Abstract
Investigators have constructed dsDNA molecules with several different base modifications and have characterized their bending and twisting flexibilities using atomic force microscopy, DNA ring closure, and single-molecule force spectroscopy with optical tweezers. The three methods provide persistence length measurements that agree semiquantitatively, and they show that the persistence length is surprisingly similar for all of the modified DNAs. The circular dichroism spectra of modified DNAs differ substantially. Simple explanations based on base stacking strength, polymer charge, or groove occupancy by functional groups cannot explain the results, which will guide further high-resolution theory and experiments.
Real double-stranded DNA molecules differ from the idealized zero-Kelvin A, B, and Z forms. They can adopt deformed average conformations, as in bent A-tract DNA or protein-DNA complexes. The path of the DNA helix axis also varies due to thermal energy, so at very long lengths DNA behaves as a random coil. The term “long lengths” is relative to the persistence length P of the wormlike chain model. P is the average offset of the end of a chain along its initial direction, or alternatively the length over which the unit vectors and tangent to the helix axis lose colinearity according to
where d12 is the contour length from point 1 to point 2, as in Fig. 1. P can be measured by hydrodynamics (1), atomic force microscopy (AFM) (2), DNA ring closure (3) or protein-DNA looping (4), tethered particle microscopy (5), or single-molecule optical tweezers experiments (6). The long-range loss of memory of DNA direction grows out of local variations in the helix axis direction specified by roll, tilt, and twist angles that parameterize changes in the helix axis direction. For harmonic bending potentials, the bending persistence length is related to roll and tilt according to
where ℓ = 3.4 Å, so for P ∼ 50 nm (147 bp) the average standard deviations in the roll and tilt angles σroll and σtilt are ∼4.7°, although in real DNA, roll varies more than tilt. Similar relationships hold for twist flexibility (7).
Figure 1.
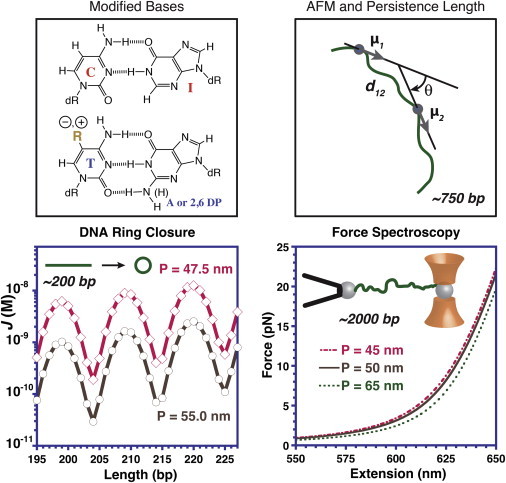
The base modifications studied by Peters et al. (13,14) affect both Watson-Crick hydrogen bonding and groove occupancy. They used AFM, DNA ring closure, single-molecule force spectroscopy, and circular dichroism spectroscopy (not shown) to characterize the resulting changes in bending and twisting flexibility. DNA molecules are not shown to scale. To see this figure in color, go online.
DNA flexibility can be studied at contour length scales from Ångstroms to microns. Flexibility at the atomic scale accessed by nuclear magnetic resonance, x-ray crystallography, cryo-electron microscopy, and molecular dynamics simulations (8) refers to many aspects of conformational variability. One active thread of research at this scale concerns interconversion among helical forms, base flipping, DNA kinking, changes in backbone torsion angles, and the sequence dependence of all of these local properties. Local fluctuations in the basepair roll, tilt, and twist angles do seem to predict the correct long-range behavior (9). A second thread asks whether the wormlike chain model holds at DNA lengths shorter than P (2,10); the active controversy concerning enhanced bendability at short lengths has recently been reviewed by Vologodskii and Frank-Kamenetskii (11). A third thread asks whether we can understand the underlying biophysical causes of long-range DNA flexibility. These presumably include base stacking, electrostatic repulsion along the backbone, changes in the counterion atmosphere (12), occupancy of the major and minor grooves by functional groups, conformational entropy, the strength of Watson-Crick hydrogen bonding, and water structure. Helical polymorphisms and the junctions between polymorphs presumably affect the sequence dependence of the persistence length.
Peters et al. (13,14) have attempted to understand bending and twisting flexibility by characterizing a variety of modified nucleic acids using DNA ring closure, AFM, and optical tweezer methods, sketched in Fig. 1. In previous work (13), they used ring closure to show that major groove substituents that alter the charge on the polymer do not have substantial effects on the bending persistence length, and that the effects were not correlated in an obvious way to the stacking propensity of the modified bases. The work described in this issue of the Biophysical Journal (14) uses all three methods to demonstrate that DNA with 2-amino-adenosine (a.k.a., 2,6-diaminopurine) substituted for adenosine has an increased persistence length, whereas inosine substitution for guanosine reduces the persistence length, as would be expected if groove occupancy (or the number of Watson-Crick hydrogen bonds) affects flexibility. However, the authors did one experiment too many—when they measured the effects of the earlier major groove substituents (13) using AFM, the correlation with groove occupancy disappeared. This could be because changes in helical geometry, as evidenced by the circular dichroism spectroscopy also reported in the article, alter the grooves sufficiently to prevent a straightforward connection to flexibility.
