRNAs in Treg-cell-derived exosomes were suppressive, we purified exosomes released from WT and Dicer–/– Treg cells. WT, but not Dicer–/–, Treg cells suppressed Th1 cell proliferation, as previously reported (Liston et al., 2008; Muljo et al., 2005). Similarly, 107 exosomes from WT, but not Dicer–/–, Treg cells suppressed Th1 cell proliferation (Figures 6A, 6B, S6E, and S6F) and IFN-γ secretion (Figure 6C), indicating that exosomes were suppressive in a Dicer-dependent manner. Although Th1 cells could transfer RNA to conventional T cells in vitro (Figure S6G), Th1-cell-derived exosomes had no impact on Th1 cell differentiation (Figure S6H). Th1 cells had reduced amounts of Let-7d, compared to naive T cells (Figure S7A), and Th1-cell-derived exosomes were devoid of Let-7d (Figure S1I), providing one explanation of why Th1-cell-derived exosomes could not suppress Th1 cells.
Figure 6.
Treg-Cell-Derived Exosomes and Exogenous Let-7d Suppress Th1 Cell Proliferation and IFN-γ Secretion
(A–C) Cell trace violet-labeled Th1 cells were cultured with WT or Dicer–/– Treg cells, as in Figure 5D, or with exosomes recovered from WT or Dicer–/– Treg cells.
(A) Cell proliferation was determined by cell trace violet dilution.
(B) Alamar blue fluorescence intensity was determined in culture supernatants.
(C) IFN-γ secretion determined was determined in culture supernatants by ELISA.
Th1 cells were transfected with miR-155, Let-7b, Let-7d miRNA mimics (100 nM), as indicated.
(D) miR-155, Let-7b, and Let-7d were measured in Th1 cells, 24 hr after transfection with miR-155, Let-7b, Let-7d miRNA mimics. miRNAs are expressed relative to mock-transfected cells.
(E) Tnf and Ifng expression was measured in Th1 cells, 24 hr after transfection with miR-155, Let-7b, Let-7d miRNA mimics. mRNAs are expressed relative to mock-transfected cells.
(F) Proliferation (top) of transfected cells was determined by Alamar blue fluorescence tin culture supernatants. IFN-γ secretion (bottom) was determined by ELISA. A representative of three experiments shown.
∗p < 0.05. Error bars are SD.
Following the observation that miR-155, Let-7b, and Let-7d were transferred from Treg cells to conventional T cells, we tested whether miR-155, Let-7b, or Let-7d, alone or in combination, were responsible for suppression of Th1 cells. Transfection of Th1-cell-polarized WT or Dicer–/– T cells with miRNA mimics of miR-155, Let-7b, Let-7d, or all three miRNAs increased the expression of each respective miRNA (Figure 6D). Consistent with previous reports, miR-155 enhanced IFN-γ (Banerjee et al., 2010) and TNF-α secretion, without any appreciable change in proliferation (Figures 6E and 6F), indicating that miR-155 promoted, rather than inhibited, Th1 cell responses. Transfection of Th1 cells with Let-7b had little impact on IFN-γ production or proliferation (Figures 6E and 6F). In contrast, transfection with Let-7d, alone or in combination with either Let-7b or miR-155, significantly reduced Tnf and Ifng mRNA (Figure 6E), Th1 cell proliferation, and IFN-γ secretion (Figure 6F), suggesting that the transfer of Let-7d from Treg cells to Th1 cells may be an important intermediary of Treg-cell-mediated suppression of Th1 cells.
Target prediction analyses (Miranda et al., 2006) identified many putative Let-7d targets involved in Th1 cells (Il12r, Tbx21, Stat1 and Stat4, and Ifng). We therefore analyzed the miRNA and mRNA profile of FACS-purified IFN-γyfp+ Th1 cells, compared to naive T cells, and identified reduced premature and mature Let-7d with an increase in many Let-7d target genes (Figure S7A). Specifically, Let-7d was predicted to target Ptgs2 (Cox-2), which was elevated in Th1 cells (Figures S6 and S7). Testing the requirement of Cox-2 using a selective Cox-2 inhibitor (celecoxib), we noted that Cox-2 inhibition ablated IFN-γ secretion (Figure S6J), indicating that Cox-2 is required for Th1 cell responses. Thus, Let-7d may target Cox-2 in Th1 cells to regulate IFN-γ secretion.
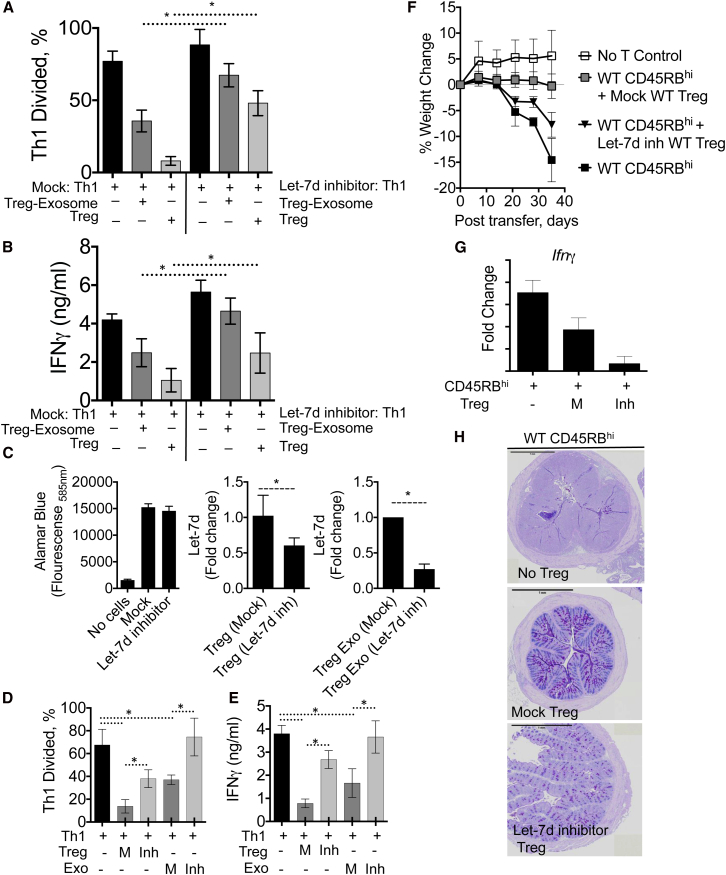
To test whether Let-7d from Treg-cell-derived exosomes contributed to the suppression of Th1 cell responses in vitro, we transfected Th1 cells with Let-7d hairpin inhibitors, to inhibit endogenous or exogenous Let-7d, and cocultured these cells with Treg cells or exosomes purified from Treg cells. Let-7d inhibitor treatment alone did not impact Th1 cell proliferation or IFN-γ secretion in the absence of Treg cells (Figures 7A, 7B, and S7E). The addition of Treg cells or Treg-cell-derived exosomes suppressed Th1 cell proliferation (Figures 7A and 7B) and IFN-γ secretion (Figure 7C). However, Treg cells and Treg-cell-derived exosomes failed to suppress Let-7d inhibitor-treated Th1 cells, indicating that sequestering endogenous or exogenous Let-7d in Th1 cells prevented Treg cell and Treg cell exosome-mediated suppression (Figures 7A–7C). To further test whether Let-7d from Treg cells contributed to Treg cell- and Treg-cell-derived exosome-mediated suppression, we transfected Treg cells with Let-7d hairpin inhibitors or control inhibitors (Figures 7D and S7). Let-7d inhibitor treatment reduced Let-7d by ∼50% in Treg cells and by ∼75% in Treg-cell-derived exosomes without any appreciable impact on cell viability (Figure 7D). Let-7d inhibitor-treated Treg cells, but not control inhibitor-treated Treg cells (Figure S7), were compromised in their ability to suppress Th1 cells, and Let-7d-depleted exosomes had completely lost their ability to suppress Th1 cells (Figures 7D and 7E). Although it remains possible that Let-7d inhibitors were transferred with Let-7d to Th1 cells in this experimental setup, taken together with the reduced Let-7d in Th1 cells (Figure S7) and the fact that Let-7d inhibitor-treated Th1 cells when cultured alone proliferated and secreted IFN-γ similar to control Th1 cells (Figures 7A and 7B, black bars), these data indicate that Let-7d contributes to Treg-cell-mediated suppression and more specifically, that Let-7d was responsible for Treg-cell-derived exosome-mediated suppression.
Figure 7.
Let-7d from Treg Cells or Treg-Cell-Derived Exosomes Is Required to Suppress Th1 Cell Responses
(A–C) Th1 cells were transfected with Let-7d miRNA short hairpin inhibitors (100 nM), or mock transfected as indicated and cocultured with Treg cells or Treg-cell-derived exosomes.
(A) Proliferation of violet cell trace-labeled Th1 cells cultured alone or in presence of Treg cells or Treg-cell-derived exosomes for 3 days.
(B) IFN-γ measured in the supernatant of cocultured cells.
(C) Freshly isolated WT Treg cells were transfected with Let-7d miRNA short hairpin inhibitors (100 nM), or mock transfected as indicated, with cell proliferation determined by Alamar blue (left) and Let-7d expression analyzed in Treg cells (middle) or in Treg-cell-derived exosomes (right).
(D) Violet cell trace-labeled Th1 cells were cocultured with mock (M)- or Let-7d inhibitor-transfected Treg cells (Inh) or with exosomes isolated from Mock (M)- or Let-7d inhibitor-transfected Treg cells (Inh), as indicated. Proliferation of violet cell trace-labeled Th1 cells was determined after 3 days. Summarized in bar chart showing the percentage of divided cells in each condition.