The magnitude of the effect of base modifications on P is the largest for the optical tweezers and the smallest for DNA ring closure, showing that no more than one of the experiments is perfect. The Supporting Material for both articles (13,14) offers valuable resources for the careful evaluation of experimental results and possible sources of error within and between experiments. For example, the DNA lengths and the ionic conditions required by the different methods differ. Ring closure results depend critically on the purity of the DNA and appropriate ligation conditions. Analysis of AFM results averaged several different statistical measures of decaying angular correlations and end-to-end distance, which did not individually always agree. In force spectroscopy there are variations in the bead attachment for each molecule, errors in the stretch modulus can affect the measured persistence length, force can induce DNA melting, and very few molecules can be observed. Rare kinking events proposed to explain enhanced bendability should affect the cyclization experiment most markedly; no evidence for enhanced flexibility was seen. Finally, Peters et al. (14) have observed that DNA twist and twisting flexibility seem to be more sensitive than the persistence length to base modifications.
Taken as a whole, this extremely thorough series of experiments shows that we still do not understand the fundamental origins of the remarkable stiffness of double-stranded DNA. There may be compensating effects that make the dissection difficult. For example, changing the charge on the polymer may induce a corresponding adjustment in the counterion condensation atmosphere, leading to a relatively constant residual charge. Groove substituents that enhance basepair stability could enhance bendability for steric reasons. Stacking thermodynamics may not change very much for the very small bend angles at any individual basepair. Locally stiff regions may introduce nearby junctions that are flexible.
The stiffness of DNA relative to other biopolymers inspired the development of DNA nanotechnology (although that field has adopted bridged synthetic constructs that are even more rigid). Further research on the biophysics, and specifically the long-range mechanical properties of DNA, will be essential as we build better models of DNA in the cell, which has evolved many proteins that act to increase apparent flexibility. The various aspects of DNA flexibility influence the protein-DNA complexes that mediate DNA’s informational role, the induction of and responses to supercoiling used for long-range communication among sites (15), and chromosome structure and genome organization.
References
- 1.Gray H.B., Jr., Hearst J.E. Flexibility of native DNA from the sedimentation behavior as a function of molecular weight and temperature. J. Mol. Biol. 1968;35:111–129. doi: 10.1016/s0022-2836(68)80041-5. [DOI] [PubMed] [Google Scholar]
- 2.Wiggins P.A., van der Heijden T., Nelson P.C. High flexibility of DNA on short length scales probed by atomic force microscopy. Nat. Nanotechnol. 2006;1:137–141. doi: 10.1038/nnano.2006.63. [DOI] [PubMed] [Google Scholar]
- 3.Shore D., Langowski J., Baldwin R.L. DNA flexibility studied by covalent closure of short fragments into circles. Proc. Natl. Acad. Sci. USA. 1981;78:4833–4837. doi: 10.1073/pnas.78.8.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gowetski D.B., Kodis E.J., Kahn J.D. Rationally designed coiled-coil DNA looping peptides control DNA topology. Nucleic Acids Res. 2013;41:8253–8265. doi: 10.1093/nar/gkt553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson S., Lindén M., Phillips R. Sequence dependence of transcription factor-mediated DNA looping. Nucleic Acids Res. 2012;40:7728–7738. doi: 10.1093/nar/gks473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bustamante C., Bryant Z., Smith S.B. Ten years of tension: single-molecule DNA mechanics. Nature. 2003;421:423–427. doi: 10.1038/nature01405. [DOI] [PubMed] [Google Scholar]
- 7.Bloomfield V.A., Crothers D.M., Tinoco I., Jr. University Science Books; Sausalito, CA: 2000. Nucleic Acids: Structures, Properties, and Functions. [Google Scholar]
- 8.Lavery R., Zakrzewska K., Sponer J. A systematic molecular dynamics study of nearest-neighbor effects on base pair and base pair step conformations and fluctuations in B-DNA. Nucleic Acids Res. 2010;38:299–313. doi: 10.1093/nar/gkp834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsumoto A., Olson W.K. Sequence-dependent motions of DNA: a normal mode analysis at the base-pair level. Biophys. J. 2002;83:22–41. doi: 10.1016/S0006-3495(02)75147-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schöpflin R., Brutzer H., Wedemann G. Probing the elasticity of DNA on short length scales by modeling supercoiling under tension. Biophys. J. 2012;103:323–330. doi: 10.1016/j.bpj.2012.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vologodskii A., Frank-Kamenetskii M.D. Strong bending of the DNA double helix. Nucleic Acids Res. 2013;41:6785–6792. doi: 10.1093/nar/gkt396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manning G.S. Poisson's ratio for a polyelectrolyte. Soft Matter. 2012;8:9334–9337. [Google Scholar]
- 13.Peters J.P., Yelgaonkar S.P., James Maher L., 3rd Mechanical properties of DNA-like polymers. Nucleic Acids Res. 2013;41:10593–10604. doi: 10.1093/nar/gkt808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peters J.P., Mogil L.S., Maher L.J., III Mechanical properties of base-modified DNA are not strictly determined by base stacking or electrostatic interactions. Biophys. J. 2014;107:448–459. doi: 10.1016/j.bpj.2014.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Travers A. Dynamic DNA underpins chromosome dynamics. Biophys. J. 2013;105:2235–2237. doi: 10.1016/j.bpj.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]