(E) IFN-γ measured in the supernatant of cocultured cells, as in (D).
(F–H) Analysis of disease in mice at week 5 after transfer of WT CD4+CD45RBhi T cells with or without mock-transfected or Let-7d inhibitor-transfected Treg cells. 105 Treg cells were adoptively transferred on week 2, 3, and 4.
(F) Weight loss measured weekly.
(G) Ifng expression measured in the colon at week 5 after cell transfer.
(H) Histopathology of large intestine 5 weeks after cell transfer. A representative of three experiments shown.
∗p < 0.05. Error bars are SD.
Finally, we tested whether Let-7d was required for Treg-cell-mediated suppression in vivo by transferring mock or Let-7d inhibitor-treated WT Treg cells into Rag2–/– hosts with WT CD45RBhi cells. Transfected Treg cells survive only 7–10 days in vivo (Kelada et al., 2013), so we transferred Treg cells weekly for 3 weeks (weeks 2, 3, and 4) before analysis at week 5. Mock-transfected Treg cells prevented weight loss, Ifng responses, and intestinal inflammation (Figures 7F–7H). In contrast, Let-7d inhibitor-treated Treg cells, which released Let-7d-depleted exosomes (Figure 7C), failed to prevent disease with significant weight loss, Ifng expression, and colonic inflammation (Figures 7F–7H). Collectively, these data support a mechanism of Treg-cell-mediated suppression through the release of miRNA-containing exosomes. Furthermore, we identified an important contribution of Let-7d in mediating this suppression in vitro and in vivo.
Discussion
The control of immune responses is critical for survival, with compromised Treg-cell-mediated control resulting in multiorgan lymphocytic infiltration, organ failure, and death (Clark et al., 1999). In this study, we identified a mechanism of Treg-cell-mediated suppression via non-cell-autonomous gene regulation, mediated by miRNA-containing exosomes. Exosome-deficient, miRNA-deficient, or Let-7d-ablated Treg cells all failed to transfer miRNAs and failed to prevent lethal systemic inflammation. These data support a growing paradigm of non-cell-autonomous gene regulation previously reported in plants (Dunoyer et al., 2010) and animals (Sarkies and Miska, 2013; Valadi et al., 2007) and now demonstrated here, functioning as an important feature of immune regulation.
The mechanism of exosome release from Treg cells appears similar to other cells, with the exception of monensin treatment (Savina et al., 2003), which reduced exosome release from Treg cells, in contrast to exosome release from the K562 cell line. This might be due to the particularly sensitive nature of T cells to calcium changes (Wei et al., 1999) or might indicate that Golgi-mediated protein secretions support exosome release. Several overlapping pathways appear to regulate Foxp3 stability and exosome release, including inactivation of HIF-1α (Dang et al., 2011), ceramide biosynthesis (Kue et al., 2013), IL-2 signaling (Fontenot et al., 2005), and Amphiregulin signaling (Zaiss et al., 2013), suggesting that Foxp3 might regulate exosome release or that exosome-associated pathways might support Foxp3+ cells. In support of the latter, we identified that miR-155, which is involved in Treg cell development (Kohlhaas et al., 2009), was transferred from Treg cell to conventional T cell with the concomitant upregulation of several Treg-cell-associated genes in recipient cells (Figure 3).
Whether exosome contents are preferentially packaged within the parent cell or whether they are randomly assorted is not well understood. We previously identified significant miRNA heterogeneity between distinct Treg cell populations (Kelada et al., 2013) and found that such heterogeneity was influenced by the cytokine microenvironment. We report here that the miRNA content of Treg-cell-derived exosomes is distinct from the miRNA content of Th1- or Th2-cell-derived exosomes, indicating that cell/context-specific miRNAs may be packaged into exosomes. It is tempting to speculate that the local cytokine environment that shapes the miRNA profile of Treg cells (Kelada et al., 2013) also influences the exosome content of divergent T cells and that specific miRNA-containing exosomes are targeted to different effector cells. Here, we identified that Let-7d was preferentially packaged and transferred to Th1 cells, suppressing Th1 cell proliferation and IFN-γ secretion; whether different miRNAs are delivered to different Th cells is unclear.
Similarly, the rate of packaging and transfer of miRNAs between cells has not been elucidated. Recent reports indicate that at the immunological synapse between T cells and APCs, a microvesicle-rich pocket is formed (Choudhuri et al., 2014), providing a focused and protected region between cells, potentially allowing mass and rapid transfer of material. Whether miRNAs are transferred in these regions between T cell and APC was unclear from this study. However, for T cell-to-T cell transfer, the precise mechanism of exosome delivery is yet to be clarified. Furthermore, the precise mechanism of exosome uptake by recipient cell remains largely unknown.
Regulatory T cells have a growing repertoire of means to inhibit and prevent immune cell activation (Shevach, 2009). With respect to suppression of conventional T cells, current mechanisms include short-range, cell-contact-dependent (cytolysis and inhibitory receptor engagement) and potentially longer-range, cell-contact-independent (IL-2 consumption and suppressive cytokine secretions, such as IL-10, TGF-β, and IL-35) mechanisms. However, a combination of cell contact and secreted mechanisms is most likely optimal for Treg-cell-mediated suppression. From this study we can add non-cell-autonomous gene silencing as a mode of cell-contact-independent Treg-cell-mediated suppression, via transfer of miRNAs. We observed that isolated Treg-cell-derived exosomes can suppress conventional T cells; however, this was not as efficient as Treg cells, indicating that exosome-mediated transfer and additional mechanisms are indeed required for optimal suppression. Although cell contact was not required for the transfer of RNA between cells, whether cell contact allows more rapid and focused transfer, as mentioned above, is currently under investigation. Together these observations suggest that exosome-mediated transfer of miRNAs may collaborate with other Treg cell mechanisms for optimal suppression. For example, Treg cells anchored to other T cells via galectins (Garín et al., 2007) or to APCs via CTLA-4 might be required for most efficient delivery of miRNA-containing exosomes to target cells. From data presented here, the ability of Treg cells to release microvesicles in a Rab27-dependent manner, and specifically transfer Let-7d to target cells, was required for optimal Treg-cell-mediated suppression in vitro and in vivo. Whether this mechanism works in concert with other previously described Treg-cell-associated mechanisms or not is currently unclear.
Of the many potential Let-7d targets in recipient Th1 cells, we identified Ptgs2 (Cox-2) as a credible candidate (Figures S6 and S7). Cox-2 is involved in Th1-cell-mediated responses and is responsible for lethal T-cell-mediated inflammation (Brewer et al., 2003; Iñiguez et al., 1999). Whether Treg-cell-derived Let-7d-containing exosomes directly target Cox-2 in Th cells, analogous to a “Cox-2 inhibitor,” is currently unclear. Supporting this pathway we observed reduced expression of Cox-2 and other Th1-cell-associated genes in suppressed T cells in vitro, concomitant with the acquisition of Let-7d from WT Treg cells. Furthermore, treatment of T cells with a COX-2 inhibitor reduced IFN-γ production by Th1 cells (Figure S6J). Given that Cox-2 inhibitors can prevent Th1-cell-mediated experimental autoimmunity (Muthian et al., 2006; Ni et al., 2007) and colitis (Paiotti et al., 2012; Zrieki et al., 2010), targeting of Cox-2 by Treg-cell-derived Let-7d may be a specific Treg-cell-mediated function to prevent lethal Th1-cell-mediated inflammation. In summary, this mechanism of Treg-cell-mediated suppression opens up the possibility that Treg cells package and deliver different proteins and RNA species, including miRNAs as we report here, to different cells at different times in a context-dependent manner.
Experimental Procedures
Animals
Female C57BL/6, Rag2–/–, CD45.1 Cd4CreDicerfl/fl R26eyfp (Dicer–/–), Rab27aashen/ashenb–/– (Rab27-DKO), miR155–/–, Il4gfpIfngyfpIl17aCre (Hirota et al., 2013), R26eFP635, Foxp3rfp, and Foxp3gfp 6- to 8-week-old (or as indicated) animals were bred and kept in the specific-pathogen-free facility at the Medical Research Council, NIMR. All animal experiments were performed according to institutional guidelines and the UK Home Office regulations. A minimum of five mice per group was used in each experiment, unless indicated.
Induction of Colitis and Systemic Inflammation
Rag2−/− mice were injected i.v. with 5 × 105 FACS-purified CD4+CD45RBhi T cells with or without 105 CD4+CD25+ Treg cells 2 weeks later, as previously described (Powrie et al., 1994).
In Vitro Cell Culture, Reagents, and Stimulation Assays
Primary cells were isolated from naive or diseased spleen, lymph nodes, or tissue, as indicated, by mechanical disruption. Full details of antibodies used can be found in Supplemental Experimental Procedures. For suppression assays, 104 Teff cells were labeled with cell trace violet (Invitrogen) as per manufacturer’s guidelines and stimulated with plate-bound anti-CD3 (1 μg/ml) and soluble CD28 (5 μg/ml) for 3 days in the presence or absence of Treg cells, at the indicated ratios.
RNA Extraction, RT-PCR, and Microarray
FACS-purified cells or isolated exosomes were stored in RLT lysis buffer at –80°C until RNA was extracted using RNeasy mini spin columns (QIAGEN). For RT-PCR, miScript RT or Quantitect RT was performed, according to manufacturer’s recommendations (QIAGEN). Real-time RT-PCR was performed on an ABI Prism 7900HT Sequence Detection System (Applied Biosystems) with relative quantities of mRNA and miRNA determined using SYBR Green PCR Master Mix (Applied Biosystems) and by the comparative threshold cycle method as described by Applied Biosystems for the ABI Prism 7700/7900HT Sequence Detection Systems. mRNA levels were normalized to HPRT and miRNA levels were normalized to RNU6B and then expressed as a relative increase or decrease compared with levels in controls, or as indicated.
ELISA
IFN-γ was measured by ELISA using cytokine capture and biotinylated detection antibodies (R&D Systems). The concentration of IFN-γ was determined from a serial-fold diluted standard curve with OD read at 450 nm in an ELISA reader.
miRNA Mimic and Hairpin Inhibitor Transfection
Cells were transfected with 100 nM of FL-dsRNA (SiGlo, Thermo Scientific Dharmacon) or BloCKiT (Invitrogen), miR-155 mimics, Let-7d mimics or hairpin inhibitors, Let-7b mimics, Cel-miR-67 hairpin inhibitors, or Cel-miR-239b hairpin inhibitors (Thermo Scientific Dharmacon) or MOCK transfected using Nucelofection reagents according to manufacturer’s recommendations (Amaxa).
Exosome Isolation and Analysis
Throughout these studies, complete media with exosome-depleted FCS (following 100,000 × g centrifugation) was used (Thery et al., 2006). Exosomes were purified by a combination of ultracentrifugation and using Exoquick solution (SBI System Bioscience) (Taylor et al., 2011), as previously reported and tested (King et al., 2012).
Full details of exosome isolation and other methods can be found in the Supplemental Experimental Procedures.
Author Contributions
M.S.W. initiated the project, carried out most of the cell biological experiments, and contributed to the experimental design and data interpretation. I.S.O., V.S.P, S.M.C., S.C., and V.P. carried out experiments and helped with transfections. T.T. and M.C.S. provided animals, helped with interpretation of experiments, and provided input for the manuscript. M.S.W supervised the project and wrote the manuscript with input from the other authors and readers.
Acknowledgments
We are grateful to A. O’Garra, V. Tybulewicz, B. Stockinger, and G. Kassiotis for critically reading the manuscript; to D. Anastasiou for help with hypoxia experiments; to A. Sessay, H. Jani, and L. Bhaw-Rosun in the Systems biology department for help with microarray experiments; to R. Mahmood and R. Anand for help with histology; to S. Kelada, Chapel Hill, UNC, for help with microarray analysis; to A. Potocnik for R26eFP635 mice; to G. Preece, W. Turnbull, and Bhavik Patel for assistance with flow cytometry-related work; to T. Norton, K. Williams, A. Adekoya, and B2, Dunkin, and building C staff for animal husbandry; and to J. Perez-Lloret for assistance with experiments. This work was supported by the Medical Research Council (MRC File Reference number MC_UP_A253_1028).
Footnotes
This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/3.0/).
Accession Numbers
The microarray data are available in the Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/gds) under the accession number GSEXXXX.
Supplemental Information
miRNAs identified in Treg-cell-derived exosomes with fold change of miRNAs relative to miRNA expression in parental Treg cells. Related to Figure 1.
A list of selected mRNAs identified in Treg-cell-derived exosomes with fold change of mRNA expression relative to mRNA expression in parental Treg cells. Related to Figure 1.
mRNA and miRNA expression in Dicer–/– T cells after culturing with WT Treg cells, relative to Dicer–/– T cells cultured alone. Related to Figure 3.
References
- Alonso M.A., Millán J. The role of lipid rafts in signalling and membrane trafficking in T lymphocytes. J. Cell Sci. 2001;114:3957–3965. doi: 10.1242/jcs.114.22.3957. [DOI] [PubMed] [Google Scholar]
- Banerjee A., Schambach F., DeJong C.S., Hammond S.M., Reiner S.L. Micro-RNA-155 inhibits IFN-gamma signaling in CD4+ T cells. Eur. J. Immunol. 2010;40:225–231. doi: 10.1002/eji.200939381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolasco G., Tracey-White D.C., Tolmachova T., Thorley A.J., Tetley T.D., Seabra M.C., Hume A.N. Loss of Rab27 function results in abnormal lung epithelium structure in mice. Am. J. Physiol. Cell Physiol. 2011;300:C466–C476. doi: 10.1152/ajpcell.00446.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyman O., Kovar M., Rubinstein M.P., Surh C.D., Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006;311:1924–1927. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- Brewer J.A., Khor B., Vogt S.K., Muglia L.M., Fujiwara H., Haegele K.E., Sleckman B.P., Muglia L.J. T-cell glucocorticoid receptor is required to suppress COX-2-mediated lethal immune activation. Nat. Med. 2003;9:1318–1322. doi: 10.1038/nm895. [DOI] [PubMed] [Google Scholar]
- Bryniarski K., Ptak W., Jayakumar A., Püllmann K., Caplan M.J., Chairoungdua A., Lu J., Adams B.D., Sikora E., Nazimek K. Antigen-specific, antibody-coated, exosome-like nanovesicles deliver suppressor T-cell microRNA-150 to effector T cells to inhibit contact sensitivity. J. Allergy Clin. Immunol. 2013;132:170–181. doi: 10.1016/j.jaci.2013.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai C.K., Zhao G.Y., Tian L.Y., Liu L., Yan K., Ma Y.L., Ji Z.W., Li X.X., Han K., Gao J. miR-15a and miR-16-1 downregulate CCND1 and induce apoptosis and cell cycle arrest in osteosarcoma. Oncol. Rep. 2012;28:1764–1770. doi: 10.3892/or.2012.1995. [DOI] [PubMed] [Google Scholar]
- Choudhuri K., Llodrá J., Roth E.W., Tsai J., Gordo S., Wucherpfennig K.W., Kam L.C., Stokes D.L., Dustin M.L. Polarized release of T-cell-receptor-enriched microvesicles at the immunological synapse. Nature. 2014;507:118–123. doi: 10.1038/nature12951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L.B., Appleby M.W., Brunkow M.E., Wilkinson J.E., Ziegler S.F., Ramsdell F. Cellular and molecular characterization of the scurfy mouse mutant. J. Immunol. 1999;162:2546–2554. [PubMed] [Google Scholar]
- Dang E.V., Barbi J., Yang H.Y., Jinasena D., Yu H., Zheng Y., Bordman Z., Fu J., Kim Y., Yen H.R. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146:772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druz A., Chu C., Majors B., Santuary R., Betenbaugh M., Shiloach J. A novel microRNA mmu-miR-466h affects apoptosis regulation in mammalian cells. Biotechnol. Bioeng. 2011;108:1651–1661. doi: 10.1002/bit.23092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunoyer P., Schott G., Himber C., Meyer D., Takeda A., Carrington J.C., Voinnet O. Small RNA duplexes function as mobile silencing signals between plant cells. Science. 2010;328:912–916. doi: 10.1126/science.1185880. [DOI] [PubMed] [Google Scholar]
- Fire A., Xu S., Montgomery M.K., Kostas S.A., Driver S.E., Mello C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- Fontenot J.D., Rasmussen J.P., Gavin M.A., Rudensky A.Y. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat. Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- Fukuda M. Rab27 effectors, pleiotropic regulators in secretory pathways. Traffic. 2013;14:949–963. doi: 10.1111/tra.12083. [DOI] [PubMed] [Google Scholar]
- Garín M.I., Chu C.C., Golshayan D., Cernuda-Morollón E., Wait R., Lechler R.I. Galectin-1: a key effector of regulation mediated by CD4+CD25+ T cells. Blood. 2007;109:2058–2065. doi: 10.1182/blood-2006-04-016451. [DOI] [PubMed] [Google Scholar]
- Hirota K., Turner J.E., Villa M., Duarte J.H., Demengeot J., Steinmetz O.M., Stockinger B. Plasticity of Th17 cells in Peyer’s patches is responsible for the induction of T cell-dependent IgA responses. Nat. Immunol. 2013;14:372–379. doi: 10.1038/ni.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutvágner G., McLachlan J., Pasquinelli A.E., Bálint E., Tuschl T., Zamore P.D. A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science. 2001;293:834–838. doi: 10.1126/science.1062961. [DOI] [PubMed] [Google Scholar]
- Iñiguez M.A., Punzón C., Fresno M. Induction of cyclooxygenase-2 on activated T lymphocytes: regulation of T cell activation by cyclooxygenase-2 inhibitors. J. Immunol. 1999;163:111–119. [PubMed] [Google Scholar]
- Josefowicz S.Z., Lu L.F., Rudensky A.Y. Regulatory T cells: mechanisms of differentiation and function. Annu. Rev. Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelada S., Sethupathy P., Okoye I.S., Kistasis E., Czieso S., White S.D., Chou D., Martens C., Ricklefs S.M., Virtaneva K. miR-182 and miR-10a are key regulators of Treg specialisation and stability during Schistosome and Leishmania-associated inflammation. PLoS Pathog. 2013;9:e1003451. doi: 10.1371/journal.ppat.1003451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King H.W., Michael M.Z., Gleadle J.M. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer. 2012;12:421. doi: 10.1186/1471-2407-12-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlhaas S., Garden O.A., Scudamore C., Turner M., Okkenhaug K., Vigorito E. Cutting edge: the Foxp3 target miR-155 contributes to the development of regulatory T cells. J. Immunol. 2009;182:2578–2582. doi: 10.4049/jimmunol.0803162. [DOI] [PubMed] [Google Scholar]
- Kosaka N., Iguchi H., Yoshioka Y., Takeshita F., Matsuki Y., Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J. Biol. Chem. 2010;285:17442–17452. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kue C.S., Lim H.X., Jung M.Y., Hong H.J., Cho D., Kim T.S. C6-ceramide in combination with transforming growth factor-β enhances Treg cell differentiation and stable FoxP3 expression in vitro and in vivo. Immunobiology. 2013;218:952–959. doi: 10.1016/j.imbio.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Liston A., Lu L.F., O’Carroll D., Tarakhovsky A., Rudensky A.Y. Dicer-dependent microRNA pathway safeguards regulatory T cell function. J. Exp. Med. 2008;205:1993–2004. doi: 10.1084/jem.20081062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda K.C., Huynh T., Tay Y., Ang Y.S., Tam W.L., Thomson A.M., Lim B., Rigoutsos I. A pattern-based method for the identification of MicroRNA binding sites and their corresponding heteroduplexes. Cell. 2006;126:1203–1217. doi: 10.1016/j.cell.2006.07.031. [DOI] [PubMed] [Google Scholar]
- Mittelbrunn M., Gutiérrez-Vázquez C., Villarroya-Beltri C., González S., Sánchez-Cabo F., González M.A., Bernad A., Sánchez-Madrid F. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun. 2011;2:282. doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montecalvo A., Larregina A.T., Shufesky W.J., Stolz D.B., Sullivan M.L., Karlsson J.M., Baty C.J., Gibson G.A., Erdos G., Wang Z. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood. 2012;119:756–766. doi: 10.1182/blood-2011-02-338004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muljo S.A., Ansel K.M., Kanellopoulou C., Livingston D.M., Rao A., Rajewsky K. Aberrant T cell differentiation in the absence of Dicer. J. Exp. Med. 2005;202:261–269. doi: 10.1084/jem.20050678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthian G., Raikwar H.P., Johnson C., Rajasingh J., Kalgutkar A., Marnett L.J., Bright J.J. COX-2 inhibitors modulate IL-12 signaling through JAK-STAT pathway leading to Th1 response in experimental allergic encephalomyelitis. J. Clin. Immunol. 2006;26:73–85. doi: 10.1007/s10875-006-8787-y. [DOI] [PubMed] [Google Scholar]
- Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- Ni J., Shu Y.Y., Zhu Y.N., Fu Y.F., Tang W., Zhong X.G., Wang H., Yang Y.F., Ren J., Wang M.W., Zuo J.P. COX-2 inhibitors ameliorate experimental autoimmune encephalomyelitis through modulating IFN-gamma and IL-10 production by inhibiting T-bet expression. J. Neuroimmunol. 2007;186:94–103. doi: 10.1016/j.jneuroim.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Nolte-’t Hoen E.N., Buermans H.P., Waasdorp M., Stoorvogel W., Wauben M.H., ’t Hoen P.A. Deep sequencing of RNA from immune cell-derived vesicles uncovers the selective incorporation of small non-coding RNA biotypes with potential regulatory functions. Nucleic Acids Res. 2012;40:9272–9285. doi: 10.1093/nar/gks658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolting J., Daniel C., Reuter S., Stuelten C., Li P., Sucov H., Kim B.G., Letterio J.J., Kretschmer K., Kim H.J., von Boehmer H. Retinoic acid can enhance conversion of naive into regulatory T cells independently of secreted cytokines. J. Exp. Med. 2009;206:2131–2139. doi: 10.1084/jem.20090639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski M., Carmo N.B., Krumeich S., Fanget I., Raposo G., Savina A., Moita C.F., Schauer K., Hume A.N., Freitas R.P. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat. Cell Biol. 2010;12:19–30. doi: 10.1038/ncb2000. 1–13. [DOI] [PubMed] [Google Scholar]
- Paiotti A.P., Ribeiro D.A., Silva R.M., Marchi P., Oshima C.T., Neto R.A., Miszputen S.J., Franco M. Effect of COX-2 inhibitor lumiracoxib and the TNF-α antagonist etanercept on TNBS-induced colitis in Wistar rats. J. Mol. Histol. 2012;43:307–317. doi: 10.1007/s10735-012-9400-8. [DOI] [PubMed] [Google Scholar]
- Powrie F., Leach M.W., Mauze S., Menon S., Caddle L.B., Coffman R.L. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1:553–562. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- Raposo G., Nijman H.W., Stoorvogel W., Liejendekker R., Harding C.V., Melief C.J., Geuze H.J. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996;183:1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101:455–458. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- Sarkies P., Miska E.A. Molecular biology. Is there social RNA? Science. 2013;341:467–468. doi: 10.1126/science.1243175. [DOI] [PubMed] [Google Scholar]
- Savina A., Furlán M., Vidal M., Colombo M.I. Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J. Biol. Chem. 2003;278:20083–20090. doi: 10.1074/jbc.M301642200. [DOI] [PubMed] [Google Scholar]
- Shevach E.M. Regulatory T cells in autoimmmunity. Annu. Rev. Immunol. 2000;18:423–449. doi: 10.1146/annurev.immunol.18.1.423. [DOI] [PubMed] [Google Scholar]
- Shevach E.M. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- Singh R.K., Mizuno K., Wasmeier C., Wavre-Shapton S.T., Recchi C., Catz S.D., Futter C., Tolmachova T., Hume A.N., Seabra M.C. Distinct and opposing roles for Rab27a/Mlph/MyoVa and Rab27b/Munc13-4 in mast cell secretion. FEBS J. 2013;280:892–903. doi: 10.1111/febs.12081. [DOI] [PubMed] [Google Scholar]
- Smyth L.A., Ratnasothy K., Tsang J.Y., Boardman D., Warley A., Lechler R., Lombardi G. CD73 expression on extracellular vesicles derived from CD4+ CD25+ Foxp3+ T cells contributes to their regulatory function. Eur. J. Immunol. 2013;43:2430–2440. doi: 10.1002/eji.201242909. [DOI] [PubMed] [Google Scholar]
- Sowinski S., Jolly C., Berninghausen O., Purbhoo M.A., Chauveau A., Köhler K., Oddos S., Eissmann P., Brodsky F.M., Hopkins C. Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nat. Cell Biol. 2008;10:211–219. doi: 10.1038/ncb1682. [DOI] [PubMed] [Google Scholar]
- Taylor D.D., Zacharias W., Gercel-Taylor C. Exosome isolation for proteomic analyses and RNA profiling. Methods Mol. Biol. 2011;728:235–246. doi: 10.1007/978-1-61779-068-3_15. [DOI] [PubMed] [Google Scholar]
- Théry C. Exosomes: secreted vesicles and intercellular communications. F1000 Biol Rep. 2011;3:15. doi: 10.3410/B3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théry C., Duban L., Segura E., Véron P., Lantz O., Amigorena S. Indirect activation of naïve CD4+ T cells by dendritic cell-derived exosomes. Nat. Immunol. 2002;3:1156–1162. doi: 10.1038/ni854. [DOI] [PubMed] [Google Scholar]
- Thery C., Amigorena S., Raposo G., Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006;Chapter 3:222. doi: 10.1002/0471143030.cb0322s30. [DOI] [PubMed] [Google Scholar]
- Trajkovic K., Hsu C., Chiantia S., Rajendran L., Wenzel D., Wieland F., Schwille P., Brügger B., Simons M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- Valadi H., Ekström K., Bossios A., Sjöstrand M., Lee J.J., Lötvall J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- Voinnet O., Baulcombe D.C. Systemic signalling in gene silencing. Nature. 1997;389:553. doi: 10.1038/39215. [DOI] [PubMed] [Google Scholar]
- Wang G.J., Liu Y., Qin A., Shah S.V., Deng Z.B., Xiang X., Cheng Z., Liu C., Wang J., Zhang L. Thymus exosomes-like particles induce regulatory T cells. J. Immunol. 2008;181:5242–5248. doi: 10.4049/jimmunol.181.8.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X., Tromberg B.J., Cahalan M.D. Mapping the sensitivity of T cells with an optical trap: polarity and minimal number of receptors for Ca(2+) signaling. Proc. Natl. Acad. Sci. USA. 1999;96:8471–8476. doi: 10.1073/pnas.96.15.8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Yu J., Yin J., Xiang Q., Tang H., Lei X. MiR-195 regulates cell apoptosis of human hepatocellular carcinoma cells by targeting LATS2. Pharmazie. 2012;67:645–651. [PubMed] [Google Scholar]
- Zaiss D.M., van Loosdregt J., Gorlani A., Bekker C.P., Gröne A., Sibilia M., van Bergen en Henegouwen P.M., Roovers R.C., Coffer P.J., Sijts A.J. Amphiregulin enhances regulatory T cell-suppressive function via the epidermal growth factor receptor. Immunity. 2013;38:275–284. doi: 10.1016/j.immuni.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Jeker L.T., Fife B.T., Zhu S., Anderson M.S., McManus M.T., Bluestone J.A. Selective miRNA disruption in T reg cells leads to uncontrolled autoimmunity. J. Exp. Med. 2008;205:1983–1991. doi: 10.1084/jem.20080707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitvogel L., Regnault A., Lozier A., Wolfers J., Flament C., Tenza D., Ricciardi-Castagnoli P., Raposo G., Amigorena S. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat. Med. 1998;4:594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- Zrieki A., Farinotti R., Buyse M. Cyclooxygenase-2 inhibitors prevent trinitrobenzene sulfonic acid-induced P-glycoprotein up-regulation in vitro and in vivo. Eur. J. Pharmacol. 2010;636:189–197. doi: 10.1016/j.ejphar.2010.03.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
miRNAs identified in Treg-cell-derived exosomes with fold change of miRNAs relative to miRNA expression in parental Treg cells. Related to Figure 1.
A list of selected mRNAs identified in Treg-cell-derived exosomes with fold change of mRNA expression relative to mRNA expression in parental Treg cells. Related to Figure 1.
mRNA and miRNA expression in Dicer–/– T cells after culturing with WT Treg cells, relative to Dicer–/– T cells cultured alone. Related to Figure 3.